Key Points
Question
Is there any difference in the characteristics of nonculprit plaques in patients with acute coronary syndromes caused by plaque erosion vs plaque rupture?
Findings
In this cohort study, patients with culprit plaque erosion had a smaller number of nonculprit plaques and a lower prevalence of rupture, macrophage accumulation, microvessels, and spotty calcium in the nonculprit lesions.
Meaning
Patients with culprit plaque erosion had lower levels of pancoronary vulnerability than those with culprit plaque rupture.
Abstract
Importance
Patients with culprit plaque rupture are known to have pancoronary plaque vulnerability. However, the characteristics of nonculprit plaques in patients with acute coronary syndromes caused by plaque erosion are unknown.
Objective
To investigate the nonculprit plaque phenotype in patients with acute coronary syndrome according to culprit plaque pathology (erosion vs rupture) by 3-vessel optical coherence tomography imaging.
Design, Setting, and Participants
In this observational cohort study, between August 2010 and May 2014, 82 patients with acute coronary syndrome who underwent preintervention optical coherence tomography imaging of all 3 major epicardial coronary arteries were enrolled at the Massachusetts General Hospital Optical Coherence Tomography Registry database. Analysis of the data was conducted between November 2016 and July 2017. Patients were classified into 2 groups based on the culprit lesion pathology: 17 patients with culprit plaque erosion and 34 patients with culprit plaque rupture. Thirty-one patients with the absence of culprit rupture or erosion were excluded from further analysis.
Exposures
Preintervention 3-vessel optical coherence tomography imaging.
Main Outcomes and Measures
Plaque characteristics at the culprit and nonculprit lesions evaluated by optical coherence tomography.
Results
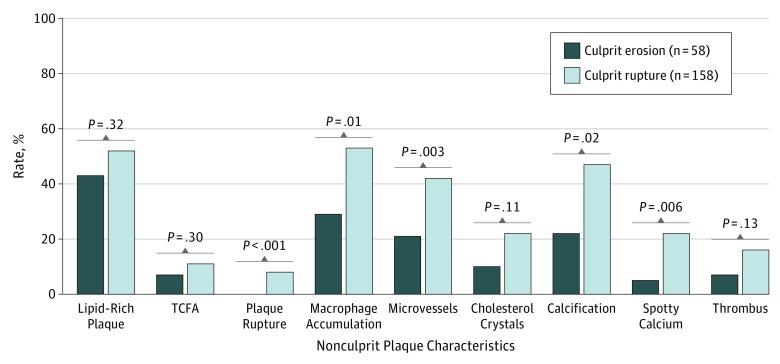
In 51 patients (37 men; mean age, 58.7 years), the characteristics of 51 culprit plaques and 216 nonculprit plaques were analyzed. In patients with culprit erosion, the mean (SD) number of nonculprit plaques per patient was smaller (3.4 [1.9] in erosion vs 4.7 [2.1] in rupture, P = .05). Patient-based analysis showed that none of 17 patients with culprit plaque erosion had nonculprit plaque rupture, whereas 26% of the patients (9 of 34) with culprit plaque rupture had nonculprit plaque rupture (P = .02). Plaque-based analysis showed that, compared with the culprit rupture group (n = 158), the culprit erosion group (n = 58) had lower prevalence of plaque rupture (0% vs 8%; P < .001), macrophage accumulation (29% vs 53%; P = .01), microvessels (21% vs 42%; P = .003), and spotty calcium (5% vs 22%; P = .006) in the nonculprit lesions. The prevalence of lipid-rich plaque, thin-cap fibroatheroma, and thrombus did not differ between the groups.
Conclusions and Relevance
Compared with those with culprit plaque rupture, patients with acute coronary syndrome caused by culprit plaque erosion had a smaller number of nonculprit plaques and the lower levels of panvascular instability, affirming that distinct pathophysiologic mechanisms operate in plaque erosion and plaque rupture.
This cohort study investigates the nonculprit plaque phenotype in patients with acute coronary syndrome according to culprit plaque pathology (erosion vs rupture) by 3-vessel optical coherence tomography imaging.
Introduction
For decades, pathology reports have shown that there are 3 major underlying mechanisms for sudden cardiac death: plaque rupture, plaque erosion, and calcified nodule. Several groups have reported in vivo diagnoses of these underlying pathologies in patients with acute coronary syndrome (ACS) using optical coherence tomography (OCT), a high-resolution intracoronary imaging modality. Previous OCT studies have also demonstrated that patients with culprit plaque rupture have greater pancoronary vulnerability than those without rupture. Compared with plaque rupture, plaque erosion is associated with a lower prevalence of vulnerable plaque features at the culprit lesion. However, to our knowledge, the characteristics of nonculprit plaques in patients with plaque erosion have not been studied. In this study, we aimed to investigate the differences in nonculprit plaque phenotype according to the culprit plaque pathology (erosion vs rupture) by 3-vessel OCT imaging.
Methods
Study Population
Between August 2010 and May 2014, 162 patients with ACS who underwent preintervention OCT imaging of all 3 major epicardial coronary arteries were identified in the Massachusetts General Hospital OCT Registry, which is an international, multicenter registry of patients who have undergone intracoronary OCT involving 20 sites across 6 countries. Patients with in-stent restenosis (n = 18), imaging after predilatation (n = 24), poor image quality (n = 12), short pullback (n = 5), and incomplete demographic, clinical, or imaging data (n = 21) were excluded. The analysis included 82 patients. Diagnosis of ACS included ST-segment–elevation myocardial infarction and non–ST-segment elevation ACS, as previously described. The culprit lesion was identified based on angiographic findings, electrocardiogram changes, and/or left ventricular wall motion abnormalities. In patients with multiple stenoses, the lesion with the most severe stenosis or with evidence of recent plaque disruption, including filling defect suggestive of thrombus on angiogram, was determined as the culprit lesion. The Massachusetts General Hospital OCT Registry was approved by the institutional review board at each participating site, and all patients provided written informed consent before enrollment.
Coronary Angiography Analysis
Quantitative coronary angiography analysis was performed using Cardiovascular Angiography Analysis System, version 5.10.1 software (Pie Medical Imaging BV). The minimal lumen diameter, reference vessel diameter, diameter stenosis, and length of the culprit and nonculprit lesions were measured. The severity and extent of coronary stenosis was assessed using the Gensini scoring system.
Optical Coherence Tomography Image Acquisition and Analysis
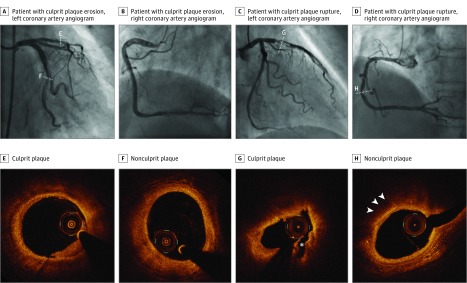
Optical coherence tomography examination was performed using either a frequency-domain (C7-XR, OCT Intravascular Imaging System; St Jude Medical) or time-domain (M2/M3 Cardiology Imaging Systems; LightLab Imaging Inc) OCT system, as previously reported. All OCT images were submitted to the Cardiology Laboratory for Integrative Physiology and Imaging at Massachusetts General Hospital and analyzed by 2 independent investigators who were blinded to clinical, angiographic, and laboratory data, using an offline review workstation (St Jude Medical). Any discordance was resolved by consensus with a third reviewer. All plaques were identified by OCT as segments with luminal narrowing and a loss of the normal 3-layered structure of the vessel wall. A distance of at least 5 mm on the longitudinal view was necessary to consider 2 plaques separated. Cross-sectional OCT images were analyzed at 1-mm intervals. Proximal and distal references were identified as the sites with the largest lumen area proximal and distal to the stenosis, but within the same segment, and mean reference lumen area was calculated. Minimal lumen area was defined as the smallest lumen area within the length of the plaque. Percent area stenosis was calculated as ([Mean Reference Lumen Area − Minimal Lumen Area] / Mean Reference Lumen Area) × 100. Lipid was defined as a signal-poor region with a poorly defined or diffuse border, and the degree of lipid arc and the overlying fibrous cap thickness (FCT) was measured in lipid plaques. Lipid length was obtained on the longitudinal view, and lipid index was calculated as the product of mean lipid arc and lipid length. Lipid-rich plaque was defined as a plaque with a maximal lipid arc greater than 90°. Thin-cap fibroatheroma (TCFA) was defined as a plaque with maximal lipid arc greater than 90° and thinnest FCT 65 μm or less. Macrophage accumulation was defined as the presence of highly backscattering focal regions within the fibrous cap. Microvessel was defined as the presence of signal-poor structures with vesicular or tubular shapes. Cholesterol crystals were identified as thin and linear regions of high signal intensity with high backscattering within a plaque. Calcification was defined as a signal-poor or heterogeneous region with a sharply delineated border. Spotty calcium was defined as the presence of lesions containing calcification arc less than 90° and extending in length less than 4 mm. Thrombus was defined as an irregular mass with minimum diameter at least 250 μm adherent to the vessel wall or floating within the lumen. Plaque rupture was defined as fibrous cap discontinuity with cavity formation. Plaque erosion was identified by the presence of attached thrombus overlying an intact and visualized plaque, luminal surface irregularity at the culprit lesion in the absence of thrombus, or attenuation of underlying plaque by thrombus without superficial lipid or calcification immediately proximal or distal to the site of thrombus. In patients with multiple stenoses, the lesion with the most severe stenosis and/or with evidence of recent plaque disruption, including thrombus, was determined as the culprit plaque. All cases were classified based on the features of the culprit lesions into 3 groups: rupture, erosion, and others, which included calcified nodule and tight stenosis in the absence of any evidence of plaque rupture or erosion. Plaque characteristics at the culprit and nonculprit lesions in all 3 major epicardial coronary arteries were compared between patients with culprit rupture and those with culprit erosion. Those that did not meet the criteria of culprit rupture or erosion were excluded from the analysis. Representative cases of ACS caused by plaque erosion and plaque rupture are shown in Figure 1.
Figure 1. Representative Cases of Culprit Plaque Erosion and Culprit Plaque Rupture.
A and B, Angiogram of a patient with culprit plaque erosion. C and D, Angiogram of a patient with culprit plaque rupture. E, Culprit plaque shows attachment of white thrombus overlying an intact plaque. F, Nonculprit plaque shows a fibrous plaque. G, Culprit plaque shows fibrous cap discontinuity with cavity formation (asterisk). H, Nonculprit plaque shows a lipid-rich plaque with macrophage accumulation (arrowheads).
Statistical Analysis
All analyses were performed using SPSS Statistics, version 21.0 software (IBM Corp). Categorical data were expressed as absolute frequencies and percentages and compared using the χ2 test or Fisher exact test, as appropriate. Continuous variables were expressed as mean (SD) for normally distributed variables and as median (interquartile range) for nonnormally distributed variables and compared using the t test, Mann-Whitney test, or 1-way analysis of variance as appropriate. Intraobserver and interobserver differences were quantified using the κ coefficient of agreement for the plaque classification. Comparisons of plaque characteristics among different groups were carried out using generalized estimating equations to take into account potential cluster effects of multiple plaques in a single patient. A 2-sided P value of less than .05 was considered statistically significant.
Results
Patient Characteristics
A total of 82 patients with ACS who underwent preintervention 3-vessel OCT imaging were enrolled. Among them, 17 patients (21%) had culprit plaque erosion, and 34 patients (41%) had culprit plaque rupture. Thirty-one patients, including 2 patients with calcified nodule and 29 patients with tight stenosis in the absence of culprit rupture or erosion, were classified as others and excluded from further analysis. Among 51 patients included in the analysis, 24 were also included in our previous study, and 27 new patients were added. Patient characteristics are summarized in Table 1. Although the difference did not reach the statistical significance, ST-segment elevation myocardial infarction tended to be more frequent in patients with culprit rupture. No significant differences were found in other baseline characteristics between patients with culprit erosion and those with culprit rupture.
Table 1. Patient Characteristics.
| Characteristic | No. (%) | P Value | |
|---|---|---|---|
| Patients With Culprit Erosion (n = 17) | Patients With Culprit Rupture (n = 34) | ||
| Male | 12 (71) | 25 (74) | >.99 |
| Age, mean (SD), y | 56.4 (11.3) | 59.8 (12.5) | .35 |
| Clinical presentation | |||
| STEMI | 6 (35) | 16 (47) | .42 |
| NSTE-ACS | 11 (65) | 18 (53) | |
| Diabetes mellitus | 7 (41) | 10 (29) | .40 |
| Hypertension | 10 (59) | 21 (62) | .84 |
| Dyslipidemia | 15 (88) | 27 (79) | .70 |
| Current smoking | 8 (47) | 12 (35) | .42 |
| Creatinine, mean (SD), mg/dL | 0.88 (0.14) | 0.84 (0.16) | .45 |
| LDL cholesterol, mean (SD), mg/dL | 99.1 (37.2) | 119.6 (41.5) | .11 |
| hs-CRP, median (IQR), mg/L | 2.0 (0.5-2.8) | 2.0 (1.0-5.0) | .28 |
| Medication | |||
| Aspirin | 7 (41) | 14 (41) | >.99 |
| Clopidogrel | 4 (24) | 8 (24) | >.99 |
| β-Blockers | 3 (18) | 7 (21) | >.99 |
| Statins | 6 (35) | 12 (35) | >.99 |
| ACE-I/ARBs | 3 (18) | 7 (21) | >.99 |
Abbreviations: ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; hs-CRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; NSTE-ACS, non–ST-segment elevation acute coronary syndrome; STEMI, ST-segment elevation myocardial infarction.
SI conversion factors: To convert C-reactive protein to nanomoles per liter, multiply by 9.524; creatinine to micromoles per liter, multiply by 88.4; LDL cholesterol to millimoles per liter, multiply by 0.0259.
Angiographic Findings
Angiographic findings are summarized in eTable 1 in the Supplement. Lesion distribution of both the culprit and nonculprit lesions was not significantly different between the groups. Patients with culprit erosion had less diameter stenosis in the nonculprit lesions than those with culprit rupture, despite similar degree of diameter stenosis in the culprit lesions. Gensini score tended to be lower in patients with culprit erosion.
Optical Coherence Tomography Findings
The mean (SD) total length of analyzed OCT pullbacks was 201 (44) mm (84 [20] mm in the right coronary artery, 66 [20] mm in the left anterior descending artery, and 50 [19] mm in the left circumflex artery). There was no difference in the length of the analyzed segments between patients with culprit plaque erosion and those with culprit plaque rupture (mean [SD], 191 [42] vs 206 [44] mm; P = .25). Among 51 patients studied, 58 nonculprit plaques were identified in 17 patients with culprit erosion, and 158 nonculprit plaques were detected in 34 patients with culprit rupture. With regard to the culprit plaques, patients with culprit erosion had a lower prevalence of TCFA (12% vs 68%; P < .001), smaller lipid burden, and thicker FCT in comparison with patients with culprit rupture (Table 2). The mean [SD] number of nonculprit plaques per patient was smaller in patients with culprit erosion than in those with culprit rupture (3.4 [1.9] vs 4.7 [2.1] plaques per patient P = .05). None of the patients with culprit erosion had nonculprit plaque rupture, whereas 9 patients with culprit rupture had nonculprit plaque rupture (prevalence of nonculprit rupture, 0% vs 26%; P = .02) The results of patient-based analysis of the nonculprit plaques are demonstrated in eTable 2 in the Supplement.
Table 2. Culprit Plaque Characteristics.
| Characteristic | Mean (SD) | P Value | |
|---|---|---|---|
| Patients With Culprit Erosion (n = 17) | Patients With Culprit Rupture (n = 34) | ||
| Lipid-rich plaque, No. (%) | 11 (65) | 29 (85) | .15 |
| Maximal lipid arc, degrees | 225.3 (65.9) | 274.5 (60.0) | .03 |
| Mean lipid arc, degrees | 169.6 (52.7) | 207.3 (52.8) | .05 |
| Lipid length, mm | 7.7 (2.9) | 9.4 (4.2) | .23 |
| Lipid index, degrees mm | 1273.5 (484.8) | 1994.4 (1096.1) | .04 |
| Thinnest FCT, μm | 143.8 (75.5) | 77.4 (71.4) | .01 |
| TCFA, No. (%) | 2 (12) | 23 (68) | <.001 |
| Minimal lumen area, mm2 | 1.41 (1.63) | 1.60 (1.18) | .62 |
| Reference lumen area, mm2 | 5.13 (1.91) | 7.53 (3.46) | .01 |
| Area stenosis, % | 74.7 (15.5) | 78.2 (11.8) | .37 |
Abbreviations: FCT, fibrous cap thickness; TCFA, thin-cap fibroatheroma.
The results of plaque-based analysis of the nonculprit plaques are shown in Figure 2 and Table 3. The culprit erosion group had a lower prevalence of plaque rupture, macrophage accumulation, microvessels, calcification, and spotty calcium in comparison with the culprit rupture group (Figure 2). The lipid arc, lipid index, FCT, and minimal lumen area did not differ significantly between the 2 groups (Table 3).
Figure 2. Comparison of Nonculprit Plaque Characteristics Between the Groups With Culprit Erosion vs Culprit Rupture.
Plaque-based analysis showed that the culprit erosion group had a lower prevalence of plaque rupture, macrophage accumulation, microvessels, calcification, and spotty calcium compared with the culprit rupture group. TCFA indicates thin-cap fibroatheroma.
Table 3. Plaque-Based Quantitative Analysis of the Nonculprit Plaques.
| Characteristic | Mean (SD) | P Value | ||||
|---|---|---|---|---|---|---|
| Culprit Erosion (n = 58) | Culprit Rupture | |||||
| Without Nonculprit Rupture (n = 145) | With Nonculprit Rupture (n = 13) | Culprit Erosion vs Culprit Rupture Without Nonculprit Rupture and Culprit Rupture With Nonculprit Rupture | Culprit Erosion vs Culprit Rupture With Nonculprit Rupture | Culprit Rupture Without Nonculprit Rupture vs Culprit Rupture With Nonculprit Rupture | ||
| Maximal lipid arc, degrees | 181.1 (53.7) | 182.4 (65.3) | 240.3 (51.5) | .43 | .001 | .001 |
| Mean lipid arc, degrees | 154.4 (48.5) | 141.9 (49.1) | 172.5 (33.4) | .31 | .14 | .008 |
| Lipid length, mm | 7.1 (5.5) | 6.5 (3.7) | 10.2 (5.1) | .88 | .07 | .004 |
| Lipid index, degrees mm | 1147.4 (968.9) | 942.0 (596.2) | 1733.2 (899.6) | .43 | .03 | .001 |
| Thinnest FCT, μm | 128.1 (72.7) | 123.0 (56.1) | 65.6 (12.6) | .46 | <.001 | .001 |
| Minimal lumen area, mm2 | 3.38 (1.85) | 3.71 (2.07) | 3.58 (1.68) | .44 | .70 | .73 |
| Reference lumen area, mm2 | 6.13 (2.30) | 7.76 (3.42) | 9.73 (2.92) | .002 | <.001 | .03 |
| Area stenosis, % | 46.4 (17.6) | 51.9 (17.0) | 62.1 (14.7) | .04 | <.001 | .008 |
Abbreviation: FCT, fibrous cap thickness.
The culprit rupture group was further divided into 2 subgroups: with nonculprit plaque rupture and without nonculprit plaque rupture. The subgroup with nonculprit plaque rupture showed more characteristics of vulnerability than those without nonculprit plaque rupture, ie, the prevalence of lipid-rich plaque, TCFA, and thrombus was higher (eFigure in the Supplement), and lipid burden was greater with thinner FCT (Table 3). Another subgroup analysis was performed in the culprit rupture group: patients with and without macrophage accumulation at the ruptured culprit plaque (eTable 3 in the Supplement). Patients with macrophage accumulation at the ruptured culprit plaque had a higher prevalence of lipid-rich plaque and TCFA in the culprit lesions and a similar trend in the prevalence of lipid-rich plaque and macrophage accumulation in the nonculprit lesions compared with those without macrophage accumulation.
Excellent intraobserver and interobserver agreement was observed in the identification of lipid-rich plaque (κ, 0.97 and 0.96, respectively), TCFA (κ, 0.97 and 0.93), plaque rupture (κ, 0.97 and 0.92), and plaque erosion (κ, 0.98 and 0.96).
Discussion
This study demonstrated that (1) patients with culprit plaque erosion had smaller number of nonculprit plaques; and (2) nonculprit plaques in patients with culprit erosion had less frequent plaque rupture, macrophage accumulation, microvessels, and spotty calcium than those in patients with culprit rupture.
Characteristics of Vulnerability in Nonculprit Plaques in Patients With Culprit Erosion
Several OCT studies have reported the differences in culprit plaque morphology and demonstrated that culprit plaques with rupture have greater lipid burden and a higher prevalence of vulnerable plaque features, such as thin fibrous cap, compared with those with erosion. Our results showed that OCT features of plaque vulnerability, such as macrophage accumulation and microvessels, were more frequently observed in the nonculprit lesions in patients with culprit plaque rupture. Pathology studies have shown that ruptured culprit lesions often harbor greater number of macrophages and microvessels, suggesting greater local inflammation. Patients with plaque rupture have elevated levels of systemic matrix metalloproteinase–9, mainly sourced from macrophages and foam cells, indicating active proinflammatory response and degradation of extracellular matrix leading to plaque instability. Furthermore, patients with culprit rupture frequently have multiple and/or nonculprit plaque ruptures. Taken together, these reports suggest more widespread inflammation and instability in patients with culprit plaque rupture vs erosion.
We previously reported a study that compared the nonculprit plaque characteristics of patients with ruptured culprit plaque and nonruptured culprit plaque. The latter group consisted of several different pathologies such as plaque erosion, calcified nodules, and others including coronary spasm, microvascular disease, and spontaneous coronary artery dissection. In contrast, this study focused on the nonculprit plaque characteristics and pancoronary vulnerability, particularly in patients with culprit plaque erosion. In the 2 major OCT consensus documents, both groups agreed the definition of an atherosclerotic plaque as a loss of the 3-layered structure. However, there has been no standardized definition of nonculprit plaque as assessed by OCT, eg, plaques with area stenosis greater than 50% as measured by OCT, plaques with diameter stenosis of 20% to 70% by quantitative coronary angiography analysis, plaques with diameter stenosis of 30% to 70% by visual estimation on angiogram, or any plaques. Angiographic diameter stenosis does not always reveal coronary atherosclerosis because atherosclerosis begins with remodeling and enlargement of the vessel. The strength of OCT imaging is its capability to detect plaques, even in the early phase of coronary atherosclerosis. Compared with culprit lesions, nonculprit lesions consist of plaques with a wide range of stenosis from mild to severe. Therefore, we did not incorporate the degree of area stenosis or diameter stenosis in the definition of nonculprit plaque in this study. Indeed, patients with culprit erosion showed less severe stenosis and less vulnerability in nonculprit plaques compared with those with culprit rupture as evidenced by the smaller number of nonculprit plaques and the lower prevalence of rupture, macrophage infiltration, microvessels, and spotty calcium. Although the difference in the prevalence of TCFA, cholesterol crystals, and thrombus in nonculprit plaques did not show statistical significance between the 2 groups, the prevalence was 2 to 3 times higher in the culprit rupture group compared with the culprit erosion group. Our results are in line with the previous reports that described the primary underlying mechanism of plaque erosion. In eroded plaques, detachment of endothelial cells and exposure of collagen initiate platelet activation and aggregation as well as recruitment of polymorphonuclear leucocytes. Recruited neutrophils can mediate the formation of neutrophil extracellular traps and amplification of thrombosis and local inflammation. Neutrophils accumulate abundantly in eroded culprit plaques and elevated levels of markers of neutrophil extracellular trap formation are associated with this plaque morphology. An OCT study demonstrated the association between the presence of plaque erosion and elevated levels of serum myeloperoxidase, a marker of neutrophil activation. These data imply that local endothelial damage rather than widespread coronary arterial inflammation initiates ACS owing to plaque erosion.
Features of Vulnerability in Nonculprit Plaques in Patients With Culprit Rupture
In this study, we performed 2 subanalyses in the culprit rupture group. In the subanalysis regarding the presence of nonculprit rupture, the subgroup with nonculprit plaque rupture showed a higher prevalence of lipid-rich plaque, TCFA, and thrombus than those without nonculprit plaque rupture. These findings are consistent with previous studies demonstrating that the presence of nonculprit plaque rupture is associated with markers of greater plaque vulnerability. In the analysis regarding macrophage accumulation at the culprit lesion, patients with macrophage accumulation had a higher prevalence of lipid-rich plaque and TCFA in the culprit lesions and a similar trend in the prevalence of lipid-rich plaque and macrophage accumulation in the nonculprit lesions compared with those without macrophage accumulation. A 2017 OCT study reported that plaque rupture with macrophage accumulation was associated with elevated levels of high-sensitivity C-reactive protein and macrophage accumulation in both culprit and remote lesions compared with those without macrophage accumulation, indicating that plaque rupture can be caused by either predominant inflammatory or noninflammatory prosesses. The main difference between the referenced study and ours was that both culprit and nonculprit plaque characteristics were evaluated in our study, whereas only culprit plaque characteristics were analyzed in the referenced study.
Clinical Implications
The underlying pathogenic mechanism of ACS may affect the outcomes in patients with ACS. Compared with patients with ruptured culprit plaque, those with eroded culprit plaques may be associated with a higher incidence of non–ST-segment elevation ACS, lower frequency of no-reflow phenomenon after stenting, less myocardial damage, and fewer adverse cardiac events during follow-up. Our study demonstrated lower levels of panvascular vulnerability within the coronary trees in patients with eroded culprit plaque. Studies conducted within the last 5 years suggest the feasibility and safety of antithrombotic therapy without stenting in selected patients with culprit plaque erosion. These observations illustrate an opportunity for more tailored treatment in patients with plaque erosion, possibly focusing on pharmacological approaches such as protection of endothelium, suppression of platelet activation and degranulation, and inhibition of neutrophil activation and neutrophil extracellular trap formation. This proposition requires rigorous testing.
Limitations
First, this is a retrospective observational study from a registry database; therefore, selection bias may have influenced the results. Second, because 3-vessel OCT imaging depends on patients’ hemodynamics and anatomy, practical difficulty in patients with ACS resulted in a limited sample size. Third, this study used 2 different OCT systems (time-domain and frequent-domain OCT). However, the distribution of plaque morphology examined by each system did not differ significantly between the 2 groups. Fourth, the ability of OCT to detect lipid has been questioned. However, the histology validation study showed high sensitivity and specificity. We also reviewed adjacent image frames to minimize the chance of misinterpretation. Fifth, the OCT system cannot image endothelial cells despite its high resolution. Therefore, diagnosis of OCT-derived erosion rested primarily on the exclusion criterion of the absence of fibrous cap rupture. Finally, this study did not evaluate clinical outcomes.
Conclusions
In this study, compared with patients with culprit plaque rupture, patients with ACS and culprit plaque erosion had a smaller number of nonculprit plaques with the lower levels of panvascular instability, affirming that distinct pathophysiologic mechanisms operate in plaque erosion and plaque rupture. These observations support the notion that different treatment strategies may apply to these 2 types of patients.
eTable 1. Angiographic Findings
eTable 2. Patient-Based Analysis of the Non-Culprit Plaque Characteristics
eTable 3. Comparison of OCT Findings Between Patients With and Without Culprit Macrophage Accumulation in the Culprit Rupture Group
eFigure. Subgroup Comparison of Non-Culprit Plaque Characteristics Between The Three Groups: Culprit Erosion, Culprit Rupture Without Non-Culprit Rupture, and Culprit Rupture With Non-Culprit Rupture
References
- 1.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20(5):1262-1275. [DOI] [PubMed] [Google Scholar]
- 2.Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation. 2003;108(14):1664-1672. [DOI] [PubMed] [Google Scholar]
- 3.Tian J, Ren X, Vergallo R, et al. Distinct morphological features of ruptured culprit plaque for acute coronary events compared to those with silent rupture and thin-cap fibroatheroma: a combined optical coherence tomography and intravascular ultrasound study. J Am Coll Cardiol. 2014;63(21):2209-2216. [DOI] [PubMed] [Google Scholar]
- 4.Jia H, Abtahian F, Aguirre AD, et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol. 2013;62(19):1748-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vergallo R, Ren X, Yonetsu T, et al. Pancoronary plaque vulnerability in patients with acute coronary syndrome and ruptured culprit plaque: a 3-vessel optical coherence tomography study. Am Heart J. 2014;167(1):59-67. [DOI] [PubMed] [Google Scholar]
- 6.Niccoli G, Montone RA, Di Vito L, et al. Plaque rupture and intact fibrous cap assessed by optical coherence tomography portend different outcomes in patients with acute coronary syndrome. Eur Heart J. 2015;36(22):1377-1384. [DOI] [PubMed] [Google Scholar]
- 7.Saia F, Komukai K, Capodanno D, et al. ; OCTAVIA Investigators . Eroded versus ruptured plaques at the culprit site of STEMI: in vivo pathophysiological features and response to primary PCI. JACC Cardiovasc Imaging. 2015;8(5):566-575. [DOI] [PubMed] [Google Scholar]
- 8.Higuma T, Soeda T, Abe N, et al. A combined optical coherence tomography and intravascular ultrasound study on plaque rupture, plaque erosion, and calcified nodule in patients with ST-segment elevation myocardial infarction: incidence, morphologic characteristics, and outcomes after percutaneous coronary intervention. JACC Cardiovasc Interv. 2015;8(9):1166-1176. [DOI] [PubMed] [Google Scholar]
- 9.Serruys PW, Foley DP, de Feyter PJ. Quantitative Coronary Angiography in Clinical Practice. Dordrecht, the Netherlands: Kluwer Academic; 1994. [Google Scholar]
- 10.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51(3):606. [DOI] [PubMed] [Google Scholar]
- 11.Kato K, Yonetsu T, Kim SJ, et al. Nonculprit plaques in patients with acute coronary syndromes have more vulnerable features compared with those with non-acute coronary syndromes: a 3-vessel optical coherence tomography study. Circ Cardiovasc Imaging. 2012;5(4):433-440. [DOI] [PubMed] [Google Scholar]
- 12.Vergallo R, Uemura S, Soeda T, et al. Prevalence and predictors of multiple coronary plaque ruptures: in vivo 3-vessel optical coherence tomography imaging study. Arterioscler Thromb Vasc Biol. 2016;36(11):2229-2238. [DOI] [PubMed] [Google Scholar]
- 13.Tearney GJ, Regar E, Akasaka T, et al. ; International Working Group for Intravascular Optical Coherence Tomography (IWG-IVOCT) . Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation [published correction appears in J Am Coll Cardiol. 2012 May 1;59(18):1662]. J Am Coll Cardiol. 2012;59(12):1058-1072. [DOI] [PubMed] [Google Scholar]
- 14.Tearney GJ, Yabushita H, Houser SL, et al. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation. 2003;107(1):113-119. [DOI] [PubMed] [Google Scholar]
- 15.Kataoka Y, Puri R, Hammadah M, et al. Spotty calcification and plaque vulnerability in vivo: frequency-domain optical coherence tomography analysis. Cardiovasc Diagn Ther. 2014;4(6):460-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ong DS, Lee JS, Soeda T, et al. Coronary calcification and plaque vulnerability: an optical coherence tomographic study. Circ Cardiovasc Imaging. 2016;9(1):e003929. [DOI] [PubMed] [Google Scholar]
- 17.Jang IK, Tearney GJ, MacNeill B, et al. In vivo characterization of coronary atherosclerotic plaque by use of optical coherence tomography. Circulation. 2005;111(12):1551-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwon JE, Lee WS, Mintz GS, et al. Multimodality intravascular imaging assessment of plaque erosion versus plaque rupture in patients with acute coronary syndrome. Korean Circ J. 2016;46(4):499-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yonetsu T, Lee T, Murai T, et al. Plaque morphologies and the clinical prognosis of acute coronary syndrome caused by lesions with intact fibrous cap diagnosed by optical coherence tomography. Int J Cardiol. 2016;203:766-774. [DOI] [PubMed] [Google Scholar]
- 20.Ozaki Y, Okumura M, Ismail TF, et al. Coronary CT angiographic characteristics of culprit lesions in acute coronary syndromes not related to plaque rupture as defined by optical coherence tomography and angioscopy. Eur Heart J. 2011;32(22):2814-2823. [DOI] [PubMed] [Google Scholar]
- 21.Kolodgie FD, Narula J, Burke AP, et al. Localization of apoptotic macrophages at the site of plaque rupture in sudden coronary death. Am J Pathol. 2000;157(4):1259-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreno PR, Purushothaman KR, Fuster V, et al. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: implications for plaque vulnerability. Circulation. 2004;110(14):2032-2038. [DOI] [PubMed] [Google Scholar]
- 23.Niccoli G, Montone RA, Cataneo L, et al. Morphological-biohumoral correlations in acute coronary syndromes: pathogenetic implications. Int J Cardiol. 2014;171(3):463-466. [DOI] [PubMed] [Google Scholar]
- 24.Chandran S, Watkins J, Abdul-Aziz A, et al. Inflammatory differences in plaque erosion and rupture in patients with ST-segment elevation myocardial infarction. J Am Heart Assoc. 2017;6(5):e005868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newby AC. Metalloproteinase expression in monocytes and macrophages and its relationship to atherosclerotic plaque instability. Arterioscler Thromb Vasc Biol. 2008;28(12):2108-2114. [DOI] [PubMed] [Google Scholar]
- 26.Yabluchanskiy A, Ma Y, Iyer RP, Hall ME, Lindsey ML. Matrix metalloproteinase-9: many shades of function in cardiovascular disease. Physiology (Bethesda). 2013;28(6):391-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prati F, Regar E, Mintz GS, et al. ; Expert’s OCT Review Document . Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J. 2010;31(4):401-415. [DOI] [PubMed] [Google Scholar]
- 28.Kataoka Y, Puri R, Hammadah M, et al. Sex differences in nonculprit coronary plaque microstructures on frequency-domain optical coherence tomography in acute coronary syndromes and stable coronary artery disease. Circ Cardiovasc Imaging. 2016;9(8):e004506. [DOI] [PubMed] [Google Scholar]
- 29.Komukai K, Kubo T, Kitabata H, et al. Effect of atorvastatin therapy on fibrous cap thickness in coronary atherosclerotic plaque as assessed by optical coherence tomography: the EASY-FIT study. J Am Coll Cardiol. 2014;64(21):2207-2217. [DOI] [PubMed] [Google Scholar]
- 30.Kataoka Y, Puri R, Hammadah M, et al. Frequency-domain optical coherence tomographic analysis of plaque microstructures at nonculprit narrowings in patients receiving potent statin therapy. Am J Cardiol. 2014;114(4):549-554. [DOI] [PubMed] [Google Scholar]
- 31.Fishbein MC, Siegel RJ. How big are coronary atherosclerotic plaques that rupture? Circulation. 1996;94(10):2662-2666. [DOI] [PubMed] [Google Scholar]
- 32.Libby P. Superficial erosion and the precision management of acute coronary syndromes: not one-size-fits-all. Eur Heart J. 2017;38(11):801-803. [DOI] [PubMed] [Google Scholar]
- 33.Quillard T, Araújo HA, Franck G, Shvartz E, Sukhova G, Libby P. TLR2 and neutrophils potentiate endothelial stress, apoptosis and detachment: implications for superficial erosion. Eur Heart J. 2015;36(22):1394-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naruko T, Ueda M, Haze K, et al. Neutrophil infiltration of culprit lesions in acute coronary syndromes. Circulation. 2002;106(23):2894-2900. [DOI] [PubMed] [Google Scholar]
- 35.Borissoff JI, Joosen IA, Versteylen MO, et al. Elevated levels of circulating DNA and chromatin are independently associated with severe coronary atherosclerosis and a prothrombotic state. Arterioscler Thromb Vasc Biol. 2013;33(8):2032-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferrante G, Nakano M, Prati F, et al. High levels of systemic myeloperoxidase are associated with coronary plaque erosion in patients with acute coronary syndromes: a clinicopathological study. Circulation. 2010;122(24):2505-2513. [DOI] [PubMed] [Google Scholar]
- 37.Xie Y, Mintz GS, Yang J, et al. Clinical outcome of nonculprit plaque ruptures in patients with acute coronary syndrome in the PROSPECT study. JACC Cardiovasc Imaging. 2014;7(4):397-405. [DOI] [PubMed] [Google Scholar]
- 38.Scalone G, Niccoli G, Refaat H, et al. Not all plaque ruptures are born equal: an optical coherence tomography study. Eur Heart J Cardiovasc Imaging. 2017;18(11):1271-1277. [DOI] [PubMed] [Google Scholar]
- 39.Hayashi T, Kiyoshima T, Matsuura M, et al. Plaque erosion in the culprit lesion is prone to develop a smaller myocardial infarction size compared with plaque rupture. Am Heart J. 2005;149(2):284-290. [DOI] [PubMed] [Google Scholar]
- 40.Jia H, Dai J, Hou J, et al. Effective anti-thrombotic therapy without stenting: intravascular optical coherence tomography-based management in plaque erosion (the EROSION study). Eur Heart J. 2017;38(11):792-800. [DOI] [PubMed] [Google Scholar]
- 41.Prati F, Uemura S, Souteyrand G, et al. OCT-based diagnosis and management of STEMI associated with intact fibrous cap. JACC Cardiovasc Imaging. 2013;6(3):283-287. [DOI] [PubMed] [Google Scholar]
- 42.Eisen A, Giugliano RP, Braunwald E. Updates on acute coronary syndrome: a review. JAMA Cardiol. 2016;1(6):718-730. [DOI] [PubMed] [Google Scholar]
- 43.Vanhoutte PM, Zhao Y, Xu A, Leung SW. Thirty years of saying NO: sources, fate, actions, and misfortunes of the endothelium-derived vasodilator mediator. Circ Res. 2016;119(2):375-396. [DOI] [PubMed] [Google Scholar]
- 44.Döring Y, Soehnlein O, Weber C. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ Res. 2017;120(4):736-743. [DOI] [PubMed] [Google Scholar]
- 45.Manfrini O, Mont E, Leone O, et al. Sources of error and interpretation of plaque morphology by optical coherence tomography. Am J Cardiol. 2006;98(2):156-159. [DOI] [PubMed] [Google Scholar]
- 46.Fujii K, Hao H, Shibuya M, et al. Accuracy of OCT, grayscale IVUS, and their combination for the diagnosis of coronary TCFA: an ex vivo validation study. JACC Cardiovasc Imaging. 2015;8(4):451-460. [DOI] [PubMed] [Google Scholar]
- 47.Yabushita H, Bouma BE, Houser SL, et al. Characterization of human atherosclerosis by optical coherence tomography. Circulation. 2002;106(13):1640-1645. [DOI] [PubMed] [Google Scholar]
- 48.Kini AS, Vengrenyuk Y, Yoshimura T, et al. Fibrous cap thickness by optical coherence tomography in vivo. J Am Coll Cardiol. 2017;69(6):644-657. [DOI] [PubMed] [Google Scholar]
- 49.Otsuka F, Joner M, Prati F, Virmani R, Narula J. Clinical classification of plaque morphology in coronary disease. Nat Rev Cardiol. 2014;11(7):379-389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Angiographic Findings
eTable 2. Patient-Based Analysis of the Non-Culprit Plaque Characteristics
eTable 3. Comparison of OCT Findings Between Patients With and Without Culprit Macrophage Accumulation in the Culprit Rupture Group
eFigure. Subgroup Comparison of Non-Culprit Plaque Characteristics Between The Three Groups: Culprit Erosion, Culprit Rupture Without Non-Culprit Rupture, and Culprit Rupture With Non-Culprit Rupture