Key Points
Question
What metabolomic signatures characterize maladaptive left ventricular remodeling in patients with aortic stenosis?
Findings
In this case series study of 44 patients with aortic stenosis undergoing valve replacement, circulating long-chain acylcarnitine levels were independently associated with measures of maladaptive cardiac remodeling. Levels of long-chain acylcarnitines improved after alleviation of cardiac pressure overload with valve replacement.
Meaning
Metabolomic profiling noninvasively assesses myocardial distress and biology; whether metabolic biomarkers can inform timing of AVR warrants further investigation.
The study evaluates if or how metabolomic signatures reflect maladaptive left ventricular remodeling in patients with end-stage aortic stenosis and assesses whether transcatheter aortic valve replacement reverses metabolomic aberrations.
Abstract
Importance
Clinical practice guidelines currently endorse a reliance on clinical symptoms of overt left ventricular (LV) failure to time aortic valve replacement for severe aortic stenosis; however, delayed aortic valve replacement can result in irreversible LV injury and adverse outcomes. Blood metabolomic signatures possess prognostic value in heart failure; this study assesses whether they are informative in aortic stenosis.
Objective
To evaluate the value of metabolomic signatures in reflecting the extent of maladaptive LV remodeling in patients with end-stage aortic stenosis undergoing transcatheter aortic valve replacement, and to assess whether this procedure reverses metabolomic aberrations.
Design, Setting, and Participants
This study of 44 patients with symptomatic severe aortic stenosis who underwent transfemoral transcatheter aortic valve replacement at a single-center tertiary care hospital. Liquid chromatography–mass spectrometry-based metabolomic profiling was performed on blood samples collected before and 24 hours after the procedure, and analyses were conducted to identify metabolites related to the measures of LV remodeling.
Main Outcomes and Measures
We evaluated LV ejection fraction, LV mass index, and relative wall thickness, as well as levels of the acylcarnitines C16, C18:1, C18:2, C18, C26, choline, and kynurenine.
Results
We enrolled 44 patients with severe aortic stenosis with a mean (SD) age of 81.9 (8.5) years, of whom 23 (52%) were women. The mean (SD) LV ejection fraction was 56.7% (18.2%), mean (SD) LV mass index was 117.3 (41.4) g/m2, and relative wall thickness was 0.53 (0.14). The mean β values of acylcarnitines C16, C18:1, C18:2, C18, and C26 were independently associated with LV mass index (C16: mean, 19.24; 95% CI, 5.48-33.01; P = .008; C18:1: mean, 26.18; 95% CI, 14.04-38.32; P < 1.0 × 10-4; C18:2: mean, 17.42; 95% CI, 3.40-31.43; P = .02; C18: mean, 25.25; 95% CI, 10.91-39.58; P = .001; C26: mean, 19.93; 95% CI, 4.41-35.45; P = .01), even after adjustments for age, sex, diabetes status, renal function, and B-type natriuretic peptide (BNP). Circulating levels of C18:2 acylcarnitine were associated with LV ejection fraction before and after multivariable adjustment (mean, −6.11; 95% CI, −10.88 to 1.34; P = .01). Blood metabolite levels did not independently relate to relative wall thickness. Within 24 hours of transcatheter aortic valve replacement, circulating levels of C16 decreased by 30.2% (P = 7.3 × 10-6), C18:1 by 42.7% (P = 3.7 × 10-8), C18:2 by 37.3% (P = 5.1 × 10-6), and C18 by 38.3% (P = 3.4 × 10-5).
Conclusions and Relevance
In symptomatic patients with severe aortic stenosis undergoing transcatheter aortic valve replacement, circulating levels of long-chain acylcarnitines were independently associated with measures of maladaptive LV remodeling, and metabolic perturbations lessened after procedure completion. Further efforts are needed to determine the clinical applicability of these novel biomarkers.
Introduction
Aortic stenosis (AS) is a progressive disease characterized by narrowing of the aortic valve, which in turn exerts a pressure load on the left ventricle (LV) and leads to reduced functional capacity, heart failure, and death. As AS worsens, maladaptive cardiomyocyte apoptosis and myocardial fibrosis result in the impairment of LV diastolic relaxation and systolic function. Clinical symptoms ensue at a late stage, at which point aortic valve replacement becomes clinically indicated. Recent data suggest altered myocardial energy metabolism in patients with end-stage heart failure. Circulating long-chain acylcarnitines (LCACs), intermediates of fatty acid metabolism, are prognostic in patients with chronic systolic dysfunction. The use of mechanical LV support alleviates metabolic derangements. We sought to evaluate LCAC metabolism within patients with AS undergoing transcatheter aortic valve replacement (TAVR).
Methods
Consecutive patients undergoing transfemoral TAVR at the Massachusetts General Hospital were enrolled as previously described. The study protocol was approved by the institutional review board at Massachusetts General Hospital. All patients provided written informed consent.
Venous blood samples were collected immediately prior to TAVR and again 24 hours after TAVR. Fasting venous blood samples were collected and processed within 30 minutes of collection. Amino acids, amino acid derivatives, urea cycle intermediates, nucleotides, and other positively charged polar metabolites were profiled through liquid chromatography–mass spectrometry-based targeted profiling. Metabolite quantification was performed by integrating peak areas for parent/daughter ion pairs using Multiquant Software, version 1.0 (Applied Biosystems/Sciex). Researchers quantified B-type natriuretic peptide (BNP) by enzyme-linked immunosorbent assay using a commercially available kit (Abcam).
Comprehensive transthoracic echocardiography was performed in all patients prior to TAVR; measurements were performed as outlined by the American Society of Echocardiography. For each patient, LV ejection fraction (LVEF) was determined using the biplane Simpson volumetric method. In addition, LV mass was determined and indexed to body surface area (LV mass index [LVMI]). Left ventricle dysfunction was defined as an LVEF less than or equal to 45%, and severe LV hypertrophy (LVH) was defined as an LVMI greater than 122 g/m2 in women and 140 g/m2 in men.
In data analysis, metabolites that did not distribute normally on visual assessment of kurtosis and skew were log-transformed. All metabolites were standardized such that the mean value was set to 0 and SD set to 1. Continuous clinical variables and metabolite levels are depicted as mean (SD), and comparisons were made using the van der Waerden test. Categorical parameters were presented as frequencies, and distributions were compared by using the Fisher exact test. Pearson correlation coefficients were generated to evaluate the correlation of metabolites with measures of LV remodeling (LVEF, LVMI, and relative wall thickness [RWT]). Univariable and multivariable linear regression analyses were used to model the association between plasma metabolites prior to TAVR and baseline parameters of LV remodeling. Multivariable adjustment was performed for age, sex, diabetes status, BNP, and estimated glomerular filtration rate (GFR) as calculated using the Modification of Diet in Renal Disease formula. We used a P value threshold of .01 for biomarker discovery; otherwise, a P < .05 was considered significant.
Results
We enrolled 44 patients with severe AS; their mean (SD) age was 81.9 (8.5) years, and 23 (52%) were women (eTable 1 in the Supplement). Diabetes mellitus was present in 16 of the 44 patients (36%), hypertension in 36 of 43 patients (84%; values were missing for 1 patient), and chronic kidney disease in 22 of 44 patients (50%). Mean (SD) estimated GFR was 58.1 (18.2) mL/min/1.73 m2. Mean (SD) LVEF was 56.7% (18.2%), mean (SD) LVMI was 117.3 (41.4) g/m2, and mean (SD) RWT was 0.53 (0.14). The average peak and mean aortic valve gradients were 84.8 (29.1) mm Hg and 50.5 (18.6) mm Hg, respectively, with a mean (SD) aortic valve area of 0.65 (0.16) cm2. Baseline mean (SD) BNP was 520 (562) pg/mL.
Several LCACs significantly correlated with LVMI, including the acylcarnitines C16 (r = 0.44; P = .003), C18:1 (r = 0.60; P < 1.0 × 10-4), C18:2 (r = 0.41; P = .007), C18 (r = 0.50; P = 6.0 × 10-4), and C26 (r = 0.38; P = .01) (eTable 2 in the Supplement). There was a negative correlation of LVEF with C18:2 acylcarnitine (r = −0.40; P = .007), as well as with choline (r = −0.43; P = .004) and kynurenine (r = −0.47; P = .001). Only C26 acylcarnitine demonstrated a significant correlation with RWT (r = 0.39; P = .01). In addition, B-type natriuretic peptide correlated significantly with LVEF (r = −0.50; P = 7.0 × 10-4) and RWT (r = −0.38; P = .01), but not LVMI (r = 0.07; P = .68).
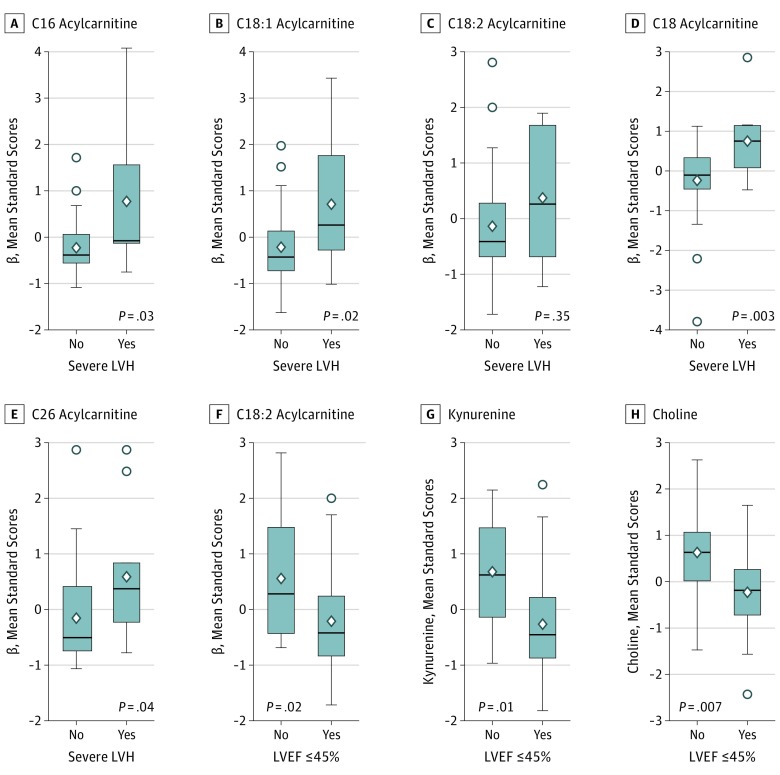
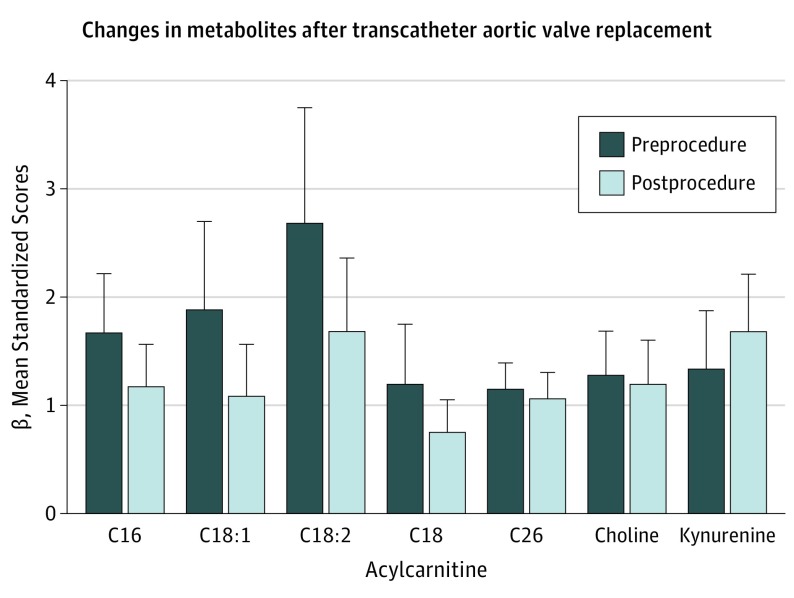
Robust relationships between the LCAC and LVMI persisted after multivariable adjustment for age, sex, diabetes status, renal function, and BNP (Table). Similarly, with the exception of C18:2 acylcarnitine, each of the LCACs distinguished between patients with and without severe LVH (Figure 1). Circulating levels of C18:2 acylcarnitine, choline, and kynurenine were associated with LVEF before and after multivariable adjustment (Table), but only C18:2 acylcarnitine was associated with LVEF after additional adjustment for BNP. Levels of each metabolite were reduced in patients with LV dysfunction (Figure 1). Within 24 hours of completing TAVR, circulating levels of LCACs (C16, C18:1, C18:2, and C18) decreased by 30.2% to 42.7% (Figure 2), and the change was significant. Mean (95% CI) decreases in β values were 0.50 (0.31-0.70) in C16, 0.22 (0.11-0.33) in C18, 1.00 (0.62-1.37) in C18:1, and 0.46 (0.26-0.65) in C18:2; for each comparison, P was less than .001. Kynurenine increased by 25.8% (mean increase in β, 0.34; 95% CI, 0.53-0.15; P = 8.9 × 10−4), although C26 acylcarnitine and choline did not change significantly after postprocedure alleviation of LV pressure overload. There was also no significant decrease of BNP within 24 hours of TAVR (pre-TAVR mean [SD] BNP, 520 [562] pg/mL; post-TAVR BNP, 410 [370] pg/mL; mean change, −110 pg/mL; range, −30 to −250 pg/mL; P = .10).
Table. Associations of Long-Chain Acylcarnitines With Left Ventricular Structure and Function.
| Characteristics | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|
| Multivariablea | Fullb | |||||
| β (95% CI) | P Value | β (95% CI) | P Value | β (95% CI) | P Value | |
| Left ventricular mass index | ||||||
| C16 | 18.04 (6.47 to 29.62) | .003 | 18.17 (5.33 to 31.01) | .007 | 19.24 (5.48 to 33.01) | .008 |
| C18:1 | 24.52 (14.21 to 34.84) | <1.0 × 10-4 | 25.44 (14.16 to 36.72) | <1.0 × 10-4 | 26.18 (14.04 to 38.32) | 1.0 × 10-4 |
| C18:2 | 16.78 (4.90 to 28.66) | .007 | 17.09 (4.21 to 29.96) | .01 | 17.42 (3.40 to 31.43) | .02 |
| C18 | 20.66 (9.52 to 31.80) | 6.0 × 10-4 | 17.14 (7.58 to 26.70) | 9.0 × 10-4 | 25.25 (10.91 to 39.58) | .001 |
| C26 | 15.44 (3.41 to 27.47) | .01 | 18.04 (3.76 to 32.32) | .0147 | 19.93 (4.41 to 35.45) | .01 |
| Left ventricular ejection fraction | ||||||
| Choline | −7.29 (−12.11 to 2.47) | .004 | −6.39 (−12.38 to 0.40) | .04 | −4.50 (−10.46 to 1.45) | .13 |
| Kynurenine | −7.99 (−12.70 to 3.27) | .001 | −7.94 (−14.61 to 1.28) | .02 | −3.31 (−11.42 to 4.79) | .41 |
| C18:2 | −6.88 (−11.76 to 2.00) | .007 | −6.97 (−11.95 to −1.99) | .007 | −6.11 (−10.88 to −1.34) | .01 |
| Relative wall thickness | ||||||
| C26 | 0.05 (0.01 to 0.09) | .01 | 0.04 (−0.01 to 0.08) | .11 | NAc | NAc |
Abbreviation: NA, not applicable.
Adjusted for age, sex, diabetes status, and estimated glomerular filtration rate.
Further adjustment includes B-type natriuretic peptide.
Since C26 did not associate significantly after partial statistical analysis, additional adjustment and testing was inappropriate.
Figure 1. Circulating Metabolites Used to Identify Maladaptive Left Ventricular Remodeling.
In patients with severe left ventricular hypertrophy (LVH), levels of C16, C18:1, C18, and C26 acylcarnitines are significantly elevated (but C18:2 acylcarnitine is not). In patients with left ventricular systolic dysfunction (defined as a left ventricular ejection fraction [LVEF] of 45% or less), levels of C18:2 acylcarnitine, kynurenine, and choline are significantly reduced. Diamond shapes indicate mean values, circles indicate outlying values, and bars indicate interquartile ranges.
Figure 2. Alleviation of Left Ventricular Pressure Overload Associated With Changes in Circulating Levels of Long-Chain Acylcarnitines.
Long-chain acylcarnitine levels decreased significantly within 24 hours of the alleviation of severe aortic stenosis via transcatheter aortic valve replacement. In specific, C16 decreased by 30.2% (P = 7.3 × 10-6), C18:1 by 42.7% (P = 3.7 × 10-8), C18:2 by 37.3% (P = 5.1 × 10-6), and C18 by 38.3% (P = 3.4 × 10-5). Kynurenine levels increased by 25.8% (P = 8.9 × 10-4). Levels of C26 and choline did not significantly change.
Discussion
Using liquid chromatography–mass spectrometry-based metabolomic profiling techniques, we demonstrated that circulating metabolites are indicative of the extent of LV remodeling in patients with severe AS. Specifically, several LCACs are associated with the extent of LVH and LV dysfunction, and these changes appeared to be independent of BNP, an established biomarker of LV distress. Circulating metabolite levels effectively differentiated patients with either severe LVH or LV systolic dysfunction. Finally, alleviation of LV pressure overload with TAVR resulted in acute reductions in LCAC levels.
Clinical practice currently relies on clinical symptoms or the presence of overt LV failure to guide the timing of aortic valve replacement for AS. This approach often identifies a late-stage cohort in whom maladaptive remodeling may be advanced and irreversible. Recent data suggest that maladaptive LV remodeling can persist in 30% to 50% of patients after aortic valve replacement, and the impact on clinical outcomes is substantial. Previously, we have demonstrated that the absence of LVEF improvement within 1 month of TAVR is associated with a tripling of the risk of 1-year all-cause mortality and a 5-fold increase in cardiac death. Similarly, diminished regression of LVH is associated with a doubling of the risk of rehospitalization in patients with heart failure. There is therefore an unmet clinical need for objective methods to identify early, reversible stages of maladaptive LV remodeling and to inform timely aortic valve replacement.
Our findings suggest that blood metabolomic signatures might reflect myocardial metabolism and distress. Cardiac substrate use shifts from fatty acids to glucose in patients with AS. Similarly, in animal models, cardiac LCACs are increased in compensated LVH and further elevated in heart failure, indicating downregulation of myocardial fatty acid oxidation. Acylcarnitine levels have recently been found to reflect perturbations in myocardial energetics; they have also been found to provide prognostic information in patients with end-stage heart failure.
Limitations
Several limitations of this study warrant attention. The sample size was limited and did not allow for validation, complete statistical adjustment, or investigation of the predictive value of plasma metabolites. These findings should consequently be considered hypothesis generating only. However, while false discovery is possible with a small sample, the marked consistency of the identified metabolites with the current heart failure literature and across different measures of LV remodeling are reassuring. Finally, acute perioperative stressors may effect circulating metabolite levels. Further efforts are needed to exclude this possibility, to confirm the myocardial origin of the identified metabolites, and to determine whether acute changes in metabolic signatures reflect improved myocardial energetics.
Conclusions
In summary, the association of circulating metabolites with cardiac remodeling supports the use of metabolomic profiling for noninvasive assessment of myocardial distress and biology. Whether metabolic biomarkers can inform the timing of aortic valve replacement warrants further investigation.
eTable 1. Baseline patient characteristics.
eTable 2. Heat map depicting Pearson correlation coefficients with measures of left ventricular remodeling
References
- 1.Weidemann F, Herrmann S, Störk S, et al. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation. 2009;120(7):577-584. [DOI] [PubMed] [Google Scholar]
- 2.Aasum E, Hafstad AD, Severson DL, Larsen TS. Age-dependent changes in metabolism, contractile function, and ischemic sensitivity in hearts from db/db mice. Diabetes. 2003;52(2):434-441. [DOI] [PubMed] [Google Scholar]
- 3.Nishimura RA, Otto CM, Bonow RO, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(22):e57-e185. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad T, Kelly JP, McGarrah RW, et al. Prognostic implications of long-chain acylcarnitines in heart failure and reversibility with mechanical circulatory support. J Am Coll Cardiol. 2016;67(3):291-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunter WG, Kelly JP, McGarrah RW III, et al. Metabolomic profiling identifies novel circulating biomarkers of mitochondrial dysfunction differentially elevated in heart failure with preserved versus reduced ejection fraction: evidence for shared metabolic impairments in clinical heart failure. J Am Heart Assoc. 2016;5(8):e003190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elmariah S, Farrell LA, Daher M, et al. Metabolite profiles predict acute kidney injury and mortality in patients undergoing transcatheter aortic valve replacement. J Am Heart Assoc. 2016;5(3):e002712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lang RM, Bierig M, Devereux RB, et al. ; American Society of Echocardiography’s Nomenclature and Standards Committee; Task Force on Chamber Quantification; American College of Cardiology Echocardiography Committee; American Heart Association; European Association of Echocardiography, European Society of Cardiology . Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7(2):79-108. [DOI] [PubMed] [Google Scholar]
- 8.Levey AS, Coresh J, Greene T, et al. ; Chronic Kidney Disease Epidemiology Collaboration . Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247-254. [DOI] [PubMed] [Google Scholar]
- 9.Elmariah S, Palacios IF, McAndrew T, et al. ; PARTNER Investigators . Outcomes of transcatheter and surgical aortic valve replacement in high-risk patients with aortic stenosis and left ventricular dysfunction: results from the Placement of Aortic Transcatheter Valves (PARTNER) trial (cohort A). Circ Cardiovasc Interv. 2013;6(6):604-614. [DOI] [PubMed] [Google Scholar]
- 10.Lindman BR, Stewart WJ, Pibarot P, et al. Early regression of severe left ventricular hypertrophy after transcatheter aortic valve replacement is associated with decreased hospitalizations. JACC Cardiovasc Interv. 2014;7(6):662-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heather LC, Howell NJ, Emmanuel Y, et al. Changes in cardiac substrate transporters and metabolic proteins mirror the metabolic shift in patients with aortic stenosis. PLoS One. 2011;6(10):e26326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai L, Leone TC, Keller MP, et al. Energy metabolic reprogramming in the hypertrophied and early stage failing heart: a multisystems approach. Circ Heart Fail. 2014;7(6):1022-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Baseline patient characteristics.
eTable 2. Heat map depicting Pearson correlation coefficients with measures of left ventricular remodeling