Key Points
Question
Do direct oral anticoagulants (DOAC) in addition to antiplatelet therapy (APT) safely reduce ischemic events after acute coronary syndromes (ACS), and are there differences according to ACS type?
Findings
In this systematic review and meta-analysis of 6 trials comprising 29 667 patients, direct oral anticoagulants with APT was associated with a reduced risk of ischemic events at the cost of an increase in major bleedings compared with APT alone. Direct oral anticoagulants was associated with a reduction in ischemic events after ST-segment elevation myocardial infarction with no effects after non–ST-segment elevation ACS, while the increased risk of major bleeding was consistent after ST-segment elevation myocardial infarction and non–ST-segment elevation ACS.
Meaning
The risk-benefit profile of direct oral anticoagulants in addition to APT appears to differ by ACS type; direct oral anticoagulants might represent an attractive strategy in patients with ST-segment elevation myocardial infarction.
Abstract
Importance
Patients with acute coronary syndrome (ACS) remain at high risk for experiencing recurrent ischemic events. Direct oral anticoagulants (DOAC) have been proposed for secondary prevention after ACS.
Objective
To evaluate the safety and efficacy of DOAC in addition to antiplatelet therapy (APT) after ACS, focusing on treatment effects stratified by baseline clinical presentation (non–ST-segment elevation ACS [NSTE-ACS] vs ST-segment elevation myocardial infarction [STEMI]).
Data Sources
PubMed, Embase, BioMedCentral, Google Scholar, and the Cochrane Central Register of Controlled Trials were searched from inception to March 1, 2017.
Study Selection
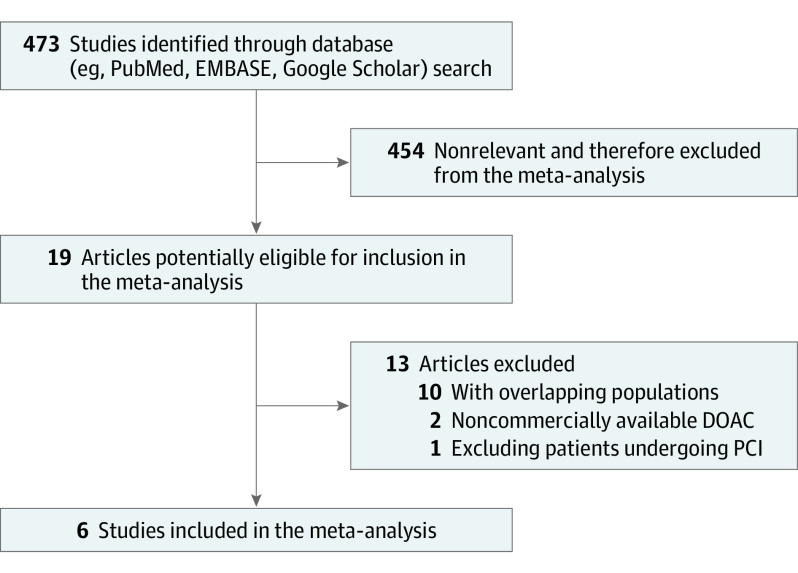
Randomized clinical trials on DOAC after ACS were evaluated for inclusion. Overall, 473 studies were screened, 19 clinical trials were assessed as potentially eligible, and 6 were included in the meta-analysis.
Data Extraction and Synthesis
Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines were used to abstract data and assess quality and validity. The risk of bias tool, version 2.0 (Cochrane) was used for risk of bias assessment. Data were pooled using random-effects models.
Main Outcomes and Measures
The prespecified primary efficacy end point was the composite of cardiovascular death, myocardial infarction, and stroke. The prespecified primary safety end point was major bleeding.
Results
Six trials that included 29 667 patients were identified (14 580 patients [49.1%] with STEMI and 15 036 [50.7%] with NSTE-ACS). The primary efficacy end point risk was significantly lower in patients who were treated with DOAC as compared with APT alone (odds ratio [OR], 0.85; 95% CI, 0.77-0.93; P < .001). This benefit was pronounced in patients with STEMI (OR, 0.76; 95% CI, 0.66-0.88; P < .001), while no significant treatment effect was observed in patients with NSTE-ACS (OR, 0.92; 95% CI, 0.78-1.09; P = .36; P for interaction = .09). With respect to safety, DOACs were associated with a higher risk of major bleeding as compared with APT alone (OR, 3.17; 95% CI, 2.27-4.42; P < .001), with consistent results in patients with STEMI (OR, 3.45; 95% CI, 1.95-6.09; P < .001) and NSTE-ACS (OR, 2.19; 95% CI, 1.38-3.48; P < .001; P for interaction = .23).
Conclusions and Relevance
To our knowledge, these findings are the first evidence to support differential treatment effects of DOAC in addition to APT according to ACS baseline clinical presentation. In patients with NSTE-ACS, the risk-benefit profile of DOAC appears unfavorable. Conversely, DOAC in addition to APT might represent an attractive option for patients with STEMI.
This meta-analysis assesses the efficacy and safety of direct oral anticoagulants in addition to background antiplatelet therapy for secondary prevention in patients with acute coronary syndrome.
Introduction
The treatment of patients with acute coronary syndrome (ACS) has significantly evolved during the last few decades, primarily because of more effective reperfusion strategies and novel antithrombotic therapies, with subsequent remarkable improvements in patients’ prognoses.1,2 Despite these advances, patients with ACS remain at high risk of experiencing recurrent ischemic events.3
Thrombosis prevention with antithrombotic agents plays a pivotal role in the treatment of patients with ACS. Dual antiplatelet therapy (APT) after mechanical reperfusion with primary percutaneous coronary intervention (PCI) represents the standard of care for patients with ACS.4,5,6,7 For a more profound antithrombotic effect, oral anticoagulants in addition to APT are an attractive option after ACS. Prior to the implementation of dual APT and PCI as the treatment of choice, vitamin K antagonists have been widely investigated with conflicting findings.8,9 Recently, direct oral anticoagulants (DOAC) in addition to APT have been shown to reduce the risk of ischemic events at the cost of a higher risk of bleeding.10,11,12,13,14,15,16,17,18
To our knowledge, the differences in terms of the safety and efficacy of DOAC in addition to APT according to ACS clinical presentation at baseline (ie, ST-segment elevation myocardial infarction [STEMI] vs non–ST-segment elevation ACS [NSTE-ACS]) have not been investigated. Therefore, we aimed to assess DOAC safety and efficacy according to ACS type to further elucidate the potential role of DOAC after ACS.
Methods
Search Strategy and Study Selection
Search strategy, study selection, data extraction, and data analysis were performed in accordance with the Cochrane Collaboration and the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines.19 The risk of bias in each study has been assessed using the revised Cochrane risk of bias tool (RoB 2.0). Three investigators (M.C., D.C., and F.C.) independently assessed 5 domains of bias for each outcome: (1) the randomization process, (2) deviations from intended interventions, (3) missing outcome data, (4) the measurement of the outcome, and (5) the selection of the reported results (eTable in the Supplement).20
Randomized clinical trials on DOAC in addition to APT after ACS (from inception to March 1, 2017) were evaluated for inclusion in the meta-analysis. To include only studies that reported data on patients who were treated with current clinical practices and to obtain results that were applicable to contemporary patients, we excluded studies in which patients who were undergoing primary PCI were not included, studies published before 1991, studies with overlapping populations, and studies that investigated DOACs that were no longer commercially available. Three authors (M.C., D.C., and F.C.) independently searched PubMed, Embase, BioMedCentral, Google Scholar, and the Cochrane Central Register of Controlled Trials. In addition, we used backward snowballing (ie, a review of references from identified articles and pertinent reviews) and searched abstracts from 2014 to 2016 that were presented at relevant scientific meetings (eg, Transcatheter Cardiovascular Therapeutics, American Heart Association, American College of Cardiology, European Society of Cardiology, EuroPCR). The search strategy for PubMed and details about included and excluded studies are available in the eAppendix in the Supplement. This study is registered with the PROSPERO International Prospective Register of Systematic Reviews (http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017058075).
Data Extraction
Three investigators (M.C., F.C., and D.C.) independently assessed studies for possible inclusion. Nonrelevant articles were excluded based on the title and abstract. The same investigators independently extracted data on study designs, measurements, patient characteristics, and outcomes using a standardized data extraction form. The inclusion and data extraction conflicts of studies were discussed and resolved with another investigator (G.G.S.). Missing data were requested by email to the corresponding author of each study. In the case of studies with overlapping populations, only the article that reported the largest number of patients was selected.
Data about authors, year of publication, inclusion and exclusion criteria, sample size, baseline features of patients, treatment features (eg, anticoagulant dosage, antiplatelet drugs, and antiplatelet treatment duration) end point definitions, effect estimates, and follow-up time were collected. To exclude dosages considered to lead to excessive bleeding risk, only data on patients who were treated with approved anticoagulant dosages have been included.21 Therefore, in the Apixaban for Prevention of Acute Ischemic Events (APPRAISE) randomized clinical trial (RCT), only data on patients who were treated with a daily dosage of apixaban at 5 mg and 10 mg have been included.
Outcomes
The prespecified primary efficacy end point was the composite of cardiovascular death, myocardial infarction, and stroke. The prespecified primary safety end point was major bleeding. Each end point was assessed according to the definitions reported in the original study protocols (eAppendix in the Supplement). Bleeding events were assessed by the International Society of Thrombosis and Hemostasis definition in the clinical trials with dabigatran, in the APPRAISE RCT, and in the APPRAISE in Japanese patients RCT. In the trials with rivaroxaban and in the APPRAISE 2 RCT, bleeding events were reported according to the Thrombolysis in Myocardial Infarction (TIMI) definition.
Statistical Analysis
Pooled odds ratios (ORs) for categorical variables (dichotomous outcomes) were calculated using a binary random-effects model to minimize the effect of clinical and methodological heterogeneity among studies, with the inverse variance weighting.22 In studies in which no events were reported within groups, the difference between groups could not be assessed. The number needed to treat (NNT) to prevent 1 primary efficacy end point event and number needed to harm (NNH) to determine 1 primary safety end point event have been derived from RCTs reporting event rates stratified by ACS type (STEMI vs NSTE-ACS). In the case of significant differences between treatment arms, 95% confidence intervals for NNT and NNH were reported. The hypothesis of statistical heterogeneity was tested by means of the Cochran Q statistic and I2 values. I2 values of less than 25%, 50%, or more than 50% indicated low, moderate, or substantial heterogeneity, respectively.23 Statistical significance was set at P < .05 (2-sided). Publication bias and small study effect were assessed for the primary efficacy and safety end points using funnel plots. The Egger linear regression method was used to detect funnel plot asymmetry. In addition to statistical tests, we visually estimated funnel plots to evaluate the possibility of publication bias.24 Subgroup analyses were performed to assess the influence of clinical presentation (STEMI vs NSTE-ACS as an index event) on the risk estimates for the outcomes, as prespecified in the meta-analysis protocol. A meta-regression analysis with a random-effects model was performed to evaluate the effect of major bleedings, clinically relevant bleedings, and the composite of major and clinically relevant bleedings on cardiovascular death and death from all causes.25 Sensitivity analyses with fixed-effect models were performed to assess consistency among effect estimates that were obtained with random- and fixed-effects models.26 Computation was performed with Review Manager, version 5.3 (Cochrane Collaboration) and Stata, version 13.1 (StataCorp). Analyses were performed according to the intention-to-treat principle.
Results
A total of 6 RCTs were identified and included in this analysis. Figure 1 shows the flowchart of the study selection process. The main features of included RCTs are presented in Table 1. A total of 29 667 patients who presented with ACS and were randomly allocated to receive DOAC or a placebo in addition to APT were analyzed.
Figure 1. Flowchart of the Study Selection Process.
DOAC indicates direct oral anticoagulant; PCI, percutaneous coronary intervention.
Table 1. Main Features of the Studies Included in the Meta-analysis.
| Source | Design | Study Population | Anticoagulant Dosages | Follow-up, mo | ||
|---|---|---|---|---|---|---|
| Overall | DOAC Group | Control Group | ||||
| APPRAISE | RCT (phase II) | 1715 | 1104 | 611 | Apixaban, 2.5 mg BID, 10 mg QD | 6 |
| APPRAISE 2 | RCT (phase III) | 7392 | 3705 | 3687 | Apixaban, 5 mg BID | 8 |
| APPRAISE J | RCT (phase II) | 150 | 99 | 51 | Apixaban, 2.5 mg BID, 10 mg QD | 6 |
| ATLAS ACS TIMI 46 | RCT (phase II) | 3491 | 2331 | 1160 | Rivaroxaban, 5, 10, 15, and 20 mg QD | 6 |
| ATLAS ACS 2 TIMI 51 | RCT (phase III) | 15 526 | 10 350 | 5176 | Rivaroxaban, 2.5 and 5 mg BID | 13 |
| REDEEM | RCT (phase II) | 1861 | 1490 | 371 | Dabigatran, 50, 75, and 110, 150 mg BID | 6 |
Abbreviations: APPRAISE, Apixaban for Prevention of Acute Ischemic Events; ATLAS ACS TIMI 46, Rivaroxaban versus Placebo in Patients With Acute Coronary Syndromes; BID, twice daily; DOAC, direct oral anticoagulant; QD, once daily; RCT, randomized clinical trial; REDEEM, Dose Finding Study for Dabigatran Etexilate in Patients With Acute Coronary Syndrome.
Baseline Features
The baseline characteristics of included patients are summarized in Table 2. The patients were primarily men with a pooled mean age of 62.5 years. A total of 14 580 patients presented with STEMI and 15 036 with NSTE-ACS as an index event (data were not available for 51 patients in the APPRAISE 2 RCT).
Table 2. Baseline Clinical Features in the Studies Included in the Meta-analysis.
| Source | Age, y | % | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Male | DM | STEMI | NSTEMI | Unstable Angina | DAPT | PCI | Prior Stroke | ||
| APPRAISE | 61 | 75 | 22 | 62.9 | 29.5 | 7.6 | 75.5 | 65 | 4 |
| APPRAISE 2 | 67 | 69 | 48 | 39.6 | 41.6 | 18.1 | 81 | 44 | 10 |
| APPRAISE J | 64.6 | 87 | 40 | 75.5 | 15.2 | 9.3 | 97.4 | 99 | 4 |
| ATLAS ACS TIMI 46 | 57.4 | 77 | 19 | 52.2 | 29.9 | 18.0 | 78.2 | 64 | NA |
| ATLAS ACS 2 TIMI 51 | 61.7 | 75 | 32 | 50.4 | 25.6 | 24.0 | 93 | 57 | NA |
| REDEEM | 61.8 | 60 | 31 | 60 | 40 | - | 99.2 | 55 | NA |
Abbreviations: APPRAISE, Apixaban for Prevention of Acute Ischemic Events; ATLAS ACS TIMI 46, Rivaroxaban versus Placebo in Patients With Acute Coronary Syndromes; CKD, chronic kidney disease; DAPT, dual antiplatelet therapy; DM, diabetes; NA, not applicable; NSTEMI, non–ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; REDEEM, Dose Finding Study for Dabigatran Etexilate in Patients With Acute Coronary Syndrome; STEMI, ST-segment elevation myocardial infarction.
Follow-up
The APPRAISE, APPRAISE-J, Rivaroxaban versus Placebo in Patients With Acute Coronary Syndromes (ATLAS ACS TIMI 46), and Dose Finding Study for Dabigatran Etexilate in Patients With Acute Coronary Syndrome (REDEEM) clinical trials reported 6-month follow-up outcomes,12,13,15,18 while APPRAISE 2 and ATLAS-ACS 2 TIMI 51 reported 8-month and 13-month follow-up, respectively.14,16
Clinical Outcomes
The risk of the primary efficacy end point was significantly lower in patients who were treated with DOAC in addition to APT as compared with those who were treated with APT alone (OR, 0.85; 95% CI, 0.77-0.93; P < .001), with no evidence of heterogeneity. Conversely, patients treated with DOAC in addition to antiplatelet therapy had a higher risk of major bleeding as compared with those treated with APT alone (OR, 3.17; 95% CI, 2.27-4.42; P < .001), without evidence of heterogeneity. Overall, the NNT to prevent a cardiovascular ischemic event by adding DOAC to APT was 84 (95% CI, 55-176), while the NNH to determine a major bleeding was 105 (95% CI, 84-139).
Figure 2 summarizes risks of the primary efficacy and safety end points with DOAC in addition to APT as compared with APT alone in studies that reported outcomes that were stratified by type of ACS. Among patients presenting with STEMI, DOAC in addition to APT resulted in a significantly lower risk of the primary efficacy end point as compared with patients who were treated with APT alone (OR, 0.76; 95% CI, 0.66-0.88; P < .001), with no evidence of heterogeneity. This reduction was achieved at the cost of a significantly higher risk of major bleeding (OR, 3.45; 95% CI, 1.95-6.09; P < .001), with evidence of low grade of heterogeneity (I2 = 11%). Despite the fact that the treatment effect estimate for bleeding appears to be more pronounced as compared with the treatment effect estimate for ischemic events, NNT results (63; 95% CI, 40-134) were lower than NNH (96; 95% CI, 72-141) in this subgroup of patients. Conversely, in patients with NSTE-ACS, DOAC in addition to APT was associated with a similar risk of the primary efficacy end point (OR, 0.92; 95% CI, 0.78-1.09; P = .361) and a considerably increased risk of major bleeding (OR, 2.19; 95% CI, 1.38-3.48; P < .001) as compared with APT alone. Therefore, among patients with NSTE-ACS, DOAC in addition to APT resulted in comparable NNT (130) and NNH (137; 95% CI, 92-263) (Figure 3). Notably, formal test results for interaction showed a trend toward a significant interaction between treatment effects and ACS type as it related to the primary efficacy end point (P for interaction = .09), while no evidence of interaction was observed for major bleeding (P for interaction = .23). Funnel plot distributions of primary efficacy and safety end points indicated the absence of publication bias and small study effect for both of the end points (eFigures 1 and 2 in the Supplement).
Figure 2. Risk of the Primary Efficacy End Point and the Primary Safety End Point With Direct Oral Anticoagulant (DOAC) in Addition to Antiplatelet Therapy (APT) as Compared With APT Alone, Overall and Stratified by Acute Coronary Syndrome Type.
A, Efficacy end point. B, Safety end point. Only studies that reported outcomes stratified for the index event are included. Weights are from a random-effects analysis. APPRAISE indicates Apixaban for Prevention of Acute Ischemic Events; ATLAS ACS TIMI 46, Rivaroxaban versus Placebo in Patients With Acute Coronary Syndromes; CV death, cardiovascular death; MI, myocardial infarction; NSTE-ACS, non–ST-segment elevation myocardial infarction; OR, odds ratio; STEMI, ST-segment elevation myocardial infarction.
Figure 3. Number Needed to Prevent an Ischemic Event and Cause a Bleeding Event.
Overall and in patients with ST-segment elevation myocardial infarction (STEMI) and non–ST-segment elevation acute coronary syndromes (NSTE-ACS).
With respect to the individual components of the primary efficacy end points, DOAC in addition to APT was associated with a trend toward a risk reduction for cardiovascular death (OR, 0.86; 95% CI, 0.73-1.01; P = .067), a significant risk reduction for myocardial infarction (OR, 0.83; 95% CI, 0.74-0.95; P = .005), and no differences for stroke (OR, 0.81. 95% CI, 0.54-1.20. P = .29) as compared with APT alone. Findings for individual components of the primary end point were consistent in patients with STEMI, whereas no differences between DOAC in addition to APT and APT alone were observed in patients with NSTE-ACS (eFigures 3-5 in the Supplement).
Finally, we performed a meta-regression to assess the effect on overall mortality risk of major bleedings and clinically relevant bleedings (eFigures 6 and 7 in the Supplement). While the effect of DOAC in addition to APT on mortality risk was not related to the effect on major bleedings (B = 0.3; P = .89), there was a nonsignificant direct relation between the positive effect on mortality risk and the negative effect on clinically relevant bleedings (B = −0.24; P = .53).
Discussion
In this meta-analysis, we assessed the efficacy and safety of DOAC in addition to background APT for secondary prevention in patients with ACS, investigating the differences in treatment effects according to ACS type. The key findings of this meta-analysis can be summarized as follows: (1) as observed in previous studies,10,11 the unrestricted use of DOAC in addition to APT after ACS is associated with a reduction in the risk of ischemic events counterbalanced by a higher risk of major bleedings; (2) in patients with NSTE-ACS, DOAC in addition to APT are associated with a nonsignificant marginal reduction in the risk of ischemic events and with a significant increase in bleeding, with an overall neutral net clinical benefit; and (3) in patients with STEMI, the risk-benefit profile of DOAC in addition to APT appears to be more favorable, with a significantly lower risk of ischemic events only partially counterbalanced by a higher risk of major bleeding.
During the last few decades, several antithrombotic drugs have been developed and implemented in treating ACS, contributing to an important reduction in the risk of mortality and recurrent ischemic events.27,28,29 Increasing antithrombotic efficacy has been shown to reduce the risk of ischemic events; however, it is at the cost of a parallel increase in the risk of bleeding. This effect has been shown with several classes of antithrombotic drugs, including novel P2Y12 inhibitors (ie, ticagrelor and prasugrel) as well as the protease activated receptor 1 antagonist (ie, vorapaxar).30,31,32,33 Similarly, DOACs, with the strongest evidence existing for rivaroxaban, seem to have a potential role in increasing the antithrombotic protection among patients with ACS without a formal indication for oral anticoagulation therapy, but they are associated with an important increase in the risk of bleeding events. Several clinical trials have evaluated the effect of DOAC in ACS, mainly using them in addition to dual APT with aspirin and clopidogrel. A favorable effect was achieved only with low-dose regimens of rivaroxaban in the ATLAS ACS 2 TIMI 51 trial. Specifically, rivaroxaban, 2.5 mg twice daily, in addition to dual APT was associated with a significant reduction in cardiovascular and all-cause mortality as compared with a standard dual APT regimen, with a 2-fold increase in the risk of major bleeding.16 To improve safety, the Study to Compare the Safety of Rivaroxaban Versus Acetylsalicylic Acid in Addition to Either Clopidogrel or Ticagrelor Therapy in Patients With ACS (GEMINI ACS) tested the replacement of aspirin in favor of rivaroxaban in addition to clopidogrel or ticagrelor. This clinical trial showed no significant differences between the 2 strategies, but a numerically higher rate of both ischemic and bleeding events was reported in the rivaroxaban arm.34 Notably, DOACs have also been evaluated in stable coronary artery disease in the Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS) clinical trial. Adding rivaroxaban to aspirin lowered the risk of major vascular events as well as cardiovascular and all-cause mortality but increased the risk of major bleeding as compared with aspirin alone. The results of the COMPASS clinical trial suggest that low-intensity simultaneous inhibition of both coagulation and platelet activity could be more effective in terms of preventing ischemic events as compared with APT alone. The positive treatment effects on major cardiovascular events were consistent whether patients were included within 2 years after having myocardial infarction (MI), 2 to 5 years after having MI, beyond 5 years after having MI, or never had an MI.35,36
The rationale for using DOAC in patients with ACS without an indication for oral anticoagulation, especially for those with STEMI, is linked to the pivotal role of thrombin in this clinical setting.37,38 After an acute MI, high thrombin levels are traceable for at least 6 months and thrombin generation is inversely correlated with recurrent cardiovascular events.39,40 Therefore, the higher thrombotic burden and coagulation cascade activation during and after STEMI compared with NSTE-ACS could help to explain, at least in part, our findings.41,42 Moreover, differences in treatment effects could also be attributed to possible intrinsic differences between patients with STEMI and NSTE-ACS, such as baseline clinical characteristics (eg, age, cardiovascular risk factors, and extent of coronary artery disease) as well as therapeutic strategies (eg, type of P2Y12 inhibitors, PCI timing, completeness of revascularization, and surgical revascularization). The relative effect of these features on DOAC treatment effects should be further investigated in future studies.
In contrast to 2 previous meta-analyses on DOAC in addition to APT after ACS, we selected major bleedings as the primary safety end point instead of the composite of major and clinically significant bleedings.10,11 As expected, the NNH reported in the present study are considerably higher than those reported in the aforementioned meta-analyses. The effect of bleeding on prognoses has been thoroughly investigated, and the correlation between bleeding events and mortality rates is well established. Our choice was because of the “dose-related” effects of bleeding events on mortality rates. Major bleedings have been consistently identified as a stronger predictor of mortality compared with minor bleedings and even more if compared with clinically relevant bleedings, irrespective of the bleeding definition used.43,44,45,46,47 Based on this evidence, we included only major bleedings as primary safety end points, excluding minor and clinically significant bleedings. This choice appears to be further supported by the results shown in our meta-regression analyses.
Overall, our results suggest a favorable net clinical benefit (defined as NNT minus NNH) when adding DOAC to APT after ACS, particularly in patients presenting with STEMI. Future RCTs that investigate the role of DOAC in secondary prevention after ACS should further investigate these hypothesis-generating findings to fully elucidate the different effect of DOAC in addition to APT in patients with STEMI.
Study Limitations
This study should be interpreted in light of some limitations. First, this is a study-level meta-analysis and the findings provide mean treatment effects. The lack of patient-level data prevents us from assessing the effect of baseline clinical characteristics and treatment strategies on DOAC treatment effects. Second, this meta-analysis shares the limits of the included RCTs. These studies mainly enrolled relatively young men with preserved renal function, who typically present a low bleeding risk. Third, different bleeding definitions have been used in the included studies, impairing the reliability of effect estimates for this end point. Moreover, in the included studies, DOAC in addition to last-generation P2Y12 inhibitors (ie, prasugrel or ticagrelor) have not been investigated. This combination could expose patients to a further increase in bleeding risk, with limited advantages in the reduction of ischemic events. Finally, a meta-regression analysis has been performed to assess the effect of different bleeding severity on the risk of mortality with interesting results. However, this analysis should be considered hypothesis-generating because it was not prespecified and included few studies.
Conclusions
The findings of this meta-analysis indicate that the clinical benefits of DOAC in addition to APT for secondary prevention after ACS might depend on the type of ACS. In patients with NSTE-ACS, the risk-benefit profile of DOAC in addition to APT appears to be unfavorable. In patients with STEMI, DOAC in addition to APT appears to improve outcomes in terms of ischemic events at the cost of a marginally increased risk of major bleeding. Future studies should focus on the role of DOAC in addition to APT in patients with STEMI who are at low bleeding risk.
eTable. Risk of Bias Assessment
eAppendix.
eFigure 1. Funnel Plot for the Primary Efficacy End Point (Egger test, P = 0.708)
eFigure 2. Funnel Plot for the Primary Safety End Point (Egger test, P = 0.787)
eFigure 3. Risk of Cardiovascular Death With DOAC Versus Alacebo in Addition to APT Stratified by ACS Type
eFigure 4. Risk of Myocardial Infarction With DOAC Versus Placebo in Addition to APT Stratified by ACS Type.
eFigure 5. Risk of Stroke With DOAC Versus Placebo in Addition to APT Stratified by ACS type.
eFigure 6. Meta-regression Analysis: Impact on Death from All Causes of Major Bleedings.
eFigure 7. Meta-regression Analysis: Impact on Death from All Causes of Clinically Relevant Bleedings
eReferences
References
- 1.Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362(23):2155-2165. [DOI] [PubMed] [Google Scholar]
- 2.Mensah GA, Wei GS, Sorlie PD, et al. Decline in cardiovascular mortality: possible causes and implications. Circ Res. 2017;120(2):366-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ibanez B, James S, Agewall S, et al. ; ESC Scientific Document Group . 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2017;2:2017.28886621 [Google Scholar]
- 4.Roffi M, Patrono C, Collet J-P, et al. ; Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology . 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(3):267-315. [DOI] [PubMed] [Google Scholar]
- 5.Steg PG, James SK, Atar D, et al. ; Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC) . ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33(20):2569-2619. doi: 10.1093/eurheartj/ehs215 [DOI] [PubMed] [Google Scholar]
- 6.Amsterdam EA, Wenger NK, Brindis RG, et al. ; ACC/AHA Task Force Members . 2014 AHA/ACC guideline for the management of patients with non–ST-segment elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130(25):e344-e426. [DOI] [PubMed] [Google Scholar]
- 7.Fihn SD, Gardin JM, Abrams J, et al. ; American College of Cardiology Foundation/American Heart Association Task Force . 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2012;126(25):e354-e471. [DOI] [PubMed] [Google Scholar]
- 8.Rothberg MB, Celestin C, Fiore LD, Lawler E, Cook JR. Warfarin plus aspirin after myocardial infarction or the acute coronary syndrome: meta-analysis with estimates of risk and benefit. Ann Intern Med. 2005;143(4):241-250. [DOI] [PubMed] [Google Scholar]
- 9.Testa L, Zoccai GB, Porto I, et al. Adjusted indirect meta-analysis of aspirin plus warfarin at international normalized ratios 2 to 3 versus aspirin plus clopidogrel after acute coronary syndromes. Am J Cardiol. 2007;99(12):1637-1642. [DOI] [PubMed] [Google Scholar]
- 10.Oldgren J, Wallentin L, Alexander JH, et al. New oral anticoagulants in addition to single or dual antiplatelet therapy after an acute coronary syndrome: a systematic review and meta-analysis. Eur Heart J. 2013;34(22):1670-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Komócsi A, Vorobcsuk A, Kehl D, Aradi D. Use of new-generation oral anticoagulant agents in patients receiving antiplatelet therapy after an acute coronary syndrome: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172(20):1537-1545. [DOI] [PubMed] [Google Scholar]
- 12.Ogawa H, Goto S, Matsuzaki M, Hiro S, Shima D; APPRAISE-J Investigators . Randomized, double-blind trial to evaluate the safety of apixaban with antiplatelet therapy after acute coronary syndrome in Japanese patients (APPRAISE-J). Circ J. 2013;77(9):2341-2348. [DOI] [PubMed] [Google Scholar]
- 13.Alexander JH, Becker RC, Bhatt DL, et al. ; APPRAISE Steering Committee and Investigators . Apixaban, an oral, direct, selective factor Xa inhibitor, in combination with antiplatelet therapy after acute coronary syndrome: results of the Apixaban for Prevention of Acute Ischemic and Safety Events (APPRAISE) trial. Circulation. 2009;119(22):2877-2885. [DOI] [PubMed] [Google Scholar]
- 14.Alexander JH, Lopes RD, James S, et al. ; APPRAISE-2 Investigators . Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med. 2011;365(8):699-708. [DOI] [PubMed] [Google Scholar]
- 15.Mega JL, Braunwald E, Mohanavelu S, et al. ; ATLAS ACS-TIMI 46 Study Group . Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS-TIMI 46): a randomised, double-blind, phase II trial. Lancet. 2009;374(9683):29-38. [DOI] [PubMed] [Google Scholar]
- 16.Mega JL, Braunwald E, Wiviott SD, et al. ; ATLAS ACS 2–TIMI 51 Investigators . Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366(1):9-19. [DOI] [PubMed] [Google Scholar]
- 17.Steg PG, Mehta SR, Jukema JW, et al. ; RUBY-1 Investigators . RUBY-1: a randomized, double-blind, placebo-controlled trial of the safety and tolerability of the novel oral factor Xa inhibitor darexaban (YM150) following acute coronary syndrome. Eur Heart J. 2011;32(20):2541-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oldgren J, Budaj A, Granger CB, et al. ; RE-DEEM Investigators . Dabigatran vs. placebo in patients with acute coronary syndromes on dual antiplatelet therapy: a randomized, double-blind, phase II trial. Eur Heart J. 2011;32(22):2781-2789. [DOI] [PubMed] [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JPT, Sterne JAC, Savović J, et al. A revised tool for assessing risk of bias in randomized trials. Cochrane Database Syst Rev. 2016;10(suppl 1):29-31. doi: 10.1002/14651858.CD201601 [DOI] [Google Scholar]
- 21.Steinberg BA, Shrader P, Thomas L, et al. ; ORBIT-AF Investigators and Patients . Off-label dosing of non-vitamin K antagonist oral anticoagulants and adverse outcomes: the ORBIT-AF II registry. J Am Coll Cardiol. 2016;68(24):2597-2604. [DOI] [PubMed] [Google Scholar]
- 22.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Fixed-effect versus random-effects models. https://www.meta-analysis.com/downloads/Meta-analysis%20Fixed-effect%20vs%20Random-effects%20models.pdf. Accessed August 8, 2017.
- 23.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323(7304):101-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baker WL, White CM, Cappelleri JC, Kluger J, Coleman CI; Health Outcomes, Policy, and Economics (HOPE) Collaborative Group . Understanding heterogeneity in meta-analysis: the role of meta-regression. Int J Clin Pract. 2009;63(10):1426-1434. [DOI] [PubMed] [Google Scholar]
- 26.Higgins J, Green S. Sensitivity analysis. http://handbook-5-1.cochrane.org/. Accessed December 8, 2017.
- 27.Puymirat E, Simon T, Steg PG, et al. ; USIK USIC 2000 Investigators; FAST MI Investigators . Association of changes in clinical characteristics and management with improvement in survival among patients with ST-segment elevation myocardial infarction. JAMA. 2012;308(10):998-1006. [DOI] [PubMed] [Google Scholar]
- 28.Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J. 2016;37(42):3232-3245. [DOI] [PubMed] [Google Scholar]
- 29.Hartley A, Marshall DC, Salciccioli JD, Sikkel MB, Maruthappu M, Shalhoub J. Trends in mortality from ischemic heart disease and cerebrovascular disease in Europe: 1980-2009. Circulation. 2016;133(20):1916-1926. [DOI] [PubMed] [Google Scholar]
- 30.Wallentin L, Becker RC, Budaj A, et al. ; PLATO Investigators . Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045-1057. [DOI] [PubMed] [Google Scholar]
- 31.Wiviott SD, Braunwald E, McCabe CH, et al. ; TRITON-TIMI 38 Investigators . Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001-2015. [DOI] [PubMed] [Google Scholar]
- 32.Morrow DA, Braunwald E, Bonaca MP, et al. ; TRA 2P–TIMI 50 Steering Committee and Investigators . Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med. 2012;366(15):1404-1413. [DOI] [PubMed] [Google Scholar]
- 33.Tricoci P, Huang Z, Held C, et al. ; TRACER Investigators . Thrombin-receptor antagonist vorapaxar in acute coronary syndromes. N Engl J Med. 2012;366(1):20-33. [DOI] [PubMed] [Google Scholar]
- 34.Ohman EM, Roe MT, Steg PG, et al. Clinically significant bleeding with low-dose rivaroxaban versus aspirin, in addition to P2Y12 inhibition, in acute coronary syndromes (GEMINI-ACS-1): a double-blind, multicentre, randomised trial. Lancet. 2017;389(10081):1799-1808. [DOI] [PubMed] [Google Scholar]
- 35.Connolly SJ, Eikelboom JW, Bosch J, et al. ; COMPASS Investigators . Rivaroxaban with or without aspirin in patients with stable coronary artery disease: an international, randomised, double-blind, placebo-controlled trial [published online November 10, 2017]. Lancet. doi: 10.1016/S0140-6736(17)32458-3 [DOI] [PubMed] [Google Scholar]
- 36.Eikelboom JW, Connolly SJ, Bosch J, et al. ; COMPASS Investigators . Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377(14):1319-1330. [DOI] [PubMed] [Google Scholar]
- 37.Angiolillo DJ, Capodanno D, Goto S. Platelet thrombin receptor antagonism and atherothrombosis. Eur Heart J. 2010;31(1):17-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brass LF. Thrombin and platelet activation. Chest. 2003;124(3)(suppl):18S-25S. [DOI] [PubMed] [Google Scholar]
- 39.Szczeklik A, Dropinski J, Radwan J, Krzanowski M. Persistent generation of thrombin after acute myocardial infarction. Arterioscler Thromb. 1992;12(5):548-553. [DOI] [PubMed] [Google Scholar]
- 40.Merlini PA, Ardissino D, Rosenberg RD, et al. In vivo thrombin generation and activity during and after intravenous infusion of heparin or recombinant hirudin in patients with unstable angina pectoris. Arterioscler Thromb Vasc Biol. 2000;20(9):2162-2166. [DOI] [PubMed] [Google Scholar]
- 41.Loeffen R, van Oerle R, Leers MPG, et al. Factor XIa and thrombin generation are elevated in patients with acute coronary syndrome and predict recurrent cardiovascular events. PLoS One. 2016;11(7):e0158355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Caterina R, Husted S, Wallentin L, et al. Oral anticoagulants in coronary heart disease (Section IV). Thromb Haemost. 2016;115(4):685-711. [DOI] [PubMed] [Google Scholar]
- 43.Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KAA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114(8):774-782. [DOI] [PubMed] [Google Scholar]
- 44.Mehran R, Pocock S, Nikolsky E, et al. Impact of bleeding on mortality after percutaneous coronary intervention results from a patient-level pooled analysis of the REPLACE-2 (randomized evaluation of PCI linking angiomax to reduced clinical events), ACUITY (acute catheterization and urgent intervention triage strategy), and HORIZONS-AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trials. JACC Cardiovasc Interv. 2011;4(6):654-664. [DOI] [PubMed] [Google Scholar]
- 45.Rao SV, O’Grady K, Pieper KS, et al. Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am J Cardiol. 2005;96(9):1200-1206. [DOI] [PubMed] [Google Scholar]
- 46.Ndrepepa G, Berger PB, Mehilli J, et al. Periprocedural bleeding and 1-year outcome after percutaneous coronary interventions: appropriateness of including bleeding as a component of a quadruple end point. J Am Coll Cardiol. 2008;51(7):690-697. [DOI] [PubMed] [Google Scholar]
- 47.Giugliano RP, Giraldez RR, Morrow DA, et al. Relations between bleeding and outcomes in patients with ST-segment elevation myocardial infarction in the ExTRACT-TIMI 25 trial. Eur Heart J. 2010;31(17):2103-2110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Risk of Bias Assessment
eAppendix.
eFigure 1. Funnel Plot for the Primary Efficacy End Point (Egger test, P = 0.708)
eFigure 2. Funnel Plot for the Primary Safety End Point (Egger test, P = 0.787)
eFigure 3. Risk of Cardiovascular Death With DOAC Versus Alacebo in Addition to APT Stratified by ACS Type
eFigure 4. Risk of Myocardial Infarction With DOAC Versus Placebo in Addition to APT Stratified by ACS Type.
eFigure 5. Risk of Stroke With DOAC Versus Placebo in Addition to APT Stratified by ACS type.
eFigure 6. Meta-regression Analysis: Impact on Death from All Causes of Major Bleedings.
eFigure 7. Meta-regression Analysis: Impact on Death from All Causes of Clinically Relevant Bleedings
eReferences