This retrospective cohort study evaluates the risk for heart disease or death in women with an infant born preterm and severely small for gestational weight.
Key Points
Question
Are women with an infant born preterm and concomitantly are severely small for gestational age at higher risk of premature heart disease or death?
Findings
This population-based retrospective cohort study comprised 710 501 singleton live births. Relative to infants who were not born preterm or were not severely small for gestational weight, the adjusted hazard ratio of the composite outcome of heart failure, dysrhythmia, or death was 1.66 (95% CI, 1.09-2.52) in women with a prior child who was born preterm and was severely small for gestational weight.
Meaning
Women with an infant born preterm and severely small for gestational weight may be at higher risk for premature cardiac disease or death.
Abstract
Importance
Women with an infant with preterm birth (PTB) or who was severely small for gestational age (SGA) are at higher future risk of premature cardiovascular disease and related death.
Objective
To determine the risk of cardiac disease or death among women with an infant with both PTB and SGA.
Design, Setting, and Participants
This population-based cohort study used electronic health records from the province of Ontario, Canada, where health care is universally available, between April 1, 2002, and March 31, 2016. All singleton live births between 23 to 42 weeks’ gestation among 710 501 nulliparous women aged 16 to 50 years without prepregnancy cardiac disease were analyzed.
Main Outcomes and Measures
Risk of a composite outcome of heart failure, atrial or ventricular dysrhythmia, or all-cause mortality, starting 30 days after the index birth. Hazard ratios were adjusted for maternal age, income quintile, and preeclampsia/eclampsia (each at the index birth), as well as diabetes, chronic hypertension, obesity, dyslipidemia, drug dependence or smoking, and kidney disease (each within 24 months before the index birth date and time-varying from the birth date onward).
Results
Of 710 501 singleton live births, 15 082 mothers (2.1%) were older than age 40 years. Relative to having an infant without PTB or severe SGA (4.1 per 10 000 person-years), the incidence rate of the composite outcome of heart failure, dysrhythmia, or death was 11.3 per 10 000 person-years among mothers with an infant with PTB-SGA (crude hazard ratio, 2.79; 95% CI, 1.85-4.21) (adjusted hazard ratio, 1.66; 95% CI, 1.09-2.52).
Conclusions and Relevance
Women who had an infant with PTB-SGA may be at higher future risk of premature cardiac disease or death.
Introduction
Infants with preterm birth (PTB) or who were small for gestational age (SGA) are likely to have experienced a pathological intrauterine environment, especially infants with severe SGA (ie, smaller than the fifth percentile).1 When PTB and SGA are present together (ie, PTB-SGA), more severe underlying placental vascular disease is seen. Women who previously gave birth to an infant with PTB or SGA are at higher future risk of premature cardiovascular disease2,3,4,5 and related death.6 The maternal metabolic syndrome may be one explanation linking placental vascular disease to future maternal cardiovascular disease.7 It is not known if women with infants with PTB-SGA are at especially high risk of premature heart disease or death, which is what this study seeks to find.
Methods
Study Sample
A retrospective cohort study was completed using linked administrative health databases for the entire province of Ontario, Canada. Residents receive universal health care under the Ontario Health Insurance Plan. We included all nulliparous mothers aged 16 to 50 years who had a singleton live birth at 23 to 42 weeks’ gestation from April 1, 2002, to March 31, 2015. The sources and details for hospitalization and outpatient data are described in the eTable in the Supplement, as previously elsewhere.4,5,7 Excluded were women with any recognized form of heart disease within 2 years before the index birth. Data sets were linked using unique encoded identifiers and analyzed at the Institute for Clinical Evaluative Sciences. Institutional review board approval was granted by the Research Ethics Board of Sunnybrook Health Sciences Centre. Patients did not need to provide informed consent because data were deidentified.
Exposures and Outcomes
The main exposure at each woman’s first delivery was 1 of 4 possible states: (1) PTB at 23 to 36 weeks’ gestation with concomitant severe SGA birth weight under the fifth percentile for sex and gestational age8 (PTB-SGA); (2) PTB at 23 to 36 weeks’ gestation without severe SGA; (3) term birth with severe SGA; or (4) neither PTB nor SGA (the reference).
There were 3 composite study outcomes: (1) hospitalization for heart failure or an atrial or ventricular dysrhythmia or all-cause mortality; (2) hospitalization for heart failure or an atrial or ventricular dysrhythmia; and (3) hospitalization for heart failure or an atrial or ventricular dysrhythmia or coronary artery disease (eTable in the Supplement). To minimize the immediate effect of the index pregnancy (eg, preeclampsia) or delivery (eg, iatrogenic fluid overload) on the risk of related cardiac events, study outcomes were assessed starting at 30 days after the index birth date. Details on cause-specific mortality were not available. The administrative data diagnostic codes used herein have largely been validated (eTable in the Supplement).
Data Analysis
Multivariable Cox proportional hazard models were used to generate hazard ratios (HRs) and 95% confidence intervals, with censoring at the end of the study period (March 31, 2016) or death (for composite outcomes 2 and 3 only). Hazard ratios were first adjusted for maternal age, income quintile, and preeclampsia/eclampsia (each at the index birth), as well as diabetes, chronic hypertension, obesity, dyslipidemia, drug dependence or smoking, and kidney disease (each within 24 months before the index birth date) (model 1). Hazard ratios were further adjusted for diabetes, chronic hypertension, obesity, dyslipidemia, drug dependence or smoking, and kidney disease, time-varying from the index birth date onward (model 2). Births with a congenital or chromosomal anomaly were then excluded from model 2, as they are more prone to be SGA and PTB and might, therefore, obscure any association between PTB-SGA and maternal cardiac disease. Statistical analyses were performed using SAS version 9.4 (SAS Institute), with significance set at a P value of less than .05.
Results
There were 710 501 singleton live births included, of which 619 311 (87.2%) were term birth without severe SGA, 43 240 (6.1%) were term birth with severe SGA, 45 134 (6.3%) were PTB without severe SGA, and 2816 (0.4%) were PTB-SGA (Table 1). At the index delivery, mothers with a PTB-SGA infant had more chronic hypertension than women with neither PTB nor SGA and more preeclampsia as well (Table 1). Dyslipidemia, kidney disease, and South Asian origin were more prevalent among the former group of women. The median follow-up of the mothers was about 7.5 years (Table 1).
Table 1. Characteristics of 710 501 Singleton Live Births and Their Nulliparous Mothersa.
| Characteristic | Term Birth Without Severe
SGA (n = 619 311) |
Term Birth With Severe
SGA (n = 43 240) |
PTB Without Severe
SGA (n = 45 134) |
PTB With Severe
SGA (n = 2816) |
|---|---|---|---|---|
| Mother | ||||
| Age at delivery, mean (SD), y | 28.3 (5.5) | 28.1 (5.7) | 28.8 (5.8) | 29.1 (6.1) |
| ≥40 y | 12 570 (2.0) | 1022 (2.4) | 1352 (3.0) | 138 (4.9) |
| Income quintile at delivery | ||||
| Quintile 1 (lowest) | 131 402 (21.2) | 11 142 (25.8) | 9939 (22.0) | 729 (25.9) |
| Quintile 5 (highest) | 100 747 (16.3) | 5889 (13.6) | 6885 (15.3) | 403 (14.3) |
| Unknown | 2730 (0.4) | 138 (0.3) | 169 (0.4) | 7 (0.2) |
| Place of residence at deliveryb | ||||
| Rural | 59 135 (9.5) | 3216 (7.4) | 4251 (9.4) | 230 (8.2) |
| World region of originb | ||||
| Canada | 472 527 (76.3) | 28 756 (66.5) | 35 273 (78.2) | 1953 (69.4) |
| Sub-Saharan Africa | 7767 (1.3) | 788 (1.8) | 563 (1.2) | 64 (2.3) |
| Caribbean | 7061 (1.1) | 897 (2.1) | 773 (1.7) | 63 (2.2) |
| East Asia and Pacific Islands | 39 430 (6.4) | 3464 (8.0) | 2495 (5.5) | 170 (6.0) |
| Latin America | 11 351 (1.8) | 1081 (2.5) | 841 (1.9) | 72 (2.6) |
| Middle East and North Africa | 13 770 (2.2) | 1049 (2.4) | 776 (1.7) | 61 (2.2) |
| South Asia | 41 109 (6.6) | 5706 (13.2) | 2877 (6.4) | 333 (11.8) |
| Westernc | 26 264 (4.2) | 1498 (3.5) | 1534 (3.4) | 100 (3.6) |
| Diagnosis received ≤24 mo before delivery or thereafter | ||||
| Diabetes | 42 432 (6.9) | 3039 (7.0) | 5180 (11.5) | 278 (9.9) |
| Chronic hypertension | 33 403 (5.4) | 3014 (7.0) | 5809 (12.9) | 818 (29.0) |
| Obesity | 18 038 (2.9) | 924 (2.1) | 1617 (3.6) | 91 (3.2) |
| Dyslipidemia | 11 561 (1.9) | 835 (1.9) | 1077 (2.4) | 69 (2.5) |
| Drug dependence or smoking | 13 247 (2.1) | 1370 (3.2) | 1357 (3.0) | 100 (3.6) |
| Kidney disease | 2457 (0.4) | 224 (0.5) | 605 (1.3) | 68 (2.4) |
| Preeclampsia or eclampsia at delivery | 6898 (1.1) | 895 (2.1) | 4077 (9.0) | 676 (24.0) |
| Infant at Birth | ||||
| Female | 304 158 (49.1) | 20 797 (48.1) | 20 075 (44.5) | 1244 (44.2) |
| Birth weight, mean (SD), g | 3472.0 (429.9) | 2618.4 (250.3) | 2415.8 (659.8) | 1571.5 (450.1) |
| Gestational age, mean (SD), wk | 39.4 (1.2) | 39.3 (1.2) | 34.1 (2.7) | 34.2 (2.7) |
| 23-31 | 0 | 0 | 6020 (13.3) | 365 (13.0) |
| 32-36 | 0 | 0 | 39 114 (86.7) | 2451 (87.0) |
| 37-42 | 619 311 (100) | 43 240 (100) | 0 | 0 |
| Congenital or chromosomal anomaly | 16 108 (2.6) | 1657 (3.8) | 4208 (9.3) | 469 (16.6) |
| Duration of maternal follow-up, starting 30 d after the index birth, median (IQR), y | 7.5 (4.2 to 10.7) | 7.3 (4.1 to 10.5) | 7.6 (4.3 to 10.7) | 7.1 (3.9 to 10.4) |
Abbreviations: IQR, interquartile range; PTB, preterm birth; SGA, small for gestational age (birth weight below the fifth percentile).
All data are presented as No. (%) unless otherwise indicated.
Totals do not add up because of unknown data.
Includes several Western nations, such as Europe, the United States, Australia, New Zealand, and Britain.
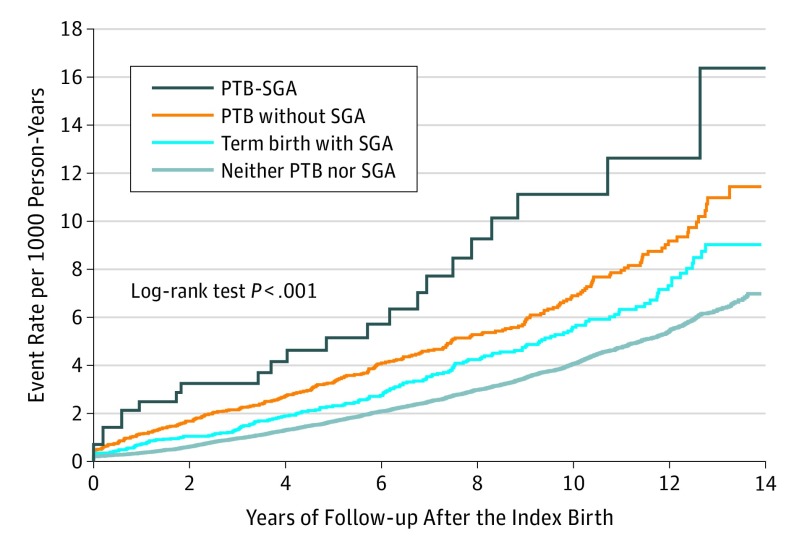
Women with a PTB-SGA infant were most likely to experience the composite outcome of hospitalization for heart failure, atrial or ventricular dysrhythmia, or death (Figure). Relative to having a term birth infant without severe SGA, the corresponding crude HR was 2.79 (95% CI, 1.85-4.21) among those with PTB-SGA (Table 2). The HRs were somewhat reduced on adjusting for baseline covariates (model 1 in Table 2) and further attenuated by adding the time-varying covariates after the index delivery (model 2 in Table 2). Excluding births with a congenital or chromosomal anomaly, the adjusted HR in model 2 for the composite of heart failure, dysrhythmia, or death was largely unchanged among women with PTB-SGA (HR, 1.66; 95% CI, 1.05-2.62).
Figure. Cumulative Probability of the Maternal Composite Cardiac Outcome Associated With Infants’ PTB or SGA Status.
The maternal composite cardiac outcomes were hospitalization for heart failure, atrial or ventricular dysrhythmia, or death. Data are presented for all singleton live births from 23 to 42 weeks’ gestation, and assessment of the study outcome started at 30 days after the index birth date. PTB indicates preterm birth at 23 to 36 weeks’ gestation; SGA, small for gestational weight below the fifth percentile.
Table 2. Risk of the Maternal Composite Cardiac Outcomes or Death Starting 30 Days After a Live Birth Pregnancya.
| Composite Outcome According to Exposure Status | No. (Incidence Rate per 10 000 Person-y) | Crude Hazard Ratio (95% CI) | Adjusted Hazard Ratio (95% CI) | |
|---|---|---|---|---|
| Model 1b | Model 2c | |||
| Heart Failure, Atrial or Ventricular Dysrhythmia, or Deathd | ||||
| Term birth without severe SGA (n = 619 311) | 1879 (4.1) | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Term birth with severe SGA (n = 43 240) | 178 (5.6) | 1.38 (1.19-1.61) | 1.33 (1.14-1.55) | 1.27 (1.09-1.48) |
| PTB without severe SGA (n = 45 134) | 246 (7.3) | 1.78 (1.56-2.03) | 1.55 (1.35-1.78) | 1.38 (1.20-1.59) |
| PTB with severe SGA (n = 2816) | 23 (11.3) | 2.79 (1.85-4.21) | 2.06 (1.35-3.13) | 1.66 (1.09-2.52) |
| Heart Failure or Atrial or Ventricular Dysrhythmiae | ||||
| Term birth without severe SGA (n = 619 311) | 585 (1.3) | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Term birth with severe SGA (n = 43 240) | 59 (1.9) | 1.47 (1.12-1.92) | 1.39 (1.06-1.82) | 1.33 (1.02-1.74) |
| PTB without severe SGA (n = 45 134) | 93 (2.7) | 2.17 (1.74-2.69) | 1.66 (1.32-2.09) | 1.44 (1.15-1.82) |
| PTB with severe SGA (n = 2816) | 8 (3.9) | 3.10 (1.55-6.24) | 1.76 (0.87-3.59) | 1.37 (0.67-2.78) |
| Heart Failure, Atrial or Ventricular Dysrhythmia, or Coronary Artery Diseasee | ||||
| Term birth without severe SGA (n = 619 311) | 844 (1.8) | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Term birth with severe SGA (n = 43 240) | 80 (2.5) | 1.38 (1.10-1.74) | 1.31 (1.04-1.65) | 1.22 (0.97-1.53) |
| PTB without severe SGA (n = 45 134) | 144 (4.3) | 2.32 (1.95-2.77) | 1.75 (1.46-2.11) | 1.38 (1.15-1.67) |
| PTB with severe SGA (n = 2816) | 12 (5.9) | 3.24 (1.83-5.73) | 1.82 (1.02-3.25) | 1.21 (0.68-2.17) |
Abbreviations: PTB, preterm birth; SGA, small for gestational age (birth weight below the fifth percentile).
Data are presented for all singleton live births from 23 to 42 weeks’ gestation.
Hazard ratios are adjusted for maternal age, income quintile, and preeclampsia/eclampsia (each at birth), as well as diabetes, chronic hypertension, obesity, dyslipidemia, drug dependence or smoking, and kidney disease (each ≤24 mo before the birth date).
Hazard ratios are further adjusted for diabetes, chronic hypertension, obesity, dyslipidemia, drug dependence or smoking, and kidney disease (time varying from the index birth date onward).
Model not censored on death.
Model censored on death.
For the composite cardiac outcome of heart failure or atrial or ventricular dysrhythmia, the HR was significantly higher in women with a PTB-SGA birth but markedly attenuated on adjusting for risk factors before and after delivery (Table 2). A similar finding was seen for the composite outcome of hospitalization for heart failure, an atrial or ventricular dysrhythmia, or coronary artery disease (Table 2).
Discussion
Although this is the first study to directly assess the association of PTB-SGA and cardiac disease or death, to our knowledge, related studies support the current findings. Among 70 182 parous women in the Nurses’ Health Study II3 who were followed for a median of 32 years, the adjusted HRs for myocardial infarction or stroke was 1.42 (95% CI, 1.16-1.72) in those with vs without PTB at less than 37 weeks and 2.01 (95% CI, 1.47-2.75) among women with very PTB at less than 32 weeks. In a Scottish data-linkage cohort study, the risk of ischemic heart disease was greatest among women who had preeclampsia with an infant both SGA and born preterm.9 We adjusted for preeclampsia and still found that mothers who had a PTB-SGA infant were at higher risk of cardiac disease or death. Among 3225 Norwegian women who underwent standardized measurements of blood pressure, serum lipids, and body mass index (calculated as weight in kilograms divided by height in meters squared) before and after pregnancy, the association between preeclampsia and postpregnancy cardiovascular risk was partly due to prepregnancy risk factors.10 It is unlikely that having a PTB-SGA infant causes future heart disease or death in the mother. Rather, the same factors that predispose severe placental vascular disease to develop in a woman,1 and hence PTB-SGA, also predispose myocardial dysfunction and coronary artery disease to prematurely develop.5 As seen elsewhere,3 this point is underscored by the observed reduction in the HR once postpregnancy risk factors were added to model 2.
Direct assessment of myocardial function before and after pregnancy and its changes over time might improve our understanding of the apparent associations between placental vascular disease, PTB-SGA, and a woman’s future risk of cardiac disease, especially in those with recurrent PTB-SGA. The reasons for spontaneous vs clinician-initiated PTB should also be evaluated, as the latter are more likely due to preeclampsia and poor fetal growth arising from placental vascular disease.11 We used a more pathological cut-point of less than the fifth percentile to denote SGA.1 At the more standard tenth percentile cut-point,8,9 many more women would qualify as having a PTB-SGA birth, but the resulting change to the current study outcomes is unknown.
Strengths and Limitations
We included more than 700 000 women in a universal health care setting. Mothers with prior cardiac disease were excluded from this cohort, and we accounted for some risk factors for PTB and SGA. In the study period, there were no notable changes in Ontario in the nature of cardiac disease diagnosis or physician reimbursement.
The median duration of follow-up was about 7.5 years, which is not long enough for adverse outcome events to develop. Furthermore, as there were only 8 events of heart failure or a dysrhythmia among women with PTB-SGA, analysis of this outcome was likely statistically underpowered and perhaps not generalizable. Another limitation was lack of availability of echocardiographic assessment of left ventricular function or electrocardiographic determination of electrical activity. Our data were limited to hospitalized cardiac events, so out-of-hospital sudden cardiac death might only be indirectly captured under all-cause mortality. A pregnancy affected by severe placental vascular disease may result in fetal growth restriction and stillbirth; yet, our study was limited to live births. Women who experience a stillbirth may be especially at high future risk for cardiac disease.12,13 Finally, there was likely residual confounding from factors not well measured in these data sets, such as body mass index and smoking.
Conclusions
Women who gave birth to an infant with PTB-SGA were at subsequent high risk of premature heart disease or death. This association was attenuated by classic cardiometabolic risk factors assessed before and after the index delivery. A clear understanding of the causes of death in women with prior PTB-SGA is needed, especially given that about two thirds of events in the main composite outcome were deaths. It should also be determined if cardiometabolic modification in women with PTB-SGA can reduce their risk of premature heart disease and death.
eTable. Variables Used to Define Cohort Entry/Exclusion Criteria and Study Exposure, Outcome, and Adjustment Variables
References
- 1.Ray JG, Park AL, Fell DB. Mortality in infants affected by preterm birth and severe small-for-gestational age birth weight. Pediatrics. 2017;140(6):e20171881. [DOI] [PubMed] [Google Scholar]
- 2.Rich-Edwards JW, Fraser A, Lawlor DA, Catov JM. Pregnancy characteristics and women’s future cardiovascular health: an underused opportunity to improve women’s health? Epidemiol Rev. 2014;36:57-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanz LJ, Stuart JJ, Williams PL, et al. Preterm delivery and maternal cardiovascular disease in young and middle-aged adult women. Circulation. 2017;135(6):578-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (CHAMPS): population-based retrospective cohort study. Lancet. 2005;366(9499):1797-1803. [DOI] [PubMed] [Google Scholar]
- 5.Ray JG, Schull MJ, Kingdom JC, Vermeulen MJ. Heart failure and dysrhythmias after maternal placental syndromes: HAD MPS Study. Heart. 2012;98(15):1136-1141. [DOI] [PubMed] [Google Scholar]
- 6.Smith GD, Whitley E, Gissler M, Hemminki E. Birth dimensions of offspring, premature birth, and the mortality of mothers. Lancet. 2000;356(9247):2066-2067. [DOI] [PubMed] [Google Scholar]
- 7.Ray JG, Vermeulen MJ, Schull MJ, McDonald S, Redelmeier DA. Metabolic syndrome and the risk of placental dysfunction. J Obstet Gynaecol Can. 2005;27(12):1095-1101. [DOI] [PubMed] [Google Scholar]
- 8.Ray JG, Sgro M, Mamdani MM, et al. Birth weight curves tailored to maternal world region. J Obstet Gynaecol Can. 2012;34(2):159-171. [DOI] [PubMed] [Google Scholar]
- 9.Smith GC, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129,290 births. Lancet. 2001;357(9273):2002-2006. [DOI] [PubMed] [Google Scholar]
- 10.Romundstad PR, Magnussen EB, Smith GD, Vatten LJ. Hypertension in pregnancy and later cardiovascular risk: common antecedents? Circulation. 2010;122(6):579-584. [DOI] [PubMed] [Google Scholar]
- 11.Morisaki N, Togoobaatar G, Vogel JP, et al. ; WHO Multicountry Survey on Maternal and Newborn Health Research Network . Risk factors for spontaneous and provider-initiated preterm delivery in high and low Human Development Index countries: a secondary analysis of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG. 2014;121(suppl 1):101-109. [DOI] [PubMed] [Google Scholar]
- 12.Flenady V, Koopmans L, Middleton P, et al. Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet. 2011;377(9774):1331-1340. [DOI] [PubMed] [Google Scholar]
- 13.Mullan Z, Horton R. Bringing stillbirths out of the shadows. Lancet. 2011;377(9774):1291-1292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Variables Used to Define Cohort Entry/Exclusion Criteria and Study Exposure, Outcome, and Adjustment Variables