Key Points
Question
Can the full extent of routine preoperative testing in patients undergoing cataract surgery be ascertained to update previous studies’ use of 30-day preoperative testing windows?
Findings
In this cross-sectional study of 440 857 Medicare beneficiaries undergoing cataract surgery, testing rates increased 41% during the interval between ocular biometry and cataract surgery, even after excluding tests occurring within 30 days before surgery. This expanded capture method estimates that Medicare expenditures for routine preoperative testing cost up to $45.4 million annually.
Meaning
This study has the limitations associated with using administrative billing databases but suggests that routine preoperative testing can be identified earlier than 30 days before surgery and is costlier than previously described.
This cross-sectional study used a data set on Medicare beneficiaries receiving cataract surgery to identify all routine preoperative testing that takes place after the decision is made to operate.
Abstract
Importance
Routine preoperative medical testing is not recommended for patients undergoing low-risk surgery, but testing is common before surgery. A 30-day preoperative testing window is conventionally used for study purposes; however, the extent of routine testing that occurs prior to that point is unknown.
Objective
To improve on existing cost estimates by identifying all routine preoperative testing that takes place after the decision is made to perform cataract surgery.
Design, Setting, and Participants
This cross-sectional study assessed preoperative care in a 50% sample of Medicare beneficiaries older than 66 years who underwent ambulatory cataract surgery in 2011. Data analysis was completed from March 2016 to October 2017.
Main Outcomes and Measures
Using ocular biometry as a procedure-specific indicator to mark the start of the routine preoperative testing window, we measured testing rates in the interval between ocular biometry and cataract surgery and compared this with testing rates in the 6 months preceding biometry. We estimated the total cost of testing that occurred between biometry and cataract surgery.
Results
A total of 440 857 patients underwent cataract surgery. A total of 423 710 (96.1%) had an ocular biometry claim before index surgery, of whom 264 514 (60.0%) were female; the mean (SD) age of the cohort was 76.1 (6.2) years. A total of 111 998 (25.4%) underwent surgery more than 30 days after biometry. Among patients with a biometry claim, the mean number of tests/patient/month increased from 1.1 in the baseline period to 1.7 in the interval between biometry and cataract surgery. Although preoperative testing peaked in all patients in the 30 days preceding surgery (1.8 tests/patient/month), the subset of patients with no overlap between postbiometry and presurgery periods experienced increased testing rates to 1.8 tests per patient per month in the 30 days after biometry, regardless of the elapsed time between biometry and surgery. The total estimated cost of routine preoperative testing in the full cohort was $22.7 million; we estimate that routine preoperative testing costs Medicare up to $45.4 million annually.
Conclusions and Relevance
In this study of Medicare beneficiaries, routine preoperative medical testing occurs more often and is costlier than has been reported previously. Extra costs are attributable to testing that occurs prior to the 30-day window preceding surgery. As a cost-cutting measure, routine preoperative medical testing should be avoided in patients with cataracts throughout the interval between ocular biometry and cataract surgery.
Introduction
Routine preoperative medical testing is not recommended for patients undergoing low-risk surgery, yet it has been shown to occur before various types of low-risk procedures, including cataract surgery, hernia repair, and other ambulatory procedures. When assessing the rate of testing preoperatively, the bulk of prior research has only counted tests occurring in the 30 days before surgery. As a result, if a surgery took place more than 30 days after the decision was made to operate, any testing that may have occurred when surgery was first contemplated would not have been reported in prior studies. Furthermore, testing that occurred in preparation for procedures that were subsequently postponed or cancelled, irrespective of whether the postponement was a reflection of test results, have not been taken into account in previous methodologies.
This extended preoperative testing period, which begins when the decision has been made to operate and continues until the patient successfully undergoes surgery, has typically been excluded by researchers assessing administrative claims because of the difficulty of identifying postponed or cancelled surgeries using claims alone. To overcome this limitation, a claim or cluster of claims that reflects a plan for surgery must appear in the data, even if surgery itself does not occur.
One type of surgery where this surrogate marker for planned surgery exists is cataract surgery. Ocular biometry is a diagnostic test performed by ophthalmologists to determine the required power of the intraocular lens implant patients will receive during cataract surgery. This test is a necessary and final step in the ophthalmologist’s diagnostic workup of a patient who will undergo cataract surgery, and it is almost never used outside of this context. Patients with cataracts who are not surgical candidates will rarely undergo biometry. In this study, we use the biometry claim as a procedure-specific indicator to mark the occasion in which an ophthalmologist first decided to operate on a patient.
To our knowledge, there have not been any prior studies using administrative claims to assess the presence of routine preoperative testing that occurs once the decision has been made to operate. To determine the extent of testing that occurs earlier than the traditional preoperative testing window, we measured the rate, prevalence, and estimated total cost of routine preoperative testing during the interval between ocular biometry and cataract surgery.
Methods
This study was approved by the institutional review board at the University of California, San Francisco. Informed consent was not obtained because the study was deemed to pose minimal risk to patients.
Data Source
We obtained research-identifiable files from the US Centers for Medicare and Medicaid Services Research Data Distribution Center for Medicare beneficiaries who underwent cataract surgery in 2011. We obtained all claims from January 1, 2010, through December 31, 2011, for each beneficiary via the following datasets: Medicare Outpatient Files, which contains data on institutional outpatient clinicians and facilities, including hospital outpatient departments; Carrier Files, which contain data on noninstitutional clinicians and facilities, including physicians, independent clinical laboratories, and freestanding ambulatory surgical centers; MedPAR Research Identifiable Files, which contain data on inpatient hospital records; and Master Beneficiary Summary Files, which contain beneficiary demographic and enrollment information. With the exception of the Master Beneficiary Summary Files, all datasets include International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes, ICD-9 or Healthcare Common Procedure Coding System procedure codes, and dates of service for services rendered.
Study Cohort
We identified patients undergoing cataract surgery between January 1, 2011, and December 31, 2011, using Current Procedural Terminology codes for cataract surgery (66982, 66983, 66984, 66850, 66920, 66930, and 66940). We included patients 66 years and older who had at least 12 months of Medicare eligibility before surgery and who were enrolled in the Medicare fee-for-service program without a concurrent health maintenance organization plan. From a total of 1 004 972 eligible individuals, we obtained a random sample of 500 000 beneficiaries (49.8%). We defined each beneficiary’s surgery date as the first date of a claim submitted by an ophthalmologist for routine cataract surgery (Current Procedural Terminology codes 66982-66984). Only the cataract surgery on the first eye, or the index surgery, was included for each patient. We excluded all beneficiaries who had an ICD-9 code indicating a prior cataract surgery; had a cataract surgery claim in 2010; had an inpatient stay in the 30 days prior to the index surgery; or could not be linked to a hospital referral region. We examined patient characteristics including age, sex, race/ethnicity, and health status using the Quan modification of the Charlson Comorbidity Index.
Identification of Ocular Biometry, Preoperative Tests, Test-Ordering Clinician, Location of Surgery, and Costs
We identified Current Procedural Terminology codes for ocular biometry (76516, 76519, and 92136). In patients with multiple biometry claims, we assumed that the first biometry claim signaled the earliest indication that an ophthalmologist had planned to schedule cataract surgery.
As in our earlier study, we identified tests that are commonly performed for preoperative workup in older patients (eTable 1 in the Supplement). We categorized the tests as routine preoperative tests if they occurred in the interval between the first biometry claim and the index surgery. If the first biometry claim occurred less than 30 days before the date of index surgery, then we defaulted to categorizing all tests that occurred in the traditional 30-day period before index surgery as routine preoperative tests.
Health system characteristics were derived with the use of the ophthalmologist’s zip code, hospital referral region, and rural–urban commuting area codes. As in our earlier study, to assess preoperative testing according to clinician, we used the operating ophthalmologist to represent the physician care team (eg, the ophthalmologist, primary care physician, and anesthesiologist) responsible for managing the preoperative evaluation of the patient. We ascertained a surgical location for 98.5% of the beneficiaries (n = 434,123) by identifying an ambulatory surgery center or hospital outpatient department claim in the carrier or outpatient file that was submitted within 1 day of the ophthalmologist-submitted surgical procedure claim.
Costs were calculated on the basis of the national limitation amount from the 2011 Medicare Clinical Laboratory Fee Schedule and the nonfacility price from the 2011 Medicare Physician Fee Schedule.
Prevalence of Ocular Biometry and Rate of Routine Preoperative Testing Among Patients Undergoing Cataract Surgery
We assessed the prevalence of biometry among patients who underwent cataract surgery. We calculated the number of days between the first biometry claim and index cataract surgery claim, and determined the percentage of patients having cataract surgery on the same day, within 30 days, and within 90 days of the biometry claim.
We subdivided beneficiaries according to the duration of time in months that had elapsed between the biometry and index surgery dates (ie, patients grouped into month 1 had a range of 1-30 days between biometry and surgery, patients in month 2 had a range of 31-60 days, month 3 had a range of 61-90 days, and so on). For the combined cohort of beneficiaries having surgery more than 30, 60, and 90 days after biometry and for each monthly subcohort, we calculated the number of tests per patient per month during the interval between biometry and surgery. We depicted the results for each subcohort graphically to provide a complete representation of the testing that took place during the interval between biometry and cataract surgery (additional details about how testing rates were derived are presented in the eMethods in the Supplement).
To determine rates of testing when cataract surgery was not being planned, we calculated the number of tests per patient per month during the 6 months prior to biometry for each subcohort of patients with the same monthly time interval between biometry and surgery and for the combined cohort of beneficiaries having surgery more than 30, 60, and 90 days after biometry. If biometry occurred less than 30 days before surgery, then the 6-month baseline period was shifted 30 days earlier than the date of index surgery to exclude any preoperative testing that occurred in the 30-day period before surgery.
Subgroup Analysis
To eliminate the possibility that the testing that occurred in the 30 days after biometry was for reasons other than for preoperative evaluation, we identified a subgroup of patients with a Charlson Comorbidity Index score of 0 and who did not undergo any preoperative tests for 6 months prior to the biometry claim. We assessed their preoperative testing rate in the months following the biometry claim.
We also analyzed a subgroup of patients with 60 or more days between biometry and surgery to highlight the characteristics and testing patterns of the subset of patients who had repeated testing in the 30 days postbiometry and presurgery. These results are available in eTable 2 and eTable 3 of the Supplement.
Calculation of Excess Routine Preoperative Testing
To calculate the excess cost, prevalence, and count of routine tests during the extended preoperative testing window, a separate baseline period of equal length that started 1 year prior to the extended preoperative testing window was identified for each patient who had any preoperative testing between the dates of biometry and surgery. For the 6479 patients (1.5%) whose time from biometry to surgery duration exceeded the number of baseline months available for direct comparison, we adjusted our calculations to reflect the ratio of the number of months included in the biometry to surgery period vs the number of months included in the shorter baseline period. We then estimated the prevalence, cost, and number of routine preoperative tests during the biometry to surgery period compared with the baseline period of equal length from 1 year prior and the traditional preoperative testing window of 30 days before surgery. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc).
Results
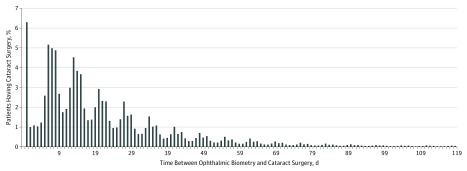
Of the 440 857 patients who had cataract surgery in 2011, 423 710 (96.1%) had a biometry claim for a procedure completed prior to index surgery (Table). Among patients with biometry, 26 639 of 423 710 (6.3%) had a biometry claim on the day of surgery, 107 812 of 423 710 (25.4%) had surgery more than 30 days after biometry, and 21 464 of 423 710 (5.1%) had surgery more than 90 days after biometry (Figure 1). The interval between biometry and surgery ranged from 0 days to 23.8 months.
Table. Characteristics of Medicare Patients Undergoing Cataract Surgery, Stratified by Preoperative Testing in the Month After Biometry.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Preoperative Testing (n = 211 034) | No Preoperative Testing (n = 212 676) | All Patients (n = 423 710) | |
| Age, mean (SD), y | 76.5 (6.3) | 75.8 (6.2) | 76.1 (6.2) |
| Sex | |||
| Male | 86 221 (40.9) | 83 903 (39.5) | 170 124 (40.2) |
| Female | 124 813 (59.1) | 128 773 (60.5) | 253 586 (59.8) |
| Race/ethnicity | |||
| White | 185 327 (87.8) | 190 627 (89.6) | 375 954 (88.7) |
| Black | 13 546 (6.4) | 12 114 (5.7) | 25 660 (6.1) |
| Other | 12 161 (5.8) | 9935 (4.7) | 22 096 (5.2) |
| Charlson Comorbidity Index | |||
| 0-1 | 112 551 (53.3) | 147 091 (69.2) | 259 642 (61.3) |
| 2 | 35 208 (16.7) | 29 565 (13.9) | 64 773 (15.3) |
| ≥3 | 63 275 (30.0) | 36 020 (16.9) | 99 295 (23.4) |
| Location of surgerya | |||
| Ambulatory surgery center | 136 784 (64.8) | 149 603 (70.3) | 286 387 (67.5) |
| Outpatient hospital department | 70 157 (33.2) | 61 319 (28.8) | 131 476 (31.0) |
| Unknown | 4093 (1.9) | 1754 (0.8) | 5847 (1.4) |
| US region of operating ophthalmologista | |||
| Northeast | 47 442 (22.5) | 24 787 (11.6) | 72 229 (17.0) |
| Midwest | 47 955 (22.7) | 54 258 (25.5) | 102 213 (24.1) |
| South | 80 327 (38.1) | 94 507 (44.4) | 174 834 (41.3) |
| Mountain West | 9329 (4.4) | 16 813 (7.9) | 26 142 (6.2) |
| Pacific West | 25 981 (12.3) | 22 311 (10.5) | 48 292 (11.4) |
| Population density of operating ophthalmologista | |||
| Urban | 177 126 (83.9) | 172 120 (80.9) | 349 246 (82.4) |
| Rural | 33 908 (16.1) | 40 556 (19.1) | 74 464 (17.6) |
| Primary care physicians per 100 000 population, rangea,b | |||
| 43.9-63.3 | 47 846 (22.7) | 59 382 (27.9) | 107 228 (25.3) |
| 63.5-69.0 | 48 181 (22.8) | 57 647 (27.1) | 105 828 (25.0) |
| 69.1-77.9 | 54 497 (25.8) | 51 203 (24.1) | 105 700 (25.0) |
| 78.4-117 | 60 510 (28.7) | 44 444 (20.8) | 104 954 (24.8) |
| Ophthalmologists per 100 000 population, rangea,b | |||
| 1.6-4.2 | 51 052 (24.2) | 62 572 (29.4) | 113 624 (26.8) |
| 4.3-4.7 | 46 872 (22.2) | 58 889 (27.7) | 105 761 (24.9) |
| 4.8-5.6 | 49 370 (23.4) | 50 994 (24.0) | 100 364 (23.7) |
| 5.7-10.6 | 63 740 (30.2) | 40 221 (18.9) | 103 961 (24.5) |
| Ophthalmologist surgical volume, 2011, rangea,b | |||
| 1-39 | 59 971 (28.4) | 42 352 (19.9) | 102 323 (24.1) |
| 40-68 | 55 797 (26.4) | 50 831 (23.9) | 106 628 (25.2) |
| 69-120 | 51 267 (24.3) | 56 099 (26.4) | 107 366 (25.3) |
| 121-662 | 43 999 (20.8) | 63 394 (29.8) | 107 393 (25.3) |
| Medicare expenditures per beneficiary in hospital referral regiona,b | |||
| 6911-8689 | 52 094 (24.7) | 54 301 (25.5) | 106 395 (25.1) |
| 8691-9674 | 51 223 (24.3) | 54 695 (25.7) | 105 918 (25.0) |
| 9693-10 311 | 55 626 (26.4) | 51 271 (24.1) | 106 897 (25.2) |
| 10 341-13 824 | 52 091 (24.7) | 52 409 (24.6) | 104 500 (24.6) |
US region, population density, primary care physicians/ophthalmologists per 100 000 population, and hospital referral regions were derived from the operating ophthalmologist's zip code.
Ranges of numbers represent the first, second, third, and fourth quartiles of the range of values in each category.
Figure 1. Relationship Between Cataract Surgery Date and Ocular Biometry Date.
Among patients with a biometry claim, 75% had cataract surgery within 30 days after biometry, 20% had surgery between 31 and 90 days after biometry, and 5% had surgery more than 90 days after biometry. Patients with more than 120 days between biometry and surgery are not shown. The periodic pattern of biometry claims relative to surgery claims reflects the typical weekly intervals between the ophthalmology office visit and cataract surgery date.
A total of 1 048 059 tests were performed during the interval between biometry and surgery. Routine preoperative testing rates were higher during the interval between biometry and surgery (1.7 tests/patient/month) compared with the baseline rate (1.1 tests/patient/month) and the rate in the months following cataract surgery (1.1 tests/patient/month). Preoperative testing peaked whether measured in relation to the 30 days after biometry (1.7 tests/patient/month) or the 30 days before surgery (1.8 tests/patient/month). After excluding the 762 608 tests that occurred in the traditional preoperative month, there was still an increase of approximately 41% in the rate of testing over baseline during the interval between biometry and surgery (285 451 tests, or 1.5 tests/patient/month).
In the subset of patients (n = 39 270) with no overlap between the postbiometry and presurgery periods, there was a spike in preoperative testing in both the postbiometry and presurgery months (1.8 and 2.2 tests/patient/month, respectively). Patients with testing in both periods were more likely to be older, have a higher Charlson Comorbidity Index score, and have an ophthalmologist in the lowest quartile of surgical volume compared with patients who did not have repeat testing (eTable 3 in the Supplement).
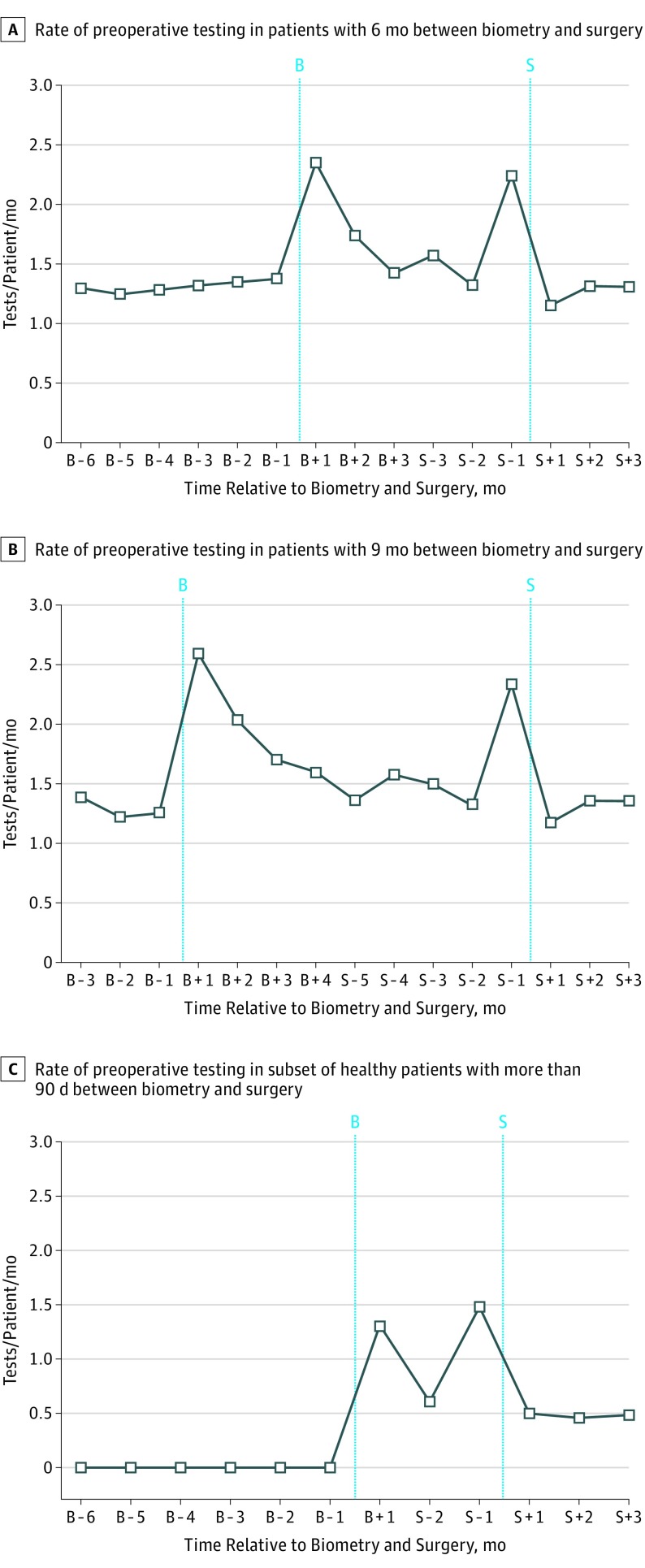
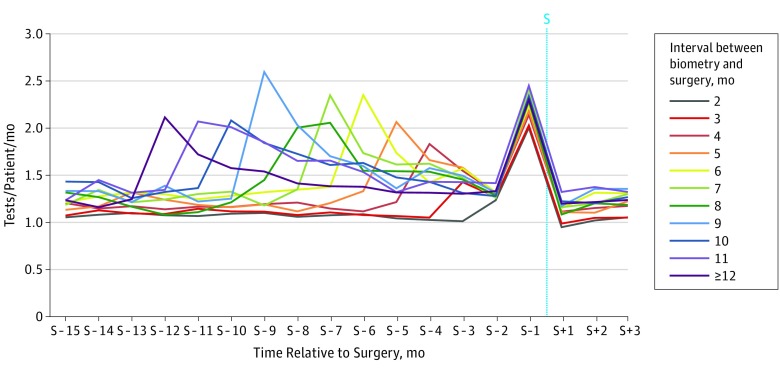
Figure 2A and B shows testing rates for the subcohorts of patients who had 6 months and 9 months between biometry and surgery, respectively; there was an increase in testing in the month immediately after biometry (2.4 and 2.6 tests/patient/month, respectively) and another increase during the month before surgery (2.2 and 2.3 tests/patient/month, respectively) compared with the baseline rate of testing (1.3 tests/patient/month in both cohorts). The same pattern was seen in the subset of patients with a Charlson Comorbidity Index score of 0 who did not undergo any preoperative testing in the 6 months prior to the biometry claim. In these patients, testing increased from a baseline of 0 to 1.1 tests/patient/month in the 30 days after biometry. To highlight the testing that occurs immediately after biometry as distinct from testing that occurs in the month before surgery, Figure 2C shows this increase in postbiometry testing in the subset of healthy patients with a greater than 90-day interval between biometry and surgery (to 1.3 tests/patient/month, up from 0 tests/patient/month). This pattern was consistent across all patient subcohorts with more than 30 days between biometry and surgery, regardless of elapsed time between biometry and surgery (Figure 3).
Figure 2. Rate of Preoperative Testing in Patients in the Interval Between Biometry and Surgery.
Preoperative testing rates in the months after ocular biometry and before cataract surgery were spliced to create a single graph of preoperative testing rates over time in relation to biometry and surgery. A, Patients with 6 months between biometry and surgery (n = 2133); B, patients with 9 months between biometry and surgery (n = 1136); C, patients with a Charlson comorbidity score of 0, an absence of preoperative testing in the 6 months prior to biometry, and a greater than 90-day interval between biometry and surgery (n = 961). The X-axis labels refer to the date of ocular biometry (B) or cataract surgery (S).
Figure 3. Rate of Preoperative Testing in Patients Having Cataract Surgery More Than 30 Days After Ocular Biometry, Stratified by Interval in Months Between Biometry and Surgery.
This figure was created using data from all patients with greater than 30 days between biometry and surgery claim dates (n = 107 812). More prebiometry data were available for patients with fewer months between biometry and surgery because of the shorter interval between biometry and surgery claim dates. For each cohort of patients, the first spike from the left represents preoperative testing that occurred in the month after ocular biometry. The second spike represents preoperative testing that occurred in the month preceding cataract surgery. The x-axis labels are approximate representations of the timing of postbiometry testing in reference to the date of surgery. Exact labeling of postbiometry periods was impossible on this single graph given the wide variation in time between biometry and surgery represented on the graph. The x-axis labels are in reference to the date of cataract surgery (S).
The total number of routine preoperative tests that were performed during the interval between biometry and surgery cost approximately $22.7 million. Expenditures on testing during the extended preoperative testing period were $7.3 million (46.9%) higher than the total spent in the 30 days before surgery and $6.1 million (36.6%) higher than the total spent on testing during the baseline period of equal length 1 year prior. The prevalence of testing during the biometry to surgery period was also higher, with 237 499 patients (56.1%) undergoing at least 1 test after biometry, compared with 223 350 (52.7%) in the 30 days before surgery and 156 301 (36.9%) during the baseline period of equal length 1 year prior (Figure 4).
Figure 4. Frequency and Cost of Routine Preoperative Testing, Grouped by Preoperative Testing Period.
The category “1 year prior to biometry” represents a baseline period of equal length starting 1 year prior to the extended preoperative testing window (ie, the time between the dates of the ocular biometry claim and the cataract surgery claim). For the 1.5% of patients whose time from biometry to surgery exceeded the number of baseline months available for direct comparison, we adjusted our frequency and cost calculations to reflect the ratio of the number of months included in the biometry to surgery period vs the number of months included in the shorter baseline period.
Discussion
In this study of routine preoperative testing coinciding with the initial decision to perform cataract surgery on Medicare patients, we show that in some patients, routine preoperative testing occurs earlier than previously described. Although three-quarters of patients had a biometry claim within 30 days of surgery, we discovered that 1 in 4 patients waited more than 30 days for cataract surgery. When we analyzed preoperative testing patterns at the time of biometry, we found that there is an increase in routine preoperative testing rates in the month after biometry similar to rates previously reported in the month before surgery. The spike in preoperative testing that occurs immediately after biometry, regardless of the interval between the biometry claim and the date of surgery, is present even among presumably healthy patients with no need for testing for 6 months before undergoing biometry.
Ocular biometry is critical for optimizing cataract surgery outcomes. The importance of biometry for surgical planning also allows its use as a procedure-specific indicator to represent the beginning of the surgical episode of care in patients with cataracts, since nearly every biometry claim in our data set was followed by a cataract surgery claim within 90 days.
Interestingly, 6.3% of patients had a biometry claim submitted on the same day as surgery. In clinical practice, this phenomenon is likely because of a subset of ophthalmologists billing for biometry that occurred at a previous office visit. Although the ocular biometry claim should ideally be submitted by these ophthalmologists in real time, these claims do not alter our study’s demonstration that claims for surrogate procedures, such as biometry, can be used as an indicator for planned surgery, even if surgery itself does not immediately follow.
When we applied this procedure-specific indicator to estimate the total cost of routine preoperative testing before index cataract surgery in Medicare patients, we found that the cost of testing during the extended preoperative testing window in our cohort increased to $22.7 million, compared with $15.4 million spent in the 30 days before surgery and $16.1 million that we had previously reported. Even when taking into account background testing that may occur in this older population, we showed that the cost of testing increased by 37% over a comparable period that occurred 1 year prior to the biometry claim, suggesting that much of the additional testing that occurred in these patients was in anticipation of undergoing low-risk elective surgery. Using this expanded capture method, given that our study cohort represents a 50% sample of Medicare patients undergoing cataract surgery in 2011, we estimate that routine preoperative testing costs Medicare up to $45.4 million annually.
Prior studies on routine preoperative testing, which usually highlight the testing that occurs during a 30-day preoperative window, have not accounted for the extended preoperative testing period that begins when the decision is first made to operate. In this context, there are likely to be more patients undergoing routine preoperative testing before low-risk surgical procedures similar to cataract surgery than previously recognized, despite the existence of guidelines recommending against such interventions. In addition, our data suggest that some patients may be undergoing routine preoperative testing more than once, which increases the cost and inconvenience of unnecessary testing to patients and clinicians. Although older, sicker patients were more likely to undergo repeated testing, we have previously shown that only a subset of physicians are responsible for ordering most of the excess testing that occurs. In fact, previous studies have indicated that the identity of the physician or care team is a much stronger predictor of getting preoperative testing before cataract surgery than all patient demographic and clinical characteristics combined.
Limitations
This study has limitations. First, the use of an administrative data set lacks clinically relevant information that can help determine the true indication for the tests performed. However, the spike in testing that occurred both in the month after biometry and in the month before cataract surgery, as well as the overall increase in testing between biometry and surgery compared with the baseline period, suggests that most of the additional tests we classified as preoperative tests were being ordered in anticipation of surgery.
Second, because our study cohort only included patients who eventually went on to have cataract surgery, we cannot be certain that biometry is followed by increased preoperative testing in patients who ultimately do not undergo surgery. Additional studies will be needed to validate the use of biometry as a procedure-specific indicator for planned cataract surgery in patients who, for whatever reason, do not end up having cataract surgery after biometry has been performed.
Finally, we do not have any information as to which member of the patient care team was responsible for ordering the individual preoperative tests performed. However, regardless of who is ordering the tests, it is the responsibility of physicians in all of the relevant specialties, not just ophthalmologists, to reduce unnecessary routine preoperative testing in patients undergoing low-risk elective surgical procedures, such as cataract surgery.
Conclusions
The present analysis shows that the traditional preoperative testing window of 30 days prior to the date of surgery does not always account for routine preoperative medical testing that occurs when surgery is first contemplated. When patients whose surgery occurs more than 30 days after initially planned are included, routine preoperative medical testing is more prevalent and more costly than previously reported. This study reinforces the importance of adhering to current guidelines that discourage the use of routine preoperative testing before low-risk surgery—regardless of their timing in relation to surgery—to minimize the perpetuation of low-value care during the perioperative period.
eMethods. Description of routine preoperative testing rates in relation to ocular biometry and index surgery
eTable 1: CPT codes used for specified preoperative tests
eTable 2: Routine preoperative tests in patients with at least 60 days between biometry and surgery, stratified by timing of testing
eTable 3: Characteristics of cataract surgery patients with at least 60 days between biometry and surgery, stratified by timing of routine preoperative testing performed
References
- 1.The Royal College of Ophthalmologists Cataract surgery guidelines. https://www.rcophth.ac.uk/wp-content/uploads/2014/12/2010-SCI-069-Cataract-Surgery-Guidelines-2010-SEPTEMBER-2010.pdf. Accessed June 20, 2016.
- 2.Apfelbaum JL, Connis RT, Nickinovich DG, et al. ; Committee on Standards and Practice Parameters; American Society of Anesthesiologists Task Force on Preanesthesia Evaluation . Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology. 2012;116(3):522-538. [DOI] [PubMed] [Google Scholar]
- 3.American Academy of Ophthalmology Routine preoperative laboratory testing for patients scheduled for cataract surgery—2014. https://www.aao.org/clinical-statement/routine-preoperative-laboratory-testing-patients-s. Accessed May 20, 2015.
- 4.American Academy of Ophthalmology Cataract and Anterior Segment Panel Preferred practice pattern guidelines: cataract in the adult eye. https://www.aao.org/preferred-practice-pattern/cataract-in-adult-eye-ppp-2016 Published 2016. Accessed December 11, 2017.
- 5.Onuoha OC, Arkoosh VA, Fleisher LA. Choosing wisely in anesthesiology: the gap between evidence and practice. JAMA Intern Med. 2014;174(8):1391-1395. [DOI] [PubMed] [Google Scholar]
- 6.Fleisher LA, Fleischmann KE, Auerbach AD, et al. ; Endorsed by the Society of Hospital Medicine . 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Nucl Cardiol. 2015;22(1):162-215. [DOI] [PubMed] [Google Scholar]
- 7.Martin SK, Cifu AS. Routine preoperative laboratory tests for elective surgery. JAMA. 2017;318(6):567-568. [DOI] [PubMed] [Google Scholar]
- 8.Chen CL, Lin GA, Bardach NS, et al. . Preoperative medical testing in Medicare patients undergoing cataract surgery. N Engl J Med. 2015;372(16):1530-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benarroch-Gampel J, Sheffield KM, Duncan CB, et al. . Preoperative laboratory testing in patients undergoing elective, low-risk ambulatory surgery. Ann Surg. 2012;256(3):518-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onuoha OC, Hatch MB, Miano TA, Fleisher LA. The incidence of un-indicated preoperative testing in a tertiary academic ambulatory center: a retrospective cohort study. Perioper Med (Lond). 2015;4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinberg EP, Javitt JC, Sharkey PD, et al. . The content and cost of cataract surgery. Arch Ophthalmol. 1993;111(8):1041-1049. [DOI] [PubMed] [Google Scholar]
- 12.Johansson T, Fritsch G, Flamm M, et al. . Effectiveness of non-cardiac preoperative testing in non-cardiac elective surgery: a systematic review. Br J Anaesth. 2013;110(6):926-939. [DOI] [PubMed] [Google Scholar]
- 13.Sahin A, Hamrah P. Clinically relevant biometry. Curr Opin Ophthalmol. 2012;23(1):47-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheard R. Optimising biometry for best outcomes in cataract surgery. Eye (Lond). 2014;28(2):118-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schein OD, Cassard SD, Tielsch JM, Gower EW. Cataract surgery among Medicare beneficiaries. Ophthalmic Epidemiol. 2012;19(5):257-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quan H, Sundararajan V, Halfon P, et al. . Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. [DOI] [PubMed] [Google Scholar]
- 17.The Dartmouth Institute for Health Policy and Clinical Practice The Dartmouth Atlas of Health Care. http://www.dartmouthatlas.org. Accessed February 11, 2014.
- 18.Rural Health Research Center Rural–urban commuting area (RUCA) codes, version 2.0. http://depts.washington.edu/uwruca/ruca-data.php. Accessed February 12, 2014.
- 19.Centers for Medicare and Medicaid Services Medicare clinical lab fee schedule 2011. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/clinlab.html. Accessed January 8, 2014.
- 20.Centers for Medicare and Medicaid Services Medicare physician fee schedule 2011. https://www.cms.gov/apps/physician-fee-schedule/overview.aspx. Accessed February 28, 2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Description of routine preoperative testing rates in relation to ocular biometry and index surgery
eTable 1: CPT codes used for specified preoperative tests
eTable 2: Routine preoperative tests in patients with at least 60 days between biometry and surgery, stratified by timing of testing
eTable 3: Characteristics of cataract surgery patients with at least 60 days between biometry and surgery, stratified by timing of routine preoperative testing performed