Key Points
Question
Is exposure to multiple vaccines through the first 23 months of life associated with an increased risk for infections not targeted by vaccines?
Findings
In this nested case-control study that included 193 cases with non–vaccine-targeted infections and 751 controls without non–vaccine-targeted infections, the estimated mean cumulative antigen exposure from birth through age 23 months was 240.6 for cases and 242.9 for controls, a difference that was not statistically significant.
Meaning
Among children from 24 through 47 months of age with non–vaccine-targeted infections, compared with children without such infections, there was no significant difference in estimated cumulative vaccine antigen exposure through the first 23 months of life.
Abstract
Importance
Some parents are concerned that multiple vaccines in early childhood could weaken their child’s immune system. Biological data suggest that increased vaccine antigen exposure could increase the risk for infections not targeted by vaccines.
Objective
To examine estimated cumulative vaccine antigen exposure through the first 23 months of life in children with and without non–vaccine-targeted infections from 24 through 47 months of age.
Design, Setting, and Participants
A nested case-control study was conducted in 6 US health care organizations participating in the Vaccine Safety Datalink. Cases were identified by International Classification of Diseases codes for infectious diseases in the emergency department and inpatient medical settings and then validated by medical record review. Cases of non–vaccine-targeted infection were matched to controls by age, sex, health care organization site, and chronic disease status. Participants were children ages 24 through 47 months, born between January 1, 2003, and September 31, 2013, followed up until December 31, 2015.
Exposures
Cumulative vaccine antigen exposure, estimated by summing the number of antigens in each vaccine dose received from birth through age 23 months.
Main Outcomes and Measures
Non–vaccine-targeted infections, including upper and lower respiratory infections and gastrointestinal infections, from 24 through 47 months of age, and the association between these infections and estimated cumulative vaccine exposure from birth through 23 months. Conditional logistic regression was used to estimate matched odds ratios representing the odds of non–vaccine-targeted infections for every 30-unit increase in estimated cumulative number of antigens received.
Results
Among the 944 patients (193 cases and 751 controls), the mean (SD) age was 32.5 (6.3) months, 422 (45%) were female, and 61 (7%) had a complex chronic condition. Through the first 23 months, the estimated mean (SD) cumulative vaccine antigen exposure was 240.6 (48.3) for cases and 242.9 (51.1) for controls. The between-group difference for estimated cumulative antigen exposure was −2.3 (95% CI, −10.1 to 5.4; P = .55). Among children with vs without non–vaccine-targeted infections from 24 through 47 months of age, the matched odds ratio for estimated cumulative antigen exposure through age 23 months was not significant (matched odds ratio, 0.94; 95% CI, 0.84 to 1.07).
Conclusions and Relevance
Among children from 24 through 47 months of age with emergency department and inpatient visits for infectious diseases not targeted by vaccines, compared with children without such visits, there was no significant difference in estimated cumulative vaccine antigen exposure through the first 23 months of life.
This nested case-control study compares cumulative vaccine antigen exposure through the first 23 months of life among children with vs without non–vaccine-targeted infections between 24 and 47 months of age.
Introduction
In the past 3 decades, the routine childhood immunization schedule in the first 2 years of life expanded from 3 vaccines against 7 diseases to 10 vaccines against 14 diseases. Some parents believe this increase in vaccine exposure is harmful to children, with specific concerns that early childhood immunization “overloads” the immune system and increases the risk for future infection. Based in part on this concern, an estimated 10% to 15% of parents are choosing delayed or alternative immunization schedules for their children.
A 2002 report by the Institute of Medicine identified biologically plausible mechanisms by which multiple antigen exposure from vaccines could induce immune dysfunction and increase the risk for infections not targeted by vaccines. This hypothesis was tested in a Danish cohort of children born between 1990 and 2001. Examining a childhood schedule that included 5 vaccines against 7 diseases, the study did not find evidence that multiple antigen exposure was associated with the risk for non–vaccine-targeted infectious diseases.
To date, the association between multiple vaccine antigen exposure and non–vaccine-targeted infections has not been tested in a US population with the current recommended immunization schedule. In addition, a 2013 Institute of Medicine committee examined existing evidence on the safety of the current schedule and concluded that additional observational safety studies were warranted. The committee specifically recommended examining the complete early childhood schedule (ages 0 through 23 months) as it relates to future adverse outcomes. The objective of this study was to examine estimated cumulative vaccine antigen exposure through the first 23 months of life in children with non–vaccine-targeted infections from ages 24 through 47 months compared with children without such infections.
Methods
Setting and Study Cohort
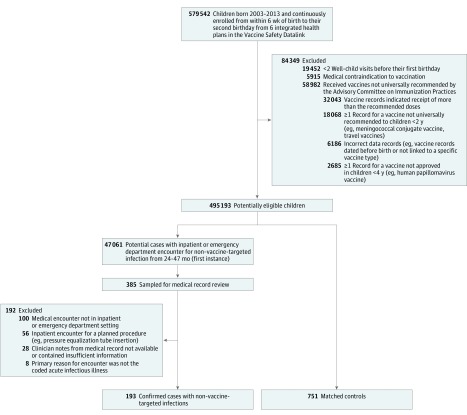
We conducted a matched case-control study that was nested within a cohort of children enrolled in 6 integrated health care organizations that are part of the Vaccine Safety Datalink (VSD). The VSD is a research collaboration funded by Centers for Disease Control and Prevention that uses electronic health record (EHR) databases to conduct epidemiological studies of vaccine safety. These health care organizations are located in Northern California, Southern California, Washington, Colorado, Oregon, and Wisconsin. Each VSD site creates standardized data sets containing demographics, membership enrollment, vaccination history, and medical encounters in outpatient, emergency department (ED), and inpatient settings. Institutional review boards at each VSD site approved the study and determined that informed consent was not required.
VSD data sets were first used to identify children born between January 1, 2003, and September 31, 2013. For inclusion, children had to be continuously enrolled in the health plan from age 6 weeks through 23 months. Children were excluded if they did not have at least 2 well-child visits before their first birthday, had a medical contraindication to vaccination, or if they had received vaccines not universally recommended by the Advisory Committee on Immunization Practices. Eligible children were followed up through age 47 months or until disenrollment from their health care organization; the final day of follow-up was December 31, 2015.
Cases of Non–Vaccine-Targeted Infection
From ages 24 through 47 months, potential non–vaccine-targeted infections were identified in VSD data sets using International Classification of Diseases, Ninth and Tenth Revisions, Clinical Modification (ICD-9/10-CM) codes in the ED and inpatient settings (eTable 1 in the Supplement). The list of outcome ICD codes was based on a Danish cohort study by Sørup et al, which tested a similar hypothesis. Non–vaccine-targeted infection outcomes included lower respiratory infections, upper respiratory infections, gastrointestinal infections, and other viral and bacterial infections. In the cohort, the first occurrence of an ICD code for a non–vaccine-targeted infection from ages 24 through 47 months was identified as a potential incident case.
A stratified, random sample of potential cases was selected for medical record review to confirm case status. The sample was stratified by VSD site and health care setting. For the medical record review, trained abstractors at each VSD site used a standardized medical record extraction form to confirm that the infectious outcome occurred, that it was an incident outcome, that the outcome was the primary reason for the medical encounter, that the outcome occurred in the inpatient or ED setting, and that there was no documentation in the medical record that the child was diagnosed as having a vaccine-preventable disease (VPD) on the same day as the non–vaccine-targeted infection. The VPDs are displayed in eTable 2 in the Supplement. Only cases that met these criteria were included in the analysis as confirmed cases. The diagnosis date for each confirmed case represented the index date for the matched case-control analysis. Cases had to be continuously enrolled in their health care organization until the index date.
Controls
A risk-set sampling approach was used to select controls. Each confirmed case was matched to up to 4 controls by age at the index date (±2 weeks), sex, VSD site, and chronic disease status. Eligible controls were randomly selected from the cohort, did not have a diagnosis for a VPD on the index date, and did not have an inpatient or ED record of a non–vaccine-targeted infection prior to the index date. Eligible controls also had to be continuously enrolled in their health care organization up until the index date. Chronic disease status at age 2 years was determined using the Pediatric Medical Complexity Algorithm.
Multiple Antigen Exposure
For cases and matched controls, vaccine antigen exposure was estimated from birth through age 23 months. Antigen exposure was measured as the number of immunogenic proteins and polysaccharides in each vaccine (eTable 3 in the Supplement). The vaccine immune response is primarily induced by these immunogenic proteins and polysaccharides. The number of antigens in recommended early childhood vaccines range from 1 to 93 per vaccine dose. During the study observation period, 5 new vaccines were added to the recommended schedule, and children who were vaccinated according to the recommended schedule could have been exposed to between 193 and 435 total cumulative antigens.
Antigen exposure was analyzed in 2 ways. For our primary exposure, we first estimated cumulative antigen exposure by summing the number of antigens in each vaccine dose through the first 23 months of life. We then compared estimated antigen exposure among children with and without non–vaccine-targeted infections from ages 24 through 47 months.
This cumulative measure of antigen exposure, however, does not account for “shot-limiting” behavior, in which parents limit the number of vaccines that their infants receive at each vaccine visit. While children on one of these alternative immunization schedules receive fewer than the recommended number of doses at each vaccine visit, they may also end up receiving all recommended doses by age 23 months. Children on such schedules may therefore have the same cumulative antigen exposure as children who receive all vaccine doses according to the Advisory Committee on Immunization Practices schedule. To account for this, we first identified the single vaccine visit with the maximum number of antigens that each child was exposed to from birth through age 23 months as a prespecified secondary exposure assessment. We then compared estimated maximum single-day antigen exposure among children with and without non–vaccine-targeted infections from ages 24 through 47 months.
Statistical Analyses
For our power analysis, we assumed an alpha of .05, an r2 value of 0.20, and an SD for estimated cumulative antigen exposure of 1.79. The r2 value represents the relationship between estimated cumulative antigen exposure and other analyzed covariates. The SD was calculated from the cohort prior to selecting cases and controls and represents the SD for estimated cumulative antigen exposure scaled to 30-unit increments. A 30-unit increment was chosen because it represents the approximate number of antigens that infants were exposed to in each of the 2-, 4-, and 6-month well-child visits, prior to the reintroduction of rotavirus vaccines in 2006.
Based on available resources, it was determined that 385 medical records of potential cases could be abstracted and manually reviewed across VSD sites. Assuming a range of confirmation rates from 25% to 80%, there would be approximately 100 to 300 confirmed cases. A case population in this range would provide 80% power to detect odds ratios (ORs) from 1.22 to 1.12, respectively. For example, with 100 confirmed cases, this study had 80% power to detect a 22% increased odds for non–vaccine-targeted infections from ages 24 through 47 months for every 30-unit increase in estimated cumulative antigen exposure from birth through age 23 months. These analyses were conducted assuming a 1:4 case to control ratio. Because the SD for cumulative vaccine antigen exposure was estimated from the entire study cohort, the power analyses were post hoc. Power analyses were conducted using PASS software (NCSS LLC).
Descriptive statistics were first conducted in the study cohort, including the sex distribution, mean estimated cumulative antigen exposure, and mean estimated maximum single-day antigen exposure. Cases and controls were analyzed with conditional logistic regression to estimate matched ORs (mORs) and 95% CIs. In the models, the dependent variable was non–vaccine-targeted infection (yes/no), and the main exposure variable was either estimated cumulative antigen exposure or maximum single-day antigen exposure before the child’s second birthday. For estimated cumulative antigen exposure, we evaluated the odds of non–vaccine-targeted infection for every 30-unit increase in cumulative antigen exposure. All models were adjusted for the number of outpatient visits from birth through age 23 months. All statistical tests were 2-sided, and P < .05 considered statistically significant. Both exposures were modeled as continuous variables, and model fit was assessed with the Hosmer-Lemeshow goodness of fit test.
We used 2 methods to evaluate the assumption of linearity of the logit. We first conducted Box-Tidwell transformations, using a P = .05 cutoff for statistical significance. We then created categorical exposure variables by dividing the distributions of estimated cumulative antigen exposure and maximum single-day antigen exposure into deciles. Logistic regression analyses were conducted with the categorical exposure variables, using the lowest decile as the referent group. The decile-specific estimates were then plotted and visually observed to detect deviations from the linearity in the logit assumption.
It is possible that some children in the study received vaccines outside their VSD health care organization, which were not captured in the study databases. This implies that estimated cumulative antigen exposure could have been misclassified for some children. To account for potential exposure misclassification, we conducted a quantitative bias analysis to determine the levels of exposure sensitivity that would have affected our conclusions on the association between estimated cumulative antigen exposure and risk of non–vaccine-targeted infections.
Secondary Analyses
In addition to assessing estimated cumulative and maximum single-day antigen exposure through age 23 months, antigen exposures were assessed up to the index date for cases and controls to account for vaccines received after 23 months of age. For these 4 different exposure assessments, we applied 2 different methods for selecting controls. The first method involved excluding potential controls who had an urgent care visit prior to the index date. It is possible that some parents of controls may have used urgent care rather than ED services for non–vaccine-targeted infections. This implies that some controls may have been false-negatives, which, if associated with vaccine antigen exposure, would create a surveillance bias. Excluding controls with an urgent care visit for a non–vaccine-targeted infection would help minimize this potential bias.
The second method involved selecting controls with an ED visit for an injury from ages 24 through 47 months. This helped ensure that controls had access to health care and were using the health care organization to receive their care, thus minimizing the potential for false-negatives.
All analyses were repeated with a case-control population matched by race/ethnicity, in addition to age, sex, VSD site, and chronic disease status. We matched on race/ethnicity to account for the possibility that it was associated with access to health care, which could affect both vaccination status and the likelihood that a child presents to the ED or inpatient setting for a non–vaccine-targeted infection. Race/ethnicity was based on parental self-report and collected from EHR and birth certificate data. We categorized these data into the following 6 groups for analysis: Hispanic, any race; white; black; Asian; multiracial; and other reported race/ethnicity. If race/ethnicity was not available, the missing data were not imputed.
As an additional set of secondary analyses, we stratified the primary analysis by chronic disease status and outcome setting (ED or inpatient). We also conducted analyses excluding children who received no vaccines from birth through age 23 months, and excluding children who did not receive the varicella vaccine. The latter analysis was conducted because varicella contains the most antigens of routinely administered early childhood vaccines. All analyses were conducted using SAS version 9.4 (SAS Institute).
Results
Study Cohort, Cases, and Controls
After exclusions, the study cohort comprised 495 193 children born between January 1, 2003, and September 31, 2013 (Figure 1). Approximately half of the cohort was female (48.8%) and 3.8% of the children had a complex chronic condition by age 24 months according to the Pediatric Medical Complexity Algorithm. The mean (SD) estimated cumulative antigen exposure was 254.6 (53.6), and the mean (SD) estimated maximum single-day antigen exposure was 102.4 (20.4) (Table 1).
Figure 1. Cohort Exclusions and Nested Case-Control Design.
A risk-set sampling approach was used to select controls. The date of diagnosis for each confirmed case of non–vaccine-targeted infection represented the index date. Each confirmed case was matched to up to 4 controls by age at the index date (±2 weeks), sex, Vaccine Safety Datalink site, and chronic disease status. Eligible controls were randomly selected from the study-eligible cohort (n = 495 193) and did not have an inpatient or emergency department record of a non–vaccine-targeted infection prior to the index date.
Table 1. Description of Cohort, Cases, and Controls.
| Characteristic | Entire Cohort (N = 495 193) | Nested Case-Control | |
|---|---|---|---|
| Cases (n = 193)a | Controls (n = 751) | ||
| Female, No. (%) | 241 551 (48.8)b | 87 (45.1) | 335 (44.6) |
| Pediatric Medical Complexity Algorithm, No. (%)c | |||
| Nonchronic | 423 529 (85.5) | 140 (72.5) | 560 (74.6) |
| Noncomplex chronic | 52 941 (10.7) | 38 (19.7) | 145 (19.3) |
| Complex chronic | 18 723 (3.8) | 15 (7.8) | 46 (6.1) |
| Estimated cumulative vaccine antigen exposure from birth through 23 mo, No. of antigens | |||
| Mean (SD) | 254.6 (53.6) | 240.6 (48.3) | 242.9 (51.1) |
| Median (range) | 266 (0-428) | 236 (5-323) | 235 (0-399) |
| Estimated maximum single-day antigen exposure from birth through 23 mo, No. of antigens | |||
| Mean (SD) | 102.4 (20.4) | 101.0 (18.4) | 100.5 (18.9) |
| Median (range) | 107 (0-162) | 104 (5-136) | 104 (0-131) |
| Age as of case date, mo | |||
| Mean (SD) | 32.5 (6.3) | 32.5 (6.2) | |
| Median (range) | 32.0 (24.0-47.0) | 31.0 (24.0-47.0) | |
| Outpatient visits from birth through 23 mo, No. | |||
| Mean (SD) | 16.6 (8.1) | 19.6 (14.0) | 17.1 (7.7) |
| Median (range) | 15.0 (2.0-350.0) | 17.0 (6.0-131.0) | 15.0 (5.0-67.0) |
Of the 193 confirmed cases, 85 (44.0%) were from an inpatient setting and 108 (56.0%) were from an emergency department setting.
Three people were missing data on sex.
The Pediatric Medical Complexity Algorithm was applied to diagnosis code data among cohort members from birth through 23 months. Children classified as having complex chronic disease had 1 or more diagnoses for a progressive medical condition, 1 or more diagnoses for a malignancy, or 2 or more diagnoses per body system for at least 2 body systems. Children classified as having noncomplex chronic disease had 2 or more diagnoses within 1 body system. Children classified as nonchronic did not fall into either of these previous 2 categories.
From ages 24 through 47 months, there were 47 061 events (ICD codes) for potential non–vaccine-targeted infections. Of these diagnoses, a stratified, random sample of 385 underwent medical record review. The characteristics of the source population of potential cases and sampled potential cases are displayed in Table 2.
Table 2. Potential and Sampled Potential Cases of Non–Vaccine-Targeted Infections in Inpatient and Emergency Department Settings.
| Potential Cases (n = 47 061) |
Sampled Potential Cases (n = 385) |
|
|---|---|---|
| Inpatient | (n = 6692) | (n = 191) |
| Age, mean (SD), mo | 32.9 (6.9) | 32.6 (6.9) |
| Female, No. (%) | 2864 (42.8) | 80 (41.9) |
| Pediatric Medical Complexity Algorithm, No. (%) | ||
| Nonchronic | 4505 (67.3) | 138 (72.3) |
| Noncomplex chronic | 1239 (18.5) | 31 (16.2) |
| Complex chronic | 948 (14.2) | 22 (11.5) |
| ICD-9-CM codes for top diagnoses, No. (%) | ||
| 382.9: Unspecified otitis media | 1128 (16.9) | 32 (16.8) |
| 486: Pneumonia | 905 (13.5) | 19 (10.0) |
| 381.4: Nonsuppurative otitis media | 575 (8.6) | 15 (7.9) |
| 465.9: Acute upper respiratory tract infection | 420 (6.3) | 15 (7.9) |
| 381.10: Chronic serious otitis media | 398 (6.0) | 14 (7.3) |
| 464.4: Croup | 294 (4.4) | 11 (5.8) |
| 008.8: Intestinal infection | 251 (3.8) | 8 (4.2) |
| 466.19: Acute bronchiolitis | 173 (2.6) | 4 (2.1) |
| Emergency Department | (n = 40 369) | (n = 194) |
| Age, mean (SD), mo | 33.1 (6.8) | 33.05 (6.6) |
| Female, No. (%) | 17 189 (42.6) | 82 (42.3) |
| Pediatric Medical Complexity Algorithm, No. (%) | ||
| Nonchronic | 32 198 (79.8) | 144 (74.2) |
| Noncomplex chronic | 5971 (14.8) | 40 (20.6) |
| Complex chronic | 2200 (5.5) | 10 (5.2) |
| ICD-9-CM codes for top diagnoses, No. (%) | ||
| 382.9: Unspecified otitis media | 11 110 (27.5) | 50 (25.8) |
| 465.9: Acute upper respiratory tract infection | 9321 (23.1) | 42 (21.7) |
| 464.4: Croup | 6063 (15.0) | 29 (15.0) |
| 486: Pneumonia | 3318 (8.2) | 20 (10.3) |
| 462: Acute pharyngitis | 1934 (4.8) | 11 (5.7) |
| 466.19: Acute bronchiolitis | 755 (1.9) | 1 (0.5) |
| 008.8: Intestinal infection | 525 (1.3) | 4 (2.1) |
Abbreviation: ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification.
Of 385 sampled potential cases, 193 (50.1%) were confirmed with medical record review. Reasons for excluding potential cases included medical encounter was not in inpatient or ED setting (26.0%), inpatient encounter was for a planned procedure (eg, pressure equalization tube insertion) rather than acute infection (14.6%), medical record was not reviewable (5.1%), encounter was not identified in medical record (2.1%), and primary reason for encounter was not the coded acute infectious illness (2.1%). Of the 193 confirmed cases, the groupings of specific infectious illnesses were upper respiratory infections (58.0%), lower respiratory infections (21.8%), gastrointestinal infections (9.3%), and other bacterial and viral infections (10.9%). Most cases were treated in the ED, as compared with the 44% that were admitted to the hospital. Cases were 45.1% female and the mean (SD) age at diagnosis was 32.5 (6.3) months. The mean (SD) estimated cumulative antigen exposure and maximum single-day antigen exposure for the cases were 240.6 (48.3) and 101.0 (18.4), respectively (Table 1).
Approximately 4 controls were matched to each case (n = 751). Controls were selected to have the same mean age, sex, and chronic condition distribution as the cases. The mean (SD) estimated cumulative antigen exposure and maximum single-day antigen exposure for the controls were 242.9 (51.1) and 100.5 (18.9), respectively. Between-group differences for estimated cumulative antigen exposure and maximum single-day antigen exposure were −2.3 (95% CI, −10.1 to 5.4; P = .55) and 0.5 (95% CI, −2.4 to 3.5; P = .72), respectively. Examples of the most common vaccine combinations are displayed by decile of estimated cumulative antigen exposure in eTable 4 in the Supplement. Because a higher proportion of cases and matched controls were identified from earlier in the follow-up period when fewer vaccines were recommended, the mean estimated cumulative antigen exposure for cases and matched controls was 5.5% lower than that of the overall study cohort (Table 1 and eTable 5 in the Supplement).
Risk of Non–Vaccine-Targeted Infection
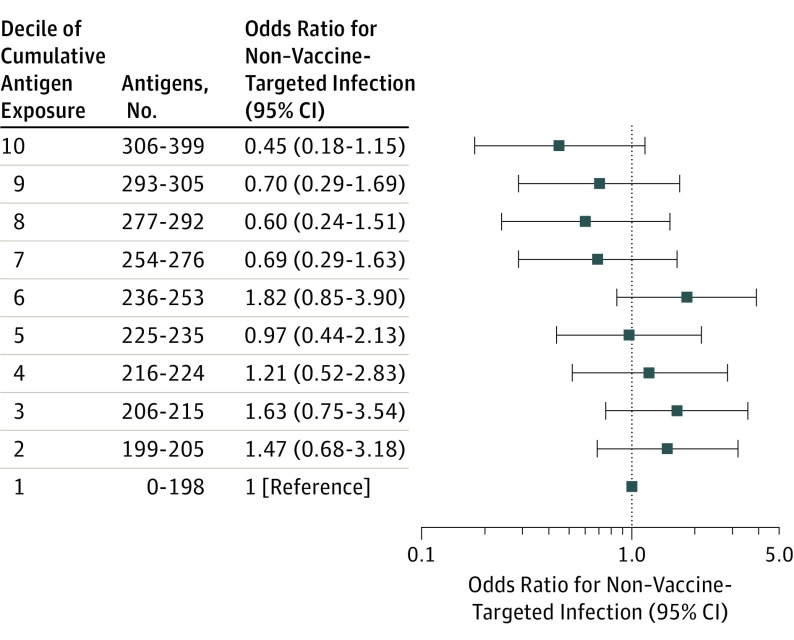
Among children with vs without non–vaccine-targeted infections from ages 24 through 47 months, the mOR for estimated cumulative antigen exposure through age 23 months was not significant (mOR, 0.94; 95% CI, 0.84-1.07). The Hosmer-Lemeshow test indicated that the model had good fit (P = .11), and the Box-Tidwell transformation indicated that the form of cumulative antigen exposure did not violate the assumption of linearity in the logit (P = .25). ORs and 95% CIs for each decile of estimated cumulative antigen exposure, compared with the lowest decile of exposure, were all nonsignificant (Figure 2).
Figure 2. Matched Odds Ratios for Non–Vaccine-Targeted Infection, by Decile of Estimated Cumulative Vaccine Antigen Exposure.
Results are from a conditional logistic regression model for 193 cases matched to 751 controls on birthdate (±2 weeks), sex, Vaccine Safety Datalink site, and chronic disease status, and adjusted for number of outpatient visits from birth through 23 months. The case or control status was modeled as the dependent (outcome) variable and decile of estimated cumulative vaccine antigen exposure from birth to age 23 months was modeled as a 10-level categorical independent variable. The reference group was the lowest decile of exposure (0-198 antigens). This decile included 17 cases and 74 controls. The unadjusted case and control distribution for other deciles was as follows: decile 2: 21 cases and 76 controls, decile 3: 24 cases and 76 controls, decile 4: 15 cases and 64 controls, decile 5: 18 cases and 87 controls, decile 6: 27 cases and 66 controls, decile 7: 19 cases and 83 controls, decile 8: 15 cases and 68 controls, decile 9: 21 cases and 73 controls, and decile 10: 16 cases and 84 controls.
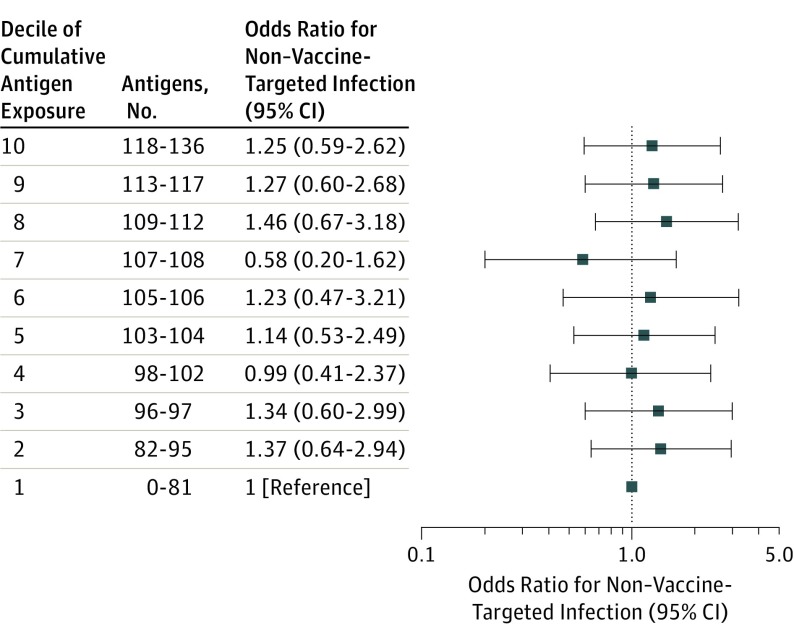
Estimated maximum single-day antigen exposure (scaled to 30-unit increments) was not significantly associated with non–vaccine-targeted infection (mOR, 1.07; 95% CI, 0.81-1.41). The model fit well (Hosmer-Lemeshow test P = .91), without violation of the linearity in the logit assumption (P = .79). ORs and 95% CIs for each decile of estimated maximum antigen exposure were all near 1.00 and nonsignificant (Figure 3).
Figure 3. Matched Odds Ratios for Non–Vaccine-Targeted Infection, by Decile of Estimated Maximum Single-Day Vaccine Antigen Exposure.
Results are from a conditional logistic regression model for 193 cases matched to 751 controls on birthdate (±2 weeks), sex, Vaccine Safety Datalink site, and chronic disease status, and adjusted for number of outpatient visits from birth through 23 months. The case or control status was modeled as the dependent (outcome) variable and decile of estimate maximum single-day vaccine antigen exposure from birth to age 23 months was modeled as a 10-level categorical independent variable. The reference group was the lowest decile of exposure (0-81 antigens). This decile included 17 cases and 77 controls. The unadjusted case and control distribution for the other deciles was as follows: decile 2: 22 cases and 75 controls, decile 3: 24 cases and 91 controls, decile 4: 11 cases and 52 controls, decile 5: 24 cases and 90 controls, decile 6: 13 cases and 47 controls, decile 7: 9 cases and 55 controls, decile 8: 20 cases and 64 controls, decile 9: 27 cases and 102 controls, and decile 10: 26 cases and 98 controls.
Quantitative Bias Analysis
For the quantitative bias analysis, we focused on an exposure group of children in the top 90% of estimated cumulative antigen exposure (199-399 antigens) and a group of children in the bottom 10% of antigen exposure (0-198 antigens). Comparing these 2 groups, the observed OR for risk of non–vaccine-targeted infection was 1.13 (95% CI, 0.65-1.97). However, it is possible that some children classified in the bottom decile received vaccines outside the VSD site and were therefore truly exposed to higher antigen levels, leading to exposure misclassification. Using quantitative bias analysis, we showed that a wide range of plausible exposure misclassification levels would not have affected our conclusion that estimated cumulative antigen exposure is not associated with non–vaccine-targeted infection (eTable 6 in the Supplement).
Secondary Analyses
None of the secondary analyses revealed a statistically significant association between estimated cumulative or maximum single-day antigen exposure and non–vaccine-targeted infections (eTables 7, 8, and 9 in the Supplement). These analyses included assessing vaccines administered up to the index date, excluding controls with a non–vaccine-targeted infection in the urgent care setting, matching cases to controls by race/ethnicity (eTable 10 in the Supplement), matching cases to controls with an ED visit for an injury, stratifying the data by chronic condition status, stratifying the data by health care setting (ED/inpatient), and excluding children who either did not receive a varicella vaccine or did not receive any vaccines through age 23 months.
Discussion
In this case-control study of children with non–vaccine-targeted infections from 24 through 47 months, compared with children without infections, there was no significant difference in estimated vaccine antigen exposure in any of the primary or secondary analyses.
The potential nonspecific effects of vaccination have been extensively studied. Several potential biological mechanisms for nonspecific vaccine effects have been proposed, but none have been established. For example, one mechanism involves the epigenetic reprograming of innate immune cells, known as “trained immunity.” Another possible mechanism—which has been well-established in animal model systems—is associated with nonspecific effects on the adaptive immune system through cross-reacting T-cell epitopes. These immunologic responses can be either beneficial or detrimental. This study did not reveal any beneficial or detrimental associations with estimated cumulative vaccine antigen exposure in young children with non–vaccine-targeted infections in ED and inpatient settings.
A Danish cohort study using national registry data for children born between 1990 and 2001 examined the association between 7 childhood vaccinations and nontargeted infectious diseases in children younger than age 5 years. The study did not find evidence that multiple vaccine exposure was associated with the risk for nontargeted infectious diseases. Despite using a different population, study period, study design, and method of defining the exposure, this current nested case-control study arrived at the same conclusion.
A key strength of this current study was the use of medical record review to confirm non–vaccine-targeted infection case status. The confirmation rate for cases was 50.1%, suggesting that approximately 50% of the potential cases of non–vaccine-targeted infection using EHR databases were false-positives. An analysis of non–vaccine-targeted infections in the automated databases without medical record review could therefore lead to significant outcome misclassification bias. The magnitude and direction of the bias would depend on whether the misclassification was differential or nondifferential with respect to antigen exposure status, and whether the misclassification was correlated with the matching variables. A medical record review or quantitative method for addressing outcome misclassification (eg, quantitative bias analysis) would therefore be recommended for all EHR-based studies of the nonspecific effects of vaccines.
Limitations
This study has several limitations. First, it is possible that the results were affected by a health care–seeking bias. When compared with parents who vaccinate their children on time, parents who intentionally undervaccinate their children may be more likely to present to the ED with acute infectious illnesses, out of concern their child has contracted a VPD. Second, physicians may be more likely to admit undervaccinated children with serious acute illnesses to the hospital than children who are fully vaccinated, thus creating a diagnostic bias. These behaviors would bias the results to the null hypothesis, if cumulative antigen exposure was truly increasing the risk of non–vaccine-targeted infection.
Third, although this study attempted to exclude VPDs from the analysis, it is possible that a proportion of the outcomes were undiagnosed VPDs. This would lead to an overestimated rate of illness among the undervaccinated (ie, those with lower vaccine antigen exposure) and may have biased the results to the null hypothesis. While this cannot be definitively ruled out, VPDs are rare in the United States, and it is unlikely that such misclassification would have changed this study’s conclusion.
Conclusions
Among children from 24 through 47 months of age with ED and inpatient visits for infectious diseases not targeted by vaccines, compared with children without such visits, there was no significant difference in estimated cumulative vaccine antigen exposure through the first 23 months of life.
eTable 1. ICD-9 and ICD-10 Codes for Infections Not Targeted by Early Childhood Vaccines.
eTable 2. ICD-9 and ICD-10 Codes for Excluded Infections Targeted by Early Childhood Vaccines.
eTable 3. Number of Antibody-Stimulating Protein and Polysaccharide Antigens in Early Childhood Vaccines Recommended by the US Advisory Committee on Immunization Practices.
eTable 4. Common Combinations of Estimated Cumulative Vaccine Antigen Exposure from Birth Through 23 Months, by Decile of Exposure.
eTable 5. Estimated Cumulative Vaccine Antigen Amounts From Birth Through 23 Months, by Birth Year Period.
eTable 6. Quantitative Bias Analysis for Potential Exposure Misclassification.
eTable 7. Association Between Estimated Vaccine Antigen Exposure and Non-Vaccine-Targeted Infections, With Exposures as Estimated Cumulative Vaccine Antigens From Birth Through 23 Months and Estimated Cumulative Vaccine Antigens From Birth Through Index Date (Primary and Secondary Analyses).
eTable 8. Association Between Estimated Vaccine Antigen Exposure and Non-Vaccine-Targeted Infections, With Exposures as Estimated Maximum Single-Day Vaccine Antigens From Birth Through 23 Months and Estimated Maximum Single-Day Vaccine Antigens From Birth Through Index Date (Primary and Secondary Analyses).
eTable 9. Association Between Estimated Vaccine Antigen Exposure and Non-Vaccine-Targeted Infections, With Exposures as Estimated Cumulative Vaccine Antigens and Estimated Maximum Single-Day Vaccine Antigens From Birth Through 23 Months, (a) Stratified by Chronic Condition Status, (b) Stratified by Outcome Setting, (c) Excluding Children Who Did Not Receive Varicella Vaccination, and (d) Excluding Children Who Received No Vaccines From Birth Through 23 Months (Secondary Analyses).
eTable 10. Race/Ethnicity of Study-Eligible Cohort Members and of Cases and Controls From Secondary Analysis Matching on Race/Ethnicity.
eReferences
References
- 1.Centers for Disease Control and Prevention Past immunization schedules. http://www.cdc.gov/vaccines/schedules/past.html. Accessed January 24, 2016.
- 2.Kennedy A, Basket M, Sheedy K. Vaccine attitudes, concerns, and information sources reported by parents of young children: results from the 2009 HealthStyles survey. Pediatrics. 2011;127(suppl 1):S92-S99. [DOI] [PubMed] [Google Scholar]
- 3.Lieu TA, Ray GT, Klein NP, Chung C, Kulldorff M. Geographic clusters in underimmunization and vaccine refusal. Pediatrics. 2015;135(2):280-289. [DOI] [PubMed] [Google Scholar]
- 4.Frew PM, Fisher AK, Basket MM, et al. Changes in childhood immunization decisions in the United States: Results from 2012 & 2014 National Parental Surveys. Vaccine. 2016;34(46):5689-5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dempsey AF, Schaffer S, Singer D, Butchart A, Davis M, Freed GL. Alternative vaccination schedule preferences among parents of young children. Pediatrics. 2011;128(5):848-856. [DOI] [PubMed] [Google Scholar]
- 6.Institute of Medicine Board on Population Health and Public Health Practice, Immunization Safety Review Committee Immunization Safety Review: Multiple Immunizations and Immune Dysfunction. Washington, DC: National Academies Press; 2002. [PubMed] [Google Scholar]
- 7.Hviid A, Wohlfahrt J, Stellfeld M, Melbye M. Childhood vaccination and nontargeted infectious disease hospitalization. JAMA. 2005;294(6):699-705. [DOI] [PubMed] [Google Scholar]
- 8.Institute of Medicine Board on Population Health and Public Health Practice, Committee on the Assessment of Studies of Health Outcomes Related to the Recommended Childhood Immunizations Schedule Childhood Immunization Schedule and Safety: Stakeholder Concerns, Scientific Evidence, and Future Studies. Washington, DC: National Academies Press; 2013. [PubMed] [Google Scholar]
- 9.Baggs J, Gee J, Lewis E, et al. The Vaccine Safety Datalink: a model for monitoring immunization safety. Pediatrics. 2011;127(suppl 1):S45-S53. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention Vaccine recommendations and guidelines of the ACIP: contraindications and precautions. https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/contraindications.html. Accessed July 17, 2017.
- 11.Sørup S, Benn CS, Poulsen A, Krause TG, Aaby P, Ravn H. Live vaccine against measles, mumps, and rubella and the risk of hospital admissions for nontargeted infections. JAMA. 2014;311(8):826-835. [DOI] [PubMed] [Google Scholar]
- 12.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd ed Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 13.Simon TD, Cawthon ML, Stanford S, et al. ; Center of Excellence on Quality of Care Measures for Children with Complex Needs (COE4CCN) Medical Complexity Working Group . Pediatric Medical Complexity Algorithm: a new method to stratify children by medical complexity. Pediatrics. 2014;133(6):e1647-e1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iqbal S, Barile JP, Thompson WW, DeStefano F. Number of antigens in early childhood vaccines and neuropsychological outcomes at age 7-10 years. Pharmacoepidemiol Drug Saf. 2013;22(12):1263-1270. [DOI] [PubMed] [Google Scholar]
- 15.Plotkin SA, Orenstein W, Offit PA, Edwards KM. Vaccines. 7th ed Philadelphia, PA: Saunders; 2017. [Google Scholar]
- 16.Offit PA, Quarles J, Gerber MA, et al. Addressing parents’ concerns: do multiple vaccines overwhelm or weaken the infant’s immune system? Pediatrics. 2002;109(1):124-129. [DOI] [PubMed] [Google Scholar]
- 17.Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB; Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) . Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2004;53(RR-6):1-40. [PubMed] [Google Scholar]
- 18.Cortese MM, Parashar UD; Centers for Disease Control and Prevention (CDC) . Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2009;58(RR-2):1-25. [PubMed] [Google Scholar]
- 19.Fiore AE, Wasley A, Bell BP; Advisory Committee on Immunization Practices (ACIP) . Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55(RR-7):1-23. [PubMed] [Google Scholar]
- 20.National Center for Immunization and Respiratory Diseases, CDC; Centers for Disease Control and Prevention (CDC) . Use of influenza A (H1N1) 2009 monovalent vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58(RR-10):1-8. [PubMed] [Google Scholar]
- 21.Nuorti JP, Whitney CG; Centers for Disease Control and Prevention (CDC) . Prevention of pneumococcal disease among infants and children: use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59(RR-11):1-18. [PubMed] [Google Scholar]
- 22.Robison SG, Groom H, Young C. Frequency of alternative immunization schedule use in a metropolitan area. Pediatrics. 2012;130(1):32-38. [DOI] [PubMed] [Google Scholar]
- 23.Daley MF, Glanz JM, Newcomer SR, et al. Assessing misclassification of vaccination status: implications for studies of the safety of the childhood immunization schedule. Vaccine. 2017;35(15):1873-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosmer DW Jr, Lemeshow S, Sturdivant RX. Applied Logistic Regression. 3rd ed Hoboken, NJ: John Wiley & Sons; 2013. [Google Scholar]
- 25.Allison PD. Logistic Regression Using SAS: Theory and Application. Cary, NC: SAS Institute; 2012. [Google Scholar]
- 26.Lash TL, Fox MP, Fink AK. Applying Quantitative Bias Analysis to Epidemiologic Data. New York, NY: Springer Science & Business Media; 2011. [Google Scholar]
- 27.Bardenheier BH, McNeil MM, Wodi AP, McNicholl JM, DeStefano F. Risk of nontargeted infectious disease hospitalizations among US children following inactivated and live vaccines, 2005-2014. Clin Infect Dis. 2017;65(5):729-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins JP, Soares-Weiser K, López-López JA, et al. Association of BCG, DTP, and measles containing vaccines with childhood mortality: systematic review. BMJ. 2016;355:i5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kandasamy R, Voysey M, McQuaid F, et al. Non-specific immunological effects of selected routine childhood immunisations: systematic review. BMJ. 2016;355:i5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blok BA, Arts RJ, van Crevel R, Benn CS, Netea MG. Trained innate immunity as underlying mechanism for the long-term, nonspecific effects of vaccines. J Leukoc Biol. 2015;98(3):347-356. [DOI] [PubMed] [Google Scholar]
- 31.Welsh RM, Che JW, Brehm MA, Selin LK. Heterologous immunity between viruses. Immunol Rev. 2010;235(1):244-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gil A, Kenney LL, Mishra R, Watkin LB, Aslan N, Selin LK. Vaccination and heterologous immunity: educating the immune system. Trans R Soc Trop Med Hyg. 2015;109(1):62-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sankoh O, Welaga P, Debpuur C, et al. The non-specific effects of vaccines and other childhood interventions: the contribution of INDEPTH Health and Demographic Surveillance Systems. Int J Epidemiol. 2014;43(3):645-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glanz JM, Wagner NM, Narwaney KJ, et al. A mixed methods study of parental vaccine decision making and parent-provider trust. Acad Pediatr. 2013;13(5):481-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roush SW, Murphy TV; Vaccine-Preventable Disease Table Working Group . Historical comparisons of morbidity and mortality for vaccine-preventable diseases in the United States. JAMA. 2007;298(18):2155-2163. [DOI] [PubMed] [Google Scholar]
- 36.Whitney CG, Zhou F, Singleton J, Schuchat A; Centers for Disease Control and Prevention (CDC) . Benefits from immunization during the vaccines for children program era: United States, 1994-2013. MMWR Morb Mortal Wkly Rep. 2014;63(16):352-355. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. ICD-9 and ICD-10 Codes for Infections Not Targeted by Early Childhood Vaccines.
eTable 2. ICD-9 and ICD-10 Codes for Excluded Infections Targeted by Early Childhood Vaccines.
eTable 3. Number of Antibody-Stimulating Protein and Polysaccharide Antigens in Early Childhood Vaccines Recommended by the US Advisory Committee on Immunization Practices.
eTable 4. Common Combinations of Estimated Cumulative Vaccine Antigen Exposure from Birth Through 23 Months, by Decile of Exposure.
eTable 5. Estimated Cumulative Vaccine Antigen Amounts From Birth Through 23 Months, by Birth Year Period.
eTable 6. Quantitative Bias Analysis for Potential Exposure Misclassification.
eTable 7. Association Between Estimated Vaccine Antigen Exposure and Non-Vaccine-Targeted Infections, With Exposures as Estimated Cumulative Vaccine Antigens From Birth Through 23 Months and Estimated Cumulative Vaccine Antigens From Birth Through Index Date (Primary and Secondary Analyses).
eTable 8. Association Between Estimated Vaccine Antigen Exposure and Non-Vaccine-Targeted Infections, With Exposures as Estimated Maximum Single-Day Vaccine Antigens From Birth Through 23 Months and Estimated Maximum Single-Day Vaccine Antigens From Birth Through Index Date (Primary and Secondary Analyses).
eTable 9. Association Between Estimated Vaccine Antigen Exposure and Non-Vaccine-Targeted Infections, With Exposures as Estimated Cumulative Vaccine Antigens and Estimated Maximum Single-Day Vaccine Antigens From Birth Through 23 Months, (a) Stratified by Chronic Condition Status, (b) Stratified by Outcome Setting, (c) Excluding Children Who Did Not Receive Varicella Vaccination, and (d) Excluding Children Who Received No Vaccines From Birth Through 23 Months (Secondary Analyses).
eTable 10. Race/Ethnicity of Study-Eligible Cohort Members and of Cases and Controls From Secondary Analysis Matching on Race/Ethnicity.
eReferences