Abstract
This interrupted time series analysis found significant changes in multiple dimensions of pain medication usage after the implementation of postoperative opioid prescription guidelines in a single hospital.
Most opioid drugs prescribed by surgeons to treat acute pain following surgery remain unused,1 which results in excess medication in the community available for diversion.2 Diversion of leftover opioid medication is a major component of the current opioid epidemic.3 Identifying strategies to curb overprescribing could mitigate this risk. We evaluated the effect of evidence-based postoperative prescribing guidelines in an effort to reduce this excess.
Methods
This protocol was deemed exempt from review by the University of Michigan institutional review board. No informed consent procedures were deemed necessary.
We identified patients who underwent elective laparoscopic cholecystectomy from January 2015 through June 2016. The amount of opioids prescribed at discharge was represented in milligrams of oral morphine equivalents (OME). Patients were queried within 12 months of operation about the number of opioid pills they had used, their use of nonopioid analgesics (specifically over-the-counter acetaminophen and ibuprofen), and their pain level during the first week after surgery. Patients were asked to measure their pain on a scale of 0 to 10, with 0 being no pain and 10 being the worst pain imaginable. Results of this telephone survey were used to develop postoperative prescribing guidelines, which were implemented at our institution in November 2016. No explicit changes in preoperative analgesia prescription practices were made in the guideline, nor were changes made in analgesia use during operations.
After guideline implementation, subsequent patients receiving laparoscopic cholecystectomy operations from November 2016 through March 2017 were administered the same survey questions. An interrupted time series analysis was conducted to evaluate the effect of these guidelines over existing trends in opioid and nonopioid prescribing.4 Preintervention and postintervention outcomes were compared using the Mann-Whitney U test and χ2 test as appropriate. P values were 2-tailed, and significance was set at P ≤ .05.
Results
All patients in the preintervention group (n = 170) received a prescription for opioids prior to the intervention; 7 of these individuals (4.1%) requested an opioid prescription refill. The median (interquartile range [IQR]) amount prescribed was 250 (200-300) mg. Of this cohort, 100 patients (58.8%) completed the survey, and they reported a median (IQR) opioid use of 30 (6-60) mg. In addition, 61 patients (61.0%) reported using acetaminophen or ibuprofen in addition to opioids. The median (IQR) pain score was 5.0 (3.5-6.5) on a scale of 0 to 10.
These data were used to develop post–laparoscopic cholecystectomy guidelines that recommended prescribing 15 tablets of hydrocodone/acetaminophen, 5/325 mg (OME, 75 mg) or 15 tablets of oxycodone, 5 mg (OME, 112.5 mg), plus acetaminophen or ibuprofen as needed. Videos and oral presentations were used to communicate these guidelines to all surgical faculty, residents, and staff.
Five months after guideline implementation, an additional 200 patients had undergone laparoscopic cholecystectomy (Table). The median amount of opioid prescribed was reduced from 250 mg to 75 mg (postintervention IQR, 75-112.5 mg) (P < .001). Despite this change, only 5 of 200 patients (2.5%) requested refills, compared with 7 of 170 patients (4.1%) prior to these guidelines (P = .40). Prescriptions for either acetaminophen or ibuprofen increased; 36 of 170 preintervention patients (21%) and 98 of 200 postintervention patients (49%) received these (P < .001). However, in a survey of 86 patients, 59 (69.0%) reported use of these medications; this was not a significant change from the preintervention use (61.0%; P = .60). Median (IQR) postoperative opioid use was significantly reduced from 30 mg to 20 mg after the intervention (postintervention IQR, 0-45 mg) (P = .04) without a change in pain score (median, 5.0; IQR, 3.1-6.5) (P = .80).
Table. Preintervention and Postintervention Comparison.
| Patient Characteristics and Prescribed Pain Medication | Preintervention (n = 170) |
Postintervention (n = 200) |
P Value |
|---|---|---|---|
| Age, mean (SD), y | 46 (14) | 48 (16) | .20 |
| Female, No. (%) | 132 (77.6) | 149 (74.5) | .50 |
| Patients receiving opioid prescription, No. (%) | 170 (100) | 185 (92.5) | < .001 |
| Prescription size, median (IQR), mg OME | 250 (200-300) | 75 (75-112.5) | < .001 |
| No. of pills, median (IQR) | 40 (30-60) | 15 (10-20) | < .001 |
| Requests for refills, No. (%) | 7 (4.1) | 5 (2.5) | .40 |
| Acetaminophen/ibuprofen prescribed, No. (%) | 36 (21.2) | 97 (48.5) | < .001 |
| Survey completion, No. (%) | 100 (58.8%) | 86 (43.0%) | .002 |
| Amount of opioid used,a median (IQR), mg OME | 30 (6-60) | 20 (0-45) | .04 |
| Patients using acetaminophen/ibuprofen,a No. (%) | 61 (61) | 59 (69) | .60 |
| Patient-reported pain score,a,b median (IQR) | 5.0 (3.5-6.5) | 5.0 (3.1-6.5) | .80 |
Abbreviations: IQR, interquartile range; OME, oral morphine equivalent.
Final 3 rows include only those individuals who completed surveys (n = 100 and n = 86 for preintervention and postintervention groups, respectively).
Pain was reported on a 0 to 10 scale, with 0 indicating no pain and 10 indicating the worst pain imaginable.
Interrupted time-series analysis revealed that, despite a preexisting decline in mean (SD) prescription size of 4 (2) mg/mo, there was a significant reduction of 119 (25) mg/mo (P < .001) after guideline implementation (Figure).
Figure. Reduction in Postoperative Opioid Prescribing After Implementation of Prescribing Guidelines.
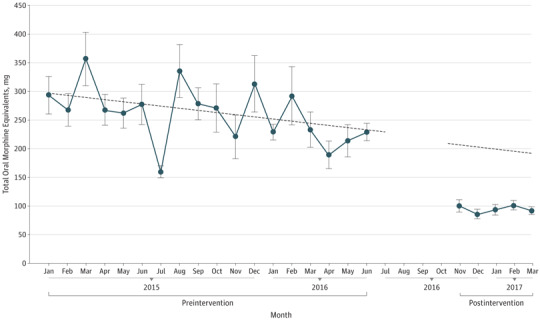
Following the implementation of evidence-based prescribing guidelines, opioid prescriptions were significantly reduced from an equivalent of approximately 45 pills of hydrocodone, 5 mg, to approximately 15 pills (P < .001). The dashed line represents the expected decline in prescribing prior to the study intervention.
Discussion
Following laparoscopic cholecystectomy, the median prescription size was 250 mg (OME), while median patient use was only 30 mg. This is equivalent to receiving 50 tablets of hydrocodone/acetaminophen, 5/325 mg, and using only 6 tablets. Evidence-based prescribing guidelines reduced prescription size by 63% without increasing the need for medication refills, thereby eliminating the excessive prescription of roughly 7000 pills. Patients also reported using fewer opioids after guideline implementation. This might be explained in part by the anchoring and adjustment heuristic,5 where the initial prescription size serves as the mental reference point for assessments of change. A recent study of cesarean delivery patients showed a significant correlation between larger prescription size and self-reported use.6
Limitations
Limitations of this study include the nonrandomized study design and the single-institution implementation. These results should also be interpreted within the context of this procedure, although the framework of this study could be applied to a variety of surgical procedures.
Conclusions
Initial work within the state of Michigan intimates a similar mismatch between prescribing and medication use. This work will be used as a template for statewide practice transformation, which may serve as a platform for other states.
References
- 1.Hill MV, McMahon ML, Stucke RS, Barth RJ Jr. Wide variation and excessive dosage of opioid prescriptions for common general surgical procedures. Ann Surg. 2017;265(4):709-714. [DOI] [PubMed] [Google Scholar]
- 2.Harris K, Curtis J, Larsen B, et al. Opioid pain medication use after dermatologic surgery: a prospective observational study of 212 dermatologic surgery patients. JAMA Dermatol. 2013;149(3):317-321. [DOI] [PubMed] [Google Scholar]
- 3.Lipari RN, Hughes A. How people obtain the prescription pain relievers they misuse: the CBHSQ Report. https://www.samhsa.gov/data/sites/default/files/report_2686/ShortReport-2686.html. Published January 12, 2017. Accessed October 19, 2017. [PubMed]
- 4.Lopez Bernal J, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2016;pii: dyw098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marchiori D, Papies EK, Klein O. The portion size effect on food intake: an anchoring and adjustment process? Appetite. 2014;81:108-115. [DOI] [PubMed] [Google Scholar]
- 6.Bateman BT, Cole NM, Maeda A, et al. Patterns of opioid prescription and use after cesarean delivery. Obstet Gynecol. 2017;130(1):29-35. [DOI] [PMC free article] [PubMed] [Google Scholar]


