Abstract
This prospective longitudinal analysis of people living in an impoverished neighborhood finds that fentanyl-positive urine samples increased rapidly during a 5-month period while opiate-positive samples declined.
Planning for the implications of nonprescribed fentanyl use relies on multisource forensic or clinical samples. Complementing these descriptions, we report a prospective longitudinal study of change in urine fentanyl prevalence in a high-risk, community-based sample.
Methods
Directly assessed participants were from a health outcomes study of people living in an impoverished neighborhood of Vancouver, Canada. For context, overall overdose deaths (Vancouver) and first responder calls (Vancouver and neighborhood-specific) were obtained from the British Columbia Coroner’s Office and Vancouver Police and Fire statistics for the study period (March 1 to July 31, 2017). Participants attended monthly visits and reported prescribed and nonprescribed drug use during the previous week, including fentanyl, buprenorphine, codeine, heroin, hydromorphone, methadone, morphine, and oxycodone. Participants (N = 237) contributed 595 urine samples that were tested for fentanyl/norfentanyl, opiates (morphine, heroin, and codeine), and methadone using detection strips (BTNX Inc). Agreement between reports and detection was assessed by κ statistic. Repeated measures logistic mixed-effects models with random intercept and slope were used to estimate associations between detection and reported opioid use in the prior week. Fixed effects of recent nonprescribed opioid use over time were estimated adjusting for age and sex. The study was approved by the institutional review boards at the University of British Columbia and Simon Fraser University, and participants provided written informed consent.
Results
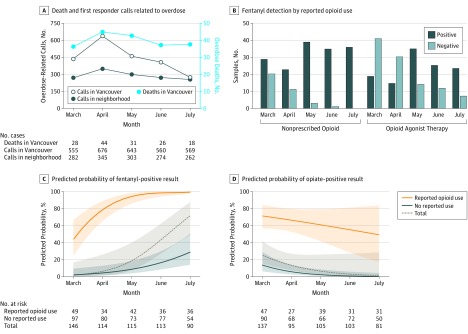
Between March and April 2017, an upsurge occurred in overdose deaths and first responder calls (Figure, A). Directly assessed participants had a mean (SD) age of 46.4 (12.2) years, were mostly men (184 of 237 [78%]), and were marginally housed or street homeless. Participants had a mean education of 10.2 years. Injection drug use in the past week was reported by 110 of 231 participants (48%). During the 5-month period, nonprescribed opioid use was reported by 91 of 237 individuals (38%), including 57 of 103 individuals (55%) who were prescribed opioid agonist therapy (hydromorphone, methadone, buprenorphine, morphine, or heroin). Fentanyl was detected in 229 of 590 urine samples (39%), including 116 of 222 samples (52%) from participants prescribed opioid agonist therapy (Figure, B). Overall, 83 of 91 participants (91%) reporting nonprescribed opioid use had at least 1 fentanyl-positive sample; 15 of these 83 (18%) reported taking fentanyl (11 of whom reported daily use). Opiates were detected in 196 of 581 urine samples (34%). Agreement between self-report and detection was low for fentanyl (κ = 0.12) and moderate or greater for other opioids (κ range, 0.54-0.84). The probability of fentanyl detection doubled each month (odds ratio, 2.28; P < .001) (Figure, C and Table). With self-reported nonprescribed opioid use, fentanyl detection probability was greater (odds ratio, 34.47; P < .001) and increased at a faster rate over time (odds ratio, 2.34; P = .03). In contrast, opiate detection decreased over time (odds ratio, 0.32; P = .003) (Figure, D and Table). By July 2017, all samples from participants reporting nonprescribed opioid use were fentanyl-positive.
Figure. Community-Based Studies of Fentanyl From March 1 to July 31, 2017.
The British Columbia Coroner’s Office reports of overdose deaths in Vancouver and overdose calls to first responders (police and fire department) for Vancouver and for the neighborhood from which participants were recruited (A). Numbers of urine samples with fentanyl detected are shown for participants using nonprescribed opioids and for those taking prescribed opioid agonist therapy (B). The probability of urine samples being positive for fentanyl (C) or opiates (heroin, morphine, or codeine) (D) changed during a 5-month period and differed according to reported use of nonprescribed opioids in the week before urinalysis. Samples were tested for fentanyl/norfentanyl (sensitivity 10 ng/mL, with cross-reactivity for acetylfentanyl, butyrylfentanyl, carfentanil, fluorofentanyl, 4-fluoroisobutyryl fentanyl, furanylfentanyl, 3-methylfentanyl, sufentanil, and thiofentanyl) and for opiates (heroin, morphine, and codeine). Shading represents the 95% confidence interval.
Table. Factors Associated With Changes in Urine Fentanyl and Opiate Detection in Nonprescribed Opioid Users Between March and July 2017.
| Characteristic | Unadjusted Models | Model 1a | Model 2b | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Urine fentanyl detected (n = 229 individuals; 575 observations) | ||||||
| Time, mo | 2.28 (1.61-3.23) | <.001 | 3.31 (1.66-6.58) | <.001 | 2.05 (1.30-3.24) | .002 |
| Intercept | ||||||
| Age, y | 0.92 (0.85-0.99) | .02 | 0.91 (0.84-0.98) | .02 | 0.98 (0.94-1.02) | .29 |
| Female | 5.22 (0.91-30.05) | .06 | 6.63 (1.03-42.62) | <.05 | 1.89 (0.72-4.97) | .19 |
| Nonprescribed opioid use | 30.64 (7.36-127.64) | <.001 | NA | NA | 34.47 (7.27-163.43) | <.001 |
| Slope | ||||||
| Nonprescribed opioid use | 2.49 (1.20-5.18) | .01 | NA | NA | 2.34 (1.11-4.93) | .03 |
| Urine opiatec detected (n = 215 individuals; 518 observations) | ||||||
| Time, mo | 0.32 (0.15-0.69) | .003 | 0.46 (0.19-1.11) | .08 | 0.39 (0.21-0.74) | .004 |
| Intercept | ||||||
| Age, y | 1.00 (0.96-1.04) | .87 | 0.99 (0.95-1.04) | .76 | 1.01 (0.98-1.05) | .44 |
| Female | 3.39 (0.93-12.31) | .06 | 3.49 (0.92-13.28) | .07 | 2.16 (0.86-5.41) | .10 |
| Nonprescribed opioid use | 14.24 (6.07-33.39) | <.001 | NA | NA | 15.89 (4.09-61.71) | <.001 |
| Slope | ||||||
| Nonprescribed opioid use | 2.21 (1.01-4.85) | <.05 | NA | NA | 2.01 (0.90-4.51) | .09 |
Abbreviations: NA, not applicable; OR, odds ratio.
Fixed effect of time in the whole cohort, adjusting for age and sex.
Fixed effects of nonprescribed opioid use on baseline and rate of change of detection over time, adjusting for age, sex, and time. To test for the possible effects of missing data, we examined associations between predictor and outcome measures and the number of visits made. There were no significant associations. The analysis was rerun 5 times with multiple imputation to assess the possible effects of missing visits; the OR and P values were similar.
Detected opiates included morphine, heroin, and codeine.
Discussion
Fentanyl-positive urine samples increased rapidly during a 5-month period while opiate-positive samples declined. In Vancouver, as elsewhere, the initial phase of the opioid epidemic was associated with diverted pharmaceuticals. This changed as nonpharmaceutical fentanyl entered the market as a heroin additive. The low concordance between reported fentanyl use and detection is consistent with unawareness of exposure. In the early months, as fentanyl-positive samples rapidly increased in the participants, an increase in overdose calls to first responders occurred in the neighborhood, and fatal overdoses increased city-wide. Some amelioration occurred by July 2017 when fentanyl-positive urine samples were ubiquitous among participants reporting nonprescribed opioid use. Tolerance to the adverse effects of higher potency opioids may be developing among users, as some individuals report actively seeking fentanyl. Fentanyl was detected in half of the participants in opioid agonist therapy programs in our study, which raises concern for increasing tolerance. Our fentanyl assay demonstrates cross-reactivity with other fentanyl analogues. Rapid and specific tests for fentanyl and related analogues are urgently needed, along with innovative treatments.
References
- 1.Dowell D, Noonan RK, Houry D. Underlying factors in drug overdose deaths. JAMA. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frank RG, Pollack HA. Addressing the fentanyl threat to public health. N Engl J Med. 2017;376(7):605-607. [DOI] [PubMed] [Google Scholar]
- 3.Arfken CL, Suchanek J, Greenwald MK. Characterizing fentanyl use in methadone-maintained clients. J Subst Abuse Treat. 2017;75:17-21. [DOI] [PubMed] [Google Scholar]
- 4.Amlani A, McKee G, Khamis N, Raghukumar G, Tsang E, Buxton JA. Why the FUSS (Fentanyl Urine Screen Study)? a cross-sectional survey to characterize an emerging threat to people who use drugs in British Columbia, Canada. Harm Reduct J. 2015;12:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Honer WG, Cervantes-Larios A, Jones AA, et al. The Hotel Study: clinical and health service effectiveness in a cohort of homeless or marginally housed persons. Can J Psychiatry. 2017;62(7):482-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciccarone D, Ondocsin J, Mars SG. Heroin uncertainties: Exploring users’ perceptions of fentanyl-adulterated and -substituted ‘heroin’. Int J Drug Policy. 2017;46:146-155. [DOI] [PMC free article] [PubMed] [Google Scholar]