This study investigates the hypothesis that brain dysconnectivity is a transdiagnostic phenotype in children and adolescents with increased susceptibility and symptoms of psychiatric disease.
Key Points
Question
Is brain structural connectivity a sensitive transdiagnostic phenotype for predicting psychiatric symptoms in youth?
Findings
This cross-sectional study of 748 children, adolescents, and young adults found that general and continuous symptom scores are associated with brain white matter patterns and that general psychopathology is a heritable trait in youth.
Meaning
This implicates brain dysconnectivity as a transdiagnostic phenotype that may be detected early in life in individuals with increased susceptibility for psychiatric disease.
Abstract
Importance
Many mental disorders emerge during adolescence, which may reflect a cost of the potential for brain plasticity offered during this period. Brain dysconnectivity has been proposed as a common factor across diagnostic categories.
Objective
To investigate the hypothesis that brain dysconnectivity is a transdiagnostic phenotype in adolescence with increased susceptibility and symptoms of psychiatric disease.
Design, Setting, and Participants
We investigated clinical symptoms as well as cognitive function in 6487 individuals aged 8 to 21 years from November 1, 2009, to November 30, 2011, in the Philadelphia Neurodevelopmental Cohort and analyzed diffusion magnetic resonance imaging brain scans for 748 of the participants.
Main Outcomes and Measures
Independent component analysis was used to derive dimensional psychopathology scores, and genome-wide complex trait analysis was used to estimate its heritability. Multimodal fusion simultaneously modeled contributions of the diffusion magnetic resonance imaging metrics fractional anisotropy, mean diffusivity, radial diffusivity, L1 (the principal diffusion tensor imaging eigen value), mode of anisotropy, as well as dominant and secondary fiber orientations, and structural connectivity density, and their association with general psychopathology and cognition.
Results
Machine learning with 10-fold cross-validation and permutation testing in 729 individuals (aged 8 to 22 years; mean [SD] age, 15.1 [3.3] years; 343 females [46%]) revealed significant association with general psychopathology levels (r = 0.24, P < .001) and cognition (r = 0.39, P < .001). A brain white matter pattern reflecting frontotemporal connectivity and crossing fibers in the uncinate fasciculus was the most associated feature for both traits. Univariate analysis across a range of clinical domains and cognitive test scores confirmed its transdiagnostic importance. Both the general psychopathology (16%; SE, 0.095; P = .05) and cognitive (18%; SE, 0.09; P = .01) factor were heritable and showed a negative genetic correlation.
Conclusion and relevance
Dimensional and heritable general cognitive and psychopathology factors are associated with specific patterns of white matter properties, suggesting that dysconnectivity is a transdiagnostic brain-based phenotype in individuals with increased susceptibility and symptoms of psychiatric disorders.
Introduction
Adolescence is a major window for individual adaptation, growth, and opportunities; however, it also coincides with the emergence of many mental disorders, which may reflect a cost of the vast potential for brain plasticity offered during this period. Several lines of evidence, including substantial shared genetic contribution to mental disorders, high rate of psychiatric comorbidity, reports of a common underlying factor across diagnostic categories on which high-scoring individuals are more likely to have cognitive impairments as well as compromised early-life brain function, all highlight the importance of identifying brain-based intermediate phenotypes that may be shared across disorders and present before the clinical manifestation of disease. Crucially, the myelinated axons of the brain white matter form the structural backbone that enables functional integration and adaptation of neural networks, together termed brain connectivity. There is a long-held notion of brain dysconnectivity as a transdiagnostic model for psychopathology, in which aberrant brain biology and synchronization modulate the risk for mental health issues. Recent demonstration of a link between a specific pattern of cross-talk between brain networks and a general positive-negative mode of population variability across a range of behavioral, demographic, and lifestyle measures supports that the level of individual adjustment can be conceptualized along a single dimension with a common brain basis.
The microstructural and connectivity properties of the human brain undergo profound changes throughout childhood and adolescence with a protracted maturation continuing well into the third decade and play a paramount role in the development of a healthy mind. Whereas the boundaries of this maturational potential are likely regulated by genetic predispositions, experiences and environmental perturbations mediate the individual development of cognitive and mental characteristics. The intricate biological processes shaping the brain connectome during neurodevelopment are highly coordinated across the brain, yet white matter development in childhood and adolescence also displays anatomically differentiated trajectories, suggesting regional variation in rates and timing of maturational changes. Hence, when considered from a system-level perspective, different brain circuits may compete for common resources, and the cognitive and clinical correlates may be modulated by the relative balance between different networks subserving distinct functions. Indeed, diffusion-weighted imaging–based indices of brain tissue organization may reflect the relative balance between differentially affected crossing fiber populations. Consequently, fusion of multimodal brain imaging indices, which allows for a joint consideration of different brain characteristics, may yield more biologically interpretable patterns of brain microstructure and connectivity sensitive to the development of cognitive and mental health.
To inform brain-based models of the development of cognitive functions and risk for psychopathology, and in line with the aims of the National Institute of Mental Health Research Domain Criteria project for mental disorders, we performed a data-driven multivariate delineation of brain and behavioral phenotypes. Scores from a diverse battery of cognitive tests and clinical questionnaires were decomposed to form and isolate general and specific phenotypic traits, including general cognitive and general psychopathology factors, using principal component analysis and independent component analysis (ICA). In a subset with available diffusion-weighted imaging data, we used linked ICA (LICA) to decompose 8 white matter maps per individual reflecting different microstructural properties into independent components. Each component is characterized by group-level spatial maps (1 per white matter index) and corresponding participant weights (1 per participant) reflecting the individual contribution to the observed brain patterns. These weights were used in multivariate cross-validated analysis for prediction of age, cognition, and psychopathology using machine learning and to test for univariate associations. We used genome-wide complex trait analysis to estimate the lower-bound heritability of the general cognitive and general psychopathology phenotypes based on variation in common single-nucleotide polymorphisms (SNPs) in a subset of individuals of European descent and estimated the genetic correlation between these traits.
Methods
Sample and Exclusion Criteria
The analysis was based on the publicly available data from the Philadelphia Neurodevelopmental Cohort. Recruitment procedures, sample characteristics, clinical, cognitive, and imaging protocols are described in detail by Satterthwaite et al and are briefly described in the eMethods in the Supplement. The institutional review boards of the University of Pennsylvania and the Children’s Hospital of Philadelphia approved all study procedures, and written informed consent was obtained from all participants. The study was conducted from November 1, 2009, to November 30, 2011. We assessed cognitive and clinical data for 6487 participants, genetic data for 4450 participants of European descent, and diffusion-weighted imaging data from 883 participants. After removal of data sets that did not pass quality assessment (n = 91) and of participants with severe medical health conditions (n = 44, rating was performed by trained personnel in the Philadelphia Neurodevelopmental Cohort study team), the final magnetic resonance imaging (MRI) sample comprised 748 individuals (343 females [46%]) aged 8.7 to 22.6 years (mean [SD] age, 15.1 [3.3] years). Of the 748 participants included in the MRI analysis, 729 (97.5%) had available clinical data.
MRI Acquisition and Analysis
Diffusion MRI scans were acquired at University of Pennsylvania on a 3-T Siemens TIM Trio scanner (Siemens Medical Solutions), acquired using a twice-refocused spin-echo single-shot echo-planar imaging sequence (field of view, 240 × 240 mm; matrix, 128 × 128 × 70; 64 diffusion-weighted directions; b = 1000 seconds/mm2; voxel resolution, 1.875 × 1.875 × 2 mm). We computed temporal-signal-to-noise ratio, motion, and entropy of the distribution of the principal diffusion directions for all data sets (eMethods in the Supplement).
Diffusion-weighted imaging data were processed using FMRIB’s (ie, Oxford Centre for Functional Magnetic Resonance Imaging of the Brain) Diffusion Toolbox, part of the FMRIB Software Library, and included correction for eddy current distortions, motion (eddy), and rotation of diffusion gradients. Fractional anisotropy, eigen-vector and eigen-value maps were then computed by fitting a tensor model to the corrected diffusion data and used to derive maps for axial (L1, the principal diffusion tensor imaging eigen value), mean and radial diffusivity, and the mode of anisotropy.
Estimation of the probability distribution of the diffusion directions was performed with the graphics processing units accelerated version of bedpostX toolbox (part of FMRIB’s Diffusion Toolbox, version 3) with up to 3 fibers estimated per voxel. The maps for the dominant (f1) and the secondary (f2) fiber population were used in further analyses.
Whole-brain probabilistic fiber tracking was performed using probtrackx2 (part of FMRIB’s Diffusion Toolbox, version 3). In line with a recent application in aging and dementia, for each participant and from each voxel inside the native space whole-brain seed mask, 100 pathways were sampled, resulting in a 3-dimensional volume tractography map per participant, which were normalized by dividing with the total number of streamlines processed. Thus, the value in each voxel in this probabilistic tractography connectivity density map represents the likelihood that any streamline will pass through that voxel.
All maps were nonlinearly transformed to the FMRIB58_FA template and skeletonized using the FMRIB’s Diffusion Toolbox Tract-Based Spatial Statistics (TBSS) pipeline (part of FMRIB Software Library, version 5.0.9). Fiber orientation maps were warped and skeletonized using tbss_x (part of FMRIB Software Library, version 5.0.9).
We performed data-driven decomposition of imaging features into independent components using FMRIB’s LICA, which models the interparticipant variability across modalities. For each participant with available diffusion-weighted imaging data (n = 748), we included 8 skeleton maps reflecting different microstructural and connectivity properties (fractional anisotropy, L1, radial diffusivity, mean diffusivity, mode of anisotropy, f1, f2, and connectivity density). The model order was heuristically chosen at 20 components based on a visual inspection of the spatial maps. Each LICA component is characterized by 8 group-level spatial pseudo z maps, 1 for each of the white matter indices, modality weights reflecting the white matter indices’ contribution to the component, as well as participant weight for each participant reflecting the individual relative contribution to the observed spatial patterns. For instance, if a voxel has positive pseudo z value for a given modality, then higher participant weight means higher values for that modality in that voxel, and vice versa. For visualization of the skeleton-based LICA modality maps, we used tbss_fill, with a threshold at absolute z score of more than 3.
Behavioral Phenotypes
All participants completed a computerized test battery. We assessed cognitive performance scores from all participants (n = 6487) on 12 tests (eTable 1 in the Supplement) spanning executive control and working memory, episodic memory, verbal and nonverbal reasoning, and social cognition. These included accuracy and reaction time measures. Scores were adjusted for age using a linear model, and the residuals were submitted to principal component analysis. The first factor was extracted as a general measure of cognitive performance (ie, fluid intelligence [Gf]).
A computerized protocol was used to assess symptoms of anxiety, mood, behavioral, eating, and psychosis spectrum disorders with collateral informants for individuals 18 years and younger. We included 129 clinical symptom score items covering 18 psychological clinical domains (eTable 2 in the Supplement) for all available participants (n = 6487), excluding all follow-up and conditional items. A total of 1627 participants had missing values on 1 or several clinical items. For all but 2 participants who had missing values on all 129 clinical items, missing values were replaced with the nearest neighbor value based on Euclidean distance. The percentage of missing values for the 129 items ranged from 0% to 7%.
All available clinical item scores were submitted to ICA using Icasso (version 1.21; MATLAB), decomposing them into 7 independent components. The model order of the ICA was chosen after testing several different model orders ranging from 3 to 15, each of which 100 permutations was run, and based on the observed independence and reliability of the resulting components. The mean participant weight across components was computed (mean ICA) and used as a general measure of psychopathology. For comparison, we submitted the clinical variables to a principal component analysis and extracted the first principal component. In addition, we computed clinical summary scores across items based on the categorical clinical domain of each questionnaire for comparison.
Statistical Analysis
Prediction of age, Gf, and mean ICA using LICA participant weights was performed using shrinkage linear regression with automatic estimation of the correlation shrinkage intensity. We performed 1000 iterations each with a separate 10-fold cross-validation before calculating the mean correlation across all iterations. Feature importance was assessed by correlation-adjusted (marginal) correlation scores. To assess significance, for each trait we performed 10 000 permutations of the 10-fold cross-validated prediction while randomly permuting the trait scores. Univariate associations between LICA participant weights and cognitive and clinical variables were performed using linear models and included age, sex, and temporal-signal-to-noise ratio as covariates and corrected for multiple comparisons using false discovery rate. All reported P values are 2-tailed unless otherwise specified. For visualization of age curves of LICA participant weights (eFigure 1 in the Supplement) for high- and low-scoring individuals (Gf and clinical ICA score), we used a cutoff of plus or minus 1 SD to form groups Gf high (n = 114), Gf low (n = 75), mean ICA high (n = 115), mean ICA low (n = 76), independent component (IC) 2 high (n = 126), IC3 high (n = 80), and IC4 high (n = 108). We then repeatedly fitted a smooth function with automatic estimation of the smoothness parameter and computed the mean (SD) across 10 000 bootstraps for each of the groups.
Genetic Analysis
Genotyping was performed by the Center for Applied Genomics at the Children’s Hospital of Philadelphia. Single-nucleotide polymorphisms filtering parameters are described in the eMethods in the Supplement. We used genome-wide complex trait analysis to create a genetic relationship matrix based on the SNPs remaining after filtering (49 774) before running univariate and bivariate restricted maximum likelihood analysis to estimate SNP heritability for Gf and mean ICA, as well as the genetic correlation between these traits, in participants with European descent with overlapping phenotypic and genetic data (n = 2946). Age, sex, and platform were included as covariates. The genetic correlation between Gf and mean ICA scores was estimated through subsampling by drawing 6000 random subsamples (n = 2746) from the full sample (n = 2945) without replacement before estimating the mean (SD) of genetic correlation across the subsamples.
Results
Clinical ICA
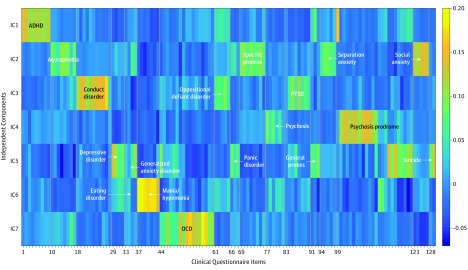
The 7 ICs reflect the empirical clustering of specific psychopathology symptoms (Figure 1 and eFigure 1 in the Supplement). Briefly, they represent attention problems (IC1); anxiety (IC2); norm-violating behavior (IC3); psychosis positive and prodromal symptoms (IC4); depression, suicide, and psychosis negative symptoms (IC5); mania (IC6); and obsessive-compulsive symptoms (IC7). The mean weights across these components (mean ICA) was highly correlated with the general factor (first principal component) from the principal component analysis on the same items (r = 0.97).
Figure 1. Weights for 129 Clinical Questionnaire Items on the Estimated Components From the Clinical Independent Component (IC) Analysis.
The color scale represents item loading on the independent components. ADHD indicates attention-deficit/hyperactivity disorder; OCD, obsessive-compulsive disorder; PTSD, posttraumatic stress disorder.
Linked Independent Component Analysis
Age Prediction
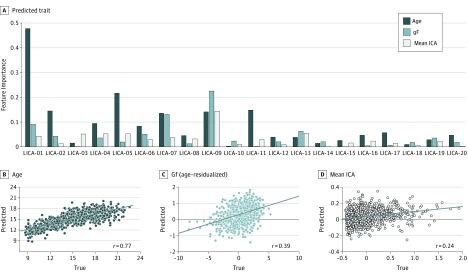
The LICA components captured distinct patterns of neurodevelopment (Figure 2A). All components combined were highly associated with age (correlation between observed and predicted age: r = 0.77, P < .001; P value obtained from nonparametric permutation testing, eFigure 2 in the Supplement). This confirms the high sensitivity of brain features for age prediction in the Philadelphia Neurodevelopmental Cohort sample and was primarily driven by the first component, capturing spatially global variance in fractional anisotropy, radial diffusivity, and mean diffusivity (LICA-01, eFigure 3 in the Supplement).
Figure 2. Linked Independent Component Analysis (LICA) Brain Components Were Robustly Associated With Age, Fluid Intelligence, and Psychopathology.
The bar graph shows the feature importance for each of the 20 components in cross-validated associations with age, fluid intelligence (Gf), and psychopathology (mean independent component analysis [ICA]) (A). Although LICA-01 is the most important feature for association with age, LICA-09 is most important for association with cognition and mental health. The scatterplots show the correlations between the true and predicted scores for age (B), Gf (C), and mean ICA (D) (P < .001 was considered significant; familywise error, 10 000 permutations).
Cognitive and Clinical Prediction
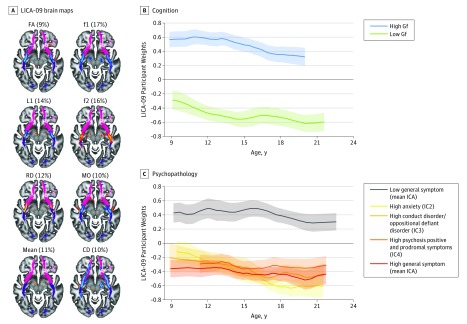
The components also allowed for significant association with the general cognitive (Gf: r = 0.39, P < .001) and psychopathology (mean ICA: r = 0.24, P < .001) factors (Figure 2). These were both strongly driven by the same component (LICA-09) of which anatomical distribution and multimodal contribution is consistent with degree of crossing fibers in frontotemporal connections. Specifically, the spatial foci of LICA-09 encompassed the intersection between the uncinate fasciculus and the inferior fronto-occipital fasciculus, with negative spatial weights for fractional anisotropy, f1, mode of anisotropy, and connectivity density and positive weights for f2 and radial diffusivity (Figure 3A). Although higher fractional anisotropy is often interpreted in terms of white matter “integrity,” fractional anisotropy may also be higher in crossing fiber regions if 1 of the fiber populations is degraded and may be inferred based on the conjunction of mode of anisotropy changes in the same region. Further, f1 and f2 models the evidence for 2 underlying fiber populations and the joint multivariate pattern revealed by LICA-09 thus is sensitive to the underlying anatomy at the intersection of 2 major white matter pathways in this region (eFigure 4 in the Supplement). Lower participant weights for LICA-09 with higher psychopathology implies less crossing fibers in individuals with increased symptom burden.
Figure 3. Linked Independent Component Analysis (LICA) Brain Components Were Robustly Associated With Cognition and Psychopathology.
Spatial maps for LICA-09, with percentages representing the relative modality contributions (A). Warm and cool colors represent positive and negative LICA weights, respectively (pseudo z threshold of ±3), overlaid on the uncinate fasciculus (pink) and inferior fronto-occipital fasciculus (purple) from the Johns Hopkins University white matter tractography atlas. Weights are plotted as a function of age and stratified by fluid intelligence (Gf) (B) and clinical independent component analysis (ICA) scores (C). The lower participant weights for participants with higher symptom burden entails increased fractional anisotropy (FA), dominant fiber population (f1), L1 (the principal diffusion tensor imaging eigen-value), connectivity density (CD), and mode of anisotropy (MO), and decreased secondary fiber population (f2) and radial diffusivity (RD) in the uncinated in the insular region, consistent with decreased crossing fibers. Curves and shaded area represent the mean (SD) of smooth function fitting across 10 000 bootstraps.
Univariate Associations
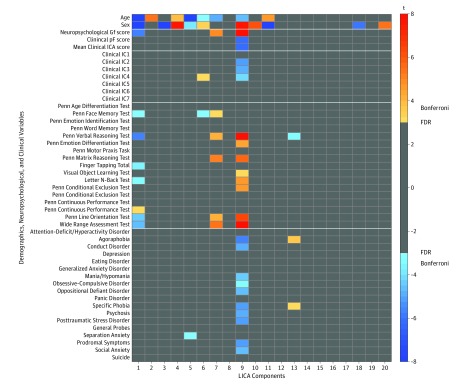
The robust clinical and cognitive relevance of LICA-09 was supported by significant univariate associations with a wide range of cognitive and clinical domain scores (Figure 4). They confirmed that the brain pattern in LICA-09 was less expressed in individuals with more severe general psychopathology and more expressed in individuals with higher cognitive performance. Although LICA-09 showed a significant negative association with age, its associations with cognitive and clinical features were relatively stable across the sampled age range (Figure 3B). Importantly, eFigure 5 in the Supplement illustrates that although the general cognitive and the general psychopathology scores showed a negative correlation (r = –0.35, P < .001), they exhibited independent associations with LICA-09. Further, the associations were robust to rigorous control for image quality indices (eFigure 6 in the Supplement), as well as projection distances from individual brains to the TBSS skeleton (eFigure 7 in the Supplement). When investigating individual clinical ICA components, each of which express more symptom specificity compared with the general psychopathology score, similar associations (eFigure 8 in the Supplement) were observed for anxiety and harm avoidance (IC2), antisocial and norm-violating behavior (IC3), and for psychosis positive and prodromal symptoms (IC4).
Figure 4. Univariate Results for the Participant Weights of the 20 Linked Independent Component Analysis (LICA) Components Support the Cognitive and Clinical Relevance of LICA-09.
The brain pattern captured by LICA-09 shows robust and inverse associations with cognitive (ie, fluid intelligence [Gf]) and clinical component scores, as well as with individual cognitive and clinical domain scores. Associations adjusted for age, sex, and temporal-signal-to-noise ratio, and corrected for multiple comparisons using false discovery rate (FDR) of q = 0.05. pF indicates first principal component for clinical items.
Genetic Analysis
Common SNPs explained 18% (P = .01; SE, 0.09) of the individual differences in general cognition and 16% (P = .05; SE, 0.095) of the differences in general psychopathology. These 2 traits also showed a negative genetic correlation (resampling mean r = –0.74; SE, 0.15; 99% CI, –1 to –0.36; eFigure 9 in the Supplement).
Discussion
Adolescence is a sensitive ontogenetic period that hosts a protracted development of brain and psychological functions, as well as the emergence of several mental health disorders. We have shown that general factors of cognition and symptoms of psychopathology in children and adolescents are heritable traits that show partly overlapping genetic architecture. Further, we demonstrated that these behavioral phenotypes, which are likely to represent proxies of critical adult-life indicators, including educational attainment and mental health, are associated with brain white matter characteristics as revealed using advanced multivariate analysis of diffusion MRI metrics. The brain pattern most critical for prediction of both cognition and psychopathology captures the developmental coordination of 2 major white matter tracts during late childhood and early adolescence. In particular, the component reflects a distinct frontotemporal pattern reflecting crossing fibers at the intersection between the uncinate fasciculus and the inferior fronto-occipital fasciculus. This white matter microstructural pattern is of particular interest with respect to mental health. Uncinate fasciculus is among the tracts with the highest heritability; it shows protracted maturation compared with other fiber tracts and is hypothesized to subserve a limbic temporo-amygdala-orbitofrontal network critically involved in integration of emotional states with cognition and behavior.
Similar associations were observed for anxiety, antisocial behavior, as well as for psychosis positive and prodromal symptoms. This confirms that the reported brain pattern is broadly associated with a range of psychopathology symptoms rather than showing specificity with a certain clinical domain. These 3 symptom categories have been shown to belong to separate empirical symptom factors, often termed internalizing, externalizing, and thought disorder symptoms; however, they also load on a general psychopathology factor, implying shared common risk. A 2016 study supported the existence of a general and heritable psychopathology factor in children and adolescents. Here, by using multivariate techniques for data reduction and integration, we replicate and extend those findings in the Philadelphia Neurodevelopmental Cohort by showing that a general cognitive and a general psychopathology factor are heritable with a shared genetic contribution and are associated with a distinct pattern of white matter microstructure and connectivity in the developing human brain.
Limitations
The current study comes with some limitations. Follow-up assessment is needed to determine the relevance of our findings for long-term outcome. Images with reverse-phase encoding were not collected but would enable better distortion correction and artifact detection, and multishell diffusion weighted data would increase the sensitivity of detecting fiber crossing. The sample size for the heritability analysis was on the lower end, and point estimates should be interpreted with caution as the confidence intervals are wide. However, the estimated negative genetic correlation between cognition and psychopathology is unlikely to occur in a population with 0 or positive values for genetic correlation, and the sign of the correlation is also in agreement with large-scale studies on cognition and psychopathology in adults. Future studies with larger samples are needed to determine whether there is also a genetic correlation between the observed brain phenotype and psychopathology.
Conclusions
The results of this study point to limbic temporo-amygdala-orbitofrontal pathways as a candidate transdiagnostic brain phenotype in psychiatric disorder. Indeed, frontotemporal connectivity has been implicated in a range of mental illnesses including schizophrenia, conduct disorder, as well as anxiety. Importantly, the present findings show that such abnormalities are present also in youth who do not have a diagnosis but are at risk for psychiatric disease.
eMethods.
eFigure 1. Distribution of Subject Weights for the 7 Clinical ICA Components, as well as the General Symptom Score (Mean ICA)
eFigure 2. Density Plots for Age, Gf, and Mean ICA
eFigure 3. LICA-01 Captures Spatially Global Brain Changes Across the Age Span
eFigure 4. Seed-based Probabilistic Tractography Confirm Involvement of UF
eFigure 5. Gf and Mean ICA Exhibit Independent Associations With LICA-09
eFigure 6. Impact of Image Quality on LICA-09 Associations
eFigure 7. Effects of Projection Distance on LICA-09 Subject Weights and its Clinical and Cognitive Associations
eFigure 8. Clinical Questionnaire Item Weights for Clinical IC2, 3, and 4 and Associations With LICA-09
eFigure 9. Distribution of rG Estimates Across Subsamples
eTable 1. List of Variables Included in the PCA to Compute the General Cognition Score (Gf)
eTable 2. List of the 129 Clinical Variables That Entered the Independent Component Analysis
References
- 1.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9(12):947-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Donovan MC, Owen MJ. The implications of the shared genetics of psychiatric disorders. Nat Med. 2016;22(11):1214-1219. [DOI] [PubMed] [Google Scholar]
- 3.Caspi A, Houts RM, Belsky DW, et al. . The p factor: one general psychopathology factor in the structure of psychiatric disorders? Clin Psychol Sci. 2014;2(2):119-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castellanos-Ryan N, Brière FN, O’Leary-Barrett M, et al. ; IMAGEN Consortium . The structure of psychopathology in adolescence and its common personality and cognitive correlates. J Abnorm Psychol. 2016;125(8):1039-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckholtz JW, Meyer-Lindenberg A. Psychopathology and the human connectome: toward a transdiagnostic model of risk for mental illness. Neuron. 2012;74(6):990-1004. [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann T, Alnæs D, Doan NT, Brandt CL, Andreassen OA, Westlye LT. Delayed stabilization and individualization in connectome development are related to psychiatric disorders. Nat Neurosci. 2017;20(4):513-515. [DOI] [PubMed] [Google Scholar]
- 7.Smith SM, Nichols TE, Vidaurre D, et al. . A positive-negative mode of population covariation links brain connectivity, demographics and behavior. Nat Neurosci. 2015;18(11):1565-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2012;60(1):340-352. [DOI] [PubMed] [Google Scholar]
- 9.Westlye LT, Walhovd KB, Dale AM, et al. . Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cereb Cortex. 2010;20(9):2055-2068. [DOI] [PubMed] [Google Scholar]
- 10.Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology. 2010;35(1):147-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douaud G, Jbabdi S, Behrens TEJ, et al. . DTI measures in crossing-fibre areas: increased diffusion anisotropy reveals early white matter alteration in MCI and mild Alzheimer’s disease. Neuroimage. 2011;55(3):880-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groves AR, Smith SM, Fjell AM, et al. . Benefits of multi-modal fusion analysis on a large-scale dataset: life-span patterns of inter-subject variability in cortical morphometry and white matter microstructure. Neuroimage. 2012;63(1):365-380. [DOI] [PubMed] [Google Scholar]
- 14.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88(1):76-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Satterthwaite TD, Connolly JJ, Ruparel K, et al. . The Philadelphia Neurodevelopmental Cohort: a publicly available resource for the study of normal and abnormal brain development in youth. Neuroimage. 2016;124(Pt B):1115-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Satterthwaite TD, Elliott MA, Ruparel K, et al. . Neuroimaging of the Philadelphia Neurodevelopmental Cohort. Neuroimage. 2014;86:544-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roalf DR, Quarmley M, Elliott MA, et al. . The impact of quality assurance assessment on diffusion tensor imaging outcomes in a large-scale population-based cohort. Neuroimage. 2016;125:903-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oguz I, Farzinfar M, Matsui J, et al. . DTIPrep: quality control of diffusion-weighted images. Front Neuroinform. 2014;8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62(2):782-790. [DOI] [PubMed] [Google Scholar]
- 21.Ennis DB, Kindlmann G. Orthogonal tensor invariants and the analysis of diffusion tensor magnetic resonance images. Magn Reson Med. 2006;55(1):136-146. [DOI] [PubMed] [Google Scholar]
- 22.Hernández M, Guerrero GD, Cecilia JM, et al. . Accelerating fibre orientation estimation from diffusion weighted magnetic resonance imaging using GPUs. PLoS One. 2013;8(4):e61892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Behrens TEJ, Berg HJ, Jbabdi S, Rushworth MFS, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage. 2007;34(1):144-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doan NT, Engvig A, Persson K, et al. . Dissociable diffusion MRI patterns of white matter microstructure and connectivity in Alzheimer’s disease spectrum. Sci Rep. 2017;7:45131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith SM, Jenkinson M, Johansen-Berg H, et al. . Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487-1505. [DOI] [PubMed] [Google Scholar]
- 26.Jbabdi S, Behrens TEJ, Smith SM. Crossing fibres in tract-based spatial statistics. Neuroimage. 2010;49(1):249-256. [DOI] [PubMed] [Google Scholar]
- 27.FLICA. FSL website. https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FLICA. Accessed December 14, 2017.
- 28.Groves AR, Beckmann CF, Smith SM, Woolrich MW. Linked independent component analysis for multimodal data fusion. Neuroimage. 2011;54(3):2198-2217. [DOI] [PubMed] [Google Scholar]
- 29.Himberg J, Hyvärinen A, Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage. 2004;22(3):1214-1222. [DOI] [PubMed] [Google Scholar]
- 30.Schäfer J, Strimmer K. A shrinkage approach to large-scale covariance matrix estimation and implications for functional genomics. Stat Appl Genet Mol Biol. 2005;4:e32. [DOI] [PubMed] [Google Scholar]
- 31.Zuber V, Strimmer K. High-dimensional regression and variable selection using CAR scores. Stat Appl Genet Mol Biol. 2011;10(1). doi: 10.2202/1544-6115.1730 [DOI] [Google Scholar]
- 32.Fjell AM, Walhovd KB, Westlye LT, et al. . When does brain aging accelerate? dangers of quadratic fits in cross-sectional studies. Neuroimage. 2010;50(4):1376-1383. [DOI] [PubMed] [Google Scholar]
- 33.Erus G, Battapady H, Satterthwaite TD, et al. . Imaging patterns of brain development and their relationship to cognition. Cereb Cortex. 2015;25(6):1676-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Passamonti L, Fairchild G, Fornito A, et al. . Abnormal anatomical connectivity between the amygdala and orbitofrontal cortex in conduct disorder. PLoS One. 2012;7(11):e48789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR. Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain. 2013;136(Pt 6):1692-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Budisavljevic S, Kawadler JM, Dell’Acqua F, et al. . Heritability of the limbic networks. Soc Cogn Affect Neurosci. 2016;11(5):746-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Catani M, Dell’acqua F, Thiebaut de Schotten M. A revised limbic system model for memory, emotion and behaviour. Neurosci Biobehav Rev. 2013;37(8):1724-1737. [DOI] [PubMed] [Google Scholar]
- 38.Neumann A, Pappa I, Lahey BB, et al. . Single nucleotide polymorphism heritability of a general psychopathology factor in children. J Am Acad Child Adolesc Psychiatry. 2016;55(12):1038-1045.e4. [DOI] [PubMed] [Google Scholar]
- 39.Sotiropoulos SN, Jbabdi S, Xu J, et al. ; WU-Minn HCP Consortium . Advances in diffusion MRI acquisition and processing in the Human Connectome Project. Neuroimage. 2013;80:125-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hagenaars SP, Harris SE, Davies G, et al. ; METASTROKE Consortium, International Consortium for Blood Pressure GWAS; SpiroMeta Consortium; CHARGE Consortium Pulmonary Group, CHARGE Consortium Aging and Longevity Group . Shared genetic aetiology between cognitive functions and physical and mental health in UK Biobank (N=112 151) and 24 GWAS consortia. Mol Psychiatry. 2016;21(11):1624-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samartzis L, Dima D, Fusar-Poli P, Kyriakopoulos M. White matter alterations in early stages of schizophrenia: a systematic review of diffusion tensor imaging studies. J Neuroimaging. 2014;24(2):101-110. [DOI] [PubMed] [Google Scholar]
- 42.Baur V, Brühl AB, Herwig U, et al. . Evidence of frontotemporal structural hypoconnectivity in social anxiety disorder: a quantitative fiber tractography study. Hum Brain Mapp. 2013;34(2):437-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Westlye LT, Bjørnebekk A, Grydeland H, Fjell AM, Walhovd KB. Linking an anxiety-related personality trait to brain white matter microstructure: diffusion tensor imaging and harm avoidance. Arch Gen Psychiatry. 2011;68(4):369-377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eFigure 1. Distribution of Subject Weights for the 7 Clinical ICA Components, as well as the General Symptom Score (Mean ICA)
eFigure 2. Density Plots for Age, Gf, and Mean ICA
eFigure 3. LICA-01 Captures Spatially Global Brain Changes Across the Age Span
eFigure 4. Seed-based Probabilistic Tractography Confirm Involvement of UF
eFigure 5. Gf and Mean ICA Exhibit Independent Associations With LICA-09
eFigure 6. Impact of Image Quality on LICA-09 Associations
eFigure 7. Effects of Projection Distance on LICA-09 Subject Weights and its Clinical and Cognitive Associations
eFigure 8. Clinical Questionnaire Item Weights for Clinical IC2, 3, and 4 and Associations With LICA-09
eFigure 9. Distribution of rG Estimates Across Subsamples
eTable 1. List of Variables Included in the PCA to Compute the General Cognition Score (Gf)
eTable 2. List of the 129 Clinical Variables That Entered the Independent Component Analysis