Abstract
In addition to its function in calcium and bone metabolism, vitamin D is neuroprotective and important for mitigating inflammation. Alzheimer’s disease (AD) is a progressive neurodegenerative disorder of the central nervous system, characterized by neuronal loss in many areas of the brain, and the formation of senile (neuritic) plaques, which increase in number and size over time. The goal of this project was to investigate whether vitamin D3 supplementation would affect amyloid plaque formation in amyloid-β protein precursor (AβPP) transgenic mice that spontaneously develop amyloid plaques within 3–4 months of birth. AβPP mice were fed control, vitamin D3-deficient or vitamin D3-enriched diets for five months, starting immediately after weaning. At the end of the study, the animals were subjected to behavioral studies, sacrificed, and examined for bone changes and brain amyloid load, amyloid-β (Aβ) peptide levels, inflammatory changes, and nerve growth factor (NGF) content. The results obtained indicate that a vitamin D3-enriched diet correlates with a decrease in the number of amyloid plaques, a decrease in Aβ peptides, a decrease in inflammation, and an increase in NGF in the brains of AβPP mice. These observations suggest that a vitamin D3-enriched diet may benefit AD patients.
Keywords: Alzheimer’s disease, amyloid-β, animal models, transgenic mice, vitamin D
INTRODUCTION
Alzheimer’s disease (AD) affects over five million patients in the United States alone and is the most common form of senile dementia associated with memory loss and failure of executive function to the point of becoming highly dependent on expensive and intensive care [1]. AD is characterized by the aggregation of amyloid-β (Aβ) peptide into neuritic plaques and hyperphosphorylated tau protein accumulating into neurofibrillary tangles (NFTs) [2]. Aβ is generated from the sequential cleavage of the amyloid-β protein precursor (AβPP) resulting in Aβ40 and Aβ42, which give rise to aggregated fibrils and plaques [3,4]. Aβ is known to be neurotoxic and is thought to play a major role in the pathogenesis of AD and accumulates as a result of either decreased turnover and/or increased production [5, 6]. Thus the reduction in Aβ levels has been the major therapeutic goal for drug development against AD [7, 8]. Understanding the mechanisms and developing potential therapeutics will help to reduce the detrimental effects of AD.
Cholecalciferol (Vitamin D3) is produced in the skin from 7-dehydrocholesterol (7-DHC) [9, 10]. Exposure of skin to sunlight in the ultraviolet B (UVB) range of the spectrum (290 to 315 nm) results in the photolytic conversion of 7-DHC to previtamin D3, which is transformed to vitamin D3 by spontaneous isomerization [10]. Vitamin D3 can be obtained from the diet (e.g., vitamin D3-fortified milk); however, its doses are much lower than synthesis and are poorly bioavailable. Vitamin D3 enters the circulation and travels to the liver where it is hydroxylated at position 25 to form 25-hydroxyvitamin D3 [25(OH)D3]. This molecule is further modified in the kidney by the addition of a second hydroxyl (-OH) group at position 1, thereby forming 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] [11]. This is the active, hormonal, and most potent form of vitamin D, which is also known as “calcitriol”. The activity of vitamin D in bone mineralization is the result of its role in promoting calcium and phosphorus absorption in the intestine [12]. Vitamin D has also a role in maintaining optimal levels of calcium in the blood and, when the calcium intake is not sufficient, vitamin D will mobilize calcium from the bones to reestablish its optimal circulating level [13]. The kidney is the only organ with endocrine function that can release 1,25(OH)2D3 into the bloodstream, thereby controlling the plasma levels of calcium and phosphorus [14, 15].
This research effort stemmed from the accumulation of scientific evidence demonstrating that vitamin D, in addition to its well-established role in the regulation of calcium levels in the blood and in bone formation, has additional and, possibly, more important functions, such as ensuring immune homeostasis (i.e., controlling the inflammatory response), and promoting the production of neuroprotective factors like nerve growth factor (NGF) [16, 17]. Calcitriol can inhibit NF-κB, a major transcription factor that induces the production of inflammatory mediators and cytokines [17, 18]. Cytokines are small proteins produced by cells of the immune system, such as macrophages, and their counterparts in the brain, microglia [19]. Chronic inflammation is particularly important in long-term processes, such as neurodegenerative diseases, that over time affect cognition. Calcitriol also antagonizes the key proinflammatory cytokine IL-1, which is thought to mediate negative behavioral effects such as the so-called “sickness behavior” [20]. Furthermore, oral treatment with 25(OH)D3 reduced production of IL-1 in the rat hippocampus [21].
The observation of widespread vitamin D deficiency in AD patients hosted in nursing homes [22], coupled with the evidence that higher levels of circulating 25(OH)D3 appear to be associated with better cognitive test performance in AD patients [23], prompted the investigation of what effects, if any, a vitamin D-enriched diet would have on genetically modified AβPP mice predisposed to show AD-like pathology and symptoms over time. Although AβPP mice do not develop the full spectrum of the disease, such as significant neuronal loss and NFT formation (deposition of hyperphosphorylated tau protein), they represent a widely used animal model for AD, especially to evaluate potential therapeutic interventions to lower Aβ peptide levels in the brain.
We report that feeding transgenic mice expressing familial AD (FAD) mutant AβPP and presenilin 1 (PS1) cDNAs (AβPP-PS1) with a vitamin D3-enriched diet for five months significantly decreases the number of amyloid plaques, levels of Aβ peptides, and increases NGF in their brains, compared to AβPP-PS1 mice fed a vitamin D3-deficient diet.
MATERIALS AND METHODS
Mice
All animal experimentation was conducted with the permission of the Institutional Animal Care and Use Committee, and according to institutional guidelines. Male AβPP-PS1 transgenic mice were bred in the Medical University of South Carolina Animal Facility. These mice express the mutant form of human PS1(DeltaE9) and the mutant chimerical mouse/human AβPP695 cDNA [21]. The DeltaE9 mutation of the human PS1 gene is a deletion of exon-9, linked to early-onset FAD. AβPP-PS1 mice start developing amyloid plaques around 3–4 months of age. Animals were housed under a 12 : 12 light/dark cycle in standard non-sterile rodent micro-isolator cages, with filtered cage tops (four mice to a cage). Mice were weaned at 3–4 weeks of age and fed identical diets, except for the presence or absence of vitamin D3 supplement [Harlan TD.98107 (control diet, 2.4 IU/g vitamin D), Harlan TD.98108 (Surplus Vitamin D Diet; VD, ~12 IU/g vitamin D) or Harlan TD.98109 (Vitamin D deficient Diet; VDD, 0 IU/g vitamin D), respectively]. About 1 week after weaning, mice were genotyped: transgenic animals were identified by polymerase-chain reaction (PCR) analysis using appropriate primers to amplify a specific segment of the AβPP gene. Mice were monitored for 5 months after weaning. Before euthanasia, mice were trained in the Morris water maze for four days and tested on the last day. Mice were euthanized at six months of age, immediately after the completion of the water maze.
Brain tissue preparations
The brain tissues of the mice were prepared soon after euthanasia in a two-day-long process. Specifically, mice were euthanized by exposure to a saturated atmosphere of isoflurane. Immediately after death, the brain of each animal was isolated. Half of the brain was removed and placed in 4% paraformaldehyde overnight; the other half was frozen immediately and stored at −80°C for later measurements of the Aβ peptide levels (see below). The hemi-brains fixed in 4% paraformaldehyde were processed and embedded in paraffin. Ten serial 30 µm-thick sections through the brain were obtained using a microtome. Cryosections of the brain hemispheres were washed three times (5 min/wash) with Tris-buffered saline (TBS) (pH 7.4) buffer, followed by washing once with 0.1% Triton X-100-TBS buffer for 5 min. Sections were then incubated in 3% H2O2 and TBS buffer for 30 min at room temperature to eliminate endogenous peroxidase activity. After 1 h of blocking with 5.0% serum (horse or goat), the sections were incubated overnight with the following primary antibodies: GFAP (1 : 200 dilution, 2E1; BD Biosciences, San Jose, CA) to detect astrocytes and Aβ (1 : 500 dilution, Aβ peptide antibody, 4 G8, Covance, Princeton, NJ) to detect amyloid deposits. The next day, sections were washed three times (5 min/wash) with 0.1% Triton X-100 and TBS buffer to remove excess primary antibody. Thereafter, primary antibodies were detected using horseradish peroxidase-conjugated IgG Vectastain ABC kit and DAB/substrate reagents (Vector Laboratories, Burlingame, CA) according to the manufacturer’s instructions. The reactions were stopped in water and coverslip mounted after treatment with xylene. Thioflavin S staining was carried out as described previously [24].
Slides with tissue samples were examined soon after they were ready for microscopic examination and the number of plaques was counted in each section. The results were expressed as the mean ± standard deviation (SD), and any significance of differences was assessed by the Student’s t-test.
Plaque counts and amyloid load determination
Plaques were measured by counting the number of plaques in each section. The amyloid area in each section was determined with a computer-assisted image analysis system, consisting of a Power Macintosh computer equipped with a Quick Capture frame grabber card, Hitachi CCD camera mounted on an Olympus microscope and camera stand. NIH Image Analysis Software v. 1.55 was used. The images were captured and the total area of amyloid was determined over the ten sections. A single operator blinded to treatment status performed all measurements. Summing the amyloid volumes of the sections and dividing by the total number of sections calculated the amyloid volume per animal.
Measurement of Aβ peptides, NGF and TNF-α in brain tissue
For quantitative analysis of Aβ peptides in the brain, an enzyme-linked immunosorbent assay (ELISA) was used to measure the levels of human Aβ40 and Aβ42 [Immuno-Biological Laboratories-America (IBL, catalog # 27718 for 1–40 and 27711 for 1–42)]. Aβ40 and Aβ42 were extracted from mouse brains as follows: frozen hemi-brains were placed in Tissue Homogenization Buffer containing Protease Inhibitor Cocktail (PIC, Sigma) 1 : 1000 dilution immediately before use, and homogenized using polytron. Tissue sample suspensions were distributed in aliquots and snap frozen in liquid nitrogen for later measurements of Aβ peptides. Content of NGF and tumor necrosis factor-α (TNF-α) in brain tissue was measured using the Chemikine NGF ELISA kit (Millipore) and Ebiosciences (San Diego, CA, USA), respectively, according to the manufacturer’s directions. For Aβ determination, samples were extracted in guanidine-HCL [24].
CTFβ and sAβPPα analyses
Western blot assays measured CTFβ and sAβPPα as previously described with the same amounts of protein in each lane [25]. CTFβ was determined in the pellet fraction from the brain extract (antibody 8717, Sigma). In addition, sAβPPα was assessed in the supernatant fraction from the brain extract (antibody 6E10, Signet Laboratories). Relative amounts of CTFβ and sAβPPα were measured by densitometry and results were expressed as percentage of the mean CTFβ and sAβPPα compared to the control group. As control, β-actin western blots (anti-β-actin from Cell Signaling Technology) monitored equal loading of the same amounts of samples (20 µg protein) in gel lanes.
Behavioral test
Morris water maze tests were conducted in a circular tank of 1.5 meters in diameter located in a room with extra maze cues. The platform (14 cm in diameter) location was kept constant for each mouse during training and was 1.5 cm beneath the surface of the water, which was maintained at 25°C throughout the duration of the testing. During 5 days of training, the mice underwent 4 trials a day, alternating among 4 pseudorandom starting points. If a mouse failed to find the platform within 60 s, it was guided to the platform by the researcher and kept there for 20 s. The inter-trial interval was 25 s, during which time each mouse was returned to its home cage. Probe trials were conducted 24 h after the last training trial. During the probe trials, the platform was removed and mice were free to swim in the tank for 60 s. The training and probe trials were recorded by a video camera mounted on the ceiling, and data were analyzed using the SMART Video tracking system (San Diego Instruments).
Bone studies
After euthanasia, the carcasses of representative mice were used for evaluation of changes in the bone by bone histology and bone mineral density (BMD) analyses. These studies were performed to verify that the special diets performed as expected. Bone specimens were fixed in 70% ethanol in phosphate-buffered saline overnight, and embedded in methyl methacrylate the following day. After several days, 7 µm-thick sections of the bone tissues were obtained using a modified Leica RM 2155 rotary microtome (Leica Microsystems, Ontario, Canada). Sections were subject to Goldner’s trichrome stain to identify mineralized bone, non-mineralized matrix or osteoid, and growth plate cartilage. BMD measurements (expressed as grams/cm2), and bone mineral content (expressed in mg) were obtained from whole animal carcasses by means of dual-energy X-ray absorptiometry (DEXA) scan. DEXA scans were performed using the GE Lunar Prodigy, Direct-Digital Densitometry, which includes normative software data.
Circulating vitamin D
Samples of blood were obtained from representative mice immediately after euthanasia and serum levels of circulating 25(OH)D3 were measured by radioimmunoassay, using a DiaSorin kit. These measurements were performed in the laboratory of Dr. Bruce Hollis (MUSC).
Statistical analysis
All values are expressed as means ± SD. Statistical analysis was performed with the analysis of variance (ANOVA) followed by Scheffe’s post hoc test. Significance level was set at p < 0.05.
RESULTS
Vitamin D levels and bone studies
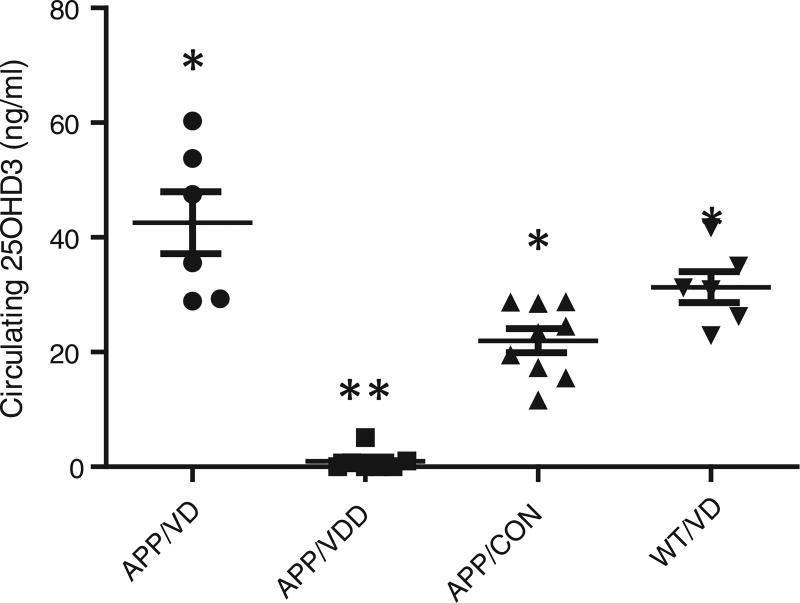
Immediately after euthanasia, blood was collected from representative mice to measure serum levels of circulating vitamin D (25(OH)D3). In addition, two each of deficient and vitamin D-treated mice were analyzed for BMD as shown in Fig. 1. The results show that the levels of circulating vitamin D were much higher in mice fed the vitamin D-enriched diet (38.53 ± 7.419 ng/ml) than those fed the control diet (21.98 ± 2.109 ng/ml) or the vitamin D-deficient diet (0.9714 ± 0.7053 ng/ml). It is worth noting that the lowest value of circulating 25(OH)D3 observed in mice fed the vitamin D enriched diet was 22.9 ng/mL of serum, while the highest value observed in mice fed a vitamin D-deficient diet was 5.1 ng/mL of serum, indicating that the special diets were generating the expected values of circulating vitamin D. Consistent with these results, it was also observed that the BMD of mice fed the vitamin-enriched diet was 27% and 14% higher than that of mice fed the vitamin D-deficient diet or wildtype mice, respectively (5.9 ± 0.30 mg/cm2 vs. 4.3 ± 0.20 mg/cm2 vs. 5.1 ± 0.21 mg/cm2) (n = 6/group).
Fig. 1.
Effects of Vitamin D-Deficient (VDD) and Vitamin D-Enriched Diet (VD 5X). The serum values of circulating vitamin D were much higher in mice fed a vitamin D-enriched diet than in mice fed a vitamin D-deficient diet, and this correlation was independent of whether the mice were transgenic or wild-type. Similarly, BMD was significantly higher in mice fed a vitamin D-enriched diet (n = 5–10 per group). *p < 0.01 compared to AβPP/VDD group; **p < 0.01 compared to AβPP/VD group.
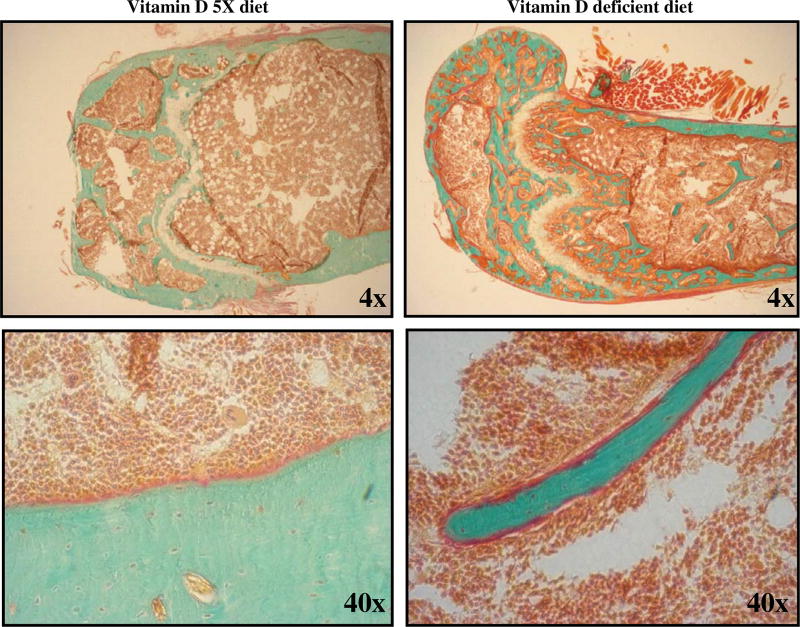
Bone specimens from the spine, femur, and tibia of select mice fed either the vitamin D-enriched or vitamin D-deficient diet were subjected to histological examination to investigate the effects of vitamin D deficiency on the microscopic structure of bone. Figure 2 shows the histology of the femur from a mouse fed a vitamin D-enriched diet (left panels) compared to the femur from a mouse fed a vitamin D-deficient diet (right panels). These histological results clearly demonstrate that the bone of the mouse fed a vitamin D-deficient diet had extensive sections of non-mineralized tissue or osteoid, demonstrating that vitamin D deficiency leads to a dramatic deficiency of calcium content in bones and supporting the conclusion vitamin D is crucial for the absorption of dietary calcium. In contrast, the bone from the mouse fed the vitamin D-enriched diet appeared very well calcified, with little evidence of non-calcified osteoid.
Fig. 2.
Effects of vitamin D deficiency on bone histology. Goldner’s trichrome stain for 7 µm thick sections of non-demineralized bone embedded in methyl methacrylate: mineralized bone stains green. Unmineralized matrix or osteoid along the mineralized bone periphery stains red. Growth plate cartilage stains light-gray and chondrocytes light-red. Bone marrow cells stain red and marrow adipocytes do not stain. Femur tissue sample from mouse fed a vitamin D-enriched diet (Vitamin D 5X diet, left column) shows few, very thin osteoid layers or seams. Femur tissue sample from mouse fed a vitamin D-deficient diet (Vitamin D deficient diet, right column) shows a thickened osteoid layer, an abnormality indicating Vitamin D deficiency.
Behavioral studies (water maze)
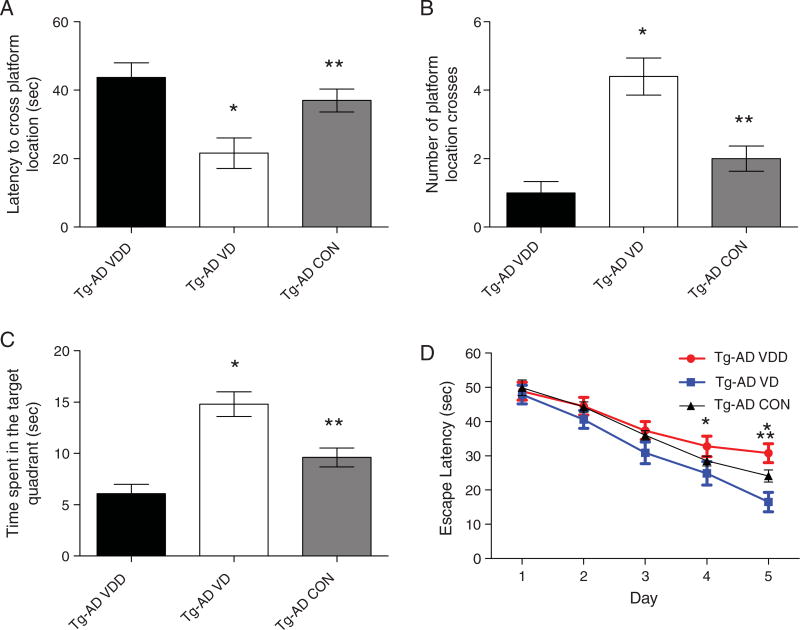
The Morris water maze was used to train and test the mice on the last week before euthanasia. We measured four parameters that correlate with memory retention (latency to cross platform, number of platform crosses, time spent in quadrant with target and the escape latency) and were recorded at the time of testing. Figure 3 illustrates the changes in behavior in mice treated with either the VDD or the VD diet. The mice on the vitamin D-enriched diet showed a significant difference in behavior compared to mice fed the vitamin D-deficient diet and the control diet. The mice on the vitamin D-enriched diet showed a decreased latency time to cross the platform (Fig. 3A. 21.6 ± 4.483 vs. 43.7 ± 4.287 vs. 37.0 ± 3.360s), increased number of platform crosses (Fig. 3B. 4.4 ± 0.5416 vs. 1.0 ± 0.3333 vs. 2.0 ± 0.3651), increased time spent in the quadrant with the target platform (Fig. 3C. 14.8 ± 1.191 vs. 6.1 ± 0.8876 vs. 9.6 ± 0.9214 s) and a decreased escape latency time to get to the platform with days 4 and 5 showing a significant difference (Fig. 3D).
Fig. 3.
Vitamin D rescues learning and memory deficits in AβPP transgenic (Tg-AD) mice. Tg-AD mice were evaluated in the spatial reference version of the Morris water maze. A–C) Reference memory, tested 24 h after last training trial, was significantly improved in the Tg-AD mice on the vitamin D-enriched diet (Tg-AD VD) compared to the vitamin D-deficient diet (Tg-AD VDD). D) Both groups showed an improvement over the 5 days of training, however, the vitamin D-treated animals at days 4 and 5 showed significantly lower levels. Data are presented as mean ± S.E. (n = 10 animals per group). *Indicates p < 0.01 compared to Tg-AD VDD group; ** indicates p < 0.01 compared to Tg-AD VD group.
Studies on amyloid deposition in the brain of AβPP mice
Brain sections of AβPP mice fed either a vitamin D-deficient diet (VDD) or a vitamin D-enriched diet (VD) were prepared immediately after euthanasia. Sections were fixed and stained to identify amyloid plaques.
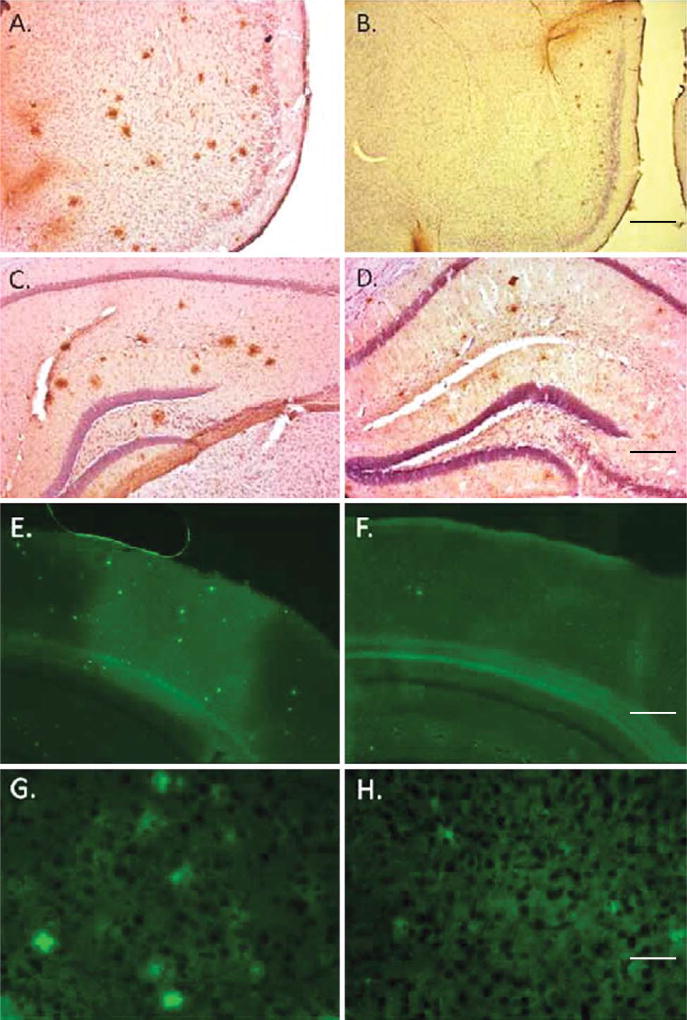
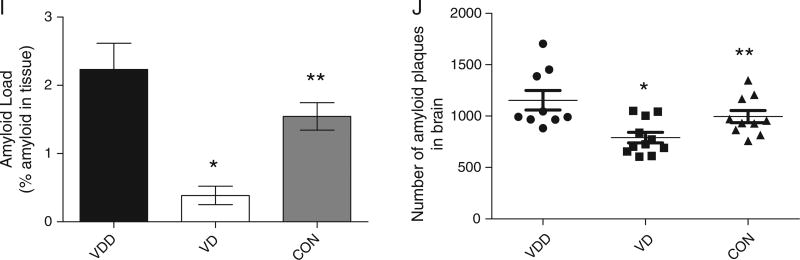
Figure 4 shows that the number and the size of the amyloid plaques in the brain of VD treated mice are decreased. Figure 4A – D illustrates the changes in amyloid plaque load and size by immunohistochemical analysis in the cortex (A and B) and the hippocampus (C and D). As indicated in Fig. 4J, not only is the number of plaques reduced in the VD treated animals, but also the sizes of the plaques are significantly smaller (Fig. 4I). Finally, we performed thioflavin S staining of the plaques in the brains of the mice. Both cortex (Fig. 4E – H) and hippocampus (not shown) show significant reductions in thioflavin S positive staining in the VD treated animals compared to VDD.
Fig. 4.
Vitamin D3 administration significantly decreases amyloid pathology. A, B) Representative micrographs of cortical regions; C, D) representative micrographs of hippocampal regions all stained with anti-Aβ antibody 4 G8, which recognizes the Aβ peptide. These results clearly indicate a decrease in Aβ plaques in mice treated with vitamin D (B, D) compared to vitamin D-deficient mice (A, C). E–H) Thioflavin S stained sections from cortical samples of mice treated with vitamin D-enriched diet (F, H) compared to mice fed a vitamin D-deficient diet (E, G). Mice on vitamin D-enriched diet had fewer and smaller plaques that were less reactive with thioflavin S. Scale bar is 350 µm for A and B, 100 µm for C and D, 400 µm for E and F and 50 µm for G and H. I) Quantitation of % amyloid in tissue from VDD and VD treated animals. Data are presented as % amyloid occupying the tissue in the brain. Data are presented as mean ± S.E. *p < 0.001; **p < 0.01. J) Number of amyloid plaques per brain hemisphere of AβPP mice. The amyloid plaque counts per brain hemisphere for eleven mice fed a vitamin D-enriched diet and nine mice fed a vitamin D-deficient diet, respectively. *p = 0.0011 (one-tailed test); **p = 0.024 (two-tailed test) (N = 9–11 per group).
Plaques were counted under a microscope and the numbers added to calculate the total of plaques per brain hemisphere (Fig. 4J ). As seen in the table, the total number of plaques in the VD animals (791 ± 51) compared to the VDD mice (1154 ± 95; p = 0.0024) and control diet mice (995 ± 59; p < 0.01) showed a significant difference. The results of these studies suggest that a diet enriched in vitamin D3 (such as can be achieved through appropriate vitamin D3 supplementation), may interfere with the factors involved in Aβ deposition and subsequent plaque formation, therefore mitigating the pathological manifestations of AD.
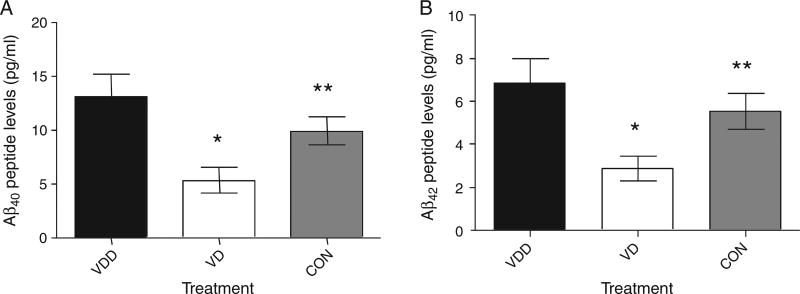
The results of the studies summarized in Fig. 4 suggested that the brain load of Aβ peptides (Aβ40 and Aβ42) in AβPP mice fed a vitamin D-enriched diet would be lower than the corresponding load of Aβ peptides in AβPP mice fed a vitamin D-deficient diet. Therefore, we measured the concentration of Aβ40 and Aβ42 in brain tissue extracts of AβPP mice fed either of the two diets for five months. Figure 5 shows the Aβ peptide determinations in the brains of the mice. As seen in the figure, both Aβ40 and Aβ42 are significantly decreased in the brains of the mice treated with vitamin D and vitamin D deficient or control mice (Aβ40 - 5.35 ± 1.190 vs. 13.11 ± 2.152 vs. 9.96 ± 1.292 pg/ml; Aβ42 - 2.89 ± 0.5805 vs. 6.84 ± 1.144 vs. 5.52 ± 0.8370 pg/ml).
Fig. 5.
Load of Aβ peptides in the brain of AβPP mice. Bar graphs show the concentration of Aβ40 (A) and Aβ42 (B) in picograms (pg) per milliliter (ml) of brain tissue extracts from AβPP mice fed a vitamin D-enriched diet (VD, white bars) versus AβPP mice fed a vitamin D-deficient diet (VDD, black bars). For both Aβ peptides the differences were statistically significant (*p = 0.0043 for Aβ40 and **p = 0.0048 for Aβ42) (n = 10 per group).
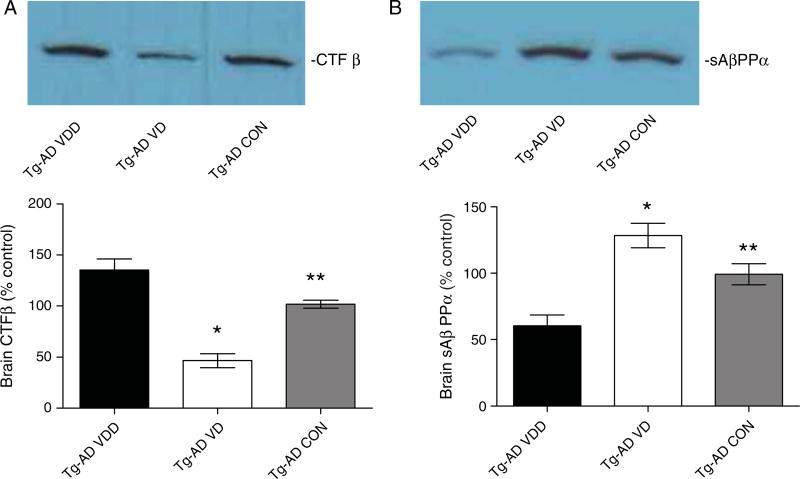
VD animals also had a reduction in brain CTFβ (Fig. 6A). The mean CTFβ levels were significantly lower (~50%) than in the control animals and the VDD animals. These data are also consistent with vitamin D mediating a reduction in β-secretase activity because CTFβ is the cleavage product of β-secreatase. Mice treated with a vitamin D-enriched diet showed an increase in brain sAβPPα (Fig. 6B). The mean sAβPPα levels were significantly higher in the VD animals (~130%) than the control animals or the VDD animals. These data also support the hypothesis that vitamin D is mediating a reduction in β-secretase activity because sAβPPα is derived from AβPPα-secretase cleavage, which competes with β-secretase cleavage of AβPP, and thus the inhibition of β-secretase activity provides more AβPP for α-secretase to produce more sAβPPα.
Fig. 6.
Vitamin D lowers and increases brain CTFβ and sAβPPα, respectively. A) Vitamin D caused a reduction in brain CTFβ. CTFβ is a proteolytic product of β-secretase cleavage of AβPP and thus these data are also consistent with vitamin D acting through the inhibition of β-secretase activity. B) In contrast, vitamin D mediated an increase in brain sAβPPα levels. These data are consistent with vitamin D mediating the inhibition of β-secretase activity because sAβPPα is derived from AβPPα-secretase cleavage, which competes with β-secretase cleavage of AβPP, and thus the inhibition of β-secretase activity provides more AβPP for β-secretase to produce more sAβPPα (*p < 0.001; ** p > 0.01).
Trophic factor and inflammation
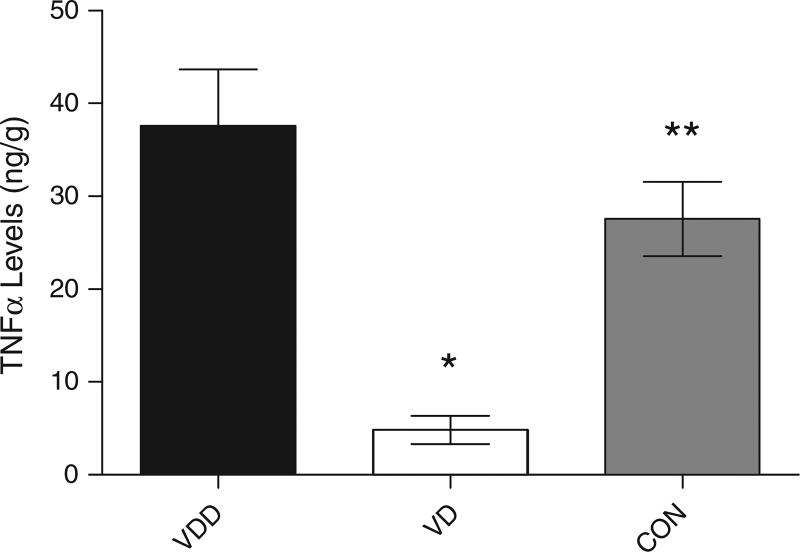
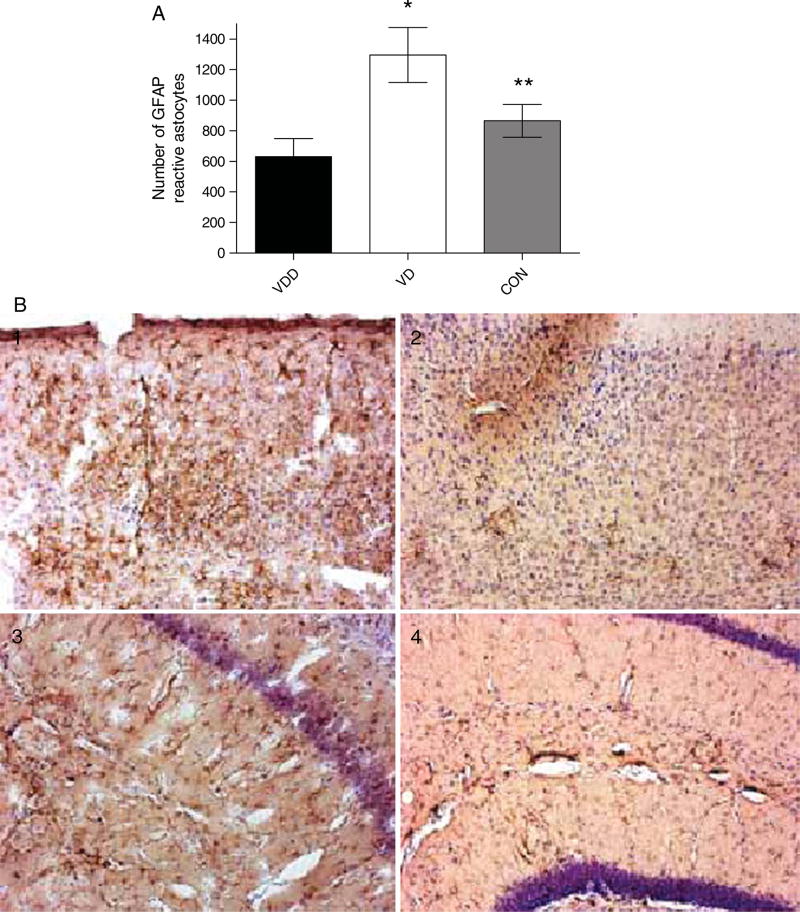
We also measured the concentration of inflammatory markers and trophic factors to determine the influence of vitamin D on inflammation and trophic factor stimulation. Figure 7 shows the results of ELISA measurement of TNF-α levels at the end of the experiment. VD animals had significantly lower levels of TNF-α compared to VDD and control diet animals (37.59 ± 6.088 vs. 4.840 ± 1.529 vs. 27.57 ± 4.005 ng/g), suggesting that vitamin D, by reducing Aβ peptide levels, can alter the inflammatory nature of AD. Interestingly, immunocytochemical analysis of glial fibrillary acidic protein (GFAP) in the brains of the mice (Fig. 8), showed a significantly higher number of activated astrocytes in VD animals compared to VDD and control diet animals (1296 ± 180.3 vs. 630.6 ± 119.0 vs. 865.0 ± 106.9).
Fig. 7.
Concentration of TNF-α in the brains of AβPP mice treated with vitamin D. ELISA measurements of TNF-α levels in the brains of mice treated with vitamin D-enriched (VD) or vitamin D-deficient diet (VDD). VD animals showed a significant reduction in TNF-α levels. Data are presented as mean ± S.E. *p < 0.001; **p < 0.01; (n = 10 per group).
Fig. 8.
GFAP immunoreactivity in the brains of AβPP mice treated with vitamin D. Brain sections of AβPP mice were immunostained with anti-mouse GFAP antibody to detect the distribution of activated astrocytes. A) AβPP mice fed a vitamin D-enriched diet (VD, white bars) versus AβPP mice fed a vitamin D-deficient diet (VDD, black bars). B) Examples of sections showing activated GFAP-positive astrocytes. 1 and 2, cortex; 3 and 4 hippocampus from AβPP mice on vitamin D-enriched diet (1 and 3) or vitamin D-deficient diet (2 and 4). Data are presented as mean ± S.E. *p < 0.001; **p < 0.01 (n = 10 per group).
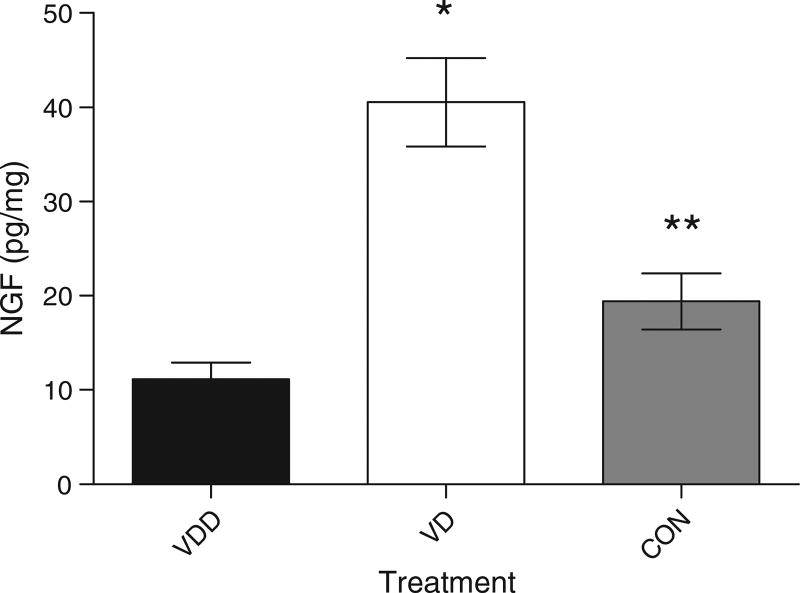
Finally, we determined the levels of NGF in brain tissue extracts from the AβPP mice fed the two different diets (Fig. 9). We observed that AβPP mice fed a vitamin D-enriched diet had a significantly higher concentration of NGF in the brain than AβPP mice fed a vitamin D-deficient diet and control diet (40.55 ± 4.687 vs. 11.13 ± 1.781 vs. 19.42 ± 2.968 pg/mg). We found that the vitamin D-enriched diet correlated with an almost four-fold increase of NGF content in the brain, and that the increase was highly significant (p < 0.0001).
Fig. 9.
Concentration of NGF in the brain of AβPP mice. Bar graphs show the concentration of NGF in picograms (pg) per milligram (mg) of brain tissue from AβPP mice fed a vitamin D-enriched diet (VD, white bars) versus AβPP mice fed a vitamin D-deficient diet (VDD, dark bars). The difference was statistically significant (*p < 0.001; **p < 0.01) (n = 10 per group).
DISCUSSION
New functions for vitamin D have been identified following the discovery of the vitamin D receptor (VDR), as it became clear that a large number of cell types, which had nothing to do with bone mineralization, express VDR [26]. The parathyroid gland, islet cells of the pancreas, bone marrow cells (lymphocytes and macrophages), and certain cells in the central nervous system (CNS) all express VDR, raising the possibility that the functions of vitamin D are much broader than previously assumed [27, 28]. The VDR is a nuclear receptor [29], that binds 1,25(OH)2D3 within the nucleus and then combines with another nuclear factor identified as the retinoid-X receptor (RXR), which serves as the vitamin D co-receptor [30]. This tri-molecular complex binds to the vitamin D response element (VDRE), which is part of the regulatory (promoter) sequences that control the expression (transcription) of the adjacent genes affected by vitamin D [31, 32].
Our results confirm that a vitamin D-deficient diet causes major deficits in bone formation and structure, compared to the healthy bone structure of the mice fed a vitamin D-enriched diet [12]. These studies were important to demonstrate that the performance of the special diets was as expected. The results of the behavioral studies showed significant difference between the groups of mice. In these studies, four specific parameters were measured: 1) latency to cross platform; 2) number of platform crosses; 3) time spent in quadrant with target; and 4) the escape latency (the average time that the mice needed to reach the platform). For all of these parameters, there were significant differences between the groups. Although the results were clearly positive, it should be noted that behavioral studies in rodents tend to be less informative than in higher mammals, such as dogs and humans. This is relevant, because new animal models of AD are being developed, with the objective of conducting behavioral studies that are more refined in nature and more informative than those being performed currently in rodents.
The results of the experiments aimed at measuring amyloid deposition in the three groups of AβPP mice were the most informative [24]. Comparative analysis revealed that the number of plaques in the brain of AβPP mice fed a vitamin D-enriched diet was significantly lower than the number of plaques observed in the brain of AβPP mice fed a control or vitamin D-deficient diet. Furthermore, we observed that the average size of the plaques was lower in the former than in the latter groups, and this difference was analyzed by statistical means and shown to be significantly different. The smaller size and number of amyloid plaques in the brain of AβPP mice fed a vitamin D-enriched diet is consistent with the mitigating effects of vitamin D supplementation on the chronic inflammatory process associated with plaque formation. Consistent with these experimental observations, we found that the vitamin D-enriched diet correlated with a significant decrease in the concentration of both Aβ40 and Aβ42 peptides in the brain of AβPP mice. This could be the result of several different possibilities. It is possible that vitamin D may alter the processing of AβPP, i.e., reduce β-secretase activity (BACE-1), or decrease Aβ peptide production by increasing the activity or reducing the inhibition of enzymes that degrade the Aβ peptide [1–7, 24]. Further studies would need to be conducted to determine the effects of vitamin D on Aβ peptide.
The CNS makes its own calcitriol [33], which in turn regulates in the expression of NGF [34]; in fact, VDRE sequences have been identified within the regulatory region of the NGF gene [32]. After synthesis within the CNS, calcitriol stays in the brain to perform its function in an autocrine and paracrine fashion [35, 36]. This function of vitamin D within the CNS is, therefore, different from the endocrine function performed by the kidney, which deploys calcitriol to its tissue targets through the blood stream. The blood-brain barrier prevents most substances carried in the bloodstream from leaving the capillaries of the brain circulatory system and entering the CNS. This mechanism provides an additional layer of protection for the brain against potentially toxic metabolites and agents that enter the blood stream while selectively transporting essential factors. 1,25(OH)2D3 and other metabolites of vitamin D have been detected in the CNS and can cross the blood-brain barrier, strongly suggesting that 1,25(OH)2D3 is needed to carry out important functions in the brain [37]. Moreover, several cell types within the CNS, including the neurons, express the 25-hydroxyvitamin D-1α-hydroxylase gene, which codes for the enzyme responsible for the final step in the synthesis of 1,25(OH)2D3, supporting the concept that the hormonal form of vitamin D3 can be produced in the CNS [38]. It is also possible that vitamin D may not only modify proteins associated with AD but may have widespread undocumented effects which could impact upon the rates of clearance of vitamin D as well as possibly liver, kidney and brain metabolism.
We also found that TNF-α levels were significantly decreased in the AβPP mice fed the vitamin D-enriched diet suggesting that, by decreasing Aβ peptide levels, vitamin D limits the expression of inflammatory markers in the brain [24]. Interestingly, we found that there was a significant increase in GFAP expression and an increase in activated astrocytes in the brains of the vitamin D-treated AβPP mice. It is possible that this activation is mediated by vitamin D and may contribute to the attenuation of Aβ peptide-mediated effects in the brain [32, 34]. We also found that the vitamin D-enriched diet correlated with a significant increase in the concentration of NGF in the brain of AβPP mice. This increase in NGF is consistent with the increase in GFAP immunoreactivity, since NGF is produced in the brain by the astrocytes [39, 40]. Thus, the activation of the astrocytes may lead to a reduction in Aβ peptides through altered processing or increased clearance as well in the generation of NGF to provide trophic support to the neurons. Taken together, these provocative results suggest that a vitamin D-enriched diet may mitigate the neuro-inflammatory process associated with Aβ peptide deposition and plaque formation. Furthermore, increased production of NGF in AβPP mice fed the vitamin D-rich diet may improve considerably neuronal function and trophism.
There are limitations to this study. For instance, AβPP mice represent a suboptimal model of AD, because they express only some of the pathologic lesions observed in human AD. Furthermore, wild-type mice do not develop amyloid plaques at old age, and mouse behavior lacks the complexity needed to perform sophisticated behavioral analyses. Although vitamin D deficiency may affect behavior due to lowered bone strength and energy, the relative increase in Aβ observed in the VDD mice argues for at least a partial role for AD pathology and the associated neurodegeneration in the poor memory scores obtained. To the best of our knowledge these provocative observations are novel; however, only the outcome of randomized prospective clinical studies in AD patients will confirm or refute the hypothesis that vitamin D3 supplementation is effective in the prevention of AD or in mitigating its pathology and associated clinical symptoms.
Acknowledgments
We thank Drs. Srinivasan Shanmugarajan, William L. Ries, and Sakamuri V. Reddy for helping with the bone-related studies; we also thank Dr. Bruce W. Hollis for measuring the serum levels of circulating vitamin D in mice. This project was funded by grants from the National Institutes of Health (MSK, ES 016774; NRB, R01 NS051575; KS, AG023055), Veterans Administration (MSK and SG-C), and the National Science Foundation EPSCoR grants (MSK, EPS-0132573 and EPS-0447660).
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=769).
References
- 1.Mayeux R. Clinical practice. Early Alzheimer’s disease. N Engl J Med. 2010;362:2194–2201. doi: 10.1056/NEJMcp0910236. [DOI] [PubMed] [Google Scholar]
- 2.Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 3.Li S, Shankar GM, Selkoe DJ. How do soluble oligomers of amyloid beta-protein impair hippocampal synaptic plasticity? Front Cell Neurosci. 2010;4:5. doi: 10.3389/fncel.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sperling RA, Dickerson BC, Pihlajamaki M, Vannini P, LaViolette PS, Vitolo OV, Hedden T, Becker JA, Rentz DM, Selkoe DJ, Johnson KA. Functional alterations in memory networks in early Alzheimer’s disease. Neuromolecular Med. 2010;12:27–43. doi: 10.1007/s12017-009-8109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh DM, Selkoe DJ. A beta oligomers – a decade of discovery. J Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 6.Lahiri DK, Zawia NH, Greig NH, Sambamurti K, Maloney B. Early-life events may trigger biochemical pathways for Alzheimer’s disease: the “LEARn” model. Biogerontology. 2008;9:375–379. doi: 10.1007/s10522-008-9162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selkoe DJ. Developing preventive therapies for chronic diseases: lessons learned from Alzheimer’s disease. Nutr Rev. 2007;65:S329–S343. doi: 10.1111/j.1753-4887.2007.tb00370.x. [DOI] [PubMed] [Google Scholar]
- 8.Sambamurti K, Granholm AC, Kindy MS, Bhat NR, Greig NH, Lahiri DK, Mintzer JE. Cholesterol and Alzheimer’s disease: clinical and experimental models suggest interactions of different genetic, dietary and environmental risk factors. Curr Drug Targets. 2004;5:517–528. doi: 10.2174/1389450043345335. [DOI] [PubMed] [Google Scholar]
- 9.MacLaughlin JA, Holick MF. Photobiology of Vitamin D3 in the Skin. Oxford Univ. Press; New York: 1983. Biochemistry and physiology of the skin; pp. 734–754. [Google Scholar]
- 10.Esvelt RP, Schnoes HK, Deluca HF. Vitamin D3 from rat skins irradiated in vitro with ultraviolet light. Arch Biochem Biophys. 1978;188:282–286. doi: 10.1016/s0003-9861(78)80010-1. [DOI] [PubMed] [Google Scholar]
- 11.Haussler MR. Vitamin D receptors: nature and function. Annu Rev Nutr. 1986;6:527–562. doi: 10.1146/annurev.nu.06.070186.002523. [DOI] [PubMed] [Google Scholar]
- 12.Nicolaysen R. Studies upon mode of action of vitamin D, III, The influence of vitamin D absorption of calcium and phosphorus in the rat. Biochem J. 1937;31:122–129. doi: 10.1042/bj0310122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlsson A. Tracer experiments on the effects of vitamin D on the skeletal metabolism of calcium and phosphorus. Acta Physiologica Scandinavica. 1952;26:212–220. doi: 10.1111/j.1748-1716.1952.tb00904.x. [DOI] [PubMed] [Google Scholar]
- 14.Fraser DR, Kodicek E. Unique biosynthesis by kidney of a biologically active vitamin D metabolite. Nature. 1970;228:764–766. doi: 10.1038/228764a0. [DOI] [PubMed] [Google Scholar]
- 15.Fraser DR, Kodicek E. Regulation of 25-hydroxycholeciferol-1-hyrdoxylase activity in kidney by parathyroid hormone. Nature (New Biology) 1973;241:163–166. doi: 10.1038/newbio241163a0. [DOI] [PubMed] [Google Scholar]
- 16.Szodoray P, Nakken B, Gaal J, Jonsson R, Szegedi A, Zold E, Szegedi G, Brun JG, Gesztelyi R, Zeher M, Bodolay E. The complex role of vitamin D in autoimmune diseases. Scand J Immun. 2008;68:261–269. doi: 10.1111/j.1365-3083.2008.02127.x. [DOI] [PubMed] [Google Scholar]
- 17.Aparna R, Subhashini J, Roy K, Reddy GS, Robinson M, Uskokovic M, Reddy GV, Reddanna P. Selective inhibition of cyclooxygenase-2 (COX-2) by 1α,25-dihydroxy-16-ene-23-yne-vitamin D3, a less calcemic vitamin D analog. J Cellular Biochem. 2008;104:1832–1842. doi: 10.1002/jcb.21749. [DOI] [PubMed] [Google Scholar]
- 18.Janjetovic Z, Zmijewski MA, Tuckey RC, DeLeon DA, Nguyen MN, Pfeffer LM, Slominski AT. 20-hydroxycholecalciferol, product of vitamin D hydroxylation by P450 scc, decreases NF-κB activity by increasing IκBα levels in human keratinocytes. PLoS ONE. 2009;4:e5988. doi: 10.1371/journal.pone.0005988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCann JC, Ames BN. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J. 2008;22:982–1001. doi: 10.1096/fj.07-9326rev. [DOI] [PubMed] [Google Scholar]
- 20.Nadjar A, Bluthe RM, May MJ, Dantzer R, Parnet P. Inactivation of the cerebral NFkappaB pathway inhibits interleukin-1 beta-induced sickness behavior and c-Fos expression in various brain nuclei. Neuropychopharmacology. 2005;30:1492–1499. doi: 10.1038/sj.npp.1300755. [DOI] [PubMed] [Google Scholar]
- 21.Savonenko A, Xu GM, Melnikova T, Morton JL, Gonzales V, Wong MPF, Price DL, Tang F, Markowska AL, Borchelt DR. Episodic-like memory deficits in the AbPPswe/PS1 dE9 mouse model of Alzheimer’s disease: relationships to β-amyloid deposition and neurotransmitter abnormalities. Neurobiol Dis. 2005;18:602–617. doi: 10.1016/j.nbd.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 22.Sato Y, Iwamoto J, Kanoko T, Satoh K. Amelioration of osteoporosis and hypovitaminosis D by sunlight exposure in hospitalized, elderly women with Alzheimer’s disease: A randomized controlled trial. J Bone Mineral Res. 2005;20:1327–1333. doi: 10.1359/JBMR.050402. [DOI] [PubMed] [Google Scholar]
- 23.Oudshoorn C, Mattace-Raso FUS, van der Velde N, Colin EM, van der Cammen TJM. Higher serum vitamin D3 levels are associated with better cognitive test performance in patients with Alzheimer’s disease. Dementia Geriatric Cog Dis. 2008;25:539–543. doi: 10.1159/000134382. [DOI] [PubMed] [Google Scholar]
- 24.El-Amouri SS, Zhu H, Yu J, Gage FH, Verma IM, Kindy MS. Neprilysin: an enzyme candidate to treat Alzheimer’s disease. Am J Pathol. 2008;172:1342–1354. doi: 10.2353/ajpath.2008.070620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hook VY, Kindy M, Hook G. Inhibitors of cathepsin B improve memory and reduce Abeta in transgenic Alzheimer’s disease mice expressing the wild-type, but not the Swedish mutant, beta-secretase AβPP site. J Biol Chem. 2008;283:7745–7753. doi: 10.1074/jbc.M708362200. [DOI] [PubMed] [Google Scholar]
- 26.Brumbaugh PF, Haussler MR. 1α,25-Dihydroxyvitamin D3 receptor: competitive binding of vitamin D analogs. Life Sci. 1973;13:1737–1746. doi: 10.1016/0024-3205(73)90120-3. [DOI] [PubMed] [Google Scholar]
- 27.Stumpf WE, Sar M, DeLuca HF. Sites of action of 1,25(OH)2 vitamin D3 in intestinal tract, stomach, kidney, skin, pituitary and parathyroid. Science. 1981;201:1188–1190. doi: 10.1126/science.505004. [DOI] [PubMed] [Google Scholar]
- 28.Prufer K, Veenstra TD, Jirikowski GF, Kumar R. Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the rat brain and spinal chord. J Chem Neuroanat. 1999;16:135–145. doi: 10.1016/s0891-0618(99)00002-2. [DOI] [PubMed] [Google Scholar]
- 29.Kream BE, Reynolds RD, Knutson JC, Eisman JA, DeLuca HF. Intestinal cytosol binders of 1,25-dihydroxyvitamin D3 and 25-hydroxyvitamin D3. Arch Biochem Biophys. 1976;176:779–787. doi: 10.1016/0003-9861(76)90222-8. [DOI] [PubMed] [Google Scholar]
- 30.Jin CH, Pike JW. Human vitamin D receptor-dependent transactivation in Saccharomyces cerevisiae requires retinoid X receptor. Mol Endocrin. 1996;10:196–205. doi: 10.1210/mend.10.2.8825559. [DOI] [PubMed] [Google Scholar]
- 31.Demay MB, Gerardi JM, DeLuca HF, Kronenberg HM. DNA sequences in the rat osteocalcin gene that bind the 1,25-dihydroxyvitamin D3 receptor in three kindreds. Mol Endocrin. 1990;90:197–201. doi: 10.1073/pnas.87.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang TT, Tavera-Mendoza LE, Laperriere D, Libby E, MacLeod NB, Nagai Y, Bourdeau V, Konstorum A, Lalle-mant B, Zhang R, Mader S, White JH. Large-scale in Silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Mol Endocrinol. 2005;19:2685–2695. doi: 10.1210/me.2005-0106. [DOI] [PubMed] [Google Scholar]
- 33.Brachet P, Neveu I, Naveilhan P, Garcion E, Wion D. Vitamin D, a neuroactive hormone: from brain development to pathological disorders. In: Feldman D, Pike JW, Glorieux FH, editors. Vitamin D. Elsevier Academic Press; San Diego: 2005. pp. 1779–1789. [Google Scholar]
- 34.Wion D, MacGrogan D, Neveu I, Jehan R, Houlgatte R, Brachet P. 1,25-Dihydroxyvitamin D3 is a potent inducer of nerve growth factor synthesis. J Neurosci Res. 1991;28:110–114. doi: 10.1002/jnr.490280111. [DOI] [PubMed] [Google Scholar]
- 35.Eyles DW, Rogers F, Buller K, McGrath JJ, Ko P, French K, Burne TH. Developmental vitamin D (DVD) deficiency in the rat alters adult behavior independently of HPA function. Pyschonueroendocrinology. 2006;31:958–964. doi: 10.1016/j.psyneuen.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Cherniack EP, Florez H, Roos BA, Troen BR, Levis S. Hypovitaminosis D in the elderly: from bone to brain. J Nutr Health Aging. 2008;12:366–373. doi: 10.1007/BF02982668. [DOI] [PubMed] [Google Scholar]
- 37.Pardridge WM, Sakiyama R, Coty WA. Restricted transport of vitamin D and A derivatives through the rat blood-brain barrier. J Neurochem. 1985;44:1138–1141. doi: 10.1111/j.1471-4159.1985.tb08735.x. [DOI] [PubMed] [Google Scholar]
- 38.Zehnder D, Bland R, Williams MC, McNich RW, Howie AJ, Stewart PM, Hewison M. Extrarenal expression of 25-hydroxyvitamin D3-1α-hydroxylase. J Clin Endocrin Metab. 2001;86:888–894. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 39.Mishra MK, Koli P, Bhowmick S, Basu A. Neuroprotection conferred by astrocytes is insufficient to protect animals from succumbing to Japanese encephalitis. Neurochem Int. 2007;50:764–773. doi: 10.1016/j.neuint.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 40.Moore ME, Piazza A, McCartney Y, Lynch MA. Evidence that vitamin D3 reverses age-related inflammatory changes in the rat hippocampus. Biochem Soc Trans. 2005;33:573–577. doi: 10.1042/BST0330573. [DOI] [PubMed] [Google Scholar]