Abstract
Background
There are varying reports of the association of basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (SCC) with mortality.
Objective
To synthesize the available information on all-cause mortality after a diagnosis of BCC or SCC in the general population.
Methods
We searched PubMed (1966-present), Web of Science (1898-present), and Embase (1947-present) and hand-searched to identify additional records. All English articles that reported all-cause mortality in patients with BCC or SCC were eligible. We excluded case reports, case series, and studies in subpopulations of patients. Random effects model meta-analyses were performed separately for BCC and SCC.
Results
Searches yielded 6538 articles, and 156 were assessed in full-text. Twelve studies met inclusion criteria, and four were included in meta-analysis (encompassing 464,230 BCC and 175,849 SCC patients), yielding summary relative mortalities of 0.92 (95% CI 0.83-1.02) in BCC and 1.25 (95% CI 1.17-1.32) in SCC.
Limitations
Only a minority of studies controlled for comorbidities. There was significant heterogeneity in meta-analysis (χ2 p<0.001, I2 >98%), but studies of SCC were qualitatively concordant: all showed statistically significant increased relative mortality.
Conclusions
We found that patients with SCC are at higher risk of death from any cause compared to the general population.
Keywords: Basal cell carcinoma, cutaneous squamous cell carcinoma, keratinocyte carcinoma, all-cause mortality, systematic review, meta-analysis
Introduction
Basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (SCC) are, taken together, the most common cancers in the United States and commonly affect older Caucasian individuals.[1] Some populations of patients, such as organ transplant recipients, are at significantly increased risk of death from BCC and SCC.[2] In the general population, however, deaths from BCCs and SCCs do not have a large impact on mortality: for example, age-adjusted morality rates for SCC are reported at approximately 1 per 100,000 person-years or less,[3-6] compared to mortality rates of 205 per 100,000 person-years for heart disease or 180 per 100,000 person years for cancer overall in the United States (US).[7] It is unclear, however, whether typical patients with a history of BCC or SCC have different risk of death from any cause compared to the general population.
Several recent studies suggest an increased risk of second primary cancers (including breast cancer, lung cancer, leukemia, and melanoma) among individuals with BCC and SCC when compared with those without.[8-16] Additionally, there is growing data that the genetic features seen in these cancers, including shortened telomeres and defective DNA repair, are associated with myocardial infarction and stroke.[17-22] Yet the few studies that evaluate all-cause mortality after a diagnosis of these skin cancers show mixed results.[23-28] Several studies group patients with BCC and SCC together, and some conclude that patients with either BCC or SCC have a decreased risk of death[27], whereas others conclude that these patients have an increased risk of death.[28] Estimates from studies that separate BCC and SCC patients suggest SCC is associated with decreased survival whereas BCC has been associated with equal or increased survival compared to the general population.[23, 25, 29] Understanding the risk of death among patients with skin cancer is important for two reasons: 1) a better understanding of disease pathogenesis, in the context of recent studies suggesting that skin cancers may be independent risk factors and markers of cancer-prone genetic phenotypes[30, 31] and 2) improving clinical care and prevention recommendations for these patients.
The aim of this study was to synthesize the available information on the risk of all-cause mortality after a diagnosis of BCC or cutaneous SCC.
Materials and Methods
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) and Meta-Analysis Of Observational Studies in Epidemiology (MOOSE) guidelines.[32, 33]
Literature Search
Studies were identified through searches of electronic databases and by scanning reference lists of articles. We searched Embase (1947 – present), PubMed (1966 – present), and Web of Science (1898 – present). Two authors (WCS and MRW) performed the search and the final search was run on February 21, 2016. Additionally, we reviewed articles and reviews on the topics of BCC and SCC and all-cause mortality closely to locate additional articles. The search strategies employed for each database are available in the Supplementary Materials.
All published articles in English that reported all-cause mortality or survival in patients with BCC and/or SCC were eligible for inclusion. Study eligibility was assessed by two authors using title and abstract for initial screening (MRW and WCS), followed by full-text review by three authors (MRW, WCS, AN). Any disagreements were settled by a fourth author (EL). We excluded articles that presented no data (review articles, editorials), as well as case reports and case series. We also excluded articles that presented data only on a subpopulation of patients (e.g. xeroderma pigmentosum patients, organ transplant patients, patients with SCC of the lip), which might have increased risks of all-cause mortality compared to all-comers with BCC and SCC. Studies that reported an effect estimate of mortality or survival for BCC and SCC separately were eligible for inclusion in quantitative meta-analysis. Studies with study population overlap were excluded from quantitative meta-analysis but were included in qualitative review if they provided additional qualitative value.
Three authors (MRW, WCS, EL) discussed studies with population overlap to determine which to include. Of the seven studies from Denmark that individually met inclusion criteria, five reported data from the Danish Cancer Registry (DCR),[25, 27, 34-36] one reported data from the Gerda Frentz Cohort (GFC),[37] and one used both DCR and GFC data.[38] Approximately 35% of patients in the GFC, however, are also found in the DCR.[38] Of these seven that analyzed overlapping data, we included Jensen et al (2008)[25] for quantitative analysis because it had the larger study population. Additionally, we included both Brondum-Jacobsen et al[27] and Jensen et al (2010)[34] in the qualitative portion of our review despite the population overlap because they provided complementary information: Brondum-Jacobsen et al[27] provided an effect estimate for BCC and SCC combined, and Jensen et al (2010)[34] compared SCC and BCC cases to gender matched controls and adjusted for socio-economic status and comorbidities, as many other studies did not. There were also two studies with overlapping data from the Cancer Registry of Norway,[39, 40] and we included the larger of the two.[39]
Data were extracted from articles using a data extraction sheet, which was developed based on the Cochrane Consumers and Communication Review Group's data extraction template.[41] We extracted the following items for each study: characteristics of study participants (including age, gender, BCC/SCC status, and comorbidities), participant inclusion/exclusion criteria, sources of mortality and BCC/SCC diagnosis, characteristics of study design (including design type, presence and characteristics of matching or standardization), statistical methods (including analysis type and variables included), and all-cause mortality or survival outcomes for BCC and/or SCC.
Statistical Methods
Hazard ratios, odds ratios, standardized mortality ratios, mortality rate ratios, and relative risks were considered equivalent measures of risk.[42, 43] Relative mortality estimates, such as standardized mortality ratios (SMR), were published for three of the studies included in the quantitative analysis. One SMR estimate was obtained from the authors of a study that assessed survival in patients with BCC and SCC but did not include relative mortality estimates in the original manuscript.[6] One SMR was calculated from the numbers of observed and expected surviving patients provided in an article.[44]
We used Stata 12 (College Station, TX) to perform random effects model meta-analysis, yielding summary relative risks and 95% confidence intervals (CIs). We analyzed data for BCC and SCC separately. To investigate heterogeneity in study outcomes, we used a χ2 test for heterogeneity and an I2 statistic. We did not statistically assess the potential for small study effects or publication bias as both of our analyses had four or fewer studies included.
Results
Figure 1 shows the study selection process. Database and hand searches yielded a total of 6538 unique publications that were screened by title and abstract. 156 articles were assessed for eligibility in full-text. 144 articles were excluded because of no relevance (n=49), reporting disease-specific, rather than all-cause mortality (n=38), only including subpopulations (n=19), no original data (n=16), language other than English (n=12), duplicate study populations (n=9), and case series (n=1).
Figure 1. PRISMA flow diagram of literature search and study selection for systematic review and meta-analysis of all-cause mortality in patients with a history of basal cell carcinoma (BCC) or squamous cell carcinoma (SCC).

* Subpopulations: articles that presented data on a subpopulation of patients (e.g. xeroderma pigmentosum patients, organ transplant patients, patients with SCC of the lip)
Twelve studies met inclusion criteria. Table 1 summarizes the study characteristics of these 12 studies. Table 2 details the outcome measures, effect estimates, and statistical adjustments for the 12 included studies. We included four studies in the quantitative meta-analysis. Excluded from the meta-analysis were studies that grouped patients with BCC and SCC,[27, 28] studies that reported effect estimates that could not be converted to a relative mortality measure,[23, 39, 45-48] studies with population overlap,[27, 34] one study that evaluated ‘malignant skin cancer, excluding melanoma’ in a cancer registry that does not include BCC,[46] and one study that did not detail confidence intervals or standard errors for its relative survival estimates.[45] The studies included in the meta-analysis analyzed data collected between1973-2011 in four different countries and represented a total of 464,230 patients with BCC and 175,849 patients with SCC.
Table 1. Summary of Study Characteristics.
| Reference | Country, publication year | Dates of data collection | Study design | Diagnosis* | No of cases | No of deaths | Age (range, mean, or percentage in years) | Percent male (%) |
|---|---|---|---|---|---|---|---|---|
| Studies included in meta-analysis | ||||||||
| Eisemann et al [44] | Germany, 2016 | 1997-2011 | Retrospective cohort using national registries | BCC | 380030 | 101848** | 68.9 | 50.8 |
| SCC | 92108 | 35185** | 75.6 | 58.1 | ||||
| Jensen et al 2008†† [25] | Denmark, 2008 | 1978-2001 | Retrospective cohort using national registries | BCC | 82837 | 28758 | 58.3% >65 | 48 |
| SCC | 13453 | 6998 | 78.3% >65 | 60.6 | ||||
| Rees et al [29] | USA, 2015 | 1993-2009 | Retrospective cohort from previous case-control study | BCC | 1363 | 169 | -- | 50.1 |
| SCC | 880 | 189 | 61.4 | |||||
| Hollestein et al [6] | Netherlands, 2012 | 1989-2008 | Retrospective cohort using national registries | SCC | 69408 | 31903 | 73.6 | 59.9 |
| Studies not included in meta-analysis | ||||||||
| Brondum-Jacobsen et al†† [27] | Denmark, 2013 | 1980-2006 | Retrospective cohort using national registries | KC | 129206 | -- | >40 | 49 |
| Clayman et al [47] | USA, 2005 | 1996-2001 | Prospective cohort | SCC | 210 | 52 | 34-94.7 | 89.1 |
| Jensen et al 2010†† [34] | Denmark, 2010 | 1990-2005 | Retrospective cohort using national registries | BCC | 72295 | -- | 8-106 | 47 |
| SCC | 11601 | -- | -- | 65 | ||||
| Kahn et al [28] | USA, 1998 | 1982-1994 | Prospective cohort | KC | 35062 | -- | M 61.5 (SD 9.1); F 60.5 (SD 10.1) | 54.5 |
| Karjalainen et al [23] | Finland, 1989 | 1967-1982 | Retrospective cohort using national registries | BCC | 23975 | -- | M=64.1; F=66.9. | 42.3 |
| SCC | 2927 | -- | M=68.5; F=72.0 | |||||
| Robsahm et al [39] | Norway, 2015 | 1963-2011 | Retrospective cohort using national registries | SCC | 30818 | -- | -- | 55.4 |
| Teppo et al [45] | Finland, 1999 | 1985-1994 | Retrospective cohort using national registries | SCC | -- | -- | -- | -- |
| Talback et al [46] | Sweden, 2004 | 1960-1998 | Retrospective cohort using national registries | SCC† | -- | -- | -- | -- |
BCC= basal cell carcinoma; SCC= squamous cell carcinoma; KC= keratinocyte carcinoma (BCC and SCC grouped together in publication)
Number of deaths was calculated using percentage of surviving patients presented in the article
Included ‘malignant skin cancer excluding melanoma’ in a cancer registry that does not collect BCC data
Jensen 2008, Brondum-Jacobsen, and Jensen 2010 have overlapping study populations. Brondum-Jacobsen was included qualitatively because it provides a combined BCC/SCC estimate. Jensen 2010 was included qualitatively because it adjusted extensively for covariates
Table 2. Summary of Outcome Effect Sizes.
| Reference | Outcome Measurea | Basal cell carcinomab | Squamous Cell Carcinomab | Adjustments/Standardization |
|---|---|---|---|---|
| Studies included in meta-analysis | ||||
| Eisemann et al [44] | SMRc | 0.87 (0.86-0.87) | 1.17 (1.16-1.18) | Age, gender |
| Relative survival %, 10 year (SD) | 105.9% (0.2%) | 91.8% (0.5%) | Age, gender | |
| Jensen et al 2008 d [25] | SMR | 0.97 (0.96-0.98); M 0.98 (0.96-0.99); F 0.95 (0.94-0.97) | 1.30 (1.26-1.33); M 1.24 (1.21-1.28); F 1.39 (1.24-1.45) | Age, gender, 5 year calendar period |
| Rees et al [29] | HR | 0.96 (0.77-1.19) | 1.25 (1.01-1.54) | Age, gender, smoking, and subsequent cancer |
| Hollestein et al [6] | SMR | -- | 1.272673 (1.272669-1.272709)e | Age, gender, calendar year |
| Studies not included in meta-analysis | ||||
| Brondum-Jacobsen et al d [27] | HR | Combined: 0.52 (0.52-0.53) | Age, gender, descent, geographical residency, educational level, estimated occupational sun exposure, estimated occupational physical activity, and baseline characteristics. | |
| Fully adjusted OR | Combined: 0.97 (0.96-0.99) | |||
| Age adjusted OR | Combined: 0.96 (0.95-0.97) | Age | ||
| Clayman et al [47] | 3 year overall survival | -- | 70% (95% CI: 62-79) | None |
| Jensen et al 2010 d [34] | Crude 10 year MRR (95% CI) | 0.93 (0.91-0.94) | By age <60 1.85 (1.70-2.01), 60-70 1.20 (1.14-1.27), >70 1.11 (1.07-1.16) | None |
| Adjusted 10 year MRR (95% CI) | 0.91 (0.89-0.92) | By age <60 1.54 (1.41-1.68); 60-70 1.17 (1.10-1.23); >70 1.11 (1.07-1.15) | Age, gender, 23 comorbidities, and socioeconomic status | |
| 10 year cml mortality, % (95% CI) | 29.3 (28.9-29.6) | By age <60 27.0 (25.3-28.9), 60-70 54.5 (52.5-56.4), >70 80.5 (79.0-82.1) | ||
| Kahn et al [28] | RR | Combinedf: M 1.03 (1.00-1.06); F 1.04 (1.00-1.09) | Age, race, education level, smoking status, BMI, alcohol use, exercise, vegetable and fat intake, aspirin use, marital status, diabetes, menopausal status, parity, use of oral contraceptive pills and estrogen replacement therapy | |
| Karjalainen et al [23] | 5 year RSR | M 98.6; F 100.1 | M 90.4 (SE 2.9); F 89.9 (SE 2.8) | Gender, age at diagnosis, calendar time of diagnosis, histologic type, and anatomic site of the tumor |
| 10 year RSR | M 98.8; F 100.3 | M 87.2 (SE 4.4); F 83.3 (SE 4.0) | ||
| Robsahm et al [39] | 5 year RSR (95% CI) | -- | Localized SCC: M 0.82 (0.80-0.84), F 0.88 (0.85-0.90)g | Gender, age, stage |
| Teppo et al [45] | 5 year RSR | -- | M 90; F 92 | Age, gender, calendar time |
| Talback et al h [46] | 5 year RSR | -- | 87.8 | Gender, age, and calendar year |
| 10 year RSR | -- | 80 | ||
MRR= Mortality rate ratio; cml= cumulative; SMR= standardized mortality ratio; RSR= relative survival rate; HR= hazard ratio; OR= odds ratio
F= female; M= male
SMR calculated using absolute and relative 10-year survival data reported in article
Jensen 2008, Brondum-Jacobsen, and Jensen 2010 have overlapping study populations. Brondum-Jacobsen was included qualitatively because it provides a combined BCC/SCC estimate. Jensen 2010 was included qualitatively because it adjusted extensively for covariates.
Unpublished estimates provided by the authors
All non-melanoma skin cancers, which may include more rare cancers such as Merkel cell carcinoma
Displayed are results from 2000-2011, as this study splits results by decade. Listed is the result for localized SCC rather than ‘advanced’ SCC
Included ‘malignant skin cancer excluding melanoma’ in a cancer registry that does not collect BCC data
Qualitatively, two studies evaluated relative mortality of BCC and SCC combined: one showed slightly increased mortality,[28] and one showed slight decreased mortality,[27] both of which were statistically significant.[27, 28]
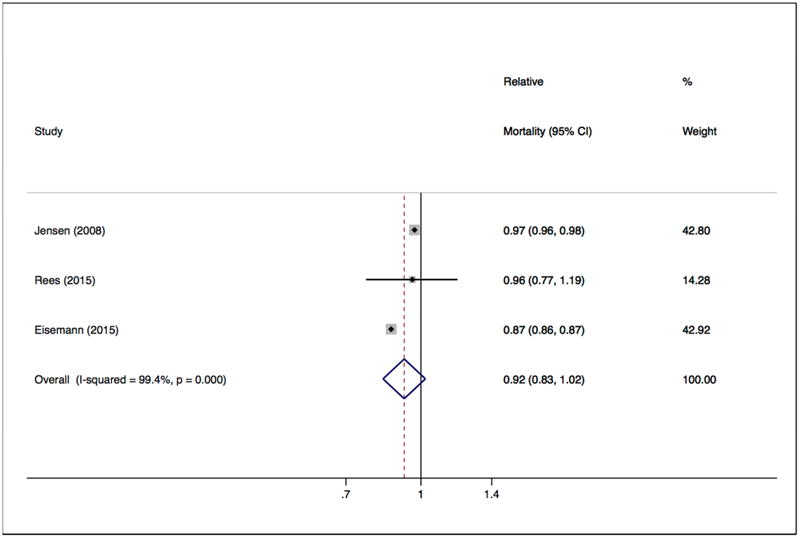
In patients with BCC, three studies included in quantitative meta-analysis showed effect estimates consistent with decreased relative mortality,[25, 29, 44] two of which were statistically significant.[25, 44] One study in the qualitative review reported relative survival rates that indicated slightly increased mortality in men and slightly decreased mortality in women but did not report statistical significance.[23] Another study in the qualitative review, which had an overlapping population with a study included in quantitative meta-analysis[25] but adjusted for 23 comorbidities and socioeconomic status, reported a statistically significant decreased mortality rate ratio[34]. The random effects meta-analysis of three studies[25, 29, 44] yielded a summary relative risk of 0.92 (95% CI 0.83-1.02, Figure 2). χ2 test for heterogeneity yielded p<0.001 and I2 statistic of 99.4%.
Figure 2. Relative all-cause mortality among patients with history of basal cell carcinoma (BCC).
In patients with SCC, all nine studies[6, 23, 25, 29, 34, 39, 44-46] that reported relative all-cause mortality (or relative survival) showed an effect estimate of increased mortality, though some of these studies did not evaluate statistical significance.[45, 46] For quantitative meta-analysis, we were able to include effect estimates for relative mortality from four studies[6, 25, 29, 44]. A random effects meta-analysis yielded a summary relative risk of 1.25 (95% CI 1.17-1.32, Figure 3). χ2 test for heterogeneity yielded p<0.001 and I2 statistic of 98.8%.
Figure 3. Relative all-cause mortality among patients with history of squamous cell carcinoma (SCC).

Discussion
In our qualitative systematic review we found no significant difference in risk of all-cause mortality in patients with BCC (relative mortality estimates showed decreased mortality or null effects). Conversely, we found consistently increased relative all cause mortality in patients with a history of SCC. Based on a quantitative meta-analysis including a total of 464,230 patients with BCC and 175,849 patients with SCC, we found that patients with SCC have statistically significantly higher (approximately 25%) all cause mortality compared to the general population.
Our findings add to the published literature by clarifying that there may be a different pattern of all-cause mortality in patients with BCC compared to those with SCC. Because these tumors often occur in the same patients and are both often caused by exposure to ultraviolet radiation, patients with BCC and SCC are often grouped together and considered to have non-melanoma skin cancers or keratinocyte carcinomas. Our data contributes to the argument that the carcinogenesis of these tumors and long-term outcomes for patients with these tumors may be distinct.
Studies that combine BCC and SCC have found varying results in relation to mortality: Brondum-Jacobsen et al[27] showed a decreased mortality while Kahn et al[28] showed a potentially increased mortality (not statistically significant). While no study has directly compared BCC and SCC patients, studies that separate BCC and SCC consistently report different relative survivals. In our systematic review, we found that most studies reported a decreased mortality or no statistically significant effect in patients with history of BCC.[23, 25, 29, 34, 44]
In patients with a history of SCC, on the other hand, every study reported an effect estimate of increased mortality, though some of these were not tested for statistical significance.[23, 25, 29, 34, 38, 44-46] The higher risk of death from any cause in patients with a history of SCC may be related to common risk factors, which our study was not able to investigate. Only two of the twelve studies in this review control for medical comorbidities,[28, 34] neither of which was included in the quantitative analysis. SCC is associated with immunosuppression, which increases risk for multiple negative health outcomes such as infection and cancer. There is also some evidence that SCC may be associated with smoking,[25, 49] which increases risk for cardiovascular disease and other cancers.[24, 26, 50-52] In addition, indoor tanning behaviors, which are associated with BCC and SCC,[53] are associated with other high risk health behaviors like smoking, alcohol and drug use.[54-56] BCC and SCC have also been associated with increased risk of subsequent cancer, including breast cancer, lung cancer, leukemia, and melanoma.[8-16] It is important to note that while SCC is associated with other factors that may be driving this increased mortality, the studies we included studied the broader general population of patients with BCC and SCC, without limiting to groups with specific comorbidities. Thus, our findings may be generalizable to all-comers with these common skin cancers.
This study is limited by the fact that it included mainly retrospective observational studies; only one of the studies was a prospective cohort study.[47] Our quantitative analysis was limited by the variation in summary measures of mortality and survival data studies reported, some of which we were not able to convert to a relative mortality measure and include in our analysis. Statistical limitations include the significant heterogeneity noted in both our quantitative meta-analyses. While random-effects methodology is the appropriate choice for heterogeneous data, the included studies may have significant clinical or methodological differences and care should be taken when using the summary estimate from this study. Some potential sources of heterogeneity that we identified were study design (one cohort study[29] was derived from a previous case-control study while the rest were national registry cohorts), location (the US, Germany, Denmark, and the Netherlands were represented in these analyses), and the lack of adjustment for potential confounders (all four used age and gender,[6, 25, 29, 44] two used calendar period,[6, 25] and one used smoking and subsequent cancer diagnosis,[29] but no other comorbidities were included). Despite this, the vast majority of studies included showed similar results to the summary estimates, and the studies of SCC in particular were all qualitatively concordant with increased relative mortalities. Therefore we believe that estimating a summary effect through meta-analysis is both useful and methodologically appropriate. Additionally, none of the included studies reported SCC-specific or BCC-specific mortality. While the published literature on mortality rates of SCC indicate that the relative contribution of the SCCs themselves to the excess mortality we observed for SCC is likely small, we were not able to further explore this.[3-6, 57] Finally, we were unable to put our findings in context with other cancers. The studies included in quantitative analysis reported all-cause mortality ratios (e.g. SMRs), which are difficult to compare to typical measures of mortality (e.g. rates) in other cancers. For example, SEER reports the 5-year relative mortality rate for melanoma at 8.5%,[58] which cannot directly be compared to our finding of an increased relative mortality ratio of 1.25 for SCC.
This study supports the growing literature that identifies BCC and SCC as distinct neoplasms with different histology, pathophysiology, and outcomes, including all-cause mortality. Patients with SCC are at increased risk of death from any cause compared to the general population, whereas patients with BCC may not have increased all-cause mortality. We believe our findings have clinical implications for patients with SCC who may need additional education and age-appropriate screening to prevent death from major diseases. While many patients get both BCC and SCC, future research should take into account that these cancers may have different long-term risks and outcomes.
Capsule summary.
There are varying reports of the association of basal cell carcinoma (BCC) or cutaneous squamous cell carcinoma (SCC) with all-cause mortality
Patients with a history of SCC have an approximately 25% increased risk of all-cause mortality compared to the general population
SCC may be a clinical marker of a decline in health
Acknowledgments
Funding/Support: This work was supported by the National Institutes of Health through the National Center for Research Resources (Grant KL2RR024130 to EL), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (Grants K24 AR052667 and R01 AR 054983 to MMC), and the Claude D. Pepper Older Americans Independence Center at the National Institute on Aging, National Institutes of Health (Grant P30 AG044281 to EL), and by Harvard Medical School's Scholars in Medicine Office (WCS). No funding sources were involved in the study design; data collection, management, analysis, and interpretation; manuscript preparation, review and approval; or the decision to submit the manuscript for publication. EL was supported by the Dermatology Foundation through a Health Care Policy Career Development Award.
Obtained funding: none. Administrative, technical, or material support: Chren and Linos. Study supervision: Linos.
We thank the librarians Min Lin Fang and Evans Whitaker, MD from the University of California, San Francisco and Julia S.Whelan from Harvard Medical School for their assistance in devising search strategy and finding manuscripts.
Abbreviations and acronyms
- BCC
basal cell carcinoma
- SCC
squamous cell carcinoma
- SMR
standardized mortality ratio
- KC
keratinocyte carcinoma
- MRR
mortality rate ratio
- RSR
relative survival rate
- HR
hazard ratio
- OR
odds ratio
Footnotes
Authors' contributions: Dr. Eleni Linos had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Wehner, Cidre, Chren, and Linos. Acquisition, analysis, and interpretation of data: Wehner, Cidre, Nosrati, Schoen, Boscardin, Linos. Drafting of the manuscript: Wehner, Cidre, Linos. Critical revision of the manuscript for important intellectual content: Wehner, Cidre, Nosrati, Boscardin, Chren, Linos.
Conflicts of Interest Disclosure: The authors have no conflict of interest to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rogers HW, et al. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol. 2015;151(10):1081–6. doi: 10.1001/jamadermatol.2015.1187. [DOI] [PubMed] [Google Scholar]
- 2.Lindelof B, et al. Mortality and clinicopathological features of cutaneous squamous cell carcinoma in organ transplant recipients: a study of the Swedish cohort. Acta Derm Venereol. 2006;86(3):219–22. doi: 10.2340/00015555-0069. [DOI] [PubMed] [Google Scholar]
- 3.Tejera-Vaquerizo A, et al. Skin Cancer Incidence and Mortality in Spain: A Systematic Review and Meta-Analysis. Actas Dermosifiliogr. 2016;107(4):318–28. doi: 10.1016/j.ad.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Ardanaz E, et al. Incidence and mortality due to cancer in Navarre, 1998-2002. Trends in the last 30 years. An Sist Sanit Navar. 2007;30(2):245–70. doi: 10.23938/ASSN.0216. [DOI] [PubMed] [Google Scholar]
- 5.Weinstock MA, et al. Nonmelanoma skin cancer mortality. A population-based study. Archives of Dermatology. 1991;127(8):1194–1197. [PubMed] [Google Scholar]
- 6.Hollestein LM, De Vries E, Nijsten T. Trends of cutaneous squamous cell carcinoma in the Netherlands: Increased incidence rates, but stable relative survival and mortality 1989-2008. European Journal of Cancer. 2012;48(13):2046–2053. doi: 10.1016/j.ejca.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention, National Center for Health Statistics. Underlying Cause of Death 1999-2014 on CDC WONDER Online Database, released 2015 Data are from the Multiple Cause of Death Files, 1999-2014, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. Accessed at http://wonder.cdc.gov/ucd-icd10.html on Nov 6, 2016 2:56:26 PM.
- 8.Chen J, et al. Nonmelanoma skin cancer and risk for subsequent malignancy. J Natl Cancer Inst. 2008;100:1215–1222. doi: 10.1093/jnci/djn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bower CP, et al. Basal cell carcinoma and risk of subsequent malignancies: A cancer registry-based study in southwest England. J Am Acad Dermatol. 2000;42:988–991. [PubMed] [Google Scholar]
- 10.Friedman GD, I, Tekawa S. Association of basal cell skin cancers with other cancers (United States) Cancer Causes Control. 2000;11:891–897. doi: 10.1023/a:1026591016153. [DOI] [PubMed] [Google Scholar]
- 11.Frisch M, et al. Risk for subsequent cancer after diagnosis of basal-cell carcinoma. A population-based, epidemiologic study. Ann Intern Med. 1996;125:815–821. doi: 10.7326/0003-4819-125-10-199611150-00005. [DOI] [PubMed] [Google Scholar]
- 12.Karagas MR, et al. Occurrence of other cancers among patients with prior basal cell and squamous cell skin cancer. Cancer Epidemiol Biomarkers Prev. 1998;7:157–161. [PubMed] [Google Scholar]
- 13.Milan T, et al. Subsequent primary cancers after basal-cell carcinoma: A nationwide study in Finland from 1953 to 1995. Int J Cancer. 2000;87:283–288. [PubMed] [Google Scholar]
- 14.Nugent Z, et al. Risk of second primary cancer and death following a diagnosis of nonmelanoma skin cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:2584–2590. doi: 10.1158/1055-9965.EPI-05-0379. [DOI] [PubMed] [Google Scholar]
- 15.Song F, et al. Risk of a second primary cancer after non-melanoma skin cancr in white men and women: a prospetive cohor study. PLoS Medicine. 2013;10:e1001433. doi: 10.1371/journal.pmed.1001433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rees JR, et al. Non-melanoma skin cancer and subsequent cancer risk. PLoS One. 2014;9(6):e99674. doi: 10.1371/journal.pone.0099674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mercer J, Mahmoudi M, Bennett M. DNA damage, p53, apoptosis and vascular disease. Mutat Res. 2007;621:75–86. doi: 10.1016/j.mrfmmm.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Mahmoudi M, Mercer J, Bennett M. DNA damage and repair in atherosclerosis. Cardiovasc Res. 2006;71(2):259–68. doi: 10.1016/j.cardiores.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Warnholtz A, et al. Increased NADH-oxidase-mediated superoxide production in the early stages of atherosclerosis: evidence for involvement of the renin-angiotensin system. Circulation. 1999;99(15):2027–33. doi: 10.1161/01.cir.99.15.2027. [DOI] [PubMed] [Google Scholar]
- 20.D'Mello MJ, et al. Association between shortened leukocyte telomere length and cardiometabolic outcomes: systematic review and meta-analysis. Circ Cardiovasc Genet. 2015;8(1):82–90. doi: 10.1161/CIRCGENETICS.113.000485. [DOI] [PubMed] [Google Scholar]
- 21.Hunt SC, et al. Leukocyte telomere length and coronary artery calcium. Am J Cardiol. 2015;116(2):214–8. doi: 10.1016/j.amjcard.2015.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang W, et al. Short telomere length in blood leucocytes contributes to the presence of atherothrombotic stroke and haemorrhagic stroke and risk of post-stroke death. Clin Sci (Lond) 2013;125(1):27–36. doi: 10.1042/CS20120691. [DOI] [PubMed] [Google Scholar]
- 23.Karjalainen S, Salo H, Teppo L. Basal cell and squamous cell carcinoma of the skin in Finland: Site distribution and patient survival. International Journal of Dermatology. 1989;28(7):445–450. doi: 10.1111/j.1365-4362.1989.tb02503.x. [DOI] [PubMed] [Google Scholar]
- 24.Johannesdottir SA, et al. Mortality in cancer patients with a history of cutaneous squamous cell carcinoma-a nationwide population-based cohort study. BMC cancer. 2012;12(1):126. doi: 10.1186/1471-2407-12-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen AO, et al. Mortality in Danish patients with nonmelanoma skin cancer, 1978-2001. British Journal of Dermatology. 2008;159(2):419–425. doi: 10.1111/j.1365-2133.2008.08698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nugent Z, et al. Risk of second primary cancer and death following a diagnosis of nonmelanoma skin cancer. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2005;14(11 Pt 1):2584–2590. doi: 10.1158/1055-9965.EPI-05-0379. [DOI] [PubMed] [Google Scholar]
- 27.Brondum-Jacobsen P, et al. Skin cancer as a marker of sun exposure associates with myocardial infarction, hip fracture and death from any cause. International Journal of Epidemiology. 2013;42(5):1486–1496. doi: 10.1093/ije/dyt168. [DOI] [PubMed] [Google Scholar]
- 28.Kahn HS, et al. Increased cancer mortality following a history of nonmelanoma skin cancer. Jama. 1998;280(10):910–912. doi: 10.1001/jama.280.10.910. [DOI] [PubMed] [Google Scholar]
- 29.Rees JR, et al. Survival after squamous cell and basal cell carcinoma of the skin: A retrospective cohort analysis. International journal of cancer. Journal international du cancer. 2015 doi: 10.1002/ijc.29436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alberg AJ, Fischer AH. Is a personal history of nonmelanoma skin cancer associated with increased or decreased risk of other cancers? Cancer Epidemiology Biomarkers & Prevention. 2014;23(3):433–436. doi: 10.1158/1055-9965.EPI-13-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wheless L, Black J, Alberg AJ. Nonmelanoma skin cancer and the risk of second primary cancers: a systematic review. Cancer Epidemiology Biomarkers & Prevention. 2010;19(7):1686–1695. doi: 10.1158/1055-9965.EPI-10-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moher D, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA StatementThe PRISMA Statement. Annals of Internal Medicine. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 33.Stroup DF, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 34.Jensen AO, et al. Non-melanoma skin cancer and ten-year all-cause mortality: a population-based cohort study. Acta Derm Venereol. 2010;90(4):362–7. doi: 10.2340/00015555-0899. [DOI] [PubMed] [Google Scholar]
- 35.Steding-Jessen M, et al. Socioeconomic status and non-melanoma skin cancer: a nationwide cohort study of incidence and survival in Denmark. Cancer epidemiology. 2010;34(6):689–695. doi: 10.1016/j.canep.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 36.Storm HH, Kejs AM, Engholm G. Improved survival of Danish cancer patients 2007–2009 compared with earlier periods. Dan Med Bull. 2011;58(12):A4346. [PubMed] [Google Scholar]
- 37.Jensen AØ, et al. Ten year mortality in a cohort of nonmelanoma skin cancer patients in Denmark. Journal of investigative dermatology. 2006;126(11):2539–2541. doi: 10.1038/sj.jid.5700433. [DOI] [PubMed] [Google Scholar]
- 38.Jensen AO, et al. Do incident and new subsequent cases of non-melanoma skin cancer registered in a Danish prospective cohort study have different 10-year mortality? Cancer Detection and Prevention. 2007;31(5):352–358. doi: 10.1016/j.cdp.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 39.Robsahm TE, Helsing P, Veierod MB. Cutaneous squamous cell carcinoma in Norway 1963-2011: increasing incidence and stable mortality. Cancer Med. 2015;4(3):472–80. doi: 10.1002/cam4.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iversen T, Tretli S. Trends for invasive squamous cell neoplasia of the skin in Norway. Br J Cancer. 1999;81(3):528–31. doi: 10.1038/sj.bjc.6690725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Consumers, C. Communication Review Group: Data Extraction Template for Cochrane Reviews. 2007 Internet. [Google Scholar]
- 42.Roerecke M, Rehm J. Cause-specific mortality risk in alcohol use disorder treatment patients: a systematic review and meta-analysis. International journal of epidemiology. 2014:dyu018. doi: 10.1093/ije/dyu018. [DOI] [PubMed] [Google Scholar]
- 43.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 44.Eisemann N, et al. Survival from non-melanoma skin cancer in Germany. British Journal of Dermatology. 2016 doi: 10.1111/bjd.14352. [DOI] [PubMed] [Google Scholar]
- 45.Teppo L, et al. Cancer Patient Survival - Patterns, Comparisons, Trends: A Population-based Cancer Registry Study in Finland. Acta Oncologica. 1999;38(3):283–294. doi: 10.1080/028418699431348. [DOI] [PubMed] [Google Scholar]
- 46.Talbäck M, Stenbeck M, Rosén M. Up-to-date long-term survival of cancer patients: an evaluation of period analysis on Swedish Cancer Registry data. European Journal of Cancer. 2004;40(9):1361–1372. doi: 10.1016/j.ejca.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 47.Clayman GL, et al. Mortality risk from squamous cell skin cancer. Journal of Clinical Oncology. 2005;23(4):759–765. doi: 10.1200/JCO.2005.02.155. [DOI] [PubMed] [Google Scholar]
- 48.Schmults CD, et al. Factors Predictive of Recurrence and Death From Cutaneous Squamous Cell Carcinoma A 10-Year, Single-Institution Cohort Study. JAMA DERMATOLOGY. 2013;149(5):541–547. doi: 10.1001/jamadermatol.2013.2139. [DOI] [PubMed] [Google Scholar]
- 49.Karagas MR, et al. Risk of subsequent basal cell carcinoma and squamous cell carcinoma of the skin among patients with prior skin cancer. Skin Cancer Prevention Study Group. JAMA: The Journal of the American Medical Association. 1992;267(24):3305–3310. [PubMed] [Google Scholar]
- 50.Askling J, et al. Is history of squamous-cell skin cancer a marker of poor prognosis in patients with cancer? Annals of internal medicine. 1999;131(9):655–659. doi: 10.7326/0003-4819-131-9-199911020-00004. [DOI] [PubMed] [Google Scholar]
- 51.Hjalgrim H, et al. Non-melanoma skin cancer may be a marker of poor prognosis in patients with non-Hodgkin's lymphoma. International journal of cancer. 2000;85(5):639–642. doi: 10.1002/(sici)1097-0215(20000301)85:5<639::aid-ijc7>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 52.Toro JR, et al. Prior history of non-melanoma skin cancer is associated with increased mortality in patients with chronic lymphocytic leukemia. haematologica. 2009;94(10):1460–1464. doi: 10.3324/haematol.2008.004721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wehner MR, et al. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:e5909. doi: 10.1136/bmj.e5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mosher CE, Danoff-Burg S. Indoor tanning, mental health, and substance use among college students: the significance of gender. Journal of health psychology. 2010 doi: 10.1177/1359105309357091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lostritto K, et al. Lifetime history of indoor tanning in young people: a retrospective assessment of initiation, persistence, and correlates. BMC Public Health. 2012;12(1):1. doi: 10.1186/1471-2458-12-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haluza D, Simic S, Moshammer H. Sunbed Use Prevalence and Associated Skin Health Habits: Results of a Representative, Population-Based Survey among Austrian Residents. International journal of environmental research and public health. 2016;13(2):231. doi: 10.3390/ijerph13020231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weinstock MA. Nonmelanoma skin cancer mortality in the United States, 1969 through 1988. Arch Dermatol. 1993;129(10):1286–90. [PubMed] [Google Scholar]
- 58. [cited 2016 November 18];SEER Stat Fact Sheets: Melanoma of the Skin. Available from: http://seer.cancer.gov/statfacts/html/melan.html.


