Abstract
Objectives
Stromal Interacting Molecule-1 (STIM1) plays a role in co-ordinating calcium signaling in different cell types. The increase or deletion of STIM1 expression in cardiomyocyte causes cardiac complication. Moreover, the deletion of STIM1 in endothelial cell causes vascular endothelial dysfunction. However, the disruption of STIM1 in SMC has no effect on endothelial function but protects vascular function when mice are infused with angiotensin-II. Nevertheless, the role of SMC-STIM1 in acute and chronic myocardial infarction induced by acute ischemia-reperfusion (I/R) injury and permanent coronary artery occlusion (PCO) is unknown.
Methods & Results
Stim1fl/fl were generated and crossed into the SM22α-Cre+ backgrounds. SM22α-Cre+ causes deletion of STIM1 floxed genes in adult SMC (Stim1SMC−/−). Control and Stim1SMC−/− mice were subjected to acute I/R injury. Hearts were then harvested and incubated with triphenyl-tetrazolium chloride to determine the infarct size.
In control mice subjected to I/R, the heart developed a significant infarct associated with an increase in STIM1 expression. Interestingly, the infarct size was substantially reduced in Stim1SMC−/− mice. The protection in Stim1SMC−/− mice against I/R injury involves the modulation of endoplasmic reticulum (ER) stress, apoptosis, oxidative stress, Akt and MAP-Kinase (ERK1/2 and p38) signaling, and inflammation. Furthermore, in another model of chronic myocardial infarction induced by PCO, SMC-STIM1 disruption significantly reduced myocardial infarct size and improved cardiac function.
Conclusion
Our results provide new evidence that SMC-STIM1 disruption is a novel mechanism that protects the heart from myocardial infarction through reduction of ER stress, oxidative stress, MAP-Kinase, apoptosis, and inflammation.
Keywords: Stromal interaction molecule 1, myocardial infarct, ER stress, MAP-Kinase, oxidative stress, inflammation, and apoptosis
INTRODUCTION
Myocardial infarction (MI) is the most common cause of morbidity and mortality in patients with cardiovascular complications.[1–3] STIM1 has been shown to play a significant role in coordinating cellular calcium signaling in different cell types including SMC.[4–7] STIM1 senses Ca2+ levels changes in endoplasmic reticulum luminal,[4,5] and directly interact with Orai channels, located at the plasma membrane, to orchestrate the opening of Ca2+ released-activated Ca2+ channels (CRAC).[8] STIM1 is also critical in the activation of store-independent Orai1/Orai3 hetero-multimeric channels.[4,5,7,9,10] STIM1 plays an essential role in vascular function and dysfunction,[11] neo-intima formation, endothelial permeability, and cardiac complications.[9–12] Balloon injury of carotid arteries in rats increases the expression of STIM1, Orai1, and Orai3, which play a crucial in the development of neo-intima hyperplasia.[10] These data indicate that STIM1 and Orai channels are essential in VSMC proliferation and migration. Additionally, two elegant studies reported that the overexpression or the deletion of STIM1 specifically in cardiomyocyte cause cardiac complications.[12–14] Together, these data indicate the differential role for STIM1 cells type-dependent. Previous studies showed communication through paracrine mechanism between vascular cells, stem cells, and cardiomyocyte in infarcted myocardium.[15–17] Nevertheless, the in vivo contribution and mechanism of SMC-STIM1 in myocardial infarction induced by I/R injury or PCO is unknown.
We previously determined that endoplasmic reticulum (ER) stress is a major factor in a variety of pathologies such hypertension and diabetes.[8,18] Indeed, the inhibition of the ER stress significantly improved vascular function in hypertension and diabetes.[8,12,18] Recently, it has been reported that not all ER stress pathways are detrimental. Thus, Dr. Glembotski’s laboratory reported that the ER stress transcription factor activating transcription factor 6 (ATF6) induction protects the cardiomyocytes from I/R injury.[19,20]
The primary goal of the present study is to determine whether SMC-STIM1 plays any role in the induction of acute myocardial infarct-induced by acute I/R injury and chronic ischemia. We also sought to determine the mechanism by which SMC-STIM1 disruption protects the heart from acute I/R injury and chronic ischemia and whether ER stress pathways, Akt and MAP kinases signaling, apoptosis, and inflammation are involved. To achieve this goal, we specifically deleted STIM1 in SMC (Stim1SMC−/−) and subjected Stim1SMC−/− and control mice to cardiac I/R injury or PCO.
METHODS
Experimental animal and treatment
All mice experimental procedures were performed according to the American Guidelines for the Ethical Care of Animals and approved by the Institutional Animal Care and Use Committee at Eastern Virginia Medical School. STIM1SMC−/− and their littermate (8–12 weeks old male) mice on the C57/Bl6 genetic background were generated by Dr. Trebak [11,21] using Stim1flx/flx mice provided by Dr. Stefan Feske, NYU.[22] Mice were divided into three groups: Group 1: C57/Bl6 (C57) and groups 2 and 3: C57 and STIM1SMC−/− subjected to acute cardiac ischemia-reperfusion injury (24 hours) or permanent coronary artery ligation for 3 weeks.
Myocardial I/R injury and PCO
We utilized mice for myocardial cardiac ischemia-reperfusion injury. We anesthetized the mice with sodium pentobarbital (50 mg/kg ip.), intubated, and then ventilated using a rodent ventilator (MiniVent Harvard Apparatus). During the procedure, the body temperature of the mouse was maintained a 37°C.
In one group of mice, We subjected hearts to ischemia for 30 seconds without any reperfusion. Then immediately we injected Evans blue into the carotid artery. This procedure is an experimental control to demonstrate that the coronary artery was ligated.
I/R injury procedures
We performed a left thoracotomy and then ligated the left anterior descending coronary artery (LAD) using 7-0 silk sutures with a section of PE-10 tubing placed over the LAD for 40 min. After 40-min ischemia, the LAD ligature was released, and then reperfusion was re-established for 24 hours. After 24 hours of reperfusion, mice were euthanized and hearts were harvested for Western blot analysis, real time PCR, and immunostaining. To determine the infarct size and area at risk, after ischemia and reperfusion for 24 hours, mice were subjected to LAD re-ligation and then injected with 1.0% of Evans blue through the carotid artery to define the in vivo area at risk (AAR). Then the heart was excised, fixed, sectioned, and stained with triphenyl tetrazolium chloride (Sigma-Aldrich, T8877). Using NIH software Image J, we determined the left ventricle area, the area of risk, and the infarct size in each heart sections as previously reported.[23,24]
PCO procedures
We performed a left thoracotomy, and then we ligated the LAD using 7-0 silk sutures for 3 weeks.
Echocardiography
Ejection fraction (EF), left ventricular end diastolic dimensions (LVEDd), left ventricular end systolic dimensions (LVESd), left ventricular posterior wall thickness dimensions (LVPWd) were measured in mice, before and 3 weeks after PCO using a Doppler echocardiograph with a 15-MHz linear transducer (Acuson c256, Mountain View, CA).[25]
Collagen determination
A 6-μm heart section was cut from each slice and then stained with Masson’s trichrome. We captured the whole heart images of the sections at low magnification. The ratio of scar length to the heart circumference was determined and expressed as a percentage to define collagen content using NIH software Image J. In control mice without PCO, there was very little collagen and therefore quantification was not performed.
Western blot analysis
Heart tissues were homogenized in RIPA protein extraction buffer buffer (50 mM HEPES, pH 7.5, 150 mM NaCl, 5 mM EDTA, 1% v/v NP-40, and 0.5% w/v deoxycholate), containing a cocktail of protease and phosphatase inhibitors (complete Mini; 11836153001, Roche Diagnostic). Western blot analysis for STIM1 (BD Transduction Laboratories; 610954; 1/1000), ER stress BIP/ATF6/CHOP (Cell Signaling Technology; 3177 and 2895; Santa Cruz Biotechnology; sc22799; 1/1000), caspase 3 (Cell Signaling Technology; 9662; 1/1000 dilution), Nox2/Nox4 (Abcam; ab80508 and ab133303; 1/1000), Orai2/Orai3 (Abcam; ab155216 and ab11558; 1/1000), and β-actin (Santa Cruz Biotechnology; sc47778; 1/1000) was performed as previously reported.[26]
In situ Apoptosis Detection (TUNEL)
The presence of apoptotic cardiomyocytes within the paraffin-embedded heart sections was determined using the DAB substrate In Situ Apoptosis Detection Kit (ab206386), following the manufacturer’s instructions. Data were expressed by the number of positive cells.
Apoptotic cardiomyocytes were detected by terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling (TUNEL) using a kit (TACS® 2 TdT-Fluor In Situ Apoptosis Detection Kit Trevigen, Inc.) as described in manufacturer’s instructions. After TUNEL labeling, sections were counterstained with 4′-6-diamidino-2-phenylindole (DAPI) to detect nuclei.
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity assay and malondialdehyde (MDA)
NADPH oxidase activity was performed as we previously described.[21] Superoxide anion levels generated by NADPH oxidase activity were measured in lysates of hearts using lucigenin chemiluminescence. Briefly, we prepared heart lysates in a sucrose buffer (KH2PO4 50 mM, EGTA 1 mM, sucrose 150 mM; pH=7.0 and the “Complete-C mini” protease inhibitor cocktail (Roche Diagnostics, IN)) in a Tissue Dounce homogenizer on the ice. We immediately used the aliquots of the homogenates. We used 100 μL of each lysate in a total volume of 1 mL PBS buffer preheated at 37°C, containing lucigenin (5 μM) and NADPH (100 μM). Blank samples contained 100 μL of sucrose buffer. We measured Lucigenin activity every 30 seconds for 10 min in a luminometer (Turner biosystem 20/20, single tube luminometer) or till enzymatic activity reached a plateau. Data are expressed as %. NADPH Oxidase activity data were normalized to protein concentration and then expressed as percentage (%) to the control.
Quantitation of reactive carbonyl compounds [Malondialdehyde (MDA)] was determined using the Thiobarbituric Acid (TBA) assay as recommended by the manufacturer protocol (TCA Assay Kit, 700870) and previously described.[27]
Immunohistochemistry
Immunohistochemistry was performed as previously reported.[26] Briefly, formalin-fixed hearts were embedded in paraffin and sectioned. Slides were heated at 58°C for 1 hour. After removal of paraffin, endogenous peroxidase activity was quenched by 5-minute incubation with 3% H2O2 in H2O. Slides were incubated with Citrate buffer (10 mmol/L, pH 6) for 10 minutes at 100°C and cooled to room temperature. After blocking with 5% normal goat serum, slides were incubated overnight with anti-CD68 (Marker for macrophages), anti-Myeloperoxidase (MPO) and anti-STIM1 (Cell Signaling) at 4°C. For every section, a negative control without the first antibody was processed. Sections were washed and incubated with biotinylated secondary antibody for 45 min, and then avidin-peroxidase conjugate (Vector Labs, Burlingame, CA) for 30 min at room temperature. The color reaction was developed with the diaminobenzidine detection kit (Vector Labs) and counterstained with hematoxylin. Image quantification was performed using Metamorph software.
Statistical Analysis
Data are presented as mean ± SEM. The analysis was performed using GraphPad Prism6 software. Statistical calculations for significant differences were performed using Student’s t-test, one-way followed by Post-Hoc test or two-way ANOVA as appropriate. The significance level was set at probability values less than 0.05.
RESULTS
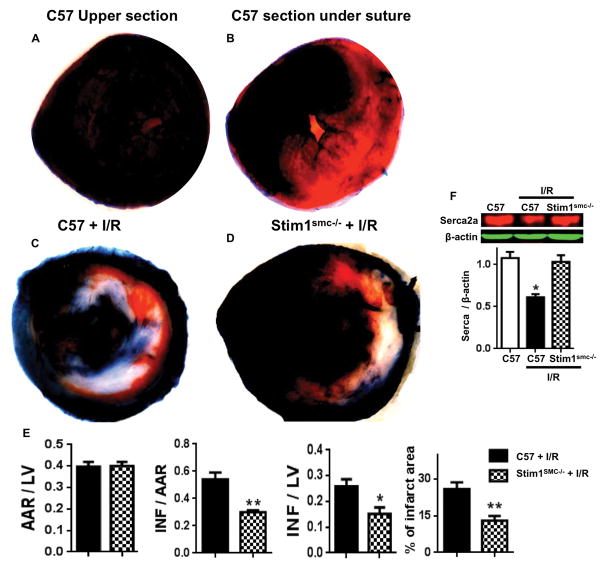
We subjected hearts to ischemia for 30 seconds without any reperfusion. Then immediately we injected Evans blue into the carotid artery. Heart sections above the ligation are well perfused, while heart sections below the suture, half of section is perfused and the other half is not perfused (Figure 1A, B). This procedure does not induce myocardial infarct and it is a control experiment to demonstrate that the coronary artery was completely ligated (Figure 1A, B).
Figure 1.
(A) Representative photographs of TTC stained sections for myocardial infarct in (A–B) C57/Bl6 (C57) subjected to ischemia for 30 seconds only “Upper section of the suture is well perfused” and “section after suture of the coronary is half perfused and half non perfused”; (C–D) photographs of TTC stained sections for myocardial infarct in C57 and Stim1SMC−/− subjected to I/R injury; (E) Area at risk (AAR) by left ventricle (LV) ratio, infarct (INF) by AAR, INF by LV, and % of infarct area; (F) Western blot analysis for Serca2 and β-actin expression in heart from C57 and C57/Stim1SMC−/− subjected to I/R injury. Data are presented by mean±SEM. *P<0.05, **<0.01 for C57 + I/R vs. Stim1SMC−/− + I/R or C57 (n=6).
We subjected the hearts of control and Stim1SMC−/− mice to 40 minutes of ischemia followed by 24 hours of reperfusion. The control mice subjected to cardiac I/R injury displayed a significant myocardial infarct size (around 27% of the left ventricle). Interestingly, Stim1SMC−/− mice subjected to cardiac I/R injury displayed a significant reduction in myocardial infarct size (Figure 1C, D, E). The area at risk was similar between the two groups of mice indicating that the ligation of the coronary was performed at the same site and therefore the hearts were subjected to comparable I/R injury (Figure 1E). The cumulative data show a reduction in myocardial infarct size determined by the area at risk, the left ventricle, and the % of infarct area (Figure 1E). Our data are supported by the recovery in SERCA2A expression (markers for myocardial infarct) in Stim1SMC−/− compared to control mice subjected to the same cardiac I/R injury (Figure 1F).
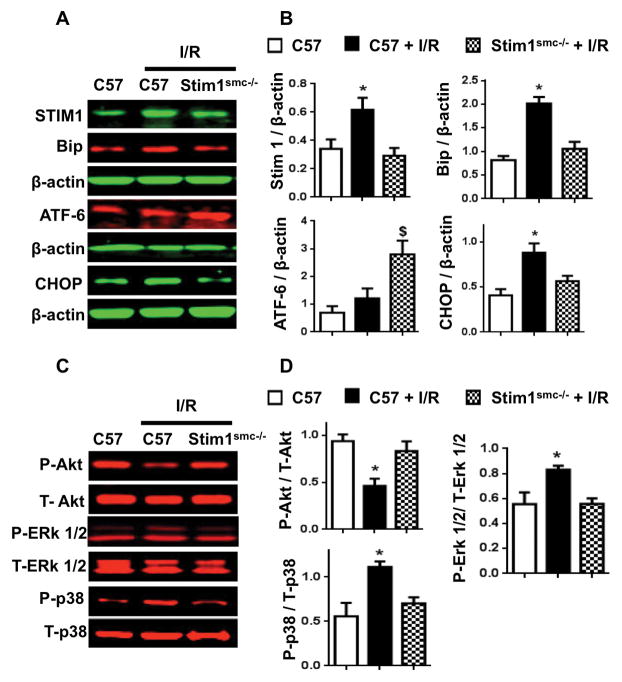
Western blot analysis revealed that STIM1 expression is augmented in the heart of control but blunted in Stim1SMC−/− mice subjected to I/R injury (Figure 2A, B). These data indicate that STIM1 in SMC dictates the expression of STIM1 in the whole heart under I/R injury. The ER stress Bip and CHOP expression were enhanced, while ATF6 expression did not change in the heart of control mice subjected to I/R injury (Figure 2A, B). Interestingly, we observed a reduction in Bip and CHOP expression associated with an increase in ATF6 expression in heart of Stim1SMC−/− mice subjected to I/R injury (Figure 2A, B). These data suggest that the increase in Bip and CHOP are detrimental and likely involved in the induction of myocardial infarct, while ATF6 is protective against the myocardial infarct. The survival pathway Akt assessed with Akt phosphorylation was reduced in control while intact in Stim1SMC−/− subjected to I/R injury (Figure 2C, D). MAP Kinases (ERK1/2 and p38) phosphorylation was increased in the heart of control mice subjected to I/R injury but blunted in Stim1SMC−/− mice (Figure 2C, D). The total Akt, ERK1/2, and p38 expressions were similar between all groups (Figure 2C, D).
Figure 2.
Western blot analysis (A, C) and cumulative data (B, D) for STIM1, Bip, ATF6, CHOP, phosphorylated (P)-Akt, total (T)-Akt, P-ERK1/2, T-ERK1/2, P-p38, T-p38, and β-actin in the heart of C57/Bl6 (C57), C57 + I/R injury, and Stim1SMC−/− + I/R injury, *P<0.05 for C57 + I/R injury vs. Stim1SMC−/− + I/R injury and C57 without I/R injury (n=4–5).
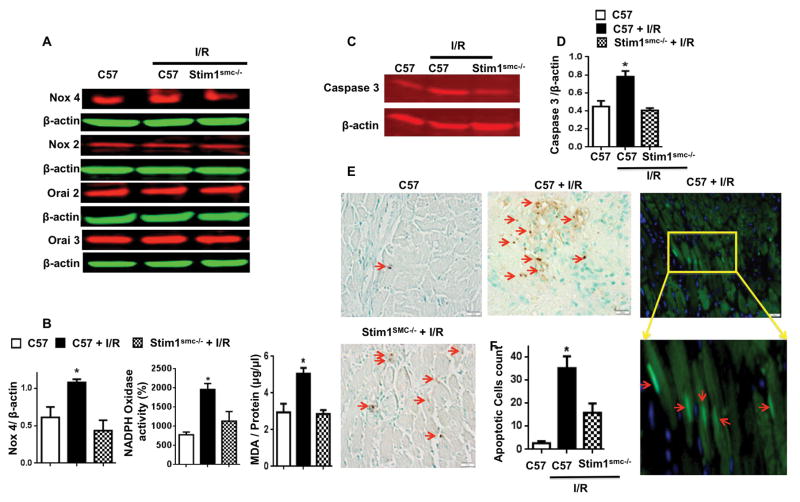
The Oxidative stress is an important factor in cardiovascular disease and augmented during cardiac complications.[28] Our results show an increase in NADPH oxidase activity, likely due to the enhanced Nox4 and other Nox but not Nox2 expression in control mice subjected to cardiac I/R injury (Figure 3A, B). However, NADPH oxidase activity and Nox4 expressions were blunted In Stim1SMC−/− (Figure 3A, B). Also, we found that MDA a product of lipid peroxidation and indicator of oxidative stress is blunted in heart of Stim1SMC−/− after I/R compare to control heart (Figure 3B). The deletion of STIM1 specifically in SMC did not affect the expression of Orai2 and Orai3 in the whole heart (Figure 3A).
Figure 3.
Western blot analysis (A) and cumulative data (B) for Nox4, Nox2, Orai2, Orai 3, and β-actin, and NADPH oxidase activity in the heart of C57/Bl6 (C57), C57 + I/R injury, and Stim1SMC−/− + I/R injury. Western blot analysis (C) and cumulative data (D) for caspase 3 in the heart of C57/Bl6 (C57), C57 + I/R injury, and Stim1SMC−/− + I/R injury; TUNEL-positive nuclei (brown staining and red arrow): Tunnel assay counterstained with methyl green; Representative immunofluorescence images of heart tissue sections stained with terminal deoxynucleotidyl transferase (TUNEL; green and red arrow, DAPI- blue) to detect apoptotic cardiomyocytes (E) and cumulative data (F) in the heart of C57/Bl6 (C57), C57 + I/R injury, and Stim1SMC−/− + I/R injury. *P<0.05 for C57 + I/R injury vs. Stim1SMC−/− + I/R injury and C57 without I/R injury, (n=4–5)
Apoptosis is one of the mechanisms involved in the induction of myocardial infarct.[29] Our results show that caspase 3 activation (Figure 3C, D) and the number of TUNEL positive cells (Figure 3E, F) were reduced in the heart from Stim1SMC−/− mice compared to heart from control mice subjected to I/R injury. The apoptotic cells are originated from the cardiomyocytes as indicated by fluorescent apoptotic assay (Figure 3E).
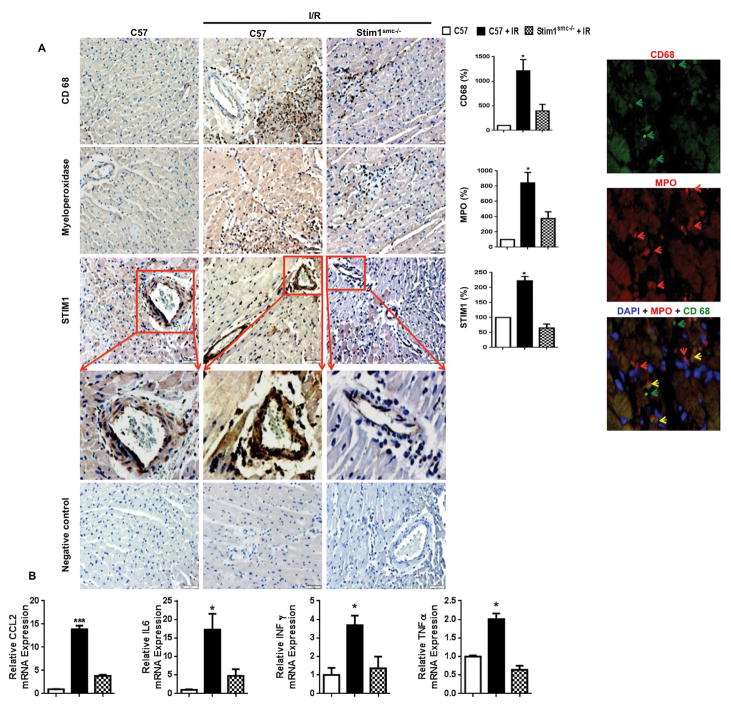
Inflammation is another factor involved in the induction of myocardial infarct.[29] We found an induction of inflammation as indicated by the increase in the infiltration of macrophages (CD68 staining) and neutrophils (Myeloperoxidase staining) into heart subjected to I/R injury from control mice, which was significantly reduced in hearts subjected to I/R injury from Stim1SMC−/− (Figure 4A). Moreover, double staining revealed inflammatory cells population that co-expresses CD68 and MPO markers, and a population that does expresses CD68 or MPO (Figure 4A). We also confirmed that STIM-1 is deleted in the Stim1SMC−/− mice within SMC only (Figure 4A). These data are correlated with STIM1 expression (Figure 4A). Together, our data illustrate the essential role of SMC-STIM1 in myocardial infarct induced by acute I/R injury. Moreover inflammatory and cytokine markers such CC chemokine ligand 2 (CCL2), interferon γ (IFN γ), Interleukin-6 (IL-6) and tumor necrosis factor alpha (TNFα) are significantly reduced in hearts subjected to I/R injury from Stim1SMC−/− compared to control mice subjected to cardiac I/R injury (Figure 4B).
Figure 4.
(A) Representative immunolabeling of paraffin-embedded heart sections stained for macrophages (CD68 specific marker for macrophages), myeloperoxidase, and STIM1 in C57/Bl6 (C57), C57 + I/R injury, and Stim1SMC−/− + I/R injury (n=4–6). The brown dark brown spots indicate immunopositive reaction to CD68, myeloperoxidase, and STIM1 antibodies. (B) Real-Time PCR for CCL2, IL-6, Interferon gamma (INFγ), and Tumor necrosis factor alpha (TNFα). *P<0.05 for C57 + I/R injury vs. Stim1SMC−/− + I/R injury and C57 without I/R injury.
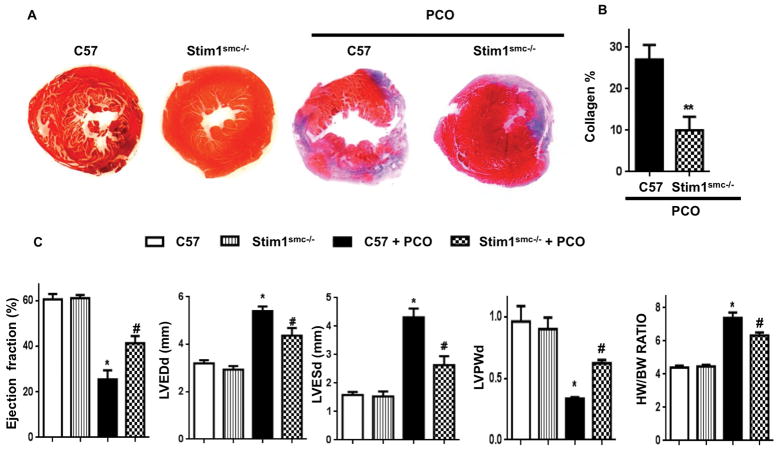
We also showed the role of SMC-STIM1 in a long-term myocardial infarct. We observed that SMC-STIM1 is an important factor in heart failure induced by chronic ischemia. Thus, we found that control mice subjected to PCO developed heart failure characterized by a significant cardiac hypertrophy, myocardial infarct, drop in ejection fraction, increase in collagen content, LVEDd, and LVESd, and reduce LVPWd (Figure 5A, B, C). Importantly, all these factors were protected in Stim1SMC−/− mice subjected to PCO (Figure 5A, B, C). These results indicate that cardiac function was partially protected and SMC-STIM1 play a critical role in heart failure induced by chronic ischemia.
Figure 5.
Representative photographs of Masson’s Trichrome staining for collagen (A) and cumulative data (A) in hearts from C57/Bl6 (C57) and Stim1SMC−/− before (n=4) and after (n=4–5) permanent coronary occlusion (PCO) for 3 weeks; (C) Echocardiographic measurements in control C57/Bl6 (C57) and Stim1SMC−/− before (n=4) and after (n=4–5) PCO [LVEDd: left ventricular end diastolic dimension; LVESd: left ventricular end systolic dimension; HW: heart weight; BW: body weight]. Data are presented by mean±SEM; *p<0.05, **<0.01 for C57 + PCO vs. C57, Stim1SMC−/−, and Stim1SMC−/− + PCO; #p<0.05 for Stim1SMC−/− + PCO vs. C57 and Stim1SMC−/−
DISCUSSION
It has been reported that the increase or deletion of STIM1 specifically in cardiomyocytes induces cardiac complication.[13,14] We previously reported that specific deletion of STIM1 in endothelial cells causes endothelial dysfunction, while specific disruption of STIM1 in SMC does not affect vascular endothelial function.[11] Moreover, we determined that SMC-STIM1 plays a crucial role in the development of hypertension and associated cardiovascular pathologies. Thus, the arterial blood pressure was significantly delayed associated with vascular endothelial function protection in Stim1SMC−/− mice chronically infused with angiotensin II.[21] In the present study, we identified a novel role for SMC-STIM1 as a critical factor in myocardial infarct induced by acute I/R injury and chronic ischemia. Cardiac I/R injury augments STIM1 expression in the whole heart and causes myocardial infarct. Importantly, the deletion of STIM1 specifically in SMC significantly reduced the myocardial infarct size through a mechanism that involves the modulation of MAP-Kinases, Akt, ER stress, oxidative stress, inflammation, and apoptosis. We also found that disruption of STIM1 in SMC protected the heart from chronic ischemia. Thus, it is likely evident that STIM1 role in physio-pathology is cell-type dependent that highlight the critical need to specifically target STIM1 in specific cells for potential therapy. Based on previous[21] and the present studies, we believe that SMC-STIM1 is a novel mechanism to target for cardiovascular protection.
STIM1 is a calcium sensor in the luminal side of the endoplasmic reticulum.[5] Mutations and up-regulation of STIM1 expression have been associated with a variety of pathologies.[9,10,12] We previously reported that deletion of STIM1 specifically in SMC does not affect vascular endothelial function. However, it reduces SMC contractility in response to sympathetic stimulation.[11] The significance of the present study relies on the fact that deletion of STIM1 specifically in SMC (Stim1SMC−/−) protected the heart against acute I/R injury and chronic ischemia. Interestingly, in Stim1SMC−/− subjected to cardiac I/R injury, the heart infarct size was significantly reduced compared to the infarct size in Stim1SMC+/+ mice subjected to I/R injury. Our results are supported by the recovery in SERCA2a expression in Stim1SMC−/− subjected to I/R injury compared to Stim1SMC+/+ subjected to cardiac I/R injury.
ER stress is involved in a variety of metabolic and cardiovascular diseases.[30,31] We previously showed that the inhibition of ER stress pathways improved vascular function in hypertension and diabetes.[18,32,33] Cardiac I/R injury causes an increase in ER stress markers “Bip and CHOP” with no change in ATF6 expression in the heart of Stim1SMC+/+ mice. Interestingly, Glembotski’s laboratory reported that the ER stress ATF6 has a protective role against I/R injury in isolated neonatal rat ventricular myocytes.[34] It would be interesting to selectively delete or overexpress ATF6 in SMC and delineate its role in myocardial infarct induced by I/R injury. The mechanisms, at the cellular level, by which STIM1 deletion specifically in SMC coordinates the ER stress activation in the whole heart and how it is related to the myocardial infarct, are yet to be elucidated.
Inflammation is one the major factor involved in myocardial infarction. Previous clinical and animal studies demonstrated that there is a correlation between increase in CCL2, IFN γ, IL-6 and TNFα levels and myocardial infarction.[35,36] These inflammatory factors are also dictated by STIM1 in SMC as indicated by their reduction in Stim1SMC−/− mice subjected to I/R injury.
Oxidative stress, inflammation, and apoptosis are key factors in the induction of myocardial infarct.[37–39] We illustrated that the deletion of STIM1 specifically in SMC blunted the induction of oxidative stress, inflammation, and apoptosis as indicated by the reduction in the expression of Nox4, the NADPH oxidase activity, the macrophages and neutrophils infiltration, and apoptotic events. Regarding the inflammation, we observed that MPO co-localize with macrophages and some do not as previously reported.[40] The blockade of these pathways is associated with cardiac protection characterized by a significant reduction in myocardial infarct size. However, the cellular and molecular mechanism by which STIM1 in SMC orchestrates the oxidative stress, inflammation, and apoptosis under acute cardiac I/R injury needs to be determined in future studies.
We utilized another model of myocardial infarct induced by cardiac chronic ischemia to determine the role of SMC-STIM1. Thus, the PCO causes heart failure in control C57/Bl6 mice characterized by infarct induction, reduction in ejection fraction and increase in LVEDd and LVESd as previously reported.[41,42] Interestingly, in we found that these echocardiographic measurements were improved in Stim1SMC−/− mice subjected to PCO. These data revealed a novel role for SMC-STIM1 in chronic myocardial infarction.
In summary, the role of SMC-STIM1 as a protein that dictates the myocardial infarct induced by acute I/R injury and PCO is very complex. STIM1 has been shown to play a differential role cells type-dependent. The present study highlights the in vivo contribution and provides proof-of-concept that disruption of SMC-STIM1 protects the heart against acute I/R injury through mechanisms that involves reduction of ERK1/2, oxidative stress, ER stress, apoptosis and inflammation. We also provided evidence that SMC-STIM1 disruption protects the heart against chronic myocardial infarct and heart failure induced by PCO. Therefore, the present study opens new avenues for exploring the cellular interaction and molecular mechanisms by which STIM1 in SMC govern the events of myocardial infarct. Also SMC-STIM1 is a potential target to protect patients with heart complications.
Perspectives and limitation
We previously reported that STIM1 plays differential roles cell-type dependent. Thus, we found that deletion of STIM1 in endothelial cells cause vascular endothelial dysfunction. However, the deletion of STIM1 in SMC does not affect vascular endothelial function but reduced vascular contractility in response to phenylephrine.[11] Our recent publication indicated that disruption of STIM1 in SMC protected the cardiovascular system in mouse chronically infused with angiotensin II.[21] Moreover, it has been reported that deletion or over-expression of STIM1 in cardiomyocytes compromises cardiac structure and function.[13,14] In the present study, we determined that STIM1 in SMC is critical in myocardial infarct induced by acute cardiac I/R injury or chronic cardiac ischemia. Thus, based on our results, we believe that developing a specific inhibitor or local gene therapy that target STIM1 in SMC will benefit patients with cardiovascular complications.
The present manuscript has limitations. First, we do not know how SMCs receive signals in response to acute I/R injury or chronic ischemia. Second, What are the factors dictated by STIM1 in SMC? Third, what are the factors released by SMCStim1−/− that protect the heart against I/R injury and chronic ischemia. These questions are important, as it will help understanding the mechanism of how VSMCs are important in myocardial infarction.
NOVELTY AND SIGNIFICANCE.
What Is Known?
Stromal Interacting Molecule 1 (STIM1) is known to play an important role in coordinating cellular calcium signaling in a variety of cells. STIM1 senses changes in endoplasmic reticulum luminal calcium levels and directly interact with Orai channels located at the plasma membrane to orchestrate the opening of calcium released-activated calcium channels (CRAC). STIM1 plays a critical role in vascular function, vascular complications, and cardiac hypertrophy. However, the role of SMC STIM1 in myocardial infarction is still unknown.
What New Information Does This Article Contribute?
Utilizing a new mouse model in which STIM1 was specifically deleted in SMCs, the results indicate that SMC-STIM1 plays a critical role in the myocardial infarct induced by acute I/R injury and chronic coronary occlusion. The deletion of SMC-STIM1 protects the heart from the adverse effect of acute I/R injury and heart failure. This article also highlights that the cardioprotection of SMC-STIM1 deletion involves the modulation of the endoplasmic reticulum stress, oxidative stress, MAP Kinases, apoptosis and inflammation-dependent mechanism. Overall, these studies identified new role for SMC-STIM1 in myocardial infarction.
Acknowledgments
Sources of funding
This work was supported by the National Institutes of Health (HL095566; PI: Dr. Matrougui) and EVMS fund (Dr. Matrougui)
N/A
Footnotes
Information about previous presentations of the whole or part of the present work
Whole or part of the present work has not been submitted to any academic journals
Conflict of Interest
There is no conflict of interest in the present study
References
- 1.Barrett-Connor E, Orchard TJ. Insulin-dependent diabetes mellitus and ischemic heart disease. Diabetes care. 1985;8(Suppl 1):65–70. doi: 10.2337/diacare.8.1.s65. [DOI] [PubMed] [Google Scholar]
- 2.Jacoby RM, Nesto RW. Acute myocardial infarction in the diabetic patient: pathophysiology, clinical course and prognosis. Journal of the American College of Cardiology. 1992;20(3):736–744. doi: 10.1016/0735-1097(92)90033-j. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, et al. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100(10):1134–1146. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- 4.Trebak M. STIM/Orai signalling complexes in vascular smooth muscle. J Physiol. 2012;590(17):4201–4208. doi: 10.1113/jphysiol.2012.233353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruhle B, Trebak M. Emerging roles for native Orai Ca2+ channels in cardiovascular disease. Curr Top Membr. 2013;71:209–235. doi: 10.1016/B978-0-12-407870-3.00009-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdullaev IF, Bisaillon JM, Potier M, Gonzalez JC, Motiani RK, Trebak M. Stim1 and Orai1 mediate CRAC currents and store-operated calcium entry important for endothelial cell proliferation. Circ Res. 2008;103(11):1289–1299. doi: 10.1161/01.RES.0000338496.95579.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motiani RK, Hyzinski-Garcia MC, Zhang X, Henkel MM, Abdullaev IF, Kuo YH, et al. STIM1 and Orai1 mediate CRAC channel activity and are essential for human glioblastoma invasion. Pflugers Arch. 2013 doi: 10.1007/s00424-013-1254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amin A, Choi SK, Galan M, Kassan M, Partyka M, Kadowitz P, et al. Chronic inhibition of endoplasmic reticulum stress and inflammation prevents ischaemia-induced vascular pathology in type II diabetic mice. J Pathol. 2012;227(2):165–174. doi: 10.1002/path.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shinde AV, Motiani RK, Zhang X, Abdullaev IF, Adam AP, Gonzalez-Cobos JC, et al. STIM1 controls endothelial barrier function independently of Orai1 and Ca2+ entry. Sci Signal. 2013;6(267):ra18. doi: 10.1126/scisignal.2003425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang W, Halligan KE, Zhang X, Bisaillon JM, Gonzalez-Cobos JC, Motiani RK, et al. Orai1-mediated I (CRAC) is essential for neointima formation after vascular injury. Circ Res. 2011;109(5):534–542. doi: 10.1161/CIRCRESAHA.111.246777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kassan M, Zhang W, Aissa KA, Stolwijk J, Trebak M, Matrougui K. Differential role for stromal interacting molecule 1 in the regulation of vascular function. Pflugers Arch. 2014 doi: 10.1007/s00424-014-1556-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi SK, Lim M, Yeon SI, Lee YH. Inhibition of endoplasmic reticulum stress improves coronary artery function in type 2 diabetic mice. Exp Physiol. 2016;101(6):768–777. doi: 10.1113/EP085508. [DOI] [PubMed] [Google Scholar]
- 13.Benard L, Oh JG, Cacheux M, Lee A, Nonnenmacher M, Matasic DS, et al. Cardiac Stim1 Silencing Impairs Adaptive Hypertrophy and Promotes Heart Failure Through Inactivation of mTORC2/Akt Signaling. Circulation. 2016;133(15):1458–1471. doi: 10.1161/CIRCULATIONAHA.115.020678. discussion 1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Correll RN, Goonasekera SA, van Berlo JH, Burr AR, Accornero F, Zhang H, et al. STIM1 elevation in the heart results in aberrant Ca(2)(+) handling and cardiomyopathy. J Mol Cell Cardiol. 2015;87:38–47. doi: 10.1016/j.yjmcc.2015.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perez-Ilzarbe M, Agbulut O, Pelacho B, Ciorba C, San Jose-Eneriz E, Desnos M, et al. Characterization of the paracrine effects of human skeletal myoblasts transplanted in infarcted myocardium. Eur J Heart Fail. 2008;10(11):1065–1072. doi: 10.1016/j.ejheart.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Chimenti I, Smith RR, Li TS, Gerstenblith G, Messina E, Giacomello A, et al. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106(5):971–980. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsunaga S, Okigaki M, Takeda M, Matsui A, Honsho S, Katsume A, et al. Endothelium-targeted overexpression of constitutively active FGF receptor induces cardioprotection in mice myocardial infarction. J Mol Cell Cardiol. 2009;46(5):663–673. doi: 10.1016/j.yjmcc.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Kassan M, Galan M, Partyka M, Saifudeen Z, Henrion D, Trebak M, et al. Endoplasmic reticulum stress is involved in cardiac damage and vascular endothelial dysfunction in hypertensive mice. Arterioscler Thromb Vasc Biol. 2012;32(7):1652–1661. doi: 10.1161/ATVBAHA.112.249318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glembotski CC. Roles for ATF6 and the sarco/endoplasmic reticulum protein quality control system in the heart. J Mol Cell Cardiol. 2014;71:11–15. doi: 10.1016/j.yjmcc.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vekich JA, Belmont PJ, Thuerauf DJ, Glembotski CC. Protein disulfide isomerase-associated 6 is an ATF6-inducible ER stress response protein that protects cardiac myocytes from ischemia/reperfusion-mediated cell death. J Mol Cell Cardiol. 2012;53(2):259–267. doi: 10.1016/j.yjmcc.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kassan M, Ait-Aissa K, Radwan E, Mali V, Haddox S, Gabani M, et al. Essential Role of Smooth Muscle STIM1 in Hypertension and Cardiovascular Dysfunction. Arterioscler Thromb Vasc Biol. 2016;36(9):1900–1909. doi: 10.1161/ATVBAHA.116.307869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma J, McCarl CA, Khalil S, Luthy K, Feske S. T-cell-specific deletion of STIM1 and STIM2 protects mice from EAE by impairing the effector functions of Th1 and Th17 cells. Eur J Immunol. 2010;40(11):3028–3042. doi: 10.1002/eji.201040614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calvert JW, Gundewar S, Jha S, Greer JJ, Bestermann WH, Tian R, et al. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes. 2008;57(3):696–705. doi: 10.2337/db07-1098. [DOI] [PubMed] [Google Scholar]
- 24.Gundewar S, Calvert JW, Elrod JW, Lefer DJ. Cytoprotective effects of N,N,N-trimethylsphingosine during ischemia-reperfusion injury are lost in the setting of obesity and diabetes. Am J Physiol Heart Circ Physiol. 2007;293(4):H2462–2471. doi: 10.1152/ajpheart.00392.2007. [DOI] [PubMed] [Google Scholar]
- 25.Peng H, Xu J, Yang XP, Dai X, Peterson EL, Carretero OA, et al. Thymosin-beta4 prevents cardiac rupture and improves cardiac function in mice with myocardial infarction. Am J Physiol Heart Circ Physiol. 2014;307(5):H741–751. doi: 10.1152/ajpheart.00129.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kassan M, Choi SK, Galan M, Bishop A, Umezawa K, Trebak M, et al. Enhanced NF-kappaB Activity Impairs Vascular Function Through PARP-1-, SP-1-, and COX-2-Dependent Mechanisms in Type 2 Diabetes. Diabetes. 2013;62(6):2078–2087. doi: 10.2337/db12-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dawn-Linsley M, Ekinci FJ, Ortiz D, Rogers E, Shea TB. Monitoring thiobarbituric acid-reactive substances (TBARs) as an assay for oxidative damage in neuronal cultures and central nervous system. J Neurosci Methods. 2005;141(2):219–222. doi: 10.1016/j.jneumeth.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Octavia Y, Brunner-La Rocca HP, Moens AL. NADPH oxidase-dependent oxidative stress in the failing heart: From pathogenic roles to therapeutic approach. Free Radic Biol Med. 2012;52(2):291–297. doi: 10.1016/j.freeradbiomed.2011.10.482. [DOI] [PubMed] [Google Scholar]
- 29.Zernecke A, Bernhagen J, Weber C. Macrophage migration inhibitory factor in cardiovascular disease. Circulation. 2008;117(12):1594–1602. doi: 10.1161/CIRCULATIONAHA.107.729125. [DOI] [PubMed] [Google Scholar]
- 30.Hou Y, Xue P, Woods CG, Wang X, Fu J, Yarborough K, et al. Association between arsenic suppression of adipogenesis and induction of CHOP10 via the endoplasmic reticulum stress response. Environ Health Perspect. 2013;121(2):237–243. doi: 10.1289/ehp.1205731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodrigo R, Prieto JC, Castillo R. Cardioprotection against ischaemia/reperfusion by vitamins C and E plus n-3 fatty acids: molecular mechanisms and potential clinical applications. Clin Sci (Lond) 2013;124(1):1–15. doi: 10.1042/CS20110663. [DOI] [PubMed] [Google Scholar]
- 32.Galan M, Kassan M, Choi SK, Partyka M, Trebak M, Henrion D, et al. A novel role for epidermal growth factor receptor tyrosine kinase and its downstream endoplasmic reticulum stress in cardiac damage and microvascular dysfunction in type 1 diabetes mellitus. Hypertension. 2012;60(1):71–80. doi: 10.1161/HYPERTENSIONAHA.112.192500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galan M, Kassan M, Kadowitz PJ, Trebak M, Belmadani S, Matrougui K. Mechanism of endoplasmic reticulum stress-induced vascular endothelial dysfunction. Biochim Biophys Acta. 2014;1843(6):1063–1075. doi: 10.1016/j.bbamcr.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chao W. Toll-like receptor signaling: a critical modulator of cell survival and ischemic injury in the heart. Am J Physiol Heart Circ Physiol. 2009;296(1):H1–12. doi: 10.1152/ajpheart.00995.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frangogiannis NG, Dewald O, Xia Y, Ren G, Haudek S, Leucker T, et al. Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation. 2007;115(5):584–592. doi: 10.1161/CIRCULATIONAHA.106.646091. [DOI] [PubMed] [Google Scholar]
- 36.Levick SP, Goldspink PH. Could interferon-gamma be a therapeutic target for treating heart failure? Heart Fail Rev. 2014;19(2):227–236. doi: 10.1007/s10741-013-9393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burke AP, Virmani R. Pathophysiology of acute myocardial infarction. Med Clin North Am. 2007;91(4):553–572. ix. doi: 10.1016/j.mcna.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Johns TN, Blalock A. Mitral insufficiency: the experimental use of a mobile polyvinyl sponge prosthesis. Ann Surg. 1954;140(3):335–341. doi: 10.1097/00000658-195409000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kubli DA, Gustafsson AB. Mitochondria and mitophagy: the yin and yang of cell death control. Circ Res. 2012;111(9):1208–1221. doi: 10.1161/CIRCRESAHA.112.265819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugiyama S, Okada Y, Sukhova GK, Virmani R, Heinecke JW, Libby P. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am J Pathol. 2001;158(3):879–891. doi: 10.1016/S0002-9440(10)64036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rafiq K, Kolpakov MA, Seqqat R, Guo J, Guo X, Qi Z, et al. c-Cbl inhibition improves cardiac function and survival in response to myocardial ischemia. Circulation. 2014;129(20):2031–2043. doi: 10.1161/CIRCULATIONAHA.113.007004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao X, Balaji P, Pachon R, Beniamen DM, Vatner DE, Graham RM, et al. Overexpression of Cardiomyocyte alpha1A-Adrenergic Receptors Attenuates Postinfarct Remodeling by Inducing Angiogenesis Through Heterocellular Signaling. Arterioscler Thromb Vasc Biol. 2015;35(11):2451–2459. doi: 10.1161/ATVBAHA.115.305919. [DOI] [PMC free article] [PubMed] [Google Scholar]