Abstract
Substantial progress has been made in the therapeutic reduction of vertebral fracture risk in patients with osteoporosis, but non-vertebral fracture risk has been improved only marginally. Human genetic studies demonstrate that the WNT16 locus is a major determinant of cortical bone thickness and non-vertebral fracture risk and mouse models with life-long Wnt16 inactivation revealed that WNT16 is a key regulator of cortical thickness. These studies, however, could not exclude that the effect of Wnt16 inactivation on cortical thickness might be caused by early developmental and/or growth effects. To determine the effect of WNT16 specifically on adult cortical bone homeostasis, Wnt16 was conditionally ablated in young adult and old mice through tamoxifen-inducible Cre-mediated recombination using CAG-Cre-ER; Wnt16flox/flox (Cre-Wnt16flox/flox) mice. First, 10-week-old Cre-Wnt16flox/flox and Wnt16flox/flox littermate control mice were treated with tamoxifen. Four weeks later, Wnt16 mRNA levels in cortical bone were reduced and cortical thickness in femur was decreased in Cre-Wnt16flox/flox mice compared to Wnt16flox/flox mice. Then, inactivation of Wnt16 in 47-week-old mice (evaluated four weeks later) resulted in a reduction of Wnt16 mRNA levels, cortical thickness and cortical bone strength with no effect on trabecular bone volume fraction. Mechanistic studies demonstrated that the reduced cortical bone thickness was caused by a combination of increased bone resorption and reduced periosteal bone formation. In conclusion, WNT16 is a crucial regulator of cortical bone thickness in young adult and old mice. We propose that new treatment strategies targeting the adult regulation of WNT16 might be useful to reduce fracture risk at cortical bone sites.
Keywords: cortical thickness, WNT16, tamoxifen, transgenic
Introduction
Osteoporosis affects hundreds of millions of people worldwide and fragility fractures cause enormous problems particularly for postmenopausal women and older men (Baron & Hesse 2012). Cortical bone is a key determinant of bone strength and non-vertebral fracture risk (Zebaze et al. 2010, Ohlsson et al. 2017). Currently available osteoporosis treatments mainly affect the trabecular bone reducing the risk of vertebral fractures by up to 70% while non-vertebral fracture risk has been improved only marginally by currently available treatments, defining an unmet medical need (Cummings et al. 2009, Baron & Hesse 2012). It is, therefore, of high clinical importance to increase the knowledge of the regulation of cortical bone mass.
Several large scale human genetic studies have demonstrated that the WNT16 locus is reproducibly associated with cortical bone thickness, bone mineral density and non-vertebral fractures (Estrada et al. 2012, Medina-Gomez et al. 2012, Zheng et al. 2012, Garcia-Ibarbia et al. 2013, Koller et al. 2013, Hendrickx et al. 2014). Subsequent translational studies using global as well as cell-specific Wnt16 inactivation in mice demonstrated that osteoblast-derived WNT16 regulates cortical bone thickness and non-vertebral fracture susceptibility (Moverare-Skrtic et al. 2014). Osteoblast-derived WNT16 affects cortical bone by inhibiting cortical osteoclast formation both by inhibiting RANK-signaling in osteoclast progenitor cells and by enhancing Opg expression in osteoblasts (Moverare-Skrtic et al. 2014). In addition, it has been described that WNT16 may also regulate periosteal bone formation rate (Wergedal et al. 2015).
Although very informative, the previous experimental studies using mouse models with life-long global or cell-specific Wnt16 inactivation could not exclude the possibility that the effect of Wnt16 inactivation on cortical bone thickness might be caused by early developmental effects (Moverare-Skrtic et al. 2014, Wergedal et al. 2015). These studies, therefore, could not separate between developmental effects of WNT16 and its effects on adult bone metabolism. As we want to determine the possible usefulness of WNT16 as an osteoporosis drug target, it is crucial to determine if WNT16 exerts important effects on cortical bone homeostasis in adult and old mice. Thus, if WNT16 only would have an effect during early development, but not in adult or old mice, this would mean that WNT16 never will become be an interesting osteoporosis drug target as osteoporosis treatment is given to relatively old subjects. Therefore, to evaluate the effect of WNT16 specifically on adult cortical bone homeostasis, we developed a mouse model with tamoxifen-inducible efficient global Wnt16 inactivation (Hayashi & McMahon 2002) and determined the effects of WNT16 on cortical bone mass in young adult and old mice. This mouse model is more similar to a systemic modulation of WNT16 activity than the previous cell-specific mouse models that we have used for detailed mechanistic studies of the effect of WNT16 on cortical bone. In these previous studies, we used Runx2-Cre and Dmp1-Cre mouse models to demonstrate that osteoblast-derived WNT16 contributes to the regulation of cortical bone thickness (Moverare-Skrtic et al. 2014). To evaluate WNT16 as a possible osteoporosis drug target, one has to consider not only osteoblast-derived WNT16 but also global WNT16 expression that may exert off target side effects, arguing for the use of an inducible global Wnt16 inactivation mouse model in the present study.
Materials and methods
Animals
Generation of Wnt16 conditional knockout mice (Wnt16flox/flox) on C57BL/6N background has been described recently (Moverare-Skrtic et al. 2014). Briefly, exon 3 of the Wnt16 gene is flanked by loxP sites in these Wnt16flox/flox mice. The following primer pairs were used for genotyping of the presence or absence of the loxP sequence: 5′-CATAAAGCCAGCTGCACTGC-3′ and 5′-AAATGTGTAACCTTCACGAG-3′. To be able to delete the floxed sequence of the Wnt16 gene in an inducible manner, we used B6.Cg-Tg(CAG-cre/Esr1*)5Amc/J (CAGGCre-ER) transgenic mice expressing a tamoxifen-inducible Cre-mediated recombination system (#004682, Jackson Laboratories (Hayashi & McMahon 2002)). To generate tamoxifen-inducible knockout mice, Wnt16flox/flox female mice were mated with CAGGCre-ER-Wnt16+/flox male mice. The tamoxifen-inducible offsprings, CAGGCre-ER-Wnt16flox/flox mice, were called Cre-Wnt16flox/flox. The control mice were littermate Wnt16flox/flox mice without CAGGCre-ER expression. The mice were housed in a standard animal facility under controlled temperature (22°C) and photoperiod (12 h of light and 12 h of darkness) and had free access to water and food pellets (RM1A, SDS Diet, UK). All experimental procedures involving animals were approved by the Ethics Committee at the University of Gothenburg and carried out in accordance with relevant guidelines.
Tamoxifen treatment
Tamoxifen (T5648, Sigma-Aldrich) was dissolved in ethanol at a concentration of 100 mg/mL and further diluted in corn oil (C8267, Sigma-Aldrich) to a concentration of 10 mg/mL. The tamoxifen suspension was administered to the Cre-Wnt16flox/flox mice and their Wnt16flox/flox littermate control mice by intraperitoneal injections during four consecutive days. Ten-week-old male mice were given 0.25 mg/mouse/day or 1 mg/mouse/day, whereas 47-week-old female mice were given 1 mg/mouse/day. Four weeks after the first tamoxifen injection, blood was collected from the axillary vein under anesthesia with Ketanest/Dexdomitor vet (Pfizer/Orion Pharma AB, Animal Health, Sollentuna, Sweden) and the mice were subsequently killed by cervical dislocation. The long bones, vertebrae and soft tissues were dissected and stored for further analyses. In addition, to determine the effect of inducible Wnt16 inactivation on cortical bone thickness in young adult female mice, 7-week-old female Cre-Wnt16flox/flox and littermate Wnt16flox/flox mice were given tamoxifen (1 mg/mouse/day) during four consecutive days and killed at 16 weeks of age.
Assessment of bone parameters
X-ray analyses
Computed tomography (CT) scans of the cortical mid-diaphyseal and trabecular epiphyseal tibia and femur were performed using pQCT XCT Research M (version 4.5B; Norland) as described previously (Windahl et al. 1999, Vidal et al. 2000). High-resolution µCT (μCT) analyses were further performed on the femur and lumbar vertebra L5 (Moverare-Skrtic et al. 2014) following the guidelines of the American Society for Bone and Mineral Research (Bouxsein et al. 2010). Femur and vertebra were imaged with an X-ray tube voltage of 50 kV, a current of 201 μA and with a 0.5-mm aluminum filter. The scanning angular rotation was 180°, and the angular increment was 0.70°. The voxel size was 4.49 µm isotropically. NRecon (version 1.6.9) was used to perform the reconstruction after the scans. In the present study, we compared the effect of inducible Wnt16 inactivation on cortical vs trabecular bone. To this end, we selected bone locations with essentially only cortical bone (mid-diaphyseal region of femur) or only trabecular bone (the inner part of the vertebral body as defined below) for µCT analyses. Cortical measurements using µCT were performed in the mid-diaphyseal region of femur starting at a distance of 5.2 mm from the distal growth plate and extending a further longitudinal distance of 134 µm in the proximal direction. In mice, this region of the femur has essentially no trabecular bone. The average cortical bone thickness is given. For trabecular bone measurements, not including cortical bone in the vertebra, the trabecular bone in the vertebral body caudal of the pedicles was selected for analysis within a conforming volume of interest (cortical bone excluded) commencing at a distance of 4.5 µm caudal of the lower end of the pedicles, and extending a further longitudinal distance of 328 µm in the caudal direction.
Static and dynamic bone histomorphometry
Femurs of the 51-week-old female mice were analyzed by PharmaTest Services, Ltd. as described previously (Moverare-Skrtic et al. 2014). Briefly, the mice were injected (i.p.) on day 4 (alizarin) and day 18 (calcein) before sacrifice. The femurs were fixed in 4% paraformaldehyde, dehydrated in 70% EtOH and embedded in methyl methacrylate. The femurs were sectioned in the transverse plane and unstained 200 μm-thick sections were analyzed for static and dynamic parameters. All parameters were measured using the OsteoMeasure histomorphometry system (OsteoMetrics, Decatur, GA, USA) following the guidelines of the American Society for Bone and Mineral Research (Dempster et al. 2013).
Biomechanical strength analyses
Humerus was loaded by three-point bending test using a mechanical testing machine (Instron 3366, Instron, Norwood, MA, USA) (Wu et al. 2016). The loading speed was 0.155 mm/s and the span length was 4.5 mm. Based on the computer recorded load deformation raw data curves, produced by Bluehill 2 software v2.6 (Instron), the results were calculated with custom-made Excel macros.
Real-time PCR
RNA was isolated from gonadal fat, liver and cortical bone (femur) using TRIzol reagent (Sigma) followed by the RNeasy Mini Kit (Qiagen). Amplifications were performed using the StepOnePlus Real-Time PCR System (PE Applied Biosystems) using Assay-on-Demand primer and probe sets (PE Applied Biosystems), labeled with the reporter fluorescent dye FAM. Predesigned primers and a probe labeled with the reporter fluorescent dye VIC, specific for 18S ribosomal RNA, were included in the reaction as an internal standard. The assay identification numbers were Wnt16 Mm00446420_m1, Catepsin K (CatK) Mm00484036_m1, Opg (osteoprotegerin, Tnfrsf11b) Mm00435452_m1, and Rankl (Tnfsf11) Mm00441908_m1.
Power calculation, blinding of investigators and randomization of mouse samples
The predesigned primary endpoint in the mouse studies was to record the effect of inducible Wnt16 inactivation on cortical bone thickness. Our power analysis suggested that when using eight WT and eight Wnt16-inactivated mice, we would have 80% power to detect a biological significant effect with a 1.51 s.d. change in cortical bone thickness at a two-sided alpha level of 0.05. Therefore, we aimed to use at least eight mice per group in the different mouse studies. All in vivo experiments and subsequent assessments of the outcomes from these experiments were done in totally blinding of the investigators. No experiments requiring randomization of sample groups were performed.
Statistical analyses
Values are given as mean ± s.e.m. The statistical difference between Cre-Wnt16flox/flox and Wnt16flox/flox mice was calculated using Student’s t test. If data were not normally distributed, they were log-transformed before statistical analyses. Pearson’s correlation coefficient (r) was calculated between cortical thickness and Wnt16 mRNA expression. A P value of <0.05 was considered statistically significant.
Results
Inducible inactivation of the Wnt16 gene
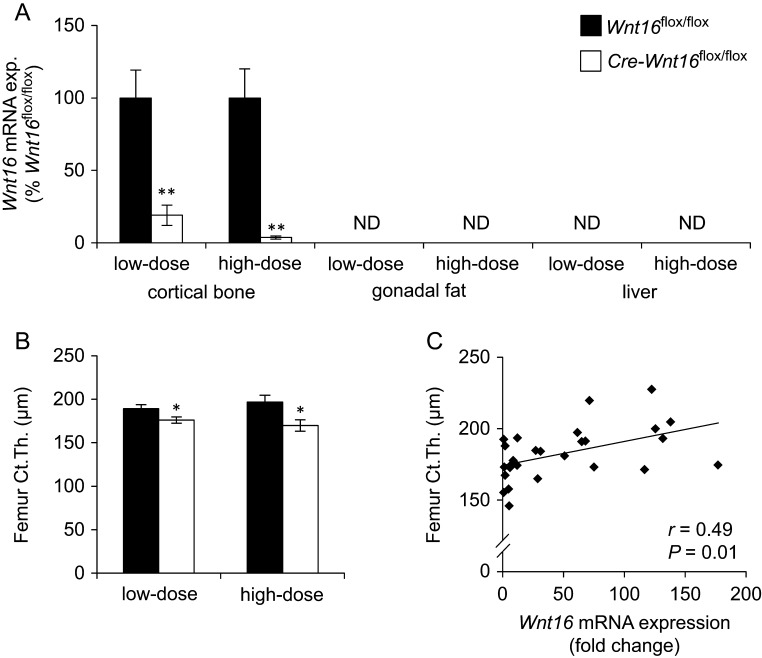
Our expression analyses confirmed that Wnt16 is abundantly expressed in cortical bone, whereas no detectable expression was observed in gonadal fat or liver (Fig. 1A). To assess the importance of WNT16 for the regulation of cortical bone thickness in adult mice, we developed a mouse model with inducible inactivation of Wnt16. To this end, we bred our recently developed mouse model having exon 3 of Wnt16 flanked by loxP sites (Wnt16flox/flox (Moverare-Skrtic et al. 2014)) with CAGGCre-ER transgenic mice (Hayashi & McMahon 2002) expressing a tamoxifen-inducible Cre-mediated recombination system. When comparing Cre-Wnt16flox/flox mice with Wnt16flox/flox littermate mice, we observed that the presence of the tamoxifen-inducible Cre-mediated recombination system without tamoxifen treatment did not influence the skeleton (Supplementary Table 1, see section on supplementary data given at the end of this article). Next, we evaluated the ability of tamoxifen to delete the floxed exon 3 of the Wnt16 gene in young adult mice with the tamoxifen-inducible Cre recombinase. Two different doses of tamoxifen were administered daily i.p. for four consecutive days to 10-week-old Cre-Wnt16flox/flox and Wnt16flox/flox mice, and the mice were examined four weeks later. The low-dose (0.25 mg/mouse/day) and high-dose (1 mg/mouse/day) tamoxifen treatment reduced the Wnt16 mRNA levels in cortical bone by 80.9 ± 7.0% (P < 0.01) and 96.3 ± 1.1% (P < 0.01), respectively in Cre-Wnt16flox/flox mice compared with Wnt16flox/flox mice (Fig. 1A). The inducible inactivation of Wnt16 did not affect body weight, weights of liver or gonadal fat, or the lengths of femur or tibia (Table 1).
Figure 1.
Inducible inactivation of Wnt16 in young adult male mice reduces cortical bone thickness. Fourteen-week-old male Cre-Wnt16flox/flox (n = 7) and littermate Wnt16flox/flox (n = 6) control mice treated for four consecutive days with either low-dose (0.25 mg/mouse/day) or high-dose (1 mg/mouse/day) tamoxifen at the age of ten weeks. (A) Expression level of Wnt16 mRNA in cortical bone of femur, gonadal fat and liver. (B) Cortical bone thickness of femur as analyzed using μCT. ND, not detectable. Values are given as mean ± s.e.m. (Wnt16flox/flox low-dose, n = 6, high-dose n = 7; Cre-Wnt16flox/flox low and high dose, n = 7). *P < 0.05, **P < 0.01, Student’s t test, Cre-Wnt16flox/flox vs Wnt16flox/flox control mice. (C) Correlation between Wnt16 mRNA levels in cortical bone of femur and cortical bone thickness in the femur diaphysis. Pearson’s correlation coefficient is given.
Table 1.
Body characteristics of young adult male Cre-Wnt16flox/flox and Wnt16flox/flox mice.
| Low-dose tamoxifen | High-dose tamoxifen | |||
|---|---|---|---|---|
| Wnt16flox/flox (n = 6) | Cre-Wnt16flox/flox (n = 7) | Wnt16flox/flox (n = 7) | Cre-Wnt16flox/flox (n = 7) | |
| Body weight (g) | 31.1 ± 0.5 | 30.1 ± 1.4 | 29.2 ± 1.3 | 32.0 ± 1.8 |
| Liver weight/body weight (%) | 4.30 ± 0.08 | 4.37 ± 0.09 | 4.45 ± 0.14 | 4.87 ± 0.16 |
| Gonadal fat weight/body weight (%) | 1.95 ± 0.24 | 2.20 ± 0.35 | 1.87 ± 0.33 | 2.56 ± 0.38 |
| Femur length (mm) | 16.4 ± 0.11 | 16.0 ± 0.20 | 16.3 ± 0.17 | 16.2 ± 0.18 |
| Tibia length (mm) | 19.0 ± 0.06 | 18.7 ± 0.11 | 19.0 ± 0.08 | 18.7 ± 0.14 |
| Tibia cortical thickness (μm) | 232 ± 7 | 216 ± 4* | 228 ± 6 | 199 ± 7** |
Body characteristics of 14-week-old Cre-Wnt16flox/flox and Wnt16flox/flox male mice treated with low-dose (0.25 mg/mouse/day) or high-dose (1 mg/mouse/day) tamoxifen during four consecutive days at the age of 10 weeks. Values are given as mean ± s.e.m.
*P < 0.056, **P < 0.01, Student’s t test, Cre-Wnt16flox/flox vs Wnt16flox/flox control mice.
Inducible inactivation of Wnt16 in young adult mice reduces cortical bone thickness
We next evaluated if the inducible Wnt16 inactivation affected cortical bone thickness in the long bones of young adult mice. Both inducible Wnt16 inactivation using low-dose (−7.0 ± 1.9%; P < 0.05) and high-dose (−13.7 ± 3.3%; P < 0.05) tamoxifen reduced the cortical bone thickness in the mid-diaphyseal region of the femur in male Cre-Wnt16flox/flox mice compared with male Wnt16flox/flox mice (Fig. 1B). Cortical cross-sectional bone area was also reduced by inducible Wnt16 inactivation (Supplementary Table 2). A similar reduction of the cortical bone thickness by inducible Wnt16 inactivation was observed in the tibia (low-dose tamoxifen, −7.0 ± 1.7%, P = 0.056; high-dose tamoxifen, −12.6 ± 3.0%, P < 0.01) of male Cre-Wnt16flox/flox mice compared with male Wnt16flox/flox mice (Table 1). Interestingly, femur cortical bone thickness was directly associated with the Wnt16 mRNA expression levels in cortical bone of femur (Pearson’s r = 0.49, P = 0.01) when evaluated in all mice, supporting a role of WNT16 in the regulation of cortical bone thickness (Fig. 1C). The finding that inducible Wnt16 inactivation reduced cortical bone thickness in young adult male mice was replicated in young adult female mice. Inducible Wnt16 inactivation reduced femur cortical thickness (−18.7 ± 6.4%; P < 0.01) in 16-week-old female Cre-Wnt16flox/flox mice compared with female Wnt16flox/flox mice.
Analyses of the trabecular bone in the lumbar vertebrae L5 demonstrated that the reduced cortical bone thickness in young adult mice with inducible inactivation of Wnt16 was not associated with reduced trabecular bone volume fraction (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th) or trabecular separation (Tb.Sp) (Table 2). In contrast, the high-dose but not the low-dose tamoxifen treatment modestly increased trabecular BV/TV in male Cre-Wnt16flox/flox mice compared with male Wnt16flox/flox mice (Table 2).
Table 2.
Trabecular bone characteristics of lumbar vertebra L5 of tamoxifen-treated Cre-Wnt16flox/flox and Wnt16flox/flox mice.
| Wnt16flox/flox | Cre-Wnt16flox/flox | |
|---|---|---|
| 14-week-old male mice (low-dose tamoxifen) | n = 6 | n = 7 |
| Bone volume/total volume (BV/TV; %) | 29.5 ± 1.6 | 27.8 ± 0.6 |
| Trabecular thickness (Tb.Th; µm) | 48.7 ± 1.1 | 46.7 ± 0.9 |
| Trabecular number (Tb.N; /mm) | 6.1 ± 0.3 | 6.0 ± 0.1 |
| Trabecular separation (Tb.Sp; µm) | 123 ± 5.7 | 125 ± 2.9 |
| 14-week-old male mice (high-dose tamoxifen) | n = 6 | n = 7 |
| Bone volume/total volume (BV/TV; %) | 25.7 ± 1.1 | 30.0 ± 1.3* |
| Trabecular thickness (Tb.Th; µm) | 46.8 ± 1.9 | 49.6 ± 1.1 |
| Trabecular number (Tb.N; /mm) | 5.3 ± 0.3 | 6.0 ± 0.2 |
| Trabecular separation (Tb.Sp; µm) | 141 ± 5.0 | 126 ± 4.6 |
| 51-week-old female mice (high-dose tamoxifen) | n = 11 | n = 9 |
| Bone volume/total volume (BV/TV; %) | 24.8 ± 2.9 | 20.6 ± 2.2 |
| Trabecular thickness (Tb.Th; µm) | 54.2 ± 2.1 | 55.1 ± 1.7 |
| Trabecular number (Tb.N; /mm) | 4.6 ± 0.6 | 3.8 ± 0.5 |
| Trabecular separation (Tb.Sp; µm) | 86.2 ± 5.1 | 90.9 ± 5.2 |
Trabecular bone µCT analyses of lumbar vertebra L5 in 14-week-old Cre-Wnt16flox/flox and Wnt16flox/flox male mice treated with low-dose (0.25 mg/mouse/day) or high-dose (1 mg/mouse/day) tamoxifen during four consecutive days at 10 weeks of age and 51-week-old Cre-Wnt16flox/flox and Wnt16flox/flox female mice treated with high-dose (1 mg/mouse/day) tamoxifen during four consecutive days at the age of 47 weeks. Values are given as mean ± s.e.m.
*P < 0.05, Student’s t test, Cre-Wnt16flox/flox vs Wnt16flox/flox control mice.
Inducible inactivation of Wnt16 in older female mice reduces cortical bone thickness
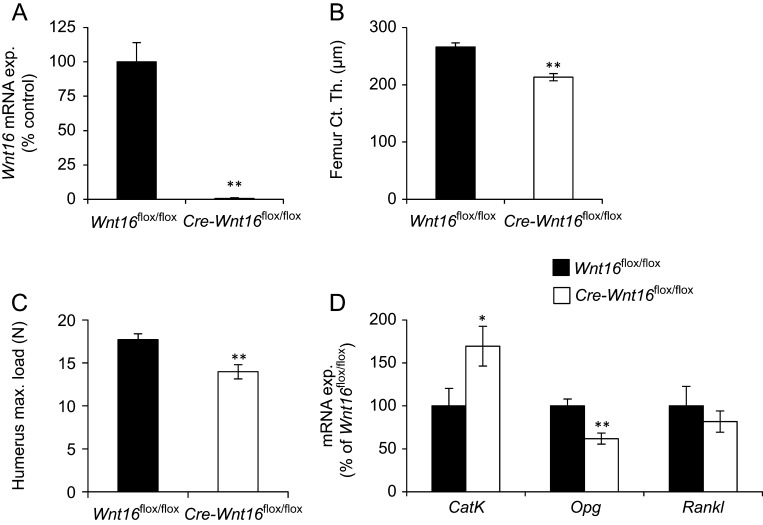
We next evaluated if WNT16 regulates cortical bone thickness also in older mice. For inducible Wnt16 inactivation, both Cre-Wnt16flox/flox and Wnt16flox/flox mice were treated by tamoxifen (1 mg/mouse/day, i.p. for four consecutive days) four weeks before killing at 51 weeks of age. Tamoxifen treatment almost completely inactivated Wnt16 expression in cortical bone (−99.3 ± 0.4%; P < 0.01) in the Cre-Wnt16flox/flox mice compared with Wnt16flox/flox mice (Fig. 2A). The inducible Wnt16 inactivation did not affect body weight, weights of liver or gonadal fat or the lengths of femur or tibia (Table 3). In contrast, the inducible Wnt16 inactivation reduced the cortical thickness of femur (−19.8 ± 2.4%, P < 0.01; Fig. 2B) and tibia as analyzed by CT (−14.5 ± 3.2%, P < 0.01; Table 3) in the Cre-Wnt16flox/flox mice compared with Wnt16flox/flox mice. Cortical cross-sectional bone area was also reduced in the femur by inducible Wnt16 inactivation (Supplementary Table 3). Static histomorphometric analyses of femur confirmed a reduced cortical bone area (−17.6 ± 6.6%, P < 0.05) and cortical thickness (−17.0 ± 5.5%, P < 0.05) in the Cre-Wnt16flox/flox mice compared with Wnt16flox/flox mice (Table 4). In contrast, no significant change was observed for total bone area or marrow cavity area (Table 4 and Supplementary Table 3). Three-point bending analysis of the humerus diaphysis revealed a substantial reduction (−21.1 ± 4.6%; P < 0.05) in maximal load at failure in the tamoxifen-treated Cre-Wnt16flox/flox mice as compared to the tamoxifen-treated Wnt16flox/flox mice, demonstrating that the decreased cortical bone thickness reduced the bone strength (Fig. 2C). The inducible Wnt16 inactivation did not affect trabecular bone parameters in the older female mice (Table 2).
Figure 2.
Inducible inactivation of Wnt16 in older female mice reduces cortical bone thickness. 51-week-old female Cre-Wnt16flox/flox and littermate Wnt16flox/flox control mice treated with tamoxifen (1 mg/mouse/day) for four consecutive days at 47 weeks of age. (A) Wnt16 mRNA levels in cortical diaphyseal bone of femur. (B) Cortical thickness of the mid-diaphysis of femur as analyzed using µCT and (C) maximal (max.) load at failure determined by three-point bending of humerus. (D) mRNA levels of CatK, Opg and Rankl in cortical diaphyseal bone of femur. Values are given as mean ± s.e.m. (Wnt16flox/flox n = 11; Cre-Wnt16flox/flox n = 9). **P < 0.01, Student’s t test, Cre-Wnt16flox/flox vs Wnt16flox/flox control mice.
Table 3.
Body characteristics of old female tamoxifen-treated Cre-Wnt16flox/flox and Wnt16flox/flox mice.
| Wnt16flox/flox (n = 11) | Cre-Wnt16flox/flox (n = 9) | |
|---|---|---|
| Body weight (g) | 33.7 ± 1.4 | 31.3 ± 2.9 |
| Liver weight/body weight (%) | 3.54 ± 0.36 | 3.74 ± 0.64 |
| Gonadal fat weight/body weight (%) | 5.43 ± 0.58 | 4.64 ± 0.87 |
| Femur | ||
| Bone length (mm) | 16.6 ± 0.11 | 16.8 ± 0.11 |
| Periosteal circumference (mm) | 4.82 ± 0.08 | 4.62 ± 0.11 |
| Endosteal circumference (mm) | 3.15 ± 0.09 | 3.28 ± 0.10 |
| Tibia | ||
| Bone length (mm) | 18.8 ± 0.11 | 18.8 ± 0.12 |
| Periosteal circumference (mm) | 4.20 ± 0.05 | 4.09 ± 0.10 |
| Endosteal circumference (mm) | 2.59 ± 0.06 | 2.71 ± 0.10 |
| Cortical thickness (µm) | 256 ± 9 | 219 ± 8** |
Body characteristics of 51-week-old female Cre-Wnt16flox/flox and Wnt16flox/flox mice treated with tamoxifen (1 mg/mouse/day) during four consecutive days at the age of 47 weeks. Values are given as mean ± s.e.m.
**P < 0.01, Student’s t test, Cre-Wnt16flox/flox vs Wnt16flox/flox control mice.
Table 4.
Histomorphometric analyses of cortical bone of old female tamoxifen-treated Cre-Wnt16flox/flox and Wnt16flox/flox mice.
| Wnt16flox/flox (n = 11) | Cre-Wnt16flox/flox (n = 8) | |
|---|---|---|
| Static histomorphometry | ||
| Total bone area (B.Ar; mm2) | 1.90 ± 0.09 | 1.77 ± 0.10 |
| Marrow cavity area (Ma.Ar; mm2) | 0.79 ± 0.05 | 0.85 ± 0.06 |
| Cortical bone area (Ct.Ar; mm2) | 1.12 ± 0.05 | 0.92 ± 0.07* |
| Cortical width (Ct.Wi; mm) | 0.28 ± 0.01 | 0.23 ± 0.01* |
| Dynamic histomorphometry | ||
| Periosteal histomorphometry | ||
| Mineral surface/bone surface (MS/BS; %) | 47.5 ± 6.3 | 28.7 ± 3.0* |
| Mineral apposition rate (MAR; µm/day) | 0.82 ± 0.08 | 0.64 ± 0.10 |
| Bone formation rate (BFR; mm3/mm2/year) | 153 ± 32 | 71.6 ± 18* |
| Endocortical histomorphometry | ||
| Mineral surface/bone surface (MS/BS; %) | 83.7 ± 5.9 | 64.7 ± 7.4 |
| Mineral apposition rate (MAR; µm/day) | 1.20 ± 0.09 | 1.33 ± 0.14 |
| Bone formation rate (BFR; mm3/mm2/year) | 374 ± 49 | 334 ± 61 |
Histomorphometric analyses of femur cortical bone of 51-week-old female Cre-Wnt16flox/flox and Wnt16flox/flox mice treated with tamoxifen at the age of 47 weeks. Values are given as mean ± s.e.m.
*P < 0.05, Student’s t test, Cre-Wnt16flox/flox vs Wnt16flox/flox control mice.
Wnt16 regulates both periosteal bone formation and bone resorption in older female mice
To investigate the mechanisms by which inducible Wnt16 inactivation reduces cortical bone thickness in older female mice, dynamic histomorphometric analyses of femur were performed (Table 4). Inducible Wnt16 inactivation decreased periosteal bone formation rate (BFR; −53.1 ± 11.8%, P < 0.05), mainly due to reduced mineralized bone surface (MS/BS; −39.6 ± 6.3%, P < 0.05) (Table 4). In contrast, at the endocortical surface, bone formation was not affected by inducible Wnt16 inactivation (Table 4). Similar to what previously have been described for global life-long Wnt16 inactivation and osteoblast-lineage-specific Wnt16 inactivation (Moverare-Skrtic et al. 2014), the mRNA levels of the anti-osteoclastogenic factor Opg in cortical bone were reduced by inducible Wnt16 inactivation (Fig. 2D). In addition, inducible Wnt16 inactivation significantly increased mRNA levels of the osteoclast marker CatK in cortical bone (+69.5 ± 23.1%, P < 0.05; Fig. 2D).
Discussion
Cortical bone mass is a major determinant of bone strength and non-vertebral fracture risk (Zebaze et al. 2010, Ohlsson et al. 2017). Although recent human genetic association studies and experimental studies using mouse models with life-long inactivation of Wnt16 have established that WNT16 is a crucial regulator of cortical bone thickness and non-vertebral fracture risk (Estrada et al. 2012, Medina-Gomez et al. 2012, Zheng et al. 2012, Garcia-Ibarbia et al. 2013, Koller et al. 2013, Hendrickx et al. 2014, Moverare-Skrtic et al. 2014), it has not been possible to determine if WNT16 exerts its effect on cortical bone mainly during development and growth or if WNT16 also is crucial for adult cortical bone homeostasis. We, herein, developed an inducible Wnt16 inactivated mouse model and demonstrated that WNT16 exerts important effects on cortical bone homeostasis both in young adult and old mice.
In the present study, we developed an inducible mouse model where the Wnt16 gene is normally expressed until it is inactivated by tamoxifen treatment (Hayashi & McMahon 2002). To this end, we used an inducible Cre-loxP transgenic system where the Cre-ER fusion protein is sequestered in the cytoplasm. In the presence of tamoxifen, Cre-ER translocates to the nucleus and drives recombination of the floxed target gene (Feil et al. 1997). Although this Cre transgenic system is well studied and known to have low background Cre activity in the absence of an inducer, previous reports indicate that the use of the tamoxifen-inducible Cre-loxP system is not without potential drawbacks (Manolagas & Kronenberg 2014, Jardí et al. 2017, Patel et al. 2017). Using correct controls are therefore fundamental. In this study, we demonstrated that the Cre recombinase expressed by the transgenic mice is not able to inactivate the floxed Wnt16 gene before tamoxifen is administered, validating the model to be inducible. The efficiency of the recombination are affected by many parameters such as the particular genomic location, the distance between the loxP sites and the ability of tamoxifen to reach different target organs (Feil et al. 2009). Using cell-specific Wnt16 inactivation, we have previously demonstrated that WNT16 in cortical bone is osteoblast derived and that the effect of WNT16 on cortical bone thickness is completely mediated by osteoblast-derived WNT16 (Moverare-Skrtic et al. 2014). Therefore, to determine the role of WNT16 for adult cortical bone metabolism, it is important to achieve an efficient inducible inactivation of Wnt16 in cortical bone, and in the present study, the efficiency of recombination of the Wnt16 gene in the cortical bone was almost complete when induced by tamoxifen treatment both in young adult and in old mice.
Tamoxifen, used for the inducible inactivation of Wnt16 in the present study, is a selective estrogen receptor modulator (SERM) that has been reported to affect the skeleton (Perry et al. 2005, Zhong et al. 2015). The possible confounding effects of tamoxifen on the skeleton were avoided by the fact that the control (Wnt16flox/flox) mice received the same dose of tamoxifen as the inducible Wnt16-knockout mice (Cre-Wnt16flox/flox) and, in addition, there was a 3.5-week wash-out period between the last tamoxifen treatment and the analyses of the skeletal phenotype. However, we cannot completely exclude the possibility that the tamoxifen treatment might have blunted or confounded some of the effects of WNT16 on bone metabolism in the present study.
The risk of osteoporosis and osteoporosis-related fractures is increasing by age and it is, therefore, of importance that candidate osteoporosis drug targets should be functional also during aging. A drawback with life-long gene inactivation using standard knockout mouse models is that it is difficult to separate effects on development and growth from effects being crucial also during aging. As we aimed to determine the possible usefulness of WNT16 as an osteoporosis drug target, it was crucial to determine if WNT16 exerts important effects on cortical bone homeostasis in adult and old mice. Thus, if WNT16 would only have had an effect during early development but not in adult mice, this would mean that WNT16 never will be an interesting osteoporosis drug target as the osteoporosis treatment should be effective in relatively old patients with osteoporosis.
WNT16 belongs to the WNT protein family and some WNTs are established regulators of skeletal development (Baron & Kneissel 2013). WNT signaling has been reported to affect all aspects of skeletal development, including craniofacial, limb and joint formation. In addition, mutations in several members of the WNT signaling pathways result in skeletal malformations in humans and mice (Balemans et al. 2001, 2002, Brunkow et al. 2001, Staehling-Hampton et al. 2002, Loots et al. 2005, Kim et al. 2011, Laine et al. 2013) and based on these findings, we could not exclude that the effect of WNT16 on the skeleton in previous studies also may be dependent on early developmental effects. As the Wnt16 inactivation used in the present study was inducible, it was possible to determine the role of WNT16 specifically for adult cortical bone homeostasis both in young adult (14-week-old) and in old (51-week-old) mice. We clearly demonstrated that WNT16 is crucial for cortical bone homeostasis both in young adult and old mice, supporting the notion that treatment strategies targeting the regulation of WNT16 might be useful to reduce fracture risk at cortical bone sites in old patients with osteoporosis. In addition, we demonstrated that inducible Wnt16 inactivation reduced cortical bone thickness in both young adult male and young adult female mice, suggesting that WNT16 is a crucial regulator of adult cortical bone thickness in both male and female mice.
Previous studies using several different mouse models with life-long global or cell-specific Wnt16 inactivation have collectively shown that osteoblast-derived WNT16 is a crucial regulator of cortical bone thickness and cortical bone strength when evaluated before sexual maturation and at young adult age (Zheng et al. 2012, Moverare-Skrtic et al. 2014, Wergedal et al. 2015). In the present study, we observed a very similar cortical bone phenotype of reduced cortical bone thickness and strength when Wnt16 was inactivated as late as in nearly one-year-old mice. Thus, WNT16 is a crucial regulator of cortical bone homeostasis during the entire lifespan in mice. In the present study, we also observed that cortical bone thickness was directly associated with the Wnt16 mRNA expression levels in cortical bone of femur supporting a role of WNT16 in the regulation of cortical bone thickness.
In contrast to the reduction of cortical bone thickness, the trabecular bone volume fraction was not decreased in the young adult or old mice with inducible Wnt16 inactivation. Inducible Wnt16 inactivation did not affect trabecular bone volume fraction in old mice while it actually modestly increased this parameter in young adult mice given high dose tamoxifen treatment. One may speculate that, in an attempt to maintain overall bone strength, the reduction in cortical bone thickness resulted in a compensatory increase in trabecular bone volume fraction in young adult mice.
The reduced cortical bone thickness in the old Wnt16-inactivated mice was associated with parameters reflecting increased bone resorption (increased CatK and reduced Opg mRNA levels in cortical bone) and reduced periosteal bone formation, supporting previous studies using mouse models with life-long global inactivation of Wnt16 (Moverare-Skrtic et al. 2014, Wergedal et al. 2015). The periosteal bone formation was reduced as a result of a reduction in mineralized bone surface while mineral apposition rate was unaffected in mice with inducible Wnt16 inactivation, suggesting a lower number of periosteal cells rather than a lower activity per cell.
In conclusion, WNT16 is a crucial regulator of cortical bone thickness in young adult and old mice. We propose that new treatment strategies targeting the adult regulation of WNT16 might be useful to reduce fracture risk at cortical bone sites.
Supplementary Material
Funding
This study was supported by the Swedish Research Council, the Swedish Foundation for Strategic Research, the European Calcified Tissue Society, the ALF/LUA research grant from the Sahlgrenska University Hospital, the IngaBritt and Arne Lundberg Foundation, the Torsten and Ragnar Söderberg’s Foundation, the Knut and Alice Wallenberg Foundation and the Novo Nordisk Foundation.
Acknowledgements
The authors thank Lotta Uggla, Biljana Aleksic and Anette Hansevi for excellent technical assistance.
References
- Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, Lacza C, Wuyts W, Van Den Ende J, Willems P, et al 2001. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Human Molecular Genetics 10 537–543. ( 10.1093/hmg/10.5.537) [DOI] [PubMed] [Google Scholar]
- Balemans W, Patel N, Ebeling M, Van Hul E, Wuyts W, Lacza C, Dioszegi M, Dikkers FG, Hildering P, Willems PJ, et al 2002. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. Journal of Medical Genetics 39 91–97. ( 10.1136/jmg.39.2.91) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R, Hesse E. 2012. Update on bone anabolics in osteoporosis treatment: rationale, current status, and perspectives. Journal of Clinical Endocrinology and Metabolism 97 311–325. ( 10.1210/jc.2011-2332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R, Kneissel M. 2013. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nature Medicine 19 179–192. ( 10.1038/nm.3074) [DOI] [PubMed] [Google Scholar]
- Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. 2010. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. Journal of Bone and Mineral Research 25 1468–1486. ( 10.1002/jbmr.141) [DOI] [PubMed] [Google Scholar]
- Brunkow ME, Gardner JC, Van Ness J, Paeper BW, Kovacevich BR, Proll S, Skonier JE, Zhao L, Sabo PJ, Fu Y, et al 2001. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. American Journal of Human Genetics 68 577–589. ( 10.1086/318811) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, et al 2009. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. New England Journal of Medicine 361 756–765. ( 10.1056/NEJMoa0809493) [DOI] [PubMed] [Google Scholar]
- Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM. 2013. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. Journal of Bone and Mineral Research 28 2–17. ( 10.1002/jbmr.1805) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada K, Styrkarsdottir U, Evangelou E, Hsu YH, Duncan EL, Ntzani EE, Oei L, Albagha OM, Amin N, Kemp JP, et al 2012. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nature Genetics 44 491–501. ( 10.1038/ng.2249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil R, Wagner J, Metzger D, Chambon P. 1997. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochemical and Biophysical Research Communications 237 752–757. ( 10.1006/bbrc.1997.7124) [DOI] [PubMed] [Google Scholar]
- Feil S, Valtcheva N, Feil R. 2009. Inducible Cre mice. Methods in Molecular Biology 530 343–363. ( 10.1007/978-1-59745-471-1_18) [DOI] [PubMed] [Google Scholar]
- Garcia-Ibarbia C, Perez-Nunez MI, Olmos JM, Valero C, Perez-Aguilar MD, Hernandez JL, Zarrabeitia MT, Gonzalez-Macias J, Riancho JA. 2013. Missense polymorphisms of the WNT16 gene are associated with bone mass, hip geometry and fractures. Osteoporosis International 24 2449–2454. ( 10.1007/s00198-013-2302-0) [DOI] [PubMed] [Google Scholar]
- Hayashi S, McMahon AP. 2002. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Developmental Biology 244 305–318. ( 10.1006/dbio.2002.0597) [DOI] [PubMed] [Google Scholar]
- Hendrickx G, Boudin E, Fijalkowski I, Nielsen TL, Andersen M, Brixen K, Van Hul W. 2014. Variation in the Kozak sequence of WNT16 results in an increased translation and is associated with osteoporosis related parameters. Bone 59 57–65. ( 10.1016/j.bone.2013.10.022) [DOI] [PubMed] [Google Scholar]
- Jardí F, Laurent MR, Dubois V, Khalil R, Deboel L, Schollaert D, Van Den Bosch L, Decallonne B, Carmeliet G, Claessens F, et al 2017. A shortened tamoxifen induction scheme to induce CreER recombinase without side effects on the male mouse skeleton. Molecular and Cellular Endocrinology 452 57–63. ( 10.1016/j.mce.2017.05.012) [DOI] [PubMed] [Google Scholar]
- Kim SJ, Bieganski T, Sohn YB, Kozlowski K, Semenov M, Okamoto N, Kim CH, Ko AR, Ahn GH, Choi YL, et al 2011. Identification of signal peptide domain SOST mutations in autosomal dominant craniodiaphyseal dysplasia. Human Genetics 129 497–502. ( 10.1007/s00439-011-0947-3) [DOI] [PubMed] [Google Scholar]
- Koller DL, Zheng HF, Karasik D, Yerges-Armstrong L, Liu CT, McGuigan F, Kemp JP, Giroux S, Lai D, Edenberg HJ, et al 2013. Meta-analysis of genome-wide studies identifies WNT16 and ESR1 SNPs associated with bone mineral density in premenopausal women. Journal of Bone and Mineral Research 28 547–558. ( 10.1002/jbmr.1796) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine CM, Joeng KS, Campeau PM, Kiviranta R, Tarkkonen K, Grover M, Lu JT, Pekkinen M, Wessman M, Heino TJ, et al 2013. WNT1 mutations in early-onset osteoporosis and osteogenesis imperfecta. New England Journal of Medicine 368 1809–1816. ( 10.1056/NEJMoa1215458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loots GG, Kneissel M, Keller H, Baptist M, Chang J, Collette NM, Ovcharenko D, Plajzer-Frick I, Rubin EM. 2005. Genomic deletion of a long-range bone enhancer misregulates sclerostin in Van Buchem disease. Genome Research 15 928–935. ( 10.1101/gr.3437105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolagas SC, Kronenberg HM. 2014. Reproducibility of results in preclinical studies: a perspective from the bone field. Journal of Bone and Mineral Research 29 2131–2140. ( 10.1002/jbmr.2293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Gomez C, Kemp JP, Estrada K, Eriksson J, Liu J, Reppe S, Evans DM, Heppe DH, Vandenput L, Herrera L, et al 2012. Meta-analysis of genome-wide scans for total body BMD in children and adults reveals allelic heterogeneity and age-specific effects at the WNT16 locus. PLoS Genetics 8 e1002718 ( 10.1371/journal.pgen.1002718) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moverare-Skrtic S, Henning P, Liu X, Nagano K, Saito H, Borjesson AE, Sjogren K, Windahl SH, Farman H, Kindlund B, et al 2014. Osteoblast-derived WNT16 represses osteoclastogenesis and prevents cortical bone fragility fractures. Nature Medicine 20 1279–1288. ( 10.1038/nm.3654) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson C, Sundh D, Wallerek A, Nilsson M, Karlsson M, Johansson H, Mellstrom D, Lorentzon M. 2017. Cortical bone area predicts incident fractures independently of areal bone mineral density in older men. Journal of Clinical Endocrinology and Metabolism 102 516–524. ( 10.1210/jc.2016-3177) [DOI] [PubMed] [Google Scholar]
- Patel SH, O'Hara L, Atanassova N, Smith SE, Curley MK, Rebourcet D, Darbey AL, Gannon AL, Sharpe RM, Smith LB. 2017. Low-dose tamoxifen treatment in juvenile males has long-term adverse effects on the reproductive system: implications for inducible transgenics. Scientific Reports 7 8991 ( 10.1038/s41598-017-09016-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry MJ, Gujra S, Whitworth T, Tobias JH. 2005. Tamoxifen stimulates cancellous bone formation in long bones of female mice. Endocrinology 146 1060–1065. ( 10.1210/en.2004-1114) [DOI] [PubMed] [Google Scholar]
- Staehling-Hampton K, Proll S, Paeper BW, Zhao L, Charmley P, Brown A, Gardner JC, Galas D, Schatzman RC, Beighton P, et al 2002. A 52-kb deletion in the SOST-MEOX1 intergenic region on 17q12-q21 is associated with van Buchem disease in the Dutch population. American Journal of Medical Genetics 110 144–152. ( 10.1002/ajmg.10401) [DOI] [PubMed] [Google Scholar]
- Wergedal JE, Kesavan C, Brommage R, Das S, Mohan S. 2015. Role of WNT16 in the regulation of periosteal bone formation in female mice. Endocrinology 156 1023–1032. ( 10.1210/en.2014-1702) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal O, Lindberg MK, Hollberg K, Baylink DJ, Andersson G, Lubahn DB, Mohan S, Gustafsson JA, Ohlsson C. 2000. Estrogen receptor specificity in the regulation of skeletal growth and maturation in male mice. PNAS 97 5474–5479. ( 10.1073/pnas.97.10.5474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windahl SH, Vidal O, Andersson G, Gustafsson JA, Ohlsson C. 1999. Increased cortical bone mineral content but unchanged trabecular bone mineral density in female ERbeta(−/−) mice. Journal of Clinical Investigation 104 895–901. ( 10.1172/JCI6730) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Moverare-Skrtic S, Borjesson AE, Lagerquist MK, Sjogren K, Windahl SH, Koskela A, Grahnemo L, Islander U, Wilhelmson AS, et al 2016. Enzalutamide reduces the bone mass in the axial but not the appendicular skeleton in male mice. Endocrinology 157 969–977. ( 10.1210/en.2015-1566) [DOI] [PubMed] [Google Scholar]
- Zebaze RM, Ghasem-Zadeh A, Bohte A, Iuliano-Burns S, Mirams M, Price RI, Mackie EJ, Seeman E. 2010. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet 375 1729–1736. ( 10.1016/S0140-6736(10)60320-0) [DOI] [PubMed] [Google Scholar]
- Zheng HF, Tobias JH, Duncan E, Evans DM, Eriksson J, Paternoster L, Yerges-Armstrong LM, Lehtimaki T, Bergstrom U, Kahonen M, et al 2012. WNT16 influences bone mineral density, cortical bone thickness, bone strength, and osteoporotic fracture risk. PLoS Genetics 8 e1002745 ( 10.1371/journal.pgen.1002745) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong ZA, Sun W, Chen H, Zhang H, Lay YA, Lane NE, Yao W. 2015. Optimizing tamoxifen-inducible Cre/loxp system to reduce tamoxifen effect on bone turnover in long bones of young mice. Bone 81 614–619. ( 10.1016/j.bone.2015.07.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a