Abstract
Objectives:
Crohn’s disease (CD) and ulcerative colitis (UC) are inflammatory bowel diseases (IBD) that compromise quality of life and may increase mortality. This study compared the mortality risk with prolonged corticosteroid use vs. antitumor necrosis factor-α (anti-TNF) drugs in IBD.
Methods:
A retrospective cohort study was conducted among Medicaid and Medicare beneficiaries from 2001 to 2013 with IBD prescribed either >3,000 mg of prednisone or equivalent within a 12-month period or new initiation of anti-TNF therapy, each treated as time-updating exposures. The primary outcome was all-cause mortality. Secondary outcomes included common causes of death. Marginal structural models were used to determine odds ratios (ORs) and 95% confidence intervals (CIs) for anti-TNF use relative to corticosteroids.
Results:
Among patients with CD, 7,694 entered the cohort as prolonged corticosteroid users and 1,879 as new anti-TNF users. Among patients with UC, 3,224 and 459 entered the cohort as prolonged CS users and new anti-TNF users, respectively. The risk of death was statistically significantly lower in patients treated with anti-TNF therapy for CD (21.4 vs. 30.1 per 1,000 person-years, OR 0.78, 0.65–0.93) but not for UC (23.0 vs. 30.9 per 1,000 person-years, OR 0.87, 0.63–1.22). Among the CD cohort, anti-TNF therapy was also associated with lower rates of major adverse cardiovascular events (OR 0.68, 0.55–0.85) and hip fracture (OR 0.54, 0.34–0.83).
Conclusions:
Compared with prolonged corticosteroid exposure, anti-TNF drug use was associated with reduced mortality in patients with CD that may be explained by lower rates of major adverse cardiovascular events and hip fracture.
Introduction
Inflammatory bowel diseases (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), affect nearly 1.5 million Americans and 3 million Europeans, with peak incidence in the second and third decades of life (1, 2, 3, 4, 5, 6). These chronic diseases reduce quality of life and overall life expectancy (7, 8, 9).
Corticosteroids (CSs) and antitumor necrosis factor-α (anti-TNF) drugs are the main therapies used to rapidly control IBD symptoms. Anti-TNF therapy use is usually chronic, whereas CSs are typically used intermittently. However, recurrence of symptoms upon weaning CSs can result in prolonged or repeated CS therapy. Neither therapy is 100% effective for IBD.
Randomized trial data directly comparing a strategy of intermittent CS therapy vs. induction and maintenance anti-TNF therapy are limited (10). However, the evidence base for efficacy is greater for anti-TNF drugs (11, 12, 13, 14) and these drugs sometimes allow for CS discontinuation (15, 16, 17). Nonetheless, CSs, alone or in combination with other medications, remain commonly used to treat IBD, in part because of fear of adverse effects from anti-TNF drugs (18, 19, 20).
Serious adverse events differ between intermittent CS use and anti-TNFs. Examples of uncommon but potentially fatal adverse reactions that have been associated with CSs and/or anti-TNF include serious infections (both drug classes) (21, 22, 23, 24, 25, 26), congestive heart failure (both drug classes) (27, 28), cancer (with anti-TNF) (29, 30), osteoporosis and fractures (with CSs) (31, 32), and pulmonary embolus (with CSs) (33). However, poorly controlled disease may also increase the risk of death and serious complications, further complicating the choice between therapies (9, 24).
Several prior studies have observed an increased risk of death among patients taking CSs for IBD (9, 34). The relationship between anti-TNF therapy and mortality is less clear. A recent group randomized trial compared protocol-driven early combined immunosuppression (anti-TNF plus an immunomodulator drug) vs. nonprotocol-driven usual care for patients with CD who had active symptoms after 4 weeks of CSs (35). This trial observed a 32% lower mortality rate in the practices following the protocol-driven use of anti-TNF therapy, but this was not statistically significant, perhaps because of the small number of deaths. In contrast, Fidder et al. (36, 37) observed a 1.9-fold increased, but not statistically significant, risk of death with anti-TNF therapy. The TREAT registry is the largest study addressing the safety of anti-TNF therapy and did not observe an increased or decreased risk of death with anti-TNF therapy, but the TREAT population was composed largely of prevalent anti-TNF users who may have already survived the period of highest mortality risk, i.e., when patients are most ill and are initially starting anti-TNF therapy (34). None of these studies specifically compared mortality rates among anti-TNF-treated patients to that of CS-treated patients. Moreover, prior studies have not addressed subpopulations such as the elderly or those with more comorbid illness who might preferentially benefit from one therapy vs. another. To address these questions, and recognizing the many potential harmful effects that different treatment strategies may be associated with, a comparative safety study was conducted of CS vs. anti-TNF therapy for IBD with death as the primary outcome and select serious adverse events as secondary outcomes.
Methods
A retrospective cohort study was conducted among US Medicare and Medicaid beneficiaries with IBD. Medicare Parts A and B cover medically necessary services and supplies, whereas Part D covers pharmacy benefits, including injectable medications for adults age ≥65 years and for individuals with certain disabilities and chronic diseases (9). Medicaid is a similar program for low-income residents. This study used Medicaid data from 2001 to 2005 and Medicare data from 2006 to 2013.
Inclusion criteria
Patients who had been treated with CSs within the prior year and subsequently received either additional CS therapy meeting the definition of prolonged CS use or newly initiated anti-TNF therapy were included in the study. New initiation of anti-TNF therapy was defined as at least 1 dispensing for an anti-TNF drug (infliximab, adalimumab, certolizumab pegol) with at least 1 filled CS prescription and no dispensing for any anti-TNF medication in the 12 months preceding the first anti-TNF dispensing. Prolonged CS use was defined as either >3,000 mg of prednisone (or equivalent) or >600 mg of budesonide divided between ≥2 prescriptions within 12 months and absence of any anti-TNF therapy during the same 12 months (see Supplementary Methods online for further details). The index date was the first date that a patient met the criteria for either prolonged CS use or new initiation of anti-TNF therapy.
The following exclusions were applied using the index date as the reference: age <18 or >90 years, <2 prior physician encounters with the diagnoses of IBD, indistinguishable IBD subtype (defined as an equal number of diagnoses of CD and UC before the index date, the diagnosis on or immediately before the index date was not the same as the most frequent of the two diagnoses before that time, or a diagnosis of fistula or ostomy any time before the index date in a patient who otherwise would be classified as having UC), <12 months of enrollment data before the index date, diagnosis of cancer other than non-melanoma skin cancer in the 12 months before the index date, and physician diagnosis before the index date with any of the following: rheumatoid arthritis, psoriasis, psoriatic arthritis, ankylosing spondylitis, systemic lupus erythematosus, Paget’s disease of bone, asthma, chronic obstructive pulmonary disease, AIDS, multiple sclerosis, or metastatic cancer. The UC cohort was limited to patients who met the entry criteria in 2007 or later as anti-TNF drugs were approved for UC in 2006.
Outcome measures
The primary outcome was all-cause mortality that was based on eligibility data recorded in the Medicare or Medicaid files (38). In addition, the following events were included as secondary outcome measures: major adverse cardiovascular event (MACE), including acute myocardial infarction, stroke, sudden death, or the need for revascularization; hip fracture, pulmonary embolus, cancer, hospitalization for serious infection, and emergency bowel resection surgery. The outcomes were selected as they are common causes of death and were included regardless of whether or not the patient died. All outcomes other than cancer were assessed at any time after the start of therapy; cancer outcomes were measured 6 months after the start of therapy given the biological implausibility that a medical therapy would cause cancer within 6 months. All secondary outcomes were determined on the basis of International Classification of Disease–Clinical Modification version 9 (ICD-9) and Current Procedural Terminology (CPT) codes. See Supplementary Methods for details.
Follow-up period
Follow-up began on the index date as defined above and continued until either the patient died, discontinued enrollment in Medicaid or Medicare Part A, B, or D, reached age 90 years, was newly diagnosed with other immune-mediated diseases or AIDS, or reached the end of the available data. Follow-up of patients with UC also ended if they were diagnosed with a fistula, as this would usually change the diagnosis to CD.
Covariates
Fifty-seven potential confounding variables thought likely to be associated with the choice between CS use or anti-TNF therapy and the outcomes of interest were measured. These included demographic characteristics, medications, diagnostic tests, comorbidities, and healthcare utilization measures. Year of cohort entry was included to account for secular trends. Given the time periods of data collection for Medicare and Medicaid, inclusion of year of cohort entry also accounts for Medicare vs. Medicaid benefits. Potential confounders were measured at baseline and as time-updating variables every 28 days (see Supplementary Methods and Supplementary Tables S1, S2 and S3 for additional details).
Statistical analyses
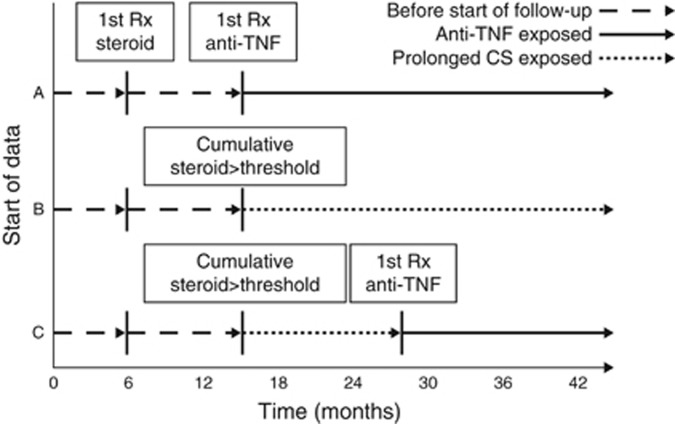
All statistical analyses were completed separately for CD and UC using SAS version 9.4 (SAS Institute, Cary, NC). Medication exposure was unidirectional time updating in the primary analysis, such that patients who initially contributed follow-up time to the prolonged CS use group could later contribute follow-up time to the anti-TNF group if they initiated therapy with an anti-TNF drug. Patients could not contribute follow-up time to the CS group once they met the anti-TNF exposure definition (Figure 1). This approach tests the hypothesis that a strategy of trying anti-TNF therapy is associated with higher or lower mortality risk even if the anti-TNF therapy is ineffective and is discontinued. Alternative approaches were used in sensitivity analyses described further below.
Figure 1.
Exposure definitions for the primary analysis. Patients were required to have at least 12 months of data before the start of follow-up. Follow-up began with the earliest of either (A) meeting the definition of new user of antitumor necrosis factor-α (anti-TNF) therapy or (B) meeting the cumulative dose threshold for prolonged corticosteroid (CS) use. A patient could switch from the prolonged CS use to anti-TNF use (C), but once a patient was a new user of anti-TNF therapy they were considered exposed to anti-TNF from that point forward even if they discontinued the anti-TNF drug.
The association between treatment and the outcomes of interest was estimated using marginal structural models with stabilized weights from inverse probability of treatment derived from propensity score models and models estimating the probability of being censored (39). Separate models were estimated for treatment at index date and for each subsequent 28 days of follow-up time. From the 57 covariates that were a priori selected as potential confounders, we excluded variables from the treatment and censoring models if any cell count for any model was <10. The other covariates, 36 baseline and 46 time varying for CD and 25 baseline and 39 time varying for UC, were included in the treatment and censoring models (Supplementary Tables S1 and S2). Patients with very high or low probability of treatment with either CS or anti-TNF at cohort entry were excluded to improve balance in covariates (40). Specifically, we excluded those with a baseline propensity score for CS treatment >0.98 for both CD (10.0%) and UC (19.8%) and <0.30 (1.8%) for CD and <0.40 (1.5%) for UC. Weights were truncated at the 2nd and 98th percentile to avoid excessive influence of patients with extremely low or high probability of receiving one of the treatments. Inverse probability treatment weights derived from the baseline model were used to estimate the balance of covariates between the treatment groups after applying the weights. Balance was assessed using standardized mean differences between the groups; standardized mean differences >0.1 are considered to reflect meaningful imbalance (41). The same weights were applied to the numerator and denominator of computed incidence rates. The inverse probability treatment weights were applied to logistic regression models to compute weighted, pooled odds ratios (ORs) and 95% confidence intervals (95% CIs) that approximate hazard ratios derived from Cox regression (39). Similar models were developed for the secondary outcomes. See Supplementary Methods for additional details.
An additional model with all-cause mortality as the outcome censored all patients when they experienced any secondary outcome event to determine whether these events explained the association between treatment and all-cause mortality. Additional analyses examined for an interaction between treatment and age, treatment and comorbidities, and treatment and insurance type (the latter only in CD). Sensitivity analyses, also using marginal structural models, were conducted to assess the impact of the exposure definition on the observed associations. These included: (i) censoring follow-up for anti-TNF-treated patients who discontinued therapy and resumed treatment with CS; (ii) allowing patients to switch bidirectionally between the treatment arms, contributing follow-up time to the treatment that they had most recently received; and (iii) using the initial treatment to define the exposure category regardless of whether the patient changed treatment, adjusted for baseline covariates and with follow-up censored at 1 year. An additional sensitivity analysis considered only those patients who received at least 3 dispensings of infliximab or 2 dispensings of adalimumab or certolizumab pegol within the first 56 days of follow-up (referred to as having received an induction course) and compared these patients with prolonged CS users with follow-up starting at day 57 for both groups so as to avoid immortal time bias. Additional sensitivity analyses adjusted for the average daily dose of prednisone used in the 12 months before the index date and used a lower threshold, 2,000 mg of traditional CS, to define prolonged CS use (see Supplementary Methods for additional details).
Ethical considerations and patient involvement
The study was approved by the institutional review boards at University of Pennsylvania and University of Alabama at Birmingham. The study question was proposed and the study was designed, including the choice of outcome measures, and implemented by the investigators. Patient stakeholders provided feedback on the study design. Results of the study will be made available to the general population through the Patient-Centered Outcomes Research Institute (PCORI) website.
Results
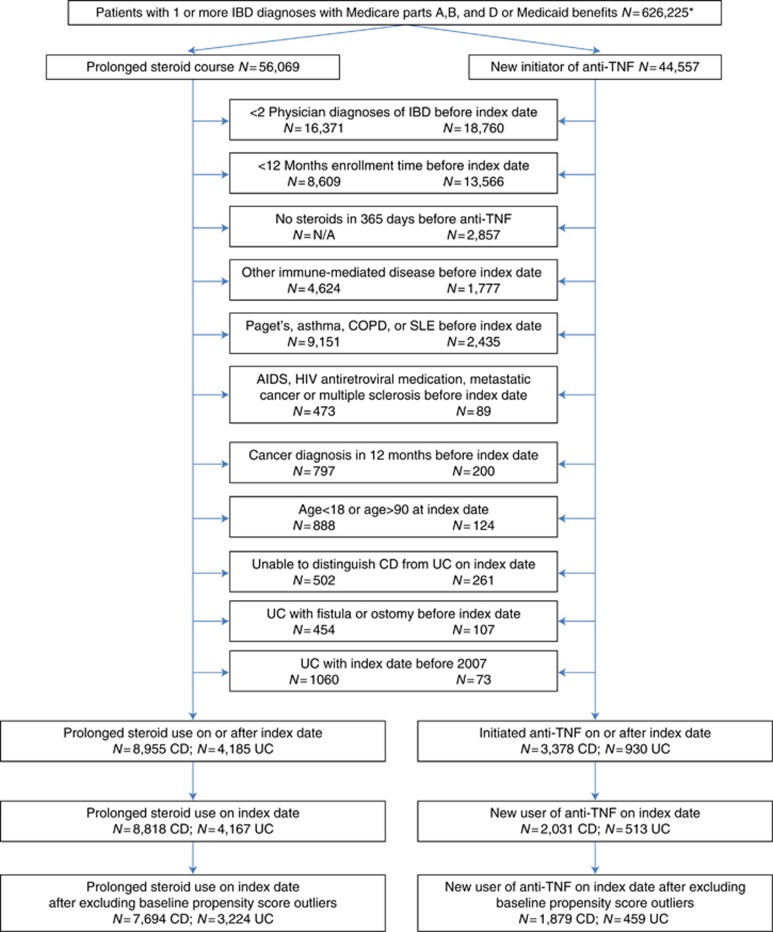
Among patients with CD, 7,694 entered the cohort as prolonged CS users and 1,879 as new anti-TNF users. Among patients with UC, 3,224 and 459 entered the cohort as prolonged CS users and new anti-TNF users, respectively (Figure 2). After the start of follow-up, 1,473 patients with CD and 462 patients with UC initiated anti-TNF such that overall there were 3,352 patients with CD and 921 with UC who initiated anti-TNF therapy during follow-up (Figure 2). The total duration of follow-up time (in person-years (median, interquartile range)) was 24,625 (2.5, 1.0–4.8) for CD initially treated with prolonged CS, 6,222 (2.6, 1.0–4.9) for CD initially treated with anti-TNF, 9,158 (2.5, 1.0–4.4) for UC initially treated with prolonged CS, and 1,213 (2.2, 0.8–4.3) for UC initially treated with anti-TNF. The weighted baseline characteristics were well balanced between the treatment groups (Table 1 and Supplementary Tables S1 and S2). There was near perfect balance on age, sex, race, and comorbidity score (Table 1). As expected, use of CS during the 12 months before the start of follow-up was greater in the patients who entered the cohort as prolonged CS users (P<0.01 for all comparisons). CS use decreased significantly in the anti-TNF group during the first 6 months and in both groups of patients during the first year of follow-up (Table 2).
Figure 2.
Creation of study cohorts. *Excludes 13,088 missing one or more of gender, date of birth, race, or zip code. Anti-TNF, antitumor necrosis factor-α CD, Crohn’s disease; COPD, chronic obstructive pulmonary disease; IBD, inflammatory bowel disease; SLE, systemic lupus erythematosus; UC, ulcerative colitis.
Table 1. Characteristics of the study population at the start of follow-upa.
|
New initiators of prolonged steroids or anti-TNF |
Final cohort excluding outliers from baseline PS |
Final weighted cohort |
||||||||
| Characteristic | Group | % | % | SMD | % | % | SMD | % | % | SMD |
| Crohn’s disease | Steroids N=8,818 | Anti-TNF N=2,031 | Steroids N=7,694 | Anti-TNF N=1,879 | Steroids N=7,694 | Anti-TNF N=1,879 | ||||
| Age at index | 18–34.9 | 19.7 | 25.9 | 0.15 | 21.1 | 24.6 | 0.08 | 22.0 | 23.5 | 0.04 |
| 35–49.9 | 24.2 | 30.5 | 0.14 | 25.9 | 30.1 | 0.09 | 26.9 | 27.8 | 0.02 | |
| 50–64.9 | 16.0 | 15.5 | 0.01 | 15.9 | 15.6 | 0.01 | 15.8 | 16.1 | 0.01 | |
| 65–69.9 | 13.7 | 11.8 | 0.06 | 13.0 | 12.2 | 0.02 | 12.8 | 12.0 | 0.02 | |
| 70–74.9 | 10.2 | 8.1 | 0.07 | 9.8 | 8.6 | 0.04 | 9.5 | 8.8 | 0.02 | |
| 75–79.9 | 7.6 | 5.0 | 0.11 | 7.3 | 5.3 | 0.08 | 6.8 | 6.5 | 0.01 | |
| 80–84.9 | 5.6 | 2.7 | 0.15 | 5.1 | 2.9 | 0.11 | 4.6 | 4.0 | 0.03 | |
| 85+ | 3.0 | 0.6 | 0.18 | 1.9 | 0.6 | 0.12 | 1.7 | 1.3 | 0.03 | |
| Mean age (s.d.) | 54.5 (19.1) | 49.5 (17.6) | 0.27 | 53.2 (18.8) | 50.1 (17.7) | 0.17 | 52.5 (18.6) | 51.4 (18.1) | 0.06 | |
| Female gender | 62.4 | 65.1 | 0.06 | 62.8 | 64.7 | 0.04 | 63.1 | 62.5 | 0.01 | |
| White race | 83.6 | 83.9 | 0.01 | 82.9 | 84.3 | 0.04 | 83.1 | 82.4 | 0.02 | |
| Comorbidity score | ≤0 | 31.8 | 25.0 | 0.15 | 31.6 | 25.9 | 0.13 | 30.4 | 30.0 | 0.01 |
| 1 | 22.0 | 22.6 | 0.01 | 22.2 | 22.8 | 0.01 | 22.3 | 23.1 | 0.02 | |
| 2–3 | 24.8 | 28.2 | 0.08 | 25.1 | 28.1 | 0.07 | 25.8 | 26.2 | 0.01 | |
| 4+ | 21.4 | 24.2 | 0.07 | 21.1 | 23.2 | 0.05 | 21.4 | 20.7 | 0.02 | |
| Medical therapyb | 5ASA | 50.7 | 51.4 | 0.01 | 51.3 | 51.3 | 0.00 | 51.5 | 53.3 | 0.04 |
| AZA/6MP | 25.6 | 34.8 | 0.20 | 27.1 | 33.7 | 0.14 | 28.5 | 30.7 | 0.05 | |
| Methotrexate | 2.1 | 3.5 | 0.08 | 2.3 | 3.4 | 0.07 | 2.2 | 3.7 | 0.09 | |
| Narcotics | 52.4 | 62.4 | 0.20 | 53.8 | 61.0 | 0.15 | 55.3 | 56.9 | 0.03 | |
| IBD hospitalizationb | 29.0 | 43.0 | 0.29 | 31.0 | 41.4 | 0.22 | 33.4 | 36.3 | 0.06 | |
| Index drug | ||||||||||
| Prednisonec | 60.0 | N/A | N/A | 65.1 | N/A | N/A | 67.7 | N/A | N/A | |
| Budesonide | 40.0 | N/A | N/A | 34.9 | N/A | N/A | 32.3 | N/A | N/A | |
| Infliximab | N/A | 70.0 | N/A | N/A | 71.4 | N/A | N/A | 77.0 | N/A | |
| Adalimumab | N/A | 23.9 | N/A | N/A | 22.8 | N/A | N/A | 18.5 | N/A | |
| Certolizumab pegol | N/A | 6.1 | N/A | N/A | 5.9 | N/A | N/A | 4.6 | N/A | |
| Ulcerative colitis | Steroids N=4,167 | Anti-TNF N=513 | Steroids N=3,224 | Anti-TNF N=459 | Steroids N=3,224 | Anti-TNF N=459 | ||||
| Age at index | 18–34.9 | 3.5 | 5.7 | 0.11 | 4.1 | 5.7 | 0.07 | 4.3 | 4.5 | 0.01 |
| 35–49.9 | 6.5 | 9.0 | 0.09 | 7.0 | 9.2 | 0.08 | 7.3 | 8.1 | 0.03 | |
| 50–64.9 | 8.9 | 9.0 | 0.00 | 9.4 | 8.9 | 0.02 | 9.3 | 9.8 | 0.02 | |
| 65–69.9 | 23.9 | 29.0 | 0.12 | 26.9 | 28.5 | 0.04 | 27.2 | 28.2 | 0.02 | |
| 70–74.9 | 20.8 | 24.6 | 0.09 | 22.8 | 25.3 | 0.06 | 23.2 | 23.4 | 0.00 | |
| 75–79.9 | 17.0 | 12.1 | 0.14 | 14.5 | 12.0 | 0.07 | 14.1 | 14.4 | 0.01 | |
| 80–84.9 | 12.2 | 7.8 | 0.15 | 10.3 | 7.6 | 0.09 | 9.9 | 7.9 | 0.07 | |
| 85+ | 7.1 | 2.9 | 0.19 | 5.1 | 2.8 | 0.12 | 4.7 | 3.8 | 0.04 | |
| Mean age (s.d.) | 69.6 (13.2) | 66.4 (14.1) | 0.24 | 68.4 (13.3) | 66.2 (14.1) | 0.16 | 68.1 (13.4) | 67.3 (12.8) | 0.06 | |
| Female gender | 57.1 | 55.2 | 0.04 | 55.0 | 54.5 | 0.01 | 55.0 | 55.5 | 0.01 | |
| White race | 92.1 | 91.8 | 0.01 | 91.4 | 91.9 | 0.02 | 91.3 | 91.9 | 0.02 | |
| Comorbidity score | ≤0 | 32.5 | 29.0 | 0.08 | 32.9 | 29.8 | 0.07 | 32.7 | 30.3 | 0.05 |
| 1 | 19.6 | 20.5 | 0.02 | 20.2 | 20.9 | 0.02 | 20.2 | 24.6 | 0.11 | |
| 2–3 | 24.9 | 28.8 | 0.09 | 25.2 | 26.8 | 0.04 | 25.4 | 25.2 | 0.00 | |
| 4+ | 23.0 | 21.6 | 0.03 | 21.7 | 22.4 | 0.02 | 21.7 | 20.0 | 0.04 | |
| Medical therapyb | 5ASA | 68.6 | 76.8 | 0.18 | 72.4 | 77.3 | 0.11 | 73.0 | 74.6 | 0.04 |
| AZA/6MP | 17.7 | 33.1 | 0.36 | 21.1 | 32.0 | 0.25 | 22.6 | 25.3 | 0.06 | |
| Methotrexate | 0.8 | 1.2 | 0.04 | 0.8 | 1.3 | 0.05 | 0.8 | 1.6 | 0.07 | |
| Narcotics | 29.9 | 26.7 | 0.07 | 28.2 | 26.6 | 0.04 | 27.8 | 27.6 | 0.00 | |
| IBD hospitalizationb | 18.4 | 35.5 | 0.39 | 21.0 | 32.0 | 0.25 | 22.6 | 25.9 | 0.08 | |
| Index drugd | ||||||||||
| Prednisonec | 74.9 | N/A | N/A | 84.1 | N/A | N/A | 85.1 | N/A | N/A | |
| Budesonide | 25.1 | N/A | N/A | 15.9 | N/A | N/A | 14.9 | N/A | N/A | |
| Infliximab | N/A | 89.1 | N/A | N/A | 88.9 | N/A | N/A | 89.2 | N/A | |
| Adalimumab | N/A | 9.7 | N/A | N/A | 9.8 | N/A | N/A | 9.9 | N/A | |
| Certolizumab pegol | N/A | 1.2 | N/A | N/A | 1.3 | N/A | N/A | 0.9 | N/A | |
Anti-TNF, antitumor necrosis factor-α 5ASA, 5-aminosalicylate; AZA/6MP, azathioprine/6-mercaptopurine; IBD, inflammatory bowel disease; PS, propensity score; N/A, not applicable; SMD, standardized mean difference.
See Supplementary Tables S1 and S2 for additional characteristics of the study population.
In preceding 183 days.
Prednisone or equivalent non-budesonide corticosteroids.
No patients were treated with golimumab at the start of follow-up.
Table 2. Steroid use in the 12 months before start of follow-up and in the first year of follow-up.
|
Chronic steroid use |
Anti-TNF use |
P value | |||
|---|---|---|---|---|---|
| Median dose (mg/day) | IQR | Median dose (mg/day) | IQR | Steroid use vs. anti-TNF | |
| Crohn's disease | |||||
| 12 months before start of follow-up | |||||
| Prednisone equivalents | 6.6 | 0.3–8.2 | 2.7 | 0.8–4.9 | <0.0001 |
| Budesonide | 0.0 | 0.0–1.5 | 0.0 | 0.0–0.0 | <0.0001 |
| Follow-up months 1–6 | |||||
| Prednisone equivalents | 7.4 | 0.0–14.5 | 0.0 | 0.0–3.6 | <0.0001 |
| Budesonide | 0.0 | 0.0–3.0 | 0.0 | 0.0–0.0 | <0.0001 |
| Follow-up months 7–12 | |||||
| Prednisone equivalents | 0.0‡ | 0.0–7.7 | 0.0‡ | 0.0–1.9 | <0.0001 |
| Budesonide | 0.0† | 0.0–0.0 | 0.0** | 0.0–0.0 | <0.0001 |
| Ulcerative colitis | |||||
| 12 months before start of follow-up | |||||
| Prednisone equivalents | 6.8 | 5.5–8.2 | 4.7 | 2.7–6.6 | <0.0001 |
| Budesonide | 0.0 | 0.0–0.0 | 0.0 | 0.0–0.0 | <0.0001a |
| Follow-up months 1–6 | |||||
| Prednisone equivalents | 9.8 | 4.9–16.4 | 0.0 | 0.0–5.5 | <0.0001 |
| Budesonide | 0.0 | 0.0–0.0 | 0.0 | 0.0–0.0 | <0.0001b |
| Follow-up months 7–12 | |||||
| Prednisone equivalents | 0.0‡ | 0.0–8.1 | 0.0‡ | 0.0–1.6 | <0.0001 |
| Budesonide | 0.0*** | 0.0–0.0 | 0.0+ | 0.0–0.0 | <0.0001c |
Anti-TNF, antitumor necrosis factor-α IQR, interquartile range.
Comparison of steroid use in months 7–12 compared with the 6 months before start of follow-up: **P=0.0020, ***P=0.0034, ‡P<0.0001, †P=0.4549, +P=0.2277.
Mean budesonide dose among patients with ulcerative colitis (UC) was 0.4 (s.d. 1.1) among chronic steroid-treated patients and 0.1 (s.d. 0.3) among anti-TNF-treated patients
Mean budesonide dose among patients with UC was 1.0 (s.d. 3.4) among chronic steroid-treated patients and 0.1 (s.d. 0.7) among anti-TNF-treated patients
Mean budesonide dose among patients with UC was 0.5 (s.d. 1.6) among chronic steroid-treated patients and 0.1 (s.d. 0.8) among anti-TNF-treated patients.
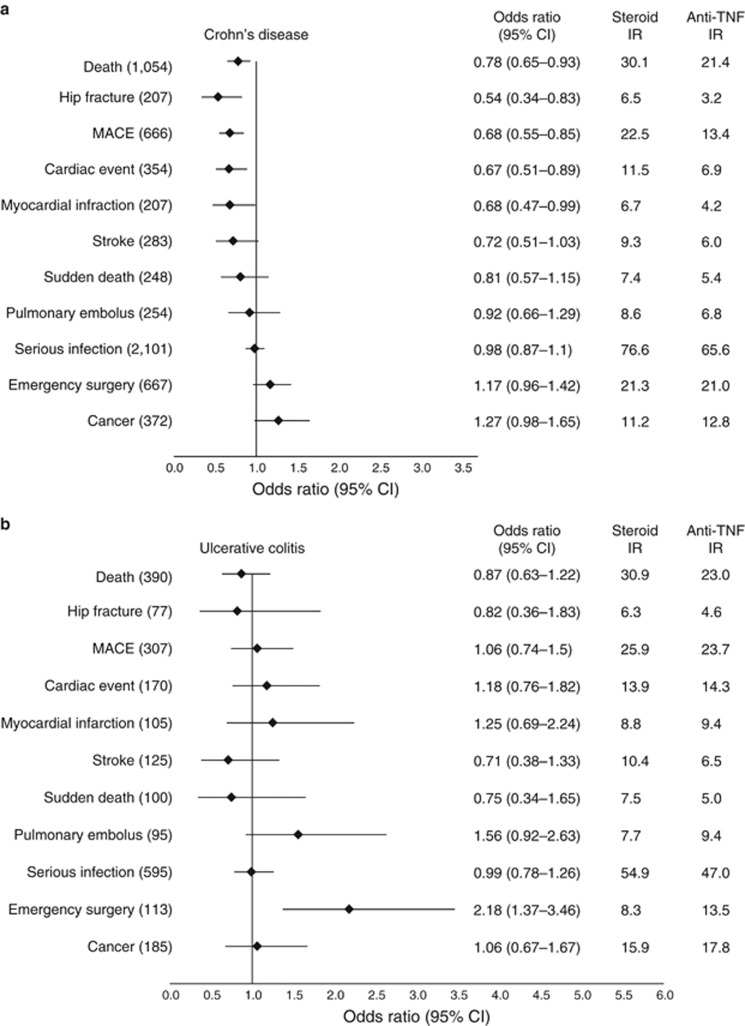
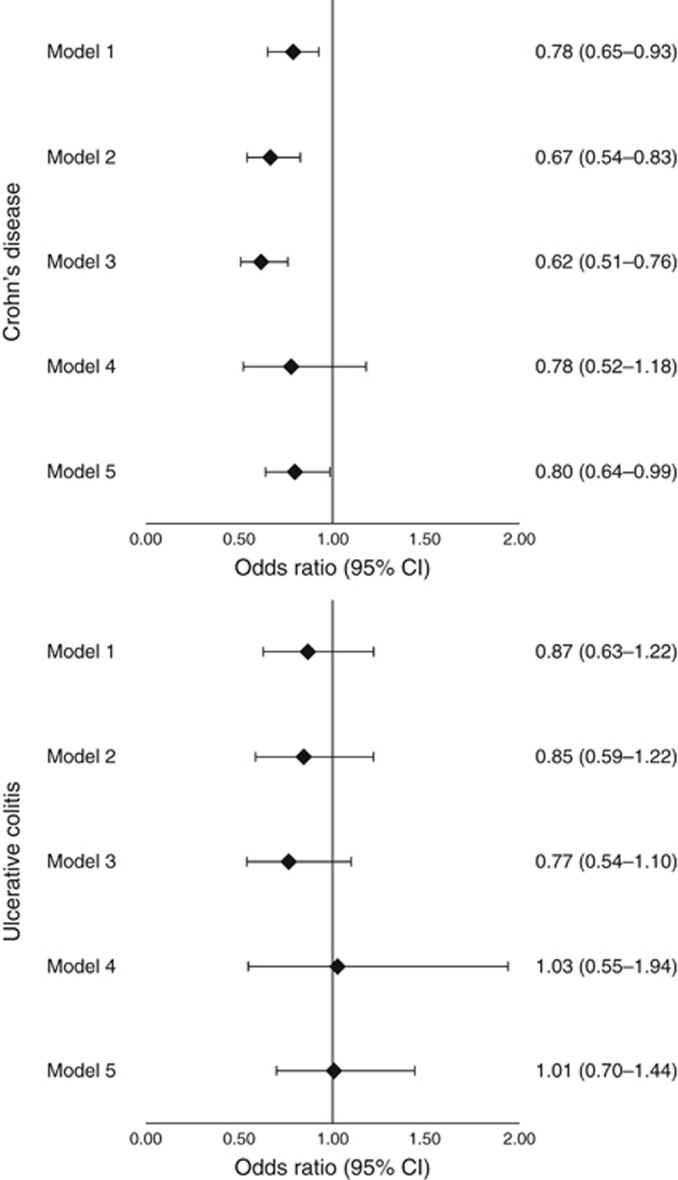
During follow-up, there were a total of 1,444 deaths. The mean age at the time of death was 67.0 (±16.2) years and 76.7 (±10.7) years in patients with CD and UC, respectively (Supplementary Table S4). The weighted annual incidence of death per 1,000 treated patients in CD patients was 21.4 (anti-TNF) vs. 30.1 (prolonged CS); in UC patients, the incidence of death was 23.0 (anti-TNF) and 30.9 (prolonged CS) (Figure 3). The risk of death was statistically significantly lower in patients treated with anti-TNF therapy for CD (OR 0.78, 95% CI 0.65–0.93) and numerically lower but not significantly different for UC (OR 0.87, 95% CI 0.63–1.22). The results were similar in sensitivity analyses modifying the exposure definition (Figure 4).
Figure 3.
Adjusted odds ratios for primary and secondary outcomes. All odds ratios are for antitumor necrosis factor-α (anti-TNF) therapy with corticosteroid therapy as the reference group. Numbers in parentheses represent the total number of outcomes in the study cohort in Crohn’s disease (a) and ulcerative colitis (b). Weighted incidence rates (IRs) are reported per 1,000 person-years. CI, confidence interval; MACE, major adverse cardiovascular event.
Figure 4.
Sensitivity analysis examining different definitions of exposure. Odds ratios and 95% confidence intervals (CIs) are reported for the antitumor necrosis factor-α (anti-TNF) therapy with prolonged corticosteroid (CS) use as the reference group. In model 1 (the primary analysis), follow-up for patients continued until either the outcome of interest occurred or they reached the end of the available data. Medication exposure was unidirectionally time updating, such that patients who initially contributed follow-up time to the prolonged CS use group could later contribute follow-up time to the anti-TNF group if they initiated therapy with an anti-TNF drug. Model 2 was the same as model 1 except that follow-up was censored if an anti-TNF-treated patient discontinued anti-TNF therapy and resumed treatment with CS. Model 3 is an as treated model in which both treatments are bidirectional time-updating variables. Participants contribute follow-up time to the treatment that the patient had most recently received. Model 4 used the initial treatment to define exposure such that patients contribute follow-up time only to the treatment arm that they were in at the time of cohort entry even if the treatment is changed. Follow-up in model 4 is censored 12 months after cohort entry for all patients. Model 5 is a sensitivity analysis limited to those having received an induction course of anti-TNF therapy in the first 56 days of follow-up compared with prolonged CS use with follow-up beginning at day 57 in both groups.
The association between anti-TNF therapy and mortality did not vary by age (Table 3, interaction P value >0.6 for both diseases). In contrast, when stratified by comorbid illness, anti-TNF therapy was associated with a reduced mortality risk only among patients who had the most comorbid illnesses (CD: OR 0.65, 95% CI 0.48–0.88; UC: OR 0.63, 95% CI 0.36–1.11), although the test for interaction was not statistically significant (P=0.17 CD; P=0.29 UC). A subgroup analysis among only those patients with CD within the Medicare data produced nearly identical lower risk of mortality with anti-TNF therapy (OR 0.76, 95% CI 0.64–0.90). The same association was not apparent in the Medicaid population (OR 1.15, 95% CI 0.46–2.88), although the CIs were wide and a test for heterogeneity was not significant (P=0.39).
Table 3. Stratified analysis of the association of anti-TNF therapy relative to CS therapy and the risk of death among patients with IBD.
| Crohn’s disease (OR, 95% CI)a | Ulcerative colitis (OR, 95% CI)a | |
|---|---|---|
| Age at cohort entry (years) | ||
| Age <65 | 0.73 (0.56–0.93) | 0.70 (0.27–1.78) |
| Age 65 or older | 0.78 (0.61–1.00) | 0.80 (0.56–1.14) |
| Interaction P value | 0.66 | 0.79 |
| Comorbidity scoreb | ||
| ≤0 | 1.11 (0.73–1.69) | 0.99 (0.49–2.00) |
| 1 | 0.94 (0.61–1.44) | 0.65 (0.21–1.99) |
| 2–3 | 0.76 (0.55–1.05) | 1.35 (0.75–2.44) |
| >3 | 0.65 (0.48–0.88) | 0.63 (0.36–1.11) |
| Interaction P value | 0.17 | 0.29 |
Anti-TNF, antitumor necrosis factor-α CI, confidence interval; CS, corticosteroid; IBD, inflammatory bowel disease; OR, odds ratio.
Odds ratios are reported for the anti-TNF therapy with prolonged CS use as the reference group.
Comorbid illness categorized according to the combined Charlson–Elixhauser index.
Among the CD cohort, anti-TNF therapy was also associated with lower rates of MACE (OR 0.68, 95% CI 0.55–0.85) and hip fracture (OR 0.54, 95% CI 0.34–0.83) (Figure 3). The risk of serious infection, pulmonary embolus, and cancer was not significantly different between treatments for CD. None of these secondary outcomes were significantly different between treatments for UC.
The difference in emergency bowel resection was not statistically significant for CD (OR 1.17, 95% CI 0.96–1.42). For UC, emergency surgery was more common in patients treated with anti-TNF therapy (OR 2.18, 95% CI 1.37–3.46). IBD-related hospitalizations were slightly more common in the anti-TNF-treated group (CD: OR 1.13, 95% CI 1.04–1.23; UC: OR 1.53, 95% CI 1.29–1.81).
Using a lower threshold to define prolonged CS use, nearly identical results were obtained for mortality (CD: 0.80, 95% CI 0.68–0.95; UC: 0.87, 95% CI 0.62–1.22) and the secondary outcomes, although anti-TNF therapy was now statistically significantly associated with a lower risk of stroke among patients with CD (Supplementary Table S4). Similarly, additional adjustment for the average daily dose of prednisone in the 12 months before index date did not appreciably affect the results for mortality (CD: OR 0.77, 95% CI 0.64–0.92; UC: OR 0.85, 95% CI 0.61–1.19) or other outcomes, although the association with myocardial infarction among patients with CD was no longer statistically significant (OR 0.74, 95% CI 0.50–1.09).
To determine whether the increased risk of death with CS therapy among patients with CD could be explained based on the measured secondary outcomes, the primary analysis was repeated censoring follow-up at the time of any of the secondary outcomes. In this model, the reduced risk for death was attenuated and very close to a null result (OR 0.97, 95% CI 0.63–1.47).
Discussion
CD and severe UC are associated with an increased risk of death compared with patients without IBD (7). Therapy for these diseases focuses on immunosuppression, either intermittently with CS or chronically often with anti-TNF drugs. Reluctance to use chronic immunosuppression is usually related to fear of serious adverse events, particularly in the elderly or patients with comorbid illnesses that further increase the risk of death (42, 43). The principle alternative is CS that can also cause serious adverse events (9, 24). This study compared the risk of death and common life-threatening events among patients treated with prolonged CS to those who initiated anti-TNF therapy. Those treated with anti-TNF therapy for CD had a risk of death that was ∼8.7 per 1,000 person-years lower, and this was most evident in those patients at greatest risk (i.e., those with the most comorbid illnesses). As demonstrated by our model censoring for any of the secondary outcomes, essentially all of the increased risk of death associated with CS therapy could be explained by the excess risk of MACE, pulmonary embolus, hip fracture, serious infection, cancer, and emergency surgery, although treatment with CS was statistically significantly associated only with higher risk of MACE and hip fracture. The risk of death was numerically but not significantly lower in the UC patients treated with TNF therapy.
In placebo-controlled trials of anti-TNF therapy there was no increased risk of short-term mortality (44). However, clinical trial populations do not reflect the broader use of therapies in clinical practice, often excluding patients with multiple comorbid illnesses and the elderly. Observational data can extend observations of clinical trials to populations who were excluded. Notably, the reduced mortality observed with anti-TNF therapy relative to CS therapy in this study was largely evident in those with the greatest burden of comorbid illness, whereas those with less severe or fewer comorbidities had comparable survival regardless of the choice of therapy. This may also explain why the association was more evident in the Medicare population, given that Medicare is composed of both elderly and disabled patients. A small study from Belgium also observed that among elderly patients with IBD, those treated with CS were more likely to die than those treated with anti-TNF therapy (43). These results, and those of a recent study examining outcomes of surgical vs. medical therapy for UC (45), suggest that prolonged CS may be the least favorable strategy for this vulnerable population.
Whether CS therapy directly increases the risk of death in CD, is a marker of inadequately controlled disease that contributes to the risk of death, or both cannot be definitively established from an observational study. To explore this hypothesis, this study examined common causes of death as secondary outcomes. Some of the observations were expected. Hospitalization and emergency surgery were more common in anti-TNF-treated patients, particularly in UC, likely because anti-TNF therapy was attempted in patients who were likely to require intravenous CS or surgery if the anti-TNF therapy was unsuccessful. Osteoporosis is a known complication of prolonged CS therapy. Unsurprisingly, higher hip fracture rates were evident with CS treatment, particularly among patients with CD. This highlights the need to minimize CS exposure and assess for bone loss in patients who received prolonged CS therapy. Among patients with CD, anti-TNF therapy was associated with lower rates of MACE. However, the same effect was not observed among patients with UC. There are limited data on the effect of anti-TNF therapy on the risk of acute cardiovascular events among patients with IBD. However, some but not all studies in rheumatoid arthritis suggest that anti-TNF therapy may reduce the risk of myocardial infarction (46), particularly if the inflammation is well controlled (47). Similar studies examining the risk of cardiovascular outcomes with CS have come to conflicting results, with a suggestion that inflammatory disease activity may be a more important risk factor than CS use (48). Because of the nature of the two diseases, systemic inflammation is more common in CD than UC (49). Whether this explains differences in associations of anti-TNF therapy with MACE between the two diseases is unknown.
Whether chronic immunosuppression with anti-TNF therapy increases the risk of cancer among patients with IBD has not been definitively answered. The incidence of cancer was not significantly higher in the anti-TNF-treated patients in this study, although the OR for cancer approached statistical significance in the CD population. In placebo-controlled clinical trials of up to 1-year duration, there was no increased incidence of cancer among anti-TNF-treated patients (44). The duration of follow-up in this study was longer, but perhaps still not long enough to fully exclude an increased risk of cancer with anti-TNF therapy. Recent observational data have implicated thiopurines, which are commonly used in combination with anti-TNF therapy, as increasing the risk of cancer (50, 51). As such, all of the analyses in this study were adjusted for thiopurine and methotrexate use as time-updating exposures to account for the potential confounding effect of these drugs.
Despite availability of anti-TNF therapy for nearly two decades, this study documents that steroid-sparing therapies are often not employed in usual care despite current treatment guidelines (52, 53). There were far more patients who met the definition of prolonged CS use than anti-TNF therapy in this population. Similar treatment patterns, including high levels of use of 5-aminosalicylate compounds that are thought to have limited activity for CD, and limited use of steroid-sparing immunomodulator and biologic drugs, have been previously described (54, 55). Whether this was related to the cost of therapy, lack of awareness of treatment guidelines, or patient preferences for other reasons could not be determined in this study.
The study has a number of unique strengths. The sample size was large, particularly for CD, allowing us to study relatively uncommon outcomes and to stratify results based on extent of comorbid illness. Because patients rarely discontinue Medicare insurance, loss to follow-up was minimized and 99% of Medicare death dates have been validated (56). Medicare covers inpatient and outpatient care and that from specialists and primary care physicians, thereby allowing capture of events that may be missed by examining only records of the treating gastroenterologists. Follow-up time was relatively long with 25% of the cohort having more than 4 years of follow-up. Although for the cancer outcome even longer follow-up may be necessary to fully rule out an association, prior research demonstrates that patients make decisions by considering a 5 year or shorter time horizon (57). The analyses were conducted with marginal structural models that are superior to standard Cox models to account for confounding by time-updating factors that are associated with treatment selection and the outcome of interest (39). Sensitivity analysis showed that the association of treatment strategy with mortality was robust to multiple assumptions and comparable during the first year and with longer duration of follow-up suggesting that the relative risk does not escalate further with longer-term exposure. Although the sensitivity analysis using the initial treatment carried forward was not statistically significant, the odds ratio was almost identical to that of the primary analysis, but with wider confidence intervals because of shorter follow-up. The shortened follow-up was necessary as switching therapy becomes common with longer follow-up and compromises interpretability. Finally, unlike the TREAT registry, this study focused on patients who were initiating therapy with anti-TNF drugs rather than prevalent users, thereby avoiding the potential for bias from depletion of susceptible subjects.
We focused on patients who were newly initiating therapy with an anti-TNF drug. Many of these patients discontinued therapy, yet we continued to follow them in the anti-TNF group for the primary analysis. This may have slightly underestimated the association between anti-TNF therapy and reduced mortality in CD as demonstrated in our “as treated” sensitivity analysis. However, this more conservative approach to the primary analysis is consistent with the decision facing clinicians and patients when deciding to start anti-TNF therapy given that only a proportion of patients will have a meaningful response to therapy and would be expected to remain on the medication for long periods of time.
The study also had several limitations. Despite adjusting for a large range of covariates in the marginal structural models, residual confounding, including confounding by indication, is possible. The strongest predictors of death in most studies are age, sex, and comorbid illnesses. Fortunately, the marginal structural models resulted in nearly perfect balance of these covariates. The claims data used in this study lack details on severity of clinical symptoms or bowel inflammation. However, we compared new users of anti-TNF therapy with patients receiving an additional prescription for CS. Both of these medications are almost always prescribed for patients with symptomatic IBD, thus lessening the potential for confounding by indication. Additionally, we adjusted for markers of disease activity such as use of colonoscopy, cross-sectional imaging, testing for Clostridium difficile, and so on. Not surprisingly, the prolonged steroid group had used more steroids in the 12 months before the index date as some patients in the anti-TNF group initiated therapy before accumulating as much steroid exposure. To account for this, we adjusted for recent steroid use in all models and demonstrated in sensitivity analyses that either adjusting for the average daily dose of prednisone or using a lower threshold to define prolong steroid use, which resulted in more similar use of steroids in the prior 12 months (data not shown), did not appreciably change the association between anti-TNF and the outcomes of interest. It was not possible to adjust for smoking, which is associated with all-cause mortality and nearly all of the major causes of death included as secondary outcomes in this study, as smoking is not reliably captured in these data. The risk of death among current smokers is estimated to be 1.5 to 2-fold greater than in non-smokers (58, 59) Smoking is also associated with more severe CD, but recent studies have demonstrated that there is relatively little difference in the proportion of smokers and nonsmokers treated with CS or anti-TNF therapy for CD (60, 61). Thus, it is unlikely that residual confounding by smoking would fully explain the observed associations.
Other residual confounding could result from physician prescribing behaviors or patient adherence to their physician’s treatment recommendations that could not be measured in these data. This would only be expected to explain the observed association between CS therapy and mortality if the unmeasured confounder was associated both with an increased risk of death and being treated with prolonged CS. We relied on administrative data to categorize comorbidities that may be imperfect. It is possible that this could have led to residual confounding, particularly at the extremes of the comorbidity index where the strongest association was seen between treatment and mortality.
Some patients categorized as CD may have had UC and vice versa. This may have been more common in the earlier years if patients with UC were coded as having CD to obtain approval for anti-TNF therapy before it was approved for UC. Based on our results, this would likely have biased the significant association observed in CD toward the null and the nonsignificant findings for UC away from the null. Thus, any misclassification of this type is unlikely to have led to the wrong interpretation. It was necessary to exclude 10–20% of patients to achieve balance in covariates. Exclusion of these outliers based on propensity scores resulted in improved balance in the proportion of patients treated with budesonide and traditional corticosteroids in the 183 days before the start of follow-up and age. These exclusions should not be viewed as a limitation, but rather a methodological approach similar to randomization or matching that increases the internal validity (40). We selected a relatively high dose of steroids to define our prolonged users. This reduced the likelihood that we would misclassify patients as prolonged steroid users because their physician wrote a prescription for a large number of prednisone tablets, many of which the patient never took. Finally, we studied patients with government-sponsored health insurance, including those with low incomes, disabilities, and the elderly. Many of the outcomes that were examined in this study are common to older populations. There was no evidence of a statistical interaction by age. Nonetheless, similar studies should be completed in younger populations and those with commercial insurance.
In conclusion, this study observed a statistically significant reduction in mortality in patients with CD treated with anti-TNF therapy relative to those with prolonged CS use. The association was in the same direction but not statistically significant among patients with UC. The reduced mortality rates among patients with CD was potentially a consequence of excess cardiovascular-related mortality and hip fractures and was largely limited to patients with multiple or serious comorbid conditions. This population, which is rarely included in clinical trials and for whom some physicians may be reluctant to treat with chronic immunosuppression, may be particularly good candidates for anti-TNF agents as CS-sparing therapy.
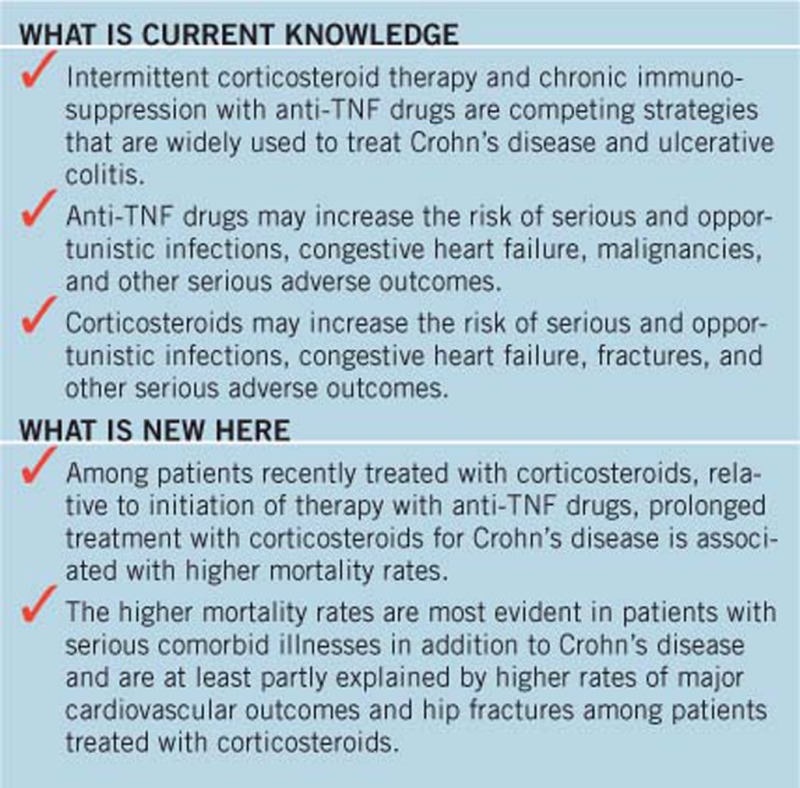
Study Highlights
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/ajg
Guarantor of the article: James D. Lewis, MD, MSCE.
Specific author contributions: J.D.L. wrote research proposal, manual of operations, interpreted the data, and drafted the manuscript. F.I.S., M.T.O., R.M., M.B., L.C., H.Y., F.X., and J.R.C. assisted in planning the study, interpreted the data, and edited the manuscript. C.M.B. assisted in planning the study, performed the data analysis,and edited the manuscript. J.A.R. assisted in designing the statistical analyses, interpreted the data, and edited the manuscript.
Financial support: The study was funded by a contract from PCORI. The funder was not involved with the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Potential competing interests: J.D.L. reports having received personal fees from Celgene, Shire, Janssen Pharmaceuticals, AbbVie, Immune Pharmaceuticals, AstraZenecca, Amgen, MedImmune, Merck, Nestle Health Science, Takeda Pharmaceuticals North America, Pfizer, Lilly, Gilead, Samsung Bioepis, and Johnson and Johnson. He has research funding from Takeda Pharmaceuticals North America and Nestle Health Science and non-financial research support from AbbVie. M.T.O. reports personal fees from Janssen, AbbVie, Takeda, Pfizer, Merck, and Lycera. He has research funding from UCB. R.M. reports personal fees from Takeda. F.I.S. reports having grants from Takeda Pharmaceuticals USA and having received personal fees from Evidera. M.B. reports research funding from Janssen, GlaxoSmithKline, and Takeda, having served as a consultant for Janssen and AbbVie, and having received honorarium for participation in a CME program sponsored by AbbVie. J.R.C. reports research funding from UCB, Janssen, Corrona, Amgen, Pfizer, BMS, and Crescendo, and personal fees from UCB, Janssen, Corrona, Amgen, Pfizer, BMS, and Crescendo. C.M.B., J.A.R., H.Y., L.C., and F.X. declare no conflict of interest.
Supplementary Material
References
- Herrinton LJ, Liu L, Lafata JE et al. Estimation of the period prevalence of inflammatory bowel disease among nine health plans using computerized diagnoses and outpatient pharmacy dispensings. Inflamm Bowel Dis 2007;13:451–461. [DOI] [PubMed] [Google Scholar]
- Herrinton LJ, Liu L, Lewis JD et al. Incidence and prevalence of inflammatory bowel disease in a Northern California managed care organization, 1996-2002. Am J Gastroenterol 2008;103:1998–2006. [DOI] [PubMed] [Google Scholar]
- Loftus EV Jr, Schoenfeld P, Sandborn WJ. The epidemiology and natural history of Crohn's disease in population-based patient cohorts from North America: a systematic review. Aliment Pharmacol Ther 2002;16:51–60. [DOI] [PubMed] [Google Scholar]
- Loftus EV Jr, Silverstein MD, Sandborn WJ et al. Crohn's disease in Olmsted County, Minnesota, 1940-1993: incidence, prevalence, and survival. Gastroenterology 1998;114:1161–1168. [DOI] [PubMed] [Google Scholar]
- Loftus EV Jr, Silverstein MD, Sandborn WJ et al. Ulcerative colitis in Olmsted County, Minnesota, 1940-1993: incidence, prevalence, and survival. Gut 2000;46:336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burisch J, Jess T, Martinato M et al. The burden of inflammatory bowel disease in Europe. J Crohns Colitis 2013;7:322–337. [DOI] [PubMed] [Google Scholar]
- Bewtra M, Kaiser LM, TenHave T et al. Crohn's disease and ulcerative colitis are associated with elevated standardized mortality ratios: a meta-analysis. Inflamm Bowel Dis 2013;19:599–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canavan C, Abrams KR, Mayberry JF. Meta-analysis: mortality in Crohn's disease. Aliment Pharmacol Ther 2007;25:861–870. [DOI] [PubMed] [Google Scholar]
- Lewis JD, Gelfand JM, Troxel AB et al. Immunosuppressant medications and mortality in inflammatory bowel disease. Am J Gastroenterol 2008;103:1428–1435 quiz 36. [DOI] [PubMed] [Google Scholar]
- D'Haens G, Baert F, van Assche G et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn's disease: an open randomised trial. [see comment]. Lancet 2008;371:660–667. [DOI] [PubMed] [Google Scholar]
- Sandborn WJ, Rutgeerts P, Feagan BG et al. Colectomy rate comparison after treatment of ulcerative colitis with placebo or infliximab. Gastroenterology 2009;137:1250–1260 quiz 520. [DOI] [PubMed] [Google Scholar]
- Lichtenstein GR, Yan S, Bala M et al. Infliximab maintenance treatment reduces hospitalizations, surgeries, and procedures in fistulizing Crohn's disease. Gastroenterology 2005;128:862–869. [DOI] [PubMed] [Google Scholar]
- Rutgeerts P, Feagan BG, Lichtenstein GR et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn's disease. [see comment]. Gastroenterology 2004;126:402–413. [DOI] [PubMed] [Google Scholar]
- Feagan BG, Panaccione R, Sandborn WJ et al. Effects of adalimumab therapy on incidence of hospitalization and surgery in Crohn's disease: results from the CHARM study. Gastroenterology 2008;135:1493–1499. [DOI] [PubMed] [Google Scholar]
- Colombel JF, Sandborn WJ, Reinisch W et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med 2010;362:1383–1395. [DOI] [PubMed] [Google Scholar]
- Colombel JF, Sandborn WJ, Rutgeerts P et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology 2007;132:52–65. [DOI] [PubMed] [Google Scholar]
- Panaccione R, Ghosh S, Middleton S et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology 2014;146:392–400 e3. [DOI] [PubMed] [Google Scholar]
- Johnson FR, Ozdemir S, Mansfield C et al. Crohn's disease patients' risk-benefit preferences: serious adverse event risks versus treatment efficacy. Gastroenterology 2007;133:769–779. [DOI] [PubMed] [Google Scholar]
- Peppercom MA. 6-mercaptopurine for the management of ulcerative colitis: a concept whose time has come [editorial; comment]. Am J Gastroenterol 1996;91:1689–1690. [PubMed] [Google Scholar]
- Sands BE, Siegel C, Ozdeir S. Gastroenterologists' tolerance for Crohn's disease treatment risks. Am J Gastroenterol 2007;102:S492. [Google Scholar]
- Grijalva CG, Chen L, Delzell E et al. Initiation of tumor necrosis factor-alpha antagonists and the risk of hospitalization for infection in patients with autoimmune diseases. JAMA 2011;306:2331–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta G, Lautenbach E, Lewis JD. Incidence and risk factors for herpes zoster among patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2006;4:1483–1490. [DOI] [PubMed] [Google Scholar]
- Toruner M, Loftus EV Jr, Harmsen WS et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology 2008;134:929–936. [DOI] [PubMed] [Google Scholar]
- Lichtenstein GR, Feagan BG, Cohen RD et al. Serious infections and mortality in association with therapies for Crohn's disease: TREAT Registry. Clin Gastroenterol Hepatol 2006;4:621–630. [DOI] [PubMed] [Google Scholar]
- Long MD, Farraye FA, Okafor PN et al. Increased risk of pneumocystis jiroveci pneumonia among patients with inflammatory bowel disease. Inflamm Bowel Dis 2013;19:1018–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long MD, Martin C, Sandler RS et al. Increased risk of pneumonia among patients with inflammatory bowel disease. Am J Gastroenterol 2013;108:240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung ES, Packer M, Lo KH et al. Anti TNFTACHFI. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the Anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation 2003;107:3133–3140. [DOI] [PubMed] [Google Scholar]
- Feenstra J, Grobbee DE, Remme WJ et al. Drug-induced heart failure. J Am Coll Cardiol 1999;33:1152–1162. [DOI] [PubMed] [Google Scholar]
- Beaugerie L, Brousse N, Bouvier AM et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet 2009;374:1617–1625. [DOI] [PubMed] [Google Scholar]
- Siegel CA, Marden SM, Persing SM et al. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn's disease: a meta-analysis. Clin Gastroenterol Hepatol 2009;7:874–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein CN, Blanchard JF, Metge C et al. The association between corticosteroid use and development of fractures among IBD patients in a population-based database. Am J Gastroenterol 2003;98:1797–1801. [DOI] [PubMed] [Google Scholar]
- van Staa TP, Cooper C, Brusse LS et al. Inflammatory bowel disease and the risk of fracture. Gastroenterology 2003;125:1591–1597. [DOI] [PubMed] [Google Scholar]
- Higgins PD, Skup M, Mulani PM et al. Increased risk of venous thromboembolic events with corticosteroid vs biologic therapy for inflammatory bowel disease. Clin Gastroenterol Hepatol 2015;13:316–321. [DOI] [PubMed] [Google Scholar]
- Lichtenstein GR, Feagan BG, Cohen RD et al. Serious infection and mortality in patients with Crohn's disease: more than 5 years of follow-up in the TREAT Registry. Am J Gastroenterol 2012;107:1409–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna R, Bressler B, Levesque BG et al. Early combined immunosuppression for the management of Crohn's disease (REACT): a cluster randomised controlled trial. Lancet 2015;386:1825–1834. [DOI] [PubMed] [Google Scholar]
- Fidder H, Schnitzler F, Ferrante M et al. Long-term safety of infliximab for the treatment of inflammatory bowel disease: a single-centre cohort study. Gut 2009;58:501–508. [DOI] [PubMed] [Google Scholar]
- Fidder HH, van de Steen K, van Assche G et al. Immortal time bias and infliximab-related mortality and malignancy incidence response. Gut 2010;59:416. [DOI] [PubMed] [Google Scholar]
- Yuan Z, Cooper GS, Einstadter D et al. The association between hospital type and mortality and length of stay: a study of 16.9 million hospitalized Medicare beneficiaries. Med Care 2000;38:231–245. [DOI] [PubMed] [Google Scholar]
- Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 2000;11:561–570. [DOI] [PubMed] [Google Scholar]
- Glynn RJ, Schneeweiss S, Sturmer T. Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol 2006;98:253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamdani M, Sykora K, Li P et al. Reader's guide to critical appraisal of cohort studies: 2. Assessing potential for confounding. BMJ 2005;330:960–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone M, Kohn A, Daperno M et al. Advanced age is an independent risk factor for severe infections and mortality in patients given anti-tumor necrosis factor therapy for inflammatory bowel disease. Clin Gastroenterol Hepatol 2011;9:30–35. [DOI] [PubMed] [Google Scholar]
- Lobaton T, Ferrante M, Rutgeerts P et al. Efficacy and safety of anti-TNF therapy in elderly patients with inflammatory bowel disease. Aliment Pharmacol Ther 2015;42:441–451. [DOI] [PubMed] [Google Scholar]
- Peyrin-Biroulet L, Deltenre P, de Suray N et al. Efficacy and safety of tumor necrosis factor antagonists in Crohn's disease: meta-analysis of placebo-controlled trials. Clin Gastroenterol Hepatol 2008;6:644–653. [DOI] [PubMed] [Google Scholar]
- Bewtra M, Newcomb CW, Wu Q et al. Mortality associated with medical therapy versus elective colectomy in ulcerative colitis: a cohort study. Ann Intern Med 2015;163:262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlake SL, Colebatch AN, Baird J et al. Tumour necrosis factor antagonists and the risk of cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology (Oxford) 2011;50:518–531. [DOI] [PubMed] [Google Scholar]
- Dixon WG, Watson KD, Lunt M et al. Reduction in the incidence of myocardial infarction in patients with rheumatoid arthritis who respond to anti-tumor necrosis factor alpha therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum 2007;56:2905–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Sijl AM, Boers M, Voskuyl AE et al. Confounding by indication probably distorts the relationship between steroid use and cardiovascular disease in rheumatoid arthritis: results from a prospective cohort study. PLoS ONE 2014;9:e87965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JD. The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterology 2011;140:1817–26 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterman MT, Haynes K, Delzell E et al. Effectiveness and safety of immunomodulators with anti-tumor necrosis factor therapy in Crohn's disease. Clin Gastroenterol Hepatol 2015;13:1293–301 e5 quiz e70, e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlyar DS, Lewis JD, Beaugerie L et al. Risk of lymphoma in patients with inflammatory bowel disease treated with azathioprine and 6-mercaptopurine: a meta-analysis. Clin Gastroenterol Hepatol 2015;13:847–58.e4 quiz e48-50. [DOI] [PubMed] [Google Scholar]
- Mowat C, Cole A, Windsor A et al. Guidelines for the management of inflammatory bowel disease in adults. Gut 2011;60:571–607. [DOI] [PubMed] [Google Scholar]
- Terdiman JP, Gruss CB, Heidelbaugh JJ et al. American Gastroenterological Association Institute guideline on the use of thiopurines, methotrexate, and anti-TNF-alpha biologic drugs for the induction and maintenance of remission in inflammatory Crohn's disease. Gastroenterology 2013;145:1459–1463. [DOI] [PubMed] [Google Scholar]
- van Deen WK, van Oijen MG, Myers KD et al. A nationwide 2010-2012 analysis of U.S. health care utilization in inflammatory bowel diseases. Inflamm Bowel Dis 2014;20:1747–1753. [DOI] [PubMed] [Google Scholar]
- Herrinton LJ, Liu L, Fireman B et al. Time trends in therapies and outcomes for adult inflammatory bowel disease, Northern California, 1998-2005. Gastroenterology 2009;137:502–511. [DOI] [PubMed] [Google Scholar]
- Death Information in the Research Identifiable Medicare Data. Research Data Assisstance Center, University of Minnesota, 2016. Accessed 3 March 2017 at https://www.resdac.org/resconnect/articles/117.
- Bewtra M, Fairchild AO, Gilroy E et al. Inflammatory bowel disease patients' willingness to accept medication risk to avoid future disease relapse. Am J Gastroenterol 2015;110:1675–1681. [DOI] [PubMed] [Google Scholar]
- Baer HJ, Glynn RJ, Hu FB et al. Risk factors for mortality in the nurses' health study: a competing risks analysis. Am J Epidemiol 2011;173:319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert C, Schottker B, Brenner H. Smoking and all-cause mortality in older people: systematic review and meta-analysis. Arch Intern Med 2012;172:837–844. [DOI] [PubMed] [Google Scholar]
- Nunes T, Etchevers MJ, Domenech E et al. Smoking does influence disease behaviour and impacts the need for therapy in Crohn's disease in the biologic era. Aliment Pharmacol Ther 2013;38:752–760. [DOI] [PubMed] [Google Scholar]
- Lawrance IC, Murray K, Batman B et al. Crohn's disease and smoking: is it ever too late to quit? J Crohns Colitis 2013;7:e665–e671. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.