Abstract
Rothmund-Thomson syndrome (RTS) is a rare autosomal recessive disorder characterized by poikiloderma, small stature, sparse hair, skeletal abnormalities, increased risk of osteosarcoma, and decreased bone mass. To date, there has not been a comprehensive evaluation of the prevalence and extent of metabolic bone disease in RTS. Furthermore, the mechanisms that result in this phenotype are largely unknown. In this report, we provide a detailed evaluation of 29 individuals with RTS with respect to their metabolic bone status including bone mineral density, calcium kinetics studies, and markers of bone remodeling. We show that individuals with RTS have decreased areal bone mineral density. Additionally, we demonstrate that the presence of pathogenic variants in RECQL4 and low bone mineral density correlate with the history of increased risk of fractures. Using a RECQL4-deficient mouse model that recapitulates skeletal abnormalities seen in individuals with RTS, we demonstrate that generalized skeletal involvement is likely due to decreased osteogenesis. Our findings are clinically relevant as they may help in the risk stratification of patients with RTS and also in the identification of individuals who may benefit from additional surveillance and management of metabolic bone disease.
Introduction
Rothmund-Thomson syndrome (RTS) is a rare autosomal recessive disorder that presents with involvement of multiple organ systems. Although the manifestations are variable, typical features include a characteristic skin rash called poikiloderma that appears in infancy, sparse hair, proportionate small stature, gastrointestinal disturbances, skeletal anomalies, and a significantly increased risk for developing osteosarcoma and skin cancers (1,2). RECQL4, which encodes an ATP-dependent DNA helicase, is the only known gene associated with RTS. Pathogenic variants in the gene are found in nearly two-thirds of affected individuals, designated as Type 2 RTS (3). The causative gene(s) for Type 1 RTS, which lacks pathogenic variants in RECQL4, has not yet been described.
Skeletal abnormalities have previously been described in individuals with RTS as well as in mouse models of RECQL4 deficiency that recapitulate the human bone phenotypes (4–7). We have previously reported that a majority (75%) of individuals with RTS have some form of skeletal abnormalities including radial aplasia or hypoplasia, synostoses, abnormal metaphyseal trabeculation, brachymesophalangy, and patellar defects (8). In addition to these localized skeletal anomalies, osteopenia, pathologic fractures, and delayed fracture healing have also been observed, suggesting that loss of RECQL4 function may lead to a more systemic skeletal involvement (1,8–11). Low areal bone mineral density (aBMD) in individuals with RTS could be due to multiple factors including a central role of RECQL4 in bone development, nutrient deficiencies due to gastrointestinal and feeding issues, and limited exposure to sunlight. Low aBMD and its associated risk for pathologic fractures can add to the significant morbidity in this disorder. However, to date, a systematic evaluation of bone remodeling, aBMD, history of fracture, and mechanisms underlying the metabolic bone disease in RTS has not been performed. Here, we report on a detailed investigation of metabolic bone disease in 29 individuals with RTS. Furthermore, using a murine model of RTS that recapitulates human skeletal disease (Prx1-Cretg/+; Recql4fl/fl), we show that decreased osteogenesis contributes to the generalized skeletal involvement in RTS.
Results
Twenty-nine individuals with RTS (13 males, 16 females) were enrolled in this study. The study population included both children (n = 20; median age 5 years) and adults (n = 9; median age 36 years). Eighteen had pathogenic variants in both alleles of RECQL4; two had a heterozygous pathogenic variant (Type 2 RTS: n = 20); and nine had no detectable pathogenic variants (Type 1 RTS: n = 9) (Table 1).
Table 1.
Characteristics of RTS subjects
| Subject | Age at enrollment (Year, month) | Sex | Pathogenic Variants in RECQL4 | Weight-for -age Z-score | Height-for-age Z-score | WB aBMD Z-score | LS aBMD Z-score | TH aBMD Z-score | FN aBMD Z-score | Fat mass/ Height2 percentile | Lean mass/ Height2 percentile | History of fractures | Number of fracturesa | Age in yrs at first fracture |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 Y, 0 m | F | (1) c.1483 + 27_1483 + 37del | −4.5 | −5.0 | NA | NA | NA | NA | NA | NA | – | 0 | NA |
| (2) c.2636del | ||||||||||||||
| 2 | 1 Y, 9 m | F | ND | 1.7 | −0.4 | NA | −2.4 | NA | NA | NA | NA | – | 0 | NA |
| 3 | 1 Y, 10 m | F | (1) c.2269C>T/p.Gln757Ter | −1.9 | −3.4 | NA | NA | NA | NA | NA | NA | – | 0 | NA |
| (2) c.2476C>T/p.Arg826Ter | ||||||||||||||
| 4 | 3 Y, 5 m | F | (1) c.1573del | −4.2 | −3.0 | NA | −2.4 | NA | NA | NA | NA | – | 0 | NA |
| (2) c.3502 + 44_3502 + 96del | ||||||||||||||
| 5 | 4 Y, 1 m | M | (1) c.2719C>T/p.Gln907Ter | −7.3 | −5.0 | NA | −4.6 | −5.1 | −4 | NA | NA | + | 1 | 1 |
| (2) c.2719C>T/p.Gln907Ter | ||||||||||||||
| 6 | 4 Y, 5 m. | M | (1) c.1573del | −0.2 | −0.2 | 0.11 | −1.0 | −1.1 | −0.1 | NA | NA | – | 0 | NA |
| (2) c.2269C>T/p.Gln757Ter | ||||||||||||||
| 7 | 4 Y, 11 m | F | (1) c.3284_3287dupAGCG | 0.9 | 2.1 | NA | NA | NA | NA | NA | NA | – | 0 | NA |
| 8 | 5 Y, 7 m | M | (1) c.118 + 27_118 + 51del | −4.4 | −3.9 | −0.39 | −2.7 | −3.3 | −2.4 | NA | NA | – | 0 | NA |
| (2) c.2886-2A>T | ||||||||||||||
| 9 | 5 Y, 9 m | F | (1) c.2269C>T/p.Gln757Ter | −0.8 | 0.6 | NA | NA | NA | NA | NA | NA | + | 2 | 5 |
| (2) c.2269C>T/p.Gln757Ter | ||||||||||||||
| 10 | 5 Y, 10 m. | F | (1) c.2161C>T/p.Arg721Ter | −1.1 | −1.4 | −1.18 | −2.8 | 0 | 0.1 | NA | NA | + | 1 | 5 |
| (2) c.2269C>T/p.Gln757Ter | ||||||||||||||
| 11 | 5 Y, 11 m | F | ND | −8.1 | −7.7 | −2.32 | −3.0 | −2.9 | −1.5 | NA | NA | – | 0 | NA |
| 12 | 6 Y, 6 m | M | (1) c.1573del | −1.6 | −3.1 | −1.33 | −2.4 | −2.6 | −2 | NA | NA | + | 2 | 3 |
| (2) c.1878 + 32_1878 + 55del | ||||||||||||||
| 13 | 8 Y, 1, | M | (1) c.2269C>T/p.Gln757Ter | −4.9 | −4.1 | NA | NA | NA | NA | NA | NA | – | 0 | NA |
| (2) c.2662C>T/p.Gln888Ter | ||||||||||||||
| 14 | 8 Y, 5 m | F | ND | −3.6 | −3.4 | −1.31 | −1.2 | −1.3 | −0.7 | 14 | 5 | + | 2 | <1 |
| 15 | 8 Y, 8 m | F | (1) c.1015_1016insC | −3.4 | −3.0 | NA | NA | NA | NA | NA | NA | – | 0 | NA |
| (2) c.2269C>T/p.Gln757Ter | ||||||||||||||
| 16 | 8 Y, 11 m | M | (1) c.2269C>T/p.Gln757Ter | 2.8 | 2.3 | NA | NA | NA | NA | NA | NA | + | 1 | 3 |
| (2) c.2269C>T/p.Gln757Ter | ||||||||||||||
| 17 | 9 Y, 1 m | M | ND# | −0.2 | −0.5 | −0.4 | 0.2 | −1.0 | −0.2 | 28 | 30 | + | 1 | 9 |
| 18 | 9 Y, 9 m | M | ND | −2.6 | −3.2 | −1.4 | −2.1 | −2.4 | −1.7 | 35 | 7 | + | 1 | 7 |
| 19 | 9 Y, 9 m | M | ND | −2.0 | −2.7 | −1.4 | −3.1 | −2.1 | −1.5 | 32 | 8 | – | 0 | NA |
| 20 | 15 Y, 0 m | F | ND | −3.0 | −1.2 | −2.2 | −2.6 | −4.4 | −4.6 | 4 | 1 | + | 3 | 6 |
| 21 | 24 Y, 7 m | F | (1) c.2492_2493del # | −2.8 | −3.8 | −0.5 | −1.2 | −1.5 | −0.7 | 36 | 16 | + | 4 | 10 |
| (2) c.3283_3284insGAGC# | ||||||||||||||
| 22 | 30 Y, 0 m | F | (1) c.3276del # | −0.9 | −1.6 | −1.2 | −1.3 | −1.4 | −1.3 | 38 | 18 | + | 9 | 2 |
| (2) c.2492_2493del # | ||||||||||||||
| 23 | 33 Y, 0 m | M | (1) c.1391-1G>A # | −2.4 | −3.7 | −2.1 | −2.3 | −2.0 | −2.2 | 53 | 13 | + | 11 | 15 |
| (2) c.1573del # | ||||||||||||||
| 24 | 34 Y, 3 m. | M | (1) c.1573del | −1.5 | −2.7 | −1.1 | 0 | −0.8 | −1.0 | 46 | 3 | + | 2 | 5 |
| (2) c.1878 + 32_1878 + 55del | ||||||||||||||
| 25 | 36 Y, 3 m | F | (1) c.1222C>T/p.Gln408Ter | 1.0 | −0.5 | 1.1 | 0.2 | 0.1 | 0 | 55 | 57 | + | 5 | 8 |
| (2) c.2555_2560del | ||||||||||||||
| 26 | 36 Y, 6 m | M | (1) c.2207_2208insC | −2.0 | −3.6 | −0.9 | −1.3 | −0.6 | −0.6 | 24 | 19 | – | 0 | NA |
| (2)c.3061C>T/p.Arg1021Trp | ||||||||||||||
| 27 | 44 Y, 4 m | M | (1) c.2492_2493del | −3.6 | −3.7 | −1.2 | −2.2 | −1.3 | −1.7 | 16 | 1 | + | 12 | 5 |
| 28 | 48 Y, 2 m | F | ND | −0.3 | −1.1 | 1.6 | 1.0 | 0.4 | 0.2 | 21 | 19 | – | 0 | NA |
| 29 | 49 Y, 11 m | F | ND | 1 | −2.1 | 0.6 | −0.2 | 0.7 | 0.8 | 73 | 68 | – | 0 | NA |
M - male; F- female; ND – mutation not detected; + and – denote presence and absence of fracture history, respectively; NA - not applicable. #Sequencing results performed by a research laboratory at Baylor College of Medicine (L.L.W.); WB – whole body; LS – lumbar spine; TH – total hip; FN – femoral neck; aNumber of reported fractures at time of enrollment.
Areal bone mineral density and fracture risk in RTS
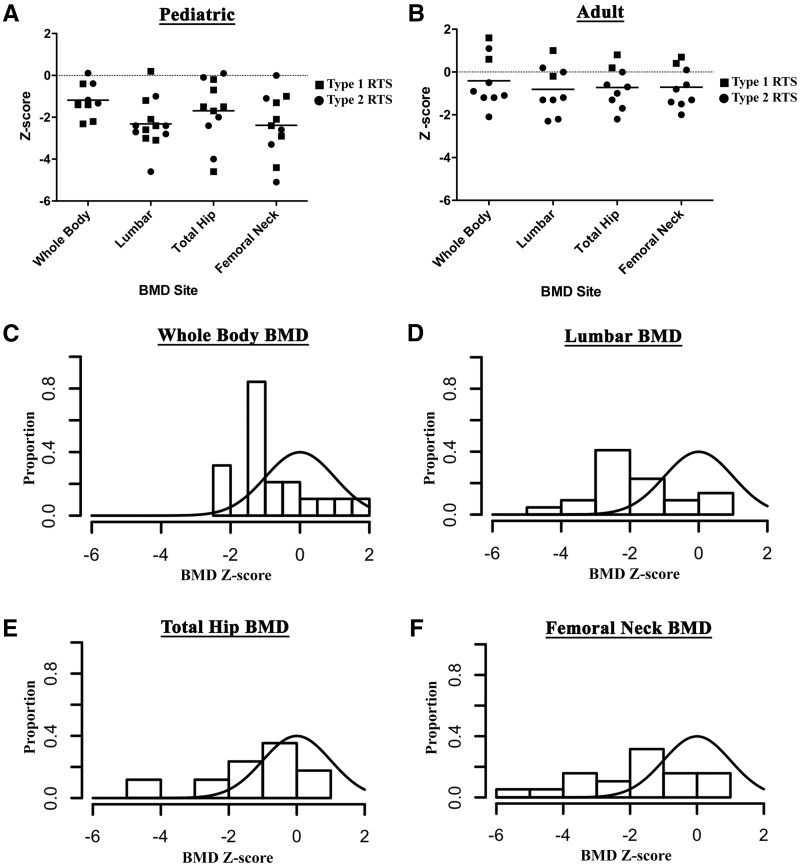
Densitometric measurements were obtained from 22 individuals (13 pediatric and 9 adult). aBMD is the most widely used diagnostic measure to assess the risk for fracture and is a surrogate measure for biomechanical strength of bone (12,13). aBMD was assessed at the whole body, lumbar spine, total hip, and femoral neck (Table 1). The median Z-scores (interquartile range) for aBMD-for-age at the various sites were: −1.3 (−1.6 to −0.4) in children and −0.9 (−1.2 to 0.94) in adults for whole body; −2.4 (−2.9 to −1.7) in children and −1.2 (−1.8 to 0.1) in adults at the lumbar spine; −1.5 (−2.4 to −0.2) in children and −0.7 (0.1 to −1.5) in adults at total hip; and −2.4 (−3.3 to −1.1) in children and −0.8 (−1.5 to 0.3) in adults at the femoral neck. The median aBMD-for-age Z-scores at all sites were statistically lower (P < 0.01) than the population reference value of zero (Fig. 1A and B), and the distribution was negatively skewed as compared to the expected distribution in the general population (Fig. 1C to F). The proportion of individuals with low bone density in RTS as defined by aBMD-for-age Z-score < −2.0 was significantly elevated from the expected ratios in the general population. More importantly, this feature was statistically significant (P< 0.05) irrespective of the site of aBMD measurement; 55, 37, 24, and 16% of the study population met the criteria for low aBMD at the lumbar spine, femoral neck, total hip, and whole body sites, respectively.
Figure 1.
aBMD-for-age Z-score in children (A) and adults (B) with RTS. Each dot represents the Z-score of a single subject at the specified site. The horizontal line represents the median of the population. Distribution of aBMD-for-age Z-scores in RTS subjects as compared to the expected normal distribution for (C) whole body, (D) lumbar spine, (E) total hip, and (F) femoral neck.
The diagnosis of osteoporosis in the pediatric population requires both aBMD-for-age Z-score ≤ −2.0 and a clinically significant fracture history (14). Since the proportion of individuals with Z-scores ≤ −2.0 in this cohort was significantly elevated, we specifically collected information about the presence or absence of fractures and total number of fractures. The fracture data comprised self-reported information by the subjects or their parent(s). In most cases, radiographs were not available to confirm the history of fractures. Forty five percent of children (9/20) and 67% (6/9) of adults reported at least one fracture by the time of enrollment (Table 1). Among the subjects with at least one reported fracture, 67% (10/15) reported two or more fractures. Whereas the cross sectional nature of the study and the wide age range of participants precludes calculation of fracture incidence, the fact that most adults enrolled in the study reported fractures in adulthood and two subjects reported greater than 10 fractures in their lifetime suggests that there is clinically significant increase in bone fragility in RTS.
The presence of pathogenic variants in RECQL4 has been previously been correlated with a severe bone phenotype in RTS (8,15). We therefore investigated the potential risk factors for fractures. The association of RECQL4 mutation status and whole body and lumbar spine aBMD-for-age Z-scores with the number of fractures at the age of enrollment was assessed by the multivariate Poisson regression model (Supplementary Material, Table S1). The age and height of the subjects were incorporated in the analysis model. RECQL4 mutation status (P = 0.0001) and lumbar spine aBMD-for-age Z-score ≤ −2.0 (P = 0.0006) were found to be statistically associated with the number of fractures. On average, when compared to another person of the same age and height, the presence of a RECQL4 mutation in an individual increased the number of fractures by about five fold (RR 5.32, 95% CI 2.27–15.68, Supplementary Material, Table S1). Similarly, a lumbar spine aBMD-for-age Z-score ≤ −2.0 in a person increased the number of fractures by about three fold (RR 3.16, 95% CI, 1.76–5.77). These data suggest that low bone mineral density is not only an important phenotypic manifestation of RTS but is also associated with a history of an increased risk for fracture.
Evaluation for causes of low aBMD
Abnormalities of calcium homeostasis due to dietary problems in RTS or decreased vitamin D synthesis due to limited sun exposure to avoid skin cancer are potential contributing factors to the low BMD. We hence measured markers that are involved in the regulation of calcium homeostasis. Serum levels of calcium, magnesium, phosphorus, and parathyroid hormone were within the normal age-adjusted limits in all subjects (Supplementary Material, Table S2). However, the level of 25-hydroxy vitamin D was low (<30ng/ml) in 52% (13 of 25) of subjects; amongst these individuals, 54% (7/13) had levels in the insufficiency range (20–30 ng/ml), and 46% (6/13 subjects) had levels in the deficiency range (<20 ng/ml). This prevalence of vitamin D deficiency, however, is comparable to the general population in the US, wherein nearly two-thirds have low vitamin D levels (16–18).
To further determine if abnormalities in calcium metabolism in RTS could be contributing to the low aBMD, particularly with respect to deposition in the bone, 13 individuals underwent calcium kinetic studies (19,20). The parameters assessed in the calcium kinetic studies included the size of the exchangeable calcium pool in bone (EP) and the rate of calcium deposition in bone (V0+) (19). The median age of participants in this substudy (6 males, 7 females) was 8 years (range 1–49 years). DXA scans were performed in eight individuals who participated in calcium kinetic studies. The median whole body aBMD-for-age Z-score in these individuals was −1.26 (IQR 0.9 to −1.4) and median lumbar spine aBMD-for-age Z-score was −2.4 (IQR −0.2 to −3.1). EP and V0+ were found to be within the age-adjusted normal ranges for all subjects (Table 2) (21–24).
Table 2.
Calcium kinetic parameters in RTS subjects
| Subject | Exchangeable pool of bone EP (g) | Bone calcium deposition Vo + (g/d) | Rate constant Ko + (d−1) |
|---|---|---|---|
| 1 | 2.5 | 1.0 | 0.4 |
| 5 | 2.6 | 1.0 | 0.4 |
| 7 | 6.7 | 2.0 | 0.3 |
| 9 | 7.8 | 1.5 | 0.2 |
| 10 | 5.4 | 1.9 | 0.4 |
| 12 | 5.0 | 1.5 | 0.3 |
| 13 | 3.7 | 1.1 | 0.3 |
| 15 | 6.5 | 1.1 | 0.2 |
| 16 | 10.8 | 3.9 | 0.4 |
| 18 | 6.1 | 1.9 | 0.3 |
| 19 | 5.5 | 1.6 | 0.3 |
| 28 | 4.3 | 0.6 | 0.1 |
| 29 | 4.6 | 0.8 | 0.2 |
| Reference: | 2.2–6.7 | 0.9–2.6 | 0.2–0.6 |
| Prepubertal(21) | |||
| Reference: | 2.9–7.7 | 0.3–1.1 | 0.1–0.2 |
| Healthy adult (age 23–39 years) (21) |
Low aBMD is typically a result of alteration in the balance between the rates of bone formation and resorption. To assess the bone remodeling status, we measured plasma levels of osteocalcin, a marker of osteoblastic activity and bone formation, and urinary levels of collagen N-telopeptide (NTX) corrected for creatinine, a marker of bone resorption (25–27). For individuals older than six years at the time of study, age-appropriate normalized values were used as the reference range for osteocalcin and NTX. In individuals six years of age or younger, where reference ranges have not been established, we used the 7–9-year age range reference values. Osteocalcin was elevated in only one individual, while it was low in 26% (6/23). NTX was not elevated in any individual, but was low in 17% (4/24) of subjects (Supplementary Material, Table S2).
Ablation of Recql4 in skeletal progenitor cells leads to low bone mass in mice
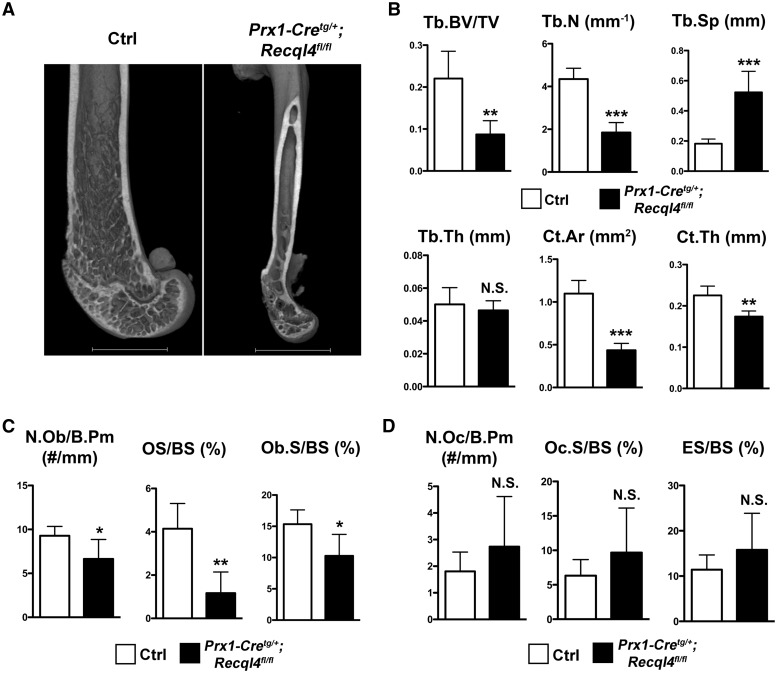
In order to test whether the low bone mass phenotype in RTS is specifically due to a primary skeletal developmental defect, we analyzed conditional knockout mice with loss of Recql4 in the skeletal progenitor cells (6). We generated these mice by breeding mice with floxed alleles of Recql4 with transgenic mice expressing Cre-recombinase under the paired-related homeoboxgene-1 (Prx1) promoter. We chose to use this specific conditional model because the skeletal anomalies observed in this murine model closely mimic those observed in humans with RTS (6). We have previously demonstrated that mice lacking Recql4 in skeletal progenitor cells (Prx1-Cretg/+; Recql4fl/fl) exhibited foreshortened limbs, digit defects, craniosynostosis, and growth plate defects at birth (6). In this study, we assessed the postnatal bone development in these mutant mice. As the Prx1-Cre transgene is not expressed in the vertebral bone (28), femora were used to study the postnatal bone development. At 3 months of age, Prx1-Cretg/+; Recql4fl/fl mice demonstrated significantly reduced bone volume (Fig. 2A). Because micro-computed tomography (µCT) showed similar findings for both Prx1-Cretg/+; Recql4fl/fl male and female mice, only male mice were used for further studies. µCT analysis demonstrated that Prx1-Cretg/+; Recql4fl/fl mice displayed more than 50% reduction of trabecular bone volume fraction (bone volume/total volume, BV/TV) and trabecular number (Tb.N) with a corresponding two-fold increase in trabecular separation (Tb.Sp) (Fig. 2B, Table 3); the trabecular thickness (Tb.Th) appeared to be similar to control mice (Fig. 2B, Table 3). In addition, Prx1-Cretg/+; Recql4fl/fl mice also showed more than 50% reduction of cortical bone area, and significantly reduced cortical thickness (Fig. 2B, Table 3). To further analyze the cellular basis of the bone phenotype, we performed bone histomorphometric studies. The number of osteoblasts per bone perimeter (N.Ob/B.Pm) in Prx1-Cretg/+; Recql4fl/fl mice was significantly reduced compared to the control mice (Fig. 2C, Supplementary Material, Table S3). The surface of osteoid and newly formed bone matrix was reduced by more than 50% in Prx1-Cretg/+; Recql4fl/fl mice (Fig. 2C, Supplementary Material, Table S3). The osteoblast surface in Prx1-Cretg/+; Recql4fl/fl mice was also significantly reduced compared to littermate controls (Fig. 2C, Supplementary Material, Table S3). Osteoclast number and function in Prx1-Cretg/+; Recql4fl/fl mice were not significantly changed compared to littermate controls (Fig. 2D, Supplementary Material, Table S3). These data suggest that loss of Recql4 in the skeletal progenitor cells causes reduced osteoblast number and reduced osteoid, which could contribute to the low bone volume in these mice.
Figure 2.
Mice lacking Recql4 in skeletal progenitor cells have low bone mass. (A) Representative 3D µCT images of distal femurs from Prx1-Cretg/+; Recql4fl/fl and littermate control (Crtl) mice. (B) µCT analyses of distal femoral trabecular bone and cortical bone showing Tb. BV/TV, Tb.N, Tb.Sp, Tb.Th, Ct.Ar, and Ct.Th parameters in Prx1-Cretg/+; Recql4fl/fl and Ctrl male mice at 3 months of age. n = 6 per group, *P < 0.05, **P < 0.01, ***P < 0.001, N. S., not significant (Student's t-test). Data are expressed as mean ± S.D. (C) Histomorphometric analyses of distal femoral trabecular bone showing osteoblast parameters: N.Ob/B.Pm, OS/BS, and Ob.S/BS. n = 5 for both groups, *P < 0.05, **P < 0.01, ***P < 0.001 (Student's t-test). Data are expressed as mean ± S.D. (D) Histomorphometric analyses of distal femoral trabecular bone showing osteoclast parameters: N.Oc/B.Pm, Oc.S/BS, and ES/BS. n = 5 for both groups, N.S., not significant (Student's t-test). Data are expressed as mean ± s.d. Tb. BV/TV = trabecular bone volume fraction; Tb.N = trabecular number; Tb.Sp = trabecular separation; Tb.Th = trabecular thickness; Ct.Ar = cortical area; Ct.Th = cortical thickness; N.Ob/B.Pm = number of osteoblasts per bone perimeter; OS/BS = osteoid surface (per bone surface); Ob.S/BS = osteoblast surface; N.Oc/B.Pm = number of osteoclasts per bone perimeter; Oc.S/BS = osteoclast surface; ES/BS = eroded surface. All the parameters have been presented in detail in Table 3 and Supplementary Material, Table S3.
Table 3.
µCT parameters for femoral trabecular and cortical bone
| Littermate Control | Prx1-Cretg/+; Recql4fl/fl | P Value | |
|---|---|---|---|
| Trabecular parameters | |||
| Femur length (mm) | 15.5±0.3 | 9.4±0.2 | <0.0001 |
| Bone volume fraction (BV/TV) | 0.22±0.065 | 0.087±0.033 | 0.0012 |
| Specific bone surface (BS/BV, mm2/mm3) | 41.31±8.44 | 43.63±5.02 | 0.577 |
| Connectivity density (Conn.D, 1/mm3) | 166.3±33.8 | 28.2±10.4 | <0.0001 |
| Trabecular number (Tb.N, 1/mm) | 4.349±0.505 | 1.847±0.468 | <0.0001 |
| Trabecular thickness (Tb.Th, mm) | 0.05±0.01 | 0.046±0.006 | 0.459 |
| Trabecular separation (Tb.Sp, mm) | 0.182±0.031 | 0.523±0.139 | 0.0002 |
| Cortical parameters | |||
| Total cross-sectional area (Tt.Ar, mm2) | 1.119±0.157 | 0.448±0.081 | <0.0001 |
| Cortical bone area (Ct.Ar, mm2) | 1.098±0.154 | 0.436±0.08 | <0.0001 |
| Cortical area fraction (Ct.Ar/Tt.Ar) | 0.982±0.002 | 0.972±0.003 | 0.0003 |
| Cortical thickness (Ct.Th, mm) | 0.225±0.023 | 0.174±0.013 | 0.0019 |
| Polar moment of inertia (J, mm4) | 0.6996±0.163 | 0.076±0.038 | <0.0001 |
Values are expressed as mean ± SD. n = 6 for both groups.
Discussion
RTS is a rare autosomal recessive disorder caused by deficiency of RECQL4, a DNA helicase belonging to the family of RecQ helicases (29,30). It is one of the three Mendelian human conditions resulting from deficiency of RecQ helicases, the other two being Bloom syndrome and Werner syndrome. Individuals with RTS exhibit significant phenotypic variability in terms of both spectrum and severity of clinical features, which include small stature, pokilodermatous skin rash, sparse hair, hyperkeratosis, dental abnormalities, gastrointestinal disturbance, and propensity for developing cancer (1,15,31,32).
Interestingly, nearly three-fourths of individuals with RTS have developmental skeletal abnormalities including absent or malformed bones, radial ray defects, and synostoses, whereas at least a fourth have osteopenia detected on skeletal surveys and history of childhood fractures (1,8). Using conditional knockout models of RECQL4 deficiency in the early skeletal progenitor cells (Prx1-Cretg/+; Recql4fl/fl) and chondrocytes (Col2a1-Cretg/+; Recql4fl/fl), we previously demonstrated that loss of RECQL4 led to increased activity of p53. This enhanced p53 activity led to decreased cell proliferation and increased apoptosis resulting in developmental anomalies of the skeleton (6). However, to date, the extent and the mechanisms behind the more generalized metabolic bone phenotype and low bone density in patients with RTS have not been systematically investigated. In this study, we conducted a detailed clinical investigation of the bone phenotype in 29 individuals with RTS. The strengths of the study are that it is the largest study to date evaluating metabolic bone disease in individuals with RTS; the assessments including DXA and calcium kinetic studies were performed in a single institution using validated methods; and the human phenotypes we observed were concordant with the mechanistic studies performed in the genetic mouse model.
While many RTS individuals had aBMD within the normal ranges (> −2.0), the median aBMD Z-scores at all sites were lower than the population reference value of zero and the distribution of aBMD was negatively skewed. These data suggest that individuals with RTS are indeed predisposed to having a lower bone mass. Detailed clinical and laboratory evaluation, including calcium kinetic studies, did not reveal any abnormalities of calcium or mineral metabolism. Markers of bone remodeling were within normal ranges in most subjects, but in about a fifth of the individuals, osteocalcin or NTX levels were below the lower limit of normal. These results suggest that a low bone turnover state may be a cause of the low bone density in RTS. Concordantly, our studies in a murine model of RTS showed that mice with loss of Recql4 in skeletal progenitor cells had reduced osteoblast numbers and osteoid surface resulting in lower bone mass in both the cortical and lamellar bone. Ng and colleagues have also shown that RECQL4 is required for osteoblast expansion (5). Our previous studies demonstrated that RECQL4 deficiency led to increased p53 activity (6). p53 has been shown to be a negative regulator of osteoblastogenesis (33,34). It is thus likely that the low bone density in RTS is secondary to decreased osteoblast numbers and function.
Self-reported fracture history was used to identify potential correlates of fracture in our RTS patient population. Even though this is the largest cohort of individuals with RTS to have evaluation of bone mass, the sample size and the power of the study are limited given the rarity of the disorder. Therefore, these analyses should be considered exploratory. RECQL4 mutation status and lumbar spine aBMD Z-score < −2.0 correlated with fracture risk. This finding of correlation of RECQL4 mutation status is consistent with our previous work, which demonstrated that presence of detectable pathogenic variants portends a more severe bone phenotype (8,15). Interestingly, lumbar spine aBMD greater than −2.0 was predicted to decrease the fracture risk in our cohort of individuals with RTS. This raises clinically important questions of how to monitor and treat the metabolic bone disease in RTS.
Based on our results, we recommend assessment of aBMD in individuals with RTS (14). DXA is a safe and non-invasive technique, and thus a baseline assessment of aBMD would be reasonable in all individuals with RTS; however, the frequency and interval for repeat testing is not known. The question of how to monitor and treat individuals with low BMD in RTS is rather difficult to address. Generally, in children with predisposition to genetic forms of low bone mass and increased bone fragility, the primary measures to optimize bone health include nutritional measures – calcium and vitamin D supplementation in particular, and physical activity. The normal calcium kinetic studies in RTS show that there is normal incorporation of calcium into the matrix, and thus vitamin supplementation consistent with recommended daily intake are reasonable to use rather than higher doses.
Pharmacotherapy to increase aBMD and decrease fracture risk generally involves treatment with antiresorptive agents that inhibit activity of osteoclasts and decrease bone resorption, or anabolic agents that stimulate bone formation. Bisphosphonates, synthetic analogs of pyrophosphate, and denosumab, a monoclonal antibody against RANKL are the more commonly used antiresorptive medications in adults with osteoporosis. Whereas the treatment guidelines for adults with osteoporosis have been established, the indications, timing, duration, risks, and benefits of bisphosphonate therapy in children are unclear (35). Bisphosphonates have been repurposed for the treatment of pediatric forms of osteoporosis (35,36). Generally, pharmacotherapy in children would be considered in the following situations: 1) children with low bone density and history of fragility fracture of long bones or vertebral fractures; and 2) children without fractures in whom quality of life would be significantly impacted by fracture. We recommend that patients or care providers keep detailed records of fracture history, including site and type of fracture, reason for fracture, healing time, and fracture-associated complications. Such history would be useful in determining whether bisphosphonate therapy would be warranted. Teriparatide is the only bone anabolic agent that is currently approved for human use by the Food and Drug Administration. Preclinical toxicological studies with teriparatide showed increased incidence of benign bone tumors and osteosarcomas with prolonged use (37,38). Though human experience has not revealed any obvious association between teriparatide and osteosarcoma, given the significantly elevated risk of osteosarcoma in RTS, this medication would be best avoided (39).
The findings of this study are to be taken in the context of the limitations of the data set. First, this was a cross sectional study on a limited study population. One time measurement of aBMD, irrespective of the accuracy of the machine and the expertise of the operator, is less informative than serial measurements. We do not have longitudinal data on subjects to show the change in aBMD with age and thus cannot make any conclusions on whether the decreased osteogenesis in RTS translates into reduced peak bone mass. Second, individuals with RTS are typically below the 5th percentile for height, and thus age-adjusted Z-scores may overestimate the magnitude of low bone mass. One way to address this would be to try to normalize the bone mineral content and density to height. Although this could be done using mathematical models, the applicability especially in a genetic form of low bone mass is unknown. Moreover, sex- and age-adjusted Z-scores are the typical parameters reported on clinical DXA reports, and thus we chose to use these parameters for statistical analyses. Third, with the varied ages of the patients and the absence of information on hormonal status, we could not assess the effect of puberty on bone mass. Fourth, the fracture history was self-reported and could not be confirmed by the radiographs or medical records for all subjects. Thus, any such retrospective data are subject to recall bias.
Overall, the results of this study have important implications for follow-up and management of low bone mass in RTS. We recommend a baseline DXA at time of diagnosis in order to establish baseline measurement of aBMD. We recommend screening for pathogenic variants in RECQL4 for any potential new patient since RECQL4 status is correlated with a more severe disease phenotype. Supplementation with calcium or vitamin D beyond the recommended daily intake is not warranted. A detailed history of fractures should be maintained. Bisphosphonates should be considered for the treatment of individuals with history of multiple fractures, particularly involving long bones or vertebral fractures.
Materials and Methods
Human subjects
The Institutional Review Board for Human Subject Research at Baylor College of Medicine, Houston, TX reviewed the study protocol and approved all study procedures.
Twenty-nine individuals with a clinically confirmed diagnosis of RTS were enrolled after signing informed consent to participate in the study. Research procedures were performed at the General Clinical Research Center at Texas Children’s Hospital, Houston, TX. Medical history was collected by interview and review of medical records. Fracture history was obtained from questionnaires administered to the subjects or their parents. Laboratory analyses including complete blood count, serum chemistries, 25-hydroxy vitamin D, intact PTH, osteocalcin, and urinary collagen N-telopeptide were performed in the CLIA- and CAP-certified clinical diagnostic laboratory of Texas Children’s Hospital, Houston, TX. RECQL4 molecular testing was performed in the Medical Genetics Laboratory of Baylor College of Medicine, a CLIA-certified laboratory (n = 25); four individuals (Subjects 17, 21–23) had sequencing done in the research laboratory in a research laboratory (L.L.W.) prior to clinical availability in a CLIA-certified commercial laboratory.
Densitometry analyses
Twenty-two individuals had aBMD assessed by DXA using a Hologic Delphi-A instrument (Bedford, MA) at the Body Composition Laboratory of the Children’s Nutrition Research Center, Houston, TX, which has extensive experience in measuring bone density and have performed over 16,000 DXA scans (40–43). Scans were performed of the whole body, lumbar spine, and proximal femur. Bone mineral content (BMC), bone area, and BMD were measured using Hologic Discovery V12.1 analysis software. For adult subjects, BMD Z-scores of the whole body, lumbar spine, left total hip, and left femoral neck were calculated using the Hologic Reference Database. Due to limitations in this for the pediatric population, validated age- and sex-matched control data from the pediatric population generated by the Body Composition Laboratory of the Children’s Nutrition Research Center, Houston, TX were used to calculate the Z-scores for pediatric subjects as previously published (40). Height-for-age and weight-for-age Z-scores were calculated using the World Health Organization reference data for children younger than two and for children and adults older than two, the Centers for Disease Control reference data was used (44,45). Percentile values for soft tissue parameters are based on gender- and ethnicity-specific normative data for subjects eight years of age or older, obtained from the National Health and Nutrition Examination Survey (NHANES) (46). References are available for Fat Mass Index (FMI: Fat Mass/Height ^2) as well as for Lean Mass/Height ^2. The NHANES reference data were modeled using the LMS curve fitting technique proposed by Cole, and the subsequent age-based LMS factors are used to produce the percentile results for each DXA scan (47).
Calcium kinetics studies
The calcium kinetic studies were performed as described previously (19,21). Subjects consumed breakfast and 180 ml of calcium-fortified orange juice to which 20 µg of 46Ca stable isotope was added. The total dietary intake of calcium with breakfast was 300 mg. Immediately after breakfast, subjects received 5 mg of 42Ca intravenously. Serum samples for calcium isotope ratio measurements were obtained at 6, 12, 20, 40, 120, 180, 240, and 480 minutes after the infusion. A 24-h urine collection was obtained on the day of calcium administration (Day 1) after which urine aliquots were collected every 8 hours for another 24 hours (Day 2). Spot urine samples were collected daily for the next five days (Days 3–8). (23). Subjects received a food scale and instructions for recording their dietary intake (i.e., weighed food record) for three days starting on Day 2. Nutrient intakes were analyzed by the study dietitian (KH) using Nutrient Data Systems for Research software developed by the Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN.
The compartmental model used for calcium kinetics was adapted for use in children and adolescents, as described previously (22). This model is based on three sequential pools before calcium deposition in the "deep" bone calcium pool. The mass of the third compartment is referred to as the exchangeable pool of bone (48). Bone calcium deposition rate is the rate of calcium flow to the final pool. Data were modeled with the use of the SAAM (Simulation, Analysis and Modeling) program. The ratio of Vo+ and Ep was determined as a rate constant to evaluate the activity of the exchangeable calcium pools (48). Endogenous fecal excretion of calcium was estimated as 1.5 mg/kg/day (49).
Generation of mouse model of RTS
The generation of Recql4 mutant mice and all experimental procedures carried out in the animals were reviewed and approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine, Houston, TX. The generation of Prx1-Cretg/+and Recql4fl/fl mice have been described previously (6,28). Prx1-Cretg/+ male mice were crossed with Recqlfl/fl females to generate Prx1-Cretg/+; Recql4fl/+ mice which were subsequently mated with Recqlfl/fl or Recql4fl/+ females to generate mice that lacked Recql4 in the skeletal progenitor cells in mouse limbs. Recqlfl/fl or Recql4fl/+ littermates were used as controls as these mice were indistinguishable from wild-type mice and did not exhibit any observable phenotype.
μCT analysis
µCT scanning of mouse femora was performed using a µCT-40 system (Scanco Medical, Wayne, PA, USA) with an isotropic voxel size of 16 µm. For femoral trabecular bone analysis, the region of interest (ROI) of control femora was manually contoured in 75 slices proximal to the distal femoral growth plate. For Prx1-Cre+; Recql4fl/fl mutants, the number of slices was reduced according to the length of mutant femora which was determined from the top of the femoral head to the bottom of the medial condyle. The trabecular parameters, including BV, TV, BS, connectivity density (Conn.D), Tb.N, Tb.Th, and Tb.Sp were determined using the Scanco software with a threshold value of 210 (50). For femoral cortical bone analysis, the ROI of control femora was contoured in 50 slices at the femoral midshaft, and the number of slices was reduced according to the length of mutant femora. The cortical parameters, including total cross-sectional area (Tt.Ar), Ct.Ar, Ct.Th, and polar moment of inertia were determined using Scanco software.
Bone histomorphometry
Non-decalcified femoral bone samples from Prx1-Cre+; Recql4fl/fl mice and control littermate mice were embedded in methacrylate and sectioned. Distal femoral metaphyses were used for histomorphometric analyses. von Kossa and van Gieson staining were used to quantify mineralized bone parameters including BV, BS, and OS. Toluidine blue staining was used to quantify osteoblast numbers, and tartrate resistant alkaline phosphatase (TRAP) staining was used for osteoclasts.
Statistical methods
Continuous variables were summarized by mean and standard deviation, or if appropriate, median and range or interquartile range. Categorical data were tabulated and summarized. Exact binomial test was used to determine if the proportion of patients with BMD ≤ 2.0 was significantly different from the expected 2.5%. The number of fractures from birth to the time of enrollment can be reasonably assumed to be distributed as Poisson with age as the time index. Hence, the Poisson regression model was used to analyze the number of fractures with potential risk factors including sex, RECQL4 mutation status, whole body, and lumbar spine aBMD Z-scores. The aBMD Z-scores were dichotomized by ≤ −2.0 and > −2.0. Due to the importance of age and height at the time of enrollment for determination of aBMD z-scores, they were always included in the model fitting process. The standard step-wise model fitting process for the univariate and multivariate models was analyzed using R (R Foundation for Statistical Computing, Vienna, Austria). In the mouse study, unpaired, two-sided Student's t-test was used to compare continuous measurements between two groups. P values less than 0.05 were considered to be statistically significant.
Supplementary Material
Supplementary Material is available at HMG online.
Supplementary Material
Acknowledgements
We gratefully acknowledge the patients and families affected by RTS without whom this work would not be possible. We also thank the following people for their contributions to this manuscript: Ta-Tara Rideau for research coordination and Michael Starbuck for technical support and advice.
Conflict of Interest statement. None declared.
Funding
L.L.W. was supported by [AR059063] from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. L.L.W. [HD42136] and B.L. [HD070394] were supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development. F.C. was supported by [CA203270] from the National Cancer Institute. Portions of this work were supported by [AI036211 and CA125123] to the Baylor College of Medicine Advanced Technology Cores, [RR000188-42] to the Baylor College of Medicine General Clinical Research Center, and [HD083092] to the Clinical Translational Core and Tissue Culture Core of the Baylor College of Medicine Intellectual and Developmental Disabilities Research Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Institute of Child Health & Human Development or the National Institutes of Health.
This work was also funded by Doris Duke Charitable Foundation Clinician Scientist Development Program, The Rolanette and Berdon Lawrence Bone Disease Program of Texas, BCM Center for Skeletal Medicine and Biology, BCM Curtis and Doris K. Hankamer Foundation Collaborative Research Grant, Amschwand Sarcoma Cancer Foundation, Carousel Young Friends of Texas Children's Cancer Center, Kurt Groten Family Research Scholar's Program, and Gillson Longenbaugh Foundation.
References
- 1. Wang L.L., Levy M.L., Lewis R.A., Chintagumpala M.M., Lev D., Rogers M., Plon S.E. (2001) Clinical manifestations in a cohort of 41 Rothmund-Thomson syndrome patients. Am. J. Med. Genet., 102, 11–17. [DOI] [PubMed] [Google Scholar]
- 2. Larizza L., Roversi G., Volpi L. (2010) Rothmund-Thomson syndrome. Orphanet J. Rare Dis., 5, 2.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kitao S., Shimamoto A., Goto M., Miller R.W., Smithson W.A., Lindor N.M., Furuichi Y. (1999) Mutations in RECQL4 cause a subset of cases of Rothmund-Thomson syndrome. Nat. Genet., 22, 82–84. [DOI] [PubMed] [Google Scholar]
- 4. Pasagadugula K.V., Chennamsetty T., Avvaru K., Chennamsetty K. (2014) Rare skeletal abnormalities in Rothmund-Thomson syndrome, a case report. Int. J. Dermatol., 55, 460–463. [DOI] [PubMed] [Google Scholar]
- 5. Ng A.J., Walia M.K., Smeets M.F., Mutsaers A.J., Sims N.A., Purton L.E., Walsh N.C., Martin T.J., Walkley C.R. (2015) The DNA helicase recql4 is required for normal osteoblast expansion and osteosarcoma formation. PLoS Genet., 11, e1005160.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lu L., Harutyunyan K., Jin W., Wu J., Yang T., Chen Y., Joeng K.S., Bae Y., Tao J., Dawson B.C.. et al. (2015) RECQL4 regulates p53 function in vivo during skeletogenesis. J. Bone Miner. Res., 30, 1077–1089. [DOI] [PubMed] [Google Scholar]
- 7. Mann M.B., Hodges C.A., Barnes E., Vogel H., Hassold T.J., Luo G. (2005) Defective sister-chromatid cohesion, aneuploidy and cancer predisposition in a mouse model of type II Rothmund-Thomson syndrome. Hum. Mol. Genet., 14, 813–825. [DOI] [PubMed] [Google Scholar]
- 8. Mehollin-Ray A.R., Kozinetz C.A., Schlesinger A.E., Guillerman R.P., Wang L.L. (2008) Radiographic abnormalities in Rothmund-Thomson syndrome and genotype-phenotype correlation with RECQL4 mutation status. Am. J. Roentgenol., 191, W62–W66. [DOI] [PubMed] [Google Scholar]
- 9. Carlson A.M., Thomas K.B., Kirmani S., Lindor N.M. (2012) Chronic tibial nonunion in a Rothmund-Thomson syndrome patient. Am. J. Med. Genet. A, 158A, 2250–2253. [DOI] [PubMed] [Google Scholar]
- 10. Beckmann N. (2015) Multiple low energy long bone fractures in the setting of Rothmund-Thomson syndrome. Case Rep. Med., 2015, 495164.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barisonek K.L., Protzman N.M., Wobst G.M., Brigido S.A. (2014) Delayed union of a Jones fracture in a patient with Rothmund-Thomson syndrome, A Case Report and Review of the Literature. J. Foot Ankle Surg., 55, 291–293. [DOI] [PubMed] [Google Scholar]
- 12. Marshall D., Johnell O., Wedel H. (1996) Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ, 312, 1254–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lotz J.C., Cheal E.J., Hayes W.C. (1991) Fracture prediction for the proximal femur using finite element models, Part I-Linear analysis. J. Biomech. Eng., 113, 353–360. [DOI] [PubMed] [Google Scholar]
- 14. Crabtree N.J., Arabi A., Bachrach L.K., Fewtrell M., El-Hajj Fuleihan G., Kecskemethy H.H., Jaworski M., Gordon C.M. and International Society for Clinical Densitometry. (2014) Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents, the revised 2013 ISCD Pediatric Official Positions. J. Clin. Densitom., 17, 225–242. [DOI] [PubMed] [Google Scholar]
- 15. Wang L.L., Gannavarapu A., Kozinetz C.A., Levy M.L., Lewis R.A., Chintagumpala M.M., Ruiz-Maldanado R., Contreras-Ruiz J., Cunniff C., Erickson R.P.. et al. (2003) Association between osteosarcoma and deleterious mutations in the RECQL4 gene in Rothmund-Thomson syndrome. J. Natl. Cancer. Inst., 95, 669–674. [DOI] [PubMed] [Google Scholar]
- 16. Wei M., Yu R., Deutsch S.C. (2014) Insignificant medium-term vitamin D status change after 25-hydroxyvitamin D testing in a large managed care population. PLoS One, 9, e105571.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ginde A.A., Liu M.C., Camargo C.A. Jr. (2009) Demographic differences and trends of vitamin D insufficiency in the US population, 1988-2004. Arch. Intern. Med., 169, 626–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mansbach J.M., Ginde A.A., Camargo C.A. Jr. (2009) Serum 25-hydroxyvitamin D levels among US children aged 1 to 11 years, do children need more vitamin D?. Pediatrics, 124, 1404–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abrams S.A. (1999) Using stable isotopes to assess mineral absorption and utilization by children. Am. J. Clin. Nutr., 70, 955–964. [DOI] [PubMed] [Google Scholar]
- 20. Abrams S.A. (2010) Calcium absorption in infants and small children, methods of determination and recent findings. Nutrients, 2, 474–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abrams S.A. (1993) Pubertal changes in calcium kinetics in girls assessed using 42Ca. Pediatr. Res., 34, 455–459. [DOI] [PubMed] [Google Scholar]
- 22. Abrams S.A., O'Brien K.O., Stuff J.E. (1996) Changes in calcium kinetics associated with menarche. J. Clin. Endocrinol. Metab., 81, 2017–2020. [DOI] [PubMed] [Google Scholar]
- 23. Abrams S.A., O'Brien K.O., Liang L.K., Stuff J.E. (1995) Differences in calcium absorption and kinetics between black and white girls aged 5–16 years. J. Bone. Miner. Res., 10, 829–833. [DOI] [PubMed] [Google Scholar]
- 24. Abrams S.A., Copeland K.C., Gunn S.K., Gundberg C.M., Klein K.O., Ellis K.J. (2000) Calcium absorption, bone mass accumulation, and kinetics increase during early pubertal development in girls. J. Clin. Endocrinol. Metab., 85, 1805–1809. [DOI] [PubMed] [Google Scholar]
- 25. Delmas P.D., Eastell R., Garnero P., Seibel M.J., Stepan J. (2000) The use of biochemical markers of bone turnover in osteoporosis. Osteoporos. Int., 11 Suppl 6, S2–17. [DOI] [PubMed] [Google Scholar]
- 26. Seregni E., Martinetti A., Ferrari L., Bombardieri E. (2001) Clinical utility of biochemical marker of bone remodelling in patients with bone metastases of solid tumors. Q. J. Nucl. Med., 45, 7–17. [PubMed] [Google Scholar]
- 27. Booth S.L., Centi A., Smith S.R., Gundberg C. (2013) The role of osteocalcin in human glucose metabolism, marker or mediator?. Nat. Rev. Endocrinol., 9, 43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Logan M., Martin J.F., Nagy A., Lobe C., Olson E.N., Tabin C.J. (2002) Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis, 33, 77–80. [DOI] [PubMed] [Google Scholar]
- 29. van Brabant A.J., Stan R., Ellis N.A. (2000) DNA helicases, genomic instability, and human genetic disease. Annu. Rev. Genomics Hum. Genet., 1, 409–459. [DOI] [PubMed] [Google Scholar]
- 30. Chakraverty R.K., Hickson I.D. (1999) Defending genome integrity during DNA replication, a proposed role for RecQ family helicases. Bioessays, 21, 286–294. [DOI] [PubMed] [Google Scholar]
- 31. Borg M.F., Olver I.N., Hill M.P. (1998) Rothmund-Thomson syndrome and tolerance of chemoradiotherapy. Australas. Radiol., 42, 216–218. [DOI] [PubMed] [Google Scholar]
- 32. Howell S.M., Bray D.W. (2008) Amelanotic melanoma in a patient with Rothmund-Thomson syndrome. Arch. Dermatol., 144, 416–417. [DOI] [PubMed] [Google Scholar]
- 33. Lengner C.J., Steinman H.A., Gagnon J., Smith T.W., Henderson J.E., Kream B.E., Stein G.S., Lian J.B., Jones S.N. (2006) Osteoblast differentiation and skeletal development are regulated by Mdm2-p53 signaling. J. Cell Biol., 172, 909–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang X., Kua H.Y., Hu Y., Guo K., Zeng Q., Wu Q., Ng H.H., Karsenty G., de Crombrugghe B., Yeh J.. et al. (2006) p53 functions as a negative regulator of osteoblastogenesis, osteoblast-dependent osteoclastogenesis, and bone remodeling. J. Cell Biol., 172, 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ward L.M., Konji V.N., Ma J. (2016) The management of osteoporosis in children. Osteoporos. Int., 27, 2147–2179. [DOI] [PubMed] [Google Scholar]
- 36. Dwan K., Phillipi C.A., Steiner R.D., Basel D. (2016) Bisphosphonate therapy for osteogenesis imperfecta. Cochrane Database Syst. Rev., 10, CD005088.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vahle J.L., Long G.G., Sandusky G., Westmore M., Ma Y.L., Sato M. (2004) Bone neoplasms in F344 rats given teriparatide [rhPTH(1-34)] are dependent on duration of treatment and dose. Toxicol. Pathol., 32, 426–438. [DOI] [PubMed] [Google Scholar]
- 38. Watanabe A., Yoneyama S., Nakajima M., Sato N., Takao-Kawabata R., Isogai Y., Sakurai-Tanikawa A., Higuchi K., Shimoi A., Yamatoya H.. et al. (2012) Osteosarcoma in Sprague-Dawley rats after long-term treatment with teriparatide (human parathyroid hormone (1-34)). J. Toxicol. Sci., 37, 617–629. [DOI] [PubMed] [Google Scholar]
- 39. Andrews E.B., Gilsenan A.W., Midkiff K., Sherrill B., Wu Y., Mann B.H., Masica D. (2012) The US postmarketing surveillance study of adult osteosarcoma and teriparatide, study design and findings from the first 7 years. J. Bone Miner. Res., 27, 2429–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ellis K.J., Shypailo R.J., Hardin D.S., Perez M.D., Motil K.J., Wong W.W., Abrams S.A. (2001) Z score prediction model for assessment of bone mineral content in pediatric diseases. J. Bone Miner. Res., 16, 1658–1664. [DOI] [PubMed] [Google Scholar]
- 41. Steinberg F.M., Murray M.J., Lewis R.D., Cramer M.A., Amato P., Young R.L., Barnes S., Konzelmann K.L., Fischer J.G., Ellis K.J.. et al. (2011) Clinical outcomes of a 2-y soy isoflavone supplementation in menopausal women. Am. J. Clin. Nutr., 93, 356–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shypailo R.J., Ellis K.J. (2005) Bone assessment in children, comparison of fan-beam DXA analysis. J. Clin. Densitom., 8, 445–453. [DOI] [PubMed] [Google Scholar]
- 43. Grover M., Brunetti-Pierri N., Belmont J., Phan K., Tran A., Shypailo R.J., Ellis K.J., Lee B.H. (2012) Assessment of bone mineral status in children with Marfan syndrome. Am J. Med. Genet. A, 158A, 2221–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Group, W.H.O.M.G.R.S. (2006) WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr., Supplement, 450, 76–85. [DOI] [PubMed] [Google Scholar]
- 45. Flegal K.M., Cole T.J. (2013) Construction of LMS parameters for the Centers for Disease Control and Prevention 2000 growth charts. Natl. Health Stat. Report, 63, 1–3. [PubMed] [Google Scholar]
- 46. Kelly T.L., Wilson K.E., Heymsfield S.B. (2009) Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS One, 4, e7038.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cole T.J. (1990) The LMS method for constructing normalized growth standards. Eur. J. Clin. Nutr., 44, 45–60. [PubMed] [Google Scholar]
- 48. Bronner F., Abrams S.A. (1998) Development and regulation of calcium metabolism in healthy girls. J. Nutr., 128, 1474–1480. [DOI] [PubMed] [Google Scholar]
- 49. Abrams S.A., Sidbury J.B., Muenzer J., Esteban N.V., Vieira N.E., Yergey A.L. (1991) Stable isotopic measurement of endogenous fecal calcium excretion in children. J. Pediatr. Gastroenterol. Nutr., 12, 469–473. [DOI] [PubMed] [Google Scholar]
- 50. Bouxsein M.L., Boyd S.K., Christiansen B.A., Guldberg R.E., Jepsen K.J., Muller R. (2010) Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res., 25, 1468–1486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.