Abstract
Glucocorticoid treatment represents a standard palliative treatment for Duchenne muscular dystrophy (DMD) patients, but various adverse effects have limited this treatment. In an effort to understand the mechanism(s) by which glucocorticoids impart their effects on the dystrophic muscle, and potentially reduce the adverse effects, we have studied the effect of prednisolone treatment in dystrophin/utrophin double knockout (dKO) mice, which exhibit a severe dystrophic phenotype due to rapid muscle stem cell depletion. Our results indicate that muscle stem cell depletion in dKO muscle is related to upregulation of mTOR, and that prednisolone treatment reduces the expression of mTOR and other pro-inflammatory mediators, consequently slowing down muscle stem cell depletion. However, prednisolone treatment was unable to improve the myogenesis of stem cells and reduce fibrosis in dKO muscle. We then studied whether glucocorticoid treatment can be improved by co-administration of an inhibitor of RhoA/ROCK signaling, which can be activated by glucocorticoids and was found in our previous work to be over-activated in dystrophic muscle. Our results indicate that the combination of RhoA/ROCK inhibition and glucocorticoid treatment in dystrophic muscle have a synergistic effect in alleviating the dystrophic phenotype. Taken together, our study not only shed light on the mechanism by which glucocorticoid imparts its beneficial effect on dystrophic muscle, but also revealed the synergistic effect of RhoA/ROCK inhibition and glucocorticoid treatment, which could lead to the development of more efficient therapeutic approaches for treating DMD patients.
Introduction
Glucocorticoids have been used as the gold standard palliative therapy for treating Duchenne muscular dystrophy (DMD) (1–3). However, besides their anti-inflammatory effect (1,4–6), little is known about the cellular and molecular mechanism(s) underlying the beneficial effects imparted by glucocorticoids in DMD patients. In addition to the well-known muscle pathological characteristics of DMD, such as muscle wasting, degeneration, and the progressive formation of fibrosis (7,8), stem cell depletion has also been described in the skeletal muscles of DMD patients and related animal models (9,10). Stem cell depletion has been associated with the inflammatory process in dystrophic muscle and is hypothesized to be responsible, at least in part, for the rapid histopathology and impaired muscle regeneration capacity seen in the dystrophic muscle of DMD patients (9,10). We therefore hypothesized that the beneficial effects of glucocorticoids may abrogate stem cell depletion in dystrophic muscle. We believe that a better understanding of the mechanism(s) of action of glucocorticoids could aid in the development of improved glucocorticoid therapies for treating DMD.
The dystrophin-deficient mdx mouse model is commonly used to study DMD; however, in contrast to DMD patients, mdx mice feature a normal life span, mild muscle damage, and an absence of stem cell depletion (9,11,12); hence, the mdx mouse has not been an optimal model for studying the effects of glucocorticoids in DMD (13). In support of this contention, mdx/mTR mice, which are dystrophin-deficient and exhibit telomere dysfunction/shortening, specifically in their muscle progenitor cells (MPCs), develop a more severe dystrophic phenotype than do standard mdx mice, and this phenotype rapidly worsens with age, due to the rapid depletion of their MPCs (9). Similarly, the dystrophin/utrophin double knockout (dKO) mouse model of DMD displays a severe phenotype similar to that of DMD patients, including a much shorter life span (∼8 weeks for dKO mice compared to ∼2 years for mdx mice), early onset of muscle necrosis and fibrosis, scoliosis/kyphosis of the spine, and severe cardiac involvement, which ultimately leads to cardiac failure (10,14–16). Moreover, dKO mice exhibit early onset of stem cell depletion and cellular senescence in their skeletal muscles, which may explain the rapid progression of the disease in this dystrophic animal model (10,16), as observed in mdx/mTR mice (9).
Mammalian target of rapamycin (mTOR) is involved in an anabolic pathway that is essential for cell proliferation and tissue growth; however, upregulation of mTOR has been found to act as a mediator of aging, and its inhibition can extend the lifespan of several murine disease models (17–19). mTOR has also been found to promote the exhaustion and senescence of stem cells (17,20–22). Calorie restriction has been shown to decrease the senescence of various stem cells, which is partially mediated by mTOR inhibition (23). Interestingly, a previous study also suggested the use of the mTOR inhibitor, rapamycin, as a potential therapeutic drug for the treatment of rare muscular dystrophies caused by a mutation in the lamin A/C gene (24).
In fact, the role of mTOR in muscular dystrophy is still unclear, because it has been shown that Wnt7a treatment reduces muscle damage by activating mTOR in mdx mice (25), while other studies have reported amelioration of the dystrophic muscle phenotype when mTOR is inhibited with rapamycin (26). mTOR is known to play a central role in cellular metabolism by promoting anabolic metabolism (27,28), and the treatment of mdx mice with anabolic steroids has been found to increase muscle damage in dystrophic muscle (29). These observations suggest that the inhibition of anabolic factors, such as mTOR, could potentially be beneficial for treating dystrophic muscle. In contrast to anabolic factors like mTOR (30) and anabolic steroids (29), glucocorticoids are catabolic steroids (i.e. prednisone, prednisolone, and dexamethasone) that can repress mTOR signaling in normal skeletal muscle (31). It has been also reported that NF-κB functions as a negative regulator of muscle stem cell myogenesis (32), and pro-inflammatory TNFα/NF-κB signaling is elevated in the skeletal muscle and muscle stem cells of dKO mice (16). We therefore posit that glucocorticoids might be involved in regulating pro-inflammatory TNFα/NF-κB signaling in dystrophic muscle, which in turn will delay stem cell depletion and delay the onset of the pathology in dystrophic mice.
In the current study, the dKO mouse model was used to first determine whether the beneficial effect of glucocorticoids in dystrophic muscle was mediated, at least in part, through a reduction in stem cell depletion, potentially via the repression of mTOR and other pro-inflammatory mediators. Our results indicate that prednisolone treatment reduces the expression of mTOR and other pro-inflammatory mediators, and consequently slows down the depletion of muscle stem cells. However, it showed that prednisolone treatment was unable to enhance myogenesis and reduce fibrosis in dKO muscle. Our previous study with dKO mice demonstrated that over-activation of RhoA signaling mediates dystrophic phenotypes in the muscle (16), and RhoA signaling is known to be involved in the inflammatory process (33–35), in myocardial, pulmonary and skeletal muscle fibrosis (16,36,37) and in repressing myogenesis (38–40). Because glucocorticoids have been shown to be able to increase RhoA activation (41,42), we hypothesize that RhoA inhibition might reduce the adverse effects and further enhance the beneficial effect of glucocorticoid in dystrophic muscle. Our results indicate that the combination of RhoA inhibition with glucocorticoid treatment was able to improve the regeneration of dystrophic muscle by promoting myogenesis while reducing fibrosis and heterotopic ossification.
Results
Muscle stem cell depletion, cell senescence, and mTOR activation are increased in the skeletal muscle of dKO mice
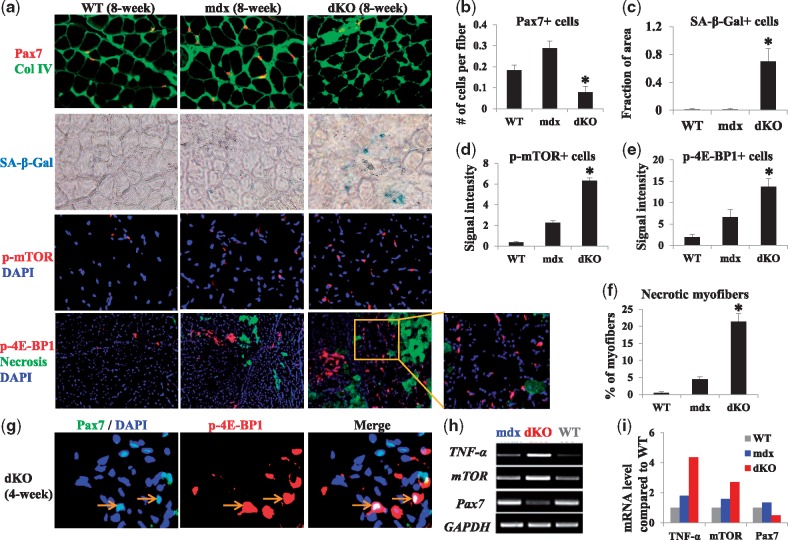
We hypothesize that the severe dystrophic phenotypes in dKO skeletal muscle might be associated with stem cell depletion/senescence through mTOR activation. Tissue sections from skeletal muscle of WT, mdx, and dKO mice were compared to evaluate muscle histopathology. Immunostaining of cryostat sections of the gastrocnemius muscle (GM) from 8-week old WT, mdx, and dKO mice revealed a reduction in the number of Pax7+ satellite cells (muscle stem cells) in the skeletal muscle of dKO mice compared to that of WT or mdx mice (Fig. 1A and B). An increased number of senescent cells (senescence-associated β-galactosidase/SA-β-gal+) was also observed in the skeletal muscle of dKO mice in contrast to WT and mdx mice (Fig. 1A and C). Meanwhile, the level of the p-mTOR protein and p-4E-BP1 protein (downstream target of mTOR) was increased in dKO mice compared to mdx and WT mice (Fig. 1A–E). A higher level of p-4E-BP1 expression reflects a higher activity of mTOR signaling in dKO skeletal muscle (43). The number of necrotic myofibers, immunostained with anti-mouse IgG, was also elevated in dKO muscle compared to either WT or mdx muscle (Fig. 1A and F). We further observed colocalization of cells expressing Pax7 and mTOR in the skeletal muscle of dKO mice (Fig. 1G). Semi-quantitative PCR analysis revealed that the expression levels of TNF-α (an inflammatory mediator) and mTOR were upregulated in the skeletal muscles of 8-week-old dKO mice compared to either WT or mdx mice, whereas the expression of Pax7 was downregulated in the skeletal muscle of dKO mice compared to the other groups (Fig. 1H and I).
Figure 1.
Detection of Pax7-positive satellite cells, cell senescence, mTOR activity, and inflammatory mediators in the skeletal muscle of dKO, WT, and mdx mice. (A) Immunostaining of Pax7 in the GM muscles of 8-week-old WT, mdx, and dKO mice revealed a reduction in the number of Pax7+ cells (red) in the dKO mice; Collagen type IV (Col IV) (green) was also immunostained to highlight myofibers. The cell senescence assay of the GM muscles of 8-week-old mice revealed the presence of senescent cells (SA-β-gal+) (arrows) in dKO mice but not in WT and mdx mice. Immunostaining of p-mTOR in the GM muscles of 8-week-old mice showed a slight increase in p-mTOR+ cells (red) in mdx mice and a significant increase in p-mTOR+ cells in dKO mice compared to WT mice. Immunostaining of p-4E-BP1 in the GM muscles of 8-week-old mice showed a slight increase in p-4E-BP1+ cells (red) in mdx mice, and a significant increase of p-4E-BP1+ cells in the dKO mice when compared to WT mice; necrotic myofibers (green), detected by immunostaining with anti-mouse IgG, were significantly higher in dKO muscle than in WT and mdx muscles. (B) The number of Pax7+ cells (cells per myofiber) was lower in the skeletal muscle of dKO mice. Error bars indicate mean ± SD; P < 0.05. (C, D, E) The numbers of SA-β-gal+, p-mTOR+, and p-4E-BP1+ cells were higher in the skeletal muscle of dKO mice than in WT and mdx mice, Error bars indicate mean ± SD; P < 0.05. (F). The percentage of necrotic myofibers (anti-mouse IgG+) was higher in the skeletal muscle of dKO mice. Error bars indicate mean ± SD; P < 0.05. (G) Immunostaining of p-4E-BP1 and Pax7 in the GM muscles of 4-week-old dKO mice indicate the presence of cells positive for both Pax7 and p-4E-BP1 (arrows). (H) RT-PCR showed upregulation of TNF-α and mTOR (mTORC1) expression, and downregulation of Pax7 expression in the GM muscles of 8-week-old dKO mice, compared to those of WT and mdx mice. (I) TNF-α, mTOR, and Pax7 gene expression (ratio compared to WT).
mTOR activation and cellular senescence are increased in isolated muscle-derived stem cells (MDSCs) from dKO mice
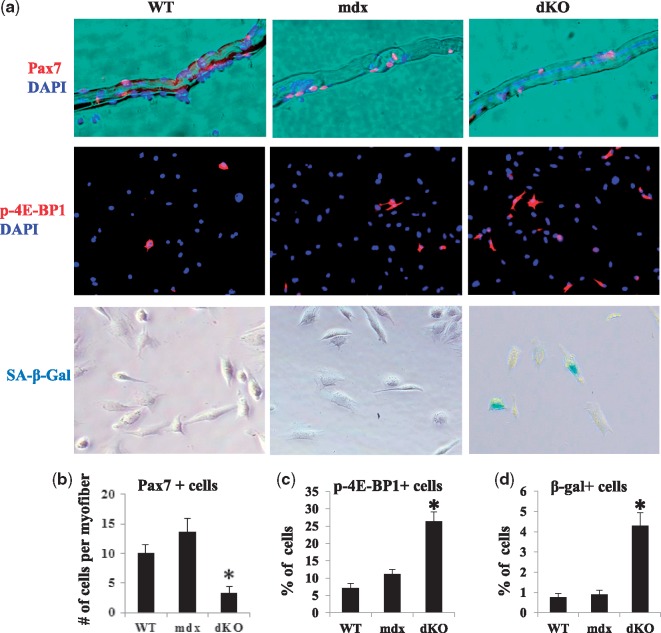
To further validate the rapid stem cell depletion in the dKO skeletal muscle, single myofibers were isolated from the tibialis anterior (TA) muscles of 8-week old WT, mdx, and dKO mice. Pax-7 immunostaining was performed to compare the number of satellite cells present in the isolated myofibers, and the results were compared among the different groups tested. More Pax7+ cells were observed in mdx and WT myofibers than in dKO myofibers (Fig. 2A and B). MDSCs were also isolated from the GM muscles of 6-week-old WT, mdx, and dKO mice, and immunostaining of p-4E-BP1 (an activated substrate of mTOR) revealed that the percentage of p-4E-BP1 expressing cells was significantly higher in dKO MDSCs compared to the WT and mdx MDSCs (Fig. 2A and C). In addition, a significantly higher number of SA-β-gal+ cells could be found in dKO MDSC cultures compared to WT and mdx MDSC cultures (Fig. 2A and D). Our previous study also revealed that the myogenic potential of dKO MDSCs was lower than that of mdx MDSCs (10). In the following set of experiments, we performed in vitro and in vivo analyses to determine whether prednisolone treatment can regulate mTOR activity in dKO MDSCs and skeletal muscles.
Figure 2.
Isolated myofiber-associated satellite cells and mTOR activity as well as cell senescence in MDSCs isolated from WT, mdx, and dKO mice. (A) Immunostaining of Pax7 in single myofibers isolated from 8-week old WT, mdx, and dKO mice revealed a lower number of Pax7+ cells (red) in the myofibers of dKO mice. Immunostaining of p-4E-BP1 in MDSCs isolated from 6-week old WT, mdx, and dKO mice showed a higher number of p-4E-BP1+ cells (red) in MDSCs from dKO mice. The cell senescence assay showed that SA-β-gal+ cells were generally absent in MDSCs isolated from WT or mdx mice, but present in dKO MDSCs. (B) The number of Pax7+ cells was lower in single myofibers isolated from dKO mice compared to those from WT and mdx mice. Error bars indicate mean ± SD; P < 0.05. (C,D) The numbers of p-4E-BP1+ and SA-β-gal+ MDSCs (percentage) were higher in MDSCs isolated from dKO mice compared to those from WT and mdx mice. Error bars indicate mean ± SD; P < 0.05.
Prednisolone treatment repressed mTOR activity and delayed cellular senescence in dKO MDSCs
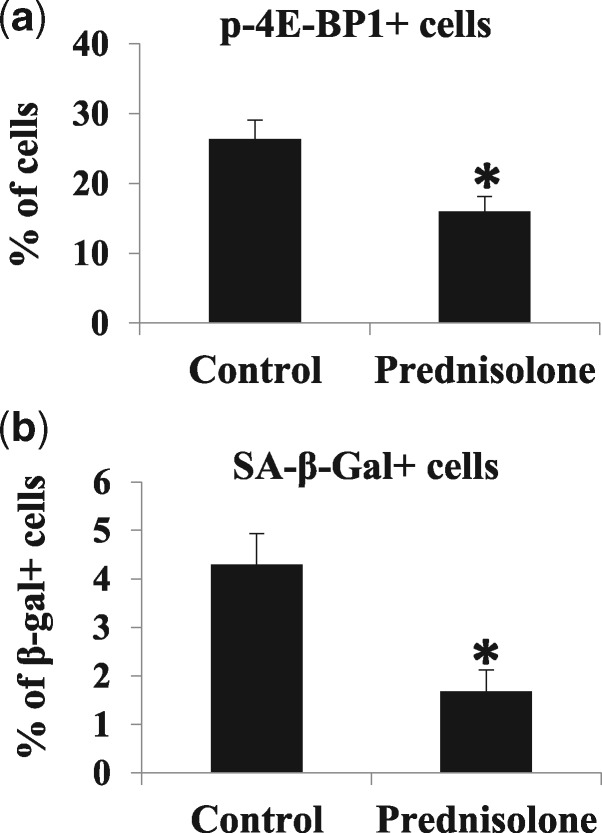
First, we evaluated the effect of prednisolone on cultured dKO MDSCs. MDSCs isolated from 6-week old dKO mice were cultured in the presence of prednisolone (10 µg/ml) for 7 days. Cells cultured in prednisolone-free medium served as a control. A significant decrease in the number of p-4E-BP1+ cells was observed in prednisolone-treated dKO MDSCs compared to non-treated cells (Fig. 3A). Similarly, a significant reduction in the percentage of SA-β-gal+ senescent cells was observed (Fig. 3B) in dKO MDSCs treated with prednisolone, compared to the control group.
Figure 3.
Effects of in vitro prednisolone treatment on dKO-MDSCs. (A) Immunostaining of p-4E-BP1 in MDSCs isolated from 6-week-old dKO mice showed that prednisolone treatment reduced the percentage of p-4E-BP1+ cells. Error bars indicate mean ± SD; P < 0.05. (B) The cell senescence assay showed that prednisolone treatment reduced the number of SA-β-gal+ cells in dKO MDSCs. Error bars indicate mean ± SD; P < 0.05.
Prednisolone treatment in dKO mice repressed mTOR and inflammatory mediators, and slowed down the depletion of muscle stem cells
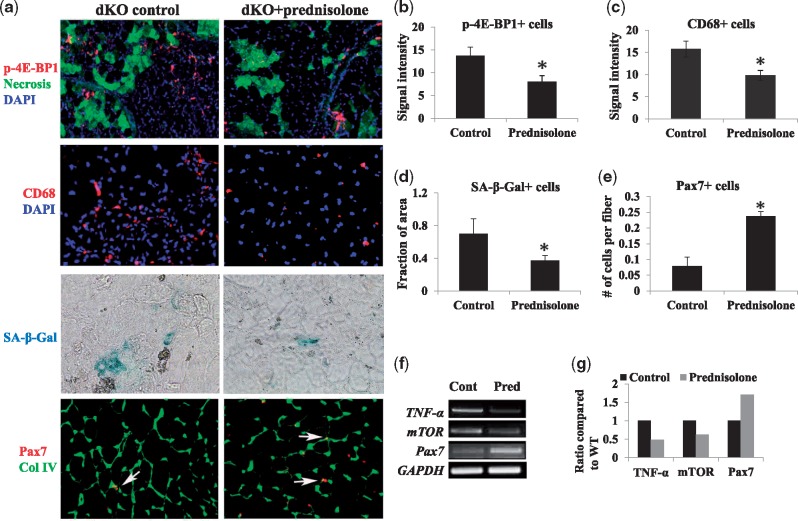
Following the in vitro effect of prednisolone on cultured dKO MDSCs, we performed experiments to determine whether the injection of prednisolone into dKO mice would also be beneficial to dystrophic muscle through reduction of stem cell depletion. Prednisolone was injected intraperitoneally into dKO mice three times a week, starting at 3 weeks of age and continuing until the mice reached 8 weeks of age. Compared to non-treated mice, a significant decrease in the number of: 1) p-4E-BP1+ cells, 2) necrotic myofibers (anti-mouse IgG+ myofibers), 3) CD68+ inflammatory cells, and 4) SA-β-gal+ senescent cells were observed in the GM muscles of prednisolone-treated dKO mice (Fig. 4A–D). In contrast, the number of Pax7+ cells was significantly increased upon prednisolone treatment (Fig. 4A and E). The expression levels of TNF-α and mTOR mRNA in the skeletal muscle of prednisolone-treated dKO mice were downregulated, while an upregulation of Pax7 mRNA was observed (Fig. 4F and G).
Figure 4.
Effects of prednisolone injection on dKO mice. (A) Immunostaining of the skeletal muscle of 8-week-old dKO mice showed that prednisolone treatment reduced the number of p-4E-BP1+ cells (red) and necrotic myofibers (anti-mouse IgG+, green). Immunostaining also revealed a reduction in the number of CD68+ cells after prednisolone treatment. The cell senescence assay showed a reduction in the number of SA-β-gal+ cells (arrows) after prednisolone treatment. Immunostaining revealed an increase in the number of Pax7+ cells (arrows) after prednisolone treatment. (B–D) The numbers of p-4E-BP1+, CD68+, SA-β-gal+ cells in the muscles were significantly reduced in dKO mice treated with prednisolone, compared to non-treated mice. Error bars indicate “mean ± SD”; P < 0.05. (E) The number of Pax7+ cells was significantly increased in dKO mice treated with prednisolone, compared to non-treated mice. Error bars indicate “mean ± SD”; P < 0.05. (F) RT-PCR assay showed that prednisolone treatment of dKO skeletal muscle downregulated the expression of TNF-α and mTOR, but upregulated Pax7 expression. (G) TNF-α, mTOR, and Pax7 gene expression (ratio compared to WT).
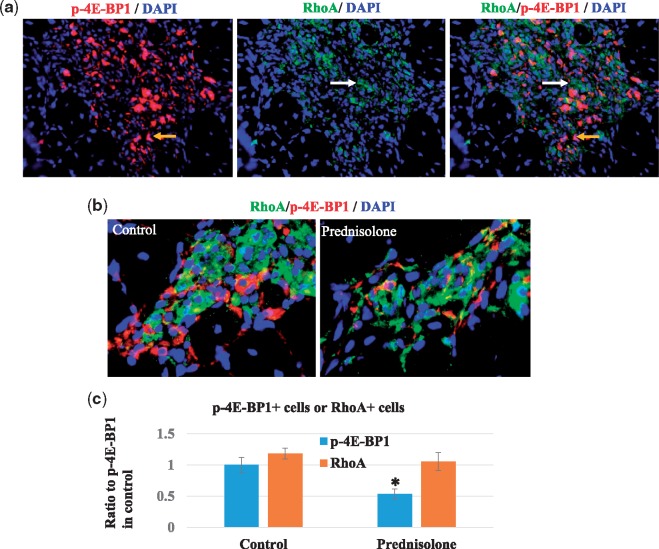
RhoA and mTOR were activated in different populations of cells in dKO skeletal muscle, and prednisolone treatment did not reduce the number of RhoA+ cells
Our previous study has demonstrated that over-activation of RhoA signaling also mediates dystrophic phenotypes in the muscle (16), and RhoA signaling is known to be involved in the inflammatory process (33–35). Here, we further evaluated whether prednisolone affects RhoA activity in dKO mice, similar to its effect on mTOR activity. First, we determined whether RhoA+ cells and mTOR+ cells represent the same cell population. Immunostaining of the skeletal muscle tissues of dKO mice showed the presence of both RhoA+ cells and mTOR+ cells in the degenerative tissue of dKO muscle; but the lack of co-localization of the two proteins suggests that they constitute two different populations of cells in the muscle (Fig. 5A). Next, we determined whether prednisolone affects RhoA activity. Or results showed that, although prednisolone treatment of dKO mice decreased the number of p-mTOR+ cells in skeletal muscle, it did not alter the number of RhoA+ cells (Fig. 5B and C). This observation suggests that prednisolone treatment has a limited effect in regulating RhoA activity in muscle cells, which may be associated with the limited beneficial effect of prednisolone in dystrophic muscle.
Figure 5.
Lack of co-localization of RhoA+ cells and p-4E-BP1+ cells. (A) Immunostaining of muscle tissues of dKO mice showed that both RhoA+ cells and mTOR+ cells accumulated in dKO muscle and were located near each other, but were generally two distinct groups of cells. (B) Activation of RhoA signaling was observed in some Pax7+ cells. (C) Prednisolone treatment of dKO mice decreased the number of p-mTOR+ cells in skeletal muscle, but did not significantly alter the number of RhoA+ cells.
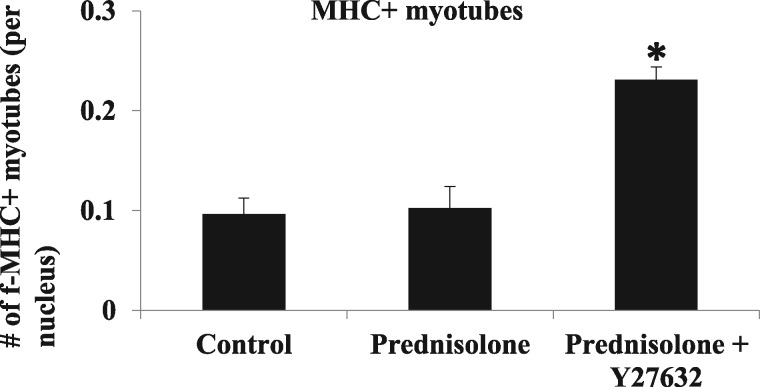
The RhoA/ROCK inhibitor Y-27632 improves the myogenic potential of dKO MDSCs in vitro, when co-administered with prednisolone
Based on the observations above, we hypothesize that RhoA inhibition may further improve the beneficial effect of prednisolone on dystrophic muscle. Activation of the myogenic potential of muscle stem cells is required for proper skeletal muscle regeneration (44); however, dKO MDSCs have much lower myogenic potential than do WT and mdx MDSCs (10). Utilizing an in vitro myogenic assay, we determined whether prednisolone, with or without co-administration of the RhoA/ROCK inhibitor Y-27632, could rescue the myogenic potential of dKO MDSCs. Our results indicate that the myogenic potential of dKO MDSCs was not improved upon prednisolone treatment alone compared to non-treated control cells (Fig. 6); however, the use of Y-27632 together with prednisolone was able to significantly improve the myogenic potential of dKO MDSCs (Fig. 6).
Figure 6.
The RhoA/ROCK inhibitor Y-27632 improves the myogenic potential of dKO MDSCs in vitro, when co-administrated with prednisolone. In vitro myogenic assay showed that prednisolone treatment did not increase the number of Myosin Heavy Chain-positive (MHC+) myotubes (red) when compared to non-treated cells. In contrast, combined treatment with prednisolone and Y-27632 was able to significantly increase the number of MHC+ myotubes formed by dKO MDSCs. Quantification of the number of MHC+ myotubes is shown (stars).
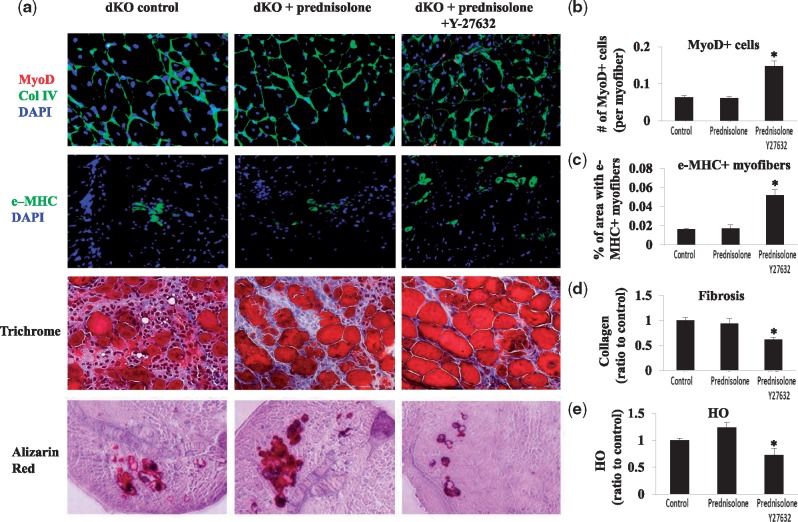
Prednisolone treatment alone did not improve muscle regeneration, whereas the co-administration of the RhoA/ROCK inhibitor Y-27632 and prednisolone improved muscle regeneration and reduced fibrosis and heterotopic ossification in dKO skeletal muscle
We observed that, despite the increase in the number of Pax-7+ cells in dKO skeletal muscle treated with prednisolone (Fig. 4), the number of MyoD+ cells (myogenic cells) (45), and embryonic myosin heavy chain (MHC)+ myofibers (regenerating myofibers) (46), were not increased (Fig. 7A–C). Also, the amount of fibrosis and HO was not obviously decreased with prednisolone treatment (Fig. 7A,D,E).
Figure 7.
The use of the RhoA/ROCK inhibitor Y-27632 with prednisolone improved muscle regeneration and reduce fibrosis and heterotopic ossification in the dKO skeletal muscle. (A) Immunostaining of the skeletal muscle of 8-week-old dKO mice showed that prednisolone treatment did not significantly increase the number of MyoD+ cells and e-MHC+ myofibers, fibrosis, and HO in dKO mice. In contrast, the combined administration of prednisolone and Y-27632 was able to significantly increase the number of MyoD+ cells and e-MHC+ myofibers, and significantly reduce fibrosis and HO in dKO skeletal muscle. (B–E) Quantification of MyoD+ cells, e-MHC+ myofibers, fibrosis, and HO in dKO muscle between the groups.
Therefore, prednisolone treatment of dKO mice appears to have a limited effect on muscle regeneration. We next investigated whether co-administration of the RhoA/ROCK inhibitor Y-27632 with prednisolone can further improve muscle regeneration in dKO mice. Both Y-27632 and prednisolone were injected into dKO mice three times a week, starting at 3 weeks of age. Our results showed that the numbers of MyoD+ cells and e-MHC+ cells increased (Fig. 7A–C), whereas fibrosis and HO decreased (Fig. 7A,D,E) in dKO muscle treated with both Y-27632 and prednisolone, compared to those of prednisolone treatment and untreated controls.
mTOR inhibition via rapamycin has a beneficial effect on dKO mice, similar to that of prednisolone
Because prednisolone seems to work through repressing mTOR activation in dystrophic muscle, we examined whether a similar beneficial effect in dystrophic muscle would be observed by directly inhibiting mTOR with rapamycin. The results showed that rapamycin treatment of dKO mice (5 mg/kg, i.p. injection, three times a week from 3 to 8 weeks of age) significantly reduced the activation of mTOR signaling and the number of CD68+ macrophages in dystrophic skeletal muscle, as well as increased the number of Pax7+ muscle stem cells (Supplementary Material, Fig. S1). This result suggests that direct mTOR inhibition in dKO mice with rapamycin is beneficial to dystrophic muscle, as observed with prednisolone treatment.
Prednisolone treatment represses the deleterious effect of mTOR activation in mdx MDSCs
To further validate the deleterious effect of mTOR activation in dystrophic muscle stem cells, and the beneficial effect of prednisolone in inhibiting mTOR activation, we performed another in vitro experiment with mdx MDSCs. We hypothesize that mTOR has a deleterious effect on mdx MDSCs in culture, and that this deleterious effect would be blocked with prednisolone. mdx MDSCs was treated with MHY1485 (an mTOR stimulator) for 3 days, with or without co-administration of prednisolone. Our results from RT-PCR showed that mTOR activation in mdx MDSCs led to a downregulation of autophagy markers (Beclin1 and LC3b) and anti-inflammatory factor (Klotho), and an upregulation of pro-inflammatory factor (IL-6) and Wnt1 (Supplementary Material, Fig. S2) expression. Importantly, these deleterious effects of mTOR activation on the expression levels of autophagy markers, anti-inflammatory factor, and pro-inflammatory factor, can be partially reversed via prednisolone co-treatment in mdx MDSCs (Supplementary Material, Fig. S2).
Discussion
Although it is well-established that glucocorticoids, such as prednisolone, are effective at improving the quality of life and delaying disease progression in DMD patients, there remain severe adverse effects (47). It is important to determine the cellular and molecular mechanisms underlying the beneficial effects of glucocorticoids, to potentially optimize glucocorticoids-based therapy for DMD, and reduce their adverse effects. Stem cell depletion has recently been reported to be responsible for the rapid onset of the histopathology (decreased muscle regeneration and increased fibrosis) of dystrophic muscle (9). We have reported that dKO mice undergo rapid muscle stem cell depletion (10), as observed in the skeletal muscle of DMD patients (9). Our current results further validate this contention, and indicate that over-activation of mTOR and pro-inflammatory mediators might be involved in the rapid depletion of stem cells in dystrophic muscle. Our results further suggest that the beneficial effect of prednisolone treatment in muscular dystrophy is not only mediated through a reduction of pro-inflammatory mediators, but also through a delay in stem cell depletion by repressing mTOR activation. More importantly, despite the delayed depletion of the stem cell pool, we observed that prednisolone failed to improve the myogenic potential of muscle stem cells and the regeneration of dystrophic muscle, an effect potentially mediated by the dystrophic micro-milieu. Because our previous study has shown that RhoA/ROCK inhibitor delays the histopathology of the dKO skeletal muscle (enhancement of myogenesis and inhibition of fibrosis and HO) (16), we posit that the beneficial effect of glucocorticoid treatment in dystrophic muscle can be potentially improved by co-administration of a RhoA/ROCK inhibitor. Indeed, our results indicate that the co-administration of a RhoA/ROCK inhibitor along with prednisolone was able to exert a synergistic effect, and further improve the beneficial effect of prednisolone treatment in dystrophic muscle by improving myogenesis and further reducing fibrosis and heterotopic ossification. Based on these results, we suggest that the RhoA/Rock inhibitor could be used to further improve the beneficial effect of steroids in DMD.
It is currently accepted that stem cells not only serve as the origin of progenitor cells, but also act as a reservoir of paracrine factors that can impart beneficial effects on the tissues in which they reside, through the production of trophic factors (48–50). Trophic factors secreted by stem cells can modify the molecular microenvironment of the resident cells and influence their activity (48–50). Stem cell therapies benefit the recipient, not only through the direct participation of the stem cells in the targeted host tissue, but also through their secretion of paracrine trophic factors (50–52). In the current study, Pax7+ satellite cell exhaustion was delayed, at least partially, in the dystrophic muscle following prednisolone treatment (Fig. 4A); however, we did not observe an increase in the number of MyoD+ cells, or obvious improvements in skeletal muscle regeneration (Fig. 5C). These results suggest that, despite the slow-down of muscle stem cell depletion with prednisone treatment, these cells did not actively participate in muscle regeneration, likely due to the dystrophic microenvironment, which consists of fibrosis, inflammatory and oxidative stressors, etc. This may also explain why there was only a slight increase in the life span of prednisolone-treated dKO mice despite the reduction in stem cell depletion (data not shown).
It is also interesting to note that, although stem cell depletion might be a contributing factor to the severely dystrophic phenotype (i.e. necrosis) in dKO mice, as indicated in the current results, stem cell depletion could also be a consequence of increased necrosis. The lack of dystrophin expression renders muscle fiber more susceptible to damage, and development of necrosis leads to a continuous cycle of myofiber degeneration/regeneration which ultimately depletes the muscle progenitor cells (MPCs) resulting in the occurrence of muscle histopathology. Therefore, the depletion of the MPC pool in dKO mice could be secondary to muscle necrosis, which consequently leads to the continuous activation of MPCs during the regenerative process. Meanwhile, it is evident that stem cell depletion can lead to impaired regeneration potential in dystrophic muscles, in both human DMD patients and mouse models (9,10). It is important to note that despite the lack of dystrophin at birth, the initiation of muscle weakness and histopathology does not occur in DMD patients until they have reached 4–8 years of age, which coincides with the onset of gradual depletion of their muscle stem cells (53,54). Also, the sparing of the extra-ocular muscles (EOM) in DMD patients appears to be related to the existence of a subpopulation of MPCs that retain their proliferative potential throughout the lives of these patients, indicating that the muscle weakness that occurs in dystrophic muscles is related to the exhaustion of MPCs as the disease progresses (55). Moreover, mdx/mTR mice, which have shortened telomeres in their MPCs, develop a more severe form of the muscular dystrophy phenotype than do mdx mice, and this phenotype progressively worsens with age due to the rapid exhaustion of MPCs (9). We suggest that stem cell depletion is closely related to and partially responsible for the severe dystrophic phenotype in dKO mice.
However, a recent discovery reported that dystrophin plays a functional role in muscle stem cells, which implies that dystrophin deficiency may also directly lead to muscle stem cell depletion (56). In fact, it was reported that in normal muscle, dystrophin is expressed in both activated muscle progenitor cells, namely satellite cells, and in differentiated myofibers (56). In myofibers, dystrophin maintains muscle membrane integrity. In activated satellite cells, localized dystrophin expression is associated with cell polarization and can promote asymmetric cell divisions, leading to the generation of satellite stem cells that lack Myf5 and committed muscle progenitors. Dumont et al. (56) show that dystrophin-null satellite cells exhibit a loss of Par-mediated cell polarity, leading to cell division errors and a decrease in asymmetric cell divisions. The resulting decrease in differentiated myocytes leads to impaired regeneration, and the impaired regeneration of dystrophin-null satellite cells, combined with the degeneration of dystrophin-null myofibers, leads to progressive muscle loss. Thus, lack of dystrophin expression may also be directly linked to the stem cell depletion and impaired muscle regeneration capacity in dystrophic muscle.
The mTOR pathway controls protein synthesis in skeletal muscle (57), and also regulates cellular energy metabolism (58–60). However, over-activation of mTOR has also been shown to contribute to the aging and senescence of various types of stem cells (22). mTOR inactivation with rapamycin has been proven effective at delaying stem cell depletion and senescence, and extending the life span of diseased animals (17,18,20). In skeletal muscle, mTOR activation was previously shown to promote both muscle cell proliferation and myogenic differentiation, and play a role in preventing muscle atrophy (61–63). In the current study, mTOR was found to be upregulated in the dystrophic muscle of dKO mice, which may play a major role in the rapid depletion of stem cell and senescence observed in the dKO mice. Although mTOR activation may not be the only factor responsible for stem cell depletion in dKO muscle, it is clear that stem cell depletion in dKO muscle is mediated, at least in part, by mTOR activation, and the inhibition of mTOR over-activation can be beneficial in delaying stem cell depletion. However, although the repression of mTOR activity by prednisolone treatment was beneficial in reducing muscle stem cell depletion and senescence in dKO mice, the myogenic potential of muscle stem cells remained unchanged. Therefore, the delay in muscle stem cell depletion by prednisolone treatment did not lead to improved muscle regeneration in dKO mice, an effect potentially driven by the dystrophic micro-milieu.
Because RhoA activation was shown to be hyper-activated in the muscle stem cells of dKO mice and the use of RhoA inhibitor improved the dystrophic micromilieu (improvement of myogenesis, and reduction in fibrosis and heterotopic ossification) (16), we studied the potential effect of the co-administration of a RhoA inhibitor with prednisolone in dKO mice. Our results further validate our hypothesis: the co-administration of a RhoA inhibitor with prednisolone was able to further improve the histopathology of dKO skeletal muscle, with enhanced muscle regeneration and reduced fibrosis and HO being observed with dual treatment, compared to prednisolone treatment only.
The current study revealed a novel mechanism by which prednisolone acts on dystrophic muscle through the repression of mTOR activity and pro-inflammatory factors (i.e. TNF-α), which consequently delayed the depletion of the muscle stem cell pool. The regeneration of dystrophic muscle, however, was not significantly improved with prednisolone treatment. Inhibition of RhoA signaling together with prednisolone treatment was able to effectively improve muscle regeneration and further reduce fibrosis in dKO mice. Therefore, we suggest that the beneficial effects of reduced inflammation and delayed stem cell depletion exerted by glucocorticoids in dystrophic muscle could be improved with RhoA/ROCK inhibition, which modifies the dystrophic micro-milieu to further improve the histopathology of dystrophic muscle.
Materials and Methods
Animals
Wild-type (C57BL/10J) mice were obtained from the Jackson Laboratories (Bar Harbor, ME). The mdx and dKO (dys-/-; utrn-/-) mice were derived from our in-house colony. Mice were housed in groups of 4 on a 12:12-h light-dark cycle at 20–23 degrees Celsius. At least six mice were used in each experimental sample group. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC).
Muscle stem cell isolation from skeletal muscle
Muscle-derived stem cells (MDSCs) were isolated from GM muscles of dKO mice (6-weeks old) via a modified preplate technique that has been previously described (64). Cells were cultured in proliferation medium [PM, consisting of DMEM (Invitrogen) supplemented with 20% fetal bovine serum (FBS) (Invitrogen), 1% penicillin-streptomycin antibiotics (Invitrogen), and 0.5% chick embryo extract (CEE) (Accurate Chemical Co)].
Single myofiber isolation
Single intact myofibers were enzymatically dissociated from the tibialis anterior (TA) muscles of 8-week old WT, mdx, and dKO mice with 0.2% type I collagenase at 37 °C for 2.5 h (65). When the muscles were sufficiently digested, they were triturated with heat-polished glass pipettes to liberate single fibers. Single myofibers were maintained in Matrigel-coated 12-well plates in PM at 5% CO2 and 37 °C.
In vitro prednisolone treatment of dKO MDSCs
MDSCs from 6-week-old dKO mice were cultured in the presence of prednisolone (Sigma, St Louis, MO) (10 µg/ml) (66,67) for 7 days. Cells cultured without prednisolone served as controls. Prednisolone treatment of the cells was refreshed every 2 days by changing the medium (PM). dKO MDSCs were then fixed with cold methanol and analysed for their expression of p-4E-BP1 and SA-β-gal.
In vitro mTOR activation and prednisolone treatment of mdx MDSCs
MDSCs from 6-week-old mdx mice were cultured in the presence of MHY1485 (synthesized 4,6-dimorpholino-N-(4-nitrophenyl)-1,3,5-triazin-2-amine) (2µM, Sigma), with or with co-treatment of prednisolone (10 µg/ml) for 3 days. mdx MDSCs were then harvested for comparing gene expression with RT-PCR between treated and non-treated cells.
In vitro myogenic differentiation assay
MDSCs from 6-week old dKO mice were cultured in myogenic differentiation medium (DM, 2% horse serum in DMEM, all from Invitrogen) with or without prednisolone (10 µg/ml). The number of myotubes [immunocytochemical staining of fast-type Myosin Heavy Chain (f-MHC)], was determined and compared between the groups, 4 days after plating in DM.
In vivo prednisolone treatment of dKO mice
Prednisolone dissolved in ethanol:H2O (1:3) was injected intraperitoneally (IP) (5 mg/kg) into dKO mice starting at 3 weeks of age, three times per week. Mice receiving an equal amount of ethanol:H2O (1:3) served as the controls. IP injections of prednisolone were continued until the mice reached 8 weeks of age, or to the end of their lifespan.
In vivo prednisolone treatment and RhoA inhibition in dKO mice
Prednisolone and RhoA/ROCK inhibitor Y-27632 were co-administered in dKO mice, with an IP injection of prednisolone (described above) followed by an IP injection of Y-27632 [5 mM in phosphate-buffered saline (PBS), 10 mg/kg per mouse] or control (PBS only) 1 h later. Injections of Y-27632 into dKO mice started at 3 weeks of age, three times per week, until the mice reached 8 weeks of age, or to the end of their lifespan.
In vivo rapamycin treatment of dKO mice
Rapamycin (Sigma) dissolved in DMSO:H2O (1:10) was injected intraperitoneally (5 mg/kg) into dKO mice, three times per week, starting at 3 weeks of age. Mice receiving an equal amount of DMSO:H2O (1:10) served as controls. IP injections of rapamycin were continued until the mice reached 8 weeks of age.
mRNA analysis via reverse transcriptase-PCR
Total RNA was obtained from MDSCs or the skeletal muscles of mice using the RNeasy Mini Kit (Qiagen, Inc., Valencia, CA) according to the manufacturer’s instructions. Reverse transcription was performed using an iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Inc., Hercules, CA). The sequences of primers are given in Table 1 for Pax7, TNF-α, mTORC1, IL6, Klotho, Beclin1, LC3b, Wnt1, and GAPDH (glyceraldehyde 3-phosphate dehydrogenase). PCR reactions were performed using an iCycler thermal cycler (Bio-Rad Laboratories, Inc.). The cycling parameters used for all primers were as follows: 95 °C for 10 min; PCR, 40 cycles of 30 s at 95 °C for denaturation, 1 min at 54 °C for annealing, and 30 s at 72 °C for extension. Products were separated by size, and were visualized on a 1.5% agarose gel stained with ethidium bromide. All data were normalized to the expression of GAPDH.
Table 1.
Primer sequences
| Gene | Primer sequence (5'-3') |
|---|---|
| GAPDH | Forward: TCCATGACAACTTTGGCATTG |
| Reverse: TCACGCCACAGCTTTCCA | |
| Pax7 | Forward: TCTCCAAGATTCTGTGCCGAT |
| Reverse: CGGGGTTCTCTCTCTTATACTCC | |
| TNF-α | Forward: GATTATGGCTCAGGGTCCAA |
| Reverse: CTCCCTTTGCAGAACTCAGG | |
| mTORC1 | Forward: CAGTTCGCCAGTGGACTGAAG |
| Reverse: GCTGGTCATAGAAGCGAGTAGAC | |
| Beclin1 | Forward: ATGGAGGGGTCTAAGGCGTC |
| Reverse: TGGGCTGTGGTAAGTAATGGA | |
| LC3b | Forward: CGCTTGCAGCTCAATGCTAAC |
| Reverse: TGCCCATTCACCAGGAGGA | |
| Klotho | Forward: CCCAAACCATCTATGAAAC |
| Reverse: CTACCGTATTCTATGCCTTC | |
| IL-6 | Forward: GGAAATCGTGGAAATGAG |
| Reverse: GCTTAGGCATAACGCACT | |
| Wnt1 | Forward: GGTTTCTACTACGTTGCTACTGG |
| Reverse: GGAATCCGTCAACAGGTTCGT |
Immunofluorescent staining
Cells or frozen tissue sections were fixed with 4% formalin. The primary antibodies used – Pax7 (DHSB), CD68 (Abcam), MyoD (Santa Cruz), p-4E-BP1 (Cell Signaling Technology), p-mTOR (Cell Signaling Technology), Embryonic Myosin Heavy Chain (e-MHC) (DHSB), fast-type Myosin Heavy Chain (f-MHC) (Abcam), anti-mouse IgG (Vector Laboratory), Collagen IV (Abcam), and RhoA (Santa Cruz) – were all applied at a 1:100 to 1:300 dilution. All slides were analysed via fluorescence microscopy (Leica Microsystems Inc., IL) and photographed at 4–40× magnification.
Cell senescence assay
A cell senescence assay was performed on muscle cells and skeletal muscle tissues using the Senescence-associated β-Galactosidase (SA-β-gal) Staining Kit (Cell Signaling Technology) following the manufacturer’s protocol. The number of cells positive for β-gal activity at pH 6 – a known characteristic of senescent cells, but not present in pre-senescent, quiescent, or immortal cells – was determined.
Measurements of results and statistical analysis
Image analysis was performed using Northern Eclipse (version 6.0; Empix Imaging, Inc., Mississauga, ON, Canada) and Image J software (version 1.32j; National Institutes of Health, Bethesda, MD). Data from at least six samples from each subject were pooled for statistical analysis. Results are given as the mean ± standard deviation (SD). The statistical significance of any difference was calculated using Student’s t-test. P values < 0.05 were considered statistically significant.
Supplementary Material
Supplementary Material is available at HMG online.
Supplementary Material
Acknowledgements
Johnny Huard was supported in part by NIH grants (PO1AG043376, and RO1AR065445), and by the department of Orthopaedic Surgery, University of Texas Health Science Center at Houston. We also acknowledge the editorial assistance of Mr. James Cummins and Dr. Lavanya Rajagopalan.
Conflict of Interest statement. None declared.
Funding
National Institutes of Health (Grant No. PO1AG043376 and RO1AR065445), and Department of Orthopaedic Surgery, University of Texas Health Science Center at Houston
References
- 1. Manzur A.Y., Kuntzer T., Pike M., Swan A. (2004) Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst Rev., 2, CD003725.. [DOI] [PubMed] [Google Scholar]
- 2. Kang J. (1996) [Glucocorticoid therapy in Duchenne muscular dystrophy]. Rinsho Shinkeigaku, 36, 1338–1340. [PubMed] [Google Scholar]
- 3. Moxley R.T. 3rd, Ashwal S., Pandya S., Connolly A., Florence J., Mathews K., Baumbach L., McDonald C., Sussman M., Wade C., et al. (2005) Practice parameter: corticosteroid treatment of Duchenne dystrophy: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology, 64, 13–20. [DOI] [PubMed] [Google Scholar]
- 4. Huynh T., Uaesoontrachoon K., Quinn J.L., Tatem K.S., Heier C.R., Van Der Meulen J.H., Yu Q., Harris M., Nolan C.J., Haegeman G., et al. (2013) Selective modulation through the glucocorticoid receptor ameliorates muscle pathology in mdx mice. J Pathol., 231, 223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rhen T., Cidlowski J.A. (2005) Antiinflammatory action of glucocorticoids–new mechanisms for old drugs. N. Engl J. Med., 353, 1711–1723. [DOI] [PubMed] [Google Scholar]
- 6. Barnes P.J. (1998) Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin. Sci. (Lond), 94, 557–572. [DOI] [PubMed] [Google Scholar]
- 7. O'Brien K.F., Kunkel L.M. (2001) Dystrophin and muscular dystrophy: past, present, and future. Mol. Genet. Metab., 74, 75–88. [DOI] [PubMed] [Google Scholar]
- 8. Hoffman E.P., Brown R.H. Jr., Kunkel L.M. (1987) Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell, 51, 919–928. [DOI] [PubMed] [Google Scholar]
- 9. Sacco A., Mourkioti F., Tran R., Choi J., Llewellyn M., Kraft P., Shkreli M., Delp S., Pomerantz J.H., Artandi S.E., et al. (2010) Short telomeres and stem cell exhaustion model Duchenne muscular dystrophy in mdx/mTR mice. Cell, 143, 1059–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lu A., Poddar M., Tang Y., Proto J.D., Sohn J., Mu X., Oyster N., Wang B., Huard J. (2014) Rapid depletion of muscle progenitor cells in dystrophic mdx/utrophin-/- mice. Hum. Mol. Genet., 23, 4786–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Janssen P.M., Hiranandani N., Mays T.A., Rafael-Fortney J.A. (2005) Utrophin deficiency worsens cardiac contractile dysfunction present in dystrophin-deficient mdx mice. Am. J. Physiol. Heart Circ. Physiol., 289, H2373–H2378. [DOI] [PubMed] [Google Scholar]
- 12. Bulfield G., Siller W.G., Wight P.A., Moore K.J. (1984) X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc. Natl Acad. Sci. U S A, 81, 1189–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sali A., Guerron A.D., Gordish-Dressman H., Spurney C.F., Iantorno M., Hoffman E.P., Nagaraju K. (2012) Glucocorticoid-treated mice are an inappropriate positive control for long-term preclinical studies in the mdx mouse. PLoS One, 7, e34204.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grady R.M., Teng H., Nichol M.C., Cunningham J.C., Wilkinson R.S., Sanes J.R. (1997) Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: a model for Duchenne muscular dystrophy. Cell, 90, 729–738. [DOI] [PubMed] [Google Scholar]
- 15. Duan D. (2006) Challenges and opportunities in dystrophin-deficient cardiomyopathy gene therapy. Hum. Mol. Genet., 15 Spec No 2, R253–2R261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mu X., Usas A., Tang Y., Lu A., Wang B., Weiss K., Huard J. (2013) RhoA mediates defective stem cell function and heterotopic ossification in dystrophic muscle of mice. Faseb J., 27, 3619–3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pani G. (2011) From growing to secreting: new roles for mTOR in aging cells. Cell Cycle, 10, 2450–2453. [DOI] [PubMed] [Google Scholar]
- 18. Harrison D.E., Strong R., Sharp Z.D., Nelson J.F., Astle C.M., Flurkey K., Nadon N.L., Wilkinson J.E., Frenkel K., Carter C.S., et al. (2009) Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature, 460, 392–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blagosklonny M.V. (2013) Big mice die young but large animals live longer. Aging (Albany NY), 5, 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blagosklonny M.V. (2008) Aging, stem cells, and mammalian target of rapamycin: a prospect of pharmacologic rejuvenation of aging stem cells. Rejuvenation Res., 11, 801–808. [DOI] [PubMed] [Google Scholar]
- 21. Iglesias-Bartolome R., Patel V., Cotrim A., Leelahavanichkul K., Molinolo A.A., Mitchell J.B., Gutkind J.S. (2012) mTOR inhibition prevents epithelial stem cell senescence and protects from radiation-induced mucositis. Cell Stem Cell, 11, 401–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Castilho R.M., Squarize C.H., Chodosh L.A., Williams B.O., Gutkind J.S. (2009) mTOR mediates Wnt-induced epidermal stem cell exhaustion and aging. Cell Stem Cell, 5, 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Blagosklonny M.V. (2010) Calorie restriction: decelerating mTOR-driven aging from cells to organisms (including humans). Cell Cycle, 9, 683–688. [DOI] [PubMed] [Google Scholar]
- 24. Ramos F.J., Chen S.C., Garelick M.G., Dai D.F., Liao C.Y., Schreiber K.H., MacKay V.L., An E.H., Strong R., Ladiges W.C., et al. (2012) Rapamycin reverses elevated mTORC1 signaling in lamin A/C-deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci. Transl Med., 4, 144ra103.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. von Maltzahn J., Renaud J.M., Parise G., Rudnicki M.A. (2012) Wnt7a treatment ameliorates muscular dystrophy. Proc. Natl Acad. Sci. U S A, 109, 20614–20619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eghtesad S., Jhunjhunwala S., Little S.R., Clemens P.R. (2011) Rapamycin ameliorates dystrophic phenotype in mdx mouse skeletal muscle. Mol. Med., 17, 917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Howell J.J., Ricoult S.J., Ben-Sahra I., Manning B.D. (2013) A growing role for mTOR in promoting anabolic metabolism. Biochem. Soc. Trans., 41, 906–912. [DOI] [PubMed] [Google Scholar]
- 28. Ochocki J.D., Simon M.C. (2013) Nutrient-sensing pathways and metabolic regulation in stem cells. J. Cell Biol., 203, 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krahn M.J., Anderson J.E. (1994) Anabolic steroid treatment increases myofiber damage in mdx mouse muscular dystrophy. J. Neurol. Sci., 125, 138–146. [DOI] [PubMed] [Google Scholar]
- 30. Iadevaia V., Huo Y., Zhang Z., Foster L.J., Proud C.G. (2012) Roles of the mammalian target of rapamycin, mTOR, in controlling ribosome biogenesis and protein synthesis. Biochem. Soc. Trans., 40, 168–172. [DOI] [PubMed] [Google Scholar]
- 31. Shimizu N., Yoshikawa N., Ito N., Maruyama T., Suzuki Y., Takeda S., Nakae J., Tagata Y., Nishitani S., Takehana K., et al. (2011) Crosstalk between glucocorticoid receptor and nutritional sensor mTOR in skeletal muscle. Cell Metab., 13, 170–182. [DOI] [PubMed] [Google Scholar]
- 32. Lu A., Proto J.D., Guo L., Tang Y., Lavasani M., Tilstra J.S., Niedernhofer L.J., Wang B., Guttridge D.C., Robbins P.D., et al. (2012) NF-kappaB negatively impacts the myogenic potential of muscle-derived stem cells. Mol. Ther., 20, 661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goto K., Chiba Y., Sakai H., Misawa M. (2009) Tumor necrosis factor-alpha (TNF-alpha) induces upregulation of RhoA via NF-kappaB activation in cultured human bronchial smooth muscle cells. J. Pharmacol. Sci., 110, 437–444. [DOI] [PubMed] [Google Scholar]
- 34. Slice L.W., Bui L., Mak C., Walsh J.H. (2000) Differential regulation of COX-2 transcription by Ras- and Rho-family of GTPases. Biochem. Biophys. Res. Commun., 276, 406–410. [DOI] [PubMed] [Google Scholar]
- 35. Nakayama Y., Komuro R., Yamamoto A., Miyata Y., Tanaka M., Matsuda M., Fukuhara A., Shimomura I. (2009) RhoA induces expression of inflammatory cytokine in adipocytes. Biochem. Biophys. Res. Commun., 379, 288–292. [DOI] [PubMed] [Google Scholar]
- 36. Zhou Y., Huang X., Hecker L., Kurundkar D., Kurundkar A., Liu H., Jin T.H., Desai L., Bernard K., Thannickal V.J. (2013) Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J. Clin. Invest., 123, 1096–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhou H., Li Y.J., Wang M., Zhang L.H., Guo B.Y., Zhao Z.S., Meng F.L., Deng Y.G., Wang R.Y. (2011) Involvement of RhoA/ROCK in myocardial fibrosis in a rat model of type 2 diabetes. Acta Pharmacol. Sin., 32, 999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Charrasse S., Comunale F., Grumbach Y., Poulat F., Blangy A., Gauthier-Rouviere C. (2006) RhoA GTPase regulates M-cadherin activity and myoblast fusion. Mol. Biol. Cell, 17, 749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Castellani L., Salvati E., Alema S., Falcone G. (2006) Fine regulation of RhoA and Rock is required for skeletal muscle differentiation. J. Biol. Chem., 281, 15249–15257. [DOI] [PubMed] [Google Scholar]
- 40. Beqaj S., Jakkaraju S., Mattingly R.R., Pan D., Schuger L. (2002) High RhoA activity maintains the undifferentiated mesenchymal cell phenotype, whereas RhoA down-regulation by laminin-2 induces smooth muscle myogenesis. J. Cell Biol., 156, 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ransom R.F., Lam N.G., Hallett M.A., Atkinson S.J., Smoyer W.E. (2005) Glucocorticoids protect and enhance recovery of cultured murine podocytes via actin filament stabilization. Kidney Int., 68, 2473–2483. [DOI] [PubMed] [Google Scholar]
- 42. Vernocchi S., Battello N., Schmitz S., Revets D., Billing A.M., Turner J.D., Muller C.P. (2013) Membrane glucocorticoid receptor activation induces proteomic changes aligning with classical glucocorticoid effects. Mol. Cell Proteomics, 12, 1764–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gingras A.C., Gygi S.P., Raught B., Polakiewicz R.D., Abraham R.T., Hoekstra M.F., Aebersold R., Sonenberg N. (1999) Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev., 13, 1422–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shi X., Garry D.J. (2006) Muscle stem cells in development, regeneration, and disease. Genes Dev., 20, 1692–1708. [DOI] [PubMed] [Google Scholar]
- 45. Berkes C.A., Tapscott S.J. (2005) MyoD and the transcriptional control of myogenesis. Semin. Cell Dev. Biol., 16, 585–595. [DOI] [PubMed] [Google Scholar]
- 46. Lyons G.E., Ontell M., Cox R., Sassoon D., Buckingham M. (1990) The expression of myosin genes in developing skeletal muscle in the mouse embryo. J. Cell Biol., 111, 1465–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Manzur A.Y., Kuntzer T., Pike M., Swan A. (2008) Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst. Rev., 2, CD003725.. [DOI] [PubMed] [Google Scholar]
- 48. Gnecchi M., Zhang Z., Ni A., Dzau V.J. (2008) Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res., 103, 1204–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Baraniak P.R., McDevitt T.C. (2010) Stem cell paracrine actions and tissue regeneration. Regen. Med., 5, 121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gharaibeh B., Lavasani M., Cummins J.H., Huard J. (2011) Terminal differentiation is not a major determinant for the success of stem cell therapy - cross-talk between muscle-derived stem cells and host cells. Stem Cell Res. Ther., 2, 31.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tang Y.L., Zhao Q., Qin X., Shen L., Cheng L., Ge J., Phillips M.I. (2005) Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann. Thorac. Surg., 80, 229–236. discussion 236-227. [DOI] [PubMed] [Google Scholar]
- 52. Lavasani M., Robinson A.R., Lu A., Song M., Feduska J.M., Ahani B., Tilstra J.S., Feldman C.H., Robbins P.D., Niedernhofer L.J., et al. (2012) Muscle-derived stem/progenitor cell dysfunction limits healthspan and lifespan in a murine progeria model. Nat. Commun., 3, 608.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Blau H.M., Webster C., Pavlath G.K. (1983) Defective myoblasts identified in Duchenne muscular dystrophy. Proc. Natl Acad. Sci. U S A, 80, 4856–4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhu C.H., Mouly V., Cooper R.N., Mamchaoui K., Bigot A., Shay J.W., Di Santo J.P., Butler-Browne G.S., Wright W.E. (2007) Cellular senescence in human myoblasts is overcome by human telomerase reverse transcriptase and cyclin-dependent kinase 4: consequences in aging muscle and therapeutic strategies for muscular dystrophies. Aging Cell, 6, 515–523. [DOI] [PubMed] [Google Scholar]
- 55. Kallestad K.M., Hebert S.L., McDonald A.A., Daniel M.L., Cu S.R., McLoon L.K. (2011) Sparing of extraocular muscle in aging and muscular dystrophies: a myogenic precursor cell hypothesis. Exp. Cell Res., 317, 873–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dumont N.A., Wang Y.X., von Maltzahn J., Pasut A., Bentzinger C.F., Brun C.E., Rudnicki M.A. (2015) Dystrophin expression in muscle stem cells regulates their polarity and asymmetric division. Nat. Med., 21, 1455–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lawrence J.C., Jr. (2001) mTOR-dependent control of skeletal muscle protein synthesis. Int. J. Sport Nutr. Exerc. Metab., 11 Suppl, S177–S185. 1. [DOI] [PubMed] [Google Scholar]
- 58. Buller C.L., Loberg R.D., Fan M.H., Zhu Q., Park J.L., Vesely E., Inoki K., Guan K.L., Brosius F.C. 3rd. (2008) A GSK-3/TSC2/mTOR pathway regulates glucose uptake and GLUT1 glucose transporter expression. Am. J. Physiol. Cell Physiol., 295, C836–C843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lai Y.C., Liu Y., Jacobs R., Rider M.H. (2012) A novel PKB/Akt inhibitor, MK-2206, effectively inhibits insulin-stimulated glucose metabolism and protein synthesis in isolated rat skeletal muscle. Biochem. J., 447, 137–147. [DOI] [PubMed] [Google Scholar]
- 60. Yue T., Yin J., Li F., Li D., Du M. (2010) High glucose induces differentiation and adipogenesis in porcine muscle satellite cells via mTOR. BMB Rep., 43, 140–145. [DOI] [PubMed] [Google Scholar]
- 61. von Maltzahn J., Bentzinger C.F., Rudnicki M.A. (2012) Wnt7a-Fzd7 signalling directly activates the Akt/mTOR anabolic growth pathway in skeletal muscle. Nat. Cell Biol., 14, 186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bodine S.C., Stitt T.N., Gonzalez M., Kline W.O., Stover G.L., Bauerlein R., Zlotchenko E., Scrimgeour A., Lawrence J.C., Glass D.J., et al. (2001) Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol., 3, 1014–1019. [DOI] [PubMed] [Google Scholar]
- 63. Ge Y., Chen J. (2012) Mammalian target of rapamycin (mTOR) signaling network in skeletal myogenesis. J. Biol. Chem., 287, 43928–43935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gharaibeh B., Lu A., Tebbets J., Zheng B., Feduska J., Crisan M., Peault B., Cummins J., Huard J. (2008) Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique. Nat. Protoc., 3, 1501–1509. [DOI] [PubMed] [Google Scholar]
- 65. Mu X., Brown L.D., Liu Y., Schneider M.F. (2007) Roles of the calcineurin and CaMK signaling pathways in fast-to-slow fiber type transformation of cultured adult mouse skeletal muscle fibers. Physiol. Genomics, 30, 300–312. [DOI] [PubMed] [Google Scholar]
- 66. Moller B., Kukoc-Zivojnov N., Koyama N., Grapenthin S., Kessler U., Klein S.A., Kalina U., Kaltwasser J.P., Hoelzer D., Ottmann O.G. (2002) Prednisolone induces interleukin-18 expression in mononuclear blood and myeloid progenitor cells. Inflamm. Res., 51, 457–463. [DOI] [PubMed] [Google Scholar]
- 67. Metzinger L., Passaquin A.C., Leijendekker W.J., Poindron P., Ruegg U.T. (1995) Modulation by prednisolone of calcium handling in skeletal muscle cells. Br. J. Pharmacol., 116, 2811–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.