Abstract
Mitochondrial diseases are a plethora of inherited neuromuscular disorders sharing defects in mitochondrial respiration, but largely different from one another for genetic basis and pathogenic mechanism. Whole exome sequencing was performed in a familiar trio (trio-WES) with a child affected by severe epileptic encephalopathy associated with respiratory complex I deficiency and mitochondrial DNA depletion in skeletal muscle. By trio-WES we identified biallelic mutations in SLC25A10, a nuclear gene encoding a member of the mitochondrial carrier family. Genetic and functional analyses conducted on patient fibroblasts showed that SLC25A10 mutations are associated with reduction in RNA quantity and aberrant RNA splicing, and to absence of SLC25A10 protein and its transporting function. The yeast SLC25A10 ortholog knockout strain showed defects in mitochondrial respiration and mitochondrial DNA content, similarly to what observed in the patient skeletal muscle, and growth susceptibility to oxidative stress. Albeit patient fibroblasts were depleted in the main antioxidant molecules NADPH and glutathione, transport assays demonstrated that SLC25A10 is unable to transport glutathione. Here, we report the first recessive mutations of SLC25A10 associated to an inherited severe mitochondrial neurodegenerative disorder. We propose that SLC25A10 loss-of-function causes pathological disarrangements in respiratory-demanding conditions and oxidative stress vulnerability.
Introduction
The mitochondrial respiratory complex I couples the transfer of electrons from NADH to ubiquinone and the translocation of protons from the mitochondrial matrix to the intermembrane space, contributing to oxidative phosphorylation. Clinical presentation of complex I deficiency is extremely heterogeneous, ranging from fatal neonatal disease to adult-onset neurodegenerative disorders (1), and often associates to epilepsy (2). The vast genetic heterogeneity of isolated complex I deficiency is caused by mutations in mitochondrial DNA (mtDNA) or, in most cases, in nuclear-DNA genes encoding structural subunits, assembly factors, or other proteins with apparently complex I-unrelated functions (see OMIM 252010). Further, the activity of complex I is susceptible to environmental factors, such as oxidative stress (3).
SLC25A10 (also known as DIC) transports dicarboxylates and phosphate across the inner mitochondrial membrane, and is conserved from yeast to mammals (4,5). As reviewed in (6), SLC25A10 inhibition has been repeatedly reported to cause reduced levels of mitochondrial glutathione (GSH) and impairment of complex I activity in rat neurons (7).
Here we report the first human mutations abolishing SLC25A10 function in a patient affected by a progressive form of epileptic encephalopathy and severe hypotonia associated with complex I deficiency.
Results
Case description
This study was approved by the Pediatric Ethics Committee of the Tuscany Region, Italy (in the context of the Project DESIRE) and informed consent was signed by the patient's parents. The proband is a 9-year-old boy born at term from non-consanguineous healthy parents of two additional healthy children (Fig. 1A). Main clinical and biochemical alterations are summarized in Table 1. Hypospadias, bilateral hydrocele and unilateral right hearing loss were noticed. At 3 months, generalized jerking and infantile spasms appeared in multiple per day episodes. Clinical examinations showed severe hypotonia, absent eye tracking and poor spontaneous movements. EEG revealed multifocal epileptiform discharges and a suppression burst pattern. MRI, initially normal, showed high signal intensity of the white matter at 1 year and also thinning of the corpus callosum at 4 years. Clinical conditions evolved in intractable tonic spasms and focal seizures and quadriparesis, which progressively became spastic and dyskinetic. Growth parameters have always been within the normal range and neuropsychiatric evaluations revealed intellectual ability in the normal-high range. Multiple metabolic investigations, cerebrospinal-fluid amino acids, neurotransmitter levels and visual evoked potentials were normal (the list of laboratory tests is reported in Supplementary Material). Blood analyses revealed microcytic anemia, which was improved after iron supplementation, and increased lactate (3.64 mM) and lactate/pyruvate (25.58) levels. The analysis of the four mitochondrial respiratory complexes from muscle homogenates, performed at 18 months, indicated reduced respiratory complex I activity (27% of the mean control value, Supplementary Material, Fig. S1) and decreased mtDNA content (40% lower than the mean control value). Multiple antiepileptic drug trials and ketogenic diet were ineffective against seizures.
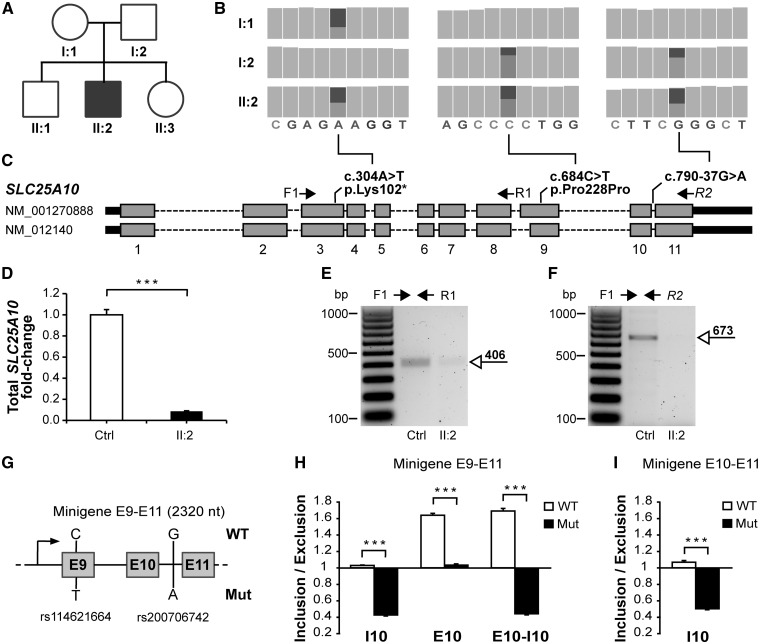
Figure 1.
SLC25A10 genetic and RNA analyses. (A) Pedigree of the affected family (filled symbol: patient; open symbols: unaffected relatives). (B) Compound heterozygous mutations identified by trio-WES, reported as the “Integrative genomic viewer” (26) output. (C) Structure of the SLC25A10 gene and location of mutations. The nucleotide and amino acid changes are indicated with respect to the reference sequences NM_001270888 and NP_001257817, respectively. (D) qRT-PCR analysis of fibroblast cDNA revealed a decrease in endogenous SLC25A10 RNA quantity in the patient (II: 2) compared with control (Ctrl). (E) Gel electrophoresis of cDNA PCR products revealed the presence of a fragment spanning exons 3-8 in patient fibroblasts (II: 2) and control fibroblasts (Ctrl), while (F) patient fibroblasts lack the fragment spanning exons 3-11. (G) Structure of the minigene construct spannig exons 9-11 (E: exon). (H) Ratio between the quantity of the minigene-derived RNA splicing fragments including and excluding intron 10 (I10), exon 10 (E10) or both of them (E10-I10) in minigene E9-E11. (I) Inclusion/Exclusion ratios in minigene E10-E11. Data are presented as mean+SEM of at least three experiments; ***: non parametric Wilcoxon test P-value<0.05.
Table 1.
Main clinical and biochemical alterations
| Analysis | Specimen | Result |
|---|---|---|
| Clinical examination | – | Severe hypotonia, intractable tonic spasms, focal seizures and quadriparesis |
| EEG | – | Multifocal epileptiform discharges |
| MRI | – | High signal intensity of the white matter (1 yo); thinning of corpus callosum (4 yo) |
| Cell count | Blood | Microcytic anemia |
| Redox state | Plasma | Increased lactate (3.64 mM) and lactate/pyruvate ratio (25.58) |
| RC activity | Skeletal muscle | Complex I deficiency (27%) |
| MtDNA content | Skeletal muscle | mtDNA depletion (40%) |
EEG: Electroencephalography; MRI: Magnetic resonance imaging; yo: years old; RC: respiratory chain; mtDNA: mitochondrial DNA.
Compound heterozygous mutations in SLC25A10
Pathogenic mutations in the mitochondrial genome were excluded by Sanger sequencing. Three independent whole exome sequencing experiments (trio-WES) were performed on the genomic DNA of the patient and his parents. Trio-WES led to a dramatic cut-off of sequence variants, compared with patient-WES, under dominant and recessive hypotheses of inheritance (Supplementary Material, Fig. S2). Compound heterozygous mutations were identified in SLC25A10 (Fig. 1B), and their co-occurrence in the healthy brother and sister was excluded by direct sequencing (Supplementary Material, Fig. S3). A heterozygous SLC25A10 nonsense mutation was inherited from the mother (NM_001270888.1: c.304A > T, p.Lys102*, Fig. 1C). This mutation, absent in the ExAC database (September 2017), generates a prematurely truncated protein lacking ∼70% of the amino acid sequence (Supplementary Material, Fig. S4). Two heterozygous mutations were inherited from the father (Fig. 1C): a synonymous mutation (NM_001270888.1: c.684C > T, p.Pro228Pro) annotated in the dbSNP146 database (rs114621664) with frequency of 0.0014 in the ExAC database and an intronic mutation (NM_001270888.1: c.790–37G > A) annotated in the dbSNP146 database (rs200706742) with frequency of 0.0011 in the ExAC database. Mutations have been submitted to the NCBI ClinVar database (www.ncbi.nlm.nih.gov/clinvar/, accessions SCV000611119 and SCV000611120).
SLC25A10 mutations cause RNA depletion and aberrant RNA splicing
The quantitative Real Time-PCR (qRT-PCR) analysis, conducted on cDNA obtained from cultured fibroblasts, showed a 10-fold decrease in SLC25A10 transcript level in the patient relative to control (Fig. 1D). PCR primer-pairs were designed to amplify two distinct portions of the SLC25A10 cDNA (Fig. 1C), which span exons 3–8 and exons 3–11, respectively. The former was detected in both the patient and control (Fig. 1E). The sequence of this fragment corresponds to the paternally inherited allele (Supplementary Material, Fig. S5), suggestive of nonsense-mediated decay of the maternally inherited one. The fragment spanning exons 3–11 is instead virtually absent in the patient cells (Fig. 1F), suggesting abnormal RNA splicing in-between exons 9 and 11. The paternally inherited mutations are predicted to break an exon splicing enhancer in exon 9 and to create a new one in intron 10, respectively (Supplementary Material, Fig. S6). Several minigene splicing reporter constructs (Fig. 1G and Supplementary Material, Fig. S7) were generated to further test the effect of these cis-acting mutations on the RNA splicing of the flanking exons/introns. The quantification of the obtained RNA splicing fragments showed that the mutant DNA promotes a shift toward shorter splicing isoforms, thus favoring the exclusion of SLC25A10 exon 10 and intron 10, when compared with the wild-type (Fig. 1H). The RNA quantitative change was also observed in other minigenes hosting the intronic mutation alone (Fig. 1I), but not in minigenes hosting the synonymous mutation alone (Supplementary Material, Fig. S7), suggesting a primary role of the intronic mutation in inducing the aberrant RNA splicing.
SLC25A10 mutations are associated with absence of protein and transport activity, and depletion of antioxidant molecules
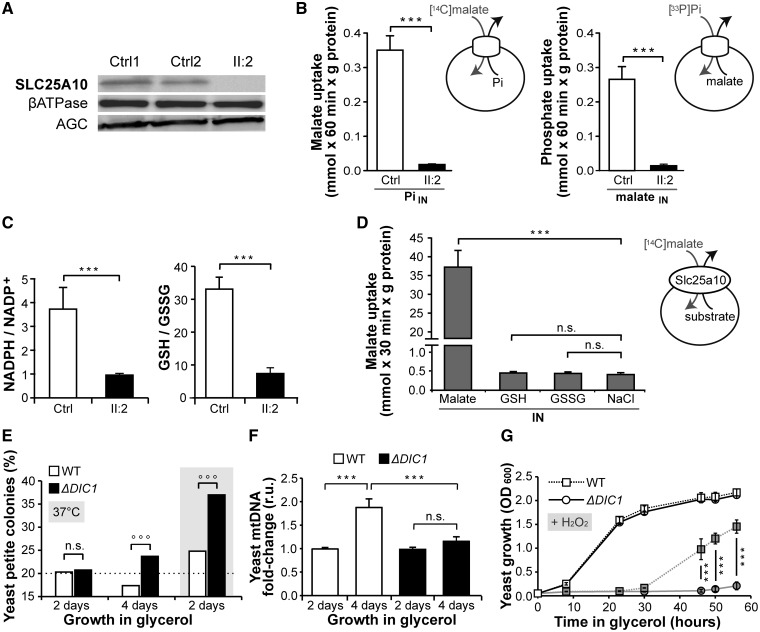
Western-blot immunodetection on mitochondrial extracts did not reveal the SLC25A10 band in the patient-derived fibroblasts, while other known mitochondria-targeted proteins, i.e. the β-subunit of the ATPase complex and the aspartate-glutamate carrier, were detected in both the patient and two controls (Fig. 2A). The SLC25A10 transport activity was determined as malate/phosphate exchange, which is the SLC25A10 defining reaction (8–11), in liposomes reconstituted with fibroblast mitochondrial extracts. Control mitochondrial extracts efficiently catalyzed the uptake of malate in exchange with phosphate, and vice versa, while negligible if any transport activity of SLC25A10 was detected in the patient (Fig. 2B) even after a long period of incubation (60 min).
Figure 2.
SLC25A10 protein and functional analyses. (A) Western-blot of primary fibroblast mitochondrial extracts (30 μg of protein) in patient (II: 2) and two control cell lines (Ctrl1 and Ctrl2). (B) Transport activity of SLC25A10 in fibroblast mitochondrial extracts of patient (II: 2) and control (Ctrl). Liposomes were reconstituted with mitochondrial extracts, preloaded with excess of Pi or malate (20 mM) and incubated with radio-labeled [14C]malate or [33P]phosphate (1 mM), respectively, for 60 min. Pi: phosphate. (C) Mass spectrometry quantification of NADPH and GSH (reduced forms) and NADP+ and GSSG (oxidized forms), reported as reduced/oxidized form ratios, in the fibroblasts of patient (II: 2) and control (Ctrl). (D) Lack of transport of GSH and GSSG by DIC (Slc25a10). Liposomes were reconstituted with the recombinant purified rat DIC, preloaded with excess of malate, GSH or GSSG or NaCl (10 mM) and incubated with radio-labeled [14C]malate (0.5 mM) for 30 min. (E) Percentage of “petite” respiratory-deficient yeast colonies generated from S. cerevisiae knockout strain (ΔDIC1) and isogenic wild-type strain (WT) after 2 or 4 days of growth in glycerol at 30 °C or 37 °C. ∼1000 colonies were screened per strain per assay. °°°: binomial test P-value<0.05, n.s.: not significant. (F) Yeast quantitative PCR analysis of relative mtDNA copy number after 4 days of growth in glycerol compared with 2 days. (G) Yeast growth curves in glycerol for WT and ΔDIC1 in absence (open symbols) or in presence (filled symbols) of H2O2 (2.5 mM). Data are presented as mean+SEM of at least three experiments except for Figure 2E, ***: non parametric wilcoxon test P-value<0.05, n.s.: not significant.
In view of the previous observation that SLC25A10 inhibition increases the amount of H2O2 released by mitochondria (7), cultured patient fibroblasts were assessed for the content of key antioxidant system molecules, GSH and Nicotinamide Adenine Dinucleotide Phosphate (NADPH). Mass spectrometry analysis of cellular extracts showed a 4-fold decrease in the NADPH/NADP+ and GSH/GSSG ratios in the patient fibroblasts compared with control (Fig. 2C), while the total quantity of these metabolites (oxidized + reduced forms) was not significantly altered (Supplementary Material, Fig. S8). To verify whether the protective role of SLC25A10 against oxidative stress depends on the proposed SLC25A10-mediated transport of GSH (7,12), rat Slc25a10 (90% identical to human SLC25A10) was expressed in bacteria, purified, incorporated into liposomes and tested for transport activity. Slc25a10 did transport malate, whereas no transport activity was observed with GSH or GSSG (Fig. 2D).
Furthermore, patient fibroblasts exhibited a growth defect, compared with control, under stress conditions induced by a low-glucose concentration (Supplementary Material, Fig. S9).
Yeast SLC25A10 ortholog lack-of-function causes impairment in mitochondrial respiration, reduced mtDNA copy number and oxidative stress vulnerability
Albeit the patient skeletal muscle revealed reduced mtDNA quantity and impaired complex I activity, cultured fibroblasts did not show any respiratory defect (Supplementary Material, Fig. S1) as frequently observed in mitochondrial disorders (13). Therefore, the model organism S. cerevisiae was employed to investigate the cellular phenotype caused by SLC25A10 lack-of-function. In these experiments the role of yeast DIC1, the ortholog and functional analog of SLC25A10 (4,5), was explored by comparing the yeast knockout strain (ΔDIC1) with the isogenic wild-type strain (WT). The “petite” frequency assay [(14) and Supplementary Material, Fig. S10] showed that the generation rate of respiratory-deficient cells increased in ΔDIC1, compared with WT, when cells were cultured in respiratory-demanding conditions, i.e. 4-day vs 2-day culture in glycerol as the only non fermentable carbon source (Fig. 2E). Yeast ΔDIC1 also showed increase in respiratory-deficient cells, compared with WT, when grown in glycerol for 2 days under environmental stress conditions (at 37° instead of 30 °C, Fig. 2E). Further, the mtDNA quantification assay showed that WT, and not ΔDIC1, increased mtDNA copy number after culturing cells for 4 days in glycerol compared with 2 days (Fig. 2F). In addition, a defective growth curve of ΔDIC1, compared with WT, emerged when H2O2 was added to the glycerol growth medium (Fig. 2G), indicating oxidative stress vulnerability.
Discussion
Here, we show the first SLC25A10 recessive mutations associated with a mitochondrial disorder with severe epileptic and progressive encephalopathy. Albeit additional SLC25A10 mutations in unrelated individuals will be required as standard proof of causality, we report genetic and functional evidence supporting that SLC25A10 is a novel disease-causing gene. Trio-WES analysis indicated SLC25A10 as the only gene encoding a mitochondria-targeted protein with mutations compatible with the patient family tree. The relevance of the detected mutations was demonstrated by the absence of maternal SLC25A10 RNA, hosting a premature stop codon, and the remarkably reduced content and impaired splicing of the paternal SLC25A10 RNA, hosting a synonymous and an intronic mutation. Consistently, the western-blot immunodetection exhibited absence of SLC25A10, and the transport assay on reconstituted mitochondrial extracts revealed no SLC25A10 activity. A S. cerevisiae strain lacking DIC1, the functional analog of SLC25A10 in yeast (4,5), showed impairment in mitochondrial respiration and decrease in mtDNA copy number, similarly to what observed in the patient skeletal muscle, when yeast cells underwent prolonged respiratory-demanding conditions.
The respiratory proficiency in permissive cell growth conditions, together with the neuromuscular patient phenotype, suggests that the respiratory-chain defect may be secondary to biochemical intracellular derangements (15) and that SLC25A10 functions cannot be accomplished by other transporters, at least in neuronal and muscle cells. Multiple literature findings reviewed in (6) indicated that SLC25A10 confers oxidative stress resistance, which was attributed to a putative SLC25A10-mediated transport of GSH into mitochondria. In rat neurons in particular, Slc25a10 inhibition has been shown to cause mitochondrial GSH depletion (7,12), release of H2O2 from mitochondria and complex I deficiency due to oxidative stress (7). Our results suggest that SLC25A10 lack-of-function might confer oxidative stress vulnerability, since the patient fibroblasts show depletion in the main antioxidant molecules NADPH and GSH, and the yeast knockout strain displays growth defect upon addiction of H2O2. Oxidative stress might induce mtDNA degradation (16) and impair mitochondrial respiration, especially at complex I, which is the greatest and more vulnerable respiratory complex (17). However, our observation that rat Slc25a10 does not transport GSH, in agreement with similar findings with yeast DIC1 (18), infers that oxidative stress resistance depends on SLC25A10 functions other than transport of GSH. The known SLC25A10 substrates malate and succinate may contribute to the cell antioxidant system, since they are donors of reducing equivalents for the production of NADPH, GSH and ubiquinol (Supplementary Material, Fig. S11). In addition to redox defense, SLC25A10 was proposed to modulate the energy metabolism of the cell, since knockdown of SLC25A10 in a cancer cell line decreases the protein content of the hypoxia inducible factor 1α (HIF-1α), a key regulator of the shift between glycolysis and oxidative phosphorylation (19). Future work is warranted to exploit the SLC25A10-mediated molecular mechanisms that protect cellular respiration and redox state.
Materials and Methods
WES and related data analyses are described in Supplementary Material. qRT-PCR was carried out (20) using Thermo Fisher assays for human SLC25A10 (Hs00201730_m1) and ACTB gene (Hs99999903_m1). Minigene expression constructs were generated using the primers listed in Supplementary Material, Table S1, from both the maternal and paternal SLC25A10 allele, and were analysed as reported in Supplementary Material. The anti-SLC25A10 polyclonal antiserum antibody (8,21) was used for western-blot immunodetection on fibroblast mitochondrial extracts. The latter were reconstituted into proteoliposomes and analysed by transport assays as described (8,22). Metabolite quantification by mass spectrometry analysis was performed as reported (23–25). Rat Slc25a10 bacterial expression, reconstitution and transport assays were performed as reported (5,22). S. cerevisiae strains were obtained from EUROSCARF (wild-type S288C strain and isogenic DIC1 knockout strain YLR348c:kanMX4), and were analysed as described in Supplementary Material.
Supplementary Material
Supplementary Material is available at HMG online.
Supplementary Material
Acknowledgements
We thank the family for partaking in this study and the “Cell line and DNA bank of Genetic Movement Disorders and Mitochondrial Diseaseas” (GMD-MD Bank), member of the Telethon Network of Genetic Biobanks [GTB12001], for providing us with specimens.
Conflict of Interest statement. None declared.
Funding
MITOCON, the Italian Association for the study and the cure of mitochondrial disorders [grant TRIO to A.D.G.]; General Medical Sciences of the National Institutes of Health [R15GM119099 to M.R.]; and European Union Seventh Framework Programme FP7/2007–2013 under the project ‘DESIRE’ [602531 to R.G.].
References
- 1. Fassone E., Rahman S. (2012) Complex I deficiency: clinical features, biochemistry and molecular genetics. J. Med. Genet., 49, 578–590. [DOI] [PubMed] [Google Scholar]
- 2. Khurana D.S., Salganicoff L., Melvin J.J., Hobdell E.F., Valencia I., Hardison H.H., Marks H.G., Grover W.D., Legido A. (2008) Epilepsy and respiratory chain defects in children with mitochondrial encephalopathies. Neuropediatrics, 39, 8–13. [DOI] [PubMed] [Google Scholar]
- 3. Musatov A., Robinson N.C. (2012) Susceptibility of mitochondrial electron-transport complexes to oxidative damage. Focus on cytochrome c oxidase. Free Radic. Res., 46, 1313–1326. [DOI] [PubMed] [Google Scholar]
- 4. Palmieri L., Palmieri F., Runswick M.J., Walker J.E. (1996) Identification by bacterial expression and functional reconstitution of the yeast genomic sequence encoding the mitochondrial dicarboxylate carrier protein. FEBS Lett., 399, 299–302. [DOI] [PubMed] [Google Scholar]
- 5. Fiermonte G., Palmieri L., Dolce V., Lasorsa F.M., Palmieri F., Runswick M.J., Walker J.E. (1998) The sequence, bacterial expression, and functional reconstitution of the rat mitochondrial dicarboxylate transporter cloned via distant homologs in yeast and Caenorhabditis elegans. J. Biol. Chem., 273, 24754–24759. [DOI] [PubMed] [Google Scholar]
- 6. Lash L.H. (2015) Mitochondrial glutathione in diabetic nephropathy. J. Clin. Med., 4, 1428–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kamga C.K., Zhang S.X., Wang Y. (2010) Dicarboxylate carrier-mediated glutathione transport is essential for reactive oxygen species homeostasis and normal respiration in rat brain mitochondria. Am. J. Physiol. Cell. Physiol., 299, 497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Palmieri L., Vozza A., Hönlinger A., Dietmeier K., Palmisano A., Zara V., Palmieri F. (1999) The mitochondrial dicarboxylate carrier is essential for the growth of Saccharomyces cerevisiae on ethanol or acetate as the sole carbon source. Mol. Microbiol., 31, 569–577. [DOI] [PubMed] [Google Scholar]
- 9. Palmieri F., Prezioso G., Quagliariello G., Klingenberg M. (1971) Kinetic study of the dicarboxylate carrier in rat liver mitochondria. Eur. J. Biochem., 22, 66–74. [DOI] [PubMed] [Google Scholar]
- 10. Palmieri F., Klingenberg M. (1979) Direct methods for measuring metabolite transport and distribution in mitochondria. Methods Enzymol., 56, 279–301. [DOI] [PubMed] [Google Scholar]
- 11. Palmieri L., Picault N., Arrigoni R., Besin E., Palmieri F., Hodges M. (2008) Molecular identification of three Arabidopsis thaliana mitochondrial dicarboxylate carrier isoforms: organ distribution, bacterial expression, reconstitution into liposomes and functional characterization. Biochem. J., 410, 621–629. [DOI] [PubMed] [Google Scholar]
- 12. Wilkins H.M., Kirchhof D., Manning E., Joseph J.W., Linseman D.A. (2013) Mitochondrial glutathione transport is a key determinant of neuronal susceptibility to oxidative and nitrosative stress. J. Biol. Chem., 288, 5091–5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rodenburg R.J.T. (2011) Biochemical diagnosis of mitochondrial disorders. J. Inherit. Metab. Dis., 34, 283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hess D.C., Myers C.L., Huttenhower C., Hibbs M.A., Hayes A.P., Paw J., Clore J.J., Mendoza R.M., Luis B.S., Nislow C.. et al. (2009) Computationally driven, quantitative experiments discover genes required for mitochondrial biogenesis. PLoS Genet., 5, e1000407.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schapira A.H. (2002) Primary and secondary defects of the mitochondrial respiratory chain. J. Inherit. Metab. Dis., 25, 207–214. [DOI] [PubMed] [Google Scholar]
- 16. Shokolenko I., Venediktova N., Bochkareva A., Wilson G.L., Alexeyev M.F. (2009) Oxidative stress induces degradation of mitochondrial DNA. Nucleic Acids Res., 37, 2539–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sharma L.K., Lu J., Bai J.L. (2009) Mitochondrial respiratory complex I: structure, function and implication in human diseases. Curr. Med. Chem., 16, 1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Booty L.M., King M.S., Thangaratnarajah C., Majd H., James A.M., Kunji E.R.S., Murphy M.P. (2015) The mitochondrial dicarboxylate and 2-oxoglutarate carriers do not transport glutathione. FEBS Lett., 589, 621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou X., Paredes J.A., Krishnan S., Curbo S., Karlsson A. (2015) The mitochondrial carrier SLC25A10 regulates cancer cell growth. Oncotarget, 6, 9271–9283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Menga A., Iacobazzi V., Infantino V., Avantaggiati M.L., Palmieri F. (2015) The mitochondrial aspartate/glutamate carrier isoform 1 gene expression is regulated by CREB in neuronal cells. Int. J. Biochem. Cell Biol., 60, 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fiermonte G., Dolce V., Arrigoni R., Runswick M.J., Walker J.E., Palmieri F. (1999) Organization and sequence of the gene for the human mitochondrial dicarboxylate carrier: evolution of the carrier family. Biochem. J., 344, 953–960. [PMC free article] [PubMed] [Google Scholar]
- 22. Palmieri F., Indiveri C., Bisaccia F., Iacobazzi V. (1995) Mitochondrial metabolite carrier proteins: Purification, reconstitution, and transport studies. Methods Enzymol., 260, 349–369. [DOI] [PubMed] [Google Scholar]
- 23. Castegna A., Scarcia P., Agrimi G., Palmieri L., Rottensteiner H., Spera I., Germinario L., Palmieri F. (2010) Identification and functional characterization of a novel mitochondrial carrier for citrate and oxoglutarate in Saccharomyces cerevisiae. J. Biol. Chem., 285, 17359–17370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Palmieri E.M., Spera I., Menga A., Infantino V., Porcelli V., Iacobazzi V., Pierri C.L., Hooper D.C., Palmieri F., Castegna A. (2015) Acetylation of human mitochondrial citrate carrier modulates mitochondrial citrate/malate exchange activity to sustain NADPH production during macrophage activation. Biochim. Biophys. Acta, 1847, 729–738. [DOI] [PubMed] [Google Scholar]
- 25. Lauderback C.M., Drake J., Zhou D., Hackett J.M., Castegna A., Kanski J., Tsoras M., Varadarajan S., Butterfield D.A. (2003) Derivatives of xanthic acid are novel antioxidants: application to synaptosomes. Free Radic. Res., 37, 355–365. [DOI] [PubMed] [Google Scholar]
- 26. Robinson J.T., Thorvaldsdóttir H., Winckler W., Guttman M., Lander E.S., Get G., Mesirov J.P. (2011) Integrative genomics viewer. Nat. Biotechnol., 29, 24–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.