Abstract
Background:
Crosstalk between cancer cells and fibroblasts is crucial for tumour progression. It has been reported that exosomes derived from cancer cells play an important role in the intracellular communications involved in the development of carcinoma. However, the role of exosomes from fibroblasts remains unclear. This study aimed to clarify the effect of exosomes from fibroblasts on the motility of gastric cancer cells.
Methods:
5 gastric cancer cell lines were used: OCUM-12, NUGC-3, MKN45, FU97 and MKN74. 2 cancer-associated fibroblasts (CAFs) were used. CD9 expression of exosomes from fibroblasts was examined by western blot. The effect of exosomes on the motility of cancer cells was analysed by migration assays. MMP2 was examined by RT-PCR or gelatin zymography. Then, CD9 and MMP2 expressions of 619 gastric cancers were analysed by immunohistochemistry.
Results:
Exosomes from CAFs were taken into scirrhous-type gastric cancer cells, namely OCUM-12 cells and NUGC-3 cells, but not into other types of gastric cancer cells. Exosomes from CAFs were positive for CD9. Exosomes from CAFs significantly stimulated the migration and invasion of OCUM-12 and NUGC-3 cells, which was inhibited by anti-CD9 antibody or CD9-siRNA. MMP2 expression of OCUM-12 and NUGC-3 cells was significantly decreased by CD9-siRNA. 116 CD9-positive cases were significantly correlated with scirrhous-type gastric cancer, lymph node metastasis and venous invasion. The 5-year survival rate of patients with CD9-positive tumours was significantly lower (P<0.001) than in those with CD9-negative tumours.
Conclusions:
CD9-positive exosomes from CAFs might stimulate the migration ability of scirrhous-type gastric cancer cells.
Keywords: exosome, gastric cancer, CD9, fibroblasts, scirrhous-type
Scirrhous-type gastric carcinoma, or Borrman type 4 gastric cancer, also known as linitis plastica carcinoma, is characterised by rapid cancer cell infiltration with abundant stromal cells. This type of gastric cancer shows an extremely poor prognosis, as compared with other types of gastric carcinoma (Japanese Gastric Cancer A, 1998; Otsuji et al, 2004; Tahara, 2004; Ikeguchi et al, 2009; Yashiro and Hirakawa, 2010). Of various stromal cells, fibroblasts constitute a major component in the tumour microenvironment of scirrhous-type gastric cancer (Otsuji et al, 2004; Semba et al, 2009).
It has been well recognised that crosstalk between cancer cells and stromal cells is crucial for tumour progression (Fuyuhiro et al, 2012; Hasegawa et al, 2014; Kasashima et al, 2014). Exosomes are small membrane vesicles containing functional biomolecules that can be horizontally transferred to recipient cells (Valadi et al, 2007). It has been reported that the exosomes play complex roles in intracellular communications (Yuana et al, 2013). Recent researches have revealed that the exosomes from cancer cells might be associated with the development of the tumour microenvironment such as metastatic niche formation and angiogenesis (Hoshino et al, 2015; Ji et al, 2015; Fujita et al, 2016). On the other hand, the role of exosomes from stromal cells in relation to cancer cells remains unclear. Fibroblasts surrounding cancer cells were reported to be cancer-associated fibroblasts (CAFs) and to have distinct features from normal fibroblasts (NFs) (Fuyuhiro et al, 2012; Kinoshita et al, 2015; Satoyoshi et al, 2015). We previously reported that CAFs stimulate the progression of scirrhous gastric cancer cells (Fuyuhiro et al, 2012). While exosomes from CAFs might influence the biologic behavior of gastric cancer cells, the effect of exosomes from CAFs on the motility of cancer cells remains unclear. It has been reported that CD9, one of surface markers of exosomes, might be associated with cancer migration and invasion (Hong et al, 2005; Zöller, 2009). However, the role of CD9-positive exosomes from CAFs has not yet been investigated. In this study, we examined the role of CD9-positive exosomes from CAFs in the invasion and migration ability of gastric cancer cells.
Materials and methods
Cell culture and cell lines
Five gastric cancer cell lines, NUGC-3 (JCRB Cell Bank, Osaka, Japan), OCUM-12(Kato et al, 2010), MKN45 (JCRB Cell Bank), FU97 (American Type Culture Collection, Rockville, MD, USA) and MKN74 (JCRB Cell Bank), and four fibroblast cell lines, CaF64, CaF65, NF64 and NF65, were used. NUGC-3 and OCUM-12 were derived from scirrhous-type gastric cancer, MKN45 and FU97 were from poorly differentiated-type gastric cancer, and MKN74 were from intestinal-type gastric cancer. Two CAFs of CaF64 and CaF65, were established from the tumoural tissue of gastric cancer. 2 NFs of NF64 and NF65, were established from non-tumoural gastric tissue. CaF64 and NaF64 were from 67-year-old man with gastric cancer, and CaF65 and NF65 were from 68-year-old man with gastric cancer. The primary culture was initiated as previously reported (Kinoshita et al, 2015). Briefly, the gastric tumour specimen was excised under aseptic conditions, and minced with forceps and scissors. The culture medium consisted of concentrations with Dulbecco’s modified Eagle medium (DMEM; Wako, Osaka, Japan) with the addition of 10% foetal bovine serum (FBS; Nichirei Biosciences Inc., Tokyo, Japan), 100 IU ml−1 penicillin (Wako), 100 mg ml−1 streptomycin (Wako), and 0.5 mM sodium pyruvate (Wako). Cells were cultured in 21%O2 at 37 °C. Fibroblasts were used from 3rd passage to 12th passage in culture, mainly at 5th passage.
Isolation of exosome from fibroblasts
Exosomes were obtained from supernatant of cells as previously described with some modifications (Yoshioka et al, 2014; Hoshino et al, 2015). In brief, after cells were grown semi-confluent in DMEM containing 10% FCS, cells were washed with PBS and incubated for an additional 2 days in 5 ml of serum-free DMEM. The supernatant was collected, and centrifuged by 300 g for 5 min to discard any floating cells. After that, the media was collected and centrifuged at 2000 g for 10 min to discard cellular debris. Then, the media was filtered using a 0.8 μm pore filter (Millipore, Billerica, MA, USA). The collected media was then ultracentrifuged at 100 000 g for 1.5 h at 4 °C. The exosomes pellet was washed with 35 ml PBS, followed by a second step of ultracentrifugation at 100 000 g for 1.5 h at 4 °C. The supernatant was discarded and stored −80 °C. The amount and number of exosomes from 10 μg protein was quantified by Nanosight Nanoparticle Tracking Analysis (QuantumDesign, Tokyo, Japan).
Western blot of exosomes
Exosomes (1 μg protein) were subjected to 10% SDS–PAGE and the separated proteins were transferred onto polyvinylidene difluoride membrane using Trans-Blo Turbo Transfer System (BIO-RAD, Hercules, CA, USA) according to the manufacture’s instruction. Then, the membrane was placed in PBS-T solution containing each primary antibody: anti-CD9 (1 : 1000; Life technologies, Carlsbad, CA, USA), anti-CD63 (1 : 1000; Life technologies), and anti-CD81 (1 : 1000; Life technologies). The blots were incubated with secondary antibody (anti-mouse HRP- conjugated antibody, 1 : 300; Cell Signaling). The bands were detected using an enhanced chemiluminescence using ECL prime (GE health care, little Chalfont, UK). Western blots were analysed using the Luminescent image analyzer LAS 4000-plus (Fuji, Tokyo, Japan).
Western blot of proteins from cell lines
OCUM-12, and NUGC-3 cells were rinsed with PBS, and incubated in DMEM with or without interfereing CD9 (CD9 siRNA). After transfection of CD9 siRNA, cell extracts (20 mg protein) were used for western blot analysis. The following primary antibodies were used: β-actin (1:300; Sigma-Aldrich, St Louis, MO, USA), anti-CD9 (1 : 1000; Life Technologies), and MMP2 (ab86607, 1 : 300; Abcam, Cambridge, UK).
Labelling of exosomes and fluorescence microscopy
For in vitro uptake assays, purified exosomes were fluorescently labelled using PKH26 (red) membrane dye (Sigma-Aldrich). We used 4 μM of PKH26 and the same volume of 10 μg/ml exosomes. PKH26-labelling exosomes (1 μg/ml) were added to gastric cancer cells, and incubated with 2% FBS at 37 °C for 24 h. After washing off excess exosomes, cancer cells were further incubated with DAPI (Wako; 1 : 1000) for 30 min at room temperature, and were viewed under a fluorescence microscope Leica TCS-SP5 (Leica, Wetzler, Germany). Excitation wave length used for DAPI and PKH26 were 405 nm, and 543 nm, respectively.
Wound healing assay
Gastric cancer cells were cultured in 96-well plates (Essen ImageLock, Essen In- struments, Birmingham, UK). After the cells reached semi-confluence, a wound was created in the cell monolayer with the 96-well Wound Maker (Essen Bioscience, Ann Arbor, MI, USA). Cancer cells were cultured in DMEM with 2% FBS in the presence of exosome (1 μg/ml) from CAFs or PBS as control. Scratched fields were taken pictured every 3 h and were monitored with Incucyte Live-Cell Imaging System and software (Essen Instruments). The degree of cell migrations was analysed as a percentage of wound confluence. The mean of eight fields was calculated as the sample value.
Invasion assay
The in vitro invasiveness was measured by two-chamber matrigel invasion assay, as previously reported (Kasashima et al, 2014). We used the chemotaxicell chambers (Millipore, Billerica, MA, USA) with a 12 μm pore membrane filter coated with 50 μg of matrigel (upper chamber) in a 24-well culture plate (lower chamber). Gastric cancer cells (2 × 103 cells/200 μl/chamber) were seeded in upper chamber, and 20 μl exosome from fibroblasts (a final concentration: 1 μg/ml exosomes with 2% FBS) were added to the upper chamber. After incubation for 72 h, cancer cells that invaded through a filter to the lower surface of the membrane were stained by the Diff- Quik (Sysmex, Kobe, Japan), and were manually counted under a microscope at × 200 magnification. Six randomly chosen fields were counted for each assay. The mean of six fields was calculated as the sample value.
Patients
A total of 619 patients who had undergone a resection of the primary gastric cancer were enrolled in this study. The pathologic diagnoses and classifications were made according to the UICC TNM classification of malignant tumours 7th edition (2009). The study protocol conformed to the ethical guidelines of the Declaration of Helsinki. This study was approved by the Osaka City University ethics committee. Written informed consent was obtained from all patients.
Inhibition of CD9
Small interfereing CD9 (CD9 siRNA) and anti-CD9 neutralising antibody were used. CD9 siRNA (Ambion, Carlsbad, CA, USA) and nontargeting siRNA (negative-siRNA; Ambion) were used. The transfection mixture was prepared by adding 150 μl of Opti-MEM including 9 μl of Lipofectamine RNA iMAX Reagent (Life technologies) to 150 μl of Opti- MEM including 90 pmol of siRNA and incubating for 5 min. The transfection mixture or anti-CD9 neutralising antibody was added to OCUM-12 cells, NUGC-3 cells, and CaF64 fibroblasts in six-well dish containing 1.7 ml of DMEM with 2% FBS. RT–PCR were performed 48 h after transfection. The exosomes from CD9 siRNA transfected CaF64 cells were collected, and then the exosomes were used for wound healing assays and invasion assays. Also, we examined the effect of the inhibition of CD9 adhesion molecule on the uptake of exosomes in cancer cells using anti-CD9 neutralising antibody (1 μg ml−1).
Quantitative real-time reverse transcription–PCR
Reverse transcription–PCR (RT-PCR) was performed using ABI Prism 7000 (Applied Biosystems, Foster City, CA, USA). The primer and probe sequences were follows. The primer and probe sequences used in this assay were Taqman Gene expression Assay, Assay ID Hs01548727 for matrix metalloproteinase-2 (MMP-2) expression. As an internal control, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (accession number: NM- 002046, NM-001256799, P 5′-CCCCTGCAAATGAGCCCCAGCCTTC-3′ Forward 5′-CCATCTTCCAGGAGCGAGATC-3′ Reverse 5′-GGCAGAGATGATGACCCTTTTG-3′) were used. RT–PCR was performed at 95 °C for 15 s and 60 °C for 60 s for 40 cycles using the ABI Prism 7000. The mRNA level of each gene was normalised by the internal control GAPDH.
Gelatin zymography
OCUM-12 cells and NUGC3 cells were prepared at 50–60% confluence in 96-well dishes. After the treatment of exosomes derived from CaF64 and/or CD9 antibody, these cells were incubated during 48 h. Then, the supernatant was used for the following gelatin zymography (Cosmo-bio, Tokyo, Japan). LAS-3000 imaging system was used for measuring the density of the band (Fuji, Tokyo, Japan).
Immunohistochemical determination of CD9 and MMP2
Immunohistochemical staining was analysed using 619 gastric cancers. Sides were deparaffinised and then heated for 10 min at 105 °C in an autoclave in Target Retrieval Solution (Dako, Carpinteria, CA, USA). After blocking endogenous peroxidase activity, the specimens were incubated with CD9 antibody (1:200; Life Technologies) and MMP2 (ab86607, 1 : 300; Abcam, Cambridge, UK) for 1 h at room temperature, and were incubated with biotinylated goat anti-rabbit IgG for 10 min. The slides were treated with streptavidin-peroxidase reagent, followed by counterstaining with Mayer’s haematoxylin. CD9 expression was evaluated at the invading tumour front. CD9 expression was evaluated by intensity of staining and percentage of stained cancer cells and stromal cells, respectively: intensity was given scores 0–3 (0=no, 1=weak, 2=moderate, 3=intense), and the percentage of immunopositive cells was given scores 0–3 (0=0%, 1=10%, 2=20–30%, 3=40–100%). The two scores were multiplied to obtain the final result of 0–9. Expressions were considered positive in tumour cells when scores were three or more and negative when scores were 0–2. Evaluation was made by two double-blinded independent observers who were unaware of clinical data and outcome.
Statistical analysis
Associations between CD9 expression and clinicopathological findings were analysed using the χ2-test. Overall survival (OS) was defined as the time from surgery to death from any cause. OS curves were estimated by the Kaplan–Meier method and compared using the log-rank test. Multivariate analysis was performed using cox proportional hazard model. As for wound healing assay, invasion assay and RT–PCR, t-test was used to determine the significance of differences between groups. All statistical analyses were performed using the JMP statistical software (version 8.0; SAS Institute, Cary, NC, USA). Two-sided probability (P) value of<0.05 was considered to be statistically significant.
Results
Uptake of exosomes from fibroblasts into gastric cancer cells.
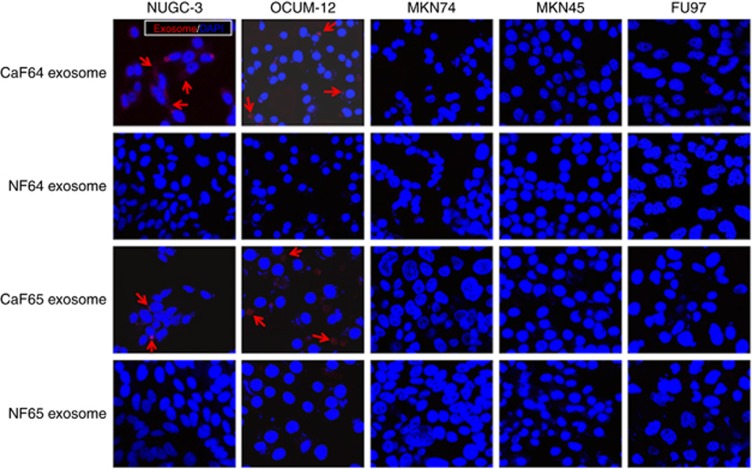
PKH26-labelled exosomes derived from CaF64 and CaF65 were distinctly observed in the cell membrane and cytoplasm of two scirrhous-type gastric cancer cell lines, NUGC-3 cells and OCUM-12 cells, but not in other type of gastric cancer cells. Meanwhile, exosomes derived from NF64 and NF65 were scarcely found in these two scirrhous-type gastric cancer cells. (Figure 1).
Figure 1.
Uptake of exosomes from fibroblasts into gastric cancer cells. Exosomes were dyed with PKH26 (red), and the nuclei of cancer cells were dyed with DAPI. PKH26-labelled exosomes derived from CaF64 were distinctly observed in the membrane and cytoplasm of NUGC-3 cells and OCUM-12 cells, but were scarcely found in that of MKN45, FU97 and MKN74 cells. Exosomes derived from NF64 were not found in any cancer cell lines. PKH26-labelled exosomes from CaF65 were also observed in NUGC-3 cells and OCUM-12 cells, but exosomes from NF65 were scarcely found. A full colour version of this figure is available at the British Journal of Cancer journal online.
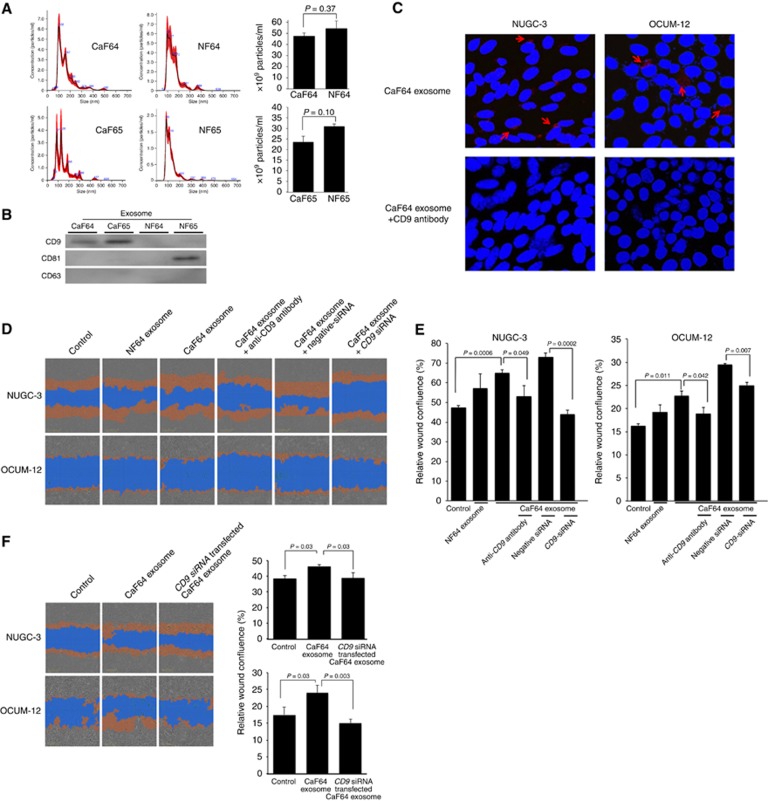
The size distribution and CD9, CD63, and CD81 expressions of exosomes from fibroblasts
The size distribution of exosomes (10 μg) from fibroblasts was shown in Figure 2A. The total number of exosomes from the same patient was not significantly different between CAF and NF. In contrast, exosomes from CaF64 and CaF65 were positive for CD9, while exosomes from NF64 and NF65 were negative for CD9. CD81 was expressed on NF65. CD63 was not found on any fibroblasts (Figure 2B). The anti-CD9 neutralising antibody abrogates the uptake of exosomes from CaF64 into both NUGC-3 cells and OCUM-12 cells (Figure 2C).
Figure 2.
Effect of exosomes from fibroblasts on the migration of gastric cancer cells. (A) The number of 1 ng of exosomes (100 μl of 10 ng ml−1) was counted by Nanosight Nanoparticle Tracking Analysis (Quantum Design, Tokyo, Japan). Black line is the mean value, and the red area is SD (n=5). The particle size of exosomes was mainly distributed at 100–200 nm, and the total number of exosomes was not significantly different between CAF and NAF. The distribution pattern of exosomes size and number was not different between CAF and NAF in a same patient. (B) The western blot analysis showed the expressions of CD9, CD63, and CD81 in exosomes from fibroblasts. CD9 was expressed in exosomes from CaF64 and CaF65. CD63 was not expressed on any exosomes. CD81 was expressed on exosomes from NF65. (C) The effect of anti-CD9 antibody to uptake of exosomes. The uptake of exosomes was abrogated by CD9 blocking antibody both in OCUM-12 cells and NUGC3 cells. (D) Representative images of wound-healing assay. Pictures shows the initial wound mask at 0 h (grey) and the wound mask at 24 h (orange). The number of cancer cells migrating across the wound was increased by the addition of exosomes derived from CaF64 compared with that in the control. CD9 siRNA or CD9 neutralisation antibody decreased the migration-stimulating activity of exosomes from CaF64. (E) Effect of CD9-positive exosomes on the migration of cancer cells. The presence of CD9-positive exosomes in the cell culture significantly increased migration of NUGC-3 cells and OCUM-12 cells. The decrease rate of migration by CD9-siRNA was evident in NUGC-3 cells. In contrast, the decrease rate of migration by the treatment of anti-CD9 antibody was evident in OCUM-12 cells. CD9 siRNA or CD9 neutralisation antibody significantly decreased the migration-stimulating activity of exosomes from CaF64. Relative wound confluence (%) was calculated as 100 × wound closure area at each time (orange)/wound area at time 0 (grey). Data are presented as means±s.d. (F) the effect of exosomes from siCD9 transfected CaF. Exosomes from siCD9 transfected CaF could not increase the migration activity of NUGC-3 cells and OCUM-12 cells. A full colour version of this figure is available at the British Journal of Cancer journal online.
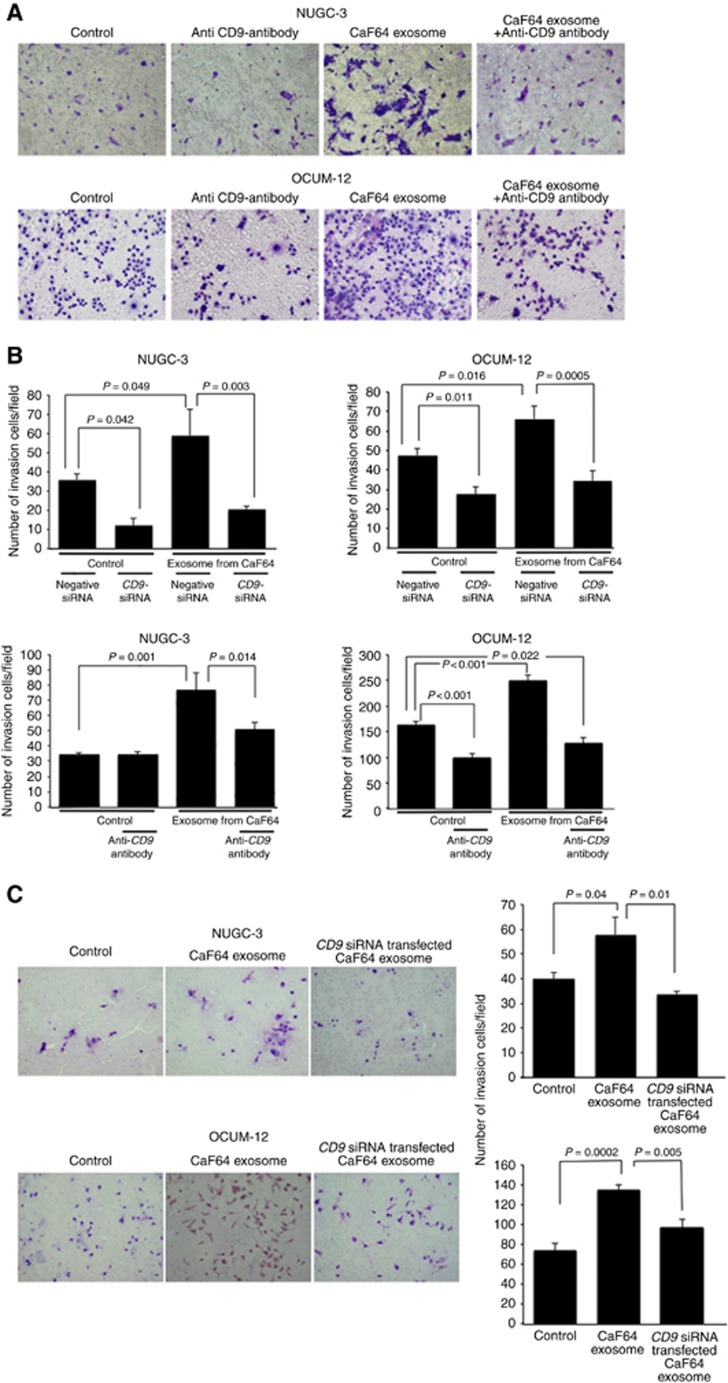
Effect of exosomes from fibroblasts on the migration and invasion abilities of gastric cancer cells
Figure 2D shows a representative phase-contrast image of scirrhous-type gastric cancer cells, NUGC-3 and OCUM-12, by wound-healing assay. The number of migrating NUGC-3 cells and OCUM-12 cells was significantly increased by the addition of exosomes derived from CaF64 (P=0.0006 and P=0.011, respectively). This migration-stimulating activity of exosomes from CaF64 was significantly decreased by treatment with CD9 siRNA (P=0.0002 and P=0.007, respectively; Figure 2E). Exosomes from CD9 siRNA transfected CaF64 did not stimulate the migration ability of NUGC-3 cells and OCUM-12 cells (Figure 2F). Figure 3A is a representative phase-contrast image of NUGC-3 cells and OCUM-12 cells that passed through the membrane filter. The number of invading NUGC-3 cells and OCUM-12 cells was significantly increased by the treatment with CD9-positive exosomes derived from CaF64. However, this migration-stimulating activity of exosomes was significantly decreased after CD9-siRNA treatment and CD9 neutralisation (Figure 3B). Exosomes from CD9 siRNA transfected CaF64 did not stimulate the invasive ability of NUGC-3 cells and OCUM-12 cells (Figure 3C).
Figure 3.
Effect of exosomes from fibroblasts on the invasion of gastric cancer cells. (A) Representative images of invading NUGC-3 cells and OCUM-12 cells by two-chamber Matrigel invasion assay. The number of cells invading the pore membrane filter increased in the presence of exosomes derived from CaF64 cells, compared with the control. CD9 siRNA or CD9 neutralisation antibody decreased the invasion-stimulating activity of exosomes from CaF64. (B) Exosomes from CaF64 cells significantly stimulated the invasive behavior of OCUM-12 and NUGC-3 cells. Anti-CD9 antibody treatment of cancer cells significantly decreased the invasion-stimulating activity of exosomes from CaF64. The decrease rate of invasion by CD9-siRNA was evident in OCUM-12 cells. In contrast, the decrease rate of invasion by the treatment of anti-CD9 antibody was evident in NUGC-3 cells. Data are presented as means and s.d. (error bars) of four experiments. (C) the effect of exosomes from siCD9 transfected CaF. Exosomes from siCD9 transfected CaF could not increase the invasive activity of NUGC-3 cells and OCUM-12 cells. A full colour version of this figure is available at the British Journal of Cancer journal online.
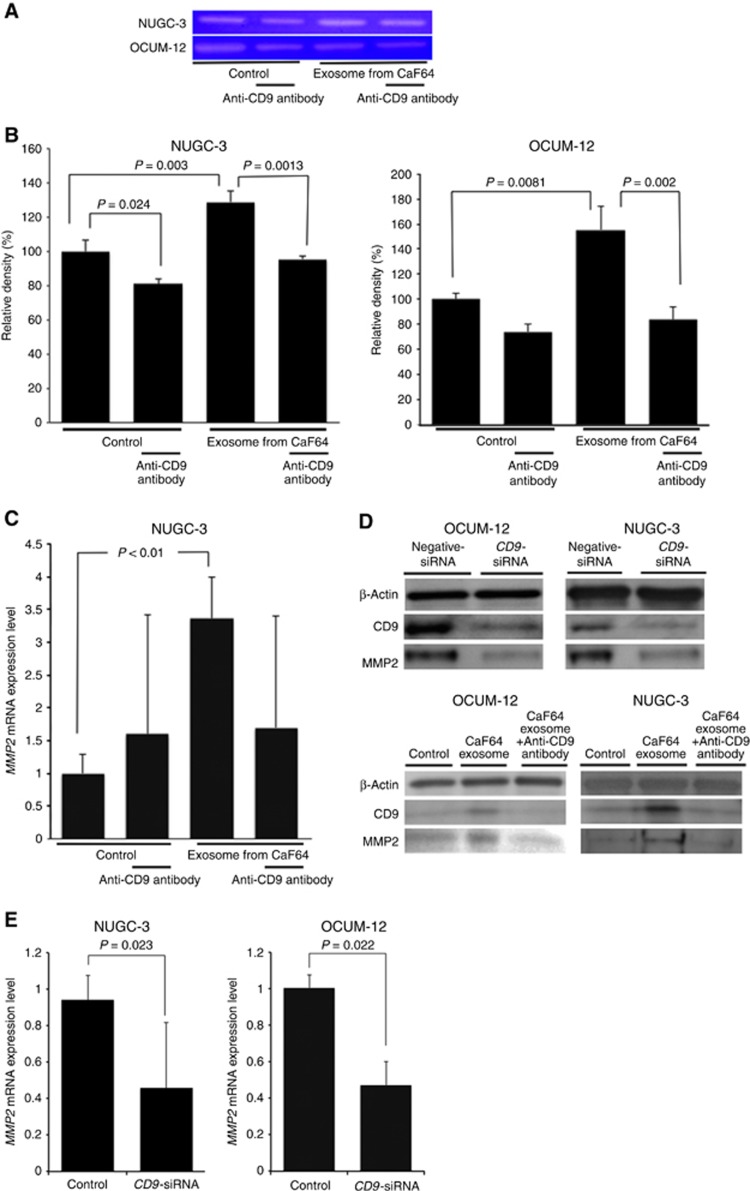
MMP2 mRNA expression and MMP2 activity
MMP2 activity, as determined by gelatin zymography, is shown in Figure 4A. After treatment of exosomes from CaF64, the MMP2 enzyme activity was increased in both NUGC-3 cells and OCUM-12 cells. Neutralisation by CD9 antibody significantly reduced the enzyme activity of MMP2 in NUGC-3 and OCUM-12 cells (Figure 4B). The level of MMP2 mRNA was significantly increased following the addition of exosomes from CaF64 in NUGC-3 cells, and was significantly decreased by anti-CD9 antibody (Figure 4C). CD9 and MMP2 expression were decreased by treatment with CD9-siRNA in both OCUM-12 cells and NUGC-3 cells (Figure 4D). The MMP2 level was significantly decreased by treatment with CD9-siRNA in both OCUM-12 cells and NUGC-3 cells (Figure 4E).
Figure 4.
MMP2 mRNA expression and MMP2 activity. (A) Gelatin zymography. (B) Effect of anti-CD9 antibody on MMP2 activity of gastric cancer cells. MMP2 activity was significantly increased by the addition of exosomes derived from CaF64, while anti-CD9 antibody decreased the MMP2 activity in OCUM-12 and NUGC-3 cells. (C) MMP2 mRNA expression level. Exosomes from CaF64 cells significantly increased the expression of MMP2 mRNA in NUGC-3 cells. Anti-CD9 antibody decreased the MMP2 mRNA level. (D) Effect of CD9 siRNA and anti-CD9 antibody on CD9 and MMP2 expression of gastric cancer cells. CD9-siRNA decreased the endogenous CD9 expression and MMP2 expression of both NUGC-3 cells and OCUM-12 cells. Anti-CD9 antibody decreased the CD9 expression, which was upregulated by CaF64 exosome. (E) Effect of CD9 siRNA on MMP2 mRNA expression of gastric cancer cells. CD9-siRNA decreased the MMP2 mRNA level of both NUGC-3 cells and OCUM-12 cells. A full colour version of this figure is available at the British Journal of Cancer journal online.
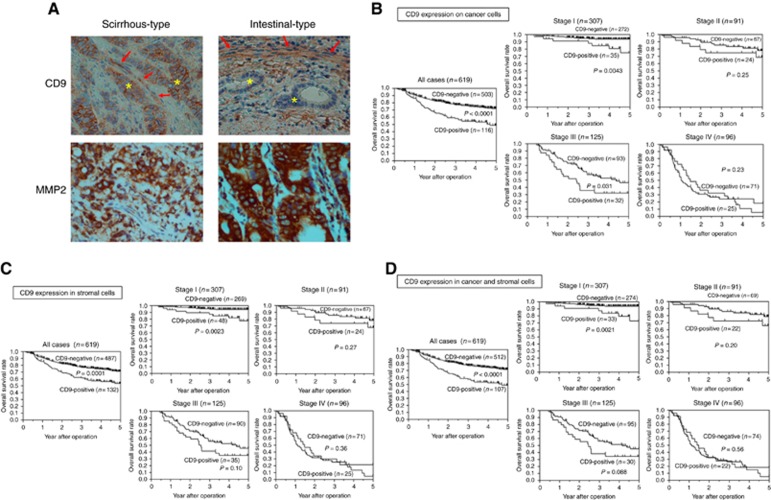
Relationship between CD9 expression and clinicopathological factors in gastric cancer
CD9 expression was mainly observed on the cell membranes of cancer cells and in the cytoplasm of stromal cells. MMP2 expression was mainly observed on the cytoplasm of cancer cells and in stromal cells. (Figure 5A). We evaluated the CD9 expressions of cancer cells and stromal cells. The correlation between CD9 expression and clinicopathological factors is shown in Table 1. The CD9 expression in stromal cells was significantly correlated with CD9 expression on cancer cells (P<0.0001). Cancer cells in 116 of 619 cases were positive for CD9. Cancer-cell CD9 positivity was significantly correlated with sex (female), scirrhous type (Type 4), tumour depth (T3-4), lymph node metastasis, lymphatic invasion and MMP2 expression. Stromal cells in 132 of 619 cases were positive for CD9. Stromal-cell CD9 positivity was significantly correlated with scirrhous type (Type 4), tumour depth (T3-4), lymph node metastasis and MMP2 expression. CD9 expression in cancer cells was significantly correlated with CD9 in stromal cells (P<0.0001). Cases with CD9 positivity in both tumour and stromal cells were significantly correlated with sex (female), scirrhous type (Type 4), tumour depth (T3-4), lymph node metastasis, lymphatic invasion and MMP2 expression. Correlations between MMP2 expression of cancer cells and clinicopathological factors are shown in Table 2. MMP2 expression was significantly associated with tumour depth (T3-4), lymph node metastasis, lymphatic invasion and venous invasion.
Figure 5.
CD9 expression and survival of patients with gastric cancer. (A) Representative pictures of CD9 and MMP2 expression. CD9 was mainly expressed in the cytoplasm of fibroblasts (arrows) and the cell membrane of scirrhous-type gastric cancer cells (asterisks). CD9 was not expressed on the intestinal-type gastric cancer cells (asterisks) even when CD9 was found in the cytoplasm of the same patient’s fibroblasts (arrows). (B) Survival of gastric cancer patients with CD9 expression on cancer cells. The Kaplan–Meier survival curve indicates that the OS of patients with CD9-positive cancer cells was significantly worse than that of patients with CD9-negative expression (P<0.0001). In patients at stage I and stage III, the OS values of patients with CD9-positive cancer cells were significantly worse than those with CD9-negative expression. (C) OS of patients with CD9-positive expression in stromal cells was significantly worse than those with CD9-negative expression, especially at stage I (P=0.0023). (D) Patients with CD9 positivity in both cancer and stromal cells had a significantly worse prognosis than the group with negative expression in one or both cell types (P<0.0001), especially at stage I (P=0.0021). A full colour version of this figure is available at the British Journal of Cancer journal online.
Table 1. Correlation between CD9 expression and clinicopathologic factors in 619 patients with gastric cancer.
|
CD9 expression on tumour cells |
CD9 expression in stromal cells |
CD9 expression in tumour and stromal cells |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinicopathological factors | Negative (n=503) | Positive (n=116) | P value | Negative (n=487) | Positive (n=132) | P value | Either negative (n=512) | Both positive (n=107) | P value |
| Age, years | |||||||||
| <65 | 222 (81.6%) | 50 (18.4%) | 216 (79.4%) | 56 (20.6%) | 224 (82.3%) | 48 (17.7%) | |||
| ⩾65 | 281 (81.0%) | 66 (19.0%) | 0.84 | 271 (55.7%) | 76 (44.3%) | 0.69 | 288 (83.0%) | 59 (17.0%) | 0.83 |
| Sex | |||||||||
| Female | 239 (86.9%) | 36 (13.1%) | 234 (85.9%) | 41 (14.9%) | 243 (88.4%) | 32 (11.6%) | |||
| Male | 264 (76.7%) | 80 (23.3%) | 0.0011 | 253 (73.6%) | 91 (26.4%) | 0.0004 | 269 (78.2%) | 75 (21.8%) | 0.0007 |
| Macroscopic type | |||||||||
| 0–3 | 447 (83.5%) | 88 (16.5%) | 435 (81.3%) | 100 (18.7%) | 456 (85.2%) | 79 (14.8%) | |||
| 4 (scirrhous-type) | 56 (66.7%) | 28 (33.3%) | 0.0005 | 52 (61.9%) | 32 (38.1%) | 0.0001 | 56 (66.7%) | 28 (33.3%) | 0.0001 |
| Histologic type | |||||||||
| Differentiated | 230 (79.3%) | 60 (20.7%) | 220 (75.9%) | 70 (24.1%) | 236 (81.4%) | 54 (18.6%) | |||
| Undifferentiated | 273 (83.0%) | 56 (17.0%) | 0.24 | 267 (81.2%) | 62 (19.8%) | 0.11 | 276 (83.9%) | 53 (16.1%) | 0.41 |
| Tumour depth | |||||||||
| T1–2 | 304 (87.6%) | 43 (12.4%) | 291 (83.9%) | 56 (16.1%) | 306 (88.2%) | 41 (11.8%) | |||
| T3–4 | 199 (73.2%) | 73 (26.8%) | <0.0001 | 196 (72.1%) | 76 (27.9%) | 0.0004 | 206 (75.7%) | 66 (24.3%) | <0.0001 |
| Lymph node metastasis | |||||||||
| Negative | 291 (86.1%) | 47 (13.9%) | 278 (82.0%) | 61 (18.0%) | 295 (87.0%) | 44 (13.0%) | |||
| Positive | 210 (75.5%) | 68 (24.5%) | 0.0008 | 207 (74.5%) | 71 (25.5%) | 0.023 | 215 (77.3%) | 63 (22.7%) | 0.0016 |
| Lymphatic invasion | |||||||||
| Negative | 235 (87.7%) | 33 (12.3%) | 223 (83.2%) | 45 (16.8%) | 237 (88.4%) | 31 (11.6%) | |||
| Positive | 268 (76.6%) | 82 (23.4%) | 0.0003 | 264 (75.4%) | 86 (24.6%) | 0.018 | 275 (78.6%) | 75 (21.4%) | 0.001 |
| Venous invasion | |||||||||
| Negative | 420 (82.2%) | 91 (17.8%) | 403 (78.9%) | 108 (21.4%) | 426 (83.4%) | 85 (16.6%) | |||
| Positive | 83 (76.9%) | 25 (23.1%) | 0.21 | 84 (77.8%) | 24 (22.2%) | 0.8 | 86 (79.6%) | 22 (20.4%) | 0.35 |
| MMP2 expression | |||||||||
| Negative | 39 (95.1%) | 2 (4.9%) | 41 (91.1%) | 4 (8.9%) | 42 (93.3%) | 3 (6.7%) | |||
| Positive | 439 (80.9%) | 104 (19.1%) | 0.012 | 420 (78.2%) | 117 (21.8%) | 0.025 | 443 (82.5%) | 94 (17.5%) | 0.038 |
| CD9 expression in stromal cells | |||||||||
| Negative | 478 (98.2%) | 9 (1.8%) | |||||||
| Positive | 25 (18.9%) | 107 (81.1%) | <0.0001 | ||||||
Values in parentheses are percentages unless indicated otherwise
Table 2. Correlation between MMP2 expression and clinicathological factors in 592 patients with gastric cancer.
|
MMP2 in tumour cells |
MMP2 and CD9 expression on tumour cells |
|||||
|---|---|---|---|---|---|---|
| Clinicopathological factors | Negative (n=45) | Positive (n=535) | P value | Either negative (n=488) | Both positive (n=104) | P value |
|
Age, years | ||||||
| <65 | 23 (8.9%) | 235 (91.1%) | 217 (82.8%) | 45 (17.2%) | ||
| ⩾65 | 22 (6.8%) | 302 (93.2%) | 0.34 | 271 (82.1%) | 59 (17.9%) | 0.82 |
|
Sex | ||||||
| Female | 22 (8.6%) | 235 (91.4%) | 230 (87.5%) | 33 (12.5%) | ||
| Male | 23 (7.1%) | 302 (92.9%) | 0.50 | 258 (78.4%) | 71 (21.6%) | 0.0037 |
|
Macroscopic type | ||||||
| 0–3 | 44 (8.4%) | 482 (91.6%) | 449 (84.2%) | 84 (15.8%) | ||
| 4 (scirrhous-type) | 1 (1.8%) | 55 (98.2%) | 0.039 | 39 (66.1%) | 20 (33.9%) | 0.0013 |
|
Histologic type | ||||||
| Differentiated | 21 (7.4%) | 262 (92.6%) | 231 (80.2%) | 57 (19.8%) | ||
| Undifferentiated | 24 (8.0%) | 275 (92.0%) | 0.78 | 257 (84.5%) | 47 (15.5%) | 0.16 |
|
Tumour depth | ||||||
| T1 and T2 | 38 (11.3%) | 298 (88.7%) | 303 (88.9%) | 38 (11.1%) | ||
| T3 and T4 | 7 (2.9%) | 239 (97.1%) | <0.0001 | 185 (73.7%) | 66 (26.3%) | <0.0001 |
|
Lymph node metastasis | ||||||
| Negative | 33 (10.0%) | 296 (90.0%) | 288 (86.8%) | 44 (13.2%) | ||
| Positive | 12 (4.8%) | 238 (95.2%) | 0.017 | 198 (77.0%) | 59 (23.0%) | 0.0022 |
|
Lymphatic invasion | ||||||
| Negative | 30 (11.5%) | 231 (88.5%) | 233 (88.6%) | 30 (11.4%) | ||
| Positive | 15 (4.7%) | 305 (95.3%) | 0.002 | 255 (77.7%) | 73 (22.3%) | 0.0004 |
|
Venous invasion | ||||||
| Negative | 42 (8.8%) | 438 (91.2%) | 406 (83.4%) | 81 (16.6%) | ||
| Positive | 3 (2.9%) | 99 (97.1%) | 0.026 | 82 (78.1%) | 23 (21.9%) | 0.20 |
Survival
The OS curves of patients by CD9 expression by the Kaplan–Meier method are shown in Figure 5B–D. The 5-year OS rates of patients with and without CD9 expression on cancer cells were 45.4% and 71.8%, respectively. Patients with CD9-positive expression on cancer cells showed significantly worse OS than patients without CD9 expression (log–rank; P<0.0001). According to the analysis for each stage, the OS rates of stage I and stage III patients with CD9 expression on cancer cells were significantly worse than those with CD9-negative expression (P=0.0043 and P=0.031, respectively; Figure 5B). Patients with CD9-positive expression in stromal cells showed significantly worse OS than patients with CD9-negative expression (P=0.0001). According to the analysis for each stage, the OS rate of stage I patients with CD9 expression on stromal cells was significantly worse than that with CD9-negative expression (P=0.0023; Figure 5C). Patients with CD9 positivity in both cancer and stromal cells had significantly worse prognosis than the patients with CD9 negative in either of cancer or stromal cells (P<0.0001). The analysis for each stage revealed that stage I patients with dual expression showed a significantly worse OS than those with negative expression in one or both cell types (P=0.0021; Figure 5D). Univariate and multivariate analyses using the proportional hazard model are shown in Table 3. Univariate analysis revealed that the overall survival of patients significantly correlated with CD9 expression on cancer cells, CD9 expression in stromal cells, both expressions, age, macroscopic type-4 cancer, undifferentiated-type cancer, tumour depth (T3–4), lymph node metastasis, distant metastasis, venous invasion and infiltration. Multivariable logistic regression analysis revealed that CD9 expression on cancer cells, age, macroscopic type-4 cancer, undifferentiated-type cancer, tumour depth (T3–4), lymph node metastasis, distant metastasis, venous invasion and infiltration, were independent predictive parameters for patient OS.
Table 3. Univariate and multivariate analyses with respect to overall survival after surgery in 619 patients with gastric cancer.
|
Univariate |
Multivariate |
|||
|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| CD9 on tumour cells (positive) | 2.09 (1.51–2.85) | <0.0001 | 1.43 (1.02–1.97) | 0.036 |
| CD9 in stromal cells (positive) | 1.99 (1.40–2.77) | 0.0001 | 1.26 (0.91–1.74) | 0.16 |
| CD9 on tumour cells and in stromal cells (Both positive) | 2.08 (1.49–2.87) | <0.0001 | 1.40 (0.99–1.95) | 0.051 |
| Age (⩾65 y.o.) | 1.52 (1.11–2.11) | 0.009 | 1.47 (1.07–2.02) | 0.015 |
| Sex (male) | 1.24 (0.90–1.72) | 0.18 | ||
| Macrosopic type (Type 4) | 7.47 (5.25–10.5) | <0.001 | 2.46 (1.70–3.56) | <0.001 |
| Histologic type (undifferentiated) | 1.94 (1.43–2.68) | <0.001 | 1.17 (0.81–1.68) | 0.38 |
| Tumour depth (T3 and T4) | 6.56 (4.57–9.64) | <0.001 | 1.90 (1.21–3.05) | 0.0044 |
| Lymph node metastasis (positive) | 8.38 (5.66–12.8) | <0.001 | 3.15 (1.98–5.17) | <0.001 |
| Distant metastasis (positive) | 6.39 (3.59–10.5) | <0.001 | 2.54 (1.48–4.11) | 0.0012 |
| Lymphatic invasion (positive) | 5.53 (3.67–8.67) | <0.001 | 1.19 (0.73–2.02) | 0.47 |
| Venous invasion (positive) | 3.29 (2.36–4.53) | <0.001 | 1.63 (1.02–1.97) | 0.0059 |
Abbreviations: CI=confidence interval; y.o.=years old.
Discussion
Scirrhous-type gastric cancer cells are surrounded by abundant CAFs. In this study, scirrhous-type gastric cancer cells took up exosomes from CAFs, but other types of gastric cancer cells did not. Scirrhous gastric carcinoma differs from other types of gastric carcinoma in its pathogenesis and biological behaviour (Japanese Gastric Cancer A, 1998; Otsuji et al, 2004; Tahara, 2004; Ikeguchi et al, 2009; Yashiro and Hirakawa, 2010), and has especially high invasive and metastasising potential compared with intestinal-type cancer (Zheng et al, 2007; Yamashita et al, 2009; Qiu et al, 2013; Chen et al, 2016). In this study, exosomes from CAFs were shown to significantly increase the migration and invasion abilities of scirrhous-type gastric cancer cells. These findings suggested that exosomes from CAFs might promote the malignant behaviours of scirrhous gastric carcinoma. Exosomes from CAFs were positive for CD9, but not for CD63 and CD81. CD9 inhibitor significantly decreased the motility-stimulating activity of CAFs exosomes. These findings suggest that CD9-positive exosomes from surrounding abundant CAFs are associated with the malignant phenotype of scirrhous gastric cancer.
Since both cancer cells and stromal cells expressed CD9 by immunohistochemistry, we analysed the relationship between clinicopathological factors and CD9 expression on cancer cells, in stromal cells and in both. CD9 expression was mainly observed on the cell membrane of cancer cells and in the cytoplasm of stromal cells. The immunohistochemical study indicated that CD9 expression on cancer cells and/or in stromal cells was positively associated with scirrhous gastric carcinoma, tumour depth, lymph node metastasis, lymphatic invasion and venous invasion. CD9 exosomes might be associated with the migration activity of scirrhous gastric cancer cells. The prognosis of patients with CD9 positivity in cancer and/or stromal cells was significantly worse than that of patients with dual CD9-negative expression. This was especially true of patients at stage I, in whom CD9 positivity was associated with significantly worse OS than that of CD9 negativity. CD9 exosomes might play an important role in the early progression of gastric cancer. Lymphatic invasion and venous invasion are important factors for distant metastasis in patients with gastric cancer at stage I (Abe et al, 1995; Lee et al, 2012). Since CD9 expression is closely associated with the invasion activity of gastric cancer in our study, CD9 expression might be associated with poor survival for patients with gastric cancer at stage I. CD9 expression in stromal cells was significantly correlated with CD9 expression on cancer cells, suggesting that most CD9 exosomes on cancer cells might be derived from surrounding stromal cells.
Cancer-cell CD9-positive patients had significantly worse OS than patients without CD9 expression. CD9 inhibition significantly decreased the invasion activity of scirrhous gastric cancer cells. It was previously reported that CD9 and CD147 double-positive exosomes were useful markers for patients with colorectal cancer (Yoshioka et al, 2014). Our study suggested that CD9 might also be useful as a prognostic marker for patients with gastric cancer. In addition, CD9 might be a promising therapeutic target in patients with gastric cancer.
The MMP2 activity of cancer cells was significantly increased by exosomes derived from CAFs, and was decreased by anti-CD9 antibody or si-CD9 RNA in this study. Previously, CD9 expression in melanoma cells has been shown to be positively associated with MMP-2 expression (Hong et al, 2005). Hong et al (2005) have reported that CD9 induces MMP-2 expression by activating c-Jun through p38 MAPK and JNK signalling pathways in human melanoma cells. When the exosomes from CAF were added in OCUM-12 and NUGC-3 cells, the MMP2 expression was increased on both cancer cells, which might indicate that CD9 in exosomes might up-regulate MMP2 in gastric cancer cells. We compared the contribution between endogenous CD9 and exosomal CD9 by the treatment of CD9-siRNA and anti-CD9 antibody. The decrease rate of migration and invasion ability by CD9-siRNA was evident in NUGC-3 cells, while that of anti-CD9 antibody was evident in OCUM-12 cells. These findings suggested that the migration and invasion ability of scirrhous-type of gastric cancer cells might be regulated by both endogenous CD9 and exosomal CD9, while the contribution rate of endogenous CD9 and exosomal CD9 was different between the cell lines.
CAFs exosomes were taken into scirrhous-type gastric cancer cells, while NFs exosomes were not. The difference between CAFs and NFs has been investigated previously. Some papers reported that CAFs stimulated the aggressiveness of cancer cells by upregulating TGFβ and lysyl oxidase-like 2 (LOXL2) but NFs did not (Fuyuhiro et al, 2012; Yeung et al, 2013; Kasashima et al, 2014). Exosomes derived from CAF were positive for CD9 while those from NFs was negative. In addition, the uptake of exosomes was abrogated by anti-CD9 neutralising antibody in both OCUM-12 cells and NUGC3 cells. These findings suggest that scirrhous-type gastric cancer cells have an affinity for CD9-positive exosomes from CAFs, as recipient cells, which may increase the motility of cancer cells. It was reported that CD9 is associated with β1 integrin adhesion receptors (Zöller, 2009; Wang et al, 2011), and We previously reported that β1 integrin was frequently expressed on the scirrhous-type gastric cancer cells and was closely associated with peritoneal metastasis (Nishimura et al, 1996; Matsuoka et al, 2000). These findings might suggest that the high expression of β1-integrin on the scirrhous-type gastric cancer cells might be one of the reasons for the selective uptake of CD-9 expressing exosomes from CAF.
Luga and Wrana (2013), reported that breast fibroblasts secrete exosomes that promote breast cancer cell (BCC) protrusive activity, motility and metastasis by activating autocrine Wnt-PCP signalling in BCCs, and suggested that this phenomenon was related to CD81-positive exosomes from breast stromal cells. On the other hand, our study showed that CD81 was not expressed in exosomes from gastric CAFs. Exosome types might differ among stromal cell types in a tumour microenvironment.
In conclusion, CD9-positive exosomes from CAFs might be associated with the migration and invasive ability of scirrhous-type gastric cancer cells through MMP2 activation.
Acknowledgments
We thank Maiko Nagai (Osaka City University Graduate School of Medicine), for technical assistance. This study is partially founded by KAKENHI Grant-in-Aid for Scientific Research, Nos. 23390329 (MY) and 26293307 (KH, MY).
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Author contribution
Study concept and design—MY; data acquisition—YM, TO, KK, GM; analysis and interpretation of data—YM, and MY; drafting of the manuscript—YM and MY; and study supervision—MY, MO, KH, and MO.
The authors declare no conflict of interest.
References
- Abe S, Yoshimura H, Nagaoka S, Monden N, Kinugasa S, Nagasue N, Nakamura T (1995) Long-term results of operation for carcinoma of the stomach in T1/T2 stages: critical evaluation of the concept of early carcinoma of the stomach. J Am Coll Surg 181(5): 389–396. [PubMed] [Google Scholar]
- Chen YC, Fang WL, Wang RF, Liu CA, Yang MH, Lo SS, Wu CW, Li AF, Shyr YM, Huang KH (2016) Clinicopathological variation of lauren classification in gastric cancer. Pathol Oncol Res 22(1): 197–202. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Yoshioka Y, Ochiya T (2016) Extracellular vesicle transfer of cancer pathogenic components. Cancer Sci 107(4): 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuyuhiro Y, Yashiro M, Noda S, Matsuoka J, Hasegawa T, Kato Y, Sawada T, Hirakawa K (2012) Cancer-associated orthotopic myofibroblasts stimulates the motility of gastric carcinoma cells. Cancer Sci 103(4): 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa T, Yashiro M, Nishii T, Matsuoka J, Fuyuhiro Y, Morisaki T, Fukuoka T, Shimizu K, Shimizu T, Miwa A, Hirakawa K (2014) Cancer-associated fibroblasts might sustain the stemness of scirrhous gastric cancer cells via transforming growth factor-beta signaling. Int J Cancer 134(8): 1785–1795. [DOI] [PubMed] [Google Scholar]
- Hong IK, Kim YM, Jeoung DI, Kim KC, Lee H (2005) Tetraspanin CD9 induces MMP-2 expression by activating p38 MAPK, JNK and c-Jun pathways in human melanoma cells. Exp Mol Med 37(3): 230–239. [DOI] [PubMed] [Google Scholar]
- Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D (2015) Tumour exosome integrins determine organotropic metastasis. Nature 527(7578): 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeguchi M, Miyake T, Matsunaga T, Yamamoto M, Fukumoto Y, Yamada Y, Fukuda K, Saito H, Tatebe S, Tsujitani S (2009) Recent results of therapy for scirrhous gastric cancer. Surg Today 39(4): 290–294. [DOI] [PubMed] [Google Scholar]
- Japanese Gastric Cancer A (1998) Japanese classification of gastric carcinoma—2nd English edition. Gastric Cancer 1(1): 10–24. [DOI] [PubMed] [Google Scholar]
- Ji R, Zhang B, Zhang X, Xue J, Yuan X, Yan Y, Wang M, Zhu W, Qian H, Xu W (2015) Exosomes derived from human mesenchymal stem cells confer drug resistance in gastric cancer. Cell Cycle 14(15): 2473–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasashima H, Yashiro M, Kinoshita H, Fukuoka T, Morisaki T, Masuda G, Sakurai K, Kubo N, Ohira M, Hirakawa K (2014) Lysyl oxidase-like 2 (LOXL2) from stromal fibroblasts stimulates the progression of gastric cancer. Cancer Lett 354(2): 438–446. [DOI] [PubMed] [Google Scholar]
- Kato Y, Yashiro M, Noda S, Tendo M, Kashiwagi S, Doi Y, Nishii T, Matsuoka J, Fuyuhiro Y, Shinto O, Sawada T, Ohira M, Hirakawa K (2010) Establishment and characterization of a new hypoxia-resistant cancer cell line, OCUM-12/Hypo, derived from a scirrhous gastric carcinoma. Br J Cancer 102(5): 898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita H, Yashiro M, Fukuoka T, Hasegawa T, Morisaki T, Kasashima H, Masuda G, Noda S, Hirakawa K (2015) Diffuse-type gastric cancer cells switch their driver pathways from FGFR2 signaling to SDF1/CXCR4 axis in hypoxic tumor microenvironments. Carcinogenesis 36(12): 1511–1520. [DOI] [PubMed] [Google Scholar]
- Lee JH, Choi MG, Min BH, Noh JH, Sohn TS, Bae JM, Kim S (2012) Predictive factors for lymph node metastasis in patients with poorly differentiated early gastric cancer. Br J Surg 99(12): 1688–1692. [DOI] [PubMed] [Google Scholar]
- Luga V, Wrana JL (2013) Tumor-stroma interaction: revealing fibroblast-secreted exosomes as potent regulators of Wnt-planar cell polarity signaling in cancer metastasis. Cancer Res 73(23): 6843–6847. [DOI] [PubMed] [Google Scholar]
- Matsuoka T, Yashiro M, Nishimura S, Inoue T, Fujihara T, Sawada T, Kato Y, Seki S, Hirakawa-Ys Chung K (2000) Increased expression of alpha2beta1-integrin in the peritoneal dissemination of human gastric carcinoma. Int J Mol Med 5(1): 21–25. [DOI] [PubMed] [Google Scholar]
- Nishimura S, Chung YS, Yashiro M, Inoue T, Sowa M (1996) Role of alpha 2 beta 1- and alpha 3 beta 1-integrin in the peritoneal implantation of scirrhous gastric carcinoma. Br J Cancer 74(9): 1406–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuji E, Kuriu Y, Okamoto K, Ochiai T, Ichikawa D, Hagiwara A, Yamagishi H (2004) Outcome of surgical treatment for patients with scirrhous carcinoma of the stomach. Am J Surg 188(3): 327–332. [DOI] [PubMed] [Google Scholar]
- Qiu MZ, Cai MY, Zhang DS, Wang ZQ, Wang DS, Li YH, Xu RH (2013) Clinicopathological characteristics and prognostic analysis of Lauren classification in gastric adenocarcinoma in China. J Transl Med 11: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoyoshi R, Kuriyama S, Aiba N, Yashiro M, Tanaka M (2015) Asporin activates coordinated invasion of scirrhous gastric cancer and cancer-associated fibroblasts. Oncogene 34(5): 650–660. [DOI] [PubMed] [Google Scholar]
- Semba S, Kodama Y, Ohnuma K, Mizuuchi E, Masuda R, Yashiro M, Hirakawa K, Yokozaki H (2009) Direct cancer-stromal interaction increases fibroblast proliferation and enhances invasive properties of scirrhous-type gastric carcinoma cells. Br J Cancer 101(8): 1365–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobin LH, Gospodarowicz M, Wittekind C (2009) International Union against Cancer (UICC). TNM-Classification of Malignant Tumoursh 7th edn. Wiley-Blackwell: Hoboken, NJ, USA. [Google Scholar]
- Tahara E (2004) Genetic pathways of two types of gastric cancer. IARC Sci Publ 157: 327–349. [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9(6): 654–659. [DOI] [PubMed] [Google Scholar]
- Wang HX, Li Q, Sharma C, Knoblich K, Hemler ME (2011) Tetraspanin protein contributions to cancer. Biochem Soc Trans 39(2): 547–552. [DOI] [PubMed] [Google Scholar]
- Yamashita K, Sakuramoto S, Katada N, Futawatari N, Moriya H, Hirai K, Kikuchi S, Watanabe M (2009) Diffuse type advanced gastric cancer showing dismal prognosis is characterized by deeper invasion and emerging peritoneal cancer cell: the latest comparative study to intestinal advanced gastric cancer. Hepatogastroenterology 56(89): 276–281. [PubMed] [Google Scholar]
- Yashiro M, Hirakawa K (2010) Cancer-stromal interactions in scirrhous gastric carcinoma. Cancer Microenviron 3(1): 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung TL, Leung CS, Wong KK, Samimi G, Thompson MS, Liu J, Zaid TM, Ghosh S, Birrer MJ, Mok SC (2013) TGF-beta modulates ovarian cancer invasion by upregulating CAF-derived versican in the tumor microenvironment. Cancer Res 73(16): 5016–5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka Y, Kosaka N, Konishi Y, Ohta H, Okamoto H, Sonoda H, Nonaka R, Yamamoto H, Ishii H, Mori M, Furuta K, Nakajima T, Hayashi H, Sugisaki H, Higashimoto H, Kato T, Takeshita F, Ochiya T (2014) Ultra-sensitive liquid biopsy of circulating extracellular vesicles using ExoScreen. Nat Commun 5: 3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuana Y, Sturk A, Nieuwland R (2013) Extracellular vesicles in physiological and pathological conditions. Blood Rev 27(1): 31–39. [DOI] [PubMed] [Google Scholar]
- Zheng H, Takahashi H, Murai Y, Cui Z, Nomoto K, Miwa S, Tsuneyama K, Takano Y (2007) Pathobiological characteristics of intestinal and diffuse-type gastric carcinoma in Japan: an immunostaining study on the tissue microarray. J Clin Pathol 60(3): 273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zöller M (2009) Tetraspanins: push and pull in suppressing and promoting metastasis. Nat Rev Cancer 9(1): 40–55. [DOI] [PubMed] [Google Scholar]