Abstract
Purpose
Reduction in inner retinal oxygen delivery (DO2) can cause retinal hypoxia and impair inner retinal oxygen metabolism (MO2), leading to vision loss. The purpose of the current study was to establish measurements of DO2 and MO2 in healthy subjects and test the hypothesis that DO2 and MO2 are reduced in sickle cell retinopathy (SCR) subjects.
Methods
Dual wavelength retinal oximetry and Doppler optical coherence tomography were performed in 12 healthy control and 12 SCR subjects. Images were analyzed to measure retinal arterial and venous oxygen content (O2A and O2V), venous diameter (DV), and total retinal blood flow (TRBF). Retinal arteriovenous oxygen content difference (O2AV), DO2, MO2, and oxygen extraction fraction (OEF) were calculated according to the following equations: O2AV = O2A − O2V; DO2 = TRBF * O2A; MO2 = TRBF * O2AV; OEF = MO2/DO2.
Results
Retinal DV and TRBF were higher in the SCR group as compared to the control group, whereas, O2A, O2V, and O2AV were lower in SCR group as compared to the control group. DO2, MO2, and OEF were not significantly different between control and SCR groups. MO2 and DO2 were linearly related, such that higher MO2 was associated with higher DO2. There was an inverse relationship between TRBF and OEF, such that lower TRBF was associated with higher OEF.
Conclusions
Increased blood flow compensated for decreased oxygen content, thereby maintaining DO2, MO2, and OEF at predominately lower stages of SCR. Quantitative assessment of these parameters has the potential to advance knowledge and improve diagnostic evaluation of retinal ischemic conditions.
Keywords: oxygen metabolism, oxygen extraction fraction, blood flow, sickle cell retinopathy
The retinal tissue requires oxygen and nutrients to maintain normal function for visual processing. Reduction in inner retinal oxygen delivery (DO2) can cause retinal hypoxia and impair inner retinal oxygen metabolism (MO2), leading to vision loss. Therefore, it is imperative to develop methods for quantitative assessment of these key parameters under normal and retinal ischemic conditions. To this end, methods have been developed for assessment of MO2 in humans by combining spectrophotometry to measure retinal vascular oxygen saturation (SO2) with Doppler optical coherence tomography (OCT) to measure total retinal blood flow (TRBF).
Various approaches to quantify TRBF using Doppler OCT have been described previously.1 In order to integrate flow in a blood vessel sectioned on an OCT B-scan, the angle between the OCT beam and the direction of blood flow was obtained using double circular scans,2,3 resampled from volume scans,4,5 or created by dual-beam OCT.6 Alternatively, to minimize the error associated with angle determination, a dual-angle Doppler OCT, dual-beam OCT with a known angular offset between beams was used.6–11 Furthermore, to obviate the need for angle calculation, an en face Doppler OCT approach employs high-speed three-dimensional volumetric OCT and integrates flow across an en face section of the blood vessel. However, to measure the high axial blood velocity in the central retinal vein or artery, high-speed (200 kHz or higher) OCT systems were necessary.12–14 In order to apply the en face method on slower Doppler OCT systems (70−100 kHz), measurements were obtained on vessel branches with lower blood velocity using tilted planes15 or multiple en face planes.16
To date, calculated measurements of DO2 have not been reported in humans. Since DO2 is the product of TRBF and arterial oxygen content, TRBF alone provides only partial information about oxygen delivery to the retina. Similarly, MO2 is the product of TRBF and arteriovenous O2 difference,17 thus measuring either SO2 or TRBF individually would likely lead to incorrect conclusions with regard to MO2. Alternatively, MO2 can be expressed as the product of DO2 and the inner retinal oxygen extraction fraction (OEF).18,19 OEF quantifies how much of the oxygen available from the retinal vasculature is extracted by the retinal tissue for metabolism. Under physiologic or pathologic challenges, compensatory changes in DO2 and OEF respond in order to maintain MO2. Conversely, alterations in MO2 can play a role in regulating DO2. In healthy subjects, MO2 was measured under normoxia and hyperoxia.20,21 Furthermore, MO2 was shown to be reduced in subjects with diabetic retinopathy.22,23 However, MO2 has not been evaluated in sickle cell retinopathy (SCR), in which vision-threatening retinal capillary occlusions, nonperfusion, ischemia, and neovascularization are known to occur.24–32 The purpose of the current study was to establish measurements of DO2 and MO2 in healthy subjects and test the hypothesis that DO2 and MO2 are reduced in SCR subjects.
Methods
Subjects
The research study was approved by an institutional review board at the University of Illinois at Chicago. Prior to subject enrollment, the research study was explained to the subjects and informed consents were obtained according to the tenets of the Declaration of Helsinki. Twelve healthy control and 12 African American SCR subjects (eight with hemoglobin C disease, three with hemoglobin SS disease, and one with hemoglobin S–beta thalassemia disease) participated in the study. One, nine, and two subjects had retinopathy stages of 1, 2, and 3, respectively. The stages were defined according to their most severe feature: peripheral arteriolar occlusions (stage 1), peripheral arteriolar-venular anastomoses (stage 2), or neovascular/fibrous proliferation (stage 3).33 Prior to imaging, subjects' pupils were dilated using 1% tropicamide and 2.5% phenylephrine. Mean arterial pressure (MAP) and hematocrit (HCT) were measured.
Image Acquisition and Analysis
Multimodal imaging was performed to derive DO2 and MO2 by measurements of TRBF and retinal vascular SO2. TRBF was measured using a commercially available 70-kHz Doppler OCT system (Avanti; Optovue Inc., Fremont, CA, USA) and a custom scan protocol that measured blood velocity in vein branches separately on multiple optimized en face planes.16 Three images covering a 2 × 2-mm area centered on the central retinal vein were acquired. Each image consisted of five consecutive volume scans. Each volume contained 80 B-scans with 500 A-lines/B-scan, and the three-dimensional volumetric data set contained 195 en face planes. TRBF was measured by our previously published method.16,34 The raw spectral data were exported and a customized software was written in MATLAB (Mathworks, Natick, MA, USA) to analyze the Doppler OCT images, detect vessels, and compute TRBF. An example of an en face OCT image of the optic nerve head in a healthy control subject is shown in Figure 1. A phase-resolved technique was used to calculate Doppler phase shift and flow within vein segments on the en face plane (Fig. 1). Veins were distinguished from arteries by the sign of the Doppler shift and direction of blood flow. TRBF was computed as the sum of flow in all individual detected retinal veins from different en face planes. To reduce measurement variability, the TRBF measurements from all volumes were averaged. A grader excluded volumes in which motion artifacts interfered with vein identification or flow calculation. Data were included from an average of nine volumes, range between 3 and 15.
Figure 1.
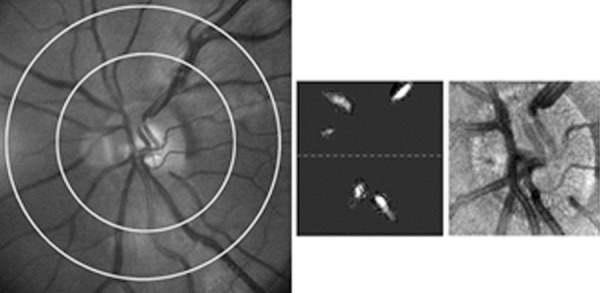
(Left) Example of a retinal image in a healthy control subject. Retinal arterial and venous diameter and oxygen saturation were measured in blood vessel segments within the concentric rings. (Middle) Blood flow was measured in the detected veins from different en face planes, projected here in one en face plane. (Right) En face OCT image of the optic nerve head acquired in the same subject.
For vascular SO2 measurements, dual wavelength (570 and 606 nm) retinal oximetry was performed using our custom-built slit lamp biomicroscope and previously published method.18,35 At each imaging wavelength, nine retinal images were acquired in a 5 × 5-mm area centered on the optic disk. An example of a retinal image acquired at 570 nm in a healthy control subject is shown in Figure 1. Retinal images were analyzed using a custom software written in MATLAB (Mathworks, Natick, MA, USA) to measure vessel diameter and SO2 in all retinal vessels within a circumpapillary region of interest extending between one and two disk radii from the perimeter of the optic disk (Fig. 1). Vessel diameter was determined by calculating the full width at half maximum of intensity profiles perpendicular to the vessel centerlines.18,36 Retinal arterial and venous diameter (DA and DV, respectively) and SO2 (SO2A and SO2V, respectively) were determined for each blood vessel by averaging multiple values along the vessel segment. Blood oxygen content of retinal arteries and veins (O2A and O2V) were determined by the following relation: O2 = O2max * Hgb * SO2, where O2max is the oxygen-binding capacity of hemoglobin37 and Hgb is the hemoglobin concentration calculated from the measured HCT values. Mean values of DV, O2A, and O2V were determined per eye by averaging measurements in all retinal arteries and veins.
Retinal arteriovenous oxygen content difference (O2AV) was calculated as O2A − O2V. DO2, MO2, and OEF were calculated according to the following equations: DO2 = TRBF * O2A; MO2 = TRBF * O2AV; OEF = MO2/DO2.
Statistical Analysis
Compiled retinal oxygen metrics (DV, TRBF, O2A, O2V, O2AV, DO2, MO2, OEF), demographic, and systemic physiologic data were compared between groups using unpaired t-tests. Linear regression analysis was performed to determine the significance of associations between metrics. Statistical analyses were performed using statistical software (SSPS Statistics, version 24; IBM Armonk, New York, USA). Statistical tests were two-sided and significance was accepted at P ≤ 0.05.
Results
Age of control and SCR subjects were 41 ± 15 years and 38 ± 14 years (mean ± standard deviation), respectively (P = 0.62). MAP of control and SCR subjects was 95 ± 10 mm Hg and 86 ± 12 mm Hg, respectively (P = 0.06). HCT of SCR subjects (33% ± 7%) was lower than that of control subjects (45% ± 6%) (P < 0.001).
The Table lists retinal oxygen metrics in control and SCR groups. Retinal DV and TRBF were higher in the SCR group as compared to the control group. O2A, O2V, and O2AV were lower in the SCR group as compared to the control group. DO2, MO2, and OEF were not significantly different between control and SCR groups. As shown in Figure 2, there was a linear relationship between MO2 and DO2, such that higher MO2 was associated with higher DO2 (r = 0.67; P < 0.001; N = 24). As shown in Figure 3, lower TRBF was associated with higher OEF, with a significant linear fit to the data (r = −0.55; P = 0.005; N = 24).
Table.
Comparison of Retinal Oxygen Metrics Between Control (N = 12) and SCR (N = 12) Groups

Figure 2.
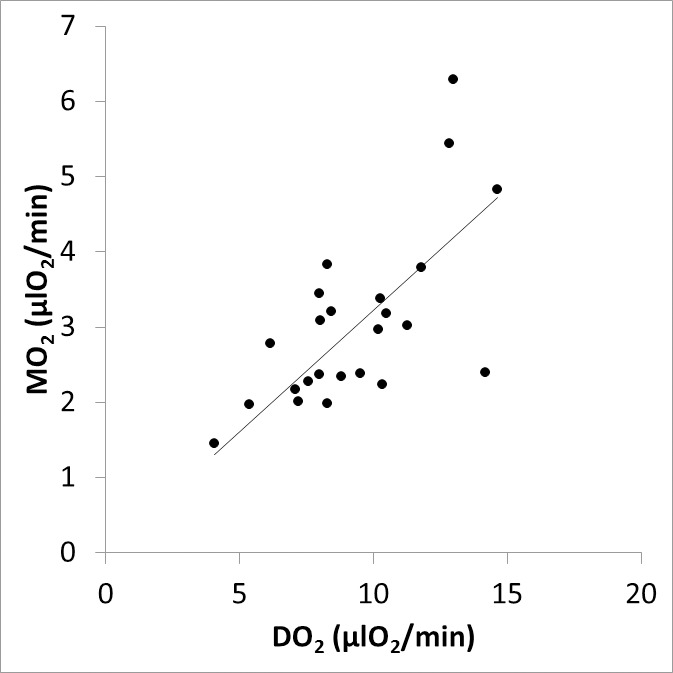
Relationship between inner retinal MO2 and DO2. The best fit line to the data is shown.
Figure 3.
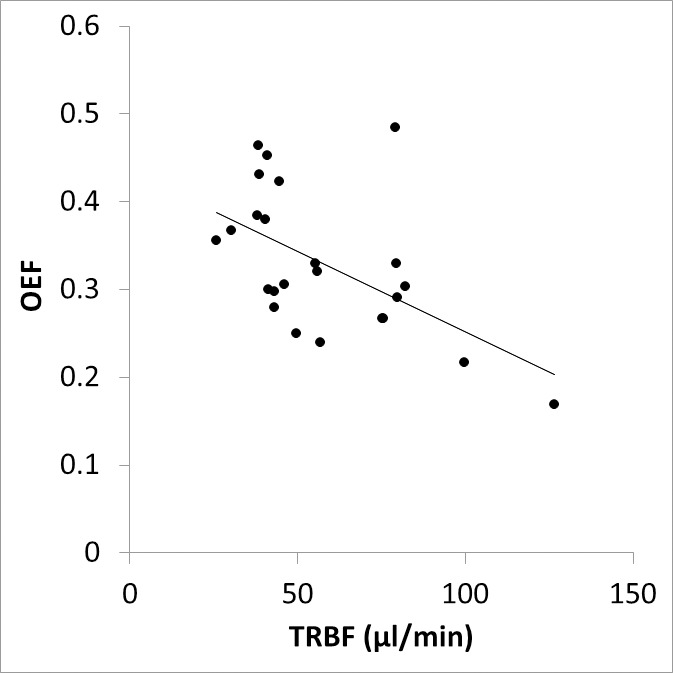
Relationship between inner retinal OEF and TRBF. The best fit line to the data is shown.
Discussion
The feasibility of our methodology to quantitatively measure retinal DO2 and MO2 in humans was demonstrated. In control subjects, diameter measurements in major retinal veins were in agreement with previously reported values.18,38–40 Similarly, TRBF was consistent with previously published values.16,20,22,23 SO2 measurements in retinal arteries and veins were in agreement with values obtained from previously published studies.18,39,41 MO2 measurements obtained in the present study were similar to those reported previously in control subjects.20,22 Of note, MO2 depends on both the tissue consumption rate and the amount of tissue supplied by the retinal circulation. In the current study, the amount of tissue supplied in healthy and SCR subjects was likely similar, thus MO2 is indicative of oxygen consumption rate. However, under certain conditions, such as hyperoxia,21,42,43 a portion of the inner retina may be supplied by the choroidal circulation, thus MO2 can decrease even if the consumption per amount of tissue is unchanged. Although measurements of TRBF and SO2 have been previously published in human subjects,20 we report here, for the first time to our knowledge, calculated values of DO2. Moreover, OEF values derived from direct measurements of MO2 and DO2 indicate approximately 35% of the available oxygen is metabolized by the retinal tissue under normal physiological condition, which is consistent with previous OEF values obtained by oximetry alone.18
As expected, blood O2 contents of retinal arteries and veins were reduced in SCR subjects due to the lower systemic HCT. The observed vasodilation and increased TRBF in SCR suggests a regulatory mechanism to compensate for the reduced O2 contents. The finding of increased TRBF is consistent with a recent study that reported increased retinal arterial and venous flow velocities measured by retinal function imaging.44 However, a previous study showed blood velocity is decreased in central retinal artery, but increased in the ophthalmic artery of sickle cell patients with no retinopathy.45 A previous study in subjects with Eisenmenger syndrome, a cardiac condition resulting in systemic hypoxia, showed arterial and venous SO2 difference was not altered46; however, no information about changes in MO2 was reported since TRBF was not measured.
In the current study, SO2 was not corrected for oxygen loss across the vessel walls and not weighted by blood flow in each vessel. Furthermore, for calculation of the blood O2 content, the low level of fraction of dissolved oxygen was not included. Nevertheless, the values of arterial and venous O2 contents obtained in control subjects (0.19 and 0.13 mL O2/mL) are similar to previously reported corrected values (0.181 and 0.128 mL O2/mL).20 The oxygen-hemoglobin dissociation curves may differ between healthy and SCR subjects, although a previous study showed the difference was small at high SO2 (>92%) but shifted to the right at lower levels.47 Finally, hemoglobin absorption spectra may not be the same in healthy and SCR subjects. Differences in healthy and sickle cell hemoglobin absorption spectra have been reported based on blood samples,48,49 although measurements under physiological conditions are not readily available. In the current study, adjustments were not made for any potential differences in hemoglobin absorption spectra.
DO2 was not found to be impaired in predominately lower stages of SCR. This finding is attributed to the increased TRBF, which adequately compensated for the decreased O2A. Similarly, MO2 was maintained due to the countereffects of increased TRBF and reduced O2AV. A reduction in MO2 was expected in SCR due to peripheral arteriolar occlusions and arteriolar-venular anastomoses (shunting). The finding of unchanged MO2 suggests a higher metabolic rate per unit volume in the central retina than in the thinner peripheral retina and potentially a limited contribution of shunting in peripheral retina to altering MO2.
A linear relationship between MO2 and DO2 was established based on compiled data from all subjects. This finding indicates the rate at which oxygen is delivered by the inner retinal circulation is highly correlated with the oxygen metabolic rate. Furthermore, TRBF and OEF were shown to be inversely related, similar to findings in the brain.50 This result demonstrates the presence of a compensatory increase in OEF under conditions of decreased TRBF in order to maintain MO2. Nevertheless, the maximum value for OEF was 0.48, which indicates OEF augmentation to supply oxygen in these SCR subjects.
Overall, our method will allow investigation of the relationships among retinal blood flow, vascular oxygen levels, and oxygen consumption under physiologically challenged and pathologic conditions. Quantitative assessment of retinal MO2, DO2, and OEF has the potential to advance knowledge of oxygen dynamics and serve as new biomarkers for disease diagnosis, progression, and treatment monitoring.
Acknowledgments
Supported by National Institutes of Health grants: DK104393 (MS), EY023285 (DH), EY001792, EY010572, Antonio Champalimaud Vision Award (DH), Senior Scientific Investigator Award (MS) and a departmental award from Research to Prevent Blindness.
Disclosure: M. Shahidi, None; A.E. Felder, None; O. Tan, Optovue, Inc. (F); N.P. Blair, None; D. Huang, Optovue, Inc. (F, I, R)
References
- 1. Leitgeb RA, Werkmeister RM, Blatter C, Schmetterer L. . Doppler optical coherence tomography. Prog Retin Eye Res. 2014; 41: 26– 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang Y, Bower BA, Izatt JA, Tan O, Huang D. . In vivo total retinal blood flow measurement by Fourier domain Doppler optical coherence tomography. J Biomed Opt. 2007; 12: 041215. [DOI] [PubMed] [Google Scholar]
- 3. Wang Y, Bower BA, Izatt JA, Tan O, Huang D. . Retinal blood flow measurement by circumpapillary Fourier domain Doppler optical coherence tomography. J Biomed Opt. 2008; 13: 064003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singh AS, Kolbitsch C, Schmoll T, Leitgeb RA. . Stable absolute flow estimation with Doppler OCT based on virtual circumpapillary scans. Biomed Opt Express. 2010; 1: 1047– 1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wehbe H, Ruggeri M, Jiao S, Gregori G, Puliafito CA, Zhao W. . Automatic retinal blood flow calculation using spectral domain optical coherence tomography. Opt Express. 2007; 15: 15193– 15206. [DOI] [PubMed] [Google Scholar]
- 6. Dai C, Liu X, Zhang HF, Puliafito CA, Jiao S. . Absolute retinal blood flow measurement with a dual-beam Doppler optical coherence tomography. Invest Ophthalmol Vis Sci. 2013; 54: 7998– 8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blatter C, Coquoz S, Grajciar B,et al. . Dove prism based rotating dual beam bidirectional Doppler OCT. Biomed Opt Express. 2013; 4: 1188– 1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doblhoff-Dier V, Schmetterer L, Vilser W,et al. . Measurement of the total retinal blood flow using dual beam Fourier-domain Doppler optical coherence tomography with orthogonal detection planes. Biomed Opt Express. 2014; 5: 630– 642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Trasischker W, Werkmeister RM, Zotter S,et al. . In vitro and in vivo three-dimensional velocity vector measurement by three-beam spectral-domain Doppler optical coherence tomography. J Biomed Opt. 2013; 18: 116010. [DOI] [PubMed] [Google Scholar]
- 10. Werkmeister RM, Dragostinoff N, Palkovits S,et al. . Measurement of absolute blood flow velocity and blood flow in the human retina by dual-beam bidirectional Doppler Fourier-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012; 53: 6062– 6071. [DOI] [PubMed] [Google Scholar]
- 11. Werkmeister RM, Dragostinoff N, Pircher M,et al. . Bidirectional Doppler Fourier-domain optical coherence tomography for measurement of absolute flow velocities in human retinal vessels. Opt Lett. 2008; 33: 2967– 2969. [DOI] [PubMed] [Google Scholar]
- 12. Baumann B, Potsaid B, Kraus MF,et al. . Total retinal blood flow measurement with ultrahigh speed swept source/Fourier domain OCT. Biomed Opt Express. 2011; 2: 1539– 1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Choi W, Baumann B, Liu JJ,et al. . Measurement of pulsatile total blood flow in the human and rat retina with ultrahigh speed spectral/Fourier domain OCT. Biomed Opt Express. 2012; 3: 1047– 1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Srinivasan VJ, Adler DC, Chen Y,et al. . Ultrahigh-speed optical coherence tomography for three-dimensional and en face imaging of the retina and optic nerve head. Invest Ophthalmol Vis Sci. 2008; 49: 5103– 5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee B, Choi W, Liu JJ,et al. . Cardiac-gated en face Doppler measurement of retinal blood flow using swept-source optical coherence tomography at 100, 000 axial scans per second. Invest Ophthalmol Vis Sci. 2015; 56: 2522– 2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tan O, Liu G, Liang L,et al. . En face Doppler total retinal blood flow measurement with 70 kHz spectral optical coherence tomography. J Biomed Opt. 2015; 20: 066004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pittman R. . Oxygen transport in normal and pathological situations: defects and compensations. : Pittman R, . Regulation of Tissue Oxygenation. San Rafael, CA: Morgan & Claypool Life Sciences; 2011: 47– 50. [PubMed] [Google Scholar]
- 18. Felder AE, Wanek J, Blair NP, Shahidi M. . Inner retinal oxygen extraction fraction in response to light flicker stimulation in humans. Invest Ophthalmol Vis Sci. 2015; 56: 6633– 6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Teng PY, Wanek J, Blair NP, Shahidi M. . Inner retinal oxygen extraction fraction in rat. Invest Ophthalmol Vis Sci. 2013; 54: 647– 651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Werkmeister RM, Schmidl D, Aschinger G,et al. . Retinal oxygen extraction in humans. Sci Rep. 2015; 5: 15763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Palkovits S, Lasta M, Told R,et al. . Retinal oxygen metabolism during normoxia and hyperoxia in healthy subjects. Invest Ophthalmol Vis Sci. 2014; 55: 4707– 4713. [DOI] [PubMed] [Google Scholar]
- 22. Fondi K, Wozniak PA, Howorka K,et al. . Retinal oxygen extraction in individuals with type 1 diabetes with no or mild diabetic retinopathy. Diabetologia. 2017; 60: 1534– 1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tayyari F, Khuu LA, Flanagan JG, Singer S, Brent MH, Hudson C. . Retinal blood flow and retinal blood oxygen saturation in mild to moderate diabetic retinopathy. Invest Ophthalmol Vis Sci. 2015; 56: 6796– 6800. [DOI] [PubMed] [Google Scholar]
- 24. Asdourian GK, Goldberg MF, Rabb MF. . Macular infarction in sickle cell B+ thalassemia. Retina. 1982; 2: 155– 158. [DOI] [PubMed] [Google Scholar]
- 25. Goldberg MF. . Retinal vaso-occlusion in sickling hemoglobinopathies. Birth Defects Orig Artic Ser. 1976; 12: 475– 515. [PubMed] [Google Scholar]
- 26. Merritt JC, Risco JM, Pantell JP. . Bilateral macular infaction in SS disease. J Pediatr Ophthalmol Strabismus. 1982; 19: 275– 278. [DOI] [PubMed] [Google Scholar]
- 27. Knapp JW. . Isolated macular infarction in sickle cell (SS) disease. Am J Ophthalmol. 1972; 73: 857– 859. [DOI] [PubMed] [Google Scholar]
- 28. Lim JI. . Ophthalmic manifestations of sickle cell disease: update of the latest findings. Current Opin Ophthalmol. 2012; 23: 533– 536. [DOI] [PubMed] [Google Scholar]
- 29. Ryan SJ., Jr. Occlusion of the macular capillaries in sickle cell hemoglobin C disease. Am J Ophthalmol. 1974; 77: 459– 461. [DOI] [PubMed] [Google Scholar]
- 30. Westrich DJ, Feman SS. . Macular arteriolar occlusions in sickle cell beta-thalassemia. Am J Ophthalmol. 1986; 101: 739– 740. [DOI] [PubMed] [Google Scholar]
- 31. Grover S, Sambhav K, Chalam KV. . Capillary nonperfusion by novel technology of OCT angiography in a patient with sickle cell disease with normal fluorescein angiogram. Eur J Ophthalmol. 2016; 26: e121– e123. [DOI] [PubMed] [Google Scholar]
- 32. Minvielle W, Caillaux V, Cohen SY,et al. . Macular microangiopathy in sickle cell disease using optical coherence tomography angiography. Am J Ophthalmol. 2016; 164: 137– 144.e1. [DOI] [PubMed] [Google Scholar]
- 33. Nagpal KC, Goldberg MF, Rabb MF. . Ocular manifestations of sickle hemoglobinopathies. Surv Ophthalmol. 1977; 21: 391– 411. [DOI] [PubMed] [Google Scholar]
- 34. Pechauer AD, Tan O, Liu L,et al. . Retinal blood flow response to hyperoxia measured with en face Doppler optical coherence tomography. Invest Ophthalmol Vis Sci. 2016; 57: OCT141– OCT145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Felder AE, Wanek J, Blair NP,et al. . The effects of diabetic retinopathy stage and light flicker on inner retinal oxygen extraction fraction. Invest Ophthalmol Vis Sci. 2016; 57: 5586– 5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moss HE, Treadwell G, Wanek J, DeLeon S, Shahidi M. . Retinal vessel diameter assessment in papilledema by semi-automated analysis of SLO images: feasibility and reliability. Invest Ophthalmol Vis Sci. 2014; 55: 2049– 2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. West J. . Pulmonary Physiology and Pathophysiology: An Integrated, Case-Based Approach. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2007: 150. [Google Scholar]
- 38. Bek T. . Diameter changes of retinal vessels in diabetic retinopathy. Curr Diab Rep. 2017; 17: 82. [DOI] [PubMed] [Google Scholar]
- 39. Blair NP, Wanek J, Felder AE,et al. . Retinal oximetry and vessel diameter measurements with a commercially available scanning laser ophthalmoscope in diabetic retinopathy. Invest. Ophthalmol Vis Sci. 2017; 58: 5556– 5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kifley A, Wang JJ, Cugati S, Wong TY, Mitchell P. . Retinal vascular caliber, diabetes, and retinopathy. Am J Ophthalmol. 2007; 143: 1024– 1026. [DOI] [PubMed] [Google Scholar]
- 41. Rilven S, Torp TL, Grauslund J. . Retinal oximetry in patients with ischaemic retinal diseases. Acta Ophthalmol. 2017; 95: 119– 127. [DOI] [PubMed] [Google Scholar]
- 42. Linsenmeier RA, Yancey CM. . Effects of hyperoxia on the oxygen distribution in the intact cat retina. Invest Ophthalmol Vis Sci. 1989; 30: 612– 618. [PubMed] [Google Scholar]
- 43. Pournaras CJ, Riva CE, Tsacopoulos M, Strommer K. . Diffusion of O2 in the retina of anesthetized miniature pigs in normoxia and hyperoxia. Exp Eye Res. 1989; 49: 347– 360. [DOI] [PubMed] [Google Scholar]
- 44. Birkhoff W, de Vries J, Dent G,et al. . Retinal microcirculation imaging in sickle cell disease patients. Microvasc Res. 2017; 116: 1– 5. [DOI] [PubMed] [Google Scholar]
- 45. Aikimbaev K, Guvenc B, Canataroglu A, Canataroglu H, Baslamisli F, Oguz M. . Value of duplex and color Doppler ultrasonography in the evaluation of orbital vascular flow and resistance in sickle cell disease. Am J Hematol. 2001; 67: 163– 167. [DOI] [PubMed] [Google Scholar]
- 46. Traustason S, Jensen AS, Arvidsson HS, Munch IC, Sondergaard L, Larsen M. . Retinal oxygen saturation in patients with systemic hypoxemia. Invest Ophthalmol Vis Sci. 2011; 52: 5064– 5067. [DOI] [PubMed] [Google Scholar]
- 47. Abdu A, Gomez-Marquez J, Aldrich TK. . The oxygen affinity of sickle hemoglobin. Respir Physiol Neurobiol. 2008; 161: 92– 94. [DOI] [PubMed] [Google Scholar]
- 48. Akuwudike A, Chikezie P, Chilaka F. . Absorption spectra of normal adult and sickle cell haemoglobins treated with hydrogen peroxide at two pH values. Adv Biores. 2010; 1: 55– 60. [Google Scholar]
- 49. Nahavandi M, Nichols JP, Hassan M, Gandjbakhche A, Kato GJ. . Near-infrared spectra absorbance of blood from sickle cell patients and normal individuals. Hematology. 2009; 14: 46– 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Watchmaker JM, Juttukonda MR, Davis LT,et al. . Hemodynamic mechanisms underlying elevated oxygen extraction fraction (OEF) in moyamoya and sickle cell anemia patients [published online ahead of print December 28, 2016]. J Cereb Blood Flow Metab. doi:10.1177/0271678X16682509. [DOI] [PMC free article] [PubMed]


