Abstract
We estimate costs and their predictors for three HIV prevention interventions in Kenya: HIV testing and counselling (HTC), prevention of mother-to-child transmission (PMTCT) and voluntary medical male circumcision (VMMC). As part of the ‘Optimizing the Response of Prevention: HIV Efficiency in Africa’ (ORPHEA) project, we collected retrospective data from government and non-governmental health facilities for 2011–12. We used multi-stage sampling to determine a sample of health facilities by type, ownership, size and interventions offered totalling 144 sites in 78 health facilities in 33 districts across Kenya. Data sources included key informants, registers and time-motion observation methods. Total costs of production were computed using both quantity and unit price of each input. Average cost was estimated by dividing total cost per intervention by number of clients accessing the intervention. Multivariate regression methods were used to analyse predictors of log-transformed average costs. Average costs were $7 and $79 per HTC and PMTCT client tested, respectively; and $66 per VMMC procedure. Results show evidence of economies of scale for PMTCT and VMMC: increasing the number of clients per year by 100% was associated with cost reductions of 50% for PMTCT, and 45% for VMMC. Task shifting was associated with reduced costs for both PMTCT (59%) and VMMC (54%). Costs in hospitals were higher for PMTCT (56%) in comparison to non-hospitals. Facilities that performed testing based on risk factors as opposed to universal screening had higher HTC average costs (79%). Lower VMMC costs were associated with availability of male reproductive health services (59%) and presence of community advisory board (52%). Aside from increasing production scale, HIV prevention costs may be contained by using task shifting, non-hospital sites, service integration and community supervision.
Keywords: HIV/AIDS prevention, costs, HIV testing and counselling, prevention of mother-to-child transmission, voluntary medical male circumcision, economics
Key Messages
In Kenya, during 2011–12, the costs per client in HIV testing and counselling (HTC) was $7, for prevention of mother-to-child transmission (PMTCT) client tested was $59 and per voluntary medical male circumcision (VMMC) procedure was $66.
Economies of scale were observed for the production of HIV prevention services particularly for PMTCT and for VMMC.
Task shifting was associated with lower costs for PMTCT and for VMMC, but not for HTC.
Incentives for good performance for staff members were not associated with higher costs.
The availability of male reproductive health services and the presence of a community advisory board were associated with lower VMMC costs.
Introduction
HIV is a serious public health problem in Kenya with 1.6 million people including 191 000 children infected (5.6% prevalence) (NACC and NASCOP 2014). In 2013 over 50 000 new HIV infections were documented among women, over 38 000 among men, and ∼13 000 among children (NACC and NASCOP 2014). The distribution shows remarkable geographical diversity with Homa Bay county in Lake Victoria region having the highest prevalence of 25.7% whereas Wajir County in north-eastern region had the lowest at 0.2% (NACC and NASCOP 2014), raising the importance of local population targeting with the most cost-effective interventions to maximize the effects of HIV prevention and treatment (Anderson et al. 2014; UNAIDS 2015b; Cassels and Camlin 2016; Chang et al. 2016; Coburn et al. 2017).
Kenya was a signatory country of the 2011 United Nations (UN) Political Declaration on HIV and AIDS (General Assembly Resolution No. 65/277) (universal access targets), adopted in June 2011 at the UN General Assembly High‐Level Meeting on AIDS, and the country has now moved towards implementing the Sustainable Development Goals (SDGs) (UN 2017). The new targets include reduced annual new HIV infections among adults by 75%, and reduced HIV transmission rates from mother to child from 14 to < 5% (NACC 2016a). Significant progress has been made in stemming the tide in Kenya (Kimanga et al. 2014), such as the reduction of national prevalence from a peak of ∼10% in the mid-1990s to 5.6% in 2014, and annual incidence from nearly 300 000 to 100 000 during the same period (NACC and NASCOP 2014; NASCOP 2014); however, a number of key national targets have not been met in part due to funding gaps (NACC 2014a). To reach the universal access targets for prevention and treatment set out in the UNAIDS Getting to Zero 2011–2015 Strategy (UNAIDS 2010), the country’s third National AIDS Strategic Plan (KNASP III, 2009/10–2012/13) estimated total resource requirement at $3.5 billion (58% treatment and care and 20% prevention) over the period, with a gap of nearly half of that ($1.7 billion) (NACC 2009). Similarly, estimates for treatment and prevention of mother-to-child transmission (PMTCT) alone for 2010–14 showed a funding gap of $1.8 billion (Government of the Republic of Kenya 2011). Furthermore, during 2010–20 the cost of the HIV response is estimated to increase by 114% with an overall funding gap of $1.75 billion or 0.3% of gross national product (GDP) by 2020 (UNAIDS 2013b). The latest published (2009/10–2011/12) National AIDS Spending Assessment (NASA) indicates that 62% of HIV expenditure was financed by donors (Republic of Kenya 2014). Overall expenditure decreased from $826 in 2009–10 to $786 million in 2011–12 (Republic of Kenya 2014) due in part to reduced PEPFAR bilateral dollars (PEPFAR 2011). A review of the KNASP III highlights the outstanding and future critical gaps in financing due to a real concern of withdrawal or termination of various donor funding agreements (NACC 2014b; NACC 2016c). Hence, in order to scale-up HIV services to achieve nationally set objectives and targets within a sustainability financing mechanism, it is paramount to optimize efficiency in resources use by HIV programs.
Like other high prevalence countries in sub-Saharan Africa, achieving an AIDS-free generation in Kenya requires aggressive programming in stopping transmission by preventing new infections (UNAIDS 2010, 2014; Goosby et al. 2012; Wamai 2014). In Kenya, however, prevention programs receive <20% of the HIV budget (Republic of Kenya 2014; NACC 2016c); thus the need to focus on a few key priority pillars with proven effectiveness. One of these is expanding HIV testing and counselling (HTC) to increase the number of people aware of their status, given that still less than half (47%) of women and about a third of men (35.8%) have received a test in the past year and know their results (NACC 2014a). In addition, to achieve virtual elimination of mother-to-child transmission (Mahy et al. 2010), the country needs to urgently improve its program for PMTCT: its coverage slipped from 86% in 2010 to about 70% in 2013 due to increased demand (NACC 2014a), and it varies widely across the counties (NACC 2016b). A third pillar is the full implementation of the on-going policy program for voluntary medical male circumcision (VMMC), which although has reached initial targets of adult men, needs to reach younger men and infant boys (Bailey et al. 2007; NASCOP 2008; Mwandi et al. 2011; Galbraith et al. 2014; WHO 2016).
Given the continued need for expanded services, while resources are diminishing, the main objectives of this paper are to document the costs of HIV prevention interventions, explore the predictors of economic efficiency, and quantify the potential economies of scale in the production of HIV prevention services. We estimate average costs at each step of the service cascade for each intervention, and then quantify the relationship between average unit cost and scale of production (i.e. the number of clients) as well as quality indicators for each type of facility. We define economic (technical) efficiency as delivering a given level of HIV services output at the lowest feasible cost (Bautista-Arredondo et al. 2008; Bertozzi et al. 2008) while holding other characteristics constant, including quality. Under this framework, economies of scale imply a reduction in the average cost of services as the number of clients scales-up (see Supplementary Technical Appendix, Section 1, for additional details on defining and measuring efficiency). The existence of economies of scale in production has been theoretically and empirically associated with decreased costs (Over 1986; Dandona et al. 2005; Guinness et al. 2005; Boily et al. 2007; Marseille et al. 2007; Chandrashekar et al. 2010; Marseille et al. 2012). Our study also includes questions relevant to evaluate costs and efficiency determinants as identified in the literature (Preyra and Pink 2001; Kasymova et al. 2009; Basinga et al. 2011; Chandrashekar et al. 2014; Siapka et al. 2014).
Documenting potential economies of scale as well as other determinants of economic efficiency is important for several reasons. First, Kenya is the country with the largest expenditure in terms of HIV prevention activities among low- and middle-income countries (LMICs) in the world (Amico et al. 2012). Second, the Getting to Zero and Fast Track: Ending the AIDS Epidemic campaigns have been formulated in a time when more people will be living with HIV/AIDS, demanding dramatic increases in funding (Hecht et al. 2010; Institute of Medicine 2011; UNAIDS 2015b). At the same time, the new WHO guidelines for universal treatment of all persons testing positive regardless of CD4 count (UNAIDS 2015a), which have been adopted by Kenya, imply an expanded demand for resources (NASCOP 2016). In this context, major shortfalls and concerns about sustainability of international financing for health exist (Medecins Sans Frontieres 2010; Quinn and Serwadda 2011; African Union 2012; UNAIDS 2012; Kates et al. 2015), stressing the need for LMICs to make the best use of resources combining best practices of targeted public and private interventions (Sinanovic and Kumaranayake 2006; Hecht et al. 2009; Anderson et al. 2014). It is within this transitionary context that the prevention program in Kenya has recognized the importance of investing in an efficiency and effectiveness framework in the current KNASP (NACC 2014b) while increasing domestic financing for HIV programming (UNAIDS 2013a; AU and UNAIDS 2014). Third, there is a dearth of empirical evaluations of costs for HIV prevention with important evaluations relying on mathematical modelling (Boily et al. 2007; Galarraga et al. 2009). Lastly, the methods to measure cost and scale have developed slowly in the HIV field over the past decade with innovations still necessary to optimize program scale and economic efficiency (Kumaranayake 2008). Mathematical modelling in costing has played an important role, but the mathematical models can only predict accurately if there is empirical measurement of costs at various scales. Most of the literature has explored costs and scale in HIV prevention relying on modelling, with only few recent exceptions (Lepine et al. 2015); thus, the technical issues of documenting costs and their relationship with scale of HIV prevention services production remain as fertile areas of research with important policy implications.
Methods
This study was part of the large multi-country ‘Optimizing the Response of Prevention: HIV Efficiency in Africa’ (ORPHEA) research project (2011–14) that was carried out in Kenya, Zambia, South Africa, Rwanda and Nigeria. The general multi-country methods are presented elsewhere (Bautista-Arredondo et al. 2014). The ORPHEA Kenya study ran from March 2012 to December 2013 and was developed following consultations with representatives of the National AIDS and STI Control Programme (NASCOP), the National AIDS Control Council (NACC), as well as other main HIV/AIDS stakeholders in the country. All research procedures were approved by the Kenyatta National Hospital/University of Nairobi Institutional Review Board and Northeastern University, Boston, USA.
The study sampled Government of Kenya (GOK) health service delivery points at all levels and private providers (i.e. for-profit and not-for-profit service providers, and faith-based facilities) at each level within the health system (hospitals, nursing and maternity homes, medical clinics, and dispensaries). We used multistage sampling techniques to select 56 sites for HTC, 57 sites for PMTCT and 31 sites for VMMC, for a total of 144 sites in 78 health facilities, with most facilities offering more than one intervention. Ten out of 47 counties in Kenya were purposively selected for inclusion in the study to ensure national representation. Data were collected at the district level and at each of the 144 sites. The study collected information through several avenues: interviews with facility in-charges and other relevant health staff; record verification; payslip checking; direct observation; client exit interviews; and provider vignettes. In addition, we gathered: district- and site-level characteristics, inputs to HIV service production, amount of services produced by each site, quality of services provided, service coverage, sources of funding, accountability-related characteristics and the potential demand of relevant HIV services in the same area. Cost data were collected retrospectively for the most recent year available: 2011 or 2012 (see Supplementary Technical Appendix, Section 1.6, for additional details on sampling).
Once the data were cleaned, coded and checked for inconsistencies, we calculated total annual facility costs, average costs and cost heterogeneity of producing each HIV prevention intervention as well as the determinants of management efficiency, namely key management aspects such as supervision, accountability, monitoring, incentives and governance. We used a micro-costing (ingredients) approach to estimate total variable costs as the product of the annual number of clients for each intervention times the price for each component of the HIV prevention service. Variable costs included specific items such as rapid tests, antiretrovirals for PMTCT prophylaxis, surgical circumcision kits, etc. Fixed costs included items such as utilities, capital, equipment, training and supervision, etc. We then calculated average costs by dividing the total costs incurred in the facility for each HIV prevention intervention by the total number of clients served for each particular service in that facility (Drummond 2005). A combination of space and time allocation was used to apportion costs to tasks jointly producing more than one HIV prevention service (Roberts 2006). (See Supplementary Technical Appendix, Section 1.7.1, for additional details on measuring costs).
The costs were collected in current Kenyan shillings, and transformed into US dollars at the constant exchange rate of 88.9 shillings per USD for the year 2011 (Central Bank of Kenya 2015). The dependent variable (average costs) was log transformed to more closely approximate a normal distribution, be able to apply linear regression methods, and to be able to interpret the scale coefficients as an elasticity (Manning and Mullahy 2001; Zhou et al. 2001). For the statistical analysis, we used linear regression methods. Based on the theory and previous literature (Over 1986; Dandona et al. 2005; Guinness et al. 2005; Boily et al. 2007; Marseille et al. 2007, 2012; Chandrashekar et al. 2010) we included a large selection of potential predictors of unit costs.
In addition, we included the following measures relevant to evaluating costs and efficiency determinants (Kasymova et al. 2009; Basinga et al. 2011; Preyra and Pink 2001; Chandrashekar et al. 2014; Siapka et al. 2014): incentives for staff performance as well as at the facility level; questions on the ownership of the hospitals and/or facilities variables related to service-integration and task-shifting; and variables related to supervision and outreach costs. (Details on the selected variables explored are presented in Supplementary Technical Appendix Exhibit A2). In addition, two indexes of quality were estimated with principal components analysis: Competence and Performance.
For the log-transformed linear regression models, we first analysed the number of clients per year (scale) as the main predictor; and then we analysed the full model adjusting for other types of variables such as those related to the service delivery model as well as the management of HIV prevention services at the facility level. We adopted an accounting identity approach to characterize the relationship between cost and its determinants (Meyer-Rath and Over 2012), assuming that scale effects were generated at the facility level only and that facility and contextual characteristics increased or decreased cost multiplicatively. Taking logarithms allowed us to estimate the identity equation via ordinary least squares regression and test for the sign of the regression coefficients, such as scale effects. (Supplementary Technical Appendix, Section 3, presents the model derivation and equations).
The aim of the model was to quantify the correlation between unit costs and scale of production, where scale was defined as the number of clients served with the specific HIV prevention service produced. The main determinants included were based on general microeconomic theory (Perloff 2017) and previous literature (Bautista-Arredondo et al. 2014; Marseille et al. 2004) adapted to HIV prevention services production so that we specifically included the most relevant factors as follows. The annual number of clients served measured the scale of production: This was done at each step of the service cascade, such that for testing for example, we first measured all clients tested, and then we measured the number of clients who tested HIV positive. We also included number of supervisions received because oversight may be important in determining efficiency. Similarly, we included measures of community-based or outreach operations, which may be more costly; or whether the facility targeted the testing of populations most at risk (PMAR), which again may require additional economic resources. We also included whether the facility had a community advisory council because appropriate guidance and leadership may affect efficiency; as would do incentives: whether staff could receive rewards for good performance. Finally, we included a measure of whether the facility performed task shifting, meaning that HIV prevention services may be produced by delegating specific tasks to less specialized personnel. The specific variables in the final model were also chosen for their initial statistical significance (P < 0.10) as well as their overall contribution for the model’s explanatory power as measured by the overall model significance (F test) and the R-squared. We used the term predictor instead of independent variable to emphasize that we did not have an experimental design, so the model measured only associations given by the direction and magnitude of the coefficients from linear regression. The main component of unit costs were staff salaries which we measured using allocations based on full time equivalents (FTE) devoted to specific HIV prevention services (see Supplementary Technical Appendix, Section 2.1). Thus, this method implicitly accounted for capacity because some facilities may have more employees, which served more or less clients depending on various aspects of (technical) efficiency, while holding other characteristics (such as quality) constant.
Results
Table 1 presents the dependent variables: the average costs per client. For HTC, the average cost per client tested was $7, while the average cost per client tested and found HIV-positive was $146. For PMTCT, the average cost per client tested was $59, while the average cost per client tested and found HIV-positive was $674. For VMMC, the average cost per procedure completed was $66.
Table 1.
Dependent variables: average cost per client across selected indicators of the HIV prevention service cascade in Kenyan facilities, 2011–2012
| N | Mean | 95% CI | Weighted mean | Median | SD | IQR | ||
|---|---|---|---|---|---|---|---|---|
| HTC | ||||||||
| Cost per client tested | 56 | 7.4 | 5.5 | 9.2 | 6.5 | 4.8 | 7.1 | 5.9 |
| Cost per client tested and positive | 56 | 145.9 | 62.6 | 229.2 | 80.2 | 54.9 | 318.0 | 74.7 |
| PMTCT | ||||||||
| Cost per client tested | 57 | 58.7 | 36.8 | 80.6 | 48.5 | 34.6 | 84.3 | 59.0 |
| Cost per client tested and positive | 51 | 673.5 | 388.8 | 958.1 | 775.8 | 256.3 | 1,037.1 | 594.5 |
| Cost per client on ART | 31 | 1,385.0 | 64.7 | 2,705.2 | 1,261.6 | 274.8 | 3,750.5 | 701.2 |
| VMMC | ||||||||
| Cost per procedure | 33 | 66.3 | 39.5 | 93.1 | 41.1 | 42.4 | 78.6 | 51.1 |
Weighted mean according to total annual patient volume.
CI, confidence interval; SD, standard deviation; IQR, interquartile range; ART, Antiretroviral therapy; HTC, HIV testing and counselling; PMTCT, Prevention of mother-to-child transmission; VMMC, Voluntary medical male circumcision.
Table 2 shows the cost predictors as characteristics of the service delivery model and management indicators affecting the costs of HIV prevention interventions in Kenya by type of facility (hospital vs non-hospital). The survey included a total of N = 56 facilities that provided HTC services to an average of 4235 clients per year in each facility (last columns). There were differences by type of facility: hospitals had a greater number of HTC clients than non-hospital facilities (5244 vs 3071); and hospital’s HTC staff were also more likely to receive rewards for good performance in comparison to staff in non-hospital facilities (40 vs 19%). Among the 57 facilities selected for PMTCT services, an average of 864 clients were tested annually in each facility; and hospitals were also more likely to have PMTCT staff who can receive rewards for good performance in comparison to non-hospital facilities (47 vs 20%). In the 33 facilities providing VMMC, an average of 869 VMMC procedures per year were conducted in each facility. Hospitals were also more likely to have VMMC staff who can receive rewards for good performance in comparison to non-hospital facilities (50 vs 17%).
Table 2.
Predictors: service delivery and management indicators for HIV prevention interventions by facility type, Kenya, 2011–12
| Hospital |
Non-hospital |
P-value | Total |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | M | 95% CI | N | M | 95% CI | N | M | 95% CI | |||||
| HTC | |||||||||||||
| Annual number of clients tested | 30 | 5,244 | 3,553 | 6,934 | 26 | 3,071 | 1,565 | 4,576 | 0.069 | 56 | 4,235 | 3,065 | 5,404 |
| Annual number of clients tested and positive | 30 | 410 | 276 | 544 | 26 | 338 | 164 | 513 | 0.522 | 56 | 377 | 269 | 484 |
| Number of supervisions received in 2011 | 30 | 13 | 9 | 17 | 26 | 18 | 4 | 32 | 0.447 | 56 | 16 | 9 | 22 |
| Facility performs community based testing | 30 | 0.40 | 0.22 | 0.58 | 26 | 0.27 | 0.10 | 0.44 | 0.311 | 56 | 0.34 | 0.21 | 0.46 |
| Facility targets testing (PMAR—symptoms) | 30 | 0.23 | 0.08 | 0.39 | 26 | 0.15 | 0.01 | 0.30 | 0.464 | 56 | 0.20 | 0.09 | 0.30 |
| Facility has a community advisory council | 30 | 0.30 | 0.13 | 0.47 | 26 | 0.38 | 0.19 | 0.58 | 0.514 | 56 | 0.34 | 0.21 | 0.46 |
| Staff can receive rewards for good performance | 30 | 0.40 | 0.22 | 0.58 | 26 | 0.19 | 0.04 | 0.35 | 0.095 | 56 | 0.30 | 0.18 | 0.43 |
| Facility performs task shifting | 30 | 0.57 | 0.39 | 0.75 | 26 | 0.58 | 0.38 | 0.77 | 0.940 | 56 | 0.57 | 0.44 | 0.70 |
| Total number of FTE (clinical) | 30 | 4.61 | 2.94 | 6.27 | 26 | 3.04 | 1.77 | 4.32 | 0.159 | 56 | 3.88 | 2.80 | 4.96 |
| Total number of FTE (non-clinical) | 30 | 0.69 | 0.18 | 1.20 | 26 | 0.69 | 0.29 | 1.08 | 0.987 | 56 | 0.69 | 0.36 | 1.02 |
| PMTCT | |||||||||||||
| Annual number of clients tested | 32 | 1,033 | 662 | 1,404 | 25 | 648 | 259 | 1,037 | 0.169 | 57 | 864 | 593 | 1,136 |
| Annual number of clients tested and positive | 29 | 63 | 34 | 92 | 22 | 67 | 37 | 96 | 0.859 | 51 | 64 | 44 | 85 |
| Number of supervisions received in 2011 | 32 | 14 | 10 | 18 | 25 | 12 | 6 | 17 | 0.479 | 57 | 13 | 10 | 16 |
| Facility targets testing (PMAR—symptoms) | 32 | 0.03 | −0.03 | 0.09 | 25 | 0.00 | 0.00 | 0.00 | 0.382 | 57 | 0.02 | −0.02 | 0.05 |
| Funding linked to facility performance | 32 | 0.31 | 0.15 | 0.48 | 25 | 0.20 | 0.04 | 0.36 | 0.347 | 57 | 0.26 | 0.15 | 0.38 |
| Staff can receive rewards for good performance | 32 | 0.47 | 0.29 | 0.64 | 25 | 0.20 | 0.04 | 0.36 | 0.035 | 57 | 0.35 | 0.23 | 0.48 |
| Facility performs task shifting | 32 | 0.47 | 0.29 | 0.64 | 25 | 0.56 | 0.36 | 0.76 | 0.503 | 57 | 0.51 | 0.38 | 0.64 |
| Total number of FTE (clinical) | 32 | 7.70 | 5.32 | 10.07 | 25 | 3.78 | 2.55 | 5.00 | 0.011 | 57 | 5.98 | 4.46 | 7.49 |
| Total number of FTE (non-clinical) | 32 | 1.41 | 0.63 | 2.19 | 25 | 0.75 | 0.28 | 1.22 | 0.188 | 57 | 1.12 | 0.63 | 1.61 |
| VMMC | |||||||||||||
| Annual number of clients tested | 10 | 855 | 611 | 1,098 | 23 | 875 | 495 | 1,255 | 0.946 | 33 | 869 | 597 | 1,141 |
| Number of supervisions received in 2011 | 10 | 15 | 8 | 22 | 23 | 17 | 2 | 32 | 0.869 | 33 | 17 | 6 | 27 |
| Facility has a community advisory council | 10 | 0.30 | 0.00 | 0.60 | 23 | 0.26 | 0.08 | 0.44 | 0.824 | 33 | 0.27 | 0.12 | 0.43 |
| Facility offers male reproductive health services | 10 | 0.90 | 0.70 | 1.10 | 21 | 0.76 | 0.58 | 0.95 | 0.380 | 31 | 0.81 | 0.67 | 0.95 |
| Staff can receive rewards for good performance | 10 | 0.50 | 0.17 | 0.83 | 23 | 0.17 | 0.02 | 0.33 | 0.056 | 33 | 0.27 | 0.12 | 0.43 |
| Facility performs task shifting | 10 | 0.30 | 0.00 | 0.60 | 23 | 0.30 | 0.11 | 0.50 | 0.981 | 33 | 0.30 | 0.14 | 0.46 |
| Total number of FTE (clinical) | 10 | 4.02 | 2.52 | 5.52 | 23 | 2.71 | 2.07 | 3.35 | 0.072 | 33 | 3.11 | 2.45 | 3.77 |
| Total number of FTE (non-clinical) | 10 | 1.43 | 0.41 | 2.46 | 23 | 0.80 | 0.35 | 1.25 | 0.204 | 33 | 0.99 | 0.55 | 1.44 |
This table presents proportions unless otherwise stated. The non-hospital category includes health centres, dispensaries and clinics. HTC, HIV testing and counselling; PMTCT, Prevention-of-mother-to-child-transmission; VMMC, voluntary medical male circumcision; N, number of sites; CI, confidence interval. The P-values are probability values for statistical comparison tests between hospital and non-hospital facilities. Annual number of supervisions received in 2011 was computed as the sum of self-reported supervisory visits by donors and national, provincial or district governments during the costing year. Facility performs community based testing is a binary indicator of facilities that reported offering testing and pre/post-test counselling for individuals, couples or groups. PMAR, Populations-most-at-risk (indicates if they reported offering HIV testing based on client screening for symptoms or having profiles characteristic of high-risk populations. Funding linked to performance indicates facilities that reported that at least part of the funding is tied to one or more of the following criteria: inputs management, inventory management, quality of care or patient volume. Staff can receive rewards for good performance indicates if the facilities reported established mechanisms such as bonuses, certificates, verbal recognition, training, preferential rotation or time-off. Facility performs task shifting indicates if care activities have delegated to nurses and other health staff, based on the time-allocation component of the study. Facility offers male reproductive health services indicates if they reported performing detection and treatment of health issues such as fertility or erectile dysfunction. FTE denotes full-time equivalent. Clinical staff includes doctors, nurses and other health staff (e.g. counsellors); non-clinical staff includes clerical, managerial and other support staff (e.g. guards).
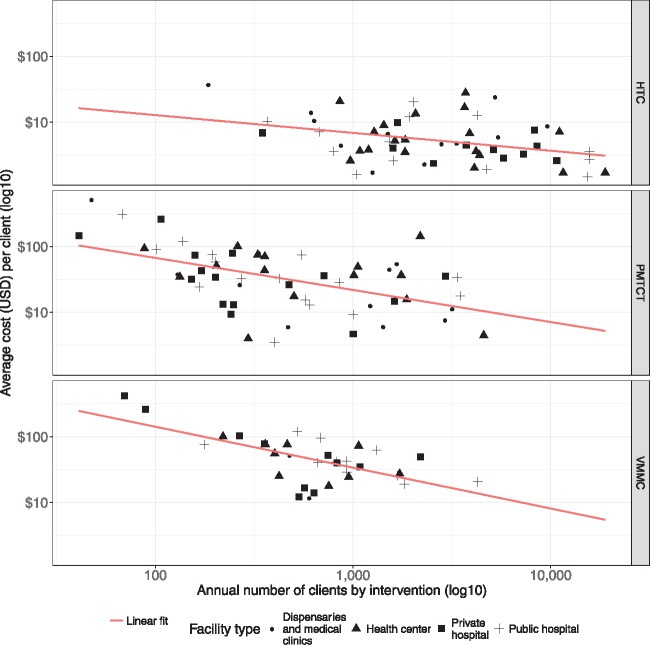
We now present graphical results. Figure 1 plots the relation between the average costs per client and the total number of clients by intervention type (HTC, PMTCT and VMMC), differentiating also by type of facility (public vs private hospital, as well as health centre, dispensaries and clinics). As the scale of production increased, the per-client costs declined for all interventions. Analysing the detailed results (Supplementary Tables S1–S3), we see that doubling the number of clients tested reduced the cost of per PMTCT client by 50%; while doubling the number of clients reduced the cost of per VMMC procedure by 45%. The scale coefficient for HTC was also negative (−0.18) but was not statistically significant. (We repeated the process using fully adjusted multivariate models which have higher predictive value, as given by higher R-square coefficients. Supplementary Figure A1 shows that the size of the scale effect is consistent between the bivariate and multivariate models).
Figure 1.
Average costs per client and number of clients, by intervention type
Figure 2 summarizes the coefficients for the other predictors of the average costs (detailed results are presented in Supplementary Tables S1–S3). For HTC, the factor most strongly correlated with costs was whether the facility targets testing based on risk factors (β = 0.79; CI 95% 0.24–1.34). The HTC costs model including scale explained about a fifth of the variation in the average costs (R-squared = 0.22). For PMTCT, costs decreased if the facility performed task shifting (β = −0.59; CI 95% −1.09 to −0.09) and also if the facility performed audits at least once per year (β =−0.56; 95% CI −1.21 to 0.09). On the other hand, PMTCT costs increased if the facility was a hospital (β = 0.56; 95% CI −0.03 to 1.14). The final PMTCT model including scale explained over a third of the variation in the average costs (R-squared = 0.35). For VMMC, costs decreased if the facility promoted the procedure through the male reproductive services (β = −0.59 95% CI −1.06 to −0.13); if the facility performed task shifting (β = −0.54; 95% CI −0.94 to −0.14); and if the facility had a community advisory council (β = −0.52; 95% CI −0.93 to −0.11). On the other hand, VMMC costs increased if some of the activities were performed outside the facility (β = 0.49; 95% CI −0.04 to 1.03), and for the facilities that had the highest performance (β = 0.67; 95% CI −0.06 to 1.40). Further, there was an interaction effect whereby facilities that were both the best in terms of competence and performance for VMMC had substantially lower costs (β = −0.90; 95% CI −1.81 to 0.01). The final VMMC costs model including scale explained most of the variation in the average costs (R-squared = 0.59).
Figure 2.
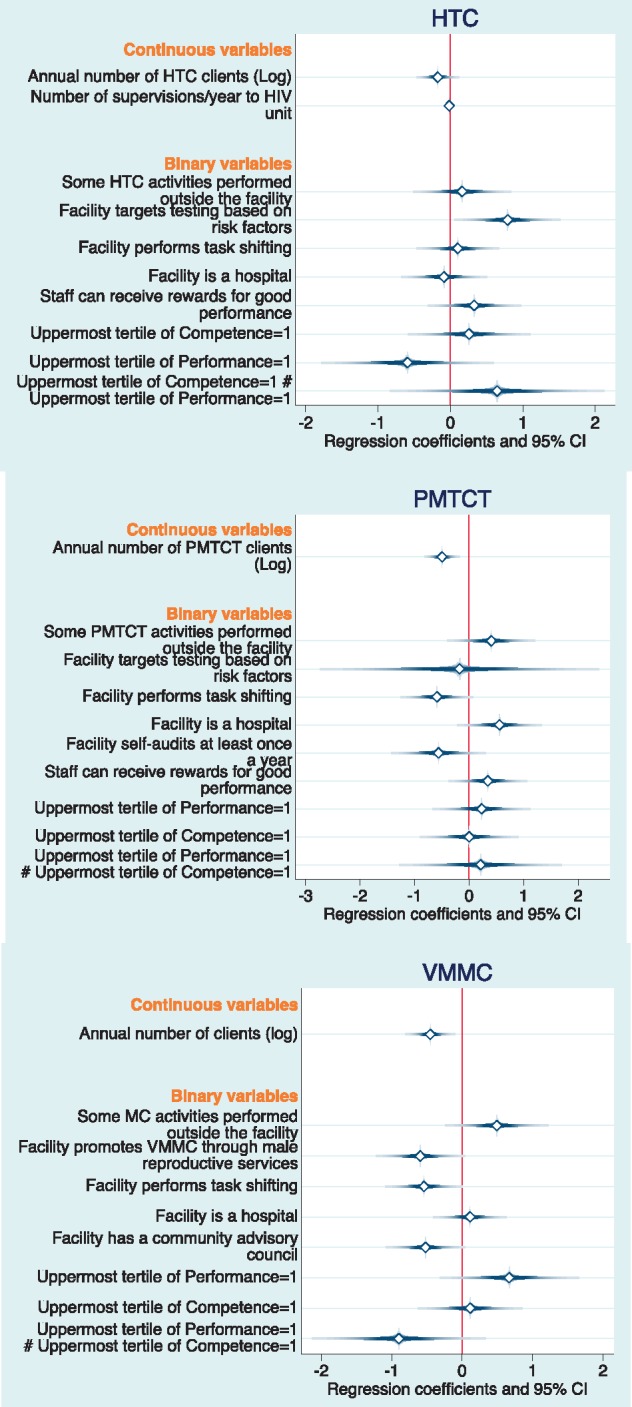
Multiple regression coefficients for Log of cost per HTC, PMTCT and VMMC client
Discussion
This is, to our knowledge, the first article examining predictors of HIV prevention costs at the national level in sub-Saharan Africa. Several points merit discussion. First, most studies in the past have been based on smaller samples, localized interventions and non-standard data collection methods (Galárraga et al. 2009; Menzies et al. 2012; Bautista-Arredondo et al. 2014). Second, this article reports the associations between the average cost of each prevention intervention (HTC, PMTCT and VMMC) and scale, as one of the most widely discussed determinant in the literature (Johns and Baltussen 2004; Kumaranayake 2008), as well as other determinants of average costs. In addition, this article also explores combination of HIV prevention services as well as integration with other services. For all interventions, we found point estimates consistent with evidence of economies of scale: Average cost tends to decrease as facilities serve more clients. Doubling the number of clients was correlated with 18% decrease in HTC costs, 51% for PMTCT and 45% for VMMC (though the HTC estimate was not significant). Third, task shifting (i.e. using qualified lower level staff instead of physicians) correlates with lower unit costs for PMTCT and VMMC, but not for HTC. This result seems logical as the tasks for PMTCT and VMMC may be more amenable to be shifted to personnel with fewer formal qualifications than HTC, which is already conducted by staff with minimum levels of formal training. The literature has provided evidence of the potential use of task-shifting alcohol interventions for HIV-positive persons in Kenya (Galárraga et al. 2017), and for PMTCT and VMMC in other countries as well (Fieno 2008; Lehmann et al. 2009; McCollum et al. 2010; Fulton et al. 2011; Aliyu et al. 2013; Siapka et al. 2014). In addition, for VMMC there was a positive association between unit costs and performing activities outside the facility (e.g. mobile units). This result may be explained by the increased costs associated with outreach (Larson et al. 2015).
Fifth, the costs of PMTCT, HTC and VMMC are largely consistent with the previous literature. The cost per HTC client of $7.4 in Kenya suggests that costs have decreased over time as scale has increased when compared to a previous estimation of $16 per client a decade earlier (Forsythe et al. 2002). The median PMTCT cost per client on ART of $275 is higher than what was found for Zambia for another project ($185) (Scott et al. 2013), and will complement estimates that have relied on modelling (Sweat et al. 2004; Gopalappa et al. 2014). The costs observed in Zambia may have been lower possibly because Scott et al. (2013) relied on a convenience sample while this article presents nationally representative results for Kenya. The cost of $66 per VMMC procedure is comparable to previous estimates of $59–74 in Swaziland, and $56–61 in Zimbabwe (Edgil et al. 2011; Njeuhmeli et al. 2014). Similarly, in Uganda the VMMC costs were $34 at fixed sites and $61–72 in mobile sites (Larson et al. 2015).
The results also show that unit costs tend to be higher in hospitals for PMTCT, but not for the other interventions (Supplementary Tables S1–S3). Two offsetting forces may be at work in this observed relationship relating to hospitals. The first may be that hospitals have overall lower unit costs because of economies of scale. As was seen in Table 2, in comparison to non-hospital based facilities, hospitals had larger numbers of clients per year for HTC (though not for PMTCT and VMMC). At the same time, a second set of variables may make hospitals less efficient as they were more likely to have funding linked to facility performance for PMTCT. Notably, incentives for staff with good performance were not associated with changes in costs. This result is unexpected as incentives for good performance usually add to the unit costs (Saronga et al. 2014).
For VMMC other factors associated with lower average costs were the presence of male reproductive health services at the facility, as well as the existence of a community advisory board. The former result provides some evidence of economies of scope (and/or integration) as related to VMMC facilities, while the latter result may be related to overall facility supervision and oversight (Gray et al. 2013; Siapka et al. 2014).
Our results suggest that there is a potential to increase efficiency within the current constraints of the health system in Kenya, both financial and structural. Specifically, we found that scale is important even across facility types, and not just comparing hospitals with clinics. In light of these results, it is important to think about scale not only as a given factor, which in many circumstances it is, but also to give importance to demand creation activities at the facility level. Our results suggest that this type of investment will probably be very productive. It may also be important to consider economies of scale when determining the size or capacity of health facilities and when selecting their location and size. The results also suggest the importance of evaluating excess supply, such as the extent to which current levels of structure and staffing are not being used to their optimum potential. This may become increasingly important to consider at the policy level as devolution of health deepens (Kibui et al. 2015). Already, counties are allocating varied financing for health programming (Maina et al. 2016), and some guidance may be needed towards achieving efficiencies.
In terms of strengths, this article contributes a specific example of applying a micro-costing approach to HIV prevention services and modelling a unit cost function in terms of its main predictors. The main weakness may be that we can only observe associations given the cross-sectional nature of the data. Thus, more rigorous research is needed to attempt to measure causal relationships in the future. In addition, there are other limitations. First, our modelling choice of a cost accounting identity imposes arithmetical consistency, which is useful for short-run budgeting discussions and enables us to make projections of incremental policies such as scale-up in coverage. However, our models are agnostic with respect to technology, as opposed to flexible cost functions. Thus, other policy concerns such as substitution between health inputs or the impact of economies of scope would necessitate other econometric approaches, which would require more degrees of freedom than those available in our data. A second concern is that the study did not account for all of the potential variables that can affect unit cost variations; other constraints, different from technology and competence, may also explain inefficiency. Finding the right balance in key management aspects such as supervision, accountability, monitoring, incentives and governance remains a challenge, especially in the fully devolved county health functions. Additional exploration of facility-level and county-level management practices and standards that explain variability in efficiency is needed. Third, our study provides evidence—and identifies gaps—on the efficiency and costs of HIV prevention services for a specific cross-section at a particular time point. Ideally, this type of information should be provided on a continued basis and even in real-time to decision makers and managers at all levels in the health system; thus, more regular evaluations are recommended. Although the results may be applicable to other settings and times with similar set of circumstances these findings are specific to Kenya. Finally, the R-squared for the intervention models, given that we have cross-sectional data, were only modestly high and therefore conclusions need to be made with caution.
Conclusion
The analysis has established that volume of service or scale of output explains considerable variation in unit costs for HIV prevention activities, but not all. This implies that an increase in the number of clients in all facilities and particularly in the lower level facilities can lead to declines in costs. Expanding the volume of services can improve levels of efficiency in the HIV prevention response particularly for PMTCT and VMMC. Other factors associated with decreased costs were: task-shifting, community oversight and service integration. Factors associated with increased costs were: hospital-based services; and outreach efforts. Aside from increasing production scale, HIV prevention costs may be further contained by using task shifting for PMTCT and VMMC. In contrast, targeted testing for HTC may require more resources. ORPHEA provides the first national-level evidence base for HIV prevention costs and their determinants for an African country, Kenya.
Supplementary Material
Acknowledgements
Claire Chaumont provided outstanding project management from headquarters at the National Institute of Public Health (INSP) in Mexico. Amilcar Isamar and Roberto Bahena provided excellent research assistance. Alvaro Canales, Victor Canales, Beatriz Godoy and Juan Muñoz designed and programmed the data collection instruments in CSPro. The authors also wish to thank the members of the Government of Kenya for their help and assistance during its implementation. In particular, we are very grateful to the members of the Kenyan Ministry of Health, Dr. Nicholas Muraguri, as well as National AIDS Control Council (NACC) and the National AIDS and STI Control Programme (NASCOP) for their support and involvement in the study.
Funding
This study was supported by the Bill and Melinda Gates Foundation.
Conflict of interest statement. None declared.
Supplementary data
Supplementary data are available at HEAPOL online
References
- African Union. 2012. Roadmap on Shared Responsibility and Global Solidarity for AIDS, TB and Malaria Response in Africa.
- Aliyu MH, Blevins M, Audet C. et al. 2013. Optimizing PMTCT service delivery in rural North-Central Nigeria: protocol and design for a cluster randomized study. Contemporary Clinical Trials 36: 187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amico P, Gobet B, Avila-Figueroa C, Aran C, De Lay P.. 2012. Pattern and levels of spending allocated to HIV prevention programs in low- and middle-income countries. BMC Public Health 12: 221.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SJ, Cherutich P, Kilonzo N. et al. 2014. Maximising the effect of combination HIV prevention through prioritisation of the people and places in greatest need: a modelling study. Lancet 384: 249–56. [DOI] [PubMed] [Google Scholar]
- AU and UNAIDS. 2014. Delivering results toward ending AIDS, Tuberculosis and Malaria in Africa: African Union accountability report on Africa–G8 partnership commitments. African Union and United Nations Joint Programme on HIV/AIDS (UNAIDS).
- Bailey RC, Moses S, Parker CB. et al. 2007. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet 369: 643–56. [DOI] [PubMed] [Google Scholar]
- Basinga P, Gertler PJ, Binagwaho A. et al. 2011. Effect on maternal and child health services in Rwanda of payment to primary health-care providers for performance: an impact evaluation. Lancet 377: 1421–8. [DOI] [PubMed] [Google Scholar]
- Bautista-Arredondo S, Gadsden P, Harris JE, Bertozzi SM.. 2008. Optimizing resource allocation for HIV/AIDS prevention programmes: an analytical framework. AIDS 22: S67–74. [DOI] [PubMed] [Google Scholar]
- Bautista-Arredondo S, Sosa-Rubi SG, Opuni M. et al. 2014. Assessing cost and technical efficiency of HIV prevention interventions in sub-Saharan Africa: the ORPHEA study design and methods. BMC Health Service Research 14: 599.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertozzi SM, Laga M, Bautista-Arredondo S, Coutinho A.. 2008. Making HIV prevention programmes work. Lancet 372: 831–44. [DOI] [PubMed] [Google Scholar]
- Boily MC, Lowndes CM, Vickerman P. et al. 2007. Evaluating large-scale HIV prevention interventions: study design for an integrated mathematical modelling approach. Sexually Transmitted Infections 83: 582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassels S, Camlin CS.. 2016. Geographical mobility and heterogeneity of the HIV epidemic. Lancet HIV 3: e339–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Central Bank of Kenya. 2015. Forex Exchange Rates. Nairobi: Central Bank of Kenya. [Google Scholar]
- Chandrashekar S, Guinness L, Kumaranayake L. et al. 2010. The effects of scale on the costs of targeted HIV prevention interventions among female and male sex workers, men who have sex with men and transgenders in India. Sexually Transmitted Infections 86 : i89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar S, Guinness L, Pickles M. et al. 2014. The costs of scaling up HIV prevention for high risk groups: lessons learned from the Avahan Programme in India. PLoS ONE 9: e106582.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LW, Grabowski MK, Ssekubugu R. et al. 2016. Heterogeneity of the HIV epidemic in agrarian, trading, and fishing communities in Rakai, Uganda: an observational epidemiological study. Lancet HIV 3: e388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn BJ, Okano JT, Blower S.. 2017. Using geospatial mapping to design HIV elimination strategies for sub-Saharan Africa. Science Translational Medicine 9: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandona L, Sisodia P, Ramesh YK. et al. 2005. Cost and efficiency of HIV voluntary counselling and testing centres in Andhra Pradesh, India. National Medical Journal India 18: 26–31. [PubMed] [Google Scholar]
- Drummond M. 2005. Methods for the Economic Evaluation of Health Care Programmes, 3rd edn.Oxford (NY: ): Oxford University Press. [Google Scholar]
- Edgil D, Stankard P, Forsythe S. et al. 2011. Voluntary medical male circumcision: logistics, commodities, and waste management requirements for scale-up of services. PLoS Medicine 8: e1001128.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieno JV. 2008. Costing adult male circumcision in high HIV prevalence, low circumcision rate countries. AIDS Care 20: 515–20. [DOI] [PubMed] [Google Scholar]
- Forsythe S, Arthur G, Ngatia G. et al. 2002. Assessing the cost and willingness to pay for voluntary HIV counselling and testing in Kenya. Health Policy and Planning 17: 187–95. [DOI] [PubMed] [Google Scholar]
- Fulton BD, Scheffler RM, Sparkes SP. et al. 2011. Health workforce skill mix and task shifting in low income countries: a review of recent evidence. Human Resources for Health 9: 1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galárraga O, Colchero MA, Wamai RG, Bertozzi SM.. 2009. HIV prevention cost-effectiveness: a systematic review. BMC Public Health 18; (9 Suppl 1:S5). doi: 10.1186/1471-2458-9-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galárraga O, Gao B, Gakinya BN. et al. 2017. Task-shifting alcohol interventions for HIV+ persons in Kenya: a cost-benefit analysis. BMC Health Service Research 17: 239.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith JS, Ochieng A, Mwalili S. et al. 2014. Status of voluntary medical male circumcision in Kenya: findings from 2 nationally representative surveys in Kenya, 2007 and 2012. Journal of Acquired Immune Deficiency Syndromes 66: S37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosby E, Dybul M, Fauci AS. et al. 2012. The United States President's Emergency Plan for AIDS Relief: a story of partnerships and smart investments to turn the tide of the global AIDS pandemic. Journal of Acquired Immune Deficiency Syndromes 60: S51–6. [DOI] [PubMed] [Google Scholar]
- Gopalappa C, Stover J, Shaffer N, Mahy M.. 2014. The costs and benefits of Option B+ for the prevention of mother-to-child transmission of HIV. AIDS 28: S5–14. [DOI] [PubMed] [Google Scholar]
- Government of the Republic of Kenya. 2011. Kenya National Health Accounts 2009/2010. Health Systems 20/20, Abt Associates Inc.
- Gray RH, Wawer MJ, Kigozi G.. 2013. Programme science research on medical male circumcision scale-up in sub-Saharan Africa. Sexually Transmitted Infections 89: 345–9. [DOI] [PubMed] [Google Scholar]
- Guinness L, Kumaranayake L, Rajaraman B. et al. 2005. Does scale matter? The costs of HIV-prevention interventions for commercial sex workers in India. Bulletin of the World Health Organization 83: 747–55. [PMC free article] [PubMed] [Google Scholar]
- Hecht R, Bollinger L, Stover J. et al. 2009. Critical choices in financing the response to the global HIV/AIDS pandemic. Health Affairs (Millwood) 28: 1591–605. [DOI] [PubMed] [Google Scholar]
- Hecht R, Stover J, Bollinger L. et al. 2010. Financing of HIV/AIDS programme scale-up in low-income and middle-income countries, 2009–31. Lancet 376: 1254–60. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. 2011. Preparing for the Future of HIV/AIDS in Africa: A Shared Responsibility. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- Johns B, Baltussen R.. 2004. Accounting for the cost of scaling-up health interventions. Health Economics 13: 1117–24. [DOI] [PubMed] [Google Scholar]
- Kasymova N, Johns B, Sharipova B.. 2009. The costs of a sexually transmitted infection outreach and treatment programme targeting most at risk youth in Tajikistan. Cost Effectiveness and Resource Allocations 7: 19.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates J, Wexler A, Lief E.. 2015. Financing the Response to HIV in Low- and Middle-Income Countries: International Assistance from Donor Countries in 2014. Kaiser Family Foundation (KFF).
- Kibui AW, Mugo RK, Nyaga G. et al. 2015. Health policies in Kenya and the new constitution for vision 2030. International Journal of Scientific Research and Innovative Technology 2: 127–34. [Google Scholar]
- Kimanga DO, Ogola S, Umuro M. et al. 2014. Prevalence and incidence of HIV infection, trends, and risk factors among persons aged 15–64 years in Kenya: results from a nationally representative study. Journal of Acquired Immune Deficiency Syndromes 66: S13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaranayake L. 2008. The economics of scaling up: cost estimation for HIV/AIDS interventions. AIDS 22: S23–33. [DOI] [PubMed] [Google Scholar]
- Larson B, Tindikahwa A, Mwidu G, Kibuuka H, Magala F.. 2015. How much does it cost to improve access to voluntary medical male circumcision among high-risk, low-income communities in Uganda? PLoS ONE 10: e0119484.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann U, Van Damme W, Barten F, Sanders D.. 2009. Task shifting: the answer to the human resources crisis in Africa? Human Resources for Health 7: 49.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepine A, Vassall A, Chandrashekar S, Blanc E, Le Nestour A.. 2015. Estimating unbiased economies of scale of HIV prevention projects: a case study of Avahan. Social Science and Medicine 131: 164–72. [DOI] [PubMed] [Google Scholar]
- Mahy M, Stover J, Kiragu K. et al. 2010. What will it take to achieve virtual elimination of mother-to-child transmission of HIV? An assessment of current progress and future needs. Sexually Transmitted Infections 86: ii48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maina T, Akumu A, Muchiri S.. 2016. Kenya County Health Accounts: Summary of Findings from 12 Pilot Counties. Futures Group, Health Policy Project.
- Manning WG, Mullahy J.. 2001. Estimating log models: to transform or not to transform? Journal of Health Economics 20: 461–94. [DOI] [PubMed] [Google Scholar]
- Marseille E, Dandona L, Marshall N. et al. 2007. HIV prevention costs and program scale: data from the PANCEA project in five low and middle-income countries. BMC Health Services Research 7: 108.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marseille E, Dandona L, Saba J. et al. 2004. Assessing the efficiency of HIV prevention around the world: methods of the PANCEA project. Health Services Research 39: 1993–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marseille E, Giganti MJ, Mwango A. et al. 2012. Taking ART to scale: determinants of the cost and cost-effectiveness of antiretroviral therapy in 45 clinical sites in Zambia. PLoS ONE 7: e51993.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollum ED, Preidis GA, Kabue MM. et al. 2010. Task shifting routine inpatient pediatric HIV testing improves program outcomes in urban Malawi: a retrospective observational study. PLoS ONE 5: e9626.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medecins Sans Frontieres. 2010. No Time to Quit: HIV/AIDS Treatment Widening in Africa. Médecins Sans Frontières Brussels Operational Centre.
- Menzies NA, Berruti AA, Blandford JM.. 2012. The determinants of HIV treatment costs in resource limited settings. PLoS ONE 7: e48726.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Rath G, Over M.. 2012. HIV treatment as prevention: modelling the cost of antiretroviral treatment—state of the art and future directions. PLoS Medicine 9: e1001247.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwandi Z, Murphy A, Reed J. et al. 2011. Voluntary medical male circumcision: translating research into the rapid expansion of services in Kenya, 2008–2011. PLoS Medicine 8: e1001130.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NACC. 2009. Kenya National AIDS Strategic Plan, 2009/10–2012/13—Delivering on Universal Access. National AIDS Control Council.
- NACC. 2014a. Kenya AIDS Response Progress Report 2014: Progress towards Zero. National AIDS Control Council (NACC).
- NACC. 2014b. Kenya National AIDS Strategic Framework, 2014/15–2018/19. National AIDS Control Council.
- NACC. 2016a. Kenya AIDS Response Progress Report 2016. National AIDS Control Council (NACC).
- NACC. 2016b. Kenya HIV County Profiles 2016. National AIDS Control Council (NACC) and National AIDS and STI Control Programme (NASCOP).
- NACC. 2016c. Kenya National AIDS Spending Assessment, 2012/13–2015/16 [Draft]. National AIDS Control Council.
- NACC and NASCOP. 2014. Kenya HIV Estimates. National AIDS Control Council (NACC); National AIDS and STI Control Progamme (NASCOP); Ministry of Health, Nairobi, Kenya.
- NASCOP. 2008. National Guidance for Voluntary Male Circumcision in Kenya. National AIDS and STI Control Programme (NASCOP); Ministry of Health, Republic of Kenya, Nairobi.
- NASCOP. 2014. Kenya AIDS Indicator Survey 2012 (KAIS). National AIDS and STI Control Programme (NASCOP).
- NASCOP. 2016. Guidelines on use of Antiretroviral Drugs for Treating and Preventing HIV Infection in Kenya 2016. Ministry of Health, National AIDS & STI Control Programme (NASCOP), Nairobi.
- Njeuhmeli E, Kripke K, Hatzold K. et al. 2014. Cost analysis of integrating the PrePex medical device into a voluntary medical male circumcision program in Zimbabwe. PLoS ONE 9: e82533.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Over M. 1986. The effect of scale on cost projections for a primary health care program in a developing country. Social Science and Medicine 22: 351–60. [DOI] [PubMed] [Google Scholar]
- PEPFAR. 2011. Partnership to Fight HIV/AIDS in Kenya. U.S. President's Emergency Plan for AIDS Relief (PEPFAR).
- Perloff JM. 2017. Microeconomics: Theory and Applications with Calculus, 4th edn.Boston: Pearson. [Google Scholar]
- Preyra C, Pink G.. 2001. Balancing incentives in the compensation contracts of nonprofit hospital CEOs. Journal of Health Economics 20: 509–25. [DOI] [PubMed] [Google Scholar]
- Quinn TC, Serwadda D.. 2011. The future of HIV/AIDS in Africa: a shared responsibility. Lancet 377: 1133–4. [DOI] [PubMed] [Google Scholar]
- Republic of Kenya. 2014. Kenya National AIDS Spending Assessment. UNAIDS.
- Roberts JA. 2006. The Economics of Infectious Disease. Oxford(N Y: ): Oxford University Press. [Google Scholar]
- Saronga HP, Duysburgh E, Massawe S. et al. 2014. Efficiency of antenatal care and childbirth services in selected primary health care facilities in rural Tanzania: a cross-sectional study. BMC Health Services Research 14: 96.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott CA, Iyer HS, Lembela Bwalya D. et al. 2013. Uptake, outcomes, and costs of antenatal, well-baby, and prevention of mother-to-child transmission of HIV services under routine care conditions in Zambia. PLoS ONE 8: e72444.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siapka M, Remme M, Obure CD. et al. 2014. Is there scope for cost savings and efficiency gains in HIV services? A systematic review of the evidence from low- and middle-income countries. Bulletin of the World Health Organization 92: 499–511AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinanovic E, Kumaranayake L.. 2006. Financing and cost-effectiveness analysis of public-private partnerships: provision of tuberculosis treatment in South Africa. Cost Effectiveness and Resource Allocation 4: 11.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweat MD, O’Reilly KR, Schmid GP, Denison J, de Zoysa I.. 2004. Cost-effectiveness of nevirapine to prevent mother-to-child HIV transmission in eight African countries. AIDS 18: 1661–71. [DOI] [PubMed] [Google Scholar]
- UN. 2017. Kenyya Sustainable Development: Knowledge Platform. New York: Division for Sustainable Development, Department of Economic and Social Affairs, United Nations Secretariat. [Google Scholar]
- UNAIDS. 2010. Getting to Zero: 2011–2015 Strategy. Geneva: Joint United Nations Programme on HIV/AIDS (UNAIDS; ). [Google Scholar]
- UNAIDS. 2012. AIDS Dependency Crisis: Sourcing African Solutions. Geneva: United National Joint Programme on HIV/AIDS (UNAIDS; ). [Google Scholar]
- UNAIDS. 2013a. Efficient and Sustainable HIV Responses: Case Studies on Country Progress .Geneva: United Nations Joint Programme on HIV/AIDS (UNAIDS; ). [Google Scholar]
- UNAIDS. 2013b. UNAIDS Report on the Global AIDS Epidemic 2013. Geneva: United Nations Joint Programme on HIV/AIDS (UNAIDS; ). [Google Scholar]
- UNAIDS. 2014. Fast Track: Ending the AIDS Epidemic by 2030. Geneva: United Nations Joint Programme on AIDS (UNAIDS; ). [Google Scholar]
- UNAIDS. 2015a. Guideline on When to Start Antiretroviral Therapy and on Pre-Exposure Prophylaxis for HIV. Geneva: Word Health Organization (WHO; ). [PubMed] [Google Scholar]
- UNAIDS. 2015b. On the Fast-Track to End AIDS by 2030: Focus on Location and Population. Geneva: UNAIDS. [Google Scholar]
- Wamai RG. 2014. Civil society organizations and the response to the HIV/AIDS crisis in Africa In: Obadare E. (ed). The Handbook of Civil Society in Africa. New York: Springer International, pp. 361–98. [Google Scholar]
- WHO. 2016. Voluntary Medical Male Circumcision for HIV Prevention in 14 Priority Countries in East and Southern Africa: Progress Brief. Geneva: World Health Organization (WHO; ). [Google Scholar]
- Zhou XH, Stroupe KT, Tierney WM.. 2001. Regression analysis of health care charges with heteroscedasticity. Journal of the Royal Statistical Society Series C-Applied Statistics 50: 303–12. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.