Abstract
Limb-girdle muscular dystrophy type 2D (LGMD2D) is a rare autosomal-recessive disease, affecting striated muscle, due to mutation of SGCA, the gene coding for α-sarcoglycan. Nowadays, more than 50 different SGCA missense mutations have been reported. They are supposed to impact folding and trafficking of α-sarcoglycan because the defective polypeptide, although potentially functional, is recognized and disposed of by the quality control of the cell. The secondary reduction of α-sarcoglycan partners, β-, γ- and δ-sarcoglycan, disrupts a key membrane complex that, associated to dystrophin, contributes to assure sarcolemma stability during muscle contraction. The complex deficiency is responsible for muscle wasting and the development of a severe form of dystrophy. Here, we show that the application of small molecules developed to rescue ΔF508-CFTR trafficking, and known as CFTR correctors, also improved the maturation of several α-sarcoglycan mutants that were consequently rescued at the plasma membrane. Remarkably, in myotubes from a patient with LGMD2D, treatment with CFTR correctors induced the proper re-localization of the whole sarcoglycan complex, with a consequent reduction of sarcolemma fragility. Although the mechanism of action of CFTR correctors on defective α-sarcoglycan needs further investigation, this is the first report showing a quantitative and functional recovery of the sarcoglycan-complex in human pathologic samples, upon small molecule treatment. It represents the proof of principle of a pharmacological strategy that acts on the sarcoglycan maturation process and we believe it has a great potential to develop as a cure for most of the patients with LGMD2D.
Introduction
Limb-girdle muscular dystrophy type 2D (LGMD2D) is an autosomal recessive disease caused by mutations in the SGCA gene coding for α-sarcoglycan (α-SG). α-SG is a single-pass transmembrane glycoprotein that together with β-, γ-, and δ-SG forms a tetrameric complex localized into the sarcolemma of striated muscle (1,2). Sarcoglycan complex, as part of the dystrophin-associated protein complex (DAPC), plays a key role in assuring sarcolemma stability during muscle contraction, and seems involved in signaling processes (3). LGMD2D, as well as the other forms of sarcoglycanopathy (LGMD2E, 2C and 2F) can be classified as loss of function (LOF) disease because defects in the specific sarcoglycan are typically responsible for the absence/strong reduction of the mutated protein with the secondary deficiency of the wild type partners (4). In the last few years, by studying the pathogenesis of LGMD2D, it has been established that the LOF condition is the consequence of the activity of the protein quality control (QC) system of the cell. In fact, the majority of LGMD2D genetic defects are missense mutations originating a folding-defective protein that is recognized by the endoplasmic reticulum-QC and delivered to degradation through the ubiquitin-proteasome system (5,6).
Moreover, different missense mutants of α-SG can be properly rescued at the plasma membrane, by targeting the degradative pathway (5–8). This evidence also suggests that, although mutated, these proteins retain their functionality and that the development of novel therapeutic strategies, aiming to reduce the disposal of the mutants, would be fruitful for patients. To this intent, being the presence of a folding-defective α-SG the main cause of pathogenicity in LGMD2D, it is conceivable a ‘repair strategy’ by means of small molecules facilitating the folding process of the mutants that can therefore pass the quality control and move at the proper site of action.
Protein misfolding is involved in hundreds of genetic diseases, including cystic fibrosis, retinitis pigmentosa, Gaucher’s disease, hypogonadotropic hypogonadism (9,10) etc. and the molecules proposed to revert this condition are also numerous. Such compounds can directly act on the improperly folded protein, as pharmacological chaperones, or indirectly by fostering the folding process, as proteostasis regulators (11–14). Among them, several compounds known as correctors of the cystic fibrosis transmembrane regulator (CFTR) protein are also included (15,16). CFTR correctors have been developed for their ability to recover at the cell surface type II mutants of the chloride channel defective in folding and trafficking (17,18).
Here, we show that CFTR correctors are effective in recovering also different mutants of α-SG. This evidence has been provided utilizing cell models expressing folding defective forms of α-SG and, more importantly, primary myogenic cells isolated from a patient with LGMD2D. Indeed, in patient’s myotubes, upon CFTR corrector treatments, the mutated sarcoglycan increased in content, assembled with the wild type partners, allowing a correct localization of the whole complex at the sarcolemma and consequently a reduction of membrane fragility. These results strongly suggest the feasibility of a protein ‘repair strategy’ to treat LGMD2D, starting from already available small molecules that act on the maturation process of the α-SG mutants.
Results
Rescue of different mutants of α-sarcoglycan by means of CFTR correctors
In the attempt to find a therapeutic approach for LGMD2D, we assessed 12 CFTR correctors (see Table 1) for their ability to improve expression and traffic of different mutants of α-SG, which undergo similar processing mechanism of type II mutants of the CFTR chloride channel (18).
Table 1.
List of the CFTR correctors with concentrations utilized in this study
| CFF namea | Original name | Compoundb | IUPAC name | Concentration µM |
|---|---|---|---|---|
| C2 | VRT-640c | C | 2-{1-[4-(4-Chloro-benzensulfonyl)-piperazin-1-yl]-ethyl}-4-piperidin-1-yl-quinazoline | 5 |
| C3 | VRT-325d | B | 4-Cyclohexyloxy-2-{1-[4-(4-methoxy-benzensulfonyl)-piperazin-1-yl]-ethyl}-quinazoline | 10 |
| C5 | corr 5ae | H | 4, 5, 7-trimethyl-N-phenylquinolin-2-amine | 15 |
| C6 | corr 5ce | I | N-(4-bromophenyl)-4-methylquinolin-2-amine | 10 |
| C9 | KM11060f | F | 7-chloro-4-(4-(4-chlorophenyl sulfonyl)piperazin-1-yl)quinoline | 10 |
| C4 | corr 4ae | E | N-[2-(5-Chloro-2-methoxy-phenylamino)-4'-methyl-[4, 5']bithiazolyl-2'-yl]-benzamide | 10 |
| C13 | corr 4ce | Q | N-(2-(3-acetylphenylamino)-4'-methyl-4, 5'-bithiazol-2'-yl)benzamide | 5 |
| C14 | corr 4de | R | N-(2'-(2-methoxyphenylamino)-4-methyl-5, 5'-bithiazol-2-yl)benzamide | 5 |
| C17 | 15jfg | A | N-(2-(5-chloro-2-methoxyphenylamino)-4'-methyl-4, 5'-bithiazol-2'-yl)pivalamide | 2 |
| C15 | corr 2be | S | N-phenyl-4-(4-vinylphenyl)thiazol-2-amine | 15 |
| VX809h Lumacaftor | U | 3 -(6-(I -(2, 2- difluorobenzo[d][l, 3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid | 6 | |
| Glafeninei | 2, 3-dihydroxypropyl 2-[(7-chloroquinolin-4-yl)amino]benzoate | 10 |
Name according to the list of the Cystic Fibrosis Foundation [https://www.cff.org/CFTR-Chemical-Compound.pdf].
Name according to [WO2014086687].
WO2004111014 A.
US20050059687 A1.
Pedemonte et al. (2005) (15).
Robert et al. (2008) (20).
Yoo et al. (2008) (21).
US2011257223.
Robert et al. (2010) (22).
We tested CFTR correctors in HEK293 cells transiently expressing either the R98H or the D97G mutant of α-SG, as well as in V247M cells, i.e. a population of HEK293 cells that constitutively expresses the V247M-α-SG. These three mutants are responsible for the development of a severe phenotype in patients with LGMD2D [http://www.dmd.nl] and are potentially recoverable as they traffic toward the plasma membrane of cell models by interfering with different steps of the degradative pathway (6–8).
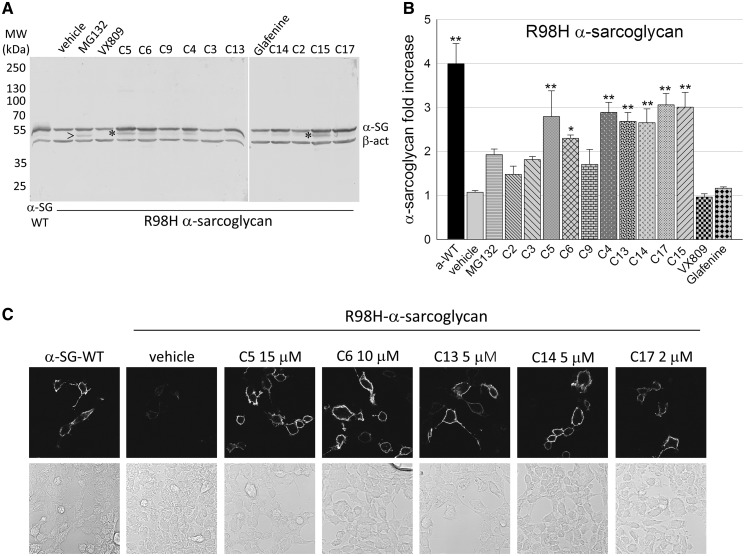
Figure 1 shows the data concerning the R98H mutant of α-SG whereas results from D97G- and V247M-α-SG are reported in Supplementary Material, Figures S1–S3. Cells expressing the α-SG mutant were incubated for 24 h with CFTR correctors at the concentrations reported in Table 1, known to rescue in vitro CFTR processing mutants (15,20–24). As negative controls, cells were incubated with compound vehicle (1‰ DMSO), whereas MG132 was utilized as positive control of rescue (6). Cells transfected with the wild type α-SG were utilized for comparison. α-SG expression in total protein lysates was analysed by western blot with a specific antibody (Fig. 1A). As already shown, α-SG antibody recognizes a band of about 55 kDa both for wild type and mutated α-SG, suggesting that the mutants, although folding-defective, are fully glycosylated (6,8). The brief treatment with MG132 (8 h), as expected, induced a modest increase of the mutant content and led to the appearance of an additional band, at lower molecular weight, representing the de-glycosylated form of the protein accumulated by proteasomal inhibition (6,8). The effect of 24 h of incubation with the diverse CFTR correctors, as visible in the western blots, was quantified by densitometric analysis (see graphs in Fig. 1B related to R98H-α-SG and in Supplementary Material, Fig. S1A and C for D97G and V247M mutants, respectively). Data are presented as fold increase of the negative control, i.e. of the mutant protein content in cells treated with the sole vehicle.
Figure 1.
CFTR correctors promoted increase and traffic of R98H-α-sarcoglycan. (A) Representative western blot of total protein lysates from HEK-293 cells transiently expressing the R98H form of α-SG and treated with the indicated CFTR correctors, at the concentrations reported in Table 1, MG132 10 µM, as in (6) or vehicle (1‰ DMSO); lysate from cells expressing wild type-α-SG was used for comparison. Membranes were incubated with primary antibodies against α-SG and β-actin, used as loading control; arrowhead indicates the de-glycosylated form of the protein (6), whereas asterisks indicate the immature form of the protein recognized by the α-SG antibody. (B) Quantification of α-SG content by densitometric analysis of western blots from at least four independent experiments. The average amount of α-SG (± SEM), is expressed as fold increase of the protein content compared with the negative control (vehicle). Statistical analysis was performed by One-way ANOVA test - multiple comparisons Dunnett test; n.s., P > 0.05; *P ≤ 0.05; **P ≤ 0.01. (C) Membrane localization of α-SG in HEK293 cells expressing R98H-α-SG treated with either vehicle or the indicated CFTR correctors. For comparison, cells expressing wild type-α-SG were also analysed. Localization was evaluated by confocal immunofluorescence analysis of intact cells immuno-decorated with an antibody recognizing an extracellular epitope of α-SG. The primary antibody was revealed with the secondary Alexa Fluor 594-conjugated anti-mouse antibody. Images were recorded with a Leica SP5 laser scanning confocal microscope at the same setting conditions and magnification. Below each image the same field in light transmission was recorded.
Many of the tested CFTR correctors (C5, C6, C4, C13, C14, C17 and C15) were effective on R98H-α-SG, inducing a fold increase of the mutant content ranging from 2.5 to 3 times the level of control (vehicle). Expression of D97G- and V247M-α-SG also raised by similar extent upon treatment with correctors C5 or C4, and C4 or C17, respectively (see Supplementary Material, S1A and C). VX809, a CFTR corrector approved for the treatment of cystic fibrosis, in accordance with its high specificity for cystic fibrosis chloride channel and related ABC proteins (25), was ineffective on α-SG (Fig. 1A and Supplementary Material, Fig. S1A and C). Finally, coherently with its mechanism of action, VX770 was unproductive when tested in V247M cells (Supplementary Material, Fig. S1C). Indeed, this small molecule is a CFTR potentiator, a pharmacological agent that increases the flow of ions through activated CFTR channels, without affecting folding and maturation of the protein (26).
To be significant in a possible therapeutic approach, mutant rescue must comprise trafficking toward the plasma membrane. Therefore, we evaluated the membrane localization of R98H-α-SG on intact cells, upon treatment with some of the most active CFTR correctors. By using an antibody recognizing an extracellular epitope of α-SG, we showed that only traces of R98H-α-SG (Fig. 1C) were present at the surface of untreated cells (vehicle). Conversely, it is possible to appreciate that C5, C6, C13, C14 and C17 successfully induced the localization at the plasma membrane of R98H-α-SG. Indeed, the cell surface signal increased to level similar to wild type control (Fig. 1C). In related experiments, D97G-α-SG localized at the plasma membrane upon incubation with corrector C5, whereas V247M-α-SG regained the ability to reach the cell surface upon treatment with both C4 and C17 (Supplementary Material, Fig. S1B and D).
Among the tested molecules, only VX809 and glafenine have been already approved for clinical use in other diseases (27,28). Therefore, the cytotoxicity of the remaining CFTR correctors was evaluated by measuring the release of the cytosolic enzyme LDH in the medium of V247M cells, upon 24 h of incubation. Results reported in Supplementary Material, Figure S4 show that these CFTR correctors, at the concentrations utilized, were safe with the only exception of C4, which produced a statistically significant, although very modest, cell damage at 10 µM.
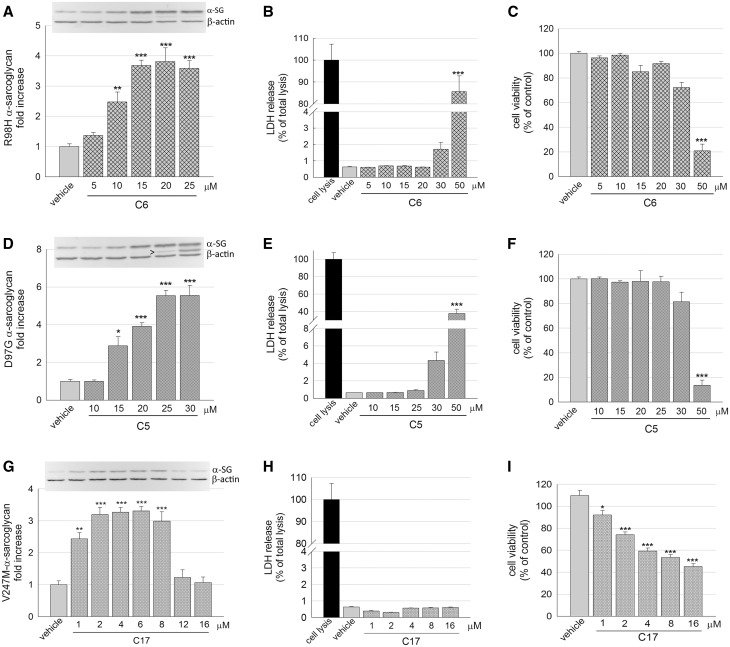
Dose-response effects of CFTR correctors on α-SG mutant content
The dose-dependent effect, in terms of increase of the α-sarcoglycan mutant content, was then evaluated for the most promising correctors. In Figure 2A, it is evident that the protein level of R98H-α-SG increases by increasing the C6 corrector concentration, to reach a plateau at the higher doses. At the same time, none or negligible signs of toxicity were evident at the most effective concentrations, whereas the highest dose tested (50 µM) was toxic for the cells. Indeed, damaged cells released the cytosolic enzyme LDH (Fig. 2B), and cell viability dropped around 20% that of the control cells, as determined by the MTT assay (Fig. 2C). A comparable trend was observed for corrector C5, as assessed in cell expressing the D97G mutant of α-SG (Fig. 2D–F). Finally, increasing concentrations of corrector C17 were tested in V247M cells. As reported in Figure 2G, this small molecule has a very narrow window of efficacy, as the level of V247M-α-SG increased in the 1 to 8 µM range of concentrations, but dropped to value similar to the untreated cells at higher concentrations (12 and 16 µM). To understand this trend, we evaluated the effect of the small molecule on cell integrity and vitality at the diverse concentrations. No statistically significant release of the cytosolic enzyme LDH was recorded at any concentration tested (Fig. 2H), implying that cells were undamaged by the treatment. However, cell viability was reduced, declining from approximately 80% at 2 µM C17 (the concentration utilized in most of the experiments), to approximately 50% of control at the highest concentrations, as determined by the MTT assay (Fig. 2I). This could be due to either a lower number of cells or a slowdown of cell metabolism. Therefore, to verify a possible effect on cell proliferation, we measured the cell number at the beginning and at the end of the incubation time with C17. We recovered a progressively reduced number of cells from wells with increasing concentrations of the corrector C17 (Supplementary Material, Fig. S5). Therefore, considering the absence of cell death (Fig. 2H) but the reduced number of cells recovered, we hypothesize that corrector C17 exerted a cytostatic effect on proliferating cells.
Figure 2.
Corrector C6, C5 and C17 induced a dose-dependent increase of different α-SG mutants without major toxic effects. (A, D, G) Quantification of α-SG content in HEK293 cells expressing R98H-α-SG (A), D97G-α-SG (D) or V247M-α-SG (G) treated for 24 h with increasing concentrations of the indicated correctors. α-SG protein content was determined by WB and densitometric analysis on total protein lysates from at least three independent experiments. Above each graph is reported a representative western blot; β-actin was used as loading control. Arrowhead indicates an extra band recognized by the α-SG antibody that probably represents an immature form of the protein. (B, E, H) Cytotoxicity of correctors evaluated as the release of the cytosolic enzyme LDH in the culture medium of cells expressing R98H-α-SG treated with increasing concentration of C6 (B), D97G-α-SG treated with increasing concentration of C5 (E) or V247M-α-SG treated with increasing concentration of C17 (H). LDH release is expressed as percentage of the total amount of enzyme in cell lysate. (C, F, I) cell viability evaluated by measuring the metabolism of cells expressing R98H-α-SG treated with increasing concentration of C6 (C), D97G-α-SG treated with increasing concentration of C5 (F) or V247M-α-SG treated with increasing concentration of C17 (I). Cell viability was expressed as percentage (± SEM) toward cells treated with vehicle. Statistical analysis was performed by One-way ANOVA test - multiple comparisons Dunnett test; n.s., P > 0.05; *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Rescue of the folding defective R77C-α-SG by CFTR correctors: single and combined administration
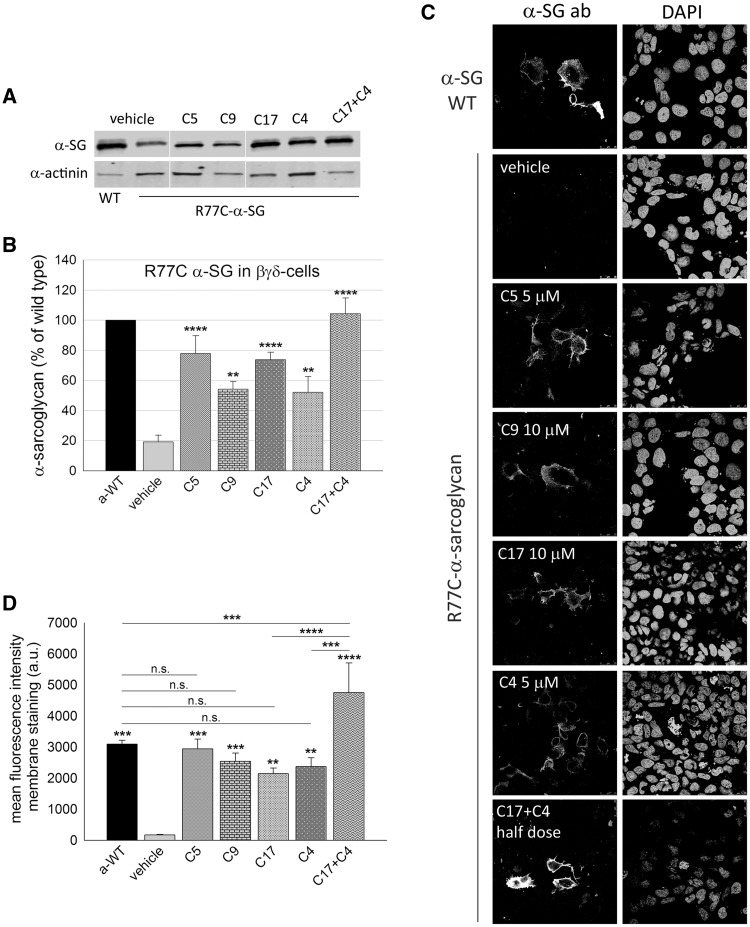
The R77C amino acid substitution is the most frequently reported mutation responsible for LGMD2D [http://www.dmd.nl], consequently it was mandatory to verify the effectiveness of CFTR corrector treatments on this mutant. This mutant tends to aggregate when expressed in HEK293 cells (29), and, probably for this reason, the incubation with MG132 failed to rescue the protein at the cell membrane (6). Therefore, we decided to seek out a different cell model to better mimics the condition observed in human subjects where R77C-α-SG is absent or strongly reduced, and the sole γ-SG is expressed in variable residual amount (30,31). As HER911 cells concomitantly transfected with β-, γ-, δ- and R77C-α-SG were utilized to successfully rescue the mutant at the cell surface upon α-mannosidases inhibition (5), we decided to adopt a similar system. However, to overcome variability associated with a quadritransfection model, we generated a cell line, hereafter named βγδ-cells, in which the cDNAs coding for the wild type β-, γ-, δ-SG were stably integrated in the genome of HER911cells by lentivirus transduction (Supplementary Material, Fig. S6). The subsequent transfection with either wild type or R77C-α-SG led to the production of the final cell model. Here, we show that the level of R77C-α-SG transfected in βγδ-cells (either untreated or treated with the sole vehicle) was very low, about 20% in comparison to wild type (Fig. 3A and B;Supplementary Material, Fig. S6D) and the protein was almost undetectable at the plasma membrane (Fig. 3C, vehicle), well mimicking the condition present in patient’s muscle cells (30,31). Therefore, we exploited this model to assess the efficacy of some of the most effective CFTR correctors, administered either as single molecule or in combination. We found that the presence of the other sarcoglycan subunits had a stabilizing effect on the rescued mutant. In fact, treatment with C5, C9, C17 and C4 induced a three to four-fold increase of R77C-α-SG protein content (Fig. 3A and B).
Figure 3.
Rescue of the folding-defective R77C-α-SG by means of CFTR correctors. (A) Western blot of protein lysates from βγδ-cells transiently expressing R77C-α-SG and treated for 24 h with corrector C5 5 µM, C9 10 µM, C17 10 µM, C4 5 µM. One sample was treated with the combination of corrector C17 and C4 (each one-half dose). Cells expressing wild type-α-SG were utilized as positive control. Membrane were probed with antibodies against α-SG and α-actinin, used as loading control. (B) quantification by densitometric analysis of α-SG protein bands on at least three independent Western blot experiments. The average amount of α-SG (± SEM) is shown as percentage of the protein content in cells expressing the wild type form. Statistical analysis was performed by One-way ANOVA test - multiple comparisons Dunnett test; **P ≤ 0.01; ****P ≤ 0.0001. (C) IF confocal analysis of βγδ-cells expressing R77C-α-SG and treated for 24 h with the indicated correctors. Intact cells (not permeabilized) were immune-decorated with an anti α-SG antibody, recognizing an extracellular epitope, revealed by the secondary Alexa Fluor 594-conjugated anti-mouse antibody. Cells expressing wild type-α-SG are shown as positive control. On the right of each image is reported the same field with nuclei stained by DAPI. Images were recorded with a Leica SP5 laser scanning confocal microscope at the same setting conditions and magnification. (D) mean fluorescence intensity of membrane staining of βγδ-cells expressing R77C-α-SG treated for 24 h with vehicle (negative control) or the indicated correctors; βγδ-cells expressing WT-α-SG were used as positive control. Fluorescence values from at least three independent experiments, performed in triplicate, were recorded by using the ImageXpress microscope system. Mean values (± SEM) were normalized for the number of cells positive for both α-SG and DAPI under permeabilization condition to consider transfection efficiency. Statistical analysis was performed by One-way ANOVA test - multiple comparisons Bonferroni test; n.s., P > 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001.
On the other hand, these compounds had no effect on the wild type form of proteins, as observed when treatments were applied in βγδ-cells transfected with either WT-α-SG (Supplementary Material, Fig. S7A) or the unrelated GFP protein (Supplementary Material, Fig. S7B). Moreover, in βγδ-cells, no effect was exerted by C5, C9, C17 and C4 on the transcription rate of both the wild type and R77C form of α-SG transiently transfected in the cell model (Supplementary Material, Fig. S8A and B, respectively), as well as on WT-β-SG constitutively expressed by the cells (Supplementary Material, Fig. S8C), as determined by quantitative RT-PCR. Altogether, these data suggest that correctors exert their effect specifically on the mutated form of proteins leaving unaltered the mRNA expression controlled by the heterologous promoter.
Remarkably, the rescued protein localized at the plasma membrane, as proven by the intense cell-surface staining of not-permeabilized cells (Fig. 3C). Notably, the combined C17 + C4 administration, each corrector at half dose of the single application, resulted in a more robust effect, as the total mutant content reached that of the wild type (Fig. 3B). In addition, the intensity of the fluorescence signal at the plasma membrane was significantly higher than the one obtained by individually applied correctors (Fig. 3C), as evaluated by using the ImageXpress microscope system (Fig. 3D). Then, we evaluated the effect of the co-administration of C17 + C5, also in this case used at halved dose. In Supplementary Material, Figure S9 is reported the quantification of the cell membrane staining upon CFTR corrector treatment. Also in this case, the co-administration resulted in the additional increase of the cell surface α-SG rescue in comparison to the single-compound administration. All this considered, we conclude that the mutant R77C-α-SG can be successfully rescued in vitro by CFTR corrector treatments and those correctors may have an additive and even a synergic effect when administered in combination.
Corrector C17 increases the α-SG mutant level in myotubes from a LGMD2D patient
To validate the data collected with cellular models, we assessed the efficacy of CFTR correctors in rescuing SG-complex directly in human samples. Indeed, we had the opportunity to use myogenic cells derived from a small bioptic fragment of a patient with LGMD2D carrying the L31P and V247M mutations on the SGCA alleles. After 7 days of differentiation, we examined the myotubes derived from these cells by immunofluorescence-confocal analysis. As expected, in comparison to the healthy subject, myotubes from the patient with LGMD2D expressed a low level of SGs, with only traces of the α-SG protein at the myotube surface (Supplementary Material, Fig. S10).
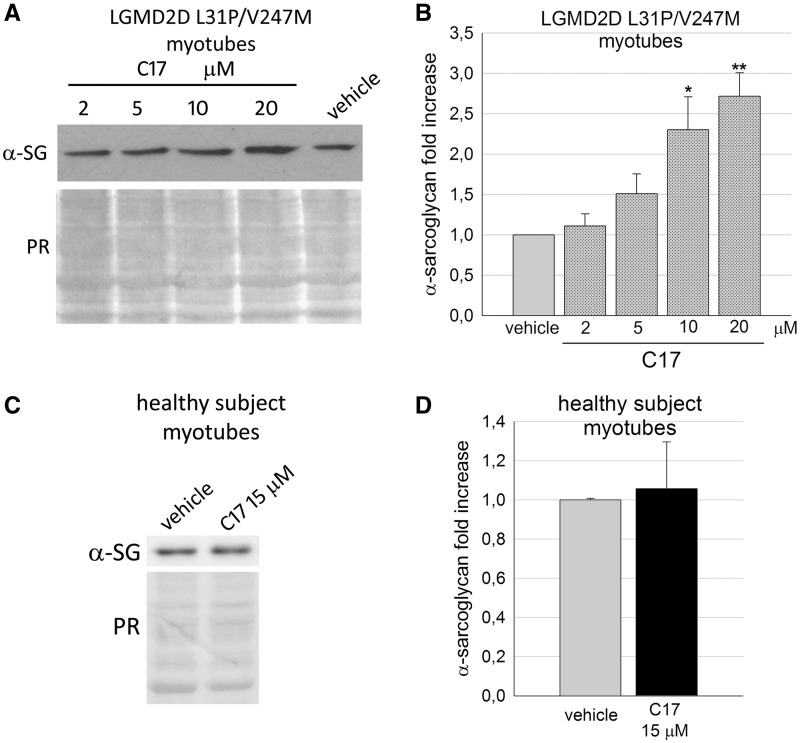
Thereafter, we treated the LGMD2D myotubes with three CFTR correctors or vehicle. Since one of the α-SG mutation is the V247M substitution, we firstly tested C17, the most effective ‘V247M-α-SG corrector’ as assessed in the cell model. Figure 4A and B shows that the small molecule induced a dose-dependent increase of α-SG mutant, with 10 µM C17 being the concentration needed to elicit a statistical significant increase of the mutant level. On the contrary, 48 h of incubation with 15 µM C17 were ineffective on the level of the endogenous, wild type form of α-SG, expressed by the healthy subject’s myotubes, used as control (Fig. 4C and D). These data confirm what already observed with the βγδ-cells (Supplementary Material, Fig. S7) and suggests that corrector C17 is either directly acting on the α-SG mutants or, more probably, on cellular pathways specifically handling the mutant, or on both.
Figure 4.
Corrector C17 induced a dose dependent increase of α-SG mutant in LGMD2D myotubes. (A) representative western blot of total protein lysates of myogenic cells from a patient carrying the L31P/V247M α-SG mutations grown and differentiated for 7 days and treated for the last 48 h with either 1‰ DMSO (vehicle) or increasing concentrations of corrector C17, as indicated. α-SG protein was revealed with specific primary antibody, the Ponceau red staining (PR) is reported and utilized to normalize the total amount of proteins loaded in each lane. (B) quantification by densitometric analysis of α-SG protein bands of three independent western blot experiments, as described in (A). The average amount of α-SG (± SEM) is expressed as fold increase of the protein content present in myotubes treated with vehicle. Statistical analysis was performed by One-way ANOVA test - multiple comparisons Dunnett test; *P ≤ 0.05; **P ≤ 0.01. (C) myogenic cells from a healthy subject were grown and differentiated for 7 days and treated for the last 48 hours with either 1‰ DMSO (vehicle) or 15 µM C17. Total protein lysates were analyzed by Western blot as described in (A). (D) quantification by densitometric analysis of wild type α-SG protein bands of three independent Western blot experiments as described in (C). Statistical analysis was performed by unpaired two-tailed Student’s t-test.
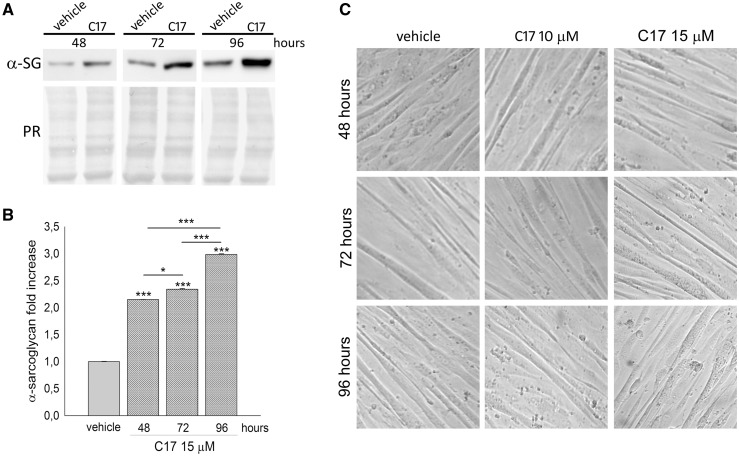
The recovery of the mutant protein elicited by C17 increased with the length of the incubation time, as shown in Figure 5A and B, reporting representative western blots and the densitometric quantification of three independent experiments over a period from 48 to 96 h. Importantly, no evident sign of toxicity was observed neither at 10 nor at 15 µM C17, and at any time point. In fact, the differentiated LGMD2D cells show no major alterations in the morphology of the elongated myotubes nor overt signs of cytotoxicity, such as membrane blebbing or cells detaching from the plate, even after 96 h of incubation (Fig. 5C).
Figure 5.
Corrector C17 induced a time dependent increase of mutated α-SG, without toxicity, in LGMD2D myotubes. (A) myogenic cells from a patient carrying the L31P/V247M α-SG mutations were grown and differentiated for 7 days and treated with 1‰ DMSO (vehicle) or 15 µM C17 for the indicated time intervals. α-SG protein content was evaluated by western blot of total myotube lysates. The Ponceau red staining (PR) is reported to normalize the total amount of proteins loaded in each lane. (B) quantification by densitometric analysis of α-SG protein bands of three independent western blot experiments, as described in (A). The average amount of α-SG (± SEM) is expressed as fold increase of the protein content present in myotubes treated with vehicle for the same incubation interval. Statistical analysis was performed by One-way ANOVA test - multiple comparisons Bonferroni test; *P ≤ 0.05; ***P ≤ 0.001. (C) phase contrast images of myotubes treated with either 1‰ DMSO (vehicle) or C17 at the concentration and time intervals indicated to evaluate possible toxic effects. All images were recorded at the same magnification.
Treatment with corrector C17 stabilized the α-SG mutant in patient’s myotubes
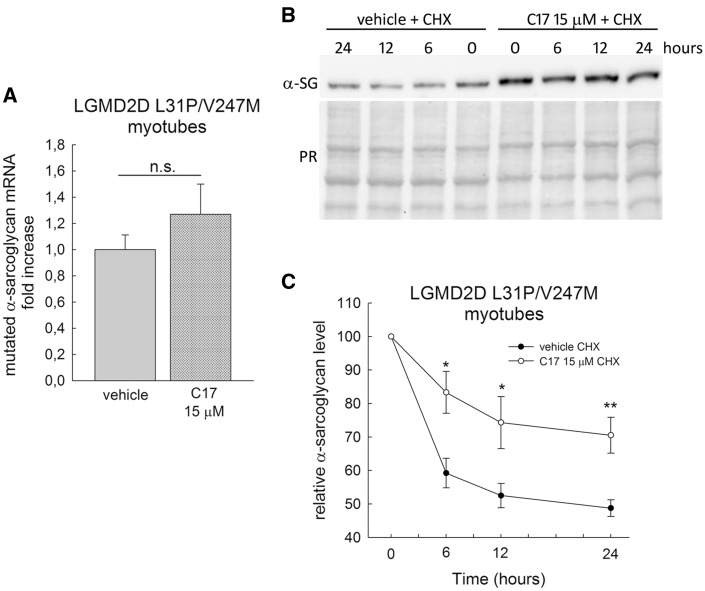
The increase of α-SG mutant level, observed in LGMD2D myotubes treated with corrector C17, could be the result of either the transcriptional upregulation of SGCA gene or the stabilization of the protein. For the first issue, we studied the effect of the small molecule on SGCA transcription by quantitative RT-PCR analysis. For the second issue, we evaluated the rate of α-SG protein disappearance after blocking protein synthesis by cycloheximide. No statistical significant effect was detected on the endogenous SGCA transcription, as shown in Figure 6A reporting the average amount of α-SG mRNA in LGMD2D myotubes after 48 h of treatment with corrector C17 compared with control (vehicle). This data on the transcription of the endogenous SGCA gene, confirms what observed when sarcoglycans were exogenously expressed in βγδ-cells (Supplementary Material, Fig. S8).
Figure 6.
Corrector C17 had no effect on the transcription of SGCA and stabilized α-SG mutant, in LGMD2D myotubes. (A) LGMD2D myotubes were treated for 48h with vehicle (1‰ DMSO) or 15 µM C17. The SGCA transcription was evaluated by quantitative real-time PCR (see M&M for details concerning normalization) and reported as mean, ± SEM, relative to DMSO treated control of two independent experiments performed in quadruplicate. Statistical analysis was performed using unpaired two-tailed Student’s t-test; n.s., P > 0.05. (B) myogenic cells from a patient carrying the L31P/V247M α-SG mutations were grown and differentiated for 7 days and treated with 1‰ DMSO (vehicle) or 15 µM C17 for 96 h. At the end of incubation, 100 µg/ml cycloheximide was added and myotubes were lysate at the indicated time points. Protein lysate were analyzed by western blot with anti α-SG antibody, the Ponceau red staining (PR) is reported to normalize the total amount of proteins loaded in each lane. (C) quantification by densitometric analysis of α-SG protein bands of three independent western blot experiments, as described in (B). The average amount of α-SG (± SEM) is expressed as percentage of the protein present at time 0. Statistical analysis was performed by unpaired two-tailed Student’s t-test; *P ≤ 0.05; **P ≤ 0.01.
On the other hand, a clear stabilizing effect was observed on the mutated α-SG expressed by the patient’s myotubes upon C17 incubation. In Figure 6B and C are reported a representative western blot and the densitometric quantification of the α-SG level at different time points after the addition of the protein synthesis inhibitor, cycloheximide. In defect of protein neosynthesis, the α-SG mutant in untreated myotubes (vehicle) rapidly diminished during the first 6 h, to reach a value of approximately 50% of the initial level after 24 h. Conversely, the decline of α-SG mutant, accumulated thanks to the C17 treatment, is slower, being only 15% after 6 h and 30% of the initial value at the last time point.
Rescue and traffic of sarcoglycan complex in patient’s myotubes upon CFTR corrector treatments
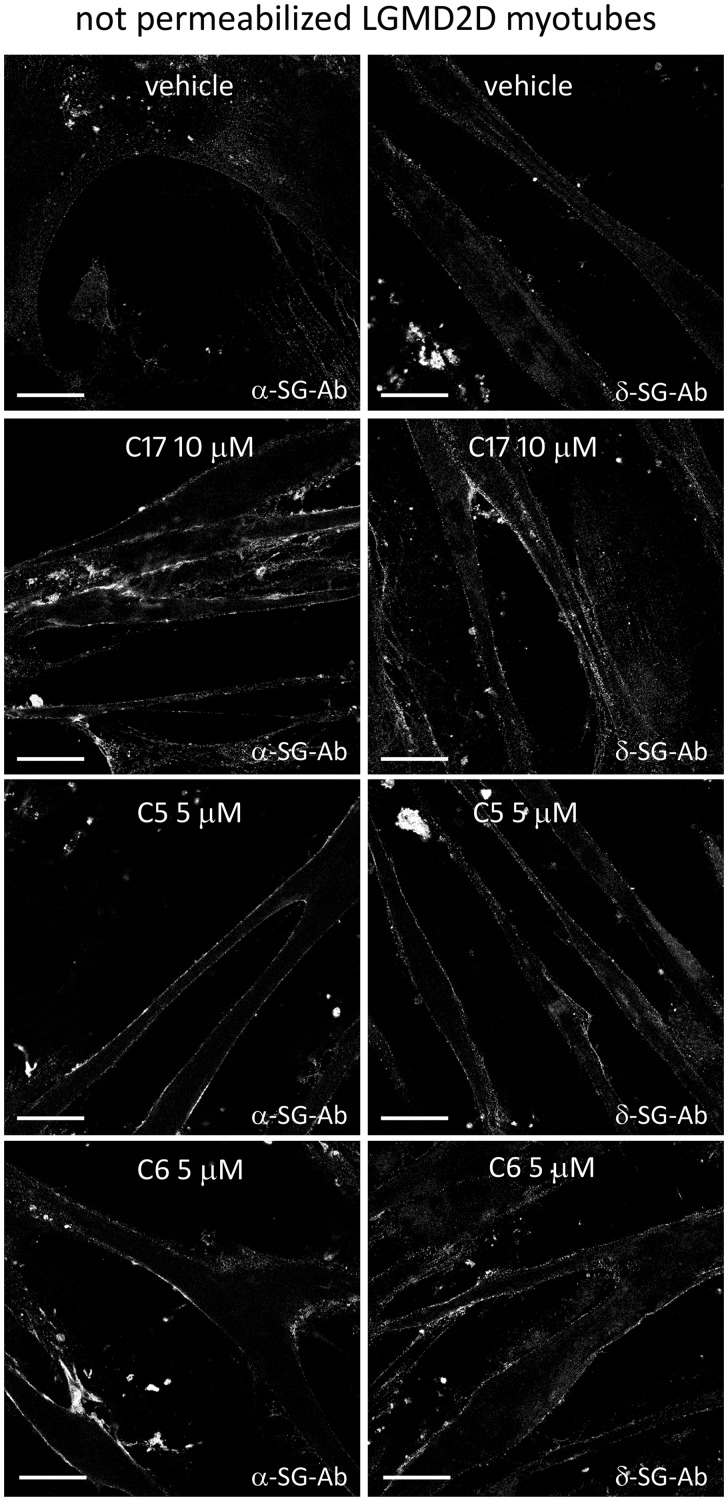
The accumulation of α-SG mutant is therapeutically effective only if followed by SG-complex assembly and localization at the sarcolemma. Therefore, we examined the localization at the myotube surface of both α-SG and δ-SG by antibodies recognizing extracellular epitopes of the two proteins. δ-SG is marker of SG-complex assembly because it is considered the key subunit that forms with β-SG the core complex to which γ- and α-SG associate later to move eventually toward the plasma membrane (32–34). In Figure 7, showing immunofluorescence-confocal images of 7-day-old myotubes, it is undoubtedly evident that the amount of the surface-resident sarcoglycans is extremely low (very faint fluorescence signal) in the absence of any treatment (vehicle). Conversely, the signal for both α- and δ-SG dramatically increased at the sarcolemma after 48 h of incubation with C17, suggesting the treatment successfully recovered the SG-complex at the proper cellular location in human pathological specimens.
Figure 7.
CFTR correctors rescued the sarcoglycan complex in LGMD2D myotubes. Myogenic cells from a patient carrying the L31P/V247M α-SG mutations were grown and differentiated for 7 days and treated for the last 48 h with 1‰ DMSO (vehicle) or the indicated CFTR correctors. At the end of incubation intact myotubes (not permeabilized) were labelled with antibodies recognizing an extracellular epitope of either α-SG (on the left) or δ-SG (on the right), as indicated, to mark the membrane resident sarcoglycans only. Primary antibodies were revealed with the secondary DyLight 488-conjugated anti-rabbit antibodies. Bars indicate 31.75 µm. Images were recorded with a Leica SP5 laser scanning confocal microscope at the same setting conditions.
Treatment with corrector C5 and C6 resulted in very similar effects. Indeed, when C5 and C6 were applied to myotubes from the patient with LGMD2D, the amount of the mutated α-SG (Supplementary Material, S11A and B) increased in comparison to the control (myotubes treated with vehicle), and the SG-complex was recognized at the surface of treated myotubes (Fig. 7).
C17 treatment restores membrane functionality to patient’s myotubes in vitro
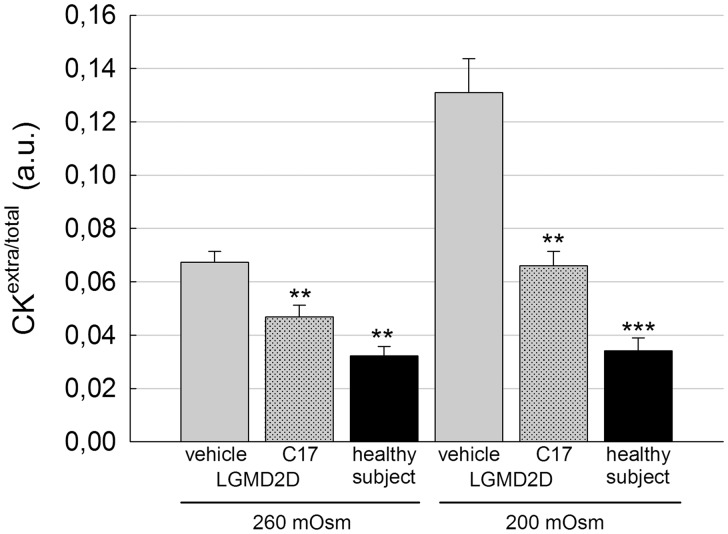
The SG-complex, interacting with dystrophin and dystroglycans, plays a protective role for the sarcolemma during muscle contraction cycles (35,36). In sarcoglycanopathy the SG-complex is severely reduced or absent from sarcolemma and one of the first clinical sign is the elevated serum content of the cytosolic protein of muscle fibers, creatine kinase (CK, more than 10-fold) (37). This feature is common to many other muscular dystrophies in which there is an alteration of the DAPC (38). There is also evidence that the lack of dystrophin increases the fragility of both cardiomyocytes and skeletal myotubes in vitro, which respond to osmotic stress by releasing elevated levels of CK (39–40). Therefore, we applied a protocol to check whether the presence of the SG-complex, rescued by CFTR corrector treatment, reduced the sarcolemma fragility of the LGMD2D myotubes under stressful conditions. Seven-day-old myotubes were treated for 96 h with vehicle or 15 µM C17. At the end of the treatment, myotubes were maintained 20 min in hypoosmolar conditions (260 and 200 mOsmol) and the release of CK was then measured in the supernatant. Figure 8 shows that myotubes from the healthy subject released very low amount of CK in comparison to the LGMD2D myotubes (treated with vehicle). This occurred at both the hypoosmotic conditions applied and the release from the pathologic myotubes increased by worsening the stress conditions. On the contrary, the release of CK from LGMD2D myotubes pretreated with C17 was significantly reduced in comparison to the vehicle treated control. This suggests the functional improvement of the sarcolemma of the patient’s myotubes when the SG-complex is restored upon CFTR corrector treatment.
Figure 8.
C17 treatment restores membrane functionality to patient’s myotubes in vitro. Myogenic cells from a patient carrying the L31P/V247M α-SG mutations were grown and differentiated for 7 days and treated for the last 96 h with 1‰ DMSO (vehicle) or 15 µM C17. At the end of the treatment, myotubes were incubated for 20 min in hypo-osmotic solutions as indicated. Then, the cytosolic protein creatine kinase (CK) was measured in the supernatant of myotubes, whereas the intracellular level of the protein was determined after cell lysis. Ratios between extra and total CK values were plotted as average ± SEM of two independent experiments performed in sextuplicate. As reference, the release of CK from myotubes from a healthy subject was assessed at the same hypo-osmotic conditions. Statistical analysis was performed using One-way ANOVA test—multiple comparisons Dunnett test; **P ≤ 0.01; ***P ≤ 0.001.
Discussion
The folding process is a key step for each protein to acquire the functional role. The presence of genetic mutations can lead to a folding-defective polypeptide, usually promptly disposed of by the QC system. The net result is a loss of function even when the folding-defective protein retains, either partially or completely, its function (13,41). This is the pathogenic mechanism of most cases of LGMD2D (7,8), a severe muscular dystrophy, in which more than 50 different missense mutations account for more than 65% of α-SG genetic defects (36). LGMD2D is a rare disease, at present incurable, that could benefit from the use of small molecules acting on either the degradative pathway of the defective mutants, as already shown (5–8), or on the folding/maturation process. In this attempt, we used CFTR correctors, small molecules that promote the recovery of folding-defective mutants of the CFTR protein (17,19). By using these compounds, we show that it is possible to skip the degradation of α-SG mutants, which re-localize at the proper site of action together with the wild type partners. Four different α-SG missense mutations were successfully rescued in cellular models, and importantly, CFTR correctors were effective in primary myogenic cells from a LGMD2D patient. In these cells, the recovered α-SG assembled into a functional SG-complex, which properly localized at, and strengthened the sarcolemma of the patient’s myotubes against stressful conditions.
To test the effectiveness of several CFTR correctors, as in vitro model we adopted the simple transfection of HEK293 cells with different α-SG mutants, such as R98H-, D97G- and V247M-α-SG. Conversely, for the R77C mutant we needed to better mimic the condition recognizable in pathologic muscle (30,31), and for this reason we generated the novel βγδ-cells, constitutively expressing three SGs in which we transfected the mutant. All the mutants checked are rescuable by CFTR corrector treatments as we measured an increase in both quantity (twofold increase was considered significant), and surface localization of the defective proteins (Table 2).
Table 2.
CFTR corrector efficacy related to specific α-SG mutant expressed in cell models
| Corrector | V247MHEK293 |
R98HHEK293 |
D97GHEK293 |
R77C βγδ-cells |
||||
|---|---|---|---|---|---|---|---|---|
| protein | surface | protein | surface | protein | surface | protein | surface | |
| C2 | ++ | n.d. | −+ | n.d. | − | n.d. | n.d. | n.d. |
| C3 | ++ | n.d. | −+ | n.d. | − | n.d. | n.d. | n.d. |
| C5 | − | − | ++ | √ | ++ | √ | +++ | √ |
| C6 | − | − | ++ | √ | ** | n.d. | n.d. | n.d. |
| C9 | ++ | n.d. | −+ | n.d. | −+ | n.d. | ++ | √ |
| C4 | ++ | √ | ++ | n.d. | ++ | √ | ++ | √ |
| C13 | ++ | n.d. | ++ | √ | ** | n.d. | n.d. | n.d. |
| C14 | ++ | n.d. | ++ | √ | −+ | n.d. | n.d. | n.d. |
| C17 | +++ | √ | +++ | √ | ** | n.d. | +++ | √ |
| C15 | − | n.d. | +++ | n.d. | −+ | n.d. | n.d. | n.d. |
| VX809 | −+ | − | − | − | − | − | n.d. | n.d. |
| Glafenine | n.d. | n.d. | − | n.d. | − | n.d. | n.d. | n.d. |
√: positive immunofluorescence, −: ineffective, −+: >1X, +: ≥ 1.5X, ++: ≥2X, +++: ≥3X, **: ≥2X but not statistically significant, n.d.: not determined.
CFTR correctors promote folding and trafficking of type II mutants of the chloride channel, such as ΔF508-CFTR, by two mechanisms. In the first one, correctors are supposed to directly bind and stabilize CFTR, acting as pharmacological chaperones. In the second one, they should modulate the biological capacity of the protein quality control network of the cells, acting as proteostasis regulators (17). In general, a pharmacological chaperone is supposed to be highly specific for a single protein or for conserved motifs of structurally related proteins; conversely, a proteostasis regulator should have a wider spectrum of action (42). α-SG has no structural similarity with CFTR, being a type I protein with a large extracellular N-terminal domain and a short cytosolic C-terminal tail. In our study, we obtained positive results with four mutations, three of them (R77C, D97G and R98H) present in the cadherin-like domain of α-SG, (43) and the last one (V247M) located near the membrane, close to a region probably involved in the interaction with the other SGs (32). Analysing the rescue of the different mutants (Table 2), we can observe that C4 and C17 were effective on all mutants, and C5 on three out of four (those located in the cadherin like domain). This is not surprising for C4 because of its broad activity profile. C4 is active on different processing mutants of CFTR (44,45) as well as on proteins both structurally-correlated and structurally-uncorrelated to CFTR (25,46–49). Moreover, this compound is a weak inhibitor of the E1-E3 ubiquitin ligase cascade (50), therefore could interfere with the degradative pathway of mutants. The effectiveness on α-SG mutants is more surprising for correctors C5 or C17, since they are supposedly specific for ΔF508-CFTR or ABC transporter family, respectively (47,51). On the other hand, both C5 and C17 successfully rescued the I661T mutant of ATP8B1, a protein responsible for the onset of the familial intrahepatic cholestasis, that lacks homology with CFTR (49). Therefore, we can argue that the positive effects exerted by certain CFTR correctors on α-SG is mainly due to a general mechanism, that could modulate the activity, composition or concentration of elements of the proteostasis network. Only VX809 was totally ineffective on α-SG mutants. However, this is in accordance with the high specificity of VX809 for CFTR (47), and ABC proteins sharing common motifs (25), and indeed this small molecule, in combination with Kalydeco (ivacaftor), has been approved by the U.S. FDA as oral treatment for CF since 2015.
Several CFTR correctors listed in Table 2 would deserve further investigation, in particular by testing different concentrations and, above all, the membrane-localization of rescued mutants. The βγδ-cells transfected with any possible α-SG mutant are a valuable tool to design such experiments, as well as to test additional compounds with improved potency such as, but not limited to, other derivatives of the bithiazole corrector C4 (52). In βγδ-cells transfected with R77C-α-SG we also checked the co-administration of corrector C17 with either C4 or C5. The experience in CF teaches that some promising correctors resulted ineffective in clinical trial when applied individually (53), fostering the research toward the combined use of such compounds. Indeed, the co-administration of C17 + C5 or C17 + C4 resulted in a more effective rescue of the α-SG mutant at the plasma membrane compared with that of either compound applied individually. While the activity of C17 + C5 seems simply additive, that of C17 + C4 suggests a possible synergic mechanism and further work will clarify this point. However, from these data we can suppose that these correctors, even those belonging to the same chemical family (C4 and C17 are two bithiazole derivatives), modulated either different and maybe complementary pathways, or sequential steps of the same pathway during folding and/or trafficking of α-SG toward the plasma membrane. This is remarkable for a future therapy for sarcoglycanopathy, as would permit to treat a wide spectrum of mutations possibly addressing problems distributed along the entire maturation process of sarcoglycans.
We also showed that in vitro protein recovery of α-SG mutants was dependent from the dose of the CFTR corrector used (C5, C6 and C17) and that the toxicity on proliferating cells became evident only at very high concentrations. Only corrector C17 showed a very narrow efficacy window (1–8 µM) probably because of a cytostatic effect on proliferating cells exerted at those concentrations. In support of this, there is the observation that no distress sign was evidenced when prolonged treatment and high concentration of C17 were applied on differentiated, non-dividing, LGMD2D myotubes. We are aware that these features are acceptable only for a proof of concept study; however, C17 could represent a lead compound, suitable for efficacy and safety improvement by chemical derivatization.
Even though the data of rescue in cellular models are extremely promising, significantly we validated and reinforced those observations with the results obtained in primary myogenic cells derived from a subject suffering from LGMD2D. Indeed, in patient’s myotubes the incubation with C17, C5 or C6 effectively rescued at the sarcolemma the whole SG-complex. The complex, although containing a mutated subunit, was functional as its presence strengthened the sarcolemma, reducing the release of the cytosolic CK protein, under stressful condition.
Experiments with myotubes allowed also to address a few initial issues concerning the mechanism of action of correctors on α-SG. Indeed, our data indicate that corrector C17 had an effect of stabilization on the mutated forms of α-SG only, and this took place without affecting the transcription of the gene. Similar evidence was recorded with βγδ-cells also with correctors C4, C5 and C9. Consequently, we suppose that, acting probably as proteostasis modulators, CFTR correctors help the processes of maturation of α-SG mutants, eventually changing the balance of the biosynthetic/degradative pathways and fostering the assembly with the endogenous partners into a tetramer competent to traffic toward the sarcolemma.
Our strategy is specifically addressing the forms of LGMD2D due to the presence of a missense mutation in the SGCA gene. More than 65% of α-SG defects are missense mutations [http://www.dmd.nl] (36) potentially recoverable helping their maturation by corrector treatments. Moreover, many LGMD2D patients are composite heterozygotes, carrying different mutations on the two SGCA alleles, and this extends the number of subjects that could benefit from the treatment. Indeed, the recovery of just one allele is predictably sufficient to ameliorate the patient’s conditions.
At present it is unknown the amount of sarcoglycan proteins required to reverse the disease phenotype. Surely, 50% of expression guarantees a healthy phenotype, being LGMD2D a recessive disease (1). On the other hand, it is important to remind that disease severity is inversely related to the level of SGs present at the sarcolemma (54–56). Therefore, even a small rescue of SG-complex is expected to ameliorate the patient’s conditions. In this framework, the reduced release of CK from pathologic myotubes treated with C17 is significant. Indeed, this is the first observation of a functional rescue of the SG-complex in a human LGMD2D sample upon a pharmacological treatment in vitro.
Once at the sarcolemma, a critical issue is the stability of the recovered complex. The presence of a defective α-SG should lead to a rapid recycling of the tetramer from the sarcolemma, and further work will clarify this point. However, we measured a reduction of the sarcolemma fragility of the LGMD2D myotubes upon corrector treatments, meaning that the SG-complex is sufficiently stable to ameliorate the disease phenotype. We believe that the main issue to overcome in sarcoglycanopathy is to skip mutant degradation, and CFTR correctors, by helping mutant folding, seem to accomplish this task. Then, once assembly and traffic of the tetramer occurred, we expect the interactions among SG-subunits and between SG-complex and elements of the DAPC (33,34) guarantee an adequate structural stability.
Several points need deepening before compounds such as C17, C5, C6, or possibly their derivatives, become actual drugs to treat LGMD2D. Nevertheless, our study represents the proof of principle of a novel pharmacological strategy applicable to a large cohort of patients with LGMD2D. Moreover, we speculate that the same approach is effective with patients suffering from LGMD2C, 2E and 2F, since they share a similar pathogenic mechanism (36). On the other hand, this study, together with other work (48,49), suggests that CFTR correctors could be a significant pharmacological option for many orphan diseases, barely considered by pharmaceutical companies, but with an unmet need to find a cure to ameliorate life condition and life expectancy of patients.
Materials and Methods
Chemicals and treatments
Cycloheximide, glafenine and MG132 were from Sigma-Aldrich, VX809 and VX770 were from Selleck Chemicals, C2, C3, C4, C9 and C17 were a kind gift of the Cystic Fibrosis Foundation, C4, C5, C6, C9, C13, C14 and C15 were from Exclusive Chemistry. All compounds were dissolved in DMSO and the working solution prepared 1000X to have the same content of vehicle (1‰) in each treatment.
Plasmids, cell culture, transfection, and treatments
The full-length cDNA encoding human α-sarcoglycan cloned in the pcDNA3 mammalian expression vector was previously described (57). Plasmids expressing missense mutants of α-sarcoglycan were previously described (6,7).
HEK-293, V247M cells (8), HER-911 and βγδ-HER were grown in Dulbecco’s modified Eagle’s medium (Sigma) supplemented with 10% fetal bovine serum (FBS) (Gibco) and maintained in a humidified atmosphere containing 5% CO2 at 37°C.
Immortalized human myoblasts (58) were from the ‘Human cell culture platform’ of the Myology Institute in Paris. Primary human myogenic cells from an LGMD2D patient were isolated from a bioptic fragment from the Telethon Genetic Bio-Bank facility (8). Myogenic cells were grown in Skeletal Muscle Cell Growth Medium (Promocell) supplemented with 15% FBS (Gibco), named skeletal growth medium (SGM). To start differentiation, myoblasts, grown at confluence, were incubated with DMEM supplemented with 2% Horse Serum (Euroclone), 10 µg/ml human recombinant insulin (Sigma), 100 µg/ml human Apotransferrin (Sigma-Aldrich), named skeletal differentiating medium (SDM). Differentiation was carried out for seven days.
For transient expression, HEK 293 cells were seeded at 50 000 cells/cm2 and transfected the day after with TransIT-293 (MirusBio) according to manufacturer’s instruction. Twenty-four hours after transfection, medium was replaced with DMEM supplemented with 2% FBS containing the indicated concentration of correctors (dissolved in DMSO) or with DMSO alone (final DMSO concentration 1‰).
HER911 or βγδ-cells cells were seeded at 50 000 cells/cm2 and transfected the day after with 9 μl of Fugene HD Transfection Reagent (E2312 from Promega) and 3 μg of plasmid coding for wild type or mutated α-SG.
MG132 was added 8 h before cell lysis, CFTR correctors were added 24 h before cell lysis or IF assay (transfected HEK293, V247M cells, HER911 and βγδ-cells) and 24–96 h before myotubes lysis, treatment with CHX or IF assay.
After treatments, cells were washed twice with ice cold PBS and lysed with 5% sodium deoxycholate supplemented with complete protease inhibitor (Sigma-Aldrich).
Establishment of a HER911 cell line stably expressing β-, γ -, and δ-sarcoglycan
The stably expressing βγδ-SG clonal cell line was obtained by integration of β-SG, γ-SG, and δ-SG cDNA using lentiviral vectors in HER911 cells. The day following plating cells in 6-well plates, an appropriate volume of lentiviral vector-expressing β-, γ- an δ-SG was added directly into each well containing 1 ml of culture medium, to infect the cells at 100 Multiplicity of Infection (MOI). After 3 h of incubation at 37°C, 1 ml of culture medium was added into each well and cells were incubated at 37°C for an additional 48 h. Each well was subcultured again in 6-well plates and the transduction was repeated two more times as explained above. After three transductions, cells were sub-cultured in a T-75 culture flask and were maintained at 37°C until 100% of confluence. Then, cells were collected, centrifuged, and resuspended in culture medium at a density of 5 × 105 cells/ml. To obtain clonal cell lines, single cells were sorted by Astrios Beckman Coulter (Brea, CA) from the cell suspension and seeded in 96-well plates in culture medium. After 10 days, a total number of 30 clonal cell lines were selected and subcultured in larger culture plates. All 30 clones were subjected to qPCR analysis to determine the copy-number/genome of each SG and to RT-qPCR analysis to monitor the expression of SGs. One positive SG expressing cell clone was chosen for the next step.
DNA and RNA extraction, qPCR and RT-qPCR from HER911 cells
Genomic DNA (gDNA) and total RNA were extracted using TRIzol reagent (Thermo Fisher Sci.) following the manufacturer’s protocol. Total RNA was used as a template for reverse transcription using the MultiScribe RT cDNA kit (Life technologies) according to manufacturer’s protocol. gDNA or cDNA from each clone were used as template for qPCR to determine SG-cDNA copy number/genome or sarcoglycan expression, respectively. Amplification was carried out using the ABsoluteQPCR ROXMix (Thermo Fisher Sci.) and the following primers and Taqman probes to β-SG (Forward: 5-CCCGCCTACCCAGTTCCT-3; Reverse: 5-TGTAGCGTACCCAGTCACCACTA-3; Probe: 5-CAGTGGAGACCAGTTGG-3), γ-SG (Forward: 5-AAGTCGGTCCCAAAATGGTAGA-3; Reverse: 5-TGCCGTCGTTGGAGTTGA-3; Probe: 5-CAGAATCAACAGTTTCAG-3), δ-SG (Forward: 5-TGCCTCAGGAGCAGTACACTCA-3; Reverse: 5-CCATAAATCCCCACCTTGTATACC-3; Probe: 5-ACCGGAGCACCATGCCTGGCT-3). The albumin gene was amplified to normalize the results of copy number/genome of SG-cDNAs, whereas the ubiquitous acidic ribosomal phosphoprotein (P0) was amplified to normalize the result of SGs expression. All PCR reactions were performed in duplicate and each quantification repeated three times.
RNA extraction from LGMD2D myotubes and RT-qPCR
Total RNA from differentiated myotubes, treated for 48h with 0.1‰ DMSO or C17 15 µM, was extracted with TRIsure reagent (Bioline) according to manufacturer’s instructions, and further purified with RNA Clean Up columns (Zymo research). 1 µg of total RNA was reverse transcribed using the SensiFast cDNA synthesis kit (Bioline). Cyclophilin A (PPIA), β-2-microglobulin (B2M), and ribosomal protein L32 (RPL32) were used as reference genes. qPCR was performed in triplicate in a IQ5 Thermal Cycler (Bio-Rad, Hercules, CA, USA) using Biorad iQ Sybr green supermix. The PCR parameters were: initial denaturation at 95°C for 3 min followed by 40 cycles of 10 s at 95°C and 30 s at 55°C for acquisition of fluorescence signal. A melting curve was generated by the iQ5 software following the end of the final cycle for each sample to confirm the specificity of the amplified product. The efficiency of each run was determined by a standard curve and used for calculations. Normalization was performed by geNorm software (https://genorm.cmgg.be/).
Primer sequences were as follows: α-SG forward primer, 5’- CCCCAGACCGTGACTTCTTG-3’, reverse primer 5’- TCTCTTCAGCCTTCCCTCCC-3’; PPIA forward primer, 5′-TTCATCTGCACTGCCAAGA-3′, reverse primer, 5′-CGAGTTGTCCACAGTCAGC-3′; B2M forward primer, 5′-TATCCAGCGTACTCCAAGA-3′, reverse primer, 5′-GACAAGTCTGAATGCTCCAC-3′; RPL32 forward primer, 5′-CATCTCCTTCTCGGCATC-3′, reverse primer, 5′-CTGGGTTTCCGCCAGTTA-3′.
Data are expressed as means ± SE of three experiments. Comparisons were made using the t test, with values of P < 0.05 considered statistically significant.
Cycloheximide treatments
LGMD2D myotubes treated for 96h with DMSO or C17 15 µM were incubated with cycloheximide 100 µg/ml for 0, 6, 12 and 24 h. At each time point, cells were lysed with 5% sodium deoxycholate supplemented with complete protease inhibitor and the lysates were stored at -80°C before immunoblot analysis. The intensity of the α-SG signals was normalized to total protein loading, assessed by Ponceau Red staining. Each experiment was performed in triplicate.
Immunoblot analysis
Proteins were quantified by the bicinchoninic-acid protein assay kit (Thermo Scientific), according to manufacturer’s instructions. Proteins were resolved by SDS-PAGE, blotted onto a nitrocellulose membrane and probed with selected antibodies (see below). Secondary antibody was horseradish peroxidase-labeled goat anti-mouse IgG (Sigma-Aldrich). Blots were developed with ECL chemiluminescent substrate (Euroclone), and chemiluminescent signals were digitally acquired with a Chemidoc Imaging System (Biorad). In alternative, total proteins lysates were resolved on NuPAGE® Novex® 4–12% Bis-Tris Protein Gels (Thermo Fisher Scientific) and transferred to nitrocellulose membranes (iBlot, Thermo Fisher Scientific) following the manufacturer’s instructions. Membranes were blocked in Odyssey Blocking Buffer (Li-Cor) for 1 h at room temperature. Incubations with primary antibodies were carried out at 4°C overnight in Odyssey Blocking Buffer. After 1-h incubation with donkey anti-rabbit IRDye680 and anti-mouse IRDye800 antibodies (EuroBio) at room temperature, proteins were detected by fluorescence in a Odissey imaging system (Li-Cor) following the manufacturer’s instructions.
Densitometry was performed with the ImageJ software. The intensities of sarcoglycan bands were normalized for the intensity of β-actin or α-actinin.
Cell viability and cytotoxicity assay
Cells were seeded in a 96-multiwell at a density of 10 000 cells per well. After 24 h, correctors dissolved in 1‰ DMSO were added at the indicated concentrations. After 24 h of treatment, cell viability was determined measuring the bioreduction of MTS (Owen’s reagent) by living cells with the Cell Titer 96 Aqueous proliferation assay (Promega) according to the manufacturer’s instruction. Cell toxicity was determined measuring the release of LDH in culture supernatant with the Cytotox 96 cytotoxicity assay (Promega) according to the manufacturer’s instruction. A maximum LDH release control was performed adding a lysis solution 45 min before adding Cytotox 96 reagent, and LDH release was calculated as (experimental LDH release-blank)/(maximum LDH release-blank)×100.
All spectrophotometric measurements were performed with a Novapath Microplate Reader (Bio-Rad). All experiments were run in sextuplicate.
Hypo-osmotic stress and CK release assay
Myotubes differentiated for 7 days and treated for 96 h with 1‰ DMSO or C17 15 µM were incubated with two hypo-osmotic solutions (260 and 200 mOsm) for 20 min at 37°C. Hypo-osmotic solutions consisted of a salt solution (5 mM HEPES, 5 mM KCl, 1 mM MgCl2, 5 mM NaCl, 1.2 mM CaCl2, 1mM glucose) supplemented with 157 mM sucrose (200 mOsm) or 213 mM sucrose (260 mOsm). Osmolarities were verified with a OM 801 osmometer (Vogel). After the treatment, the supernatant containing the released CK was removed and an equal volume of ice-cold hypo-osmotic solution was added to the cells. The cells were recovered by scraping and lysed by three cycles of freeze-thawing. Released and intra-cellular Creatine Kinase were measured in sextuplicate using the Creatine Kinase Activity Colorimetric Assay Kit (BioVision) according to the manufacturer’s instructions.
Confocal immunofluorescence
Immunofluorescence-confocal analyses were performed either in intact cells (not permeabilized) or in permeabilized cells. For the former condition, cells, grown on polylysine-treated glasses, at the end of treatments were incubated for 30 min at 4°C, then gently washed twice with ice-cold PBS and incubated with primary antibodies for 5 h at 4°C. After three gentle washings with ice-cold PBS, cells were incubated with fluorescently labeled secondary antibodies for 2 h at 4°C. Primary and secondary antibodies were diluted in PBS supplemented with 0.5% BSA. After secondary antibody incubation, cells were washed with PBS and then fixed for 15 min with 4% paraformaldehyde in PBS (PFA). After incubation with 50 mmol/L NH4Cl for 15 min and washing with PBS, nuclei were stained with Hoechst or DAPI. For analysis in permeabilized cells, cells grown and treated as above, were washed with PBS, fixed for 15 min in PBS 3.7% formaldehyde (Sigma-Aldrich) at room temperature. Slides were rinsed in PBS and permeabilized with PBS 0.5% Triton X-100 (Sigma-Aldrich) for 5 min and then blocked for 30 min with PBS containing 10% SVF to prevent non-specific staining. Incubation with primary and secondary antibody was performed as above described. Cells were examined with a Leica SP5 confocal laser scanning microscope.
Quantification of the mean fluorescence intensity of membrane staining in βγδ-cells transfected with R77C-α-SG was performed by using ImageXpress microscope system (Molecular Devices). Normalization was performed by counting the number of cells positive for both DAPI and α-SG in permeabilization conditions to consider possible differences in transfection efficiency.
Antibodies
Mouse monoclonal antibody specific for α-SG (NCL-a-SARC) was from Leica Biosystem; rabbit monoclonal anti α-SG (AB189254) was from Abcam; mouse monoclonal antibody specific for β-SG, δ-SG, γ-SG and β-actin were from Sigma, rabbit polyclonal antibody specific for α-and δ-SG were produced as previously described (8), rabbit polyclonal antibody specific for α-actinin was from Santa Cruz. Alexa fluor 488, Alexa fluor 594- and DyLight 488-conjugated goat anti-mouse and goat anti-rabbit were from Life Technologies.
Statistical analysis
Data are expressed as means ± SEM. Statistical differences among groups were determined by One-way ANOVA test, followed by either Dunnett test for simultaneous multiple comparisons with control, or Bonferroni test for simultaneous comparisons of all possible contrasts (pairs). When only two groups were considered, statistical analysis was performed by the unpaired two-tailed Student’s t-test. A level of confidence of P < 0.05 was used for statistical significance.
Supplementary Material
Supplementary Material is available at HMG online.
Supplementary Material
Acknowledgements
Giulia Rossetto is kindly thanked for her precious help with primary myogenic cells. Cécile Patissier and Marine Faivre from Genethon INTEGRARE, Evry, are gratefully thanked for their work in generation and characterization of the βγδ-cells; Anne-Laure Egesipe and Julie Pernelle, from Istem, AFM Telethon, are gratefully thanked for the valuable help in setup of cell surface fluorescence quantification by ImageXpress. Vincent Mouly and the ‘Human cell culture platform’ of the Myology Institute Université Pierre et Marie Curie, Paris 6 are gratefully thanked for providing immortalized human myoblasts derived from a healthy subject. Elena Pegoraro and the Telethon Genetic Bio-Bank facility, University of Padova are gratefully thanked for providing the skeletal muscle biopsy of a LGMD2D patient. A special acknowledgement to the Cystic Fibrosis Foundation for providing the first stock of CFTR correctors.
Conflict of Interest statement. None declared.
Funding
Association Françoise contre les Myopathies (18620 to D.S. and I.R.), Italian Telethon Foundation (GEP12058 and GGP15140 to D.S.), University of Padova (CPDA149821/14 to D.S.). Funding to pay the Open Access publication charges for this article was provided by Italian Telethon Foundation.
References
- 1. Nigro V., Savarese M. (2014) Genetic basis of limb-girdle muscular dystrophies: the 2014 update. Acta Myol., 33, 1–12. [PMC free article] [PubMed] [Google Scholar]
- 2. Tarakci H., Berger J. (2016) The sarcoglycan complex in skeletal muscle. Front. Biosci. (Landmark Ed.), 21, 744–756. [DOI] [PubMed] [Google Scholar]
- 3. Sandonà D., Betto R. (2009) Sarcoglycanopathies: molecular pathogenesis and therapeutic prospects. Expert Rev. Mol. Med., 11, e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kirschner J., Lochmüller H. (2011) Sarcoglycanopathies. Handb. Clin. Neurol., 101, 141. [DOI] [PubMed] [Google Scholar]
- 5. Bartoli M., Gicquel E., Barrault L., Soheili T., Malissen M., Malissen B., Vincent-Lacaze N., Perez N., Udd B., Danos O., Richard I. (2008) Mannosidase I inhibition rescues the human alpha-sarcoglycan R77C recurrent mutation. Hum. Mol. Genet., 17, 1214–1221. [DOI] [PubMed] [Google Scholar]
- 6. Gastaldello S., D'Angelo S., Franzoso S., Fanin M., Angelini C., Betto R., Sandonà D. (2008) Inhibition of proteasome activity promotes the correct localization of disease-causing alpha-sarcoglycan mutants in HEK-293 cells constitutively expressing beta-, gamma-, and delta-sarcoglycan. Am. J. Pathol., 173, 170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Soheili T., Gicquel E., Poupiot J., N'Guyen L., Le Roy F., Bartoli M., Richard I. (2012) Rescue of sarcoglycan mutations by inhibition of endoplasmic reticulum quality control is associated with minimal structural modifications. Hum. Mutat., 33, 429–439. [DOI] [PubMed] [Google Scholar]
- 8. Bianchini E., Fanin M., Mamchaoui K., Betto R., Sandona D. (2014) Unveiling the degradative route of the V247M α-sarcoglycan mutant responsible for LGMD-2D. Hum. Mol. Genet., 23, 3746–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Denny R.A., Gavrin L.K., Saiah E. (2013) Recent developments in targeting protein misfolding diseases. Bioorg. Med. Chem. Lett., 23, 1935–1944. [DOI] [PubMed] [Google Scholar]
- 10. Valastyan J.S., Lindquist S. (2014) Mechanisms of protein-folding diseases at a glance. Dis. Model.Mech., 7, 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Y.J., Di X.J., Mu T.W. (2014) Using pharmacological chaperones to restore proteostasis. Pharmacol. Res., 83, 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Powers E.T., Morimoto R.I., Dillin A., Kelly J.W., Balch W.E. (2009) Biological and chemical approaches to diseases of proteostasis deficiency. Annu. Rev. Biochem., 78, 959–991. [DOI] [PubMed] [Google Scholar]
- 13. Chaudhuri T.K., Paul S. (2006) Protein-misfolding diseases and chaperone-based therapeutic approaches. febs J., 273, 1331–1349. [DOI] [PubMed] [Google Scholar]
- 14. Leidenheimer N.J., Ryder K.G. (2014) Pharmacological chaperoning: a primer on mechanism and pharmacology. Pharmacol. Res., 83, 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pedemonte N., Lukacs G.L., Du K., Caci E., Zegarra-Moran O., Galietta L.J., Verkman A.S. (2005) Small-molecule correctors of defective DeltaF508-CFTR cellular processing identified by high-throughput screening. J. Clin. Invest., 115, 2564–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cai Z., Liu J., Li H., Sheppard D.N. (2011) Targeting F508del-CFTR to develop rational new therapies for cystic fibrosis. Acta Pharmacol. Sin., 32, 693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Birault V., Solari R., Hanrahan J., Thomas D.Y. (2013) Correctors of the basic trafficking defect of the mutant F508del-CFTR that causes cystic fibrosis. Curr. Opin. Chem. Biol., 17, 353–360. [DOI] [PubMed] [Google Scholar]
- 18. Bell S.C., De Boeck K., Amaral M.D. (2015) New pharmacological approaches for cystic fibrosis: promises, progress, pitfalls. Pharmacol. Ther., 145, 19–34. [DOI] [PubMed] [Google Scholar]
- 19. Rowe S.M., Verkman A.S. (2013) Cystic fibrosis transmembrane regulator correctors and potentiators. Cold Spring Harb. Perspect. Med., 3, a009761. pii: a009761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Robert R., Carlile G.W., Pavel C., Liu N., Anjos S.M., Liao J., Luo Y., Zhang D., Thomas D.Y., Hanrahan J.W. (2008) Structural analog of sildenafil identified as a novel corrector of the F508del-CFTR trafficking defect. Mol. Pharmacol., 7, 478–489. [DOI] [PubMed] [Google Scholar]
- 21. Yoo C.L., Yu G.J., Yang B., Robins L.I., Verkman A.S., Kurth M.J. (2008) Methyl-4, 5'-bithiazole-based correctors of defective delta F508-CFTR cellular processing. Bioorg. Med. Chem. Lett., 18, 2610–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Robert R., Carlile G.W., Liao J., Balghi H., Lesimple P., Liu N., Kus B., Rotin D., Wilke M., de Jonge H.R.. et al. (2010) Correction of the Delta phe508 cystic fibrosis transmembrane conductance regulator trafficking defect by the bioavailable compound glafenine. Mol. Pharmacol., 77, 922–930. [DOI] [PubMed] [Google Scholar]
- 23. Loo T.W., Bartlett M.C., Wang Y., Clarke D.M. (2006) The chemical chaperone CFcor-325 repairs folding defects in the transmembrane domains of CFTR-processing mutants. Biochem. J., 395, 537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Loo T.W., Bartlett M.C., Clarke D.M. (2013) Corrector VX-809 stabilizes the first transmembrane domain of CFTR. Biochem. Pharmacol., 86, 612–619. [DOI] [PubMed] [Google Scholar]
- 25. Sabirzhanova I., Lopes Pacheco M., Rapino D., Grover R., Handa J.T., Guggino W.B., Cebotaru L. (2015) Rescuing trafficking mutants of the ATP-binding Cassette Protein, ABCA4, with small molecule correctors as a treatment for Stargardt eye disease. J. Biol. Chem., 290, 19743–19755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Van Goor F., Hadida S., Grootenhuis P.D., Burton B., Cao D., Neuberger T., Turnbull A., Singh A., Joubran J., Hazlewood A. (2009) Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc. Natl. Acad. Sci. U S A, 106, 18825–18830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wainwright C.E., Elborn J.S., Ramsey B.W., Marigowda G., Huang X., Cipolli M., Colombo C., Davies J.C., De Boeck K., Flume P.A., TRAFFIC Study Group; TRANSPORT Study Group. et al. (2015) Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N. Engl. J. Med., 373, 220–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ginsberg F., Bourguignon R.P., Smets P., Famaey J.P. (1983) Tiapride versus glafenine: a double-blind comparative study in the management of acute rheumatic pain. Curr. Med. Res. Opin., 8, 562–569. [DOI] [PubMed] [Google Scholar]
- 29. Draviam R.A., Wang B., Shand S.H., Xiao X., Watkins S.C. (2006a) Alpha-sarcoglycan is recycled from the plasma membrane in the absence of sarcoglycan complex assembly. Traffic, 7, 793–810. [DOI] [PubMed] [Google Scholar]
- 30. Carrié A., Piccolo F., Leturcq F., de Toma C., Azibi K., Beldjord C., Vallat J.M., Merlini L., Voit T., Sewry C.. et al. (1997) Mutational diversity and hot spots in the alpha-sarcoglycan gene in autosomal recessive muscular dystrophy (LGMD2D). J. Med. Genet., 34, 470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duggan D.J., Gorospe J.R., Fanin M., Hoffman E.P., Angelini C. (1997) Mutations in the sarcoglycan genes in patients with myopathy. N. Engl. J. Med., 336, 618–624. [DOI] [PubMed] [Google Scholar]
- 32. Shi W., Chen Z., Schottenfeld J., Stahl R.C., Kunkel L.M., Chan Y.-M. (2004) Specific assembly pathway of sarcoglycans is dependent on beta- and delta-sarcoglycan. Muscle Nerve, 29, 409–419. [DOI] [PubMed] [Google Scholar]
- 33. Draviam R.A., Shand S.H., Watkins S.C. (2006b) The beta-delta-core of sarcoglycan is essential for deposition at the plasma membrane. Muscle Nerve, 34, 691–701. [DOI] [PubMed] [Google Scholar]
- 34. Allikian M.J., McNally E.M. (2007) Processing and assembly of the dystrophin glycoprotein complex. Traffic, 8, 177–183. [DOI] [PubMed] [Google Scholar]
- 35. Straub V., Campbell K.P. (1997) Muscular dystrophies and the dystrophin-glycoprotein complex. Curr. Opin. Neurol., 10, 168–175. [DOI] [PubMed] [Google Scholar]
- 36. Carotti M., Fecchio C., Sandonà D. (2017) Emerging therapeutic strategies for sarcoglycanopathy. Exp. Opin. Orphan Drugs, 5, 381–396. [Google Scholar]
- 37. Bushby K. (2009) Diagnosis and management of the limb girdle muscular dystrophies. Pract. Neurol., 9, 314–323. [DOI] [PubMed] [Google Scholar]
- 38. Nigro V., Piluso G. (2015) Spectrum of muscular dystrophies associated with sarcolemmal-protein genetic defects. Biochim. et Biophys. Acta, 1852, 585–593. [DOI] [PubMed] [Google Scholar]
- 39. Menke A., Jockusch H. (1995) Extent of shock-induced membrane leakage in human and mouse myotubes depends on dystrophin. J. Cell Sci., 108, 727–733. [DOI] [PubMed] [Google Scholar]
- 40. Young C.S., Hicks Michael R., Ermolova Natalia V., Nakano Haruko., Jan Majib., Younesi Shahab., Karumbayaram Saravanan., Kumagai-Cresse Chino., Wang Derek., Zack Jerome A.. et al. (2016) A single CRISPR-Cas9 deletion strategy that targets the majority of DMD patients restores dystrophin function in hiPSC-derived muscle cells. Cell Stem Cell, 18, 533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Noack J., Brambilla Pisoni G., Molinari M. (2014) Proteostasis: bad news and good news from the endoplasmic reticulum. Swiss Med. Wkly, 144, w14001. [DOI] [PubMed] [Google Scholar]
- 42. Balch W.E., Morimoto R.I., Dillin A., Kelly J.W. (2008) Adapting proteostasis for disease intervention. Science, 319, 916–919. [DOI] [PubMed] [Google Scholar]
- 43. Dickens N.J., Beatson S., Ponting C.P. (2002) Cadherin-like domains in alpha-dystroglycan, alpha/epsilon-sarcoglycan and yeast and bacterial proteins. Curr. Biol., 12, R197–R199. [DOI] [PubMed] [Google Scholar]
- 44. Grove D.E., Rosser M.F., Ren H.Y., Naren A.P., Cyr D.M. (2009) Mechanisms for rescue of correctable folding defects in CFTR Delta F508. Mol. Biol. Cell, 20, 4059–4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Caldwell R.A., Grove D.E., Houck S.A., Cyr D.M. (2011) Increased folding and channel activity of a rare cystic fibrosis mutant with CFTR modulators. Am. J. Physiol. Lung Cell Mol. Physiol., 301, L346–L352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang Y., Bartlett M.C., Loo T.W., Clarke D.M. (2006) Specific rescue of cystic fibrosis transmembrane conductance regulator processing mutants using pharmacological chaperones. Mol. Pharmacol., 70, 297–302. [DOI] [PubMed] [Google Scholar]
- 47. Van Goor F., Hadida S., Grootenhuis P.D., Burton B., Stack J.H., Straley K.S., Decker C.J., Miller M., McCartney J., Olson E.R.. et al. (2011) Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc. Natl. Acad. Sci. U S A, 108, 18843–18848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sampson H.M., Lam H., Chen P.C., Zhang D., Mottillo C., Mirza M., Qasim K., Shrier A., Shyng S.L., Hanrahan J.W., Thomas D.Y. (2013) Compounds that correct F508del-CFTR trafficking can also correct other protein trafficking diseases: an in vitro study using cell lines. Orphanet. J. Rare Dis., 8, 11.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. van der Woerd W.L., Wichers C.G., Vestergaard A.L., Andersen J.P., Paulusma C.C., Houwen R.H., van de Graaf S.F. (2016) Rescue of defective ATP8B1 trafficking by CFTR correctors as a therapeutic strategy for familial intrahepatic cholestasis. J. Hepatol., 64, 1339–1347. [DOI] [PubMed] [Google Scholar]
- 50. Jurkuvenaite A., Chen L., Bartoszewski R., Goldstein R., Bebok Z., Matalon S., Collawn J.F. (2010) Functional stability of rescued delta F508 cystic fibrosis transmembrane conductance regulator in airway epithelial cells. Am. J. Respir. Cell. Mol. Biol., 42, 363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Loo T.W., Bartlett M.C., Shi L., Clarke D.M. (2012) Corrector-mediated rescue of misprocessed CFTR mutants can be reduced by the P-glycoprotein drug pump. Biochem. Pharmacol., 83, 345–354. [DOI] [PubMed] [Google Scholar]
- 52. Ye L., Hu B., El-Badri F., Hudson B.M., Phuan P.W., Verkman A.S., Tantillo D.J., Kurth M.J. (2014) ΔF508-CFTR correctors: synthesis and evaluation of thiazole-tethered imidazolones, oxazoles, oxadiazoles, and thiadiazoles. Bioorg. Med. Chem. Lett., 24, 5840–5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Clancy J.P., Rowe S.M., Accurso F.J., Aitken M.L., Amin R.S., Ashlock M.A., Ballmann M., Boyle M.P., Bronsveld I.nez., Campbell P.W.. et al. (2012) Results of a phase IIa study of VX-809, an investigational CFTR corrector compound, in subjects with cystic fibrosis homozygous for the F508del-CFTR mutation. Thorax, 67, 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Barresi R., Confalonieri V., Lanfossi M., Di Blasi C., Torchiana E., Mantegazza R., Jarre L., Nardocci N., Boffi P., Tezzon F.. et al. (1997) Concomitant deficiency of beta- and gamma-sarcoglycans in 20 alpha-sarcoglycan (adhalin)-deficient patients: immunohistochemical analysis and clinical aspects. Acta Neuropathol., 94, 28–35. [DOI] [PubMed] [Google Scholar]
- 55. Vainzof M., Passos-Bueno M.R., Pavanello R.C., Marie S.K., Oliveira A.S., Zatz M. (1999) Sarcoglycanopathies are responsible for 68% of severe autosomal recessive limb-girdle muscular dystrophy in the Brazilian population. Neurol. Sci., 164, 44–49. [DOI] [PubMed] [Google Scholar]
- 56. Boito C., Fanin M., Siciliano G., Angelini C., Pegoraro E. (2003) Novel sarcoglycan gene mutations in a large cohort of Italian patients. J. Med. Genet., 40, e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sandona D., Gastaldello S., Martinello T., Betto R. (2004) Characterization of the ATP-hydrolysing activity of alpha-sarcoglycan. Biochem. J., 381, 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhu C.H., Mouly V., Cooper R.N., Mamchaoui K., Bigot A., Shay J.W., Di Santo J.P., Butler-Browne G.S., Wright W.E. (2007) Cellular senescence in human myoblasts is overcome by human telomerase reverse transcriptase and cyclin-dependent kinase 4: consequences in aging muscle and therapeutic strategies for muscular dystrophies. Aging Cell, 6, 515–523. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.