Abstract
Glycogen storage disease type-Ib (GSD-Ib), deficient in the glucose-6-phosphate transporter (G6PT), is characterized by impaired glucose homeostasis, myeloid dysfunction, and long-term risk of hepatocellular adenoma (HCA). We examined the efficacy of G6PT gene therapy in G6pt−/− mice using recombinant adeno-associated virus (rAAV) vectors, directed by either the G6PC or the G6PT promoter/enhancer. Both vectors corrected hepatic G6PT deficiency in murine GSD-Ib but the G6PC promoter/enhancer was more efficacious. Over a 78-week study, using dose titration of the rAAV vectors, we showed that G6pt−/− mice expressing 3–62% of normal hepatic G6PT activity exhibited a normalized liver phenotype. Two of the 12 mice expressing < 6% of normal hepatic G6PT activity developed HCA. All treated mice were leaner and more sensitive to insulin than wild-type mice. Mice expressing 3–22% of normal hepatic G6PT activity exhibited higher insulin sensitivity than mice expressing 44–62%. The levels of insulin sensitivity correlated with the magnitudes of hepatic carbohydrate response element binding protein signaling activation. In summary, we established the threshold of hepatic G6PT activity required to prevent tumor formation and showed that mice expressing 3–62% of normal hepatic G6PT activity maintained glucose homeostasis and were protected against age-related obesity and insulin resistance.
Introduction
Glycogen storage disease type Ib (GSD-Ib, MIM232220) is caused by a deficiency in the ubiquitously expressed glucose-6-phosphate (G6P) transporter (G6PT or SLC37A4) that translocates G6P from the cytoplasm into the lumen of the endoplasmic reticulum (ER) (1,2). Inside the ER, G6P is hydrolyzed to glucose and phosphate by either the liver/kidney/intestine-restricted glucose-6-phosphatase-α (G6Pase-α or G6PC) or the ubiquitously expressed G6Pase-β. The G6PT and G6Pase are functionally co-dependent and form the G6PT/G6Pase complexes. The G6PT/G6Pase-α complex maintains interprandial blood glucose homeostasis. A deficiency of either protein results in an abnormal metabolic phenotype characterized by fasting hypoglycemia, hepatomegaly, nephromegaly, hyperlipidemia, hyperuricemia, lactic acidemia, and growth retardation (1,2). The G6PT/G6Pase-β complex maintains neutrophil/macrophage homeostasis and function, and a deficiency of either protein results in neutropenia and myeloid dysfunction (1,2). Therefore, GSD-Ib is not only a metabolic but also an immune disorder, characterized by impaired glucose homeostasis, neutropenia, and myeloid dysfunction (1,2). Untreated GSD-Ib is juvenile lethal. Strict compliance with dietary therapies (3,4) along with granulocyte colony-stimulating factor (G-CSF) therapy (5,6) have enabled GSD-Ib patients to attain near normal growth and pubertal development. However, no current therapy is able to address the long-term complication of hepatocellular adenoma (HCA) that develops in 75% of GSD-I patients over 25 years old (1,2,7,8).
We have generated GSD-Ib (G6pt−/−) mice that manifest both the metabolic and myeloid dysfunction characteristic of human GSD-Ib (9). When left untreated, the G6pt−/− mice rarely survive weaning, reflecting the juvenile lethality seen in human patients. We have previously shown that systemic administration of a recombinant adeno-associated virus (rAAV) pseudotype 2/8 vector expressing human (h) G6PT directed by the chicken β-actin (CBA) promoter/CMV enhancer, delivers the hG6PT transgene primarily to the liver (10). In doing so, it normalizes metabolic abnormalities in murine GSD-Ib (10). However, of the five treated G6pt−/− mice that survived for 51–72 weeks, two (40%) developed multiple HCAs with one undergoing malignant transformation.
Studies have shown that the choice of transgene promoter can impact targeting efficiency, tissue-specific expression, and the level of immune response or tolerance to the therapy (11,12). Indeed, for the related disease, GSD-Ia, caused by a deficiency in the G6Pase-α enzyme activity, we have shown that a G6Pase-α-expressing rAAV vector directed by the native 2.8-kb hG6PC promoter/enhancer (GPE) provides sustained correction of metabolic abnormalities in murine GSD-Ia with no evidence of HCA (13,14). Moreover, the gluconeogenic tissue-specific GPE does not elicit the humoral response we observed using the CBA promoter/CMV enhancer (15). For GSD-Ib, we sought to use a similar promoter strategy and compared the hG6PC promoter/enhancer (GPE) with the minimal hG6PT promoter/enhancer (miGT) consisting of nucleotides -610 to -1 upstream of the +1 nucleotide of the G6PT coding sequence (16).
In this study, we examined the safety and efficacy of liver-directed gene therapy in G6pt−/− mice using rAAV-GPE-G6PT and rAAV-miGT-G6PT, rAAV8 vectors directed by the human hG6PC and hG6PT promoter/enhancer, respectively. We also examined the threshold of hepatic G6PT activity required to prevent tumor formation. In a 60–78-week study, we showed that while both vectors delivered the hG6PT transgene to the liver and corrected metabolic abnormalities in murine GSD-Ib, the rAAV-GPE-G6PT vector had greater efficacy. By grouping mice according to levels of hepatic G6PT activity restored, we showed that rAAV-treated G6pt−/− mice expressing 3–62% of normal hepatic G6PT activity maintained glucose homeostasis, tolerated a long fast, and did not elicit anti-hG6PT antibodies. However, G6pt−/− mice with < 6% of normal hepatic G6PT activity restored were at risk of developing hepatic tumors. We also showed that restoration of hepatic G6PT expression up to 62% of wild type activity appeared to confer protection against developing age-related obesity and insulin resistance that is seen in wild-type mice.
Results
rAAV infusion delivers the hG6PT transgene to the liver
GSD-Ib mice suffer from frequent hypoglycemic seizures and despite glucose therapy to control hypoglycemia, less than 10% mice survive past weaning (9). For gene therapy, each vector was administered to G6pt−/− mice in two doses, one neonatal and one at age 4 weeks, to both provide early therapy and to allow for the developmental increase in liver mass. Initially, we used two hG6PT-expressing vectors: rAAV-GPE-G6PT, a single-stranded vector directed by the 2.8-kb hG6PC promoter/enhancer (15,17) and rAAV-GT-G6PT, a single-stranded vector directed by the analogous 1.62 kb hG6PT promoter/enhancer. In contrast to the efficacy seen with rAAV-GPE-G6PT, as described below, rAAV-GT-G6PT infusion failed to sustain the survival of G6pt−/− mice, and only 4 of the 40 infused G6pt−/− mice survived to age 12 weeks. Following further promoter analysis, we constructed an alternative vector construct, rAAV-miGT-G6PT, directed by the 610-bp hG6PT promoter/enhancer, yielding a double-stranded vector to ensure proper packaging of the rAAV virus. It was also anticipated that this vector construct would also benefit from an increased transduction efficiency (18) which arises from bypassing the rate-limiting conversion of single-stranded to double-stranded vector genomes during transduction (19). Preliminary experiments showed that the rAAV-GPE-G6PT vector was still more efficacious than the rAAV-miGT-G6PT vector. Accordingly, in this study, we adjusted the dosages of the two vectors administrated to the G6pt−/− mice to yield comparable levels of restoration of hepatic G6PT activity.
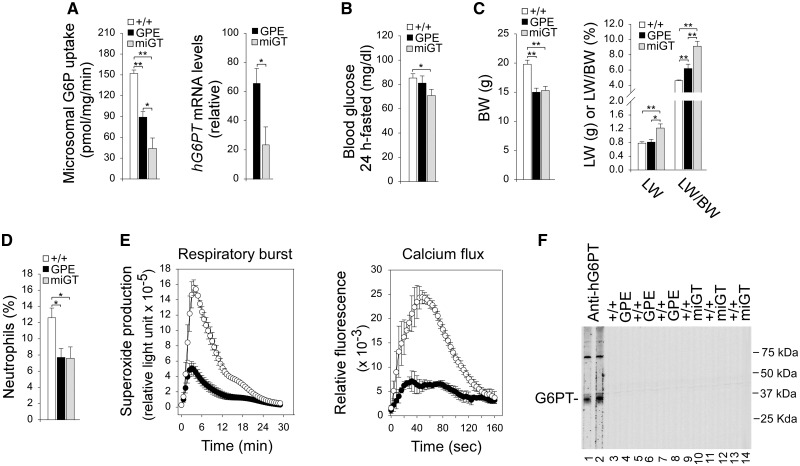
Since the GSD-Ib mice die young, early therapeutic intervention is required. However, because of the vector dilution that occurs during the rapid growth of transduced neonatal liver, two serial doses were required to treat the mice effectively. For rAAV-GPE-G6PT the first, neonatal dose was 0.7 × 1013 viral particles (vp)/kg followed at 4 weeks with a second dose of 2 × 1013 vp/kg. These were called “GPE” mice. For rAAV-miGT-G6PT, the first, neonatal dose was 1.4 × 1013 vp/kg followed at 4 weeks with a second dose of 4 × 1013 vp/kg. These were called “miGT” mice. Both vectors delivered the hG6PT transgene to the liver of G6pt−/− mice and markedly improved their survival. Hepatic microsomes isolated from 6 week-old mice (n = 12 per therapy) had G6P uptake activity of 60% (GPE) and 30% (miGT), respectively of wild-type hepatic G6P uptake activity at 152 ± 5 units (or pmole/min/mg) (Fig. 1A), indicating that the rAAV-GPE-G6PT vector expresses ∼4 fold more activity than the rAAV-miGT-G6PT vector on a dose (vp/kg) basis. Likewise, the rAAV-GPE-G6PT vector also directed significantly higher levels of hG6PT mRNA expression as compared to the rAAV-miGT-G6PT vector (Fig. 1A). Notably, both GPE and miGT mice could sustain 24 h of fasting (Fig. 1B). While the 24-h fasted blood glucose levels of GPE were consistently lower than those of wild-type mice they were not statistically different. Similarly the 24-h fasted blood glucose levels of miGT mice were also lower but still within the normal range of 70 to 100 mg/dl (Fig. 1B). Both GPE and miGT mice were significantly leaner than their wild-type control littermates (Fig. 1C). While the liver weights of GPE mice were similar to that of wild-type mice, the liver weights of miGT mice were significantly higher (Fig. 1C). Because the rAAV-treated mice were leaner, the ratios of liver weight to body weight (LW/BW) in both mouse groups were higher than that of wild-type littermates (Fig. 1C). GSD-Ib is also characterized by neutropenia and neutrophil dysfunction (1,2). We have previously shown that rAAV-CBA/CMV-G6PT infusion corrects neutropenia in G6pt−/− mice transiently for 2 weeks (10). In this study, the 6-week-old GPE and miGT mice continued manifesting neutropenia (Fig. 1D) and neutrophil dysfunction (Fig. 1E), reflecting most likely the different cellular tropisms of the AAV2/8 serotype. To determine whether a humoral response directed against hG6PT is generated in the infused mice, we performed Western blot analysis using sera obtained from 6-week-old wild-type and rAAV-treated G6pt−/− mice mice. A polyclonal anti-hG6PT antibody (20) was used as a positive control. We detected no antibodies against hG6PT in the sera of control, GPE, and miGT mice (Fig. 1F).
Figure 1.
Phenotype analysis of 6-week-old wild-type and rAAV-treated G6pt−/− mice. (A) Liver microsomal G6P uptake activity and hG6PT mRNA expression. The data were obtained from wild-type (+/+, n = 8), GPE (n = 12) and miGT (n = 12) mice. (B) Blood glucose levels. (C) BW, LW, and LW/BW of mice. The data were obtained from wild-type (+/+, n = 24), GPE (n = 13) and miGT (n = 15) mice. (D) Blood neutrophils counts expressed as % of white blood cells. The data were obtained from wild-type (+/+, n = 16), GPE (n = 6) and miGT (n = 7) mice. (E) Bone marrow neutrophil respiratory burst activity in response to 200 ng/mL of PMA and calcium flux activity in response to 10−6 M of fMLP. Wild-type (○, n = 3); GPE/miGT (•). The data from GPE (n = 2) and miGT (n = 2) were indistinguishable and grouped together. (F) Antibodies against hG6PT. Microsomal proteins from Ad-hG6PT (20) infected COS-1 cells were electrophoresed through a single 12% polyacrylamide-SDS gel and transferred onto a PVDF membrane. Membrane strips, representing individual lanes on the gel were individually incubated with the appropriate mouse serum at 1: 50 dilution. A polyclonal anti-hG6PT antibody (20) was used as a positive control. Lanes 1, 2: anti-hG6PT antiserum; lanes 3, 5, 7, 9, 11, 13: serum samples from wild-type mice; serum samples from GPE (lanes 4, 6, 8) or miGT (lanes 10, 12, 14) mice. Data represent the mean ± SEM. *P < 0.05, **P < 0.005.
rAAV infusion directs long-term hepatic hG6PT expression
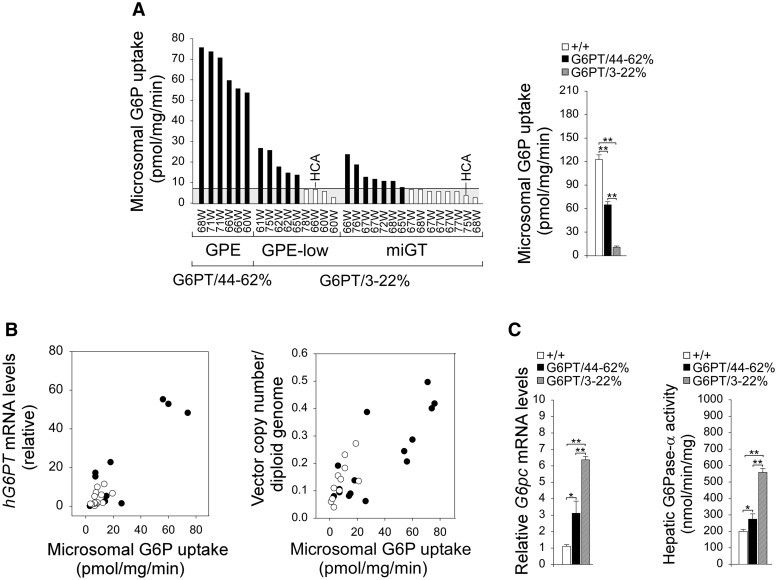
We then examined the dosage of the rAAV vectors required to maintain glucose homeostasis and prevent HCA development in G6pt−/− mice over a 78-week study. For the rAAV-GPE-G6PT studies, all neonatal mice (n =15) received 0.7 × 1013 vp/kg followed at 4 weeks by either 2 × 1013 vp/kg (GPE mice, n = 6) or 0.7 × 1013 vp/kg (GPE-low mice, n = 9). For the rAAV-miGT-G6PT studies, all neonatal mice received 1.4 × 1013 vp/kg neonatally, then 4 × 1013 vp/kg at age 4 weeks (miGT mice, n = 15). Hepatic G6PT activity was examined in wild-type and rAAV-treated mice sacrificed after a 24-h fast. For the 60–78 week-old wild-type mice, the mean hepatic microsomal G6P uptake activity was 123 +/− 6 units (representing 100% normal hepatic G6PT activity). The GPE mice had 44–62% of wild-type hepatic G6PT activity and named G6PT/44–62% mice (Fig. 2A). The GPE-low and miGT mice had 3–22% of wild-type hepatic G6PT and named G6PT/3–22% mice (Fig. 2A). There was no HCA in any of the 60–78 week-old wild-type or G6PT/44–62% mice (Fig. 2A). Among the 24 G6PT/3–22% mice, 12 had ≤ 5.7% of normal hepatic G6PT activity (≤ 7 units of microsomal G6P uptake activity). One GPE-low and one miGT mouse with 5.7% and 3.2% of normal hepatic G6P uptake activity, respectively, in the non-tumor liver tissues developed HCA (Fig. 2A). This suggests that 5.7% of normal hepatic G6PT activity is on the threshold of HCA formation in GSD-Ib. Hepatic microsomal G6P uptake activity correlated with hepatic hG6PT mRNA expression and the vector genome copy number (Fig. 2B). In summary, the rAAV-treated G6pt−/− mice with < 6% of normal hepatic G6PT activity restored are at risk of developing HCA.
Figure 2.
Biochemical analyses of 60–78 week-old wild-type and rAAV-treated G6pt−/− mice. (A) Liver microsomal G6P uptake activity in the rAAV-treated G6pt−/− mice is shown at the indicated ages in weeks (W). The mice were grouped based on the gene construct and viral dosages: GPE (n = 6), GPE-low (n = 9), and miGT (n = 15) mice. Two major subgroups emerge for mice expressing 44–62% (G6PT/44–62%, n = 6) and 3–22% (G6PT/3–22%, n = 24), respectively of normal hepatic G6PT activity. The G6PT/44–62% mice included GPE mice and the G6PT/3–22% mice included GPE-low and miGT mice. Hepatic microsomal G6P uptake activity in 60–78 week-old wild-type mice (n = 30) averaged 123 ± 6 units (pmol/min/mg). (B) Hepatic microsomal G6P uptake activity and its relationship to hG6PT mRNA expression and vector genome copy numbers in rAAV-GPE-G6PT- (•, GPE and GPE-low, n = 11–15) and rAAV-miGT-G6PT- (○, miGT, n = 15) treated G6pt−/− mice. (C) Hepatic G6pc mRNA expression and microsomal G6Pase-α enzymatic activity of 60–78-week-old wide-type (+/+, n = 30), G6PT/44–62% (n = 6), and G6PT/3–22% (n = 24) mice. Data represent the mean ± SEM. *P < 0.05, **P < 0.005.
During fasting, blood glucose homeostasis is maintained by hydrolysis of G6P to glucose by the G6PT/G6Pase-α complex in the terminal step of gluconeogenesis and glycogenolysis in the liver (1,2). We showed that levels of hepatic G6pc mRNA were increased in all rAAV-treated G6pt−/− mice relative to wild-type mice (Fig. 2C). In parallel, levels of hepatic G6Pase-α enzymatic activity in all rAAV-treated mice were increased 1.4- to 2.7-fold over that of wild-type controls (Fig. 2C). The G6PT-mediated hepatic microsomal G6P uptake activity is the rate-limiting step in endogenous glucose production (21) but it is co-dependent on G6Pase-α activity (22). Previously we have shown that hepatic microsomes prepared from GSD-Ia mice which lack G6Pase-α but express wild-type G6PT, exhibit markedly lower G6P uptake activity compared to wild-type hepatic microsomes (22). That phenotype can be reversed if G6Pase-α activity is restored via gene transfer (23). In rAAV-treated G6pt−/− mice, the increase in hepatic G6Pase-α activity was inversely correlated to hepatic microsomal G6P uptake activity (compare Fig. 2A and C).
rAAV infusion corrects metabolic abnormalities in GSD-Ib
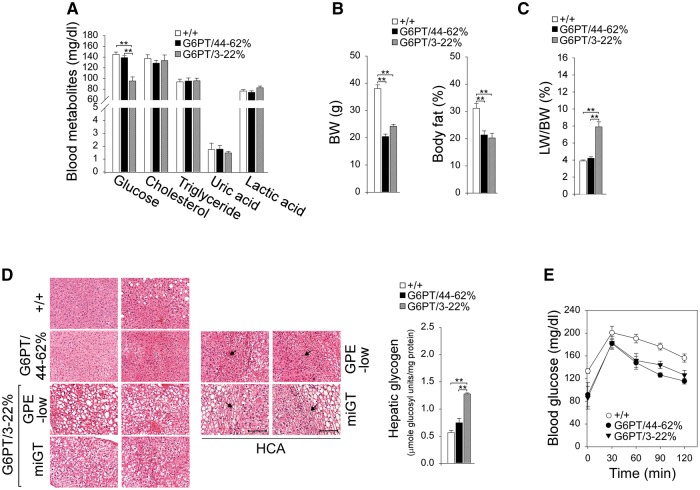
GSD-Ib is characterized by hypoglycemia, hyperlipidemia, hyperuricemia, and lactic acidemia (1,2). None of the 60–78-week-old rAAV-treated G6pt−/− mice suffered from hypoglycemic seizures. The basal blood glucose levels of G6PT/44–62% and wild-type mice were indistinguishable (Fig. 3A). Despite the ability of the G6PT/3–22% mice to maintain normoglycemia, their basal blood glucose levels were significantly lower than the levels in wild-type mice (Fig. 3A). Gene therapy normalized serum cholesterol, triglyceride, uric acid, and lactic acid profiles in all treated mice (Fig. 3A). The average BW and body fat (Fig. 3B) values of the treated G6pt−/− mice were significantly lower than those of their age-matched control mice, suggesting the treated mice were protected against age-related obesity. GSD-Ib is also characterized by hepatomegaly (1,2). The liver to body weight ratios were similar between G6PT/44–62% and wild-type mice, although G6PT/3–22% mice continued manifesting hepatomegaly (Fig. 3C).
Figure 3.
Phenotype analysis and fasting blood glucose tolerance profiles of 60–78-week-old wild-type and rAAV-treated G6pt−/− mice. The data were analyzed from wide-type (+/+, n = 30), G6PT/44–62% (n = 6), and G6PT/3–22% (GPE-low, n = 9 and miGT, n = 15) mice. (A) Blood glucose, cholesterol, triglyceride, uric acid, and lactic acid levels. (B) BW and body fat values. (C) LW/BW ratios. (D) H&E stained liver sections and hepatic glycogen contents. Each plate represents an individual mouse, so two mice are shown for each treatment. Two representative H&E stained HCA are shown in the GPE-low and the miGT mice. Scale bar = 200 µm. The arrow denotes HCA. (E) Glucose tolerance test profiles. Data represent the mean ± SEM. *P < 0.05, **P < 0.005.
Aside from hepatomegaly and instances of HCA, the hepatic tissue histology was unremarkable, even in the non-tumor regions of the two HCA-bearing mice (Fig. 3D). One HCA nodule of 1 cm in diameter was identified in a GPE-low mouse expressing 5.7% of normal hepatic G6PT activity, and 4 HCA nodules of 1, 0.7, 0.3, and 0.3 cm in diameter were identified in a miGT mouse expressing 3.2% of normal hepatic G6PT activity. The HCAs were well circumscribed with increased glycogen storage in both HCA and non-HCA tissues (Fig. 3D). While the 24-h fasted hepatic glycogen contents of G6PT/44–62% and wild-type mice were statistically similar, the G6PT/3–22% mice exhibited marked increases in fasting glycogen storage (Fig. 3D). The blood glucose tolerance profiles of all treated mice were indistinguishable from those of wild-type littermates (Fig. 3E).
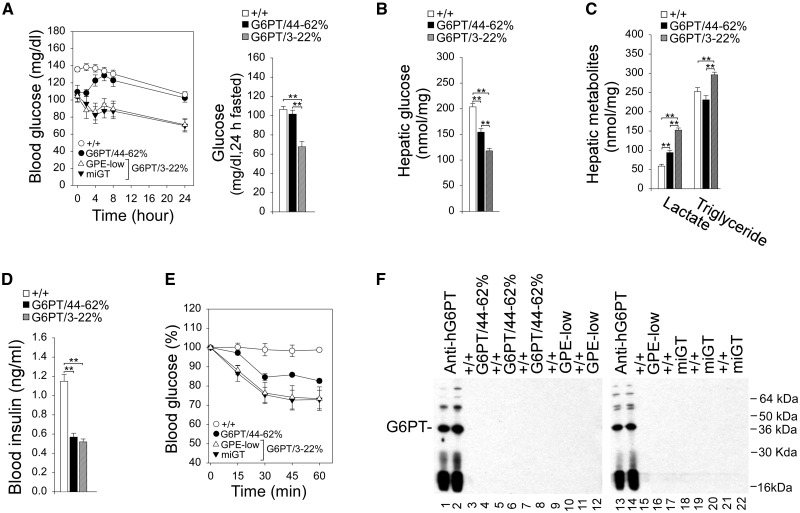
The fasting blood glucose profiles of G6PT/44–62% and wild-type mice were indistinguishable (Fig. 4A). The fasting blood glucose profiles of GPE-low and miGT (G6PT/3–22%) mice paralleled those of the control mice but blood glucose levels were consistently lower (Fig. 4A). In summary, G6pt−/− mice expressing more than 3% of normal hepatic G6PT activity no longer suffered from the fasting hypoglycemia characteristic of GSD-Ib.
Figure 4.
Phenotype, glucose tolerance, insulin tolerance, and anti-hG6PT antibodies analysis of 60–78 week-old wild-type and rAAV-treated G6pt−/− mice. The data were analyzed from wide-type (+/+, n = 30), G6PT/44–62% (n = 6), and G6PT/3–22% (GPE-low, n = 9 and miGT, n = 15) mice. (A) Fasting glucose tolerance profiles and the 24-h fasted blood glucose levels. (B) Twenty-four hour fasted hepatic glucose levels. (C) Twenty-four hour fasted hepatic lactate and triglyceride contents. (D) Twenty-four hour fasted blood insulin levels. (E) Insulin tolerance test profiles. Values are reported as a percent of respective level of each group at zero time. (F) Antibodies against human G6PT. Microsomal proteins from Ad-hG6PT (20) infected COS-1 cells were electrophoresed through a 12% polyacrylamide-SDS gel, transferred onto a PVDF membrane, and incubated with the appropriate mouse serum at 1: 50 dilution. A polyclonal anti-hG6PT antibody (20) was used as a positive control. Lanes 1, 2, 13, 14: anti-hG6PT antiserum; lanes 3, 5, 7, 9, 11, 15, 17, 19, 21: serum samples from wild-type mice, or serum samples from G6PT/44–62% (lanes 4, 6, 8), GPE-low (lanes 10, 12, 16), or miGT (lanes 18, 20, 22) mice. Data represent the mean ± SEM. *P < 0.05, **P < 0.005.
Biochemical phenotype of the rAAV-treated G6pt−/− mice
The G6pt−/− mice, lacking a functional G6PT, are incapable of producing endogenous glucose via the G6PT/G6Pase-α complex. All the rAAV-treated G6pt−/− mice could tolerate a long fast. Indeed, after 24 h of fasting, hepatic free glucose levels in G6PT/3–22% and G6PT/44–62% mice were 58% and 76%, respectively of wild-type hepatic glucose levels (204 ± 6 nmole/mg) (Fig. 4B). Furthermore, all rAAV-treated G6pt−/− mice had higher hepatic lactate levels compared to wild-type mice; however, hepatic lactate levels in the G6PT/44–62% mice were significantly lower than those in the G6PT/3–22% mice (Fig. 4C). While hepatic triglyceride contents were similar between G6PT/44–62% and wild-type mice, hepatic triglyceride levels in G6PT/3–22% mice were significantly increased compared to the controls (Fig. 4C).
Fasting blood insulin levels in the 60–78 week-old wild-type mice were 1.15 ± 0.07 ng/ml (Fig. 4D). The fasting blood insulin levels were significantly lower in all rAAV-treated G6pt−/− mice (Fig. 4D), which were closer to the levels in 10–20 week-old young adult mice than those in the old wild-type mice (24). The rAAV-treated G6pt−/− mice exhibit increased insulin sensitivity and a reduced insulin dose of 0.25 IU/kg was chosen to monitor blood insulin tolerance profiles. Following an intraperitoneal insulin injection, blood glucose levels in the old wild-type failed to decrease (Fig. 4E), reflecting age-related decrease in insulin sensitivity (25). While all treated mice exhibited increased insulin sensitivity as compared to wild-type mice, the increase in insulin sensitivity was more pronounced in the G6PT/3–22% mice (Fig. 4E). Western-blot analysis again showed that antibodies against hG6PT were not detected in the sera of 60–78-week-old control and rAAV-treated G6pt−/− mice (Fig. 4F).
Activation of hepatic ChREBP signaling
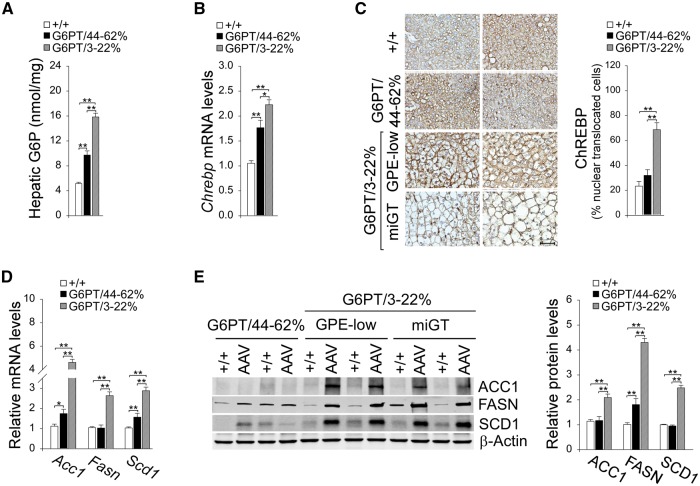
Studies have shown that mice over-expressing hepatic carbohydrate response element binding protein (ChREBP) exhibit improved glucose tolerance compared to controls (26). We have shown that activation of ChREBP signaling is one pathway that protects the rAAV-treated GSD-Ia mice from developing age-related insulin resistance (14). The ChREBP signaling can be activated by G6P, which promotes ChREBP nuclear translocation (27). In this study of rAAV-treated G6pt−/− mice, hepatic levels of G6P in G6PT/3–22% and G6PT/44–62% mice were 3.1- and 1.9-fold, respectively higher than the control mice (Fig. 5A). This was accompanied by increased hepatic Chrebp transcripts in all rAAV-treated G6pt−/− mice (Fig. 5B). Compared to wild-type mice, hepatic nuclear ChREBP protein contents were markedly increased in G6PT/3–22% mice but the increase in hepatic nuclear ChREBP protein contents was not statistically significant in G6PT/44–62% mice (Fig. 5C). Consistently, levels of mRNA and protein of ChREBP-regulated hepatic genes (26,27), acetyl-CoA carboxylase isoform-1 (ACC1), fatty acid synthase (FASN), stearoyl-CoA desaturase 1 (SCD1) were markedly increased in the G6PT/3–22% mice but only moderately and inconsistently increased in the G6PT/44–62% mice (Fig. 5D and E).
Figure 5.
Analysis of hepatic ChREBP signaling in 60–78-week-old wild-type and rAAV-treated G6pt−/− mice. For quantitative RT-PCR and hepatic G6P levels, the data represent the mean ± SEM for 60–78-week-old wild-type (n = 30), G6PT/44–62% (n = 6), and G6PT/3–22% (GPE-low, n = 9 and miGT, n = 15) mice. (A) Hepatic G6P levels. (B) Quantification of ChREBP mRNA by real-time RT-PCR. (C) Immunohistochemical analysis of hepatic ChREBP nuclear localization and quantification of nuclear ChREBP-translocated cells. Scale bar = 50 µm. The data represent the mean ± SEM for wild-type (+/+, n = 7), G6PT/44–62% (n = 4), and G6PT/3–22% (n = 15) mice. (D) Quantification of mRNA for Acc1, Fasn, and Scd1 by real-time RT-PCR. (E) Western blot analysis of ACC1, FASN, and SCD1, β-actin and quantification of protein levels by densitometry of wild-type (+/+, n = 17), G6PT/44–62% (n = 5), and G6PT/3–22% (n = 12) mice. Data represent the mean ± SEM. *P < 0.05, **P < 0.005.
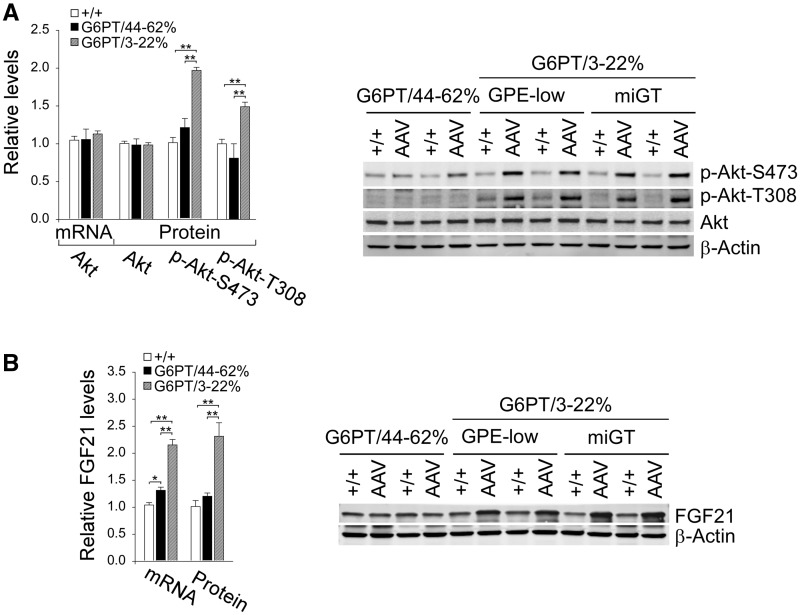
Studies have shown that mice overexpressing hepatic ChREBP along with increased SCD1 exhibit improved insulin signaling that correlates with phosphorylation and activation of protein kinase B/Akt (26). We showed that hepatic Akt mRNA and total Akt protein were similar between wild-type and rAAV-treated G6pt−/− mice (Fig. 6A). In parallel with the increase in hepatic levels of nuclear translocation of ChREBP protein, hepatic levels of the active, phosphorylated forms of Akt (28), p-Akt-S473 and p-Akt-T308, were statistically similar between the wild-type and G6PT/44–62% mice. However for the G6PT/3–22% mice, while the Akt protein levels remained wild-type, p-Akt-S473 and p-Akt-T308, were 2.1- and 1.5-fold higher, respectively (Fig. 6A).
Figure 6.
Analysis of hepatic Akt and FGF21 in 60–78-week-old wild-type and rAAV-treated G6pt−/− mice. For quantitative RT-PCR, the data represent the mean ± SEM for 60–78-week-old wild-type (n = 30), G6PT/44–62% (n = 6), and G6PT/3–22% (GPE-low, n = 9 and miGT, n = 15) mice. (A) Quantification of mRNA for Akt, Western blot analysis of Akt, p-Akt-S473, p-Akt-T308, and β-actin and quantification protein levels by densitometry of wild-type (+/+, n = 17), G6PT/44–62% (n = 5), and G6PT/3–22% (n = 12) mice. (B) Quantification of mRNA for FGF21, Western blot analysis of FGF21, β-actin and quantification protein levels by densitometry of wild-type (+/+, n = 17), G6PT/44–62% (n = 5), and G6PT/3–22% (n = 12) mice. Data represent the mean ± SEM. *P < 0.05, **P < 0.005.
Fibroblast growth factor 21 (FGF21) is a major regulator of energy homeostasis and insulin sensitivity (29) and is a target of ChREBP (30). The administration of FGF21 reverses hepatic steatosis, counteracts obesity, and alleviates insulin resistance in both rodents and nonhuman primates (29). Again, consistent with the increase in hepatic levels of nuclear translocation of ChREBP protein, hepatic levels of FGF21 transcript and protein were markedly higher only in G6PT/3–22% mice, compared to the controls (Fig. 6B).
Discussion
Previous gene therapy studies have shown that an hG6PT-expressing rAAV2/8 vector directed by the CBA promoter/CMV enhancer delivered the transgene to the liver and achieved metabolic correction in murine GSD-Ib (10). While that study showed promise, hepatic G6PT activities restored in the 52–72-week-old G6pt−/− mice were low, averaging ∼3% of normal hepatic G6PT activity, and 2 of the 5 transduced mice developed multiple HCAs with one undergoing malignant transformation (10). Previous studies in hepatic disease have also shown that the use of tissue-specific promoter/enhancer elements can improve expression efficiency and reduce the level of immune response that reduces long-term transgene expression (11,12). Indeed, we have shown that the gluconeogenic tissue-specific hG6PC promoter/enhancer is significantly more effective than CBA/CMA in directing persistent hepatic G6Pase-α expression in murine GSD-Ia and that an inflammatory immune response elicited by the vector containing the CBA/CMA elements reduced hepatic transgene expression (15). In this study, we used tissue-specific promoter/enhancer and compared the efficacy of rAAV-GPE-G6PT, a single-stranded rAAV vector directed by the hG6PC promoter/enhancer (GPE) (13–15) or rAAV-miGT-G6PT, a double-stranded rAAV vector directed by the native hG6PT promoter/enhancer (miGT) (16). While both vectors directed persistent hepatic hG6PT expression, the vector using the hG6PC promoter/enhancer was ∼ 4 fold more efficient in transgene expression, on a dose basis, than the vector using the hG6PT promoter/enhancer. We showed that the rAAV-treated G6pt−/− mice expressing 3–62% of normal hepatic G6PT activity grew normally for up to 78 weeks, displayed a normalized metabolic phenotype, had no detectable anti-hG6PT antibodies, and were protected against age-related obesity and insulin resistance. Significantly, we showed that G6pt−/− mice with < 6% of normal hepatic G6PT activity restored were at risk of developing hepatic tumors, establishing the threshold of hepatic G6PT activity required to prevent tumor formation.
In contrast to GSD-Ib patients (1,2) and G6pt knock-out mice (9), which cannot tolerate a short fast, the mice expressing 3–62% of normal hepatic G6PT activity could sustain 24 h of fasting. The hydrolysis of cytoplasmic G6P depends upon the functional co-dependence of G6PT and G6Pase-α in the G6PT/G6Pase-α complex (1). In gene therapy studies of murine GSD-Ia lacking G6Pase-α, we have shown that when 3–63% of normal hepatic G6Pase-α activity was reconstituted, the levels of hepatic G6PT mRNA became elevated 2.2-fold over wild-type (13). We have proposed that this represents a feedback response which attempts to increase the amount of rate limiting G6PT protein to offset the lower G6Pase-α activity. In line with those observations, we now showed a 1.4- to 2.8-fold increase in G6Pase-α expression when G6PT activity was reconstituted to 44–62% and 3–22%, respectively of normal hepatic G6PT activity. The treated GSD-Ib mice produced hepatic endogenous glucose averaging 58% to 76% of control littermates, enabling them to maintain glucose homeostasis during prolonged fasts. Therefore, there appears to be a functional feedback mechanism in which the expression levels of G6Pase-α and G6PT are regulated such that a decrease in one is offset by an increase in the other. This partially compensates for the overall decrease in the G6PT/G6Pase-α complex that occurs in type I GSDs. This extends our understanding of the nature of functional co-dependence of the two components of the G6PT/G6Pase-α complex that maintains interprandial blood glucose homeostasis.
In rAAV-treated GSD-Ia mice, we have shown that G6Pase-α is distributed unevenly in the liver, with many hepatocytes exhibiting minimal activity, but with some foci containing markedly higher levels of activity (13). Presently, we are unable to analyze G6PT expression in liver tissues by immunohistochemistry. However, it is likely that a similar mosaic pattern of G6PT expression will be seen in rAAV-treated G6pt−/− mice and a substantial proportion of hepatocytes will harbor little or no G6PT. Our data suggest that a uniform hepatic G6PT expression is not required for rescuing the GSD-Ib phenotype. The fact that only 2 of the 12 rAAV-treated G6pt−/− mice expressing < 6% of normal hepatic G6PT activity developed HCA, suggests that additional alterations associated with G6PT deficiency contribute to HCA formation. Studies have shown that autophagy-deficient mice develop HCA (31). We have recently shown that G6Pase-α deficiency leads to defective autophagy in the liver (32). Future study should focus on whether hepatic G6PT deficiency will lead to defective autophagy and the mechanism underlying HCA development in GSD-Ib.
The abnormal metabolic liver phenotype of GSD-Ib is characterized by fasting hypoglycemia, hepatomegaly, hyperlipidemia, hyperuricemia, and lactic acidemia (1,2). The G6PT/3–22% mice exhibited a normalized metabolic liver phenotype but continued exhibiting hepatomegaly. They also had increased hepatic glycogen and triglyceride contents along with reduced basal and 24-h fasted blood glucose levels. On the other hand, the G6PT/44–62% mice exhibited a metabolic liver phenotype indistinguishable from that of the wild-type mice, including normal levels of blood glucose and metabolites, normal levels of hepatic glycogen and triglyceride, normal LW/BW, and normal glucose tolerance and fasting glucose tolerance profiles. However, unlike wild-type mice that gain fat and lose insulin sensitivity with age, all treated mice were protected against age-related obesity and insulin resistance, although GSD-Ib mice with 3–22% reconstituted hepatic G6PT activity were more insulin sensitive than the mice with 44–62% of reconstituted hepatic G6PT activity.
Studies have shown that mice overexpressing hepatic ChREBP exhibit improved glucose and lipid metabolism resulting from Akt activation and an increase in the expression of SCD1 that converts saturated fatty acids into the beneficial mono-unsaturated fatty acids (26,33). Moreover, FGF21 that improves insulin sensitivity, ameliorates hepatic steatosis and enhances energy expenditure (29) is a target of ChREBP (30). We showed that hepatic ChREBP signaling is activated in the 60–78-week-old G6PT/3–22% mice, evident by increased nuclear translocation of ChREBP proteins, along with increased levels of FGF21, SCD1, the active p-Akt-S473 and p-Akt-T308, providing one underlying mechanism for the improved metabolic phenotype of the G6PT/3–22% mice. GSD-Ib is an autosomal recessive disorder. It is therefore not surprising that the G6PT/44–62% mice displayed a metabolic liver phenotype indistinguishable from that of wild-type mice. Indeed, ChREBP signaling in G6PT/44–62% and wild-type mice appeared to be similar. Supporting this, the components of the ChREBP signaling pathways, including nuclear translocated ChREBP proteins, activated forms of Akt, and levels of SCD1 and FGF21 were statistically similar between G6PT/44–62% and wild-type mice. This may explain the reduced insulin sensitivity of these mice, compared to G6PT/3–22% mice expressing lower levels of normal hepatic G6PT activity. Our results suggest that semi-optimal levels of hepatic G6PT activity might be beneficial. This reflects a similar observation seen in the GSD-Ia mice (14) and perhaps not surprising given the link between increases in hepatic G6Pase-α/G6PT activity and diabetes (34,35).
To develop therapeutic approaches for future clinical translation, a gene therapy strategy needs to consider practical vector designs to reduce genotoxicity. We selected rAAV as the gene delivery vector instead of the integrating retrovirus and lentivirus vectors because of the potential genotoxicity of insertional mutagenesis associated with the integrating vectors (36). Moreover, the episomal rAAV vectors are considered nonpathogenic and have demonstrated efficacy in clinical trials (37). We have also employed tissue-specific promoter/enhancer to potentially improve expression efficiency and decrease the level of immune response. However, studies have shown that insertional mutagenesis can also be associated with rAAV-mediated gene delivery. Using a murine model of β-glucuronidase deficiency treated neonatally with a rAAV vector, Donsante et al. have documented an increased rate of HCC formation (38). This was recently confirmed and extensively characterized by Chandler et al. (39). Following therapeutic rAAV gene delivery, Chandler et al. showed that insertional mutagenesis into the Rian (RNA imprinted and accumulated in nucleus) locus is associated with HCC development (39). The authors further showed that the AAV-mediated insertional mutagenesis and subsequent genotoxicity are influenced by the vector serotype and dose, choice of promoter/enhancer, the age of treatment, and the genome species. Therefore, future clinical trial designs should take into consideration of all factors that affect genotoxicity associated with the rAAV-mediated gene delivery.
In summary, we have demonstrated that G6pt−/− mice receiving G6PT gene therapy titrated to express at least 3% of normal hepatic G6PT activity maintain glucose homeostasis and are protected against age-related insulin resistance and obesity. We further show that one underlying mechanism responsible for the beneficial metabolic phenotype of the treated mice arises from activation of hepatic ChREBP signaling pathway. We also show that hepatocytes harboring less than 6% of normal hepatic G6PT activity are at risk of HCA development. Our study suggests that full restoration of normal G6PT activity will not be required to confer significant therapeutic benefits in liver-directed gene therapy for the metabolic disease in murine GSD-Ib. However, the liver-directed gene therapy does not correct myeloid and renal dysfunction in GSD-Ib. Recently, Rocca et al. have shown that rAAV2/9 kidney transduction could be improved using a retrograde renal vein injection of the virus in mice (40). While this may hold future promise for metabolic correction for GSD-Ib, neither vector targets hematopoietic stem cells effectively. Identification of viral serotypes that effectively transduce all affected tissue types remains one avenue to be explored further.
Materials and Methods
Construction of rAAV vectors and infusion of G6pt−/− mice
The pTR-GPE-G6PT plasmid, containing hG6PT under the control of the 2.8-kb hG6PC promoter/enhancer was constructed by replacing hG6PC at 5’-SbfI and 3’NotI sites in pTR-GPE-G6PC (15) with the hG6PT cDNA at 5’-NsiI and 3’NotI sites. The pTR-miGT-G6PT plasmid, containing hG6PT under the control of the hG6PT minimal promoter/enhancer was constructed by replacing GPE at 5’-KpnI and 3’HindIII sites in pTR-GPE-G6PT with the miGT at 5’-KpnI and 3’HindIII sites. Both plasmids were verified by DNA sequencing. The rAAV-GPE-G6PT and rAAV-miGT-G6PT vectors were produced at the University of Florida Powell Gene Therapy Center Vector Core Laboratory. All animal studies were conducted under an animal protocol approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Animal Care and Use Committee. For gene therapy, each vector was administered to the G6pt−/− mice in two doses - neonatally via the temporal vein and age 4 weeks via the retro-orbital sinus. Age-matched G6pt+/+/G6pt+/- mice with indistinguishable phenotype were used as controls (referred collectively as wild-type or control mice).
Microsomal G6P uptake and phosphohydrolase assays
Microsomal preparations, G6P uptake and phosphohydrolase measurements were performed as described previously (9,22). In G6P uptake assays, microsomes isolated from liver were incubated for 3 min at 30 °C in a reaction mixture (100 μl) containing 50 mM sodium cacodylate buffer, pH 6.5, 250 mM sucrose, and 0.2 mM [U-14 C]G6P (50 μCi/μmol, American Radiolabeled Chemicals, St Louis, MO, USA). The reaction was stopped by filtering through a nitrocellulose membrane (Millipore, Billerica, MA). Microsomes permeabilized with 0.2% deoxycholate, to abolish G6P uptake, were used as negative controls. One unit of G6PT activity represents the uptake of one pmol G6P per minute per mg microsomal protein.
In phosphohydrolase assays, reaction mixtures (50 μl) containing 50 mM sodium cacodylate buffer, pH 6.5, 2 mM EDTA, 10 mM G6P, and appropriate amounts of microsomal preparations were incubated at 30 °C for 10 min. Disrupted microsomal membranes were prepared by incubating intact membranes in 0.2% deoxycholate for 20 min at 4 °C. Non-specific phosphatase activity was estimated by pre-incubating disrupted microsomal preparations at pH 5 for 10 min at 37 °C to inactivate the acid labile G6Pase-α.
Flow cytometry and functional analysis of bone marrow neutrophils
Heparinized mouse peripheral blood cells were erythrocyte-depleted and fixed in Lysis/Fix buffer (BD Biosciences, San Jose, CA, USA). The resulting leukocytes were stained with a FITC-conjugated mouse monoclonal Gr-1 antibody (eBiosciences, San Diego, CA, USA) and a PE-conjugated CD11b antibody (eBiosciences), and analyzed by flow cytometry using a Guava EasyCyte Mini System (Millipore).
Bone marrow cells were isolated from the femurs and tibiae of 6-week-old wild-type and rAAV-treated G6pt−/− mice, and neutrophils were purified from the bone marrow cells using the MACS separation columns system (Miltenyi Biotec, San Diego, CA, USA) with Gr-1 MicroBead Kit (Miltenyi Biotec). The respiratory burst of bone marrow neutrophils was monitored by luminal-amplified chemiluminescence using the LumiMax Superoxide Anion Detection kit (Agilent Technologies, Santa Clara, CA, USA) and Victor Light 1420 Luminescence counter (PerkinElmer Life & Analytical Sciences, American Fork, UT, USA) as described previously (41). Neutrophils in LumiMax SOA assay medium were activated with 200 ng/ml of phorbol myristate acetate (PMA) (Sigma-Aldrich, St. Louis, MO, USA). The calcium flux of bone marrow neutrophils in response to 10−6 M f-Met-Leu-Phe (fMLP) (Sigma-Aldrich) was measured using the FLIPER calcium 3 assay kit component A (Molecular Devices, Sunnyvale, CA, USA) and analyzed in a Flexstation II Fluorimeter (Molecular Devices) set at 37 °C as described previously (41).
Phenotype analysis
Body composition was assessed using the Bruker minispec NMR analyzer (Karlsruhe, Germany). The presence of HCA nodules in mice was confirmed by histological analysis of liver biopsy samples, using 5 or more separate sections per liver. Blood levels of glucose, cholesterol, triglyceride, lactate, and urate along with hepatic levels of glucose, triglyceride, lactate, and G6P were determined as described previously (13,14).
Glucose tolerance testing of mice consisted of fasting for 6 h, prior to blood sampling, followed by intraperitoneal injection of a glucose solution at 2 mg/g body weight, and repeated blood sampling via the tail vein for 2 h (13). Insulin tolerance testing of mice consisted of a 4-h fast, prior to blood sampling, followed by intraperitoneal injection of insulin at 0.25 IU/kg, and repeated blood sampling via the tail vein for 1 h (14).
Quantitative real-time RT-PCR and western-blot analysis
The mRNA expression was quantified by real-time RT-PCR in an Applied Biosystems 7300 Real-Time PCR System using Applied Biosystems TaqMan probes (Foster City, CA, USA). Data were normalized to Rpl19 RNA. Western-blot images were detected using the LI-COR Odyssey scanner and the Image studio 3.1 software (Li-Cor Biosciences, Lincoln, NE, USA). Mouse monoclonal used was β-actin (Santa Cruz Biotechnology, Dallas, TX, USA). Rabbit monoclonals used were: p-Akt-S473 and p-Akt-T308 (Cell Signaling, Danvers, MA, USA); FGF21 (Abcam, Cambridge, MA, USA). Rabbit polyclonals used were: ChREBP (Novus Biologicals, Littleton, CO, USA); Akt, ACC and SCD-1 (Cell Signaling); FASN (Abcam). Protein expression was quantified by densitometry using the ImageJ 1.51a software (NIH, Bethesda, MD, USA).
Analysis of ChREBP nuclear localization
The nuclear location of ChREBP in mouse liver sections was performed as described previously (14). Mouse liver paraffin sections (10 μm thickness) were treated with 0.3% hydrogen peroxide in methanol to quench endogenous peroxidases, then blocked with the Avidin/Biotin Blocking Kit (Vector Laboratories, Burlingame, CA, USA). For ChREBP detection, liver sections were incubated serially with a rabbit antibody against ChREBP and a biotinylated anti-rabbit IgG (Vector Laboratories). The resulting complexes were detected with an ABC kit using the DAB Substrate (Vector Laboratories). Sections were counterstained with hematoxylin (Sigma-Aldrich) and visualized using a Zeiss Axioskop2 plus microscope equipped with 40X/0.50 NA objectives (Carl Zeiss MicroImaging, Jena, Germany). Images were acquired using a Nikon DS-Fil digital camera and NIS-Elements F3.0 imaging software (Nikon, Tokyo, Japan). The percentage of cells in 10 randomly selected fields containing ChREBP positive nuclei was recorded.
Statistical analysis
The unpaired t-test was performed using the GraphPad Prism Program, version 4 (GraphPad Software, San Diego, CA, USA). Values were considered statistically significant at P < 0.05.
Conflict of Interest statement. None declared.
Funding
Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Jonah Pournazarian Fund to Support Glycogen Storage Disease Ib, Delano Fund for a Cure, and Team Tallulah for GSD Type Ib Research Fund.
References
- 1. Chou J.Y., Matern D., Mansfield B.C., Chen Y.T. (2002) Type I glycogen storage diseases: disorders of the glucose-6-phosphatase complex. Curr. Mol. Med., 2, 121–143. [DOI] [PubMed] [Google Scholar]
- 2. Chou J.Y., Jun H.S., Mansfield B.C. (2010) Glycogen storage disease type I and G6Pase-β deficiency: etiology and therapy. Nat. Rev. Endocrinol., 6, 676–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Greene H.L., Slonim A.E., O'Neill J.A., Burr I.M. (1976) Continuous nocturnal intragastric feeding for management of type 1 glycogen-storage disease. N. Engl. J. Med., 294, 423–425. [DOI] [PubMed] [Google Scholar]
- 4. Chen Y.T., Cornblath M., Sidbury J.B. (1984) Cornstarch therapy in type I glycogen-storage disease. N. Engl. J. Med., 310, 171–175. [DOI] [PubMed] [Google Scholar]
- 5. Visser G., Rake J.P., Fernandes J., Labrune P., Leonard J.V., Moses S., Ullrich K., Smit G.P. (2000) Neutropenia, neutrophil dysfunction, and inflammatory bowel disease in glycogen storage disease type Ib: results of the European Study on Glycogen Storage Disease type I. J. Pediatr., 137, 187–191. [DOI] [PubMed] [Google Scholar]
- 6. Visser G., Rake J.P., Labrune P., Leonard J.V., Moses S., Ullrich K., Wendel U., Groenier K.H., Smit G.P.A. (2002) Granulocyte colony-stimulating factor in glycogen storage disease type 1b. Results of the European Study on Glycogen Storage Disease Type 1. Eur. J. Pediatr., 161, S83–S87. [DOI] [PubMed] [Google Scholar]
- 7. Rake J.P., Visser G., Labrune P., Leonard J.V., Ullrich K., Smit G.P. (2002) Glycogen storage disease type I: diagnosis, management, clinical course and outcome. Results of the European Study on Glycogen Storage Disease Type I (ESGSD I). Eur. J. Pediatr., 161, S20–S34. [DOI] [PubMed] [Google Scholar]
- 8. Franco L.M., Krishnamurthy V., Bali D., Weinstein D.A., Arn P., Clary B., Boney A., Sullivan J., Frush D.P., Chen Y.-T., Kishnani P.S. (2005) Hepatocellular carcinoma in glycogen storage disease type Ia: a case series. J. Inherit. Metab. Dis., 28, 153–162. [DOI] [PubMed] [Google Scholar]
- 9. Chen L.-Y., Shieh J.-J., Lin B., Pan C.-J., Gao J.-L., Murphy P.M., Roe T.F., Moses S., Ward J.M., Lee E.J. (2003) Impaired glucose homeostasis, neutrophil trafficking and function in mice lacking the glucose-6-phosphate transporter. Hum. Mol. Genet., 12, 2547–2558. [DOI] [PubMed] [Google Scholar]
- 10. Yiu W.H., Pan C.J., Mead P.A., Starost M.F., Mansfield B.C., Chou J.Y. (2009) Normoglycemia alone is insufficient to prevent long term complications of hepatocellular adenoma in glycogen storage disease type Ib mice. J. Hepatol., 51, 909–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ziegler R.J., Cherry M., Barbon C.M., Li C., Bercury S.D., Armentano D., Desnick R.J., Cheng S.H. (2007) Correction of the biochemical and functional deficits in fabry mice following AAV8-mediated hepatic expression of alpha-galactosidase A. Mol. Ther., 15, 492–500. [DOI] [PubMed] [Google Scholar]
- 12. Franco L.M., Sun B., Yang X., Bird A., Zhang H., Schneider A., Brown T., Young S.P., Clay T.M., Amalfitano A., Chen Y.T., Koeberl D.D. (2005) Evasion of immune responses to introduced human acid alpha-glucosidase by liver-restricted expression in glycogen storage disease type II. Mol. Ther., 12, 876–884. [DOI] [PubMed] [Google Scholar]
- 13. Lee Y.M., Jun H.S., Pan C.J., Lin S.R., Wilson L.H., Mansfield B.C., Chou J.Y. (2012) Prevention of hepatocellular adenoma and correction of metabolic abnormalities in murine glycogen storage disease type Ia by gene therapy. Hepatology, 56, 1719–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim G.Y., Lee Y.M., Cho J.H., Pan C.J., Jun H.S., Springer D.A., Mansfield B.C., Chou J.Y. (2015) Mice expressing reduced levels of hepatic glucose-6-phosphatase-alpha activity do not develop age-related insulin resistance or obesity. Hum. Mol. Genet., 24, 5115–5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yiu W.H., Lee Y.M., Peng W.T., Pan C.-J., Mead P.A., Mansfield B.C., Chou J.Y. (2010) Complete normalization of hepatic G6PC deficiency in murine glycogen storage disease type Ia using gene therapy. Mol. Ther., 18, 1076–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hiraiwa H., Chou J.Y. (2001) Glucocorticoids activate transcription of the gene for glucose-6-phosphate transporter, deficient in glycogen storage Disease type 1b. DNA Cell. Biol., 20, 447–453. [DOI] [PubMed] [Google Scholar]
- 17. Lee Y.M., Pan C.J., Koeberl D.D., Mansfield B.C., Chou J.Y. (2013) The Upstream enhancer elements of the G6PC promoter are critical for optimal G6PC expression in murine glycogen storage disease type Ia. Mol. Genet. Metab., 110, 275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McCarty D.M. (2008) Self-complementary AAV vectors; advances and applications. Mol. Ther., 16, 1648–1656. [DOI] [PubMed] [Google Scholar]
- 19. Fisher K.J., Gao G.P., Weitzman M.D., DeMatteo R., Burda J.F., Wilson J.M. (1996) Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J. Virol., 70, 520–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen L.-Y., Pan C.-J., Shieh J.-J., Chou J.Y. (2002) Structure-function analysis of the glucose-6-phosphate transporter deficient in glycogen storage disease type Ib. Hum. Mol. Genet., 11, 3199–3207. [DOI] [PubMed] [Google Scholar]
- 21. Arion W.J., Lange A.J., Ballas L.M. (1976) Quantitative aspects of relationship between glucose 6-phosphate transport and hydrolysis for liver microsomal glucose-6-phosphatase system. Selective thermal inactivation of catalytic component in situ at acid pH. J. Biol. Chem., 251, 6784–6690. [PubMed] [Google Scholar]
- 22. Lei K.J., Chen H., Pan C.J., Ward J.M., Mosinger B., Lee E.J., Westphal H., Mansfield B.C., Chou J.Y. (1996) Glucose-6-phosphatase dependent substrate transport in the glycogen storage disease type 1a mouse. Nat. Genet., 13, 203–209. [DOI] [PubMed] [Google Scholar]
- 23. Zingone A., Hiraiwa H., Pan C.-J., Lin B., Chen H., Ward J.M., Chou J.Y. (2000) Correction of glycogen storage disease type 1a in a mouse model by gene therapy. J. Biol. Chem., 275, 828–832. [DOI] [PubMed] [Google Scholar]
- 24. Flatt P.R., Bailey C.J. (1981) Development of glucose intolerance and impaired plasma insulin response to glucose in obese hyperglycaemic (ob/ob) mice. Horm. Metab. Res., 13, 556–560. [DOI] [PubMed] [Google Scholar]
- 25. Barzilai N., Huffman D.M., Muzumdar R.H., Bartke A. (2012) The critical role of metabolic pathways in aging. Diabetes, 61, 1315–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Benhamed F., Denechaud P.-D., Lemoine M., Robichon C., Moldes M., Bertrand-Michel J., Ratziu V., Serfaty L., Housset C., Capeau J.. et al. (2012) The lipogenic transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans. J. Clin. Invest., 122, 2176–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Filhoulaud G., Guilmeau S., Dentin R., Girard J., Postic C. (2013) Novel insights into ChREBP regulation and function. Trends Endocrinol. Metab., 24, 257–268. [DOI] [PubMed] [Google Scholar]
- 28. Danielpour D., Song K. (2006) Cross-talk between IGF-I and TGF-beta signaling pathways. Cytokine Growth Factor Rev., 17, 59–74. [DOI] [PubMed] [Google Scholar]
- 29. Fisher F.M., Maratos-Flier E. (2016) Understanding the physiology of FGF21. Annu. Rev. Physiol., 78, 223–241. [DOI] [PubMed] [Google Scholar]
- 30. Iizuka K., Takeda J., Horikawa Y. (2009) Glucose induces FGF21 mRNA expression through ChREBP activation in rat hepatocytes. FEBS Lett., 583, 2882–2886. [DOI] [PubMed] [Google Scholar]
- 31. Takamura A., Komatsu M., Hara T., Sakamoto A., Kishi C., Waguri S., Eishi Y., Hino O., Tanaka K., Mizushima N. (2011) Autophagy-deficient mice develop multiple liver tumors. Genes Dev., 25, 795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cho J.-H., Kim G.-Y., Pan C.-J., Anduaga J., Choi E.-J., Mansfield B.C., Chou J.Y., Plagnol V. (2017) Downregulation of SIRT1 signaling underlies hepatic autophagy impairment in glycogen storage disease type Ia. PLOS Genet., 13, e1006819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Flowers M.T., Ntambi J.M. (2008) Role of stearoyl-coenzyme A desaturase in regulating lipid metabolism. Curr. Opin. Lipidol., 19, 248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Antinozzi P.A., Berman H.K., O'Doherty R.M., Newgard C.B. (1999) Metabolic engineering with recombinant adenoviruses. Annu. Rev. Nutr., 19, 511–544. [DOI] [PubMed] [Google Scholar]
- 35. Clore J.N., Stillman J., Sugerman H. (2000) Glucose-6-phosphatase flux in vitro is increased in type 2 diabetes. Diabetes, 49, 969–974. [DOI] [PubMed] [Google Scholar]
- 36. David R.M., Doherty A.T. (2017) Viral vectors: the road to reducing genotoxicity. Toxicol Sci., 155, 315–325. [DOI] [PubMed] [Google Scholar]
- 37. Kotterman M.A., Schaffer D.V. (2014) Engineering adeno-associated viruses for clinical gene therapy. Nat. Rev. Genet., 15, 445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Donsante A., Miller D.G., Li Y., Vogler C., Brunt E.M., Russell D.W., Sands M.S. (2007) AAV vector integration sites in mouse hepatocellular carcinoma. Science, 317, 477. [DOI] [PubMed] [Google Scholar]
- 39. Chandler R.J., LaFave M.C., Varshney G.K., Trivedi N.S., Carrillo-Carrasco N., Senac J.S., Wu W., Hoffmann V., Elkahloun A.G., Burgess S.M., Venditti C.P. (2015) Vector design influences hepatic genotoxicity after adeno-associated virus gene therapy. J. Clin. Invest., 125, 870–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rocca C.J., Ur S.N., Harrison F., Cherqui S. (2014) rAAV9 combined with renal vein injection is optimal for kidney-targeted gene delivery: conclusion of a comparative study. Gene Ther., 21, 618–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jun H.S., Lee Y.M., Cheung Y.Y., McDermott D.H., Murphy P.M., De Ravin S.S., Mansfield B.C., Chou J.Y. (2010) Lack of glucose recycling between endoplasmic reticulum and cytoplasm underlies cellular dysfunction in glucose-6-phosphatase-β-deficient neutrophils in a congenital neutropenia syndrome. Blood, 116, 2783–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]