Synopsis
Seasonality is a critically important aspect of environmental variability, and strongly shapes all aspects of life for organisms living in highly seasonal environments. Seasonality has played a key role in generating biodiversity, and has driven the evolution of extreme physiological adaptations and behaviors such as migration and hibernation. Fluctuating selection pressures on survival and fecundity between summer and winter provide a complex selective landscape, which can be met by a combination of three outcomes of adaptive evolution: genetic polymorphism, phenotypic plasticity, and bet-hedging. Here, we have identified four important research questions with the goal of advancing our understanding of evolutionary impacts of seasonality. First, we ask how characteristics of environments and species will determine which adaptive response occurs. Relevant characteristics include costs and limits of plasticity, predictability, and reliability of cues, and grain of environmental variation relative to generation time. A second important question is how phenological shifts will amplify or ameliorate selection on physiological hardiness. Shifts in phenology can preserve the thermal niche despite shifts in climate, but may fail to completely conserve the niche or may even expose life stages to conditions that cause mortality. Considering distinct environmental sensitivities of life history stages will be key to refining models that forecast susceptibility to climate change. Third, we must identify critical physiological phenotypes that underlie seasonal adaptation and work toward understanding the genetic architectures of these responses. These architectures are key for predicting evolutionary responses. Pleiotropic genes that regulate multiple responses to changing seasons may facilitate coordination among functionally related traits, or conversely may constrain the expression of optimal phenotypes. Finally, we must advance our understanding of how changes in seasonal fluctuations are impacting ecological interaction networks. We should move beyond simple dyadic interactions, such as predator prey dynamics, and understand how these interactions scale up to affect ecological interaction networks. As global climate change alters many aspects of seasonal variability, including extreme events and changes in mean conditions, organisms must respond appropriately or go extinct. The outcome of adaptation to seasonality will determine responses to climate change.
Introduction
Seasonality represents the strongest and most ubiquitous source of external variation influencing human and natural systems (Levins 1968; Fretwell 1972; Wingfield and Kenagy 1991). The combined effects of Earth’s tilt and rotation result in annual sine wave variations in day-length, with downstream effects on temperature, rainfall, and resource availability (Lisovski et al. 2017). In the temperate and polar zones, winters are characterized by short days, cold air temperatures, moisture in the form of ice and snow, and reduced or suspended primary production and activity (Williams et al. 2015). Summers are characterized by long days and conditions that are permissive for growth and development, including warm air temperatures, elevated primary production, and increased animal activity often including reproduction. In the tropics, seasonality consists of wet and dry seasons, which drive changes in morphology (including leaf senescence), physiology, and behavior that contribute to seasonal shifts in operative temperatures (e.g., Christian and Bedford 1995). Seasonal changes thus impact organisms both directly, through the effects of light, temperature, precipitation, and other abiotic variables on their physiology, and indirectly, via biotic interactions.
Seasonal environments impose fluctuating selection on life history traits that can elicit adaptive responses (Varpe 2017). Morphological and physiological traits are frequently plastic, described by functions termed reaction norms that relate a trait value to an environmental variable (Kingsolver et al. 2015). The shape and intercept of these functions may evolve in response to changing seasonality, for example, the shape of the temperature versus survivorship curve. Alternatively, organism may evolve life cycles with distinct life stages, each with different reaction norms “tuned” to the seasons in which they occur (McNamara and Houston 2008; Wingfield 2008). This strategy is also an evolutionary solution to constraints caused by trade-offs among life history traits that compete for resources (Zera and Harshman 2001). For example, selection may favor high reproductive investment during favorable periods associated with population growth (e.g., summer), but reduced reproductive (and increased somatic) investment during unfavorable periods characterized by stress (e.g., winter) (Schluter et al. 1991; Betini et al. 2017). Organisms often evolve distinct reproductive and dormant or migratory life history stages in response to these contrasting selection pressures (Varpe 2017).
Organisms in seasonal environments must integrate information from multiple environmental cues to time transitions between life-history stages. Phenology, the timing of biological events, must synchronize both with environmental conditions, and with interacting organisms from the same and other species. Synchronizing cues must predict future selective environments, and are most reliable when cues are themselves drivers of selection (e.g., temperature, precipitation, and food resources). However, drivers of selection are frequently not used as cues, when there is a long time lag between cue sensing and readiness to respond (Levins 1968; Visser et al. 2010). For example, many organisms need to complete development, undergo morphological changes, or migrate before beginning feeding and reproduction (Koštál 2006; Tombre et al. 2008). Such time lags can reduce the adaptive value of plastic responses (Padilla and Adolph 1996). Therefore, many organisms use cues, frequently day-length, that are not themselves drivers of selection to provide advance notice of seasonal transitions.
Global climate change is shifting the relationship between day-length and drivers of selection, fundamentally altering seasonal cycles. Spring is coming earlier, and fall later, extending the growing season and causing many organisms to alter their phenology (Parmesan 2006). Earlier spring phenology is exposing organisms to increased risk of damaging cold snaps on vulnerable life stages in spring. Environmentally cued phenology and physiological reaction norms exhibit predictable genetic variation that responds rapidly to selection (Bradshaw and Holzapfel 2001; Menzel et al. 2006; Parmesan 2006; Diamond et al. 2017). Thus, the legacy of past adaptation to seasonal environments will impact future responses to global climate change, making it a high priority to understand the evolutionary impacts of seasonality.
Outstanding questions
The ecological and evolutionary impacts of seasonality on organisms have long been a topic of interest to biologists (Levins 1968; Fretwell 1972; Dobzhansky and Ayala 1973), but our understanding of these impacts is still incomplete. We have identified four pressing questions that together promise to advance our understanding of evolutionary impacts of seasonality. In the following sections, we give background on each question, outline gaps in knowledge, and suggest how these gaps can be addressed.
How does seasonally fluctuating selection impact evolutionary trajectories?
If large body size is an adaptation to cold (…), what size is optimum in an environment which is sometimes hot and sometimes cold? (Levins 1968).
Background
Seasonally fluctuating selective pressures complicate the selective environment in comparison to stable or weakly seasonal environments. Evolutionary responses to seasonality include (1) the maintenance of genetic polymorphism, (2) phenotypic plasticity, and (3) bet-hedging (Fig. 1). Genetic polymorphism refers to the presence of two or more distinct gene variants (alleles) at a single locus within a population. When the selective drivers change across generations, natural selection can cause cyclic changes in allele frequencies in genes associated with adaptation to distinct seasons (Dobzhansky and Ayala 1973). “Winter” alleles rise throughout the winter and reach a peak in spring due to differential survival of individuals with those alleles, which are then gradually replaced by individuals bearing “summer” alleles during the growing season (Fig. 1B;Carvalho and Crisp 1987; Bergland et al. 2014; Cogni et al. 2014). Seasonal variation is thus one class of temporal variation that can maintain genetic variation within populations (Haldane and Jayakar 1963), potentially maintaining polymorphisms at many loci across the genome (Wittmann et al. 2017). Seasonal changes in frequency at polymorphic loci may be generated by life history trade-offs in resource allocation or acquisition (Schluter et al. 1991; Betini et al. 2017). Poleward phenotypes and genotypes tend to resemble winter phenotypes and genotypes, suggesting that seasons can in some ways be considered the time-analog of spatial environmental clines. Variation in the extent and magnitude of seasonality can also be one mechanism by which spatial clines are generated (e.g., the seasonal phase cline model, Rhomberg and Singh 1988). For example, populations of Drosophila melanogaster collected in Pennsylvania orchards at the end of the growing season are similar genetically and phenotypically to southern populations, while populations emerging after winter are similar to northern populations (Cogni et al. 2014; Behrman et al. 2015; Cogni et al. 2015). Adaptation to seasonal fluctuations, therefore, contributes to adaptation across a geographic range (Conover 1992).
Fig. 1.
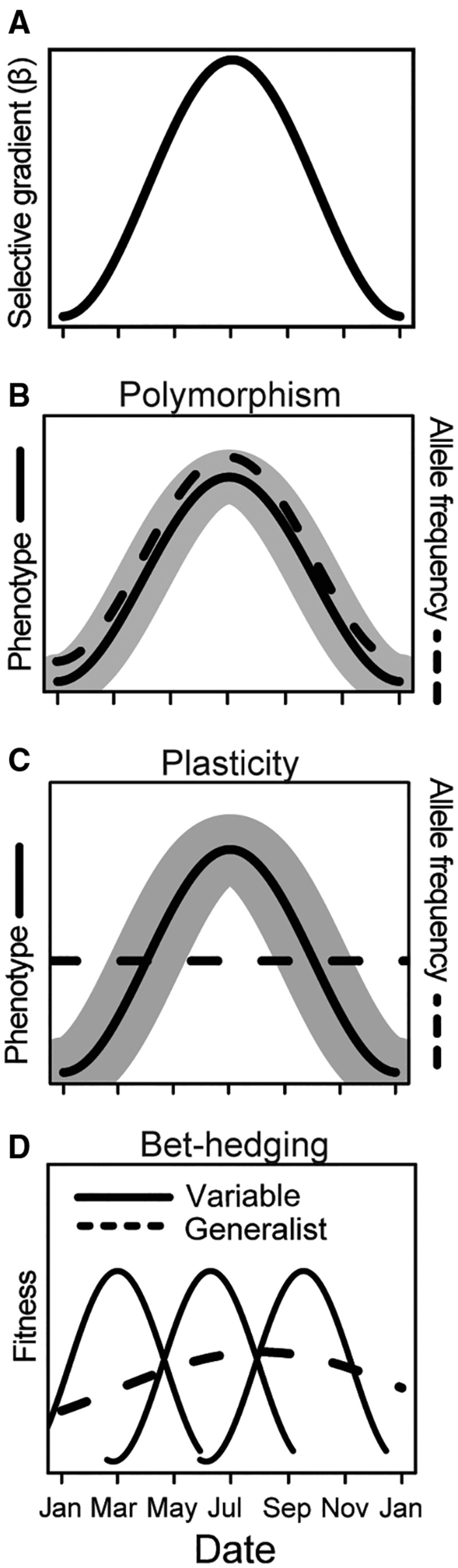
Outcomes of adaptation to seasonality. (A) Selective gradients on life history traits fluctuate seasonally. (B) For species with short generation times relative to season length, these fluctuating selective gradients can result in cyclic fluctuations in both phenotype (solid line) and allele frequency (broken line) at polymorphic loci, leading to maintenance of genetic polymorphisms within populations under certain conditions. (C) Seasonal fluctuations can also be accommodated through phenotypic plasticity, whereby a single genotype produces multiple phenotypes in response to environmental variation. (D) Unpredictable fluctuations will favor the evolution of bet-hedging, whereby a single genotype either produces multiple variable phenotypes whose fitness varies across the season (solid lines), or a single generalist phenotype whose fitness in summer is decreased but which has higher cumulative fitness across the year than a specialist phenotype.
Phenotypic plasticity enables genotypes to express diverse phenotypes in response to environmental variation, which are adaptive when they improve fitness in a given environment (Fig. 1C;Van Tienderen 1991; Schlichting and Pigliucci 1998). Plasticity is heritable and responds to selection (Scheiner and Lyman 1991). Seasonality influences the evolution of adaptive plasticity, which is determined by genetic and physiological properties of the organism (e.g., costs and limits to plasticity, genetic architecture of plastic responses) and characteristics of the environment (e.g., predictability and reliability of cues, grain of environmental variation relative to generation time) (Levins 1968; Van Tienderen 1991; DeWitt et al. 1998; Schlichting and Pigliucci 1998;).
Two leading hypotheses for the evolution of plasticity of thermal hardiness in seasonal environments make opposing predictions: the latitudinal hypothesis predicts that plasticity will increase with increasing seasonality due to increased environmental variation (Janzen 1967), while the trade-off hypothesis predicts that plasticity will be lower in seasonal environments due to a trade-off between inherent and inducible hardiness (Cavicchi et al. 1995; Stillman 2003; Overgaard et al. 2011). Support for both hypotheses is mixed. The latitudinal hypothesis is most often supported in situations where behavioral thermoregulation is limited (thus increasing selection on physiological sensitivity), such as for dormant life stages or aquatic habitats where spatial thermal heterogeneity is reduced (Gunderson and Stillman 2015; Shah et al. 2017). The trade-off hypothesis is supported in some groups of animals and not others, suggesting that our knowledge of the costs and mechanisms of thermal plasticity are incomplete (Stillman 2003).
Phenotypic plasticity can be expressed within a single generation (at timescales ranging from rapid hardening responses through developmental acclimatization) or across generations (aka parental environmental effects, Mousseau and Fox 1998); the rate of environmental change compared with the generation time of organisms is pertinent to which evolves more readily (Gilchrist 1995). Within- and across-generation plasticity can sometimes evolve in concert, with one sometimes influencing the adaptive value and evolution of the other (Ezard et al. 2014; Kuijper and Hoyle 2015). Phenological plasticity is an important class of plasticity, and will be discussed further in “How do selection on phenology and the physiological niche interact?”.
Some aspects of seasonal fluctuations are unpredictable, such as frequency of extreme events, which can favor the evolution of bet-hedging (Seger and Brockman 1987; Van Tienderen 1991). Bet-hedging describes a form of risk-spreading, whereby the fitness in benign environments is decreased in order to increase fitness across all environments (formally, the mean arithmetic fitness of a strategy is reduced in order to decrease variance in fitness, thus increasing geometric mean fitness). For example, variation in insect diapause or seed dormancy may diversify the environments experienced by individuals (Hopper 1999; Venable 2007). Bet-hedging can occur through genotypes producing variable phenotypes (e.g., variation in germination time; Venable 2007), or genotypes producing a single generalist phenotype (Fig. 1D;Van Tienderen 1991).
Gaps in knowledge
The specific ecological and genetic conditions that maintain seasonal polymorphisms over long periods of time are unclear, as is the extent to which polymorphisms that are maintained by seasonal fluctuations in turn contribute to adaptation to geographically variable environments. Regarding plasticity, how do within and across-generational plasticity jointly evolve in response to seasonality? How does the interaction of predictable seasonal variation and unpredictable extreme events influence the evolution of plasticity versus bet-hedging? How will intrinsic and extrinsic factors influence the evolution of each strategy? At the ecophysiological level, the presence of seasonally fluctuating polymorphisms is a tantalizing hint of genetic trade-offs between stress hardiness and reproduction. Functionally characterizing the physiological pathways linking specific environmental drivers to selection on these loci will further our understanding of the pathways and processes underlying seasonal adaptation.
Significance and future prospects
Contemporary climate change is pushing environmental variation beyond the boundaries of past selection in a variety of ways: altering seasonal amplitude, shifting means and variation, and changing the onset of seasonal events. Adaptation to highly seasonal environments potentially impacts responses to environmental change in two ways: (1) if seasonality increases genetic polymorphisms in populations, the increased genetic variation may increase the adaptive response to climate change (Schmidt and Conde 2006); and (2) adaptive phenotypic plasticity and bet-hedging could promote population persistence during periods of rapid environmental change, potentially altering selective gradients (Bay et al. 2017). Determining more broadly the relative contributions of these three outcomes of adaptation in the response to seasonal environments in diverse animal and plant populations will enable broad-scale insights into the potential for adaptive responses to climate change. These outcomes will also affect how population dynamics respond to environmental change, and thus how ecological feedbacks will shape evolutionary processes, promoting adaptation or increasing risk of extinction (Winder and Schindler 2004; Kokko and Lopez-Sepulcre 2007; Schoener 2011; Betini et al. 2017).
An increase in extreme weather events (Easterling et al. 2000) may alter the predictability of seasonal environments and shift the outcome of adaptation toward bet-hedging or a fixed generalist strategy. Similarly, phenotypic plasticity may become less reliable if the relationship between cues and environmental drivers of selection become decoupled, which will lead to directional selection on cue sensitivity. Plasticity may either promote or impede adaptation to sustained environmental change, depending on the magnitude and direction of the plastic response compared with the optimal phenotype (Lynch and Lande 1993; Chevin et al. 2010; Bay et al. 2017). Including seasonality in models for organisms with multiple generations per season suggests that phenotypic plasticity (including the evolution of plasticity) will contribute relatively more than genetic evolution to climate change responses in more seasonal environments, and that plasticity can facilitate evolution by buffering seasonal variation in selection (Kingsolver and Buckley 2017).
How do selection on phenology and the physiological niche interact?
Background
When a single organism experiences seasonal fluctuation within its lifetime, that organism must be able to withstand the full range of seasonal environmental variation. Despite the fitness disadvantages associated with delaying reproduction, organisms often respond to temporal variation by limiting reproduction to specific seasons (Tuljapurkar 1990). Environmentally cued phenology is critical to matching each life stage to seasonal environments within the limits of physiological performance. Conversely, variation in physiological traits underlies phenology (Hereford 2017). Differences in phenology across latitude can buffer thermal exposure of different life stages across seasonal changes in the environments, effectively preserving the thermal niche across gradients in seasonality. For example, organisms frequently shorten growing seasons (with concomitant lengthening of dormancy) at high relative to low latitudes such that the reproductive stages of different populations experience relatively similar thermal environments for reproduction despite strong gradients in seasonality (Bradshaw and Lounibos 1977; Ragland and Kingsolver 2008; Sheldon and Tewksbury 2014). Any modifications to life cycle timing therefore alter the selective environment experienced by a given life stage or critical life-history event (Donohue et al. 2010; Donohue 2014). Phenological shifts will have the maximum impact on fitness in the spring and fall when temperatures are crossing thresholds for activity, relative to mid-season when temperatures are uniformly hot (Levy et al. 2016).
In the above examples, behavior and phenology serve to homogenize natural selection on some focal trait linked to fitness, for example, the thermal optimum for performance traits such as sprint speed or flight duration, by allowing organisms to remain within their optimal or “pejus” temperatures (Pörtner 2010). However, changes in behavior and phenology can also increase heterogeneity of selective environments on other traits. For example, different thermoregulatory behaviors may lead to different exposure to predation (Huey and Slatkin 1976), and changes in phenology may change the availability of seasonally fluctuating food resources (Visser et al. 2006). Even considering only the thermal environment, changes in phenology cannot completely buffer against changing climates. For example, estimates of development rate based on the thermal sensitivity of insect development suggest that phenological shifts have partially, but incompletely buffered exposure to warmer temperatures associated with climate change (Buckley et al. 2017). The consequences of phenological shifts may also span generations, in cases where parental phenology determines the selective environment experienced by offspring (Crozier et al. 2008; Sheriff et al. 2015; Edwards et al. 2017).
In addition to altering phenology, spatial or behavioral adjustments can also determine an organism’s exposure to seasonal environmental stresses (Williams et al. 2016). Changes in thermoregulatory behavior across latitude or altitude can lead to similar body temperatures despite strong environmental clines (Adolph 1990; Huey et al. 2003). Differences in thermoregulatory ability across life stages can alter the strength of selection on physiological sensitivities, with less mobile life stages experiencing stronger selection on physiology, while mobile life stages can accommodate changing environmental conditions through shifts in thermoregulatory behavior (Kingsolver et al. 2011). Together with shifts in phenology, these forms of habitat selection will impact the selective environment and the degree of stress hardiness required by a given life stage.
Gaps in knowledge
A first step toward understanding the impacts of phenological shifts on the physiological niche is to incorporate stage-specific physiological sensitivity into models predicting responses to environmental change. Any phenological shift that decouples a hardy life stage from a stressful period should increase mortality. There is increasing evidence that considering the physiological sensitivity of multiple life history stages may significantly improve forecasts of climate change impacts compared with predictions based on single life stages. Negative effects on a sensitive life stage can counter benefits in more robust life stages, and in some cases reverse predictions of relative susceptibility of species or populations to climate-induced declines (Radchuk et al. 2013; Levy et al. 2015). Moreover, different components of fitness differ in their thermal sensitivity (Huey and Berrigan 2001; Bestion et al. 2015), making it a challenge to determine which are the most appropriate fitness components to measure for any given system.
Another gap in knowledge lies in predicting when phenology versus physiological sensitivity will respond to selection. Bradshaw and Holzapfel (2008) argue that the majority of known responses to contemporary climate change involve changes in phenology, not thermal physiology. This may partially reflect that phenology is more commonly measured. There are examples of rapid evolution of thermal physiology (Angilletta et al. 2007; Higgins et al. 2014; Diamond et al. 2017), suggesting that as more data become available we may see more instances of evolution of thermal physiology. Alternatively, precipitation changes may be more important than temperature in driving evolution in response to climate change (Siepielski et al. 2017). In some cases, phenological and physiological traits are genetically correlated, and will thus evolve jointly (Scheiner and Istock 1991; Wilczek et al. 2010). Finally, the degree to which phenological cuing predicts selective environments at different life stages and thereby alters adaptive outcomes is also important. Empirical studies could test how changes in phenology influence adaptive dynamics in other traits, such as physiological sensitivities. To our knowledge, there are currently no predictive models of the joint evolution of phenology and physiological sensitivity.
Significance and future prospects
A key next step is to identify sets of conditions under which phenology versus physiological sensitivity should evolve so that empirical studies of the evolutionary potential of particular traits could be prioritized. Comparing evolution in systems with constrained phenology (due to day-length cues, snow melt constraints, etc.) to those where environmental conditions (e.g., temperature, water availability) both determine phenology and exert selection is one potentially powerful approach. It is important to determine when evolution of phenology is sufficient to maintain fitness in the face of changing climates, versus when physiological adaptation is also required. One hypothesis is that phenological shifts will be the primary evolutionary response to changing environments when developmental transitions between stress-hardy and stress-susceptible life stages coincide with seasonal transitions (e.g., spring and fall). Conversely, phenological shifts are unlikely to affect stage-specific environmental exposure when developmental transitions occur mid-season. For example, for organisms with multiple generations during the growing season, all life-stages will experience summer temperatures, so phenological shifts are unlikely to buffer thermal exposure in mid-summer, suggesting that evolution of physiological sensitivity may be more important in these cases (Levins 1968).
What are the critical physiological mechanisms governing seasonal responses, and are they genetically constrained?
Background
A core set of key phenotypes facilitate seasonal adaptation, including environmental sensing and downstream responses, thermal hardiness and thermoregulation, and dormancy. These complex seasonal phenotypes consist of coordinated modules of independent, but functionally related, traits. For example, during preparation for dormancy, mammals and insects must down-regulate reproduction, up-regulate fat accumulation and stress hardiness, then down-regulate metabolism (Koštál 2006; Staples 2016). These complex phenotypes are jointly regulated by single cues or the integration of multiple cues. Linking environmental changes to organismal responses requires elucidating the pathways through which environmental cues are sensed and transduced into physiological responses (Jennings et al. 2017) and how multiple traits may be coordinated by a single cue (Stager et al. 2015). One way that a single cue can control multiple traits is through pleiotropy, wherein one gene regulates multiple processes. Pleiotropy has been documented for the environmental regulation of multiple phenological transitions in plants, sometimes with the gene functioning in the same pathways and sometimes not (Chiang et al. 2009; Jiang et al. 2012; Auge et al. 2017). Pleiotropy may both promote and hinder multi-trait adaptation (Griswold and Whitlock 2003; Brakefield 2006; Wagner et al. 2008). On the one hand, placing the control of multiple traits under the influence of a single master regulator can help to better integrate whole-organism responses. If different traits are regulated by the same cue, changes in the seasonal coordination of cues may not disrupt the integration of functionally related phenotypes (Sinclair et al. 2013). On the other hand, negative or antagonistic pleiotropic effects can constrain the expression of optimal phenotypes of individual traits, compared with those that are more modular in their expression. For example, increases in stress resistance may trade-off against investment in other life history traits, such as fecundity (Schmidt et al. 2005). However, pleiotropic genes do not always regulate multiple traits through concordant pathways or modes of gene regulation (Auge et al. 2017). For example, genetic correlations between thermal hardiness of larval and adult Drosophila melanogaster flies are weak or absent, with associated genes mainly affecting hardiness in only one life stage (Freda et al. 2017). This may be important if different life stages inhabit distinct thermal environments (Kingsolver et al. 2011; Woods et al. 2015). Traits are also integrated at the physiological, morphological, and behavioral levels, and these types of constraints can also constrain evolutionary pathways (Ghalambor et al. 2003).
If traits are regulated by different cues—for example, if temperature affects one suite of traits and day-length another—disruption of the seasonal coordination of these environmental cues may also disrupt the integrated organismal response (Moyes et al. 2011; Kristensen et al. 2015). Both genetic modularity and modular responses to different seasonal cues may allow for fine-tuning of individual trait responses, but this potential may come at a cost of reduced robustness in the integrated response if environmental cues that were once synchronized become strongly asynchronous.
Knowledge gaps
We currently lack a detailed picture of the functional linkages between critical sensory systems, the physiological and developmental changes these sensory systems induce, and the underlying genetic architecture of integrated seasonal phenotypes for any species (Caro et al. 2013; Meuti and Denlinger 2013). Our understanding of the degree of evolutionary conservation of mechanisms governing seasonal adaptation across species or populations is still at the anecdotal stage, lacking general principles. For example, some genes that regulate flowering time are conserved across flowering plants, but some are not (Simpson 2004). Similarly, while regulatory network structure seems to be largely conserved across species of songbirds that diverged roughly 45 million years ago, only a subset of the genes involved in those networks respond similarly to changing seasons across species (Cheviron and Swanson 2017). Another outstanding question relates to the degree and nature of genetic constraint for traits that underlie seasonal adaptations. Potential genetic constraints stem from insufficient genetic variation either for single traits, or for multi-trait combinations (Arnold 1992). Similarly, constraints due to pleiotropic effects on seasonally adaptive trait complexes are understudied, but are now receiving increasing attention. Assessing magnitudes and causes of genetic correlations among traits associated with adaptation to seasonality would provide important data on the evolutionary potential of responses to climate change (Shaw and Etterson 2012). An important next step is to incorporate genetic architectures of responses into models predicting responses to environmental change (Bay et al. 2017).
Significance and future prospects
In taxa in which physiological determinates of phenology are well understood, it will be important to determine the degree to which common genes and pathways regulate responses to the environment, and then use a comparative approach to assess conservation of function in related species. The inherent plasticity of phenological traits will complicate this effort; genotype-by-environment interactions can only be assessed by measuring genotypes, or the effects of specific alleles, across multiple environments. For example, Genome Wide Association Studies (GWAS) for phenology phenotypes may need to be applied across different thermal environments (Gienapp et al. 2017). Moreover, complex traits, such as those relevant to seasonal adaptation, are likely underlain by many genetic variants of small effect, which are challenging to discover using traditional GWAS alone (Rockman 2012). Physiological traits are environmentally labile and often technically demanding to measure, requiring “low-throughput” acclimation experiments and extensive phenotyping efforts (Cobb et al. 2013). However, even lacking specific knowledge of physiological mechanisms, quantitative genetic approaches can inform predictions of evolutionary responses to changing climates, and as a result, these studies represent important next steps (Reed et al. 2016).
A detailed understanding of the constraints on the evolution of the dynamic traits that underlie seasonal adaptation can enable predictions of evolutionary responses to changing climates (N.R. Senner, M. Stager, and Z.A. Cheviron, manuscript in review). As a result, mechanistic studies of seasonal adaptations not only inform basic questions on the evolution of complex traits, but also provide key insights into the robustness of species and populations in a changing world. Knowledge of the mechanisms and genes regulating phenological and behavioral adaptations, combined with knowledge of stage-specific physiological sensitivity, can be applied to predicting the geographic range of species (Morin et al. 2007).
How is changing seasonality impacting ecological interaction networks?
Background
Every individual of every species comprising a biotic community pursues its own seasonal schedules of maintenance and reproduction, manifested as annual routines—the scheduling of activities in a regular way over the year (McNamara and Houston 2008). The degree to which species vary in the environmental regulation of their phenology shapes seasonal patterns of presence, abundance, and trophic status, which in turn shapes the seasonality of food webs and other interactions (McMeans et al. 2015). As climate change decouples cues and drivers of selection, organisms are shifting phenology to differing degrees (Edwards and Richardson 2004), because the underlying norms of reaction for responses to cues differ among organisms. This is leading to mismatches in ecological interactions, such as trophic and competitive interactions that affect fitness (Visser and Holleman 2001; Winder and Schindler 2004). As an example, breeding birds that rely on insects to feed nestlings have not altered their breeding time to match the advanced date of insect emergence (Visser and Holleman 2001), resulting in avian population declines in temperate regions (Both et al. 2009). In some cases, natural selection may act to retain synchrony between partners (van Asch et al. 2013), but in other cases heritable variation in reaction norms will not be sufficient to keep pace with climate change (Visser 2008).
Gaps in knowledge
In order to predict how changing seasonality will impact ecological interaction networks, we need to move beyond simple dyadic interactions (i.e., species vs. an abiotic condition, or one species vs. another species). To understand how network interactions are affected by shifts in phenology due to climate change, we need to assess how interaction strengths change systematically across gradients in seasonality (Humphries et al. 2017). One option for tackling these problems would be to use large-scale, coordinated sampling of interaction strengths in a relatively simple ecosystem replicated across a seasonal gradient with a known trajectory of environmental change. The interacting partners would need to be amenable to common garden experiments and to laboratory study in order to characterize their reaction norms in response to cues. Leveraging a system where long-term information on phenology exists (e.g., Long-Term Ecological Research sites [LTER], funded by US National Science Foundation) could be fruitful. Ideally, the system would also allow for a deeper understanding of the conditions under which top-down versus bottom-up processes dominate responses to climate change.
Evolutionary responses could allow species to maintain synchronization with the critical resources they require. The timing of life history events is heritable traits that are subject to selection (Savolainen et al. 2007). However, the strength of selection will vary with the strength of the temporal overlap of the ecological interaction. A major gap in our knowledge is how changes in temporal overlap among interacting species will alter selection and evolutionary responses to climate change, including the extent to which evolutionary responses may restore mismatched interactions and thereby stabilize interaction networks.
Phenological synchronization is believed to be especially critical for species in more seasonal environments where resources tend to be available during narrow windows of time and where species specialize on one phenological stage of their host (Varpe 2012). However, tropical species may also suffer from phenological mismatches, but we know little about recent shifts in phenology in the tropics (Chambers et al. 2013). Thus, how changing seasonality impacts interaction networks is a question beyond the much studied seasonality of boreal and temperate environments.
Significance and future prospects
Climate change will have direct physiological impacts on species that will alter their phenology and ecological interactions. Predicting which species are most vulnerable to climate change thus requires an integration across levels from the individual to the community. Given the strong linkages between cues, individual states, timing of life history events, and fitness (McNamara and Houston 2008), the biological impacts of seasonality may be a particularly fruitful arena for working toward the close integration of physiology, chronobiology, evolutionary ecology, and interaction networks. This work should merge proximate and ultimate perspectives and provide more mechanistic hypotheses about species potential to respond to climate change.
Conclusions
The questions raised here provide several important pathways forward for better understanding adaptation to seasonality. First, we suggest that understanding the relative contributions of genetic polymorphism, adaptive phenotypic plasticity, and bet-hedging in response to seasonality is essential for understanding the capacity for and outcome of adaptive responses to climate change in seasonal environments. Identifying the cues that elicit plasticity, the environmental factors that exert selection on plastic phenotypes, and the probabilistic relationship between them is a major priority for predicting the adaptive value of plastic phenotypes. Next, in order to understand the evolutionary potential for adaptive change, we need to identify under which conditions phenology versus direct physiological changes evolve. This would allow us to determine the amount of genetic constraint on the physiological mechanisms of seasonal responses, particularly by examining genetic architecture across multiple seasonally-linked traits. Finally, we need to link the individual and its annual routine to the community by examining the strength of interactions across gradients of seasonality. This will allow us to determine the relative importance of bottom-up versus top down effects on ecological networks. Several ideal systems for addressing these goals exist—the key now is to concentrate our efforts on these questions. These issues are becoming increasingly pressing within the context of climate change.
Given the scale of the task, efforts aimed at mechanistic dissection of seasonal phenotypes should perhaps be concentrated on a handful of strategically chosen model systems that are investigated from multiple perspectives by a collaborative research community. Ideally, information from these model systems may be leveraged in related species. At present, model systems including the great tit Parus major, the pitcher plant mosquito Wyeomyia smithii, Tephritid flies (Rhagoletis sp.), Drosophila melanogaster, and Arabidopsis sp. stand out as among the best-developed, owing to large research communities and a mature research infrastructure. It continues to be a challenge to combine demanding physiological experiments with genetic approaches, which require large sample sizes and research infrastructure beyond what is available for most species (e.g., genetic mapping of populations). In addition, the relationship between genotype and phenotype is complex and inferences differ depending on genetic background and whether studies are conducted in the field or laboratory (Sarup et al. 2011), highlighting the importance of having a broad and ecologically relevant context for studies of seasonal adaptation.
Acknowledgments
We wish to acknowledge the intellectual contributions of participants in the Evolutionary Impacts of Seasonality workshop during the SICB 2017 meeting in New Orleans, LA. Daniel A. Hahn helped facilitate the workshop and contributed to development of these ideas. Lori Strong, Brett Burk, Ruedi Birenheide, and Richard Blob provided technical and administrative support. We thank Cameron Ghalambor and an anonymous reviewer for helpful comments on a previous draft.
Funding
This work was supported by the Society of Integrative and Comparative Biology (SICB), the Company of Biologists, the National Science Foundation [IOS 1637201 to C.M.W. and G.J.R.; IOS 1558159 to C.M.W.; IOS 1700773 to G.J.R.; DEB 1020963 and IOS 1146383 to K.D.], the National Institutes of Health [NIH R01GM100366 to P.S.S.] and the Fulbright Arctic Initiative [to Ø.V.].
References
- Adolph SC. 1990. Influence of behavioral thermoregulation on microhabitat use by two Sceloporus lizards. Ecology 71:315–27. [Google Scholar]
- Angilletta MJ Jr, Wilson RS, Niehaus AC, Sears MW, Navas CA, Ribeiro PL.. 2007. Urban physiology: city ants possess high heat tolerance. PLoS One 2:e258.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SJ. 1992. Constraints on phenotypic evolution. Am Nat 140:S85–S107. [DOI] [PubMed] [Google Scholar]
- Auge GA, Blair LK, Neville H, Donohue K. Forthcoming 2017. Maternal vernalization and vernalization-pathway genes influence progeny seed germination. New Phytol. (doi: 10.1111/nph.14520). [DOI] [PubMed] [Google Scholar]
- Bay RA, Rose N, Barrett R, Bernatchez L, Ghalambor CK, Lasky JR, Brem RB, Palumbi SR, Ralph P.. 2017. Predicting responses to contemporary environmental change using evolutionary response architectures. Am Nat 189:463–473. [DOI] [PubMed] [Google Scholar]
- Behrman EL, Watson SS, O’Brien KR, Heschel MS, Schmidt PS.. 2015. Seasonal variation in life history traits in two Drosophila species. J Evol Biol 28:1691–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergland AO, Behrman EL, O’Brien KR, Schmidt PS, Petrov DA.. 2014. Genomic evidence of rapid and stable adaptive oscillations over seasonal time scales in Drosophila. PLoS Genet 10:e1004775.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestion E, Teyssier A, Richard M, Clobert J, Cote J.. 2015. Live fast, die young: experimental evidence of population extinction risk due to climate change. PLoS Biol 13:e1002281.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betini GS, McAdam AG, Griswold CK, Norris DR.. 2017. A fitness trade-off between seasons causes multigenerational cycles in phenotype and population size. Elife 6:e18770.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both C, vsan Asch M, Bijlsma RG, Burg AB, Visser ME.. 2009. Climate change and unequal phenological changes across four trophic levels: constraints or adaptations? J Anim Ecol 78:73–83. [DOI] [PubMed] [Google Scholar]
- Bradshaw WE, Holzapfel CM.. 2001. Genetic shift in photoperiodic response correlated with global warming. Proc Natl Acad Sci U S A 98:14509–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw WE, Holzapfel CM.. 2008. Genetic response to rapid climate change: it’s seasonal timing that matters. Mol Ecol 17:157–66. [DOI] [PubMed] [Google Scholar]
- Bradshaw WE, Lounibos LP.. 1977. Evolution of dormancy and its photoperiodic control in pitcher-plant mosquitoes. Evolution 31:546–67. [DOI] [PubMed] [Google Scholar]
- Brakefield PM. 2006. Evo–devo and constraints on selection. Trends Ecol Evol 21:362–8. [DOI] [PubMed] [Google Scholar]
- Buckley LB, Arakiki AJ, Cannistra AF, Kharouba HM, Kingsolver JG.. 2017. Insect development, thermal plasticity and fitness implications in changing, seasonal environments. Integr Comp Biol (doi:10.1093/icb/icx032). [DOI] [PubMed] [Google Scholar]
- Caro SP, Schaper SV, Hut RA, Ball GF, Visser ME.. 2013. The case of the missing mechanism: how does temperature influence seasonal timing in endotherms? PLoS Biol 11:e1001517.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho GR, Crisp DJ.. 1987. The clonal ecology of Daphnia magna (Crustacea: Cladocera): I. Temporal changes in the clonal structure of a natural population. J Anim Ecol 56:453–68. [Google Scholar]
- Cavicchi S, Guerra D, La Torre V, Huey RB.. 1995. Chromosomal analysis of heat-shock tolerance in Drosophila melanogaster evolving at different temperatures in the laboratory. Evolution 49:676–84. [DOI] [PubMed] [Google Scholar]
- Chambers LE, Altwegg R, Barbraud C, Barnard P, Beaumont LJ, Crawford RJM, Durant JM, Hughes L, Keatley MR, Low M, et al. 2013. Phenological changes in the southern hemisphere. PLoS One 8:e75514.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevin L-M, Lande R, Mace GM.. 2010. Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biol 8:e1000357.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheviron ZA, Swanson DL.. 2017. Comparative transcriptomics of seasonal phenotypic flexibility in two North American songbirds. Integr Comp Biol (doi:10.1093/icb/icx118). [DOI] [PubMed] [Google Scholar]
- Chiang GCK, Barua D, Kramer EM, Amasino RM, Donohue K.. 2009. Major flowering time gene, flowering locus C, regulates seed germination in Arabidopsis thaliana. Proc Natl Acad Sci U S A 106:11661–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian KA, Bedford GS.. 1995. Seasonal changes in thermoregulation by the Frillneck lizard, Chlamydosaurus kingii, in tropical Australia. Ecology 76:124–32. [Google Scholar]
- Cobb JN, Declerck G, Greenberg A, Clark R, McCouch S.. 2013. Next-generation phenotyping: requirements and strategies for enhancing our understanding of genotype–phenotype relationships and its relevance to crop improvement. Theor Appl Genet 126:867–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogni R, Kuczynski C, Koury S, Lavington E, Behrman EL, O’Brien KR, Schmidt PS, Eanes WF.. 2014. The intensity of selection acting on the couch potato gene—spatial–temporal variation in a diapause cline. Evolution 68:538–48. [DOI] [PubMed] [Google Scholar]
- Cogni R, Kuczynski K, Lavington E, Koury S, Behrman EL, O’Brien KR, Schmidt PS, Eanes WF.. 2015. Variation in Drosophila melanogaster central metabolic genes appears driven by natural selection both within and between populations. Proc R Soc Lond B Biol Sci 282, published online (doi: 10.1098/rspb.2014.2688). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover DO. 1992. Seasonality and the scheduling of life history at different latitudes. J Fish Biol 41:161–78. [Google Scholar]
- Crozier LG, Hendry AP, Lawson PW, Quinn TP, Mantua NJ, Battin J, Shaw RG, Huey RB.. 2008. Potential responses to climate change in organisms with complex life histories: evolution and plasticity in Pacific salmon. Evol Appl 1:252–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt TJ, Sih A, Wilson DS.. 1998. Costs and limits of phenotypic plasticity. Trends Ecol Evol 13:77–81. [DOI] [PubMed] [Google Scholar]
- Diamond SE, Chick L, Perez A, Strickler SA, Martin RA.. 2017. Rapid evolution of ant thermal tolerance across an urban–rural temperature cline. Biol J Linn Soc 121:248–57. [Google Scholar]
- Dobzhansky T, Ayala FJ.. 1973. Temporal frequency changes of enzyme and chromosomal polymorphisms in natural populations of Drosophila. Proc Natl Acad Sci U S A 70:680–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue K. 2014. Why ontogeny matters during adaptation: developmental niche construction and pleiotropy across the life cycle in Arabidopsis thaliana. Evolution 68:32–47. [DOI] [PubMed] [Google Scholar]
- Donohue K, Rubio de Casas R, Burghardt LT, Kovach K, Willis CG.. 2010. Germination, postgermination adaptation, and species ecological ranges. Annu Rev Ecol Evol Syst 41:293–319. [Google Scholar]
- Easterling DR, Meehl GA, Parmesan C, Changnon SA, Karl TR, Mearns LO.. 2000. Climate extremes: observations, modeling, and impacts. Science 289:2068–74. [DOI] [PubMed] [Google Scholar]
- Edwards BE, Burghardt LT, Kovach K, Donohue K.. 2017. Canalization of seasonal phenology in the presence of developmental variation: seed dormancy cycling in an annual weed. Integr Comp Biol (doi: 10.1093/icb/icx065). [DOI] [PubMed] [Google Scholar]
- Edwards M, Richardson AJ.. 2004. Impact of climate change on marine pelagic phenology and trophic mismatch. Nature 430:881–4. [DOI] [PubMed] [Google Scholar]
- Ezard THG, Prizak R, Hoyle RB.. 2014. The fitness costs of adaptation via phenotypic plasticity and maternal effects. Funct Ecol 28:693–701. [Google Scholar]
- Freda PJ, Alex JT, Morgan TJ, Ragland GJ.. 2017. Genetic decoupling of thermal hardiness across metamorphosis in Drosophila melanogaster. Integr Comp Biol (doi:10.1093/icb/icx102). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fretwell SD. 1972. Populations in a seasonal environment. Monogr Popul Biol 5:1–217. [PubMed] [Google Scholar]
- Ghalambor CK, Walker JA, Reznick DN.. 2003. Multi-trait selection, adaptation, and constraints on the evolution of burst swimming performance. Integr Comp Biol 43:431–8. [DOI] [PubMed] [Google Scholar]
- Gienapp P, Laine VN, Mateman AC, van Oers K, Visser ME.. 2017. Environment-dependent genotype-phenotype associations in avian breeding time. Front Genet 8:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist GW. 1995. Specialists and generalists in changing environments. I. Fitness landscapes of thermal sensitivity. Am Nat 146:252–70. [Google Scholar]
- Griswold CK, Whitlock MC.. 2003. The genetics of adaptation: the roles of pleiotropy, stabilizing selection and drift in shaping the distribution of bidirectional fixed mutational effects. Genetics 165:2181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson AR, Stillman JH.. 2015. Plasticity in thermal tolerance has limited potential to buffer ectotherms from global warming. Proc R Soc Lond B Biol Sci 282:20150401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldane JBS, Jayakar SD.. 1963. Polymorphism due to selection in varying directions. J Genet 58:237–42. [Google Scholar]
- Hereford J. 2017. Thermal performance curves reveal variation in the seasonal niche of a short-lived annual. Integr Comp Biol (doi:10.1093/icb/icx089). [DOI] [PubMed] [Google Scholar]
- Higgins JK, MacLean HJ, Buckley LB, Kingsolver JG.. 2014. Geographic differences and microevolutionary changes in thermal sensitivity of butterfly larvae in response to climate. Funct Ecol 28:982–9. [Google Scholar]
- Hopper KR. 1999. Risk-spreading and bet-hedging in insect population biology. Annu Rev Entomol 44:535–60. [DOI] [PubMed] [Google Scholar]
- Huey RB, Berrigan D.. 2001. Temperature, demography, and ectotherm fitness. Am Nat 158:204–10. [DOI] [PubMed] [Google Scholar]
- Huey RB, Hertz PE, Sinervo B.. 2003. Behavioral drive versus behavioral inertia in evolution: a null model approach. Am Nat 161:357–66. [DOI] [PubMed] [Google Scholar]
- Huey RB, Slatkin M.. 1976. Cost and benefits of lizard thermoregulation. Q Rev Biol 51:363–84. [DOI] [PubMed] [Google Scholar]
- Humphries MM, Studd EK, Menzies AK, Boutin S.. 2017. To everything there is a season: summer-to-winter food webs and the functional traits of keystone species. Integr Comp Biol (doi:10.1093/icb/icx119). [DOI] [PubMed] [Google Scholar]
- Janzen DH. 1967. Why mountain passes are higher in the tropics. Am Nat 101:233–49. [Google Scholar]
- Jennings KJ, Chasles M, Cho H, Mikkelsen J, Bentley GE, Keller M, Kriegsfeld LJ.. 2017. The preoptic area and the RFamide-related peptide neuronal system gate seasonal changes in chemosensory processing. Integr Comp Biol (doi:10.1093/icb/icx099). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Kumar S, Eu YJ, Jami SK, Stasolla C, Hill RD.. 2012. The Arabidopsis mutant, fy-1, has an ABA-insensitive germination phenotype. J Exp Bot 63:2693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsolver JG, Arthur Woods H, Buckley LB, Potter KA, MacLean HJ, Higgins JK.. 2011. Complex life cycles and the responses of insects to climate change. Integr Comp Biol 51:719–32. [DOI] [PubMed] [Google Scholar]
- Kingsolver JG, Buckley LB.. 2017. Evolution of plasticity and adaptive responses to climate change along climate gradients. Proc Biol Sci 284, published online (doi: 10.1098/rspb.2017.0386.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsolver JG, Diamond SE, Gomulkiewicz R.. 2015. Curve-thinking: understanding reaction norms and developmental trajectories as traits In: Martin LB, Ghalambor CK, Woods HA, editors. Integrative organismal biology. Hoboken (NJ: ): Wiley Blackwell. p. 39–54. [Google Scholar]
- Kokko H, Lopez-Sepulcre A.. 2007. The ecogenetic link between demography and evolution: can we bridge the gap between theory and data? Ecol Lett 10:773–82. [DOI] [PubMed] [Google Scholar]
- Koštál V. 2006. Eco-physiological phases of insect diapause. J Insect Physiol 52:113.. [DOI] [PubMed] [Google Scholar]
- Kristensen NP, Johansson J, Ripa J, Jonzén N.. 2015. Phenology of two interdependent traits in migratory birds in response to climate change. Proc R Soc Lond B Biol Sci 282, published online (doi: 10.1098/rspb.2015.0288). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijper B, Hoyle RB.. 2015. When to rely on maternal effects and when on phenotypic plasticity? Evolution 69:950–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levins R. 1968. Evolution in changing environments: some theoretical explorations. Princeton (NJ: ): Princeton University Press. [Google Scholar]
- Levy O, Buckley LB, Keitt TH, Angilletta MJ.. 2016. Ontogeny constrains phenology: opportunities for activity and reproduction interact to dictate potential phenologies in a changing climate. Ecol Lett 19:620–8. [DOI] [PubMed] [Google Scholar]
- Levy O, Buckley LB, Keitt TH, Smith CD, Boateng KO, Kumar DS, Angilletta MJ.. 2015. Resolving the life cycle alters expected impacts of climate change. Proc R Soc Lond B Biol Sci 282, published online (doi: 10.1098/rspb.2015.0837). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisovski S, Ramenofsky M, Wingfield JC.. 2017. Defining the degree of seasonality and its significance for future research. Integr Comp Biol (doi:10.1093/icb/icx040). [DOI] [PubMed] [Google Scholar]
- Lynch M, Lande R.. 1993. Evolution and extinction in response to environmental change In: Kareiva P, Kingsolver JG, Huey RB, editors. Biotic interactions and global change. Sunderland (MA: ): Sinauer. [Google Scholar]
- McMeans BC, McCann KS, Humphries M, Rooney N, Fisk AT.. 2015. Food web structure in temporally-forced ecosystems. Trends Ecol Evol 30:662–72. [DOI] [PubMed] [Google Scholar]
- McNamara JM, Houston AI.. 2008. Optimal annual routines: behaviour in the context of physiology and ecology. Philos Trans R Soc B Biol Sci 363:301–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel A, Sparks TH, Estrella N, Koch E, Aasa A, Ahas R, Alm-KÜBler K, Bissolli P, BraslavskÁ OG, Briede A, et al. 2006. European phenological response to climate change matches the warming pattern. Glob Change Biol 12:1969–76. [Google Scholar]
- Meuti ME, Denlinger DL.. 2013. Evolutionary links between circadian clocks and photoperiodic diapause in insects. Integr Comp Biol 53:131–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin X, Augspurger C, Chuine I.. 2007. Process-based modeling of species’ distributions: what limits temperate tree species’ range boundaries? Ecology 88:2280–91. [DOI] [PubMed] [Google Scholar]
- Mousseau TA, Fox CW.. 1998. Maternal effects as adaptations. New York (NY: ): Oxford University Press. [Google Scholar]
- Moyes K, Nussey DH, Clements MN, Guinness FE, Morris A, Morris S, Pemberton JM, Kruuk LEB, Clutton-Brock TH.. 2011. Advancing breeding phenology in response to environmental change in a wild red deer population. Glob Change Biol 17:2455–69. [Google Scholar]
- Overgaard J, Kristensen TN, Mitchell KA, Hoffmann AA.. 2011. Thermal tolerance in widespread and tropical Drosophila species: does phenotypic plasticity increase with latitude? Am Nat 178(Suppl 1):S80–96. [DOI] [PubMed] [Google Scholar]
- Padilla DK, Adolph SC.. 1996. Plastic inducible morphologies are not always adaptive: the importance of time delays in a stochastic environment. Evol Ecol 10:105–17. [Google Scholar]
- Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37:637–69. [Google Scholar]
- Pörtner H-O. 2010. Oxygen- and capacity-limitation of thermal tolerance: a matrix for integrating climate-related stressor effects in marine ecosystems. J Exp Biol 213:881–93. [DOI] [PubMed] [Google Scholar]
- Radchuk V, Turlure C, Schtickzelle N.. 2013. Each life stage matters: the importance of assessing the response to climate change over the complete life cycle in butterflies. J Anim Ecol 82:275–85. [DOI] [PubMed] [Google Scholar]
- Ragland GJ, Kingsolver JG.. 2008. Evolution of thermotolerance in seasonal environments: the effects of annual temperature variation and life-history timing in Wyeomyia smithii. Evolution 62: 1345–57. [DOI] [PubMed] [Google Scholar]
- Reed TE, Gienapp P, Visser ME.. 2016. Testing for biases in selection on avian reproductive traits and partitioning direct and indirect selection using quantitative genetic models. Evolution 70:2211–25. [DOI] [PubMed] [Google Scholar]
- Rhomberg LR, Singh RS.. 1988. Evidence for a link between local and seasonal cycles in gene frequencies and latitudinal gene clines in a cyclic parthenogen. Genetica 78:73–9. [DOI] [PubMed] [Google Scholar]
- Rockman MV. 2012. The QTN program and the alleles that matter for evolution: all that’s gold does not glitter. Evolution 66:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarup P, Sørensen JG, Kristensen TN, Hoffmann AA, Loeschcke V, Paige KN, Sørensen P.. 2011. Candidate genes detected in transcriptome studies are strongly dependent on genetic background. PLoS One 6:e15644.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savolainen O, Pyhäjärvi T, Knürr T.. 2007. Gene flow and local adaptation in trees. Annu Rev Ecol Evol Syst 38:595–619. [Google Scholar]
- Scheiner SM, Istock CA.. 1991. Correlational selection on life history traits in the pitcher-plant mosquito. Genetica 84:123–8. [Google Scholar]
- Scheiner SM, Lyman RF.. 1991. The genetics of phenotypic plasticity. II. Response to selection. J Evol Biol 4:23–50. [Google Scholar]
- Schlichting C, Pigliucci M.. 1998. Phenotypic evolution: a reaction norm perspective. Sunderland (MA: ): Sinauer. [Google Scholar]
- Schluter D, Price TD, Rowe L.. 1991. Conflicting selection pressures and life history trade-offs. Proc R Soc Lond B Biol Sci 246:11–7. [Google Scholar]
- Schmidt PS, Conde DR.. 2006. Environmental heterogeneity and the maintenance of genetic variation for reproductive diapause in Drosophila melanogaster. Evolution 60:1602–11. [PubMed] [Google Scholar]
- Schmidt PS, Paaby AB, Heschel MS.. 2005. Genetic variance for diapause expression and associated life histories in Drosophila melanogaster. Evolution 59:2616–25. [PubMed] [Google Scholar]
- Schoener TW. 2011. The newest synthesis: understanding the interplay of evolutionary and ecological dynamics. Science 331:426–9. [DOI] [PubMed] [Google Scholar]
- Seger J, Brockman HJ.. 1987. What is bet-hedging? In Harvey PH, Partridge L, editors. Oxford surveys in evolutionary biology. Oxford: Oxford University Press; p. 182–211. [Google Scholar]
- Shah AA, Funk WC, Ghalambor CK.. 2017. Thermal acclimation ability varies in temperate and tropical aquatic insects from different elevations. Integr Comp Biol (doi:10.1093/icb/icx101). [DOI] [PubMed] [Google Scholar]
- Shaw RG, Etterson JR.. 2012. Rapid climate change and the rate of adaptation: insight from experimental quantitative genetics. New Phytol 195:752–65. [DOI] [PubMed] [Google Scholar]
- Sheldon KS, Tewksbury JJ.. 2014. The impact of seasonality in temperature on thermal tolerance and elevational range size. Ecology 95:2134–43. [DOI] [PubMed] [Google Scholar]
- Sheriff MJ, Buck CL, Barnes BM.. 2015. Autumn conditions as a driver of spring phenology in a free-living arctic mammal. Clim Chang Response 2:4. [Google Scholar]
- Siepielski AM, Morrissey MB, Buoro M, Carlson SM, Caruso CM, Clegg SM, Coulson T, DiBattista J, Gotanda KM, Francis CD, et al. 2017. Precipitation drives global variation in natural selection. Science 355:959–62. [DOI] [PubMed] [Google Scholar]
- Simpson GG. 2004. The autonomous pathway: epigenetic and post-transcriptional gene regulation in the control of Arabidopsis flowering time. Curr Opin Plant Biol 7:570–4. [DOI] [PubMed] [Google Scholar]
- Sinclair BJ, Ferguson LV, Salehipour-shirazi G, MacMillan HA.. 2013. Cross-tolerance and cross-talk in the cold: relating low temperatures to desiccation and immune stress in insects. Integr Comp Biol 53:545–56. [DOI] [PubMed] [Google Scholar]
- Stager M, Swanson DL, Cheviron ZA.. 2015. Regulatory mechanisms of metabolic flexibility in the dark-eyed junco (Junco hyemalis). J Exp Biol 218:767–77. [DOI] [PubMed] [Google Scholar]
- Staples JF. 2016. Metabolic flexibility: hibernation, torpor, and estimation. Compr Physiol 6:737–71. [DOI] [PubMed] [Google Scholar]
- Stillman JH. 2003. Acclimation capacity underlies susceptibility to climate change. Science 301:65.. [DOI] [PubMed] [Google Scholar]
- Tombre IM, Høgda KA, Madsen J, Griffin LR, Kuijken E, Shimmings P, Rees E, Verscheure C.. 2008. The onset of spring and timing of migration in two arctic nesting goose populations: the pink-footed goose Anser brachyrhynchus and the barnacle goose Branta leucopsis. J Avian Biol 39:691–703. [Google Scholar]
- Tuljapurkar S. 1990. Delayed reproduction and fitness in variable environments. Proc Natl Acad Sci U S A 87:1139–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Asch M, Salis L, Holleman LJM, van Lith B, Visser ME.. 2013. Evolutionary response of the egg hatching date of a herbivorous insect under climate change. Nat Clim Chang 3:244–8. [Google Scholar]
- Van Tienderen PH. 1991. Evolution of generalists and specialist in spatially heterogeneous environments. Evolution 45:1317–31. [DOI] [PubMed] [Google Scholar]
- Varpe Ø. 2012. Fitness and phenology: annual routines and zooplankton adaptations to seasonal cycles. J Plankton Res 34:267–76. [Google Scholar]
- Varpe Ø. 2017. Life history adaptations to seasonality: concepts, examples, and perspectives. Integr Comp Biol (doi:10.1093/icb/icx123). [DOI] [PubMed] [Google Scholar]
- Venable DL. 2007. Bet hedging in a guild of desert annuals. Ecology 88:1086–90. [DOI] [PubMed] [Google Scholar]
- Visser ME. 2008. Keeping up with a warming world; assessing the rate of adaptation to climate change. Proc R Soc Lond B Biol Sci 275:649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser ME, Caro SP, van Oers K, Schaper SV, Helm B.. 2010. Phenology, seasonal timing and circannual rhythms: towards a unified framework. Philos Trans R Soc Lond B Biol Sci 365:3113–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser ME, Holleman LJM.. 2001. Warmer springs disrupt the synchrony of oak and winter moth phenology. Proc R Soc Lond B Biol Sci 268:289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser ME, Holleman LJM, Gienapp P.. 2006. Shifts in caterpillar biomass phenology due to climate change and its impact on the breeding biology of an insectivorous bird. Oecologia 147:164–72. [DOI] [PubMed] [Google Scholar]
- Wagner GP, Kenney-Hunt JP, Pavlicev M, Peck JR, Waxman D, Cheverud JM.. 2008. Pleiotropic scaling of gene effects and the ‘cost of complexity’. Nature 452:470–2. [DOI] [PubMed] [Google Scholar]
- Wilczek AM, Burghardt LT, Cobb AR, Cooper MD, Welch SM, Schmitt J.. 2010. Genetic and physiological bases for phenological responses to current and predicted climates. Philos Trans R Soc B Biol Sci 365:3129–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CM, Buckley LB, Sheldon KS, Vickers M, Portner HO, Dowd WW, Gunderson AR, Marshall KE, Stillman JH.. 2016. Biological impacts of thermal extremes: mechanisms and costs of functional responses matter. Integr Comp Biol 56:73–84. [DOI] [PubMed] [Google Scholar]
- Williams CM, Henry HAL, Sinclair BJ.. 2015. Cold truths: how winter drives responses of terrestrial organisms to climate change. Biol Rev 90:214–35. [DOI] [PubMed] [Google Scholar]
- Winder M, Schindler DE.. 2004. Climate change uncouples trophic interactions in an aquatic ecosystem. Ecology 85:2100–6. [Google Scholar]
- Wingfield JC. 2008. Organization of vertebrate annual cycles: implications for control mechanisms. Philos Trans R Soc B Biol Sci 363:425–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield JC, Kenagy GJ.. 1991. Natural regulation of reproductive cycles In: Schreibman M, Jones RE, editors. Vertebrate endocrinology: fundamentals and biomedical implications. New York (NY: ): Academic. [Google Scholar]
- Wittmann MJ, Bergland AO, Feldman MW, Schmidt PS, Petrov DA.. 2017. Segregation lift: a general mechanism for the maintenance of polygenic variation under seasonally fluctuating selection. bioRxiv published online (doi10.1101/115444). [Google Scholar]
- Woods HA, Dillon ME, Pincebourde S.. 2015. The roles of microclimatic diversity and of behavior in mediating the responses of ectotherms to climate change. J Therm Biol 54:86–97. [DOI] [PubMed] [Google Scholar]
- Zera AJ, Harshman LG.. 2001. The physiology of life history trade-offs in animals. Annu Rev Ecol Syst 32:95. [Google Scholar]


