Abstract
Sepsis and trauma are both leading causes of death in the United States and represent major public health challenges. Murine models have largely been used in sepsis and trauma research to better understand the pathophysiological changes that occur after an insult and to develop potential life-saving therapeutic agents. Mice are favorable subjects for this type of research given the variety of readily available strains including inbred, outbred, and transgenic strains. In addition, they are relatively easy to maintain and have a high fecundity. However, pharmacological therapies demonstrating promise in preclinical mouse models of sepsis and trauma often fail to demonstrate similar efficacy in human clinical trials, prompting considerable criticism surrounding the capacity of murine models to recapitulate complex human diseases like sepsis and traumatic injury. Fundamental differences between the two species include, but are not limited to, the divergence of the transcriptomic response, the mismatch of temporal response patterns, differences in both innate and adaptive immunity, and heterogeneity within the human population in comparison to the homogeneity of highly inbred mouse strains. Given the ongoing controversy, this narrative review aims to not only highlight the historical importance of the mouse as an animal research model but also highlight the current benefits and limitations of the model as it pertains to sepsis and trauma. Lastly, this review will propose future directions that may promote further use of the model.
Keywords: animal models, inflammation, mouse, murine, sepsis, shock, translational research, trauma
Introduction
Despite significant advances in the diagnosis and management of sepsis and trauma over the past few decades, severe infection and injury continue to represent major public health challenges (Cohen et al. 2015; El Mestoui et al. 2015; MacKenzie et al. 2006; Vincent et al. 2014; Weir et al. 2010). Collectively, the estimated cost of treating major adult trauma and sepsis in the United States is $51 billion annually (Lagu et al. 2012; Weir et al. 2010). In addition to the considerable costs sepsis and trauma impose on the health care system, they are both significant sources of morbidity and mortality. Although in-hospital mortality to sepsis has declined over the past few decades, mortality remains unacceptably high, estimated at approximately 25–30% overall and as great as 40–50% when shock is present (Cohen et al. 2015; Vincent et al. 2014). The declining in-hospital mortality has been attributed, in part, to improvements in the early recognition of sepsis with prompt initiation of supportive measures such as the administration of intravenous fluids and antibiotics (Rivers et al. 2001). However, others have proposed the improved survival may be due to an increase in reporting of less severe illness with the introduction of new diagnostic codes rather than a true change in the outcome of the disease (Rhee et al. 2014). Regardless, 1-year mortality from sepsis has remained unchanged with these alterations in the hospital course of the patient, such that physicians may have replaced early mortality with late mortality rather than improving long-term outcomes (Iwashyna et al. 2012).
Severe injury has followed a similar course to sepsis over the last decade regarding mortality. Traumatic injury, including suicides, is the leading cause of death in people <45 years of age, and despite our efforts to streamline trauma care, in-hospital mortality resulting from polytrauma remains exorbitantly high, ranging from 7% to 19% (El Mestoui et al. 2015; MacKenzie et al. 2006). Similar to sepsis, recent studies demonstrate a modest improvement in survival over the past decade, which has been primarily attributed to a more rapid transport to a trauma center and early resuscitation of these patients (Brown et al. 2010).
Unfortunately, the incidence of both sepsis and trauma is not declining, and their impact is not solely limited to in-hospital mortality (Lagu et al. 2012; Rhee et al. 2014; Xiao et al. 2006). Patients who survive their initial injury or septic insult frequently succumb to late complications including chronic critical illness and multi-organ failure (Gentile et al. 2012). There is also increasing evidence of emerging phenotypes within the sepsis, burn, and trauma populations, characterized by persistent low-grade organ dysfunction and long-term resource utilization with a failure to return to a previous functional baseline, referred to as chronic critical illness, “induced frailty,” and a “persistent inflammatory-immunosuppressive and catabolic syndrome” (Mira et al. 2016).
Given the significant morbidity, mortality, and costs associated with sepsis and trauma, clinicians and investigators have made considerable efforts to attain a better understanding of the underlying pathophysiology of these disease processes, often through the use of animal models. The most common species used for trauma and sepsis research has been the mouse (Mus musculus) (Fink 2014). From preclinical mouse studies, more than a hundred promising therapies have been developed that target either inflammatory or immunosuppressive pathways in an attempt to prevent or dampen the inflammatory response or stimulate protective immunity. Much to the dismay of the research community, these therapeutic agents have universally failed to demonstrate efficacy in humans despite demonstrating promise in preliminary animal models (Dyson and Singer 2009; Fink 2014; Zanotti-Cavazzoni and Goldfarb 2009). Not only are the proposed therapies largely ineffective, but in some cases, the therapies paradoxically worsen patient outcomes, prompting early termination of clinical trials. To date, not a single therapeutic agent demonstrating efficacy in preclinical animal models for sepsis or trauma is currently utilized in clinical practice, forcing several researchers to question the value of animal models (Efron et al. 2015; Fink 2014; Gentile et al. 2014a; Seok et al. 2013).
The murine model has been appropriately challenged in regards to its ability to replicate complex human disease processes. Not surprisingly, considerable controversy surrounds the topic, given the mouse is the most common animal model used in research, particularly in sepsis and trauma research. Therefore, the aim of this review is to discuss the advantages as well as the current limitations of the murine model in translational research as it pertains to sepsis and trauma. This review will also highlight the historical importance of the mouse as a model organism and discuss recent advances that may increase the relevance of the murine model and further promote its use.
Origin of the Mouse as a Model Organism
To gain a better understanding of the complex cellular and molecular mechanisms that underlie disease processes, researchers often use model organisms, since studying these processes in humans is often not feasible. Several nonmammal model organisms have been used, including but not limited to E. coli, yeast, C. elegans, Drosophila, and zebrafish (Muller and Grossniklaus 2010). While these organisms are suitable models for studying individual developmental processes and cellular functions such as the cell cycle, they are not ideal for studying complex human physiological systems such as the immune, nervous, cardiovascular, and endocrine systems, necessitating mammalian models (Muller and Grossniklaus 2010). Of the mammalian model systems, the mouse has risen as the premier model organism over the past century due to its genetic and physiological similarities to humans. Furthermore, the species provides an integrated biological system to study complex pathological processes such as sepsis and traumatic injury. The foundation of using mammalian model organisms such as the mouse to study human physiology and pathology, however, heavily relies on the similarities between species; hence, significant efforts have been put forth to characterize both the human and mouse genome.
In 1990, the Human Genome Project was launched with the goal of providing a complete and accurate sequence of the human genome. Not long after, in 1999, the Mouse Genome Sequencing Consortium was established to sequence the entire mouse genome. Collaboration between four large sequencing centers including the Broad Institute/Massachusetts Institute of Technology, Center for Genomic Research, the Washington University Genome Sequencing Center, the Wellcome Trust Sanger Institute, and the Baylor College of Medicine Human Genome Sequencing Center along with the international database, Ensembl, provided the entire genomic sequence of the C57BL/6 strain of Mus musculus. The total length of the euchromatic mouse genome was determined to be 2.5 Gb, which is approximately 14% smaller in comparison to the human genome, which contains 2.9 Gb (Mouse Genome Sequencing Consortium et al. 2002). Despite this difference, there is a high degree of conserved synteny between the two species (90%), and alignment of the two genomes reveals several shared nucleotide sequences (40%) (Mouse Genome Sequencing Consortium et al. 2002).
In addition to comparing the entire genomic sequences of these two species, researchers have performed multiple studies to examine specific differences in the genomic expression of mice and humans in response to different forms of systemic inflammation such as endotoxinemia, polymicrobial sepsis, burns, and trauma/hemorrhage (Brownstein et al. 2006; Calvano et al. 2005; Copeland et al. 2005; Lederer et al. 2008; Xiao et al. 2011). Most of this data has been collected and is available through the Inflammation and the Host Response to Injury large-scale collaborative research program, otherwise known as the “Glue Grant.” This data repository serves as a resource to compare transcriptomic, proteomic, and pathway similarities across species that are relevant to the fields of sepsis and trauma. The Mouse Genome Informatics database has also been established as a resource to compare and mine mouse data for primary and translational research (Eppig et al. 2017). This comprehensive data set includes genetic, genomic, and biological data.
Advantages of Using the Murine Model
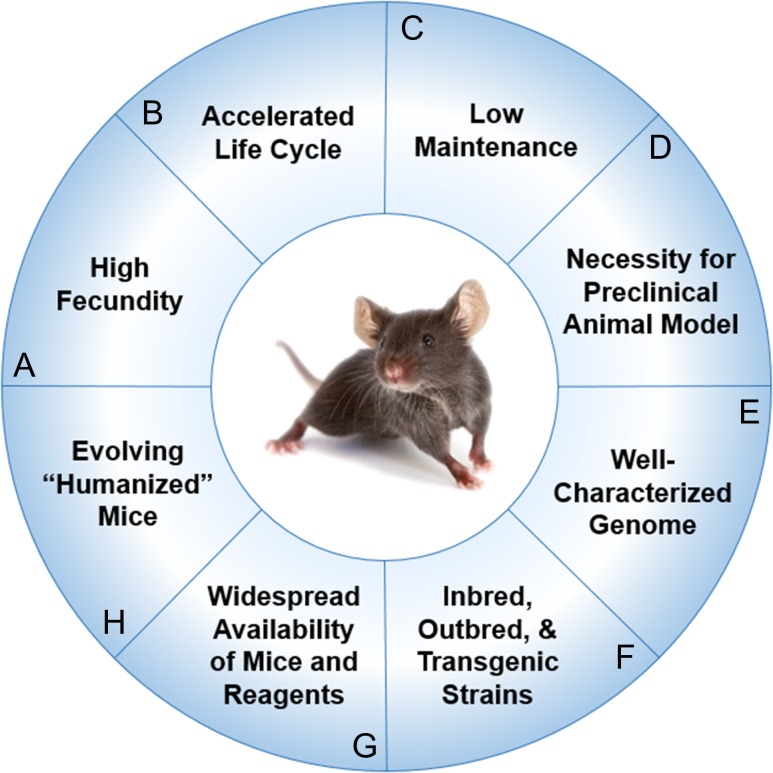
The mouse is the most common preclinical model for sepsis and trauma, as its use has several advantages (Figure 1) (Deitch 1998; Efron et al. 2015; Fink 2014; Fink and Heard 1990; Frink et al. 2011). Mice are widely accessible through multiple commercial and private entities, with literally thousands of inbred, outbred, and transgenic strains having been generated. Spontaneous as well as induced mutations have given rise to specific strains of clinically applicable mice. Similar to humans, mice develop natural diseases with aging such as cancer, hypertension, diabetes, glaucoma, and atherosclerosis. Much of the knowledge gained about the immunological response to these disease processes was first established in murine models, so its impact on and value to sepsis and trauma research cannot be overstated. Furthermore, most regulatory agencies, including the FDA, require demonstration of successful outcomes of new therapies in animals prior to conducting clinical trials in humans, necessitating the existence of a preclinical animal model (Mak et al. 2014). The uniform genetic background of the inbred mouse makes it an ideal study model, allowing for minimal bias to be introduced into an experiment. The genetic homogeneity of highly inbred strains may also imply a more consistent response to acquired illness or traumatic insult. For some experiments, particularly those that require a large sample size or number of conditions (i.e., test gradient doses of a therapeutic agent), using the mouse is desirable given its relatively low level of maintenance and its high fecundity. This is especially relevant to outcome studies in sepsis and trauma research.
Figure 1.
Advantages of using murine models for preclinical experiments (A) High fecundity. Mice reach sexual maturity at 6–8 weeks (Silver 1995) and females have approximately 8 pups per litter at birth (Pritchett-Corning et al. 2009). (B) Accelerated life cycle. Life expectancy of the average laboratory mouse is approximately 24 months. Correlates between the lifespans of humans and mice reveal 1 human year is equivalent to approximately 9 mice days (Dutta and Sengupta 2016). (C) Low maintenance. The mouse has a docile temperament that lends to ease of handling. Mice are also fairly low-maintenance, requiring minimal routine husbandry. (D) Necessity for preclinical animal model. Most regulatory agencies including the U.S. Food and Drug Administration (FDA) require demonstration of safety and efficacy of new therapies in preclinical animal models prior to conducting clinical trials. (E) Well-characterized genome. The entire genomic sequence of the C57BL/6 strain of Mus musculus was sequenced in the 1990s through the collaborative efforts of the Mouse Genome Sequencing Consortium (Mouse Genome Sequencing et al. 2002). (F) Inbred, outbred, and transgenic strains. Thousands of inbred, outbred, and transgenic strains have been generated. Spontaneous and induced mutations have given rise to specific strains of clinically applicable mice that can be used to study complex human disease processes like diabetes or Alzheimer’s disease and rare genetic disorders such as amyotrophic lateral sclerosis or myotonia (Julien et al. 1998; Lithner et al. 2011). (G) Widespread availability of mice and reagents. Frequently, the availability of immunological reagents for mice exceeds that for humans. (H) Evolving “humanized mice.” Researchers are engrafting human CD34+ hematopoietic cord blood stem cells into gamma-irradiated neonatal NSG mice to create “humanized” mouse models to study human disease (Pearson et al. 2008). During this process, the engrafted stem cells undergo negative selection during differentiation into T and B cells in mice, ultimately leading to a complement of mature human T and B cells that are tolerant of the mouse host.
Although human and mouse lineages diverged approximately 75 million years ago, a significant proportion of mouse genes, estimated at approximately 80%, have a single identifiable orthologue in the human genome (Mouse Genome Sequencing Consortium et al. 2002). Parallel research between the mouse and human species has supported the value of the mouse in the study of diverse human disease, as manipulation of corresponding mouse genes has provided unique insights into the intricacies of various human genes. These genetically modified animals have proven to be extremely useful in studying mechanisms of disease and have been critical in elucidating cellular and molecular processes as well as metabolic pathways.
With the recent widespread utilization of the Cre-Lox, TALON, and CRISPR-Cas9 gene editing tools, the creation of highly tissue-specific inducible transgenic animals has become routine in most laboratories. Instead of understanding the functions and actions of individual genes at the level of the entire animal, strains of mice can be created with tissue-specific alterations in gene expression that may be regulated externally. Laboratories are also pushing the limits by creating “humanized mouse models” in which entire murine immune cell populations are being replaced with their human homologues (Pearson et al. 2008). In many cases, the human cells being used have undergone earlier modification by TALON or CRISPR-Cas9 gene editing programs. To expand on this concept, some cancer researchers are actually using human cells from specific individuals to create murine avatars for drug development and personalized medicine (Malaney et al. 2014). Hence, the future of murine models for the study of human disease is virtually unlimited. It is clear the murine model is not just of historical importance, but remains one of the key tools by which the underlying pathophysiology of disease can be studied with the potential of developing life-saving therapies.
The Ethical and Humane Use of Mice in Models of Trauma and Sepsis
The Office of Laboratory Animal Welfare, NIH, requires the humane use of living animals in research for all federally funded activities. The American Association for the Accreditation of Laboratory Animal Care inspects and reviews animal research programs nationwide, and more recently throughout the world. Both organizations rely on the Guide for the Care and Use of Laboratory Animals, 10thEdition, published by the National Research Council of the National Academies of Science. Although mice are not a USDA-covered species, severe sepsis and trauma research would be categorized USDA Category E, which identifies animals that have pain or distress that is not adequately relieved with anesthetics, analgesics, and/or tranquilizer drugs or other methods for relieving pain or distress. Furthermore, many of these studies frequently use mortality as an outcome, and it is not uncommon to see investigators allowing animals to die spontaneously.
Historically, investigators have refrained from using analgesics in these models, the primary explanation being that they may alter the host response to sepsis or trauma. As an example, nonsteroidal anti-inflammatory agents are frequently contraindicated due to their known immunomodulatory effects, but alternatives do exist. Other justifications include the inability to compare current results treated with analgesics with historical controls, and the cost and complexity of using these agents. In addition, allowing animals to die spontaneously was supported by the very rare occurrence of a moribund animal achieving full recovery. Since investigators now have a better understanding of what the animal experiences, practice standards have changed. Most ethics committees follow the Guide and require that the decision to withhold analgesia must be supported by experimental evidence. In 2016, it is no longer acceptable to deny analgesic support to an injured or septic animal without direct experimental evidence that providing analgesic support would invalidate the experimental results.
Several years ago, investigators transitioned to a routine analgesic protocol where subcutaneous lidocaine is provided for relief of incisional pain, and buprenorphine (a synthetic opioid) or another adequate analgesic for prolonged systemic pain relief. Such a routine does not significantly impact outcome or the magnitude of the inflammatory response (Cotroneo et al. 2012). In addition, appropriate euthanasia can be considered as a means to prevent unnecessary pain and distress. Since inception of the protocol, animals are no longer allowed to die spontaneously. Using a predetermined set of physiological criteria, animals are euthanized after reaching a level of morbidity associated with irreversible death. At the present time, there is a large push for investigators using murine models of trauma and sepsis to be required to demonstrate that their procedures and study designs are humane and minimize pain and distress. This can be readily achieved with only modest effect on the scientific outcomes.
Mouse Models for Trauma and Hemorrhagic Shock
Experimental murine models to simulate traumatic injury emerged decades ago and have significantly evolved over time. One of the first trauma models was developed by researchers Noble and Collip in 1942. They developed a circular metal drum that was used to induce blunt trauma in mice. As described in their paper, a mouse that did not receive anesthesia was placed in the metal drum and subsequently tumbled repeatedly. This ultimately led to intra-abdominal organ injury, muscle and soft tissue damage, traumatic brain injury, and often death (Noble and Collip 1942). This rudimentary model has since been replaced by more controlled, reproducible models. Several of these models focus on isolated organ or tissue injury such as traumatic brain injury, thoracic trauma with resulting blunt lung injury, intra-abdominal organ injury, long bone fractures with and without soft tissue injury, or hemorrhagic shock (Frink et al. 2011). Models simulating blunt trauma often employ either a pendulum or weight dropped from a predetermined height or a pneumatic device that can deliver an impact with a predictable force in an effort to optimize the reproducibility of the injury. In a similar fashion, murine trauma models of hemorrhagic shock are performed in either a pressure-controlled or volume-controlled setting to standardize the blood loss. Each of these models has been extensively used to better understand the immunological effects of trauma and the process of wound healing (Table 1).
Table 1.
Examples of murine trauma models
| Trauma Models | Examples | Description |
|---|---|---|
| Hemorrhagic Shock | Pressure-controlled hemorrhagic shock | Following anesthesia, catheter is placed in femoral artery and blood is withdrawn until BP 35 ± 5 mm Hg for 30–90 min (Pfeifer et al. 2013) |
| Volume-controlled hemorrhagic shock | Following anesthesia, catheter is placed in femoral artery and 0.025–0.05 mL/g body weight of blood is withdrawn (35–60%) (Claridge et al. 2001) | |
| Traumatic Brain Injury | Lateral fluid percussion (LFP) | Involves craniotomy over left parietal bone followed by injury using a fluid percussion device producing a pressure of 3.6 ± 1 atm (Carbonell et al. 1998) |
| Controlled cortical impact (CCI) | Craniotomy performed followed by deployment of pneumatically driven impactor measuring 3 mm at a velocity of 5–6 m/sec (Smith et al. 1995) | |
| Weight drop model | Impact using gravitational forces from a 250 g metal rod striking the exposed skull or dura matter from a 2–3 cm height (Flierl et al. 2009) | |
| Long Bone Fracture Models | Open femoral fracture | Femur is exposed and fractured via osteotomy or by weakening bone with several drill holes (Cheung et al. 2003) |
| Closed femoral fracture | Fracture of the femur usually followed by placement of an intramedullary pin, locking nail, or intramedullary compression screw (Holstein et al. 2007; Manigrasso and O’Connor 2004) | |
| Tibial fracture | Involves the creation of a closed fracture of the distal tibial (Holstein et al. 2009) | |
| Pseudofracture model | Bilateral muscle crush injury to the hindlimbs with injection of a bone solution into the injured muscles (Darwiche et al. 2011) | |
| Thoracic Trauma | Blunt trauma via weight drop method | Uses a defined weight at a predetermined height to create gravitational forces delivering an energy of 1.8–2.7 J to the thoracic cavity (Raghavendran et al. 2005) |
| Blunt trauma via cortical contusion impactor or captive bolt gun | Cortical contusion impactor strikes lateral chest with a velocity of 5.8 m/s and an energy of 152 J/m2 to cause pulmonary contusion (Hoth et al. 2007) | |
| Blast injury by laser-induced stress wave | Blast generator creates laser-induced stress waves with a peak pressure of 0.75 bar at a distance of 2 cm for 3.4 ms (Satoh et al. 2010) | |
| Ventilation with high tidal volumes | Ventilate with tidal volumes of 15–45 mL/kg body weight to induce lung injury (Kuiper et al. 2011; Wilson et al. 2003) | |
| Polytrauma | Noble and Collip’s metal drum | Mouse which is not anesthetized is placed in a metal drum and tumbled repeatedly, producing variable injury patterns (Noble and Collip 1942) |
| Original polytrauma model | Pressure-controlled hemorrhagic shock plus laparotomy (Wang et al. 1993) | |
| Modified polytrauma model | Pressure-controlled hemorrhagic shock, laparotomy, and long bone fracture (Tsukamoto and Pape 2009) | |
| Modern polytrauma model | Pressure-controlled hemorrhagic shock, long-bone fracture, soft tissue injury, and laparotomy with cecectomy (Gentile et al. 2013) |
Examining isolated, single-compartment organ injury may be of benefit; however, this does not accurately replicate human trauma, which often involves multiple organ systems in the presence of hemorrhagic shock. In an effort to better mimic multisystem trauma with hemorrhagic shock in humans, we devised a murine trauma model that is multi-compartmental and produces injury equivalent to an Injury Severity Score of >15 in humans, which reflects severe injury with increased susceptibility to post-injury infection (Gentile et al. 2013). The model combines pressure-controlled hemorrhagic shock, long bone fracture, soft-tissue injury, and laparotomy with cecectomy. By augmenting the severity of trauma and incorporating multiple organ systems, the resulting inflammatory changes may more closely resemble those of a human in response to trauma. Based on this rationale, we hypothesized the new polytrauma model would outperform existing murine models in reproducing the systemic inflammatory response observed in humans. The model was compared with two frequently used murine trauma models, including the trauma-hemorrhage model and hemorrhage plus femur fracture model, neither of which include laparotomy with cecectomy to simulate intra-abdominal trauma requiring surgical intervention. Analysis of plasma cytokine and chemokine concentrations revealed the inflammatory response was more robust in the proposed polytrauma model when compared to the traditional trauma models (Gentile et al. 2013). Polytrauma not only led to a greater systemic inflammatory response but also caused sustained leukocytosis with neutrophilia, expansion of myeloid-derived suppressor cells, a reduction in major histocompatibility complex class II expression on CD14+ monocytes, and early lymphocyte activation, as observed in human studies (Gentile et al. 2013). Genome-wide expression was performed using the RNA from circulating mouse leukocytes of the various models, which demonstrated a greater magnitude of the fold changes in gene expression in the polytrauma model, further supporting its ability to produce a greater inflammatory response. The polytrauma model has also been shown to lead to transcriptomic changes that are similar to the changes that occur in septic mice, with activation of genes involved in pathogen-associated and damage-associated molecular pattern signaling (Mira et al. 2016).
While this new model more accurately depicted the early plasma inflammatory cytokine and chemokine responses as well as the phenotypic leukocyte changes expected to be seen in human trauma, the transcriptomic changes were disappointing when compared to humans. Using data from the Glue Grant, the total blood leukocyte genome-wide expression from 167 trauma patients was compared to the genomic expression of blood leukocytes in a murine model of trauma and burn injury (Seok et al. 2013). Genomic analysis revealed mouse injury poorly reflected human trauma with <10% of the variation in human gene expression after trauma being predicted by changes in the mouse genome (Gentile et al. 2014a). Interestingly, increasing the severity of trauma in mice only modestly improved the correlation with humans, while altering the age of the mice appeared to have no effect (Gentile et al. 2014a). However, it would be an oversimplification to conclude that the murine and human responses were completely different. Instead of focusing on the entire transcriptome, there were numerous individual signaling pathways involved in early inflammation and innate/adaptive immunity where gene expression appeared to be strongly correlated between mice and humans (Takao and Miyakawa 2015).
Current Mouse Models for Sepsis and Septic Shock
Several experimental models of sepsis have been developed in mice, each of which have their limitations. These models can be classified into three broad categories based on mechanism, which include administration of an exogenous toxin, administration of a viable pathogen, and disruption of the animal’s endogenous protective barrier allowing for bacterial invasion (Buras et al. 2005) (Table 2). Earlier models of sepsis often involved direct administration of toxins such as lipopolysaccharide (LPS) into the blood, peritoneum, or lung. This induces a strong immediate inflammatory response that mimics activation of the innate immune system in human sepsis. The advantage of using this approach is its technical ease and reproducible response following injection of a quantifiable dose of toxin. However, studies using this model demonstrate that the temporal kinetics and the magnitude of the immunological response differ dramatically from murine and human sepsis arising from a nidus of infection. A bolus injection of endotoxin (LPS) produces a transient but exaggerated increase in the concentration of proinflammatory cytokines like tumor necrosis factor-alpha, far higher but much briefer than in models of sepsis arising from a nidus of infection (Copeland et al. 2005; Remick et al. 2000).
Table 2.
Examples of murine models of sepsis
| Types of Murine Sepsis Models | Examples | Advantages | Disadvantages |
|---|---|---|---|
| Exogenous Administration of Toxin |
Bolus injection of toll-like receptor (TLR) agonist such as LPS or zymosan |
|
|
| Continuous IV administration of LPS |
|
|
|
| Exogenous Administration of a Viable Pathogen | Inoculation of live bacteria such as E. coli, S. aureus, or Pseudomonas aeruginosa |
|
|
| Intraperitoneal implantation of fibrin clot impregnated with viable bacteria |
|
|
|
| Cecal slurry (CS) |
|
|
|
| Disruption of the Endogenous Protective Barrier | Cecal ligation and puncture (CLP) |
|
|
| Colon ascendens stent peritonitis (CASP) |
|
|
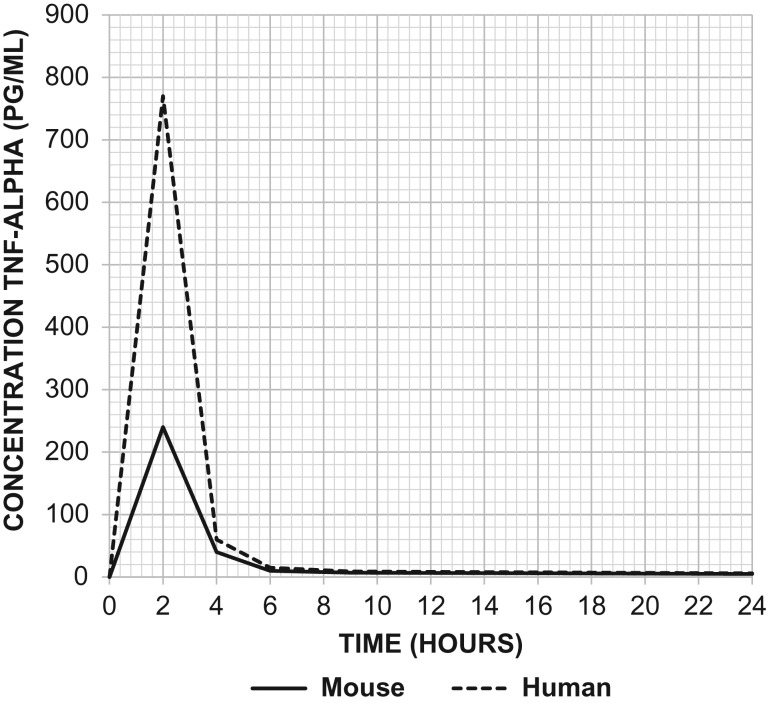
One concern about comparing results between humans and mice when endotoxin is administered are the marked differences in sensitivity and concentrations of plasma cytokines such as tumor necrosis factor-alpha (Figure 2). While humans are exquisitely sensitive to the effects of LPS, mice appear to be relatively resistant. The LD50 dose for a mouse ranges from 5 to 15 mg/kg, which is approximately 1,000 times greater than the estimated lethal dose of LPS administered to humans (Barber et al. 1995; Mahieu et al. 2006; Mestas and Hughes 2004; Warren et al. 2010). Besides interspecies variation in the sensitivity to LPS, another shortcoming of the model is its inability to reproduce the hyperdynamic cardiovascular state observed in human sepsis (Fink and Heard 1990; Wichterman et al. 1980). One of the proposed reasons as to why this model may not simulate sepsis in humans is that patients are rarely challenged with a single large bolus of endotoxin but rather experience a slow showering of bacteria from a septic focus (Wichterman et al. 1980). There are some clinical conditions associated with an overwhelming and exaggerated inflammatory response, such as toxic-shock syndrome and meningococcal sepsis, but these are often gram-positive infections and do not involve endotoxin per se but rather super-antigens and peptidoglycans.
Figure 2.
TNF-α levels following injection of LPS at 500 ng/kg body weight in mice versus 2 ng/kg body weight in humans. Injection of LPS produced much greater elevations in TNF-α in the serum of humans (dashed line) in comparison to mice (solid line). Adapted from Copeland et al. Clin Diagn Lab Immunol. 2005 Jan; 12(1):60–67.
To ameliorate the limitations of a single bolus, a continuous infusion of low-dose LPS has been utilized as a model of severe infection (Traber et al. 1988). Although this model was able to reproduce the hyperdynamic cardiovascular state associated with sepsis, it was not able to reproduce important aspects of the immune response. Moreover, its inflammatory effects were short-lived, comparable to a bolus administration, and thus the model has largely been abandoned (Buras et al. 2005; Fink and Heard 1990; Fish et al. 1986). In contrast, endotoxin administration to the lungs, either by nebulization or direct instillation, has produced a reproducible form of acute lung injury in mice, characterized by a massive influx of neutrophils (Rittirsch et al. 2008). The criticism of this model is comparable to the systemic administration of endotoxin; the quantities required to produce acute lung injury are again nearly 10,000 times the dose used in humans (O’Grady et al. 2001).
Another rodent model of toxin administration is the peritoneal injection of zymosan. Zymosan is a fungal cell surface glucan that elicits a strong inflammatory response (Rao et al. 1994; Volman et al. 2005). Unlike LPS or other toll-like receptor (TLR) agonists, the host response to zymosan is trimodal. There is an early proinflammatory response lasting several days that induces some dose-dependent mortality. This is followed by a quiescent period of chronic low-grade inflammation that progresses to a final stage characterized by organ failure and death (von Asmuth et al. 1990). Depending on the dose employed, the mortality in the early and late inflammatory periods can be titrated. The model offers the advantage of a semblance of human sepsis where the sepsis event is prolonged and multi-organ failure is both an early and late phenomenon (Volman et al. 2005). Closer examination suggests that this is a model of an early exaggerated inflammatory response with persistent inflammation, ultimately leading to organ injury.
Intravenous administration of single or multiple strains of live bacteria into murine hosts has not been found to reproduce human sepsis, since this does not lead to colonization and replication of bacteria necessary for the production of a nidus of infection (Buras et al. 2005). Furthermore, in many cases, the degree of bacteremia is 6-logs greater than seen in patients with bacteremic sepsis. Like administration of endotoxin, the early inflammatory response to a bolus of live bacteria is massively greater than seen in either human sepsis or murine models of sepsis derived from a nidus of infection (Cross et al. 1993).
Since the direct administration of toxins and bacteria has produced a model that overexaggerates the early inflammatory response, does not replicate human sepsis that arises from a nidus of infection, and generally fails to produce a protracted host inflammatory response, additional murine sepsis models have been used. The two most common sources of sepsis in the human population are intra-abdominal infections and pneumonia, so not surprisingly, intra-abdominal sepsis models and pneumonia models have been developed and are frequently used in mice. Presently, three commonly used intra-abdominal sepsis models exist, including cecal ligation and puncture (CLP), cecal slurry (CS), and colon ascendens stent peritonitis (CASP).
CLP was initially developed in the 1980s and is considered the gold-standard model for intra-abdominal sepsis (Parker and Watkins 2001; Wichterman et al. 1980). This model combines tissue necrosis and polymicrobial sepsis secondary to autologous fecal spillage. The procedure is performed through a midline laparotomy and involves externalization of the cecum, ligation of the cecum, and puncture of the ligated portion (Cuenca et al. 2010). CLP intends to mimic perforated hollow viscus in humans with resulting fecal contamination, similar to perforated diverticulitis or perforated appendicitis. Studies demonstrate this method of polymicrobial sepsis produces a resulting immune, hemodynamic, and biochemical response in the murine host that is similar in many regards to the septic response seen in humans (Deitch 1998; Wichterman et al. 1980). An advantage of using this model is that the operator can modulate the quantity of fecal spillage as well as the magnitude of ischemic insult by altering the needle size or the length of cecum ligated, respectively. This, in turn, affects sepsis severity and resulting mortality. As one would expect, physiological outcomes using this model can vary based on operator technique and the presence of liquid versus solid stool in the cecum (Remick et al. 2000). After CLP, some investigators administer antibiotics and resuscitate the mouse with fluids; hence the severity of the insult must be modified. One notable limitation of this model is the tendency of the host to respond to CLP by walling off the infected area, forming an intra-abdominal abscess (Maier et al. 2004; Wichterman et al. 1980). By containing the infection, some mice do not progress to septic shock and convert from an acute inflammatory response to a chronic and persistent inflammatory state. Delano et al. (2007) have followed these mice out to 12 weeks and reported continued runting, elevated inflammatory cytokines, and altered myelopoiesis (Delano et al.).
CS is another commonly used sepsis model. This method is particularly useful for the induction of polymicrobial sepsis in neonatal mice, given their small size, which is prohibitive to performing more invasive procedures like CLP or CASP. CS involves intraperitoneal injection of a measurable amount of cecal contents from a donor mouse into a recipient mouse. It shares some qualities of a bolus poly-infection model, but also has a particulate component that permits seeding of the peritoneum with bacteria resulting frequently in persistent infection. This method has been shown to produce a greater earlier inflammatory response than CLP, but the effects do not persist as long as CLP (Gentile et al. 2014b; van der Poll 2012). Furthermore, each of these sepsis models triggers unique gene expression changes with CS favoring activation of signaling pathways involved in the innate inflammatory response, while CLP favors inhibition of the adaptive immune response (Gentile et al. 2014b).
The CASP model involves implantation of a stent of a predefined diameter into the colon ascendens of the mouse via a midline laparotomy (Zantl et al. 1998). The newly inserted stent remains patent, allowing persistent leakage of fecal material containing intestinal bacteria into the peritoneal cavity, resulting in polymicrobial sepsis and peritonitis. In contrast to CLP, some authors propose this method produces more of a diffuse peritonitis, as opposed to a focal peritonitis that the host can contain in the form of an abscess (Buras et al. 2005). It also fails to contain a component of necrotic tissue. Several studies demonstrate this model produces bacteremia, which can be observed as soon as 12 hours after stent implantation, and elevated circulating LPS levels, which can be observed in the serum as early as 2 hours after stent implantation (Maier et al. 2004; Zantl et al. 1998). Akin to adjusting the needle gauge with CLP, adjusting the stent diameter can affect disease severity and the magnitude of inflammation, measured by systemic serum cytokine levels. While this model is appealing, CASP is technically more challenging to perform than CLP, which has limited its application.
Finally, intra-abdominal sepsis has been less frequently induced through implantation of a fibrin clot impregnated with pathogen. This creates a persistent nidus of infection with progression to systemic dissemination, modeling human sepsis (Mathiak et al. 2000). The benefit of introducing bacteria into the fibrin clot is that the fibrin delays systemic absorption of the entrapped bacteria, which promotes development of a local septic focus. Furthermore, this method reduces early mortality, and the operator is able to select for the particular strain of bacteria to be introduced into the murine host (Fink and Heard 1990). This limitation of this model is the use of a single organism culture, which differs from the polymicrobial infection seen in intra-abdominal sepsis in humans.
Limitations of the Murine Model in Sepsis and Trauma Research
While murine models of sepsis and trauma have provided researchers with valuable insight, these models have significant limitations (Table 3). It is clear no perfect model exists, despite multiple attempts to refine existing models or create new ones. The purpose of employing these murine models is 2-fold: first, to emulate human disease to better understand the underlying pathophysiology, and second, to evaluate pharmaco-therapeutic agents and other interventions to reduce mortality and improve patient outcomes. Billions of dollars have been spent in both the care and research of trauma and sepsis with the hopes of developing an intervention that will substantially impact patient care. Still, long-term outcomes for these disease processes are dismal (Gentile et al. 2012), mortality remains unacceptably high, and the development of successful therapeutic agents is lacking.
Table 3.
Limitations of murine models
| Limitations of Murine Models | Description |
|---|---|
| Interspecies Differences |
|
| Differences Between Strains |
|
| Effect of the Environment |
|
| Age |
|
| Gender |
|
| Mouse Homogeneity |
|
| Comorbid Disease |
|
| Ability to Provide Supportive Care |
|
| Mismatch in Disease Severity and Temporal Response |
|
| Variability within Murine Models |
|
Not surprisingly, it has been remarkably easy for investigators to “cure” sepsis and trauma in the laboratory mouse. There are at least 100 compounds that have proven beneficial in reducing mortality in mouse models, which have subsequently been tested in clinical trials. At the present time, however, not a single drug developed for the treatment of sepsis or trauma with efficacy in murine models has proven successful in clinical trials (Fink 2014). Several reasons exist as to why these murine models may not accurately reflect their human counterpart, and these limitations warrant discussion. While some of the issues with murine models may be reconciled, others may persist due to the fundamental differences between species and their response to sepsis and trauma.
Differences Between Species
The most obvious limitation of translational research is that we are comparing two different species, which diverged from one another millions of years ago. Although a comparison of their genomes reveals sets of highly conserved regulatory programs, critical differences have been noted between several genes implicated in reproduction, immunity, and olfaction (Mouse Genome Sequencing Consortium et al. 2002). There are known discrepancies in both the innate and adaptive immune system of the mouse and human (Table 4). A few of the differences include the balance of leukocyte subsets, production of defensins, regulation of inducible nitric oxide synthase (iNOS), B cell and T cell signaling pathway components, Th1/Th2 differentiation, costimulatory molecule expression and function, and chemokine and chemokine receptor expression (Doeing et al. 2003; Mestas and Hughes 2004).
Table 4.
A few differences in the innate and adaptive immune systems of mice versus humans
| Innate Immunity | Predominant circulating leukocyte in humans is the neutrophil (50–70%) versus lymphocyte (75–90%) in the mouse (Doeing et al. 2003) |
| Neutrophils in humans are an abundant source of defensins, while mouse neutrophils do not express defensins (Risso 2000) | |
| Mouse macrophages express iNOS when stimulated with LPS and INF-γ, unlike human macrophages (Risso 2000; Weinberg 1998) | |
| Adaptive Immunity | FcR expression and Ig isotypes differ between species (Daeron 1997; Monteiro and Van De Winkel 2003) |
| Differences exist in antibody class switching and B cell development related to variation between signaling molecules in the two species (Martin and Lew 1998; Mestas and Hughes 2004) | |
| TCR signaling and costimulation vary between species, leading to alterations in the development, regulation, and activation of T cells (Elder et al. 2001; Farrar et al. 2000; Lenschow et al. 1996; Mestas and Hughes 2004) |
While the predominant circulating leukocyte in humans is the neutrophil, accounting for 50–70% of the total circulating leukocyte population in the blood, the predominant circulating leukocyte in mice is the lymphocyte, comprising 75–90% of the circulating leukocyte population (Doeing et al. 2003). Interestingly, neutrophils in humans are an abundant source of antimicrobial peptides called defensins, while neutrophils in mice do not express defensins (Risso 2000). Additionally, stimulation of mouse macrophages with LPS and interferon-gamma induces expression of iNOS, whereas human macrophages fail to produce the same results when stimulated with the same inflammatory mediators (Bogdan 2001).
FcR expression and Ig isotypes also differ between species, although these alterations may not be significant enough to affect host response to an infectious challenge (Daeron 1997; Mestas and Hughes 2004; Monteiro and Van De Winkel 2003). There are also differences in antibody class switching and B cell development related to alternate signaling molecules (Gordon et al. 2001; Mestas and Hughes 2004). The development, regulation, and activation of T cells varies between species as well, with intrinsic differences observed in TCR signaling and costimulation (Mestas and Hughes 2004). Finally, variation within the mouse population itself is present with certain strains of mice demonstrating a Th1 versus Th2 versus Th17 preponderance.
Aside from differences in innate and adaptive immunity, the species vary in genetic makeup, with an estimated 300 human-specific genes. These are genes found in humans that have no known murine equivalent. To compound this difference, there is also increasing evidence that the leukocyte genome-wide transcriptional response to sepsis and trauma is fundamentally different between mice and humans, as noted by Seok et al. and Gentile et al. (Gentile et al. 2014a; Seok et al. 2013).
Differences Between Strains
In addition to interspecies differences, individual strains of mice are genetically dissimilar to one another, introducing further complexity. While both inbred and outbred strains are readily available, inbred strains are more commonly used in sepsis and trauma research. Outbred strains, which are genetically ill defined, are used in areas of research such as toxicology and pharmacology, but are not widely used in sepsis and trauma research. Although well-characterized, highly inbred strains may vary in their immunological response patterns due to genetic mutations and polymorphisms affecting both innate and adaptive immunity (De Maio et al. 2005; Sellers et al. 2012). These mutations can affect toll-like receptors, nucleotide-binding oligomerization domain (NOD)-like receptors, and complement factors, among other receptors and proteins (Sellers et al. 2012). Interestingly, adaptive immunity in mice varies among different mouse strains due to selective polarization of helper T cells. While C57BL/6 mice demonstrate a Th1-predominant response, which favors activation of macrophages and cell-mediated immunity, other strains such as BALB/c, A/J, and DBA/2 mice exhibit a Th2-predominant response leading to antibody production and eosinophil activation, which has been shown to affect outcomes in studies examining susceptibility to pathogens (Mills et al. 2000; Sellers et al. 2012).
Effect of the Environment
Not surprisingly, the environment has a significant effect on the immune system and microbiome of the mouse. Unlike humans, which are constantly challenged by surrounding pathogens, most laboratory mice used for research are housed in specific pathogen-free (SPF) barrier facilities and as a result are exposed to significantly fewer pathogens than a free living animal. The immature immune system of a SPF laboratory mouse can be equated to that of a neonate, which lacks effector-differentiated and mucosally distributed memory T cells (Beura et al. 2016). Studies have demonstrated that environmental changes such as cohabitation with pet store mice can significantly alter the innate and adaptive immune system of SPF laboratory mice. The effects of cohousing include induction of memory T cells, altered resistance to infection, T-cell differentiation in response to de novo viral infection, and changes in leukocyte expression patterns that more closely resemble that of an adult human rather than a neonate (Beura et al. 2016). Hence, the use of SPF laboratory mice may have implications in the immunological response of the mouse, particularly when challenged in situations such as polymicrobial sepsis.
Age
In constructing a murine model, the goal is to replicate human disease. However, too much emphasis is placed on development of the model itself and not enough on the population to which it is being applied. Whether animal subjects accurately reflect their human counterparts is often not assessed. Generally, they do not. Most sepsis and trauma models use a population of mice that inappropriately represents the human population targeted by these disease processes. As several epidemiological studies have demonstrated, sepsis is largely a disease that occurs at the extremes of age and has a tendency to affect the elderly, particularly those over the age of 65 (Girard et al. 2005). Despite this fact, most researchers use 6- to 10-week-old healthy, young mice to carry out their studies, which are equivalent to young teenage adults in the human population.
Some researchers have addressed this issue by applying the various sepsis models, such as the CLP model, to aged mice. Not surprisingly, both we and Turnbull found a higher mortality rate in the septic aged mouse population in comparison to septic young mice (Turnbull et al. 2003). To expand on this, Turnbull et al. (2004) examined sepsis-induced death of splenic and gut epithelial cells in young versus aged mice. Their group discovered increasing age results in accelerated apoptosis, which may account for the increase mortality seen with aging in sepsis (Turnbull et al. 2004). Some studies also report the increased sensitivity of elderly mice to LPS (Chorinchath et al. 1996; Tateda et al. 1996). We reported in severe blunt trauma that aged mice are more susceptible to a secondary pseudomonas pneumonia than young animals (Nacionales et al. 2015). While using aged mice in existing trauma and sepsis models to better mimic the human scenario seems practical, the increased cost and reduced availability of aged mice may be barriers to its application. Surprisingly, these dramatic differences in outcomes of aged mice are not reflected in their blood leukocyte transcriptomic response (Gentile et al. 2014a).
Gender
One significant bias in experiments is the predominant use of male mice, since gender may affect disease outcome. In the literature, there is evidence to suggest hormonal differences account for the improved survival of females with sepsis in both animal and clinical models (Kher et al. 2005; Knöferl et al. 2002; Merkel et al. 2001; Zellweger et al. 1997). With respect to traumatic injury, the trauma population is certainly not limited to young healthy males. Although the morbidity and mortality related to traumatic injury may not be influenced by gender, there is evidence of sex-related differences in the leukocyte genomic response during severe injury with over 300 genes being differentially expressed between males and females (Lopez et al. 2016). Interestingly, none of these genes was mapped to the X or Y chromosomes (Lopez et al. 2016). Gender disparity in research has been addressed, as rigor and transparency in research has been emphasized by the NIH. Recently, there has been a push to require sex as a biologic variable with provided justification if a single sex model is used (Collins and Tabak 2014).
Heterogeneity of the Human Population and Comorbid Disease
While gender may account for some differences in the immunological response to sepsis and trauma, one of the greatest disparities between animal models and their human counterparts is the heterogeneity of the human population and the presence of comorbid disease. In both the sepsis and trauma population in humans, comorbidities are common. Comorbidities may include diabetes, hypertension, cancer, acute or chronic kidney disease, pulmonary insufficiency, and cardiovascular disease, among others. Due to the aging baby boomers, the human population has experienced a significant increase in the proportion of elderly patients, who are fraught with comorbid disease. Obesity is a controversial variable, since some studies have shown no differences in mortality in the obese, while others have shown an increased risk of secondary infections (Trivedi et al. 2015; Winfield et al. 2010). Despite comorbidities being common in humans who suffer from trauma or sepsis, this is not accounted for in most murine models. As one would presume, studying sepsis or trauma in healthy young animals probably does not accurately reflect the clinical picture in the human population. The use of highly inbred strains of mice to perform these experiments only magnifies this difference, since humans have dramatically greater genetic variability than common inbred strains. This is unfortunate, as the presence of these conditions may significantly impact a patient’s ability to respond to an inflammatory insult or traumatic injury.
Another issue that has yet to be resolved is that, in clinical practice, patients with multiple comorbidities who develop sepsis or suffer from a traumatic insult often have prolonged hospital courses and develop complications resulting in poor clinical outcomes. Complications may include acute kidney injury or the development of multiple organ failure and chronic critical illness. Currently, no murine model exists that duplicates these long-standing effects following trauma or sepsis. These disease states may be difficult to replicate in the animal models but are important to study, as chronic critical illness is becoming the predominant phenotype in the ICU.
Differences in Supportive Care
A major limitation of replicating human trauma and sepsis in mice is our limited ability to perform hemodynamic monitoring and to administer supportive care to correct organ failure, although this is routinely provided to patients in the clinical setting. Some of these supportive measures include mechanical ventilation, renal replacement therapy, infusion of vasopressors and/or inotropic agents, surgical source control, administration of antibiotics, resuscitation with intravascular fluids, and delivery of enteral or parenteral nutrition. While some of these interventions are possible in the mouse, including administration of crystalloid fluids and antibiotics, the more complex interventions are generally not available and are certainly not ideal to perform, given the mouse’s small size (Fink 2014). It should also be noted that the small size of the mouse may impose technical challenges while performing certain operative techniques, limiting its use. This is especially important in the highly lethal murine models where the cause of death is frequently hypovolemic shock, cardiovascular collapse, and pulmonary failure. This is occurring much less frequently in humans due to earlier sepsis recognition, better accident scene recovery, and the use of standardized protocols to adequately resuscitate these patients and provide cardiovascular and pulmonary support.
Another critique of murine models involving hemorrhagic shock is the use of heparin to maintain the patency of the catheter used in the model. In human trauma, patients are not heparinized at the time of injury. Not surprisingly, the introduction of heparin prior to hemorrhage was proposed to have confounding effects on the results obtained from mouse studies using heparin. This was confirmed by Rana et al., who demonstrated improved microvascular patency in the liver, spleen, and kidney of rodents pretreated with heparin (Deitch 1998; Rana et al. 1992).
Mismatch in Temporal Response
An additional consideration is the difference in temporal response patterns evident in some of the animal models of sepsis and trauma. In humans, sepsis generally develops over hours to days while it is induced within minutes in mice. Furthermore, the progression of sepsis and trauma to multi-organ failure is delayed in humans due to immediate interventional support. Multiple organ failure is often observed over the span of days to weeks, while the progression in mice is relatively rapid, occurring within hours to days. Another example of the variation in temporal response is supported by a study conducted by Seok et al. (2013). This group evaluated the gene expression profile for murine trauma or burns and found that the time to recovery, that is, time to restore normal gene expression levels, required days in mice in comparison to humans, which required months, in some cases in excess of a year (Seok et al. 2013).
Abundance of Murine Models with Varying Immunological Effects
Although strain differences may play a role in the resulting immunological effects, another confounding factor is variation among sepsis and trauma models. Several murine models for both trauma and sepsis exist, and there does not appear to be a uniform consensus on which model is best in replicating the human condition. In addition to the lack of agreement on the ideal model, similar murine models have been shown to produce markedly different host responses, both from an immunological standpoint and in terms of lethality. For example, comparison of the CLP and CASP models of intra-abdominal sepsis reveals the CASP model produces greater elevations in inflammatory cytokines and bacterial counts in the blood of the host mouse when compared to CLP (Maier et al. 2004). The abundance of available murine models for sepsis and trauma with varying host responses only creates further confusion for researchers.
Should We Negate the Murine Model Altogether?
While eliminating murine models of sepsis and trauma may seem rational due to several inherent flaws, this will likely not be possible. Some researchers have attempted to use larger animals, which may be more phenotypically similar to humans to study sepsis and trauma. Animals that have been studied include the rat, rabbit, sheep, pig, and nonhuman primates. Even though these animals have physiologic responses which more closely resemble that of humans, none of these animal models have proven to be successful in clinical application for the development of biological response modifiers. However, pigs, sheep, and nonhuman primates have been frequently helpful in the design of interventions aimed at physical support mechanisms to trauma, hemorrhagic shock, burns, and inhalation injury (Marshall et al. 2005).
Another reason murine models will remain the foundation for studying sepsis and trauma is out of ethical necessity. As outlined by the Nuremberg Code and Belmont Report, studies must first be performed in animals prior to using human subjects for clinical research. Furthermore, regulatory agencies such as the FDA mandate preclinical animal study data and toxicity data prior to initiation of clinical trials. Thus, animal models are necessary, and the mouse is arguably the easiest to work with. The mouse remains the model of choice for many chronic infections, autoimmunity, and rare genetic diseases.
Bridging the Gap
As scientists attempt to bridge the gap between the two species, we must first recognize and accept the limitations of our current murine models in sepsis and trauma. It is also important to understand that sepsis and trauma are complex polygenic disease processes, which are not uniform in their distribution or magnitude of insult. Furthermore, the immunological response involves a “genomic storm” with the complex interplay of literally thousands of genes (Tsalik et al. 2014), which further complicates their ability to be studied. Due to the heterogeneity of these disease processes, using a reductionist approach may not be appropriate, and the idea of creating a “magic bullet” is most likely unachievable. Rather than focusing on a single target, the sepsis and trauma fields are shifting their focus towards understanding complex pathways in disease processes. Integration of these pathways may lend to identification of common targets that can affect several downstream signaling events. There is also a growing interest in the study of stem cells and progenitor cells involved in “emergency myelopoiesis” in sepsis and trauma, as early alterations in these cells may promote the development of dysfunctional cells that are pathologically activated, leading to T-cell immune suppression (Gabrilovich and Nagaraj 2009; Manz and Boettcher 2014).
Since using animal models that are genetically more similar to humans such as nonhuman primates is often not possible due to ethical concerns and availability of reagents, efforts should be made to further refine the existing murine models. One way mice may be better “adapted” to humans is through the development of “humanized” mice, which has been performed by Unsinger and colleagues (Unsinger et al. 2009). This involves adoptive transfer of human CD34+ hematopoietic cord blood stem cells into gamma-irradiated neonatal NOD-scid2rɤnull (NSG) mice. The term “humanized” is actually a misnomer, since these mice are generally chimeras of murine and human protective immunity. The use of an NSG mouse almost undoubtedly assures an absence of functional T and B cells, whereas the irradiation protocols rarely eliminate myeloid populations completely. As a result, the mice acquire a functional chimeric mouse-human innate and adaptive immune system. Induction of sepsis through CLP can then be performed, resulting in marked elevations in human and murine pro- and anti-inflammatory cytokines and increases in human T and B cell apoptosis (Unsinger et al. 2009). While the development of the “humanized” mouse is attractive, the process of generating these mice is costly, time-consuming, and rather challenging, which may limit the availability of these mice and their overall use. Furthermore, it remains unclear whether this model will adequately emulate sepsis in humans and prove useful in preclinical trials for drug development.
In addition to modifying existing murine models, investigators from different laboratories should collaborate to standardize murine models across laboratories. This reduction in methodological variability will allow for pragmatic comparison of data. Investigators will need to take into consideration whether the existing protocols should be further refined to more closely resemble the care that human patients receive. For example, in regards to sepsis management, patients are often administered intravenous antibiotics and intravenous fluids; however, in several mouse models these therapies are lacking. Thus, discrepancies in postsepsis and posttrauma care will need to be addressed.
The future of murine models will also rely on advances in technology that further allow researchers to manipulate the mouse genome. With the advent of CRISPR/Cas9 gene editing tools, our ability to modify the murine genome has significantly expanded. Discovery of additional editing systems may further promote the ease with which we can implement genetic modifications to more closely resemble humans.
Conclusions
Although murine models are less than ideal in recapitulating complex human disease processes such as sepsis and trauma, it appears the mouse is here to stay. Most likely, experiments using mouse models will remain a vital pathway for the development of new therapies. However, in the future it will be important to identify factors that may increase the clinical relevancy of the model. This may include altering the mechanisms producing sepsis or traumatic injury in existing models, more appropriate selection of animal subjects and human targets, and the development of novel murine models that have been populated with human cells and tissues. The research community will ultimately have to accept that while the mouse may not be the optimal animal model for sepsis and trauma research, at this time it is arguably the animal with the most potential. The key to adapt will be to continue to learn and appreciate its strengths and weaknesses and to be cautious in the interpretation of its results.
Acknowledgments
This work was supported in part by grants from the National Institute of General Medical Sciences: R01 GM040586-26 (NIH/NIGMS), R01 GM104481-03 (NIH/NIGMS), T32 GM08721-17 (NIH/NIGMS), P50 GM111152-01 (NIH/NIGMS), and R01 GM097531-05 (NIH/NIGMS). The Jackson Laboratory in Bar Harbor, Maine should also be acknowledged for its contributions to the mouse image provided in Figure 1.
References
- Barber SA, Fultz MJ, Salkowski CA, Vogel SN. 1995. Differential expression of interferon regulatory factor 1 (IRF-1), IRF-2, and interferon consensus sequence binding protein genes in lipopolysaccharide (LPS)-responsive and LPS-hyporesponsive macrophages. Infect Immun 63(2):601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, Thompson EA, Fraser KA, Rosato PC, Filali-Mouhim A, Sekaly RP, Jenkins MK, Vezys V, Haining WN, Jameson SC, Masopust D. 2016. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 532(7600):512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan C. 2001. Nitric oxide and the immune response. Nat Immunol 2(10):907–916. [DOI] [PubMed] [Google Scholar]
- Brown JB, Stassen NA, Bankey PE, Sangosanya AT, Cheng JD, Gestring ML. 2010. Helicopters and the civilian trauma system: National utilization patterns demonstrate improved outcomes after traumatic injury. J Trauma 69(5):1030–1034; discussion 1034–1036. [DOI] [PubMed] [Google Scholar]
- Brownstein BH, Logvinenko T, Lederer JA, Cobb JP, Hubbard WJ, Chaudry IH, Remick DG, Baker HV, Xiao W, Mannick JA. 2006. Commonality and differences in leukocyte gene expression patterns among three models of inflammation and injury. Physiol Genomics 24(3):298–309. [DOI] [PubMed] [Google Scholar]
- Buras JA, Holzmann B, Sitkovsky M. 2005. Animal models of sepsis: Setting the stage. Nat Rev Drug Discov 4(10):854–865. [DOI] [PubMed] [Google Scholar]
- Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, Chen RO, Brownstein BH, Cobb JP, Tschoeke SK, Miller-Graziano C, Moldawer LL, Mindrinos MN, Davis RW, Tompkins RG, Lowry SF Inflamm, Host Response to Injury Large Scale Collab. Res P . 2005. A network-based analysis of systemic inflammation in humans. Nature 437(7061):1032–1037. [DOI] [PubMed] [Google Scholar]
- Carbonell WS, Maris DO, McCall T, Grady MS. 1998. Adaptation of the fluid percussion injury model to the mouse. J Neurotrauma 15(3):217–229. [DOI] [PubMed] [Google Scholar]
- Cheung KM, Kaluarachi K, Andrew G, Lu W, Chan D, Cheah KS. 2003. An externally fixed femoral fracture model for mice. J Orthop Res 21(4):685–690. [DOI] [PubMed] [Google Scholar]
- Chorinchath BB, Kong LY, Mao L, McCallum RE. 1996. Age-associated differences in TNF-alpha and nitric oxide production in endotoxic mice. J Immunol 156(4):1525–1530. [PubMed] [Google Scholar]
- Claridge JA, Weed AC, Enelow R, Young JS. 2001. Laparotomy potentiates cytokine release and impairs pulmonary function after hemorrhage and resuscitation in mice. J Trauma 50(2):244–252. [DOI] [PubMed] [Google Scholar]
- Cohen J, Vincent JL, Adhikari NK, Machado FR, Angus DC, Calandra T, Jaton K, Giulieri S, Delaloye J, Opal S, Tracey K, van der Poll T, Pelfrene E. 2015. Sepsis: A roadmap for future research. Lancet Infect Dis 15(5):581–614. [DOI] [PubMed] [Google Scholar]
- Collins FS, Tabak LA. 2014. Policy: NIH plans to enhance reproducibility. Nature 505(7485):612–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland S, Warren HS, Lowry SF, Calvano SE, Remick D Inflammation, the Host Response to Injury I . 2005. Acute inflammatory response to endotoxin in mice and humans. Clin Diagn Lab Immunol 12(1):60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotroneo TM, Hugunin KM, Shuster KA, Hwang HJ, Kakaraparthi BN, Nemzek-Hamlin JA. 2012. Effects of buprenorphine on a cecal ligation and puncture model in C57BL/6 mice. J Am Assoc Lab Anim Sci 51(3):357–365. [PMC free article] [PubMed] [Google Scholar]
- Cross AS, Opal SM, Sadoff JC, Gemski P. 1993. Choice of bacteria in animal models of sepsis. Infect Immun 61(7):2741–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenca AG, Delano MJ, Kelly-Scumpia KM, Moldawer LL, Efron PA. 2010. Cecal ligation and puncture. Curr Protoc Immunol Chapter 19:Unit 19.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daeron M. 1997. Fc receptor biology. Annu Rev Immunol 15:203–234. [DOI] [PubMed] [Google Scholar]
- Darwiche SS, Kobbe P, Pfeifer R, Kohut L, Pape HC, Billiar T. 2011. Pseudofracture: An acute peripheral tissue trauma model. J Vis Exp 50(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maio A, Torres MB, Reeves RH. 2005. Genetic determinants influencing the response to injury, inflammation, and sepsis. Shock 23(1):11–17. [DOI] [PubMed] [Google Scholar]
- Deitch EA. 1998. Animal models of sepsis and shock: A review and lessons learned. Shock 9(1):1–11. [DOI] [PubMed] [Google Scholar]
- Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O’Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, Swan R, Chung CS, Atkinson MA, Ramphal R, Gabrilovich DI, Reeves WH, Ayala A, Phillips J, Laface D, Heyworth PG, Clare-Salzler M, Moldawer LL. 2007. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med 204(6):1463–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doeing DC, Borowicz JL, Crockett ET. 2003. Gender dimorphism in differential peripheral blood leukocyte counts in mice using cardiac, tail, foot, and saphenous vein puncture methods. BMC Clin Pathol 3(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S, Sengupta P. 2016. Men and mice: Relating their ages. Life Sci 152:244–248. [DOI] [PubMed] [Google Scholar]
- Dyson A, Singer M. 2009. Animal models of sepsis: Why does preclinical efficacy fail to translate to the clinical setting. Crit Care Med 37(1 Suppl):S30–S37. [DOI] [PubMed] [Google Scholar]
- Efron PA, Mohr AM, Moore FA, Moldawer LL. 2015. The future of murine sepsis and trauma research models. J Leukoc Biol 98(6):945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Mestoui Z, Jalalzadeh H, Giannakopoulos GF, Zuidema WP. 2015. Incidence and etiology of mortality in polytrauma patients in a Dutch level I trauma center. Eur J Emerg Med 24(1):49–54. [DOI] [PubMed] [Google Scholar]
- Elder ME, Skoda-Smith S, Kadlecek TA, Wang F, Wu J, Weiss A. 2001. Distinct T cell developmental consequences in humans and mice expressing identical mutations in the DLAARN motif of ZAP-70. J Immunol 166(1):656–661. [DOI] [PubMed] [Google Scholar]
- Eppig JT, Smith CL, Blake JA, Ringwald M, Kadin JA, Richardson JE, Bult CJ. 2017. Mouse Genome Informatics (MGI): resources for mining mouse genetic, genomic, and biological data in support of primary and translational research. Methods Mol Biol 1488:47–73. [DOI] [PubMed] [Google Scholar]
- Farrar JD, Smith JD, Murphy TL, Leung S, Stark GR, Murphy KM. 2000. Selective loss of type I interferon-induced STAT4 activation caused by a minisatellite insertion in mouse Stat2. Nat Immunol 1(1):65–69. [DOI] [PubMed] [Google Scholar]
- Fink MP. 2014. Animal models of sepsis. Virulence 5(1):143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink MP, Heard SO. 1990. Laboratory models of sepsis and septic shock. J Surg Res 49(2):186–196. [DOI] [PubMed] [Google Scholar]
- Fish RE, Lang CH, Spitzer JA. 1986. Regional blood flow during continuous low-dose endotoxin infusion. Circ Shock 18(4):267–275. [PubMed] [Google Scholar]
- Flierl MA, Stahel PF, Beauchamp KM, Morgan SJ, Smith WR, Shohami E. 2009. Mouse closed head injury model induced by a weight-drop device. Nat Protoc 4(9):1328–1337. [DOI] [PubMed] [Google Scholar]
- Frink M, Andruszkow H, Zeckey C, Krettek C, Hildebrand F. 2011. Experimental trauma models: An update. J Biomed Biotechnol 2011:797383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. 2009. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9(3):162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile LF, Cuenca AG, Efron PA, Ang D, Bihorac A, McKinley BA, Moldawer LL, Moore FA. 2012. Persistent inflammation and immunosuppression: A common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg 72(6):1491–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile LF, Nacionales DC, Cuenca AG, Armbruster M, Ungaro RF, Abouhamze AS, Lopez C, Baker HV, Moore FA, Ang DN, Efron PA. 2013. Identification and description of a novel murine model for polytrauma and shock. Crit Care Med 41(4):1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile LF, Nacionales DC, Lopez MC, Vanzant E, Cuenca A, Cuenca AG, Ungaro R, Baslanti TO, McKinley BA, Bihorac A, Cuschieri J, Maier RV, Moore FA, Leeuwenburgh C, Baker HV, Moldawer LL, Efron PA. 2014. a. A better understanding of why murine models of trauma do not recapitulate the human syndrome. Crit Care Med 42(6):1406–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile LF, Nacionales DC, Lopez MC, Vanzant E, Cuenca A, Szpila BE, Cuenca AG, Joseph A, Moore FA, Leeuwenburgh C, Baker HV, Moldawer LL, Efron PA. 2014. b. Host responses to sepsis vary in different low-lethality murine models. PLoS One 9(5):e94404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard TD, Opal SM, Ely EW. 2005. Insights into severe sepsis in older patients: From epidemiology to evidence-based management. Clin Infect Dis 40(5):719–727. [DOI] [PubMed] [Google Scholar]
- Gordon CJ, Grafton G, Wood PM, Larche M, Armitage RJ. 2001. Modelling the human immune response: can mice be trusted? Commentary. Curr Opin Pharmacol 1(4):431–435. [DOI] [PubMed] [Google Scholar]
- Holstein JH, Garcia P, Histing T, Kristen A, Scheuer C, Menger MD, Pohlemann T. 2009. Advances in the establishment of defined mouse models for the study of fracture healing and bone regeneration. J Orthop Trauma 23(5 Suppl):S31–S38. [DOI] [PubMed] [Google Scholar]
- Holstein JH, Menger MD, Culemann U, Meier C, Pohlemann T. 2007. Development of a locking femur nail for mice. J Biomech 40(1):215–219. [DOI] [PubMed] [Google Scholar]
- Hoth JJ, Hudson WP, Brownlee NA, Yoza BK, Hiltbold EM, Meredith JW, McCall CE. 2007. Toll-like receptor 2 participates in the response to lung injury in a murine model of pulmonary contusion. Shock 28(4):447–452. [DOI] [PubMed] [Google Scholar]
- Iwashyna TJ, Cooke CR, Wunsch H, Kahn JM. 2012. Population burden of long-term survivorship after severe sepsis in older Americans. J Am Geriatr Soc 60(6):1070–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien JP, Couillard-Despres S, Meier J. 1998. Transgenic mice in the study of ALS: The role of neurofilaments. Brain Pathol 8(4):759–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kher A, Wang M, Tsai BM, Pitcher JM, Greenbaum ES, Nagy RD, Patel KM, Wairiuko GM, Markel TA, Meldrum DR. 2005. Sex differences in the myocardial inflammatory response to acute injury. Shock 23(1):1–10. [DOI] [PubMed] [Google Scholar]
- Knöferl MW, Angele MK, Diodato MD, Schwacha MG, Ayala A, Cioffi WG, Bland KI, Chaudry IH. 2002. Female sex hormones regulate macrophage function after trauma-hemorrhage and prevent increased death rate from subsequent sepsis. Ann Surg 235(1):105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper JW, Plotz FB, Groeneveld AJ, Haitsma JJ, Jothy S, Vaschetto R, Zhang H, Slutsky AS. 2011. High tidal volume mechanical ventilation-induced lung injury in rats is greater after acid instillation than after sepsis-induced acute lung injury, but does not increase systemic inflammation: An experimental study. BMC Anesthesiol 11:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagu T, Rothberg MB, Shieh MS, Pekow PS, Steingrub JS, Lindenauer PK. 2012. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med 40(3):754–761. [DOI] [PubMed] [Google Scholar]
- Lederer JA, Brownstein BH, Lopez MC, Macmillan S, Delisle AJ, Macconmara MP, Choudhry MA, Xiao W, Lekousi S, Cobb JP, Baker HV, Mannick JA, Chaudry IH Inflammation, the Host Response to Injury Collaborative Research Program P . 2008. Comparison of longitudinal leukocyte gene expression after burn injury or trauma-hemorrhage in mice. Physiol Genomics 32(3):299–310. [DOI] [PubMed] [Google Scholar]
- Lenschow DJ, Walunas TL, Bluestone JA. 1996. CD28/B7 system of T cell costimulation. Annu Rev Immunol 14:233–258. [DOI] [PubMed] [Google Scholar]
- Lithner CU, Hedberg MM, Nordberg A. 2011. Transgenic mice as a model for Alzheimer’s disease. Curr Alzheimer Res 8(8):818–831. [DOI] [PubMed] [Google Scholar]
- Lopez MC, Efron PA, Ozrazgat-Baslanti T, Zhang J, Cuschieri J, Maier RV, Minei JP, Baker HV, Moore FA, Moldawer LL, Brakenridge SC. 2016. Sex-based differences in the genomic response, innate immunity, organ dysfunction, and clinical outcomes after severe blunt traumatic injury and hemorrhagic shock. J Trauma Acute Care Surg 81(3):478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie EJ, Rivara FP, Jurkovich GJ, Nathens AB, Frey KP, Egleston BL, Salkever DS, Scharfstein DO. 2006. A national evaluation of the effect of trauma-center care on mortality. N Engl J Med 354(4):366–378. [DOI] [PubMed] [Google Scholar]
- Mahieu T, Park JM, Revets H, Pasche B, Lengeling A, Staelens J, Wullaert A, Vanlaere I, Hochepied T, van Roy F, Karin M, Libert C. 2006. The wild-derived inbred mouse strain SPRET/Ei is resistant to LPS and defective in IFN-beta production. Proc Natl Acad Sci USA 103(7):2292–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier S, Traeger T, Entleutner M, Westerholt A, Kleist B, Huser N, Holzmann B, Stier A, Pfeffer K, Heidecke CD. 2004. Cecal ligation and puncture versus colon ascendens stent peritonitis: Two distinct animal models for polymicrobial sepsis. Shock 21(6):505–511. [DOI] [PubMed] [Google Scholar]
- Mak IW, Evaniew N, Ghert M. 2014. Lost in translation: Animal models and clinical trials in cancer treatment. Am J Transl Res 6(2):114–118. [PMC free article] [PubMed] [Google Scholar]
- Malaney P, Nicosia SV, Dave V. 2014. One mouse, one patient paradigm: New avatars of personalized cancer therapy. Cancer Lett 344(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manigrasso MB, O’Connor JP. 2004. Characterization of a closed femur fracture model in mice. J Orthop Trauma 18(10):687–695. [DOI] [PubMed] [Google Scholar]
- Manz MG, Boettcher S. 2014. Emergency granulopoiesis. Nat Rev Immunol 14(5):302–314. [DOI] [PubMed] [Google Scholar]
- Marshall JC, Deitch E, Moldawer LL, Opal S, Redl H, van der Poll T. 2005. Preclinical models of shock and sepsis: What can they tell us. Shock 24(Suppl 1):1–6. [DOI] [PubMed] [Google Scholar]
- Martin RM, Lew AM. 1998. Is IgG2a a good Th1 marker in mice. Immunol Today 19(1):49. [DOI] [PubMed] [Google Scholar]
- Mathiak G, Szewczyk D, Abdullah F, Ovadia P, Feuerstein G, Rabinovici R. 2000. An improved clinically relevant sepsis model in the conscious rat. Crit Care Med 28(6):1947–1952. [DOI] [PubMed] [Google Scholar]
- Merkel SM, Alexander S, Zufall E, Oliver JD, Huet-Hudson YM. 2001. Essential role for estrogen in protection against Vibrio vulnificus-induced endotoxic shock. Infect Immun 69(10):6119–6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestas J, Hughes CC. 2004. Of mice and not men: Differences between mouse and human immunology. J Immunol 172(5):2731–2738. [DOI] [PubMed] [Google Scholar]
- Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. 2000. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 164(12):6166–6173. [DOI] [PubMed] [Google Scholar]
- Mira JC, Szpila BE, Nacionales DC, Lopez MC, Gentile LF, Mathias BJ, Vanzant EL, Ungaro R, Holden D, Rosenthal MD, Rincon J, Verdugo PT, Larson SD, Moore FA, Brakenridge SC, Mohr AM, Baker HV, Moldawer LL, Efron PA. 2016. Patterns of gene expression among murine models of hemorrhagic shock/trauma and sepsis. Physiol Genomics 48(2):135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro RC, Van De Winkel JG. 2003. IgA Fc receptors. Annu Rev Immunol 21:177–204. [DOI] [PubMed] [Google Scholar]
- Mouse Genome Sequencing Consortium Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P, Agarwala R, Ainscough R, Alexandersson M, An P, Antonarakis SE, Attwood J, Baertsch R, Bailey J, Barlow K, Beck S, Berry E, Birren B, Bloom T, Bork P, Botcherby M, Bray N, Brent MR, Brown DG, Brown SD, Bult C, Burton J, Butler J, Campbell RD, Carninci P, Cawley S, Chiaromonte F, Chinwalla AT, Church DM, Clamp M, Clee C, Collins FS, Cook LL, Copley RR, Coulson A, Couronne O, Cuff J, Curwen V, Cutts T, Daly M, David R, Davies J, Delehaunty KD, Deri J, Dermitzakis ET, Dewey C, Dickens NJ, Diekhans M, Dodge S, Dubchak I, Dunn DM, Eddy SR, Elnitski L, Emes RD, Eswara P, Eyras E, Felsenfeld A, Fewell GA, Flicek P, Foley K, Frankel WN, Fulton LA, Fulton RS, Furey TS, Gage D, Gibbs RA, Glusman G, Gnerre S, Goldman N, Goodstadt L, Grafham D, Graves TA, Green ED, Gregory S, Guigo R, Guyer M, Hardison RC, Haussler D, Hayashizaki Y, Hillier LW, Hinrichs A, Hlavina W, Holzer T, Hsu F, Hua A, Hubbard T, Hunt A, Jackson I, Jaffe DB, Johnson LS, Jones M, Jones TA, Joy A, Kamal M, Karlsson EK, Karolchik D, Kasprzyk A, Kawai J, Keibler E, Kells C, Kent WJ, Kirby A, Kolbe DL, Korf I, Kucherlapati RS, Kulbokas EJ, Kulp D, Landers T, Leger JP, Leonard S, Letunic I, Levine R, Li J, Li M, Lloyd C, Lucas S, Ma B, Maglott DR, Mardis ER, Matthews L, Mauceli E, Mayer JH, McCarthy M, McCombie WR, McLaren S, McLay K, McPherson JD, Meldrim J, Meredith B, Mesirov JP, Miller W, Miner TL, Mongin E, Montgomery KT, Morgan M, Mott R, Mullikin JC, Muzny DM, Nash WE, Nelson JO, Nhan MN, Nicol R, Ning Z, Nusbaum C, O’Connor MJ, Okazaki Y, Oliver K, Overton-Larty E, Pachter L, Parra G, Pepin KH, Peterson J, Pevzner P, Plumb R, Pohl CS, Poliakov A, Ponce TC, Ponting CP, Potter S, Quail M, Reymond A, Roe BA, Roskin KM, Rubin EM, Rust AG, Santos R, Sapojnikov V, Schultz B, Schultz J, Schwartz MS, Schwartz S, Scott C, Seaman S, Searle S, Sharpe T, Sheridan A, Shownkeen R, Sims S, Singer JB, Slater G, Smit A, Smith DR, Spencer B, Stabenau A, Stange-Thomann N, Sugnet C, Suyama M, Tesler G, Thompson J, Torrents D, Trevaskis E, Tromp J, Ucla C, Ureta-Vidal A, Vinson JP, Von Niederhausern AC, Wade CM, Wall M, Weber RJ, Weiss RB, Wendl MC, West AP, Wetterstrand K, Wheeler R, Whelan S, Wierzbowski J, Willey D, Williams S, Wilson RK, Winter E, Worley KC, Wyman D, Yang S, Yang SP, Zdobnov EM, Zody MC, Lander ES. 2002. Initial sequencing and comparative analysis of the mouse genome. Nature 420(6915):520–562. [DOI] [PubMed] [Google Scholar]
- Muller B, Grossniklaus U. 2010. Model organisms—A historical perspective. J Proteomics 73(11):2054–2063. [DOI] [PubMed] [Google Scholar]
- Nacionales DC, Gentile LF, Vanzant E, Lopez MC, Cuenca A, Cuenca AG, Ungaro R, Li Y, Baslanti TO, Bihorac A, Moore FA, Baker HV, Leeuwenburgh C, Moldawer LL, Efron PA. 2014. Aged mice are unable to mount an effective myeloid response to sepsis. J Immunol 192(2):612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacionales DC, Szpila B, Ungaro R, Lopez MC, Zhang J, Gentile LF, Cuenca AL, Vanzant E, Mathias B, Jyot J, Westerveld D, Bihorac A, Joseph A, Mohr A, Duckworth LV, Moore FA, Baker HV, Leeuwenburgh C, Moldawer LL, Brakenridge S, Efron PA. 2015. A detailed characterization of the dysfunctional immunity and abnormal myelopoiesis induced by severe shock and trauma in the aged. J Immunol 195(5):2396–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble RL, Collip JB. 1942. A quantitative method for the production of experimental traumatic shock without h ae morrhage in unanaesthetized animals. Q J Exp Physiol Cogn Med Sci 31:187–199. [Google Scholar]
- O’Grady NP, Preas HL, Pugin J, Fiuza C, Tropea M, Reda D, Banks SM, Suffredini AF. 2001. Local inflammatory responses following bronchial endotoxin instillation in humans. Am J Respir Crit Care Med 163(7):1591–1598. [DOI] [PubMed] [Google Scholar]
- Parker SJ, Watkins PE. 2001. Experimental models of gram-negative sepsis. Br J Surg 88(1):22–30. [DOI] [PubMed] [Google Scholar]
- Pearson T, Greiner DL, Shultz LD. 2008. Creation of “humanized” mice to study human immunity. Curr Protoc Immunol Chapter 15:Unit 15.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer R, Lichte P, Schreiber H, Sellei RM, Dienstknecht T, Sadeghi C, Pape HC, Kobbe P. 2013. Models of hemorrhagic shock: Differences in the physiological and inflammatory response. Cytokine 61(2):585–590. [DOI] [PubMed] [Google Scholar]
- Pritchett-Corning KR, Chang FT, Festing MF. 2009. Breeding and housing laboratory rats and mice in the same room does not affect the growth or reproduction of either species. J Am Assoc Lab Anim Sci 48(5):492–498. [PMC free article] [PubMed] [Google Scholar]
- Raghavendran K, Davidson BA, Helinski JD, Marschke CJ, Manderscheid P, Woytash JA, Notter RH, Knight PR. 2005. A rat model for isolated bilateral lung contusion from blunt chest trauma. Anesth Analg 101(5):1482–1489. [DOI] [PubMed] [Google Scholar]
- Rana MW, Singh G, Wang P, Ayala A, Zhou M, Chaudry IH. 1992. Protective effects of preheparinization on the microvasculature during and after hemorrhagic shock. J Trauma 32(4):420–426. [DOI] [PubMed] [Google Scholar]
- Rao TS, Currie JL, Shaffer AF, Isakson PC. 1994. In vivo characterization of zymosan-induced mouse peritoneal inflammation. J Pharmacol Exp Ther 269(3):917–925. [PubMed] [Google Scholar]
- Remick DG, Newcomb DE, Bolgos GL, Call DR. 2000. Comparison of the mortality and inflammatory response of two models of sepsis: Lipopolysaccharide vs. cecal ligation and puncture. Shock 13(2):110–116. [DOI] [PubMed] [Google Scholar]
- Rhee P, Joseph B, Pandit V, Aziz H, Vercruysse G, Kulvatunyou N, Friese RS. 2014. Increasing trauma deaths in the United States. Ann Surg 260(1):13–21. [DOI] [PubMed] [Google Scholar]
- Risso A. 2000. Leukocyte antimicrobial peptides: Multifunctional effector molecules of innate immunity. J Leukoc Biol 68(6):785–792. [PubMed] [Google Scholar]
- Rittirsch D, Flierl MA, Day DE, Nadeau BA, McGuire SR, Hoesel LM, Ipaktchi K, Zetoune FS, Sarma JV, Leng L, Huber-Lang MS, Neff TA, Bucala R, Ward PA. 2008. Acute lung injury induced by lipopolysaccharide is independent of complement activation. J Immunol 180(11):7664–7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M Early Goal-Directed Therapy Collaborative G . 2001. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 345(19):1368–1377. [DOI] [PubMed] [Google Scholar]
- Rubins JB, Pomeroy C. 1997. Role of gamma interferon in the pathogenesis of bacteremic pneumococcal pneumonia. Infect Immun 65(7):2975–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh Y, Sato S, Saitoh D, Tokuno S, Hatano B, Shimokawaji T, Kobayashi H, Takishima K. 2010. Pulmonary blast injury in mice: A novel model for studying blast injury in the laboratory using laser-induced stress waves. Lasers Surg Med 42(4):313–318. [DOI] [PubMed] [Google Scholar]
- Sellers RS, Clifford CB, Treuting PM, Brayton C. 2012. Immunological variation between inbred laboratory mouse strains: Points to consider in phenotyping genetically immunomodified mice. Vet Pathol 49(1):32–43. [DOI] [PubMed] [Google Scholar]
- Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, Lopez CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG Inflammation, Host Response to Injury LSCRP. 2013. Genomic responses in mouse models poorly mimic human inflammatory diseases . Proc Natl Acad Sci USA 110(9):3507–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelley O, Murphy T, Paterson H, Mannick JA, Lederer JA. 2003. Interaction between the innate and adaptive immune systems is required to survive sepsis and control inflammation after injury. Shock 20(2):123–129. [DOI] [PubMed] [Google Scholar]
- Silver LM. 1995. Mouse Genetics: Concepts and Applications. Oxford: Oxford University Press. [Google Scholar]
- Smith DH, Soares HD, Pierce JS, Perlman KG, Saatman KE, Meaney DF, Dixon CE, McIntosh TK. 1995. A model of parasagittal controlled cortical impact in the mouse: Cognitive and histopathologic effects. J Neurotrauma 12(2):169–178. [DOI] [PubMed] [Google Scholar]
- Takao K, Miyakawa T. 2015. Genomic responses in mouse models greatly mimic human inflammatory diseases. Proc Natl Acad Sci USA 112(4):1167–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateda K, Matsumoto T, Miyazaki S, Yamaguchi K. 1996. Lipopolysaccharide-induced lethality and cytokine production in aged mice. Infect Immun 64(3):769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traber DL, Redl H, Schlag G, Herndon DN, Kimura R, Prien T, Traber LD. 1988. Cardiopulmonary responses to continuous administration of endotoxin. Am J Physiol 254(5 Pt 2):H833–H839. [DOI] [PubMed] [Google Scholar]
- Trivedi V, Bavishi C, Jean R. 2015. Impact of obesity on sepsis mortality: A systematic review. J Crit Care 30(3):518–524. [DOI] [PubMed] [Google Scholar]
- Tsalik EL, Langley RJ, Dinwiddie DL, Miller NA, Yoo B, van Velkinburgh JC, Smith LD, Thiffault I, Jaehne AK, Valente AM, Henao R, Yuan X, Glickman SW, Rice BJ, McClain MT, Carin L, Corey GR, Ginsburg GS, Cairns CB, Otero RM, Fowler VG, Rivers EP, Woods CW, Kingsmore SF. 2014. An integrated transcriptome and expressed variant analysis of sepsis survival and death. Genome Med 6(11):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto T, Pape HC. 2009. Animal models for trauma research: what are the options. Shock 31(1):3–10. [DOI] [PubMed] [Google Scholar]
- Turnbull IR, Buchman TG, Javadi P, Woolsey CA, Hotchkiss RS, Karl IE, Coopersmith CM. 2004. Age disproportionately increases sepsis-induced apoptosis in the spleen and gut epithelium. Shock 22(4):364–368. [DOI] [PubMed] [Google Scholar]
- Turnbull IR, Wlzorek JJ, Osborne D, Hotchkiss RS, Coopersmith CM, Buchman TG. 2003. Effects of age on mortality and antibiotic efficacy in cecal ligation and puncture. Shock 19(4):310–313. [DOI] [PubMed] [Google Scholar]
- Unsinger J, McDonough JS, Shultz LD, Ferguson TA, Hotchkiss RS. 2009. Sepsis-induced human lymphocyte apoptosis and cytokine production in “humanized” mice. J Leukoc Biol 86(2):219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Poll T. 2012. Preclinical sepsis models. Surg Infect (Larchmt) 13(5):287–292. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Marshall JC, Namendys-Silva SA, Francois B, Martin-Loeches I, Lipman J, Reinhart K, Antonelli M, Pickkers P, Njimi H, Jimenez E, Sakr Y. 2014. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med 2(5):380–386. [DOI] [PubMed] [Google Scholar]
- Volman TJ, Hendriks T, Goris RJ. 2005. Zymosan-induced generalized inflammation: Experimental studies into mechanisms leading to multiple organ dysfunction syndrome. Shock 23(4):291–297. [DOI] [PubMed] [Google Scholar]
- von Asmuth EJ, Maessen JG, van der Linden CJ, Buurman WA. 1990. Tumour necrosis factor alpha (TNF-alpha) and interleukin 6 in a zymosan-induced shock model. Scand J Immunol 32(4):313–319. [DOI] [PubMed] [Google Scholar]
- Wang P, Ba ZF, Burkhardt J, Chaudry IH. 1993. Trauma-hemorrhage and resuscitation in the mouse: effects on cardiac output and organ blood flow. Am J Physiol 264(4 Pt 2):H1166–H1173. [DOI] [PubMed] [Google Scholar]
- Warren HS, Fitting C, Hoff E, Adib-Conquy M, Beasley-Topliffe L, Tesini B, Liang X, Valentine C, Hellman J, Hayden D, Cavaillon JM. 2010. Resilience to bacterial infection: Difference between species could be due to proteins in serum. J Infect Dis 201(2):223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg JB. 1998. Nitric oxide production and nitric oxide synthase type 2 expression by human mononuclear phagocytes: A review. Mol Med 4(9):557–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir S, Salkever DS, Rivara FP, Jurkovich GJ, Nathens AB, Mackenzie EJ. 2010. One-year treatment costs of trauma care in the USA. Expert Rev Pharmacoecon Outcomes Res 10(2):187–197. [DOI] [PubMed] [Google Scholar]
- Wichterman KA, Baue AE, Chaudry IH. 1980. Sepsis and septic shock—a review of laboratory models and a proposal. J Surg Res 29(2):189–201. [DOI] [PubMed] [Google Scholar]
- Wilson MR, Choudhury S, Goddard ME, O’Dea KP, Nicholson AG, Takata M. 2003. High tidal volume upregulates intrapulmonary cytokines in an in vivo mouse model of ventilator-induced lung injury. J Appl Physiol (1985) 95(4):1385–1393. [DOI] [PubMed] [Google Scholar]
- Winfield RD, Delano MJ, Dixon DJ, Schierding WS, Cendan JC, Lottenberg L, Lopez MC, Baker HV, Cobb JP, Moldawer LL, Maier RV, Cuschieri J. 2010. Differences in outcome between obese and nonobese patients following severe blunt trauma are not consistent with an early inflammatory genomic response. Crit Care Med 38(1):51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn JL, Scumpia PO, Winfield RD, Delano MJ, Kelly-Scumpia K, Barker T, Ungaro R, Levy O, Moldawer LL. 2008. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood 112(5):1750–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H, Siddiqui J, Remick DG. 2006. Mechanisms of mortality in early and late sepsis. Infect Immun 74(9):5227–5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Brownstein BH, Mason PH, Baker HV, Finnerty CC, Jeschke MG, Lopez MC, Klein MB, Gamelli RL, Gibran NS, Arnoldo B, Xu W, Zhang Y, Calvano SE, McDonald-Smith GP, Schoenfeld DA, Storey JD, Cobb JP, Warren HS, Moldawer LL, Herndon DN, Lowry SF, Maier RV, Davis RW, Tompkins RG Inflammation, Host Response to Injury Large-Scale Collaborative Research P . 2011. A genomic storm in critically injured humans. J Exp Med 208(13):2581–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanotti-Cavazzoni SL, Goldfarb RD. 2009. Animal models of sepsis. Crit Care Clin 25(4):703–719, vii–viii. [DOI] [PubMed] [Google Scholar]
- Zantl N, Uebe A, Neumann B, Wagner H, Siewert JR, Holzmann B, Heidecke CD, Pfeffer K. 1998. Essential role of gamma interferon in survival of colon ascendens stent peritonitis, a novel murine model of abdominal sepsis. Infect Immun 66(5):2300–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellweger R, Wichmann MW, Ayala A, Stein S, DeMaso CM, Chaudry IH. 1997. Females in proestrus state maintain splenic immune functions and tolerate sepsis better than males. Crit Care Med 25(1):106–110. [DOI] [PubMed] [Google Scholar]