Abstract
The use of mouse models in biomedical research and preclinical drug evaluation is on the rise. The advent of new molecular genome-altering technologies such as CRISPR/Cas9 allows for genetic mutations to be introduced into the germ line of a mouse faster and less expensively than previous methods. In addition, the rapid progress in the development and use of somatic transgenesis using viral vectors, as well as manipulations of gene expression with siRNAs and antisense oligonucleotides, allow for even greater exploration into genomics and systems biology. These technological advances come at a time when cost reductions in genome sequencing have led to the identification of pathogenic mutations in patient populations, providing unprecedented opportunities in the use of mice to model human disease. The ease of genetic engineering in mice also offers a potential paradigm shift in resource sharing and the speed by which models are made available in the public domain. Predictively, the knowledge alone that a model can be quickly remade will provide relief to resources encumbered by licensing and Material Transfer Agreements. For decades, mouse strains have provided an exquisite experimental tool to study the pathophysiology of the disease and assess therapeutic options in a genetically defined system. However, a major limitation of the mouse has been the limited genetic diversity associated with common laboratory mice. This has been overcome with the recent development of the Collaborative Cross and Diversity Outbred mice. These strains provide new tools capable of replicating genetic diversity to that approaching the diversity found in human populations. The Collaborative Cross and Diversity Outbred strains thus provide a means to observe and characterize toxicity or efficacy of new therapeutic drugs for a given population. The combination of traditional and contemporary mouse genome editing tools, along with the addition of genetic diversity in new modeling systems, are synergistic and serve to make the mouse a better model for biomedical research, enhancing the potential for preclinical drug discovery and personalized medicine.
Keywords: mouse, CRISPR/Cas9, preclinical
Introduction
The use of mice as tools in biomedical research is well established. Their presence offers the ability to evaluate disease etiology and therapeutic profiling in a low cost, easy to maintain, rapidly reproducing mammalian model. For many years, attention has turned away from the forward genetics approach of studying spontaneous and chemically induced mouse models toward the reverse genetics approach to studying gene function via knockouts by genetic engineering. Through standard genetic engineering technologies, a tremendous amount of information has been gained with respect to gene function, pathways, and pathophysiology of disease by studying both constitutive and tissue-specific loss of function mutations. However, progress has been hampered by the costs associated with traditional genetic engineering, many of which are related to the time required to target embryonic stem (ES) cells, establish germline transmission, and breed away from selectable markers. Furthermore, the lack of efficiency in ES cells from different inbred strains has limited our exploration of phenotypes on different genetic backgrounds. This limitation is an important point to consider when comparing disease models on a single inbred background to a heterogeneous patient population with possibly differential disease penetrance.
The development of new technologies in mutant mouse development, such as CRISP/Cas9 mediated genome engineering, offers exciting opportunities to explore genetic engineering in unprecedented ways. The technology has been rapidly and efficiently adopted by transgenic core facilities in comparison with traditional technologies. Genome editing offers considerable cost savings, avoiding the use of ES cells and selection markers, with benefits of high rates of germ line transmission. The concern regarding off-target mutation events, while still valid, has not occurred with a frequency that significantly hampers progress. This, in part, is due to the efficiency of the targeting and the number of founder lines that can be produced per injection. These gains allow us to explore the generation of an allelic series in a given disease in mice, engineering multiple pathogenic variants and point mutations for a single gene as seen in the patient population. Arguably, one of the most exciting advantages of CRISPR technology is the ability to readily produce mutations on different inbred and outbred backgrounds, opening up a whole new frontier of exploration in phenotyping. Thus, a goal is to develop animal disease models tailored to specific individuals and to understand how these mutations modulate biochemical and physiologic pathways in the context of genetic heterogeneity. These data provide a resource for the evaluation of patient-specific therapies under the umbrella of personalized medicine.
The Process of Drug Development
Developing a new drug does not come without significant investments in time and financial resources. It can take between 10 and 15 years and hundreds of millions of dollars to take a new drug from laboratory concept to regulatory approval by the Food and Drug Administration (FDA). It is estimated that up to 75% of a research and development budget is spent developing candidate drugs that are not approved. Even after regulatory approval is granted, drugs can be withdrawn mostly due to one of two major decisive factors: lack of sufficient efficacy or unanticipated toxicity associated with adverse drug reactions (ADR). A listing of withdrawn and discontinued drugs publically accessible at http://cheminfo.charite.de/withdrawn (Siramshetty et al. 2016) indicates the scale of the challenges associated with drug development.
In many cases, analysis of these failures leads to a greater understanding of the nature of interaction between the drug, the disease of medical condition being treated, and how these relate to ADR. It is clear that genetic variation between individuals is a significant contributor to ADR. Significantly more research is needed to determine how drug-genome interactions occur and how to predict ADR by genetically prescreening disease patients prior to treatment to avoid ADR. The challenge is to determine how to incorporate pharmacogenetic screening into the preclinical drug development process.
There are several stages to a typical drug discovery and development pipeline. The initial basic research on clinical disease and biomarker identification leads to a greater understanding of disease diagnosis, progression, and outcomes. Animal models are identified or generated to characterize some of the earliest events associated with the disease and to identify potential drug targets that may be amenable to manipulation. The next stage involves chemists and biologists working together developing candidate therapeutic agents. These are typically evaluated for effectiveness in cell culture-based high-throughput screening assays. Small molecules, therapeutic proteins/antibodies, antisense oligonucleotides, and off-target effects of currently available pharmaceuticals are considered. Novel compounds may be recovered from libraries containing millions of phytochemicals derived from plants and biomaterial collected from around the world. This is followed by an in vivo assessment of these candidates to determine how a living system reacts to the drug. Pharmacokinetic and pharmacodynamic studies are performed during which gastrointestinal absorption, body distribution, drug metabolism, and drug excretion (ADME) parameters are determined for each candidate drug. Some are eliminated due to poor ADME characteristics or extreme toxicity. Lead candidates move forward to the next step in safety and efficacy studies using animal models prior to approval by the FDA to begin human clinical trials. The pipeline tends to be flexible in that for many potential drugs, small-scale animal efficacy studies can be performed during the in vivo assessment phase before large-scale ADME studies. This helps eliminate some of the candidates at an earlier stage, thus generating significant costs savings. Subsequent large scale efficacy studies are performed to identify the therapeutic range and high-dose effects.
Maximizing Preclinical–Clinical Synergy
An increasing change in the drug development pipeline that has emerged in recent years is the customized generation of animals that are surrogates for human disease. Historically, the process was limited to the study of drug efficacy in animal models generated by spontaneous mutation, transgenesis, or embryonic stem cell-derived mutagenesis. The recent discovery and use of CRISPR/Cas9-mediated genome alteration has revolutionized our ability to generate animal models containing human pathogenic genetic variants in the animal orthologues of disease associated genes. This technology holds great promise in increasing the correlation between preclinical and clinical disease progression and treatment translatability.
The mouse has emerged as the premier mammalian model organism. Despite more than 65 million years since primate and rodent phylogenetic lineages diverged, comparative sequence analysis of the human and mouse genomes reveal that 99% of the genes are evolutionarily conserved (Capecchi 1994). Several attributes make the mouse an excellent model organism for biomedical research: they are small, have relatively short life spans, are cost effective, and easy to breed. The mouse is also an excellent tool to generate and study animal models of human disease. Mice are susceptible to the same monogenic and polygenic diseases found in humans, including obesity, diabetes, insulin resistance, cardiovascular disease, cancer, autoimmunity, hearing and vision loss, and muscular dystrophies. They manifest anxiety, epilepsy, neurodegeneration, and addiction to alcohol and cocaine and develop aging-associated disorders similar to Parkinson's and Alzheimer's.
Inbred Mouse Strains
A strain is defined as inbred if all offspring can be traced back to a single ancestral breeding pair after 20 or more consecutive generations of brother-sister mating. Because all individuals of a specific inbred mouse strain are essentially genetically identical, the presence of multiple data replicates allows for a determination of the magnitude and range of responses associated with any treatment or intervention within a genetically defined biological system.
Each inbred strain is genetically distinct from other inbred strains. Comparison among inbred strains demonstrates differential susceptibility to many of the common conditions observed in humans, including but not limited to cancer;, preference for diet, weight gain, and obesity; altered behavior; and differences in immune function, both in a disease state or in response to environmental challenge or infection.
The mouse Phenome Database (http://www.phenome.jax.org) details the phenotypic and genotypic variations associated with 40 widely used inbred mouse strains. Mining this large dataset is a good starting point to identify mouse strains that are susceptible and resistant to specific human diseases. To date, the genomes of 17 inbred strains have been sequenced and single nucleotide polymorphisms (SNPs) identified in many others (Keane et al. 2011). These datasets provide a comprehensive resource with which to examine natural genetic variation between mouse strains and to perform extensive data mining to correlate gene sequence with phenotypic variables.
Spontaneous Mouse Mutants
There are 22,851 genes in the murine genome (assembly GRCm38.4 http://www.ensembl.org), of which only a small percentage are associated with spontaneous mutations. Spontaneous mutations are commonly found through gross observation of an abnormal phenotypic trait arising in an otherwise normal animal population. The low proportion of spontaneous mutations could be attributable to the inherent bias associated with mutant genes that are found this way. Viability is a requirement. Mutant phenotypes that are pronounced are less likely to be overlooked than mutations exerting more subtle effects. Thus, mutations affecting body weight, limb development, eye and ear deletion or malformation, and behavioral deficiencies such as circling, nonlethal seizures, aggression, or passivity tend to be overrepresented in the population of spontaneous mouse mutants.
Transgenic Mouse Models
Transgenic overexpression or modified expression of a gene or cDNA in the mouse genome is a valuable tool to characterize the effects on murine development and disease (reviewed in Palmiter and Brinster 1986). It can be applied to mouse or human genes, either harboring no mutations or containing any specified mutation. It can be used to screen for the effects of expanded repeat sequences within nontranslated DNA on protein abundance and disease or dominant negative effects can be characterized. Where transgenic mice manifest disease symptoms, the disease progression can be studied and compared with recognized clinical features of the disease. If similarities exist, then the animal model could be considered a surrogate for a known disease. Varying the choice of promoters being used to drive transgene expression can allow for the modulation of the disease in a tissue or temporal specific manner.
Embryonic Stem Cell Models
The next major technological revolution in the ability to generate specific models of human disease arose through the advent of ES cell manipulation (reviewed in Hall et al. 2009). This technology relied on the parallel discovery of homologous recombination in mammalian systems and the isolation of murine ES cells. These cells are pluripotent and can differentiate into all cell types, resulting in the recovery of mutant mouse strains containing specific engineered mutations generated in the original ES cell population.
Whereas the goal of transgenic technology is to overexpress a gene of interest, the early goal of homologous recombination was to disrupt a gene, creating a loss of function by disrupting the open reading frame or blocking expression of a specific gene in the mouse. These so-called knockout mouse mutants are valuable tools to discern the role of a gene in growth, development, behavior, and other physiological processes. When a human disease is associated with loss of function, the corresponding mouse knockout can be an important resource to understand and examine the biological effects associated with gene absence. If a human disease mutation is discovered to map to a gene of unknown biological function, the phenotype of a specific knockout mouse can provide valuable clues as to role of that gene.
Genome Engineering with Cre-LoxP
Continued research into the mechanisms and regulation of homologous recombination in mammalian, bacterial, and viral systems led to the development of the next major technological milestone in murine genome manipulation. Bacteriophage P1 undergoes a cyclization of its linear genome as part of its normal life cycle. This process is mediated via the action of the P1 expressed Cre recombinase enzyme on two paired recognition sites termed lox sequences. These lox sequences, commonly referred to as LoxP sites when used in genome remodeling, are 34-bp-long sequences consisting of two 13-bp palindromic repeats separated by an 8-bp asymmetric core spacer sequence that confers directionality. Insertion of two loxP sequences flanking a gene or critical exon in the mouse genome using ES cell-mediated targeting would initially be expected to generate a “floxed” mouse with no mutant phenotype. However, transgenic expression of Cre results in a specific deletion of DNA between the two loxP sites.
By selectively breeding these “floxed” mice to a panel of strains expressing Cre from different promoters, gene mutations could be directed to any tissues or developmental time point desired to help investigate the tissue-specific biological effects of genes.
A significant value of the use of Cre-LoxP genome engineering is that gene mutations causing embryonic lethality can be characterized in adult mice by delaying the expression of Cre until after birth and/or weaning. This approach is being applied on a genome-wide basis. The knockout mouse project, developed as a major trans-NIH initiative, is an international collaboration of scientists that aims to generate a comprehensive and public resource of mouse ES cells from which null mutations in every gene in the mouse genome, can be derived (http://www.komp.org).
A system conceptually similar to Cre-LoxP was found in Saccharomyces cerevisiae. The recombinase enzyme (Flp; termed flippase) bound to and initiated double-strand DNA breaks at FRT sequence-containing sites. These 34-bp FRT sequences are similar but distinct from loxP sequences. Although not as commonly used as cre-loxP for targeted mutagenesis of the mouse genome, the FLP-FRT system can be useful in instances where more than one genome rearrangement is attempted at the same time in the same mouse.
CRISPR-Cas9-Mediated Genome Engineering
Once in a while, truly transformative technologies emerge that completely disrupt established paradigms. Whereas formerly, mouse mutant strain generation was the domain of the few privileged laboratories or universities with outstanding core facilities, the discovery and implementation of the CRISPR-Cas9 genome engineering technology to achieve the same goal is now available to nearly all. The technology introduces discrete genetic mutations into the genome at high frequency and low cost and requires only standard molecular and cellular skills beyond a standard zygote microinjection and oocyte transfer facility. Although still predominantly in the research discovery stage, the use of genome editing as a tool to prevent expression of deleterious genes, correct mutations, inactivate viral entry or replication, or alter the epigenetic environment holds significant promise for the future of clinical disease treatment (Jang et al. 2016).
CRISPR-Cas9 adopts the adaptive immunity present in bacteria to resist viruses and plasmids (reviewed in Doudna and Charpentier 2014). Almost any location in the genome can now be targeted using a simple two-component system. Cas9 is a large multifunctional protein with two DNA cleavage sites that functions as an RNA guided endonuclease. It is directed to specific genomic targets via association with a specific guide RNA sequence that is complementary to the DNA sequence of the target region. Provided this target sequence is positioned immediately adjacent to a protospacer adjacent motif, efficient double-strand endonuclease cleavage follows.
Subsequent DNA repair occurs using the nonhomologous end joining or the homology-directed repair (HDR) end joining pathways (reviewed in Singh et al. 2015). The former is error prone and often results in the generation of insertions or deletions ranging from 1 nucleotide to several hundred nucleotides in length. Cas9-mediated double-stranded DNA breaks can also be repaired by the high fidelity HDR mechanism provided a homologous repair template is present. This can either be supplied by the nontargeted chromosome during replication or experimentally provided. Thus, co-injection of a synthesized DNA oligonucleotide or a plasmid template containing a desired mutation along with Cas9 and guide RNA into a one-cell stage mouse embryo can result in the desired mutation being transferred onto the genome as part of the HDR pathway. All that remains is the transfer of the injected zygotes into mice and screening progeny for the desired CRISPR allele.
Although other genome manipulation technologies have been developed, such as the use of Zinc finger nucleases and TAL effector nucleases, their use requires significant protein engineering to optimize targeting and they are not as widely utilized (Ousterout et al. 2015; Wefers et al. 2014). The simplicity and specificity of CRISPR-Cas9, requiring only the synthesis of an appropriate guide RNA and donor DNA sequences, is such that it has successfully been used to introduce mutations into mice, rats, fruit flies, nematodes, frogs, monkeys, rice, wheat, tobacco, and human cell lines.
The current use of CRISPR/Cas9 is to generate precise preclinical models containing the same mutation in a disease gene as found in a patient. These models allow for allele-specific therapeutic outcome evaluation. A more challenging goal of CRISPR/Cas9 is to repair mutations in vivo directly in patients with genetic changes causing diseases that are not currently amenable to treatment. Two distinct scientific challenges must first be overcome to meet this objective: 1) the association of nonhomologous end joining mutations with CRISPR/Cas9 means that unintended mutations are often introduced into the targeted gene, and 2) the ability of CRISPR/Cas9 to target a gene is completely dependent on the sequence of the short guide RNA. Targeting gene family members runs a significant risk of mutagenesis of genes with homology to the guide RNA. Increasing the effectiveness of HR while decreasing the likelihood of off-target mutations are challenging goals not only to make the tool even more efficient in preclinical model generation but also to develop new treatment modalities in humans. These improvements in clinical effectiveness would have to occur concomitantly with public discussion and education to define limits to the use of such technology.
Pharmacogenomics: Towards Understanding the ADR
In its 2011 strategic plan (FDA 2011), the FDA proposed the need for new methods to assess and characterize molecular targets and host genetic factors that may be associated with rare and unexpected adverse drug events. As discussed earlier, the most common reason for a drug withdrawal from the market post approval is ADR. Preclinical studies performed in a single inbred mouse strain or utilizing a single genetically homogeneous animal model may fail to identify toxicity potential, because these studies are not geared to explore the relationship between patient response and ADR. Even in clinical phases, the limited sizes of the phase I, II, and III cohorts may be insufficient to screen for every eventuality.
It is likely that the predominant contributor to ADR is genetic variation between patients on the drug treatment. The goal is to identify at-risk patients through predictive genetic association. This would allow for drug approval when associated with pharmacogenetic diagnostic testing, the ability to screen for patients susceptible to ADR. The situation is not dissimilar to the need to screen for histocompatibility between recipients and donors prior to an organ or tissue transplant.
There are two ways to approach an understanding of the mechanisms contributing to ADR. One is to collect DNA from all unrelated patients that experienced an ADR. Genome scanning of these individuals may identify a common previously undiagnosed genetic mutation or gene variant in one or more loci. In theory, these candidate genes could then be tested in animal models to ascertain if genetic variation of this potential “modifier” alters the drug response, when both the gene variant and drug are presented together in the same preclinical disease model. This approach may be limited by the small number of patients that experienced an ADR or the withdrawal of the drug as a rapid response to small number of ADR patients. Thus, an alternative and more practical approach is to introduce greater genetic diversity into the preclinical testing phase of new drug development.
Two mouse resources have been developed in order to investigate this latter strategy. Both offer maximal allelic variation, approaching that observed in the human population.
Collaborative Cross Mice
While traditional inbred strains of mice offer some advantages, such as reduced variance and the ability to repeat experiments using the same genetic background, they lack genetic diversity, which is a critical source of phenotypic variation in the human population. The lack of genetic diversity in many models may explain many of the difficulties in translating results to the patient population. The Collaborative Cross (CC) is a large panel of new recombinant inbred mouse strains derived from an eight-way cross of JAX inbred strains including three wild derived inbred lines (Churchill et al. 2004; Iraqi et al 2014; Threadgill and Churchill 2012). The strains selected represented the three major Mus musculus subspecies: M. m. musculus, M. m. domesticus, and M. m. castaneus. Each CC strain is a unique inbred mixture of the eight founders, and together the strains capture almost 90% of the genetic variation present in the laboratory mouse. The CC lines represent a panel of inbred strains that can be studied repeatedly and independently across treatments.
Diversity Outbred Mice
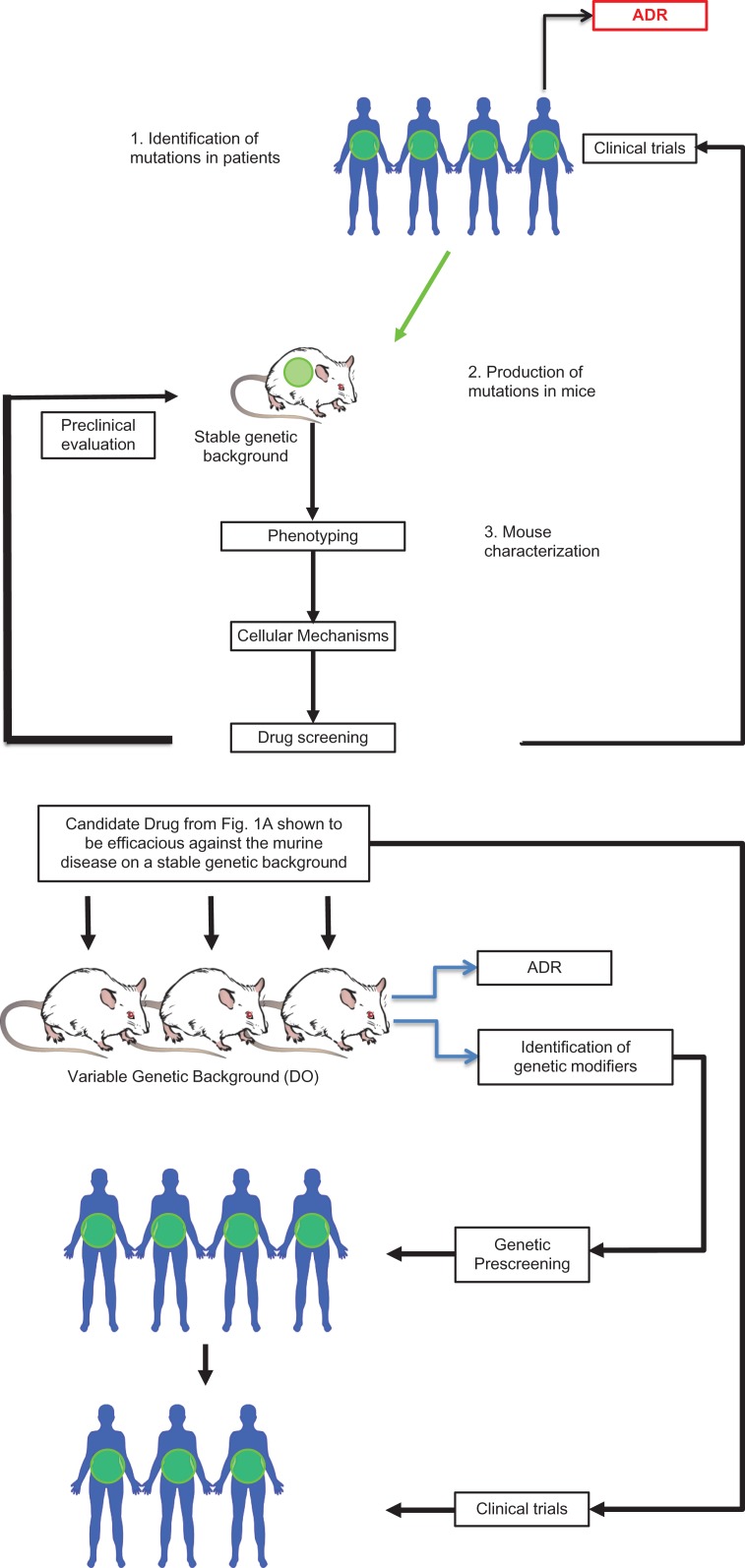
The CC panel is ideal for data integration and trait correlation. However, to find the sources of genetic variation and co-variation underlying trait similarity, a high-precision mapping population is required. For this, the Diversity Outbred (DO) mice were created. The DO mice are produced via use of a sophisticated breeding strategy that maintains a balanced mixture of founder genomes and avoids allelic loss and inbreeding. Mice are selected from 160 of the CC lines (Bogue et al 2015; Chesler et al 2008; Churchill et al. 2012). The SNPs and other variants in the DO mice perturb transcript levels and protein structure across the entire genome and increase the chances of discovering new disease- or treatment-modifier genes that have not been discovered in commonly used inbred strain experiments. Each DO mouse is genetically unique, and therefore preclinical drug testing in this model mimics human phase I clinical trials. Issues such as the toxicology associated with dosage variation and ADR can be identified and characterized in these mice leading to the development of genetic diagnostics before seeking FDA approval and moving the drug into the clinical phase (Figure 1). In addition, mutations generated in the CC background have the potential to produce more pronounced or clinically relevant phenotypes that might otherwise be masked on a typical inbred background such as C57BL/6. Collectively, the CC and DO mouse populations offer high mapping resolution and broad allelic diversity, carrying 45 million SNPs (Svenson et al. 2012).
Figure 1.
(A) A typical current preclinical drug development pipeline used to evaluate and treat a disease-causing gene (in green). (B) How drug screening of DO mice can identify at-risk ADR patients from the same population as 1A prior to clinical treatment.
Humanized Mice: Reconstituting the Human Immune System
Many human diseases are associated with immune dysfunction. These include cancers such as lymphomas and leukemia; autoimmune diseases such as type 1 diabetes, lupus, and rheumatoid arthritis; and other medical conditions such as asthma, eczema, hay fever, and other allergic reactions. Generalized immunodeficiency increases the risk of infection and disease from known disease-causing microbes and opportunistic pathogens alike. An active host immune system is directly responsible for tissue transplant rejection even when tissue-matched in the absence of immune suppressing drugs. Even residual immune cells, if present in donor tissue, can also induce life-threatening graft versus host response as a complication of transplantation.
The immune system can also be a tool in drug therapy. Therapeutic monoclonal antibodies and Fc fusion proteins are increasingly being utilized or developed for treatments against autoimmunity, inflammation, cancer, and lipid management (Chan and Carter 2010; Feinstein and Lloyd-Jones 2016; Scott et al. 2012; Weiner et al. 2010). However, species-specific differences in immune architecture pose special challenges. Although functionally equivalent, the human HLA and murine MHC molecules differ significantly in structure. There are also species differences in the growth factors and cytokines required for normal hematopoietic and immune system development.
Several mouse models exist to support therapeutic antibody preclinical studies. One of the most useful is the NSG mouse (Shultz et al. 2012). This mouse is an excellent model to reconstitute the human immune system in the mouse and for engraftment studies. NSG mice contain a mutation in scid, to prevent T and B cell development, and in the gamma chain of interleukin 2 receptor to block signaling from six distinct interleukins and prevent immune natural killer cell development. The genetic background of these mice also further reduces the endogenous immune system by eliminating hemolytic complement and reducing dendritic cells and macrophage functions. NSG mice support superior engraftment of human hematopoietic stem cells and coengraftment of multiple human tissues, including tumors without immune rejection, leading to the development of Patient Derived Xenograft or Avatar mouse models to evaluate drug therapy effective on specific patient tumors in a living system. The advantage is that the genetic and phenotypic heterogeneity associated with human cancers can be preserved within the mouse model. Over time, the collection of deep sequencing and expression data from large collections of tumors can help to profile tumors and their response to therapeutic agents.
Therapeutics with FcRn Null Mice Expressing Human FcRn
Therapeutic monoclonal antibodies and Fc fusion proteins demonstrate longer half-lives in patients relative to therapeutic proteins. The difference is considerable with therapeutic proteins, growth hormone, erythropoietin, and granulocyte colony stimulating factor showing half-lives of up to two days compared with 10 to 30 days for monoclonal antibodies and Fc-fusion proteins (Black et al. 2010; Hernández-Bernal et al. 2005; López et al. 2010; Martins et al. 2016; McMahon et al. 1990; Tanaka et al. 1999). This longer half-life has been attributed to the protective role of neonatal Fc receptor (FcRn).
First detected in the gut of rodent pups, FcRn is a heterodimer of a class I MHC-like protein and β2-microglobulin. FcRn is responsible for the early transfer of protective IgG antibodies from the dam to the pup. The IgG-FcRn complex is transported into the endosome and recycled back to the cell surface for release into the plasma. As FcRn-deficient mice demonstrate significant reductions in IgG antibody half-life, this supports an additional adult role for FcRn in maintaining IgG levels in the circulation.
To design a better preclinical model for therapeutic studies with human monoclonal antibodies and Fc containing-fusion proteins, human FcRn was expressed from a transgene in mice that are genetically deficient in the expression of murine FcRn. As expected, human antibodies are protected by human FcRn to a greater extent than by the murine FcRn. (Haraya et al. 2014; Petkova et al. 2006). The use of human FcRn-expressing mice in pharmacokinetic studies has been highly predicative of the clinical half-life of circulating antibodies, decreasing the extent of which nonhuman primate studies need to be employed.
The Efficient Use of the Mouse
While the mouse is well established as one of the premier model organisms for genetic manipulation, disease modeling, and preclinical drug testing, there remain many obstacles to their effective and efficient use. The lack of reproducibility in many preclinical studies is of growing concern, and new initiatives by the NIH call for more rigorously designed studies to ensure that data can be reproduced. These initiatives involve more thoughtful and robust statistical analysis and transparency in data reporting as well as sharing of materials. The use of public mouse repositories is a key element in promoting reproducibility. Investigators who deposit their models in repositories satisfy the NIH policy for the sharing of resources. In addition, mouse repositories apply rigorous genetic quality control and pathogen testing to ensure that the mice distributed are of high quality. By placing mouse models into the public domain, the scientific community has rapid access to the best models and key characteristics and disease progression rates of a particular model that can be rapidly validated in independent studies. An inconvenient truth is that the academic environment is not designed to foster resource sharing. For most laboratories, genetic engineering of mouse models represents a significant investment in time and money. The return on this investment comes in the form of papers and grants that keep the laboratory running and students graduating. Thus, there exists a significant disincentive to give away resources, especially for smaller laboratories or new investigators. As a result, there is often a significant lag time before investigators deposit mice in public repositories. Additional hurdles to getting the right mouse for the study can be the time-consuming and expensive licensing agreement negotiation process, required in order to make some models available to “for-profit” companies. More expedient sharing of resources and a community consensus on standardized material transfer agreements and licensing requirements would significantly improve the rate at which models are distributed and therapies developed. As it now stands, many for-profit entities and frustrated academics can end up duplicating resources by using CRISPR/Cas9-mediated genome engineering to essentially recreate published models. In the future, the ease by which we can make models using CRISPR/Cas9 technology may lower the barrier for resource sharing and work to encourage more collaboration.
Conclusions
At first glance, the continuing increase in the use of the mouse in mouse models for biomedical and preclinical research is contrary to the goals associated with the three Rs (reduce, refine, replace) as it applies to the use of animals in research. However, it can be argued that the development of newer techniques in mouse mutant development, such as CRISP/Cas9-mediated genome engineering, offers the ability to significantly increase our understanding of disease initiation and progression and identify therapeutically relevant intervention in refined mouse models containing clinically relevant disease alleles. Potentially, these disease-specific mouse models will drive a significant reduction, or replacement, of higher order mammals, including primates, being part of the future drug discovery pipeline.
The mouse is a useful and valuable experimental model. For all of the reasons given above, mice represent an economically viable, disease susceptible orthologue of human conditions. Mice can be genetically manipulated, and many of the treatments devised to work in human can be modified to evaluate the effect throughout the complete life-cycle of mice within a relatively short time span. However, it is critically important to bear in mind that a mouse is not a human, and we must understand the limitations of the models. Scientists and drug developers often search for the perfect model in a given disease area. However, the most successful areas have benefited from a number of mouse models, all of which have informed the field in different ways. Some models have been useful in the development and use of molecular and early phenotypic biomarkers. Other models manifest phenotypes associated with the disease and have been used to assess therapeutic endpoints in therapeutic testing. Still other models may not directly be used in preclinical testing, but their value in contributing to our understanding of disease mechanism has been instrumental in developing therapeutics.
The CRISPR/Cas9 technology will undoubtedly increase the efficiency and pace of genetic engineering adding to our downstream knowledge of gene function, systems biology, and models for improving safety and preclinical testing.
Importantly, there are instances where mice are not ideal or limited in their relevance to the human condition being investigated (Justice and Dhillon 2016). Caution should be exercised in interpreting preclinical data to the relevance in human trials. Data that may be statistically significant in a mouse may not be therapeutic relevance in a patient. Likewise, there are many reasons why clinical trials fail, all of which should be evaluated before deducing the preclinical model was poor. With that said, the versatility of the mouse, its similarities to human with respect to disease susceptibility and progression, the ease of access to established models, and the ease of custom model development mean that the use of the mouse as a tool in basic science research and preclinical drug development is going to be with us for a long time.
References
- Black RS, Sperling RA, Safirstein B, Motter RN, Pallay A, Nichols A, Grundman M. 2010. A single ascending dose study of bapineuzumab in patients with Alzheimer disease. Alzheimer Dis Assoc Disord 24:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogue MA, Churchill GA, Chesler EJ. 2015. Collaborative Cross and Diversity Outbred data resources in the mouse phenome database. Mamm Genome 26:9–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi MR. 1994. Targeted gene replacement. Sci Am 270:52–59. [DOI] [PubMed] [Google Scholar]
- Chan AC, Carter PJ. 2010. Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol 10:301–316. [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Miller DR, Branstetter LR, Galloway LD, Jackson BL, Philip VM, Voy BH, Culiat CT, Threadgill DW, Williams RW, Churchill GA, Johnson DK, Manly KF. 2008. The Collaborative Cross at Oak Ridge National Laboratory: developing a powerful resource for systems genetics. Mamm Genome 19:382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill GA, Airey DC, Allayee H, Angel JM, Attie AD, Beatty J, Beavis WD, Belknap JK, Bennett B, Berrettini W, Bleich A, Bogue M, Broman KW, Buck KJ, Buckler E, Burmeister M, Chesler EJ, Cheverud JM, Clapcote S, Cook MN, Cox RD, Crabbe JC, Crusio WE, Darvasi A, Deschepper CF, Doerge RW, Farber CR, Forejt J, Gaile D, Garlow SJ, Geiger H, Gershenfeld H, Gordon T, Gu J, Gu W, de Haan G, Hayes NL, Heller C, Himmelbauer H, Hitzemann R, Hunter K, Hsu HC, Iraqi FA, Ivandic B, Jacob HJ, Jansen RC, Jepsen KJ, Johnson DK, Johnson TE, Kempermann G, Kendziorski C, Kotb M, Kooy RF, Llamas B, Lammert F, Lassalle JM, Lowenstein PR, Lu L, Lusis A, Manly KF, Marcucio R, Matthews D, Medrano JF, Miller DR, Mittleman G, Mock BA, Mogil JS, Montagutelli X, Morahan G, Morris DG, Mott R, Nadeau JH, Nagase H, Nowakowski RS, O'Hara BF, Osadchuk AV, Page GP, Paigen B, Paigen K, Palmer AA, Pan HJ, Peltonen-Palotie L, Peirce J, Pomp D, Pravenec M, Prows DR, Qi Z, Reeves RH, Roder J, Rosen GD, Schadt EE, Schalkwyk LC, Seltzer Z, Shimomura K, Shou S, Sillanpää MJ, Siracusa LD, Snoeck HW, Spearow JL, Svenson K, Tarantino LM, Threadgill D, Toth LA, Valdar W, de Villena FP, Warden C, Whatley S, Williams RW, Wiltshire T, Yi N, Zhang D, Zhang M, Zou F, Complex Trait Consortium . 2004. The Collaborative Cross, a community resource for the genetic analysis of complex traits. Nature 36:1133–1137. [DOI] [PubMed] [Google Scholar]
- Churchill GA, Gatti DM, Munger SC, Svenson KL. 2012. The Diversity Outbred mouse population. Mamm Genome 23:9–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudna JA, Charpentier E. 2014. The new frontier of genome engineering with CRISPR-Cas9. Science 346:1077–1086. [DOI] [PubMed] [Google Scholar]
- FDA 2011. Available online (http://www.fda.gov/downloads/Science-Research/SpecialTopics/RegulatoryScience/UCM268225.pdf), accessed on August 21, 2016
- Feinstein MJ, Lloyd-Jones DM. 2016. Monoclonal antibodies for lipid management. Curr Atheroscler Rep 18:39–46. [DOI] [PubMed] [Google Scholar]
- Hall B, Limaye A, Kulkarni AB. 2009. Overview: generation of gene knockout mice. Curr Proc Cell Bio 19:1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraya K, Tachibana T, Nanami M, Ishigai M. 2014. Application of human FcRn transgenic mice as a pharmacokinetic screening tool of monoclonal antibody. Xenobiotica 44:1127–1134. [DOI] [PubMed] [Google Scholar]
- Hernández-Bernal F, García-García I, González-Delgado CA, Valenzuela-Silva C, Soto-Hernández R, Ducongé J, Cervantes-Llano M, Blanco-Garcés E, Rodríguez V, García-Vega Y, Bello-Rivero I, Olivera-Ruano L, López-Saura P. 2005. Bioequivalence of two recombinant granulocyte colony stimulating factor formulations in healthy male volunteers. Biopharm Drug Dispos 26:151–159. [DOI] [PubMed] [Google Scholar]
- Iraqi FA, Athamni H, Dorman A, Salymah Y, Tomlinson I, Nashif A, Shusterman A, Weiss E, Houri-Haddad Y, Mott R, Soller M. 2014. Heritability and coefficient of genetic variation analyses of phenotypic traits provide a strong basis for high resolution QTL mapping in the collaborative cross mouse genetic reference population. Mamm Genome 25:109–119. [DOI] [PubMed] [Google Scholar]
- Jang YY, Cai L, Ye Z. 2016. Genome editing systems in novel therapies. Discov Med 21:57–64. [PubMed] [Google Scholar]
- Justice MJ, Dhillon P. 2016. Using the mouse to model human disease: increasing validity and reproducibility. Disease Model Mech 9:101–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane TM, Goodstadt L, Danecek P, White MA, Wong K, Yalcin B, Heger A, Agam A, Slater G, Goodson M, Furlotte NA, Eskin E, Nellåker C, Whitley H, Cleak J, Janowitz D, Hernandez-Pliego P, Edwards A, Belgard TG, Oliver PL, McIntyre RE, Bhomra A, Nicod J, Gan X, Yuan W, van der Weyden L, Steward CA, Bala S, Stalker J, Mott R, Durbin R, Jackson IJ, Czechanski A, Guerra-Assunção JA, Donahue LR, Reinholdt LG, Payseur BA, Ponting CP, Birney E, Flint J, Adams DJ. 2011. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature 477:289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López EL, Contrini MM, Glatstein E, González Ayala S, Santoro R, Allende D, Ezcurra G, Teplitz E, Koyama T, Matsumoto Y, Sato H, Sakai K, Hoshide S, Komoriya K, Morita T, Harning R, Brookman S. 2010. Safety and pharmacokinetics of urtoxazumab, a humanized monoclonal antibody, against Shiga-like toxin 2 in healthy adults and in pediatric patients infected with Shiga-like toxin-producing Escherichia coli. Antimicrob Agents Chemother 54:239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins JP, Kennedy PJ, Santos HA, Barrias C, Sarmento B. 2016. A comphrehensive review of the neonatal FcRn receptor and its application in drug delivery. Pharmacol Ther 161:22–39. [DOI] [PubMed] [Google Scholar]
- McMahon FG, Vargas R, Ryan M, Jain AK, Abels RI, Perry B, Smith IL. 1990. Pharmacokinetics and meffects of recombinant human erythropoietin after intravenous and subcutaneous injections in healthy volunteers. Blood 76:1718–1722. [PubMed] [Google Scholar]
- Ousterout DG, Kabadi AM, Thakore PI, Perez-Pinera P, Brown MT, Majoros WH, Reddy TE, Gersbach CA. 2015. Correction of dystrophin expression in cells from Duchenne muscular dystrophy patients through genomic excision of exon 51 by zinc finger nucleases. Mol Ther. 23:523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD, Brinster RL. 1986. Germ-line transformation of mice. Ann Rev Genet 20:465–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkova SB, Akilesh S, Sproule TJ, Christianson GJ, Al Khabbaz H, Brown AC, Presta LG, Meng YG, Roopenian DC. 2006. Enhanced half-life of genetically engineered human IgG1 antibodies in a humanized FcRn mouse model: potential application in humorally mediated autoimmune disease. Int Immunol 18:1759–1769. [DOI] [PubMed] [Google Scholar]
- Scott AM, Wolchok JD, Old LJ. 2012. Antibody therapy of cancer. Nat Rev Cancer 12:278–287. [DOI] [PubMed] [Google Scholar]
- Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. 2012. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol 12:786–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Schimenti JC, Bolcun-Filas E. 2015. A mouse geneticist's practical guide to CRISPR applications. Genetics 199:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siramshetty VB, Nickel J, Omieczynski C, Gohlke B-O, Drwal MN, Preissner R. 2016. WITHDRAWN – a resource for withdrawn and discontinued drugs. Nucl Acid Res 44:D1080–D1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenson KL, Gatti DM, Valdar W, Welsh CE, Cheng R, Chesler EJ, Palmer AA, McMillan L, Churchill GA. 2012. High-resolution genetic mapping using the mouse diversity outbred population. Genetics 190:437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Seino Y, Fujieda K, Igarashi Y, Yokoya S, Tachibana K, Ogawa Y. 1999. Pharmacokinetics and metabolic effects of high-dose growth hormone administration in healthy adult men. Endocr J 46:605–612. [DOI] [PubMed] [Google Scholar]
- Threadgill DW, Churchill GA. 2012. Ten years of the Collaborative Cross. Genetics 190:291–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefers B, Ortiz O, Wurst W, Kühn R. 2014. Generation of targeted mouse mutants by embryo microinjection of TALENs. Methods. 69:94–101. [DOI] [PubMed] [Google Scholar]
- Weiner LM, Surana R, Wang S. 2010. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol 10:317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]