Abstract
Maternal stress can prenatally influence offspring phenotypes and there are an increasing number of ecological studies that are bringing to bear biomedical findings to natural systems. This is resulting in a shift from the perspective that maternal stress is unanimously costly, to one in which maternal stress may be beneficial to offspring. However, this adaptive perspective is in its infancy with much progress to still be made in understanding the role of maternal stress in natural systems. Our aim is to emphasize the importance of the ecological and evolutionary context within which adaptive hypotheses of maternal stress can be evaluated. We present five primary research areas where we think future research can make substantial progress: (1) understanding maternal and offspring control mechanisms that modulate exposure between maternal stress and subsequent offspring phenotype response; (2) understanding the dynamic nature of the interaction between mothers and their environment; (3) integrating offspring phenotypic responses and measuring both maternal and offspring fitness outcomes under real-life (either free-living or semi-natural) conditions; (4) empirically testing these fitness outcomes across relevant spatial and temporal environmental contexts (both pre- and post-natal environments); (5) examining the role of maternal stress effects in human-altered environments—i.e., do they limit or enhance fitness. To make progress, it is critical to understand the role of maternal stress in an ecological context and to do that, we must integrate across physiology, behavior, genetics, and evolution.
Introduction
Maternally-derived glucocorticoid (GC) hormones can influence the phenotype of developing offspring in both the laboratory (reviewed in Barbazanges etal. 1996; Gluckman etal. 2005; Meaney etal. 2007) and the natural world (reviewed in Meylan etal. 2012; Love etal. 2013). Here we define such phenomena as maternal stress effects, where exposure to an environmental stressor (e.g., predation risk, low food availability, social instability, and weather) elevates maternal GCs (in vertebrates), which in-turn influence offspring phenotype. Short-term examinations of these phenotypic responses in offspring have often previously been interpreted as unavoidable negative outcomes of exposure to maternally-derived GCs (i.e., smaller birth/hatch masses, slower growth; Love etal. 2013). However, integrative ecologists have proposed and begun empirically testing the environmental/maternal-matching hypotheses (Gluckman and Hanson 2004; Love and Williams 2008; Monaghan 2008; Sheriff and Love 2013), which states that maternal stress has the potential to be adaptive if the maternal and offspring environment match (i.e., mothers and offspring share a common environmental stressor, either spatially or temporally). This hypothesis has challenged the traditional negative-outcome perspective and evidence has been provided that maternal stress effects may adaptively prepare offspring for a more stressful or rigorous future environment (Marshall and Uller 2007; Uller 2008; Sheriff and Love 2013; but see Uller etal. 2013).
In order to determine whether maternal stress effects can be adaptive it is useful to consider the particular past, present, and future environments that mothers and their offspring are likely to experience (sensuMarshall and Uller 2007; Sheriff and Love 2013). For example, the fitness value of offspring phenotypes depends on the future environments within which they will interact. Similarly, the life history of the organism will influence how stress-induced signals shape offspring phenotypic responses. Finally, it is useful to consider how maternal stress influences the relative fitness of both mothers and offspring across the different, naturally-occurring contexts they might encounter.
In this paper we aim to provide researchers with greater ecological and evolutionary context within which the adaptive role of maternal stress effects can be evaluated in the laboratory and field (Fig. 1). Although we focus mainly on vertebrates, the concepts within are broadly applicable across taxa. Specifically, we discuss the intrinsic and extrinsic factors that can influence maternal stress and therefore the maternal stress–offspring phenotype relationship, how an appreciation for the integration of offspring phenotypic responses across relevant spatial and temporal environments will increase our understanding of the potential adaptive value of maternal stress effects, and, finally, the role that maternal stress effects can play within human-induced rapidly changing environments.
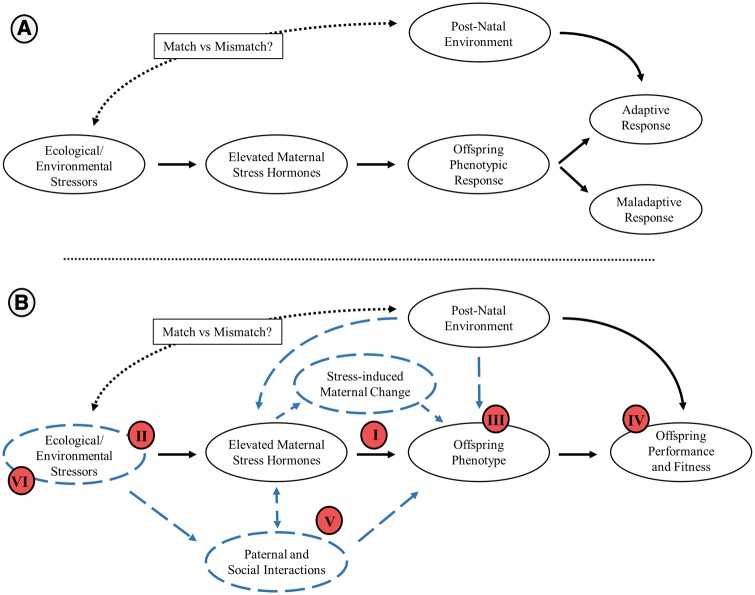
Fig. 1.
The potential pathway of an ecological/environmental stressor on offspring performance and fitness, acting through maternal stress effects. (A) Generally it is thought that an ecological or environmental stressor will increase maternal stress hormones, which in turn will alter offspring phenotype in an adaptive or maladaptive manner depending upon the post-natal environment experienced by the offspring. (B) Building upon this general framework we propose many avenues of future research that may provide novel insights into our understanding of maternal stress effects (highlighted by the blue hashed lines). (I) Although traditionally thought that maternal stress effects were controlled by the mother and her hormone levels, there is new emerging evidence that offspring may play a significant role in this relationship. Further, maternally-derived stress hormones may not be the only mediator of this relationship. (II)The ecological and environmental stressors experienced by the mother are likely dynamic in nature, and may change how and when mothers perceive and respond to them. (III) Offspring’s phenotypic response does not occur along a single axis, but may be a sex-specific integration of their behavioral, physiological, and morphological changes. Changes that do not stop at birth/hatching but likely continue through development, being influenced by both pre-natal and post-natal cues. (IV) To fully appreciate the adaptive value of maternal stress effects, the offspring phenotype’s performance and fitness must not only be considered within the ecological context it occurs but its relative value among both stressful and unstressful future environments must be compared. (V) In many cases mothers and offspring do not exist in a simple dyadic relationship, but may be part of a larger social unit that may influence the mother–environment and mother–offspring relationship. Further, new evidence is emerging on the influence of paternal and grandparental stress effects that may themselves directly influence offspring phenotype. (VI) Lastly, maternal stress effects may play a critical role in how organisms respond to a changing world. Maternal stress effects may result in evolutionary traps if mothers do not perceive novel stressors as stressful or if they perceive unstressful events as stressful. Alternatively, maternal stress effects may increase the magnitude of an adaptive response if environmental changes lead to an increase within the mean of variation of a known stressor. Clearly, there is much work to be done in this exciting field.
Mechanisms of maternal stress effects: moving beyond maternal programming
The mechanisms by which offspring phenotype is shaped by maternal stress can act both pre- and post-natally, and as such, how they act depends on whether the animal is egg-laying or placental, and the amount of subsequent parental care (see Matthews 2002; Love etal. 2013; and Monaghan and Haussmann 2015, for review). In oviparous species, discrete and finite levels of maternally-derived GCs (cortisol and corticosterone) are deposited into eggs as a function of the relative stressfulness of the mother’s environment during egg production (e.g., Saino etal. 2005; Almasi etal. 2012; Sopinka etal. 2017). In placental viviparous species, offspring exposure to maternally-derived GCs can fluctuate throughout gestation in relation to current maternal levels, producing a dynamic exposure (Matthews 2002). Nevertheless, a short-term, acute environmental insult to a mother during gestation may also be sufficient to alter offspring phenotype, depending upon the stage of gestation when the stressor occurs (e.g., Kapoor and Matthews 2005; Kapoor etal. 2009). Offspring may also be directly exposed to maternally-derived GCs post-natally, for example, through milk in mammals (Sullivan etal. 2011). Regardless of whether in ovo, in utero, or post-natal, GC exposure can have both activational and organizational effects on offspring morphology, physiology, and behavior. Laboratory studies have shown these effects to be potentially mediated by differences in methylation patterns and epigenetic changes throughout the offspring genome (Heijmans etal. 2008; Mueller and Bale 2008; Love etal. 2013; Cao-Lei etal. 2014).
Effects of maternal stress on offspring have often been described as “maternal programming”, which assumes that the phenotypic outcome of offspring was primarily under maternal control (Monaghan and Spencer 2014). Indeed, since GCs are important regulators of many key developmental pathways it is plausible that mothers may have co-opted these control systems over evolutionary time (Love etal. 2005). However, the developing embryo may not be a passive, downstream recipient of a mother’s hormonal dictates. Recent evidence suggests that mothers may not have complete control of these effects and offspring do indeed possess a number of mechanisms which may dampen or buffer the costs of GC exposure (see below), while still being able to use the signal of maternal stress as a reliable predictor of the future environment (Love etal. 2013). For example, offspring can modulate GC exposure by embryonic metabolic processes via the sulfonation pathway (Paitz and Bowden 2013). Likewise, three-spined stickleback (Gasterosteus aculeatus) embryos can rapidly efflux maternally-derived GCs from the egg via ATP-binding cassette transporters (Paitz etal. 2016). Laboratory studies in rodents have found that offspring can buffer their exposure to maternally-derived GCs via placental 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2), which converts GCs to inert forms (Seckl 2004). Nonetheless, increases in maternal stress are not accompanied by an associated increase in 11β-HSD2 levels, resulting in potentially greater offspring GC exposure as maternal stress levels increase beyond a particular threshold (Lesage etal. 2001; Lucassen etal. 2009). Thus, mothers may not have complete control of the maternal stress–offspring phenotype relationship and offspring may play an active role in their GC exposure and response to maternally-derived GCs. Indeed, this idea of “parent–offspring conflict” (Crespi and Semeniuk 2004; Uller and Pen 2011) has been examined recently with regards to exposure to maternally-derived hormones in general (Müller etal. 2007). Exploring this conflict with specific regards to maternal stress should provide significant enlightenment as to the evolutionary pathways of this maternal effect.
In addition to direct exposure to maternally-derived GCs, offspring phenotype may be indirectly influenced by stress-induced changes to maternal behavior, condition, and physiology (beyond just GC levels). For example, in the viviparous common lizard (Lacerta vivipara), maternal GC levels and body condition interact to influence dispersal propensity in offspring (e.g., Meylan etal. 2002). Mothers with elevated GC levels may also alter pre-natal nutrient allocation or provisioning (Cottrell etal. 2012), or reduce post-natal parental care (Herrenkohl and Whitney 1976; Silverin 1986; Baker etal. 2008), both of which can influence offspring phenotype. For example, nutritional restriction in early-life has been shown to alter brain development, song repertoire, growth, and energy expenditure in song sparrows (Schmidt etal. 2012, 2013, 2014). Ultimately, the mechanisms driving maternal stress effects are likely a combination of direct and indirect offspring exposure to maternal stress. Cross-fostering experiments in organisms that provide substantial post-natal care or experimental manipulations in oviparous organisms, where egg-GC-dosing effects could be compared against maternal GC effects, may help elucidate the relative contribution of direct versus indirect exposure. Clearly, a better understanding of these multiple interactive effects is needed.
Environmental regulation of the strength of maternal stress effects
Increasing attention to the evolution of transgenerational (across multiple generations), maternal effects (non-genetic inheritance) has suggested a unifying theoretical perspective that organisms should make use of relevant environmental cues to adaptively adjust their phenotype (e.g., Levins 1968; Moran 1992; Jablonka etal. 1995; Stamps and Krishnan 2014; Dall etal. 2015; Leimar and McNamara 2015; Box 1). These ideas have consistently suggested focusing on the importance of spatial and temporal stability of the environment, the “reliability” of the stressor, and the subsequent match between conditions experienced by the mother and those experienced by the offspring to examine the evolutionary role of maternal effects. While maternally-derived GCs can provide a potential cue of the future environment and a mechanism of phenotypic adjustment, maternal stress effects should only evolve when environmental stressors experienced by the mother are likely to be experienced by the offspring, but importantly, that stressors within the environment generally fluctuate among generations (Box 1). For example, if the environment is highly stable among generations, selection should favor a more fixed (rather than plastic) phenotype (Levins 1968; DeWitt etal. 1998; Boonstra 2013; Kuijper and Hoyle 2015). Yet, few empirical studies have explicitly examined how differences in the predictability and magnitude of an environmental stressor may influence the strength and ultimate impact of maternal stress effects (Burgess and Marshall 2014). Evidence suggesting that greater exposure to maternally-derived GCs increases the degree of phenotypic response in offspring (e.g., Sheriff etal. 2009, 2010), leads to the prediction that the magnitude of stressor experienced, and the variation around that mean, has the potential to influence the strength of maternal stress effects.
Box 1.
Testing maternal stress effects in free-living systems
We expect that maternal stress effects should evolve most strongly within species that experience: (i) relative consistency between the environment experienced by gestating mothers and that experienced by their offspring, but high variation in environmental stressors generally; and (ii) relatively high costs of producing an unmodified offspring in a stressful environment compared with the costs of producing a modified offspring in a benign environment (although this may be difficult to observe in nature given the selection pressures of these variable costs; Sheriff etal., submitted for publication). Further, if the focal interest is on pre-natal stress-induced effects then systems with low post-natal parental care should be selected, given this care may reduce the importance of pre-natal stress effects (with the opposite if the focal interest is on post-natal stress effects).
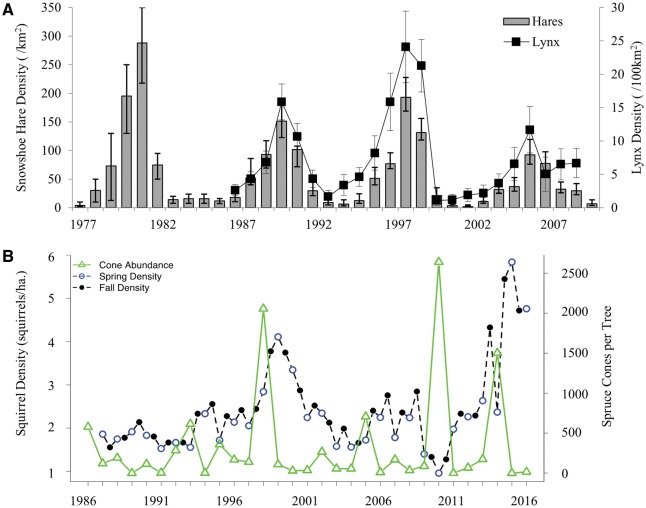
Species that exhibit cyclic population dynamics are ideal field-based models given their large, relatively predictable inter-annual variation (e.g., Fig. 2). Maternal effects have been invoked or found in the demographic patterns in cyclic systems of insects (Ginzburg and Taneyhill 1994; Rossiter 1994), birds (Martınez-Padilla etal. 2014), and mammals (voles—Boonstra and Boag 1992; Boonstra etal. 1998a; Inchausti and Ginzburg 1998; snowshoe hares—Boonstra etal. 1998b; Sheriff etal. 2011, 2015). However, most of the later studies focus only on the central herbivore within the system, yet their predators are also cycling and strong maternal stress effects are predicted to occur there as well.
In systems that do not cycle, strong maternal stress effects are also predicted to occur if species dynamics are driven by relatively consistent (if not exactly predictable) and large, variations in stressors. For example, mast years in conifer and deciduous trees driven by large scale weather patterns (Pearse etal. 2016) cause large changes in food supply every 3–8 years that cascade upwards to the herbivore populations that depend on them (e.g., Ostfeld and Keesing 2000; Fig. 2). Such fluctuations in the food supply can then either directly or indirectly cause maternal stress (because of increasing population density) resulting in an offspring phenotypic response (e.g., Dantzer etal. 2013).
Fig. 2.
Density changes over time (year) in (A) the 10-year population cycle of snowshoe hares and lynx (±95% CI; adapted from Krebs et al. 2014) and (B) the mast-density relationship between red squirrels and white spruce (adapted from Dantzer et al. 2012).
The severity of the stressor is also likely to influence the strength of maternal stress effects. For example, a potentially lethal stressor, such as the risk of predation, may have a greater effect on offspring phenotypic responses than one that is more benign, such as density or extreme temperature. As such, understanding the ecological context within which the organism has evolved is key in interpreting how a particular stressor will affect the offspring’s phenotypic response (sensuMarshall and Uller 2007; Uller 2008; Sheriff and Love 2013). It is also important to appreciate that there may be a potential disconnect between factors that mothers perceive as stressful and those their offspring perceive as stressful. For example, differential vulnerability to certain predators might occur between adults and offspring due to differences in size and morphology, habitat use, anti-predator defenses, etc. (e.g., Vitt and Cooper 1986; Reimchen 1991; Fuiman and Magurran 1994; Mattingly and Butler 1994; Benard 2004). Studies using egg predator cues to increase general perception of risk provide some evidence that mothers may respond to juvenile-specific predators (e.g., McCormick 1998; Zanette etal. 2011). However, within these studies mothers interact directly with this risk while they are still caring for the eggs or young (making it difficult to separate direct and indirect effects). A mother’s future reproductive potential and life expectancy may also influence how she responds to a stressor (Monclús etal. 2011; Gélin etal. 2015). Ultimately, the response a mother exhibits toward a stressor is likely determined by her own vulnerability and life expectancy, yet it is unclear how individual mothers may perceive and integrate stressors that may only impact her offspring, and how this may more generally influence the evolution of maternal stress effects.
An additional underappreciated and unexplored area in this field is whether the timing of maternal experience has the capacity to influence the magnitude and timing of the offspring phenotypic response. One prediction is that a mother’s current experience should have stronger immediate effects relative to past experiences since current conditions are likely to provide the most up-to-date information about the offspring’s current and future environment. However, this might not always be the case, and offspring may be better off responding to an environment experienced in the mother’s distant past or not responding until later in their life, particularly if the stressors are age-specific (e.g., Zimmer etal. 2017). For example, if mothers experience an environment that is only stressful for adults, the outcomes of maternal stress effects in the offspring may not be seen until adulthood. Empirical studies examining the dynamic nature of environmental stressors, and how they are experienced by the mother and translated to offspring would help us better understand the role of maternal stress effects in ecological and evolutionary processes.
Beyond a simple mother–offspring dyadic relationship
Mothers and offspring frequently do not exist in simple dyadic interactions, but are at least temporarily part of social units, which may include fathers, siblings, and, in more complex groups, conspecifics of varying relatedness. The social context in which stressors occur likely influences the magnitude and direction of maternal stress effects (Beery and Kaufer 2015). For example, if there is more than one provisioning parent, the consequences of stress-induced reductions in maternal care and provisioning may be overcome by the partner, though the extent to which partners are likely to compensate is itself context-dependent (Hinde 2006; Johnstone and Hinde 2006). Alternatively, if both parents are subject to a similar general environmental stressor, effects on the offspring may be enhanced, for example, if both parents reduce provisioning rates (Zanette etal. 2011). In cooperatively breeding species, mothers may be buffered from a stressful experience and their relative post-natal influence on offspring phenotype may be further reduced. In cooperative fairy wrens, for example, helper individuals can fully compensate for reductions in maternal egg investment (Russell etal. 2007) and for reductions in offspring provisioning (Wright and Dingemanse 1999; Russell etal. 2008; Brouwer etal. 2014). Thus, a consideration of the social context and mating system will provide further insights into our understanding of maternal stress effects.
The importance of fathers
The effect fathers can have on offspring phenotype has generally been neglected relative to that of the mother (Braun and Champagne 2014; Crean and Bonduriansky 2014). It has routinely been assumed that there is greater opportunity for maternal stress effects due to the intimate contact between mothers and offspring in utero or in ovo. However, laboratory-based empirical evidence suggests that fathers can also influence their offspring via stress-induced changes in sperm (e.g., Rodgers etal. 2013; Evans etal. 2017). For example, in laboratory mice where there is no paternal care, males trained to associate an odor with a stressor produced offspring that behaved differently toward the odor, even though the offspring had never been exposed to the stressor themselves (Dias and Ressier 2014). In addition, female mice mated to chronically stressed males provided less parental care compared with female mice mated to control males (Mashoodh etal. 2012). In species with paternal care, males can influence offspring directly through their parenting behavior (McGhee and Bell 2014), which can be sensitive to their own stressful experiences (Stein and Bell 2014), as well as those of their mate (McGhee etal. 2015).
This growing laboratory-based evidence for paternally-mediated stress effects on offspring prompts questions about the cumulative impact of both parents’ experience. Indeed, offspring may receive simultaneous signals pertaining to environmental quality from both parents, signals that might not always be in agreement (i.e., sexual conflict; Chapman etal. 2003; Arnqvist and Rowe 2005). This is an unexplored, yet tractable, area of research with regards to maternal stress effects. A simple prediction is that we should observe the most extreme offspring phenotypic responses when information from both parents is in agreement (Leimar and McNamara 2015). More generally, there is a need for further studies investigating how offspring manage and integrate conflicting information from mothers versus fathers. One prediction is that offspring should always respond to a parental cue about the likelihood of danger, regardless of whether information comes from one or both parents, if the costs of failing to respond are high. Another possibility is that offspring should favor information from their same-sex parent because they are more likely to experience similar environments. It will therefore be critical moving forward to test for these effects in naturally-occurring systems.
The importance of grandparents
A rapidly burgeoning field of research explores how stressors experienced by parents may persist beyond the F1 generation (reviewed in Rando 2012, Burton and Metcalfe 2014; Gapp etal. 2014). Multigenerational effects of stress are likely to occur in mammals given that gametogenesis occurs in embryos, and eggs that will produce the F2 generation are exposed to the stressor inside the F0 mother. The effect of this exposure may be enhanced or suppressed by the F1 generation’s experience. Thus, the first truly unexposed generation would be the F3 generation (Skinner etal. 2008). Interestingly, multigenerational studies often find sex-specific lineage effects (Anderson etal. 2006; Dunn etal. 2011; Bygren etal. 2014), e.g., grandsons were influenced by their paternal grandfather while grand-daughters were influenced by their maternal grandmother (Pembrey etal. 2006). Given the potential for an acute stressor to influence ecosystem processes for generations (Sheriff etal. 2015), there is a clear need to understand the prevalence and mechanisms of multigenerational (parental) stress effects in ecological contexts (Furrow and Feldman 2013; Herman etal. 2014).
The expression of offspring phenotypes
Studies of maternal stress effects have primarily examined individual aspects of offspring morphology, physiology, and behavior. As noted previously, laboratory-based studies in model rodents suggest that maternal stress results in smaller, slower-growing offspring, with increased stress reactivity and anxiety-like behaviors (see biomedical review by Meaney etal. 2007). However, ecological studies suggest that the direction and magnitude of phenotypic responses are often species- and context-specific. For example, density-induced maternal stress in red squirrels (Tamiasciurus hudsonicus) results in faster-growing offspring (Dantzer etal. 2013). In wild house wrens (Troglodytes aedon), elevated maternal GCs increased maternal investment in reproduction resulting in offspring with greater prefledging body condition (Bowers etal. 2016). Studies in sticklebacks suggest that behavioral responses in maternally-stressed offspring vary depending upon the environmental, including social, context (Giesing etal. 2011 vs. McGhee etal. 2012; Roche etal. 2012 vs. Feng etal. 2015). Given the complexity of environmental stressors within natural systems and how these may further influence and interact with offspring phenotypes, there is need for more studies investigating maternal stress effects in free-living systems across species and environmental gradients to better build our general predictions regarding maternal stress effects.
Integrating offspring phenotypic responses
In addition to understanding the relative impact of maternal stress effects within an ecological context, a greater appreciation for the integrated nature of an offspring’s global phenotypic response is needed. For example, the initial size of offspring at hatch or birth is commonly used as a proxy for fitness, and is often associated with reduced survival (Reimchen 1991; Allen etal. 2008). However, in response to greater predation risk, stress-induced reductions in offspring body size may be beneficial if they are coupled with increased hiding behavior reducing energetic needs and risky foraging behavior. Further, the cost/benefit assessment of integrated phenotypic responses must be expanded across an individual’s lifetime; processes that impact juveniles may differ substantially from those that impact adults or other life-stages (e.g., McCormick and Hoey 2004; Gagliano and McCormick 2009). There may also be compensatory and dynamic changes in offspring phenotype over time, such that an induced-trait may not remain static from development to adulthood. For example, in house wrens (T. aedon) hatchlings from corticosterone-injected eggs were lighter at hatching, but because of compensatory growth, were heavier at fledging compared with control offspring (Strange etal. 2016). Current studies in wild animals focus primarily on offspring phenotype and performance in early-life, but it is largely unknown how these change over time and translate to future performance and fitness in later life, and what compensatory mechanisms in phenotypic plasticity are in place (and if so under what circumstances they occur) (but see Blas etal. 2007).
Sex-specific susceptibility to maternal stress
Maternally-derived GCs may also have sex-specific effects on offspring characteristics, with males often more susceptible to elevated maternal stress exposure when such effects arise (e.g., Love etal. 2005; but see Montano etal. 1993). There may also be sex specific responses to maternal stress (St-Cyr etal. 2017), as evident from studies on early life stress (e.g., Schmidt etal. 2012). Biases in the primary or secondary sex ratios in species that produce more than one offspring at a time have also been shown (Pike and Petrie 2006; Bonier etal. 2007; Navara 2010, 2013; Khan etal. 2016). To date, most work on sex-specific effects of maternal stress has either investigated underlying mechanisms, such as sex-specific placental regulation (Bronson and Bale 2016), without appreciating the adaptive significance (e.g., Bale and Epperson 2015), or investigated the adaptive significance of such effects without considering the mechanisms (e.g., Trivers and Willard 1973; Veller etal. 2016; but see Cameron 2004). Future studies of maternal stress effects therefore have a unique opportunity to simultaneously examine both the proximate mechanisms and the ultimate significance of sex-specific effects.
Studies investigating sex-specific effects can provide further insights into the relative control mothers or offspring have in regulating maternal stress effects. Theoretical models suggest that if there is parent–offspring conflict in the optimal offspring phenotype (Uller and Pen 2011), mothers may attempt to maximize their own fitness by reducing offspring phenotypic quality, but offspring should attempt to resist such effects. If the valence of these effects is sex-specific, selection may favor the evolution of mechanisms that enable the more-at-risk sex, the one that experiences the biggest cost of maternal stress exposure, to better resist those effects (Love etal. 2005; Love and Williams 2008). Future studies are needed to both investigate the sex-specificity of maternal stress effects, and the impact of such effects on both maternal and sex-specific offspring fitness.
The relative influence of the post-natal environment
Offspring development does not stop at birth. Indeed, post-natal, early-life experiences that result in phenotypic or developmental plasticity can allow organisms to better cope with environmental variation later in life (Relyea 2003; West-Eberhard 2003; Snell-Rood 2012). Offspring likely continually adjust their phenotype in response to environmental cues they experience beginning in utero (or in ovo) to the end of their respective developmental window, and likely still into adulthood. Laboratory- and field-based studies have shown that post-natal maternal care can enhance or negate in utero stress-induced phenotypic responses (e.g., Francis etal. 1999; Love and Williams 2008) and environmental enrichment during adolescence can reverse the effects of prenatal stress (Morley-Fletcher etal. 2003). However, studies have also shown that early exposure to maternal stress can nonetheless have long-lasting consequences to phenotypes even if offspring are exposed to benign early-life conditions (e.g., Bian etal. 2015; Sheriff 2015). These carryover effects may drive the evolution of early-life stress as a maternal effect (i.e., the balance between costs and benefits to offspring and maternal fitness) providing a rich area for future exploration. A recent theoretical model suggested that the period of developmental sensitivity is driven by the degree of variability in the environment (Panchanathan and Frankenhuis 2016). As such, we would expect that the influence of pre- versus post-natal environment on offspring phenotypic response should depend upon the relative costs associated with not responding to either environment, the need for an individual to remain phenotypically plastic during early life given the life history of the species (Snell-Rood 2012; Panchanathan and Frankenhuis 2016), and the relative predictability/stability of the future environment (Uller 2008; Moore etal. 2015). For organisms that remain phenotypically plastic during early life a particularly interesting avenue for future research would be to investigate how offspring phenotype is influenced by the relative quality of information gained during the pre- versus post-natal period, particularly if the temporal nature of the information provides contradictory cues (i.e., your post-natal experience contradicts the cues provided by your mother pre-natally; Kuijper and Hoyle 2015).
Toward general predictions of offspring phenotypicresponses
Although many have examined individual aspects of offspring phenotypic response to maternally-derived GCs, a predictive general theory on which phenotypic traits can be expected after exposure to maternal stress has yet to be formally articulated. The biomedical literature suggests that offspring exposure to maternally-derived GCs results in smaller, slower-growing, anxious offspring (Meaney etal. 2007), but growing evidence from natural populations suggests that offspring phenotypic responses are often species-, life-history- and context-specific (Marshall and Uller 2007; Love etal. 2009; Sheriff and Love 2013). But is there a generalized outcome that can be predicted based on the taxon, the life history, and the type of stressor experienced by an organism, or are responses truly individually, context-specific? For example, in free-living mammals and birds, maternal exposure to predation risk has been shown to reduce offspring body size and weight (e.g., Sheriff etal. 2009; Zanette etal. 2011; Coslovsky and Richner 2011), whereas exposure in wild lizards to risk cues or risk-induced maternal GC levels has been shown to increase offspring body size, particularly tail length (e.g., Bestion etal. 2014). We expect offspring’s general phenotypic response is a hierarchical integration across factors, and suggest that meta-analyses across taxa and context could provide testable hypotheses to increase our understanding of maternal stress effects as a general phenomenon.
Maternal stress effects in a changing world
Maternal stress effects may play a critical role in organismal responses to human-induced rapid environmental change (i.e., HIREC; Sih 2010) with two potential outcomes. Given that maternal stress effects are species-specific responses likely to have been optimized by natural selection in response to expected environmental variation (Gluckman etal. 2005), if HIREC leads to organisms increasingly exposed to novel stressors maternal stress effects have the strong potential to result in evolutionary traps (Schlaepfer etal. 2002). This scenario can occur if mothers fail to perceive novel stressors as stressful (sensuSih etal. 2010), or if mothers perceive unstressful events as stressful (sensuTrimmer etal. 2017).
Alternatively, if HIREC results in an increase in the mean or variation of a known stressor, currently experienced within an organism’s life history, maternal stress effects may increase the magnitude of an adaptive offspring phenotypic response and increase maternal and offspring fitness. For example, Chinook salmon experience periodic droughts during reproduction, and a recent study has shown that pre-natal exposure of Chinook salmon to maternal stress results in phenotypes that perform better in low water (i.e., drought-simulated) conditions at the fry stage (Capelle 2016). This scenario may also occur if HIREC introduces a novel stressor that is within the realm of that experienced by the organism. For example, prey are under intense selection pressure to respond adaptively to predators, often a suite of predators, and it has been shown that prey can generalize their perception and antipredator responses from current predators to closely related novel predators (Griffin etal. 2001; Ferrari etal. 2007). Yet, if a novel predator has an unfamiliar hunting mode, although it may be perceived as a stressor, the offspring phenotypic response may be maladaptive. We expect the adaptive potential of maternal stress effects to novel stressors to depend upon the interactive effects of maternal perception ability, relatedness of novel stressors, and the plasticity of offspring phenotypic response.
Conclusion
The study of how maternal stress shapes offspring phenotype has intensified over the past decade and is currently within a period of extreme interest and excitement. A genuine shift from viewing maternal stress as a unanimous cost to mothers and offspring has given way to an appreciation that altered phenotypes have the potential to perform better under certain future environmental circumstances. Nevertheless, this new perspective and set of accompanied approaches is still in its infancy and much still has to be developed theoretically and then tested empirically. We have synthesized five primary areas for further research examining the adaptive potential of maternal stress where substantial progress can be made:
Identifying the mechanisms that allow offspring to modulate exposure to maternal stress and examining the indirect, alternate maternal mechanisms of influence are needed as these are the mechanistic scaffolds on which natural selection can shape the evolution of maternal stress effects.
Examining the environmental regulation and dynamic nature of the environment–mother interaction will be insightful to understanding how they drive different offspring phenotype responses and the evolution of adaptive maternal stress effects.
Measuring fitness outcomes under real-life (either free-living or semi-natural) conditions to adequately assess the adaptive potential of stress-induced phenotypes.
Empirically-testing fitness outcomes across pre- and post-natal environments, and across spatial and temporal scales, is likely to be insightful for understanding the interaction between altered offspring phenotype and environmental variation.
Examining the potential for evolved, adaptive maternal stress effects to either limit or enhance fitness outcomes (and therefore population viability) under novel, HIREC scenarios.
Critical in going forward is that future studies integrate across biological disciplines from physiology to ecology and evolution, to investigate and test the adaptive potential of maternal stress in naturally-occurring systems. We must examine maternal stress effects as a continuum; integrating studies at lower levels, that delineate the machinery, with studies at higher levels, that assess the functioning and evolution (sensuBartholomew 1986).
Acknowledgments
The authors thank C. J. Krebs, S. Boutin, A. G. McAdam, and M. M. Humphries for access to long-term data regarding the snowshoe hare—lynx population cycle and red squirrel and spruce cone abundance in the Yukon.
Funding
This work was supported by the National Science Foundation [NSF-IOS 1456655 to M.J.S.]; the National Institutes of Health [NIH R01 GM082937 to A.B.]; the Fyssen Foundation Post-Doctoral Fellowship [to L.W.].
References
- Allen RM, Buckley YM, Marshall DJ.. 2008. Offspring size plasticity in response to intraspecific competition: an adaptive maternal effect across life-history stages. Am Nat 171:225–37. [DOI] [PubMed] [Google Scholar]
- Almasi H, Rettenbacher SM, Nüeller C, Brill S, Wagner H, Jenni L.. 2012. Maternal corticosterone is transferred into the egg yolk. Gen Comp Endocrinol 178:139–44. [DOI] [PubMed] [Google Scholar]
- Anderson LM, Riffle L, Wilson R, Travlos GS, Lubomirski MS, Alvord WG.. 2006. Preconceptional fasting of fathers alters serum glucose in offspring of mice. Nutrition 22:327–31. [DOI] [PubMed] [Google Scholar]
- Arnqvist G, Rowe L.. 2005. Sexual conflict. Princeton (NJ: ): Princeton University Press. [Google Scholar]
- Baker S, Chebli M, Rees S, Lemarec N, Godbout R, Bielajew C.. 2008. Effects of gestational stress: 1. Evaluation of maternal and juvenile offspring behavior. Brain Res 1213:98–110. [DOI] [PubMed] [Google Scholar]
- Bale TL, Epperson CN.. 2015. Sex differences and stress across the lifespan. Nat Neurosci 18:1413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbazanges A, Piazza PV, Le Moal M, Maccari S.. 1996. Maternal glucocorticoid secretion mediates long-term effects of prenatal stress. J Neurosci 16:3943–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew GA. 1986. The role of natural history in contemporary biology. BioScience 36:324–9. [Google Scholar]
- Beery AK, Kaufer D.. 2015. Stress, social behavior, and resilience: insights from rodents. Neurobiol Stress 1:116–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benard MF. 2004. Predator-induced phenotypic plasticity in organisms with complex life histories. Annu Rev Ecol Evol Syst 35:651–73. [Google Scholar]
- Bestion E, Teyssier A, Aubret F, Clobert J, Cote J.. 2014. Maternal exposure to predator scents: offspring phenotypic adjustment and dispersal. Proc R Soc Lond B Biol Sci 281 published online (doi: 10.1098/rspb.2014.0701). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian J-H, Du S-Y, Wu Y, Cao Y-F, Nie X-H, He H, You Z-B.. 2015. Maternal effects and population regulation: maternal density-induced reproduction suppression impairs offspring capacity in response to immediate environment in root voles Microtus oeconomus. J Anim Ecol 84:326–36. [DOI] [PubMed] [Google Scholar]
- Blas J, Bortolotti GR, Tella JL, Baos R, Marchant TA.. 2007. Stress response during development predicts fitness in a wild, long lived vertebrate. Proc Natl Acad Sci U S A 104:8880–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonier F, Martin PR, Wingfield JC.. 2007. Maternal corticosteroids influence primary offspring sex ratio in a free-ranging passerine bird. Behav Ecol 18:1045–50. [Google Scholar]
- Boonstra R. 2013. Reality as the leading cause of stress: rethinking the impact of chronic stress in nature. Funct Ecol 27:11–23. [Google Scholar]
- Boonstra R, Boag PT.. 1992. Spring declines in Microtus pennsylvanicus and the role of steroid hormones. J Anim Ecol 61:339–52. [Google Scholar]
- Boonstra R, Krebs CJ, Stenseth NC.. 1998a. Population cycles in mammals: the problem of explaining the low phase. Ecology 79:1479–88. [Google Scholar]
- Boonstra R, Hik D, Singleton GR, Tinnikov A.. 1998b. The impact of predator-induced stress on the snowshoe hare cycle. Ecol Monogr 68:371–94. [Google Scholar]
- Bowers EK, Bowden RM, Thompson CF, Sakaluk SK.. 2016. Elevated corticosterone during egg production elicits increase maternal investment and promotes nestling growth in a wild songbird. Horm Behav 83:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun K, Champagne FA.. 2014. Paternal influences on offspring development: behavioural and epigenetic pathways. JNeuroendocrinol 26:697–706. [DOI] [PubMed] [Google Scholar]
- Bronson SL, Bale TL.. 2016. The placenta as a mediator of stress effects on neurodevelopmental reprogramming. Neuropsychopharmacology 41:207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer L, Van de Pol M, Cockburn A.. 2014. The role of social environment on parental care: offspring benefit more from the presence of female than male helpers. J Anim Ecol 83:491–503. [DOI] [PubMed] [Google Scholar]
- Burgess SC, Marshall DJ.. 2014. Adaptive parental effects: the importance of estimating environmental predictability and offspring fitness appropriately. Oikos 123:769–76. [Google Scholar]
- Burton T, Metcalfe NB.. 2014. Can environmental conditions experienced in early life influence future generations?. Proc R Soc Lond B Biol Sci 281 published online (doi: 10.1098/rspb.2014.0311). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bygren L, Tinghog P, Carstensen J, Edvinsson S, Kaati G, Pembrey M, Sjostrom M.. 2014. Change in paternal grandmothers’ early food supply influenced cardiovascular mortality of the female grandchildren. BMC Genet 15:12.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron EZ. 2004. Facultative adjustment of mammalian sex ratios in support of the Trivers–Willard hypothesis: evidence for a mechanism. Proc R Soc Lond B Biol Sci 271:1723–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao-Lei L, Massart R, Suderman MJ, Machnes Z, Elgbeili G, Laplante DP, Szyf M, King S.. 2014. DNA methylation signatures triggered by prenatal maternal stress exposure to a natural disaster: project ice storm. PLoS One 9:e107653.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capelle PM. 2016. Interactive effects of pre- and post-natal stressors on Chinook salmon performance and fitness [dissertation]. [Windsor (ON, Canada)]: University of Windsor.
- Chapman T, Arnqvist G, Bangham J, Rowe L.. 2003. Sexual conflict. Trends Ecol Evol 18:41–7. [Google Scholar]
- Coslovsky M, Richner H.. 2011. Predation risk affects offspring growth via maternal effects. Funct Ecol 25:878–88. [Google Scholar]
- Cottrell EC, Holmes MC, Livingstone DE, Kenyon CJ, Seckl JR.. 2012. Reconciling the nutritional and glucocorticoid hypotheses of fetal programming. FASEB J 26:1866–74. [DOI] [PubMed] [Google Scholar]
- Crean AJ, Bonduriansky R.. 2014. What is a paternal effect? Trends Ecol Evol 29:554–49. [DOI] [PubMed] [Google Scholar]
- Crespi B, Semeniuk C.. 2004. Parent–offspring conflict in the evolution of vertebrate reproductive mode. Am Nat 163:635–53. [DOI] [PubMed] [Google Scholar]
- Dall SRX, McNamara JM, Leimar O.. 2015. Genes as cues: phenotypic integration of genetic and epigenetic information from a Darwinian perspective. Trends Ecol Evol 30:327–33. [DOI] [PubMed] [Google Scholar]
- Dantzer B, Boutin S, Humphries MM, McAdam AG.. 2012. Behavioral responses of territorial red squirrels to natural and experimental variation in population density. Behav Ecol Sociobiol 66:865–78. [Google Scholar]
- Dantzer B, Newman AEM, Boonstra R, Palme R, Boutin S, Humphries MM, McAdam AG.. 2013. Density cues trigger maternal stress hormones that increase adaptive offspring growth in a wild mammal. Science 340:1215–7. [DOI] [PubMed] [Google Scholar]
- DeWitt TJ, Sih A, Wilson DS.. 1998. Costs and limits of phenotypic plasticity. Trends Ecol Evol 13:77–81. [DOI] [PubMed] [Google Scholar]
- Dias BG, Ressier KJ.. 2014. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat Neurosci 17:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GA, Morgan CP, Bale TL.. 2011. Sex-specificity in transgenerational epigenetic programming. Horm Behav 59:290–5. [DOI] [PubMed] [Google Scholar]
- Evans JP, Lymbery RA, Wiid KS, Rahman MM, Gasparini C.. 2017. Sperm as moderators of environmentally induced paternal effects in a livebearing fish. Biol Lett 13:20170087.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, McGhee KE, Bell AM.. 2015. Effect of maternal predator exposure on the ability of stickleback offspring to generalize a learned colour–reward association. Anim Behav 107:61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari MCO, Gonzalo A, Messier F, Chivers DP.. 2007. Generalization of learned predator recognition: an experimental test and framework for future studies. Proc R Soc Lond B Biol Sci 274:1853–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ.. 1999. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science 286:1155–8. [DOI] [PubMed] [Google Scholar]
- Fuiman LA, Magurran AE.. 1994. Development of predator defences in fishes. Rev Fish Biol Fisher 4:145–83. [Google Scholar]
- Furrow RE, Feldman MW.. 2013. Genetic variation, environmental variability, and the evolution of epigenetic regulation. Evolution 68:673–83. [DOI] [PubMed] [Google Scholar]
- Gagliano M, McCormick MI.. 2009. Hormonally mediated maternal effects shape offspring survival potential in stressful environments. Oecologia 160:657–65. [DOI] [PubMed] [Google Scholar]
- Gapp K, Jawaid A, Sarkies P, Bohacek J, Pelczar P, Prados J, Farinelli L, Miska E, Mansuy IM.. 2014. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat Neurosci 17:667–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesing ER, Suski CD, Warner RE, Bell AM.. 2011. Female sticklebacks transfer information via eggs: effects of maternal experience with predators on offspring. Proceedings of the Royal Society of London, Series B 278:1753–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gélin U, Wilson ME, Coulson GC, Festa-Bianchet M.. 2015. Experimental manipulation of female reproduction demonstrates its fitness costs in kangaroos. J Anim Ecol 84:239–48. [DOI] [PubMed] [Google Scholar]
- Ginzburg LR, Taneyhill DE.. 1994. Population cycles of forest Lepidoptera: a maternal effect hypothesis. J Anim Ecol 63:79–92. [Google Scholar]
- Gluckman PD, Hanson MA, Spencer HG, Bateson P.. 2005. Environmental influences during development and their later consequences for health and disease: implications for the interpretation of empirical studies. Proc R Soc Lond B Biol Sci 272:671–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA.. 2004. Living with the past: evolution, development, and patterns of disease. Science 305:1733–6. [DOI] [PubMed] [Google Scholar]
- Griffin AS, Evans CS, Blumstein DT.. 2001. Learning specificity in acquired predator recognition. Anim Behav 62:577–89. [Google Scholar]
- Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, Slagboom PE, Lumey LH.. 2008. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A 105:17046–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JJ, Spencer HG, Donohue K, Sultan SE.. 2014. How stable ‘should’ epigenetic modifications be? Insights from adaptive plasticity and bet hedging. Evolution 68:632–43. [DOI] [PubMed] [Google Scholar]
- Herrenkohl LR, Whitney JB.. 1976. Effects of prepartal stress on postpartal nursing behavior, litter development and adult sexual behavior. Physiol Behav 17:1019–21. [DOI] [PubMed] [Google Scholar]
- Hinde CA. 2006. Negotiation over offspring care?—A positive response to partner-provisioning rate in great tits. Behav Ecol 17:6–12. [Google Scholar]
- Inchausti P, Ginzburg LR.. 1998. Small mammal cycles in northern Europe: pattern and evidence for a maternal effect hypothesis. J Anim Ecol 67:180–94. [Google Scholar]
- Jablonka E, Oborny B, Molnar I, Kisdi E, Hofbauer J, Czaran T.. 1995. The adaptive advantage of phenotypic memory in changing environments. Philos Trans R Soc Lond B Biol Sci 350:133–41. [DOI] [PubMed] [Google Scholar]
- Johnstone RA, Hinde CA.. 2006. Negotiation over offspring care—how should parents respond to each other’s efforts? Behav Ecol 17:818–27. [Google Scholar]
- Kapoor A, Matthews SG.. 2005. Short periods of prenatal stress affect growth, behaviour and hypothalamo–pituitary–adrenal axis activity in male guinea pig offspring. J Physiol 566:967–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A, Kostaki A, Janus C, Matthews SG.. 2009. The effects of prenatal stress on learning in adult offspring is dependent on the timing of the stressor. Behav Brain Res 197:144–9. [DOI] [PubMed] [Google Scholar]
- Khan N, Peters RA, Richardson E, Robert KA.. 2016. Maternal corticosterone exposure has transgenerational effects on grand-offspring. Biol Lett 12 published online (doi: 10.1098/rsbl.2016.0627). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs CJ, Boonstra R, Boutin S, Sinclair ARE, Smith JNM, Gilbert BS, Martin K, O’Donoghue M, Turkington R.. 2014. Trophic dynamics of the boreal forests of the Kluane Region. Arctic 67, Supplement 1:71–81. [Google Scholar]
- Kuijper B, Hoyle RB.. 2015. When to rely on maternal effects and when on phenotypic plasticity? Evolution 69:950–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leimar O, McNamara JM.. 2015. The evolution of transgenerational integration of information in heterogeneous environments. Am Nat 185:E55–69. [DOI] [PubMed] [Google Scholar]
- Lesage J, Blondeau B, Grino M, Bréant B, Dupouy JP.. 2001. Maternal undernutrition during late gestation induces fetal overexposure to glucocorticoids and intrauterine growth retardation, and disturbs the hypothalamo-pituitary adrenal axis in the newborn rat. Endocrinology 142:1692–702. [DOI] [PubMed] [Google Scholar]
- Levins R. 1968. Evolution in changing environments: some theoretical explorations. Princeton (NJ: ): Princeton University Press. [Google Scholar]
- Love OP, Chin EH, Wynne-Edwards KE, Williams TD.. 2005. Stress hormones: a link between maternal condition and sex-biased reproductive investment. Am Nat 166:751–66. [DOI] [PubMed] [Google Scholar]
- Love OP, Gilchrist HG, Bêty J, Wynne-Edwards KE, Berzins L, Williams TD.. 2009. Using life-histories to predict and interpret variability in yolk hormones. Gen Comp Endocrinol 163:169–74. [DOI] [PubMed] [Google Scholar]
- Love OP, McGowan PO, Sheriff MJ.. 2013. Maternal adversity and ecological stressors in natural populations: the role of stress axis programming in individuals, with implications for populations and communities. Funct Ecol 27:81–92. [Google Scholar]
- Love OP, Williams TD.. 2008. Plasticity in the adrenocortical response of a free-living vertebrate: the role of pre-and post-natal developmental stress. Horm Behav 54:496–505. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Bosch OJ, Jousma E, Kramer SA, Andrew R, Seckl JR, Neumann ID.. 2009. Prenatal stress reduces postnatal neurogenesis in rats selectively bred for high, but not low, anxiety: possible key role of placental 11b-hydroxysteroid dehydrogenase type 2. Eur J Neurosci 29:97–103. [DOI] [PubMed] [Google Scholar]
- Marshall DJ, Uller T.. 2007. When is a maternal effect adaptive? Oikos 116:1957–63. [Google Scholar]
- Martınez-Padilla J, Redpath SM, Zeineddine M, Mougeot F.. 2014. Insights into population ecology from long-term studies of red grouse Lagopus lagopus scoticus. J Anim Ecol 83:85–98. [DOI] [PubMed] [Google Scholar]
- Mashoodh R, Franks B, Curley JP, Champagne FA.. 2012. Paternal social enrichment effects on maternal behavior and offspring growth. Proc Natl Acad Sci U S A 109:17232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews SG. 2002. Early programming of the hypothalamo–pituitary–adrenal axis. Trends Endocrinol Metab 13:373–80. [DOI] [PubMed] [Google Scholar]
- Mattingly HT, Butler MJ.. 1994. Laboratory predation on the Trinidadian guppy: implications for the size-selective predation hypothesis and guppy life history evolution. Oikos 69:54–64. [Google Scholar]
- McCormick MI. 1998. Behaviorally induced maternal stress in a fish influences progeny quality by a hormonal mechanism. Ecology 79:1873–83. [Google Scholar]
- McCormick MI, Hoey AS.. 2004. Larval growth history determines juvenile growth and survival in a tropical marine fish. Oikos 106:225–42. [Google Scholar]
- McGhee KE, Bell AM.. 2014. Paternal care in a fish: epigenetics and fitness enhancing effects on offspring. Proc R Soc Lond B Biol Sci 281 published online (doi: 10.1098/rspb.2014.1146). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee KE, Pintor LM, Suhr EL, Bell AM.. 2012. Maternal exposure to predation risk decreases offspring antipredator behaviour and survival in three-spined stickleback. Funct Ecol 26:932–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee KE, Feng S, Leasure S, Bell AM.. 2015. A female’s past experience with predators affects male courtship and the care her offspring will receive from their father. Proc R Soc Lond B Biol Sci 282 published online (doi: 10.1098/rspb.2015.1840). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M, Seckl JR.. 2007. Epigenetic mechanisms of perinatal programming of hypothalamic–pituitary–adrenal function and health. Trends Mol Med 13:269–77. [DOI] [PubMed] [Google Scholar]
- Meylan S, Belliure J, Clobert J, de Fraipont M.. 2002. Stress and body condition as prenatal and postnatal determinants of dispersal in the common lizard (Lacerta vivipara). Horm Behav 42:319–26. [DOI] [PubMed] [Google Scholar]
- Meylan S, Miles DB, Clobert J.. 2012. Hormonally mediated maternal effects, individual strategy and global change. Philos Trans R Soc Lond B Biol Sci 367:1647–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan P. 2008. Early growth conditions, phenotypic development and environmental change. Philos Trans R Soc Lond B Biol Sci 363:1635–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan P, Spencer KA.. 2014. Stress and life history. Curr Biol 10:408–12. [DOI] [PubMed] [Google Scholar]
- Monaghan P, Haussmann MF.. 2015. The positive and negative consequences of stressors during early life. Early Hum Dev 91:643–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monclús R, Tiulim J, Blumstein DT.. 2011. Older mothers follow conservative strategies under predator pressure: the adaptive role of maternal glucocorticoids in yellow-bellied marmots. Horm Behav 60:660–5. [DOI] [PubMed] [Google Scholar]
- Moore MP, Landberg T, Whiteman HH.. 2015. Maternal investment mediates offspring life history variation with context-dependent fitness consequences. Ecology 96:2499–509. [DOI] [PubMed] [Google Scholar]
- Moran N. 1992. The evolutionary maintenance of alternative phenotypes. Am Nat 139:971–89. [Google Scholar]
- Morley-Fletcher S, Rea M, Maccari S, Laviola G.. 2003. Environmental enrichment during adolescence reverses the effects of prenatal stress on play behaviour and HPA axis reactivity in rats. Eur J Neurosci 18:3367–74. [DOI] [PubMed] [Google Scholar]
- Montano MM, Wang MH, vom Saal FS.. 1993. Sex differences in plasma corticosterone in mouse fetuses are mediated by differential placental transport from the mother and eliminated by maternal adrenalectomy or stress. J Reprod Fertil 99:283–90. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL.. 2008. Sex-specific programming of offspring emotionality after stress early in pregnancy. JNeurosci 28:9055–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W, Lessells CM, Korsten P, von Engelhardt N.. 2007. Manipulative signals in family conflict? On the function of maternal yolk hormones in birds. Am Nat 169:E84. [DOI] [PubMed] [Google Scholar]
- Navara KJ. 2010. Programming of offspring sex ratios by maternal stress in humans: assessment of physiological mechanisms using a comparative approach. J Comp Physiol B 180:785–96. [DOI] [PubMed] [Google Scholar]
- Navara KJ. 2013. Hormone-mediated adjustments of sex ratio in vertebrates. Integr Comp Biol 53:877–87. [DOI] [PubMed] [Google Scholar]
- Ostfeld RS, Keesing F.. 2000. Pulsed resources and community dynamics of consumers in terrestrial ecosystems. Trends Ecol Evol 15:232–7. [DOI] [PubMed] [Google Scholar]
- Paitz RT, Bowden RM.. 2013. Sulfonation of maternal steroids is a conserved metabolic pathway in vertebrates. Integr Comp Biol 53:895–901. [DOI] [PubMed] [Google Scholar]
- Paitz RT, Bukhari SA, Bell AM.. 2016. Stickleback embryos use ATP-binding cassette transporters as a buffer against exposure to maternally derived cortisol. Proc R Soc Lond B Biol Sci 283 published online (doi: 10.1098/rspb.2015.2838). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchanathan K, Frankenhuis WE.. 2016. The evolution of sensitive periods in a model of incremental development. Proc R Soc Lond B Biol Sci 283 published online (doi: 10.1098/rspb.2015.2439). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse IS, Koenig WD, Kelly D.. 2016. Mechanisms of mast seeding: resources, weather, cues, and selection. New Phytol 212:546–62. [DOI] [PubMed] [Google Scholar]
- Pembrey ME, Bygren LO, Kaati G, Edvinsson S, Northstone K, Sjostrom M, Golding J.. 2006. Sex-specific, male-line transgenerational responses in humans. Eur J Hum Genet 14:159–66. [DOI] [PubMed] [Google Scholar]
- Pike TW, Petrie M.. 2006. Experimental evidence that corticosterone affects offspring sex ratios in quail. Proc R Soc Lond B Biol Sci 273:1093–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando OJ. 2012. Daddy issues: paternal effects on phenotype. Cell 151:702–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimchen TE. 1991. Foraging failures and the evolution of body size in stickleback. Copeia 4:1098–104. [Google Scholar]
- Relyea RA. 2003. Predators come and predators go: the reversibility of predator-induced traits. Ecology 84:1840–8. [Google Scholar]
- Roche DP, McGhee KE, Bell AM.. 2012. Maternal predator-exposure has lifelong consequences for offspring learning in threespined sticklebacks. Biol Lett 8:932–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL.. 2013. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. JNeurosci 33:9003–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossiter MC. 1994. Maternal effects hypothesis of herbivore outbreak: a framework for the inclusion of population-quality variables as central features of herbivore population-dynamics models. BioScience 44:752–63. [Google Scholar]
- Russell AF, Langmore NE, Cockburn A, Astheimer LB, Kilner RM.. 2007. Reduced egg investment can conceal helper effects in cooperatively breeding birds. Science 317:941–4. [DOI] [PubMed] [Google Scholar]
- Russell AF, Langmore NE, Gardner JL, Kilner RM.. 2008. Maternal investment tactics in superb fairy-wrens. Proc R Soc B Biol Sci 275:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saino N, Romano M, Ferrari RP, Martinelli R, Møller AP.. 2005. Stressed mothers lay eggs with high corticosterone levels which produce low-quality offspring. J Exp Zool 303:998–1006. [DOI] [PubMed] [Google Scholar]
- Schlaepfer MA, Runge MC, Sherman PW.. 2002. Ecological and evolutionary traps. Trends Ecol Evol 17:474–80. [Google Scholar]
- Schmidt KL, MacDougall-Shackleton EA, Soma KK, MacDougall-Shackleton SA.. 2014. Developmental programming of the HPA and HPG axes by early-life stress in male and female sparrows. Gen Comp Endocrinol 196:72–80. [DOI] [PubMed] [Google Scholar]
- Schmidt KL, MacDougall-Shackleton EA, MacDougall-Shackleton SA.. 2012. Developmental stress has sex-specific effects on nestling growth and adult metabolic rates but no effect on adult body size or body composition in song sparrows. J Exp Biol 215:3207–17. [DOI] [PubMed] [Google Scholar]
- Schmidt KL, Moore SD, MacDougall-Shackleton EA, MacDougall-Shackleton SA.. 2013. Early-life stress affects song complexity, song learning, and the volume of RA in adult male song sparrows. Anim Behav 86:25–35. [Google Scholar]
- Seckl JR. 2004. Prenatal glucocorticoids and long-term programming. Eur J Endocrinol 151:U49–62. [DOI] [PubMed] [Google Scholar]
- Sheriff MJ. 2015. The adaptive potential of maternal stress exposure in regulating population dynamics. J Anim Ecol 84:323–5. [DOI] [PubMed] [Google Scholar]
- Sheriff MJ, Krebs CJ, Boonstra R.. 2009. The sensitive hare: sublethal effects of predator stress on reproduction in snowshoe hares. J Anim Ecol 78:1249–58. [DOI] [PubMed] [Google Scholar]
- Sheriff MJ, Krebs CJ, Boonstra R.. 2010. The ghosts of predators past: population cycles and the role of maternal programming under fluctuating predation risk. Ecology 91:2983–94. [DOI] [PubMed] [Google Scholar]
- Sheriff MJ, Krebs CJ, Boonstra R.. 2011. From process to pattern: how fluctuating predation risk impacts the stress axis of snowshoe hares during the 10-year cycle. Oecologia 166:593–605. [DOI] [PubMed] [Google Scholar]
- Sheriff MJ, Love OP.. 2013. Determining the adaptive potential of maternal stress. Ecol Lett 16:271–80. [DOI] [PubMed] [Google Scholar]
- Sheriff MJ, McMahon EK, Krebs CJ, Boonstra R.. 2015. Predator-induced maternal stress and population demography in snowshoe hares: the more severe the risk, the longer the generational effect. J Zool 296:305–10. [Google Scholar]
- Sih A. 2010. Understanding variation in behavioural responses to human-induced rapid environmental change: a conceptual overview. Anim Behav 85:1077–88. [Google Scholar]
- Sih A, Bolnick DI, Luttbeg B, Orrock JL, Peacor SD, Pinto LM, Preisser E, Rehage JS, Vonesh JR.. 2010. Predator-prey naïveté, antipredator behavior, and the ecology of predator invasions. Oikos 119:610–21. [Google Scholar]
- Silverin B. 1986. Corticosterone-binding proteins and behavior effects of high plasma levels of corticosterone during the breeding period in the pied flycatcher. Gen Comp Endocrinol 64:67–74. [DOI] [PubMed] [Google Scholar]
- Skinner MK, Anway MD, Savenkova MI, Gore AC, Crews D.. 2008. Transgenerational epigenetic programming of the brain transcriptome and anxiety behavior. PLoS One 3:e3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell-Rood EC. 2012. Selective processes in development: implications for the costs and benefits of phenotypic plasticity. Integr Comp Biol 52:31–42. [DOI] [PubMed] [Google Scholar]
- Sopinka NM, Capelle PM, Semeniuk CAD, Love OP.. 2017. Glucocorticoids in fish eggs: variation, interactions with the environment, and the potential to shape offspring fitness. Physiol Biochem Zool 90:15–33. [DOI] [PubMed] [Google Scholar]
- St-Cyr S, Abuaish S, Sivanathan S, McGowan PO.. 2017. Maternal programming of sex-specific responses to predator odor stress in adult rats. Horm Behav 94:1–12. [DOI] [PubMed] [Google Scholar]
- Stamps JA, Krishnan VV.. 2014. Combining information from ancestors and personal experiences to predict individual differences in developmental trajectories. Am Nat 184:647–57. [DOI] [PubMed] [Google Scholar]
- Stein LR, Bell AM.. 2014. Paternal programming in sticklebacks. Anim Behav 95:165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange MS, Bowden RM, Thompson CF, Sakaluk SK.. 2016. Pre- and postnatal effects of corticosterone on fitness-related traits and the timing of endogenous corticosterone production in a songbird. J Exp Zool A Ecol Genet Physiol 325:347–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EC, Hinde K, Mendoza SP, Capitanio JP.. 2011. Cortisol concentrations in the milk of rhesus monkey mothers are associated with confident temperament in sons, but not daughters. Dev Psychobiol 53:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimmer PC, Ehlman SM, Sih A.. 2017. Predicting behavioural responses to novel organisms: state-dependent detection theory. Proc R Soc Lond B Biol Sci 284 published online (doi: 10.1098/rspb.2016.2108). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivers RL, Willard DE.. 1973. Natural selection of parental ability to vary the sex ratio of offspring. Science 179:90–2. [DOI] [PubMed] [Google Scholar]
- Uller T. 2008. Developmental plasticity and the evolution of parental effects. Trends Ecol Evol 23:432–8. [DOI] [PubMed] [Google Scholar]
- Uller T, Pen I.. 2011. A theoretical model for the evolution of maternal effects under parent–offspring conflict. Evolution 65:2075–84. [DOI] [PubMed] [Google Scholar]
- Uller T, Nakagawa S, English S.. 2013. Weak evidence for anticipatory parental effects in plants and animals. J Evol Biol 26:2161–70. [DOI] [PubMed] [Google Scholar]
- Veller C, Haig D, Nowak MA.. 2016. The Trivers–Willard hypothesis: sex ratio or investment? Proc R Soc Lond B Biol Sci 283 published online (doi: 10.1098/rspb.2016.0126). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitt LJ, Cooper WE.. 1986. Tail loss, tail color, and predator escape in Eumeces (Lacertilia: Scincidae): age-specific differences in costs and benefits. Can J Zool 64:583–92. [Google Scholar]
- West-Eberhard MJ. 2003. Developmental plasticity and evolution. New York (NY: ): Oxford University Press. [Google Scholar]
- Wright J, Dingemanse NJ.. 1999. Parents and helpers compensate for experimental changes in the provisioning effort of others in the Arabian babbler. Anim Behav 58:345–50. [DOI] [PubMed] [Google Scholar]
- Zanette LY, White AF, Allen MC, Clinchy M.. 2011. Perceived predation risk reduces the number of offspring songbirds produce per year. Science 334:1398–401. [DOI] [PubMed] [Google Scholar]
- Zimmer C, Larriva M, Boogert NJ, Spencer KA.. 2017. Transgenerational transmission of a stress-coping phenotype programmed by early-life stress in the Japanese quail. Sci Rep 7:46125. [DOI] [PMC free article] [PubMed] [Google Scholar]