Abstract
Substance use disorders (i.e., drug addiction) constitute a global and insidious public health issue. Preclinical biomedical research has been invaluable in elucidating the environmental, biological, and pharmacological determinants of drug abuse and in the process of developing innovative pharmacological and behavioral treatment strategies. For more than 70 years, nonhuman primates have been utilized as research subjects in biomedical research related to drug addiction. There are already several excellent published reviews highlighting species differences in both pharmacodynamics and pharmacokinetics between rodents and nonhuman primates in preclinical substance abuse research. Therefore, the aim of this review is to highlight three advantages of nonhuman primates as preclinical substance abuse research subjects. First, nonhuman primates offer technical advantages in experimental design compared to other laboratory animals that afford unique opportunities to promote preclinical-to-clinical translational research. Second, these technical advantages, coupled with the relatively long lifespan of nonhuman primates, allows for pairing longitudinal drug self-administration studies and noninvasive imaging technologies to elucidate the biological consequences of chronic drug exposure. Lastly, nonhuman primates offer advantages in the patterns of intravenous drug self-administration that have potential theoretical implications for both the neurobiological mechanisms of substance use disorder etiology and in the drug development process of pharmacotherapies for substance use disorders. We conclude with potential future research directions in which nonhuman primates would provide unique and valuable insights into the abuse of and addiction to novel psychoactive substances.
Keywords: addiction, animal models, baboon, nonhuman primate, rhesus monkey, squirrel monkey, substance abuse, translational
Introduction
Substance use disorders constitute a class of mental health disorders that pose an insidious public health problem. For example, an estimated 246 million people worldwide between the ages of 15 to 64 years have used an illicit drug (UNODC 2015). Of these users, approximately 10% meet diagnostic criteria for a substance use disorder (American Psychological Association 2013; UNODC 2015). Furthermore, the estimated annual economic burden of illicit drug use in the United States related to crime, lost work productivity, and health care was 193 billion US dollars in 2011 (National Drug Intelligence Center 2011). These epidemiological and economic data support biomedical research efforts to improve our mechanistic understanding of the biological, environmental, and pharmacological determinants of illicit drug use and to develop innovative and more effective treatment strategies for substance use disorders.
In biomedical research, nonhuman primates account for 0.5% of all animals utilized as subjects (Foundation for Biomedical Research 2017). Preclinical substance abuse research utilizing nonhuman primates as research subjects has been conducted since the early 1930s. In his seminal study, Spragg investigated the effects of morphine dependence and subsequent withdrawal on choice between an intramuscular morphine injection and fruit by chimpanzees (Spragg 1940). These results demonstrated that choice behavior was significantly influenced by the morphine withdrawal state, such that chimpanzees were more likely to choose morphine when they were in withdrawal. Although chimpanzees are no longer used as research subjects in substance abuse research, these results provided the empirical foundation that translationally relevant substance abuse research could be conducted in nonhumans.
To date, there are four species of nonhuman primates that have been predominantly used in substance abuse research: rhesus monkeys (Macaca mulatta), cynomolgus monkeys (Macaca fascicularis), squirrel monkeys (Saimiri sciureus), and baboons (Papio anubis). To quantify the relative prevalence of nonhuman primates in substance abuse research, a database search was conducted using the National Institutes of Health research portfolio online reporting tools (i.e., NIH RePORTER) on January 19, 2017 using the keywords “drug self-administration” and “rat,” “mouse,” or “nonhuman primate.” Results were further limited to only active projects funded by the National Institute on Drug Abuse. Using the search terms drug self-administration and rat resulted in 247 hits, drug self-administration and mouse resulted in 91 hits, and drug self-administration and nonhuman primate resulted in 41 hits. Thus, nonhuman primates served as research subjects in approximately 10% of National Institute on Drug Abuse-funded preclinical substance abuse research studies using the drug self-administration procedures that will be described in more detail below under “technical advantages.”
The use of nonhuman primates as research subjects has been instrumental in improving our mechanistic understanding of substance use disorders in at least four areas. First, there are published species differences between rats and nonhuman primates related to the pharmacokinetics of abused drugs and the pharmacodynamic profiles that maintain drug self-administration. For example, the primary metabolite of 3,4-methylenedioxymethamphetamine (MDMA, ecstasy, molly) in rhesus monkeys (Banks et al. 2007), baboons (Goodwin et al. 2013), and humans (Pardo-Lozano et al. 2012) is 3,4-dihydroxymethamphetamine, which does not appear to be behaviorally active in rats (Schindler et al. 2014) and its behavioral activity is unknown in monkeys and humans. In contrast, the primary metabolite of MDMA in rats is 3,4-methylenedioxyamphetamine (Baumann et al. 2009). 3,4-Methylenedioxyamphetamine is behaviorally active, producing both reinforcing and neurotoxic effects (Markert and Roberts 1991). These issues of pharmacokinetics will not be addressed further here; the reader is encouraged to read the review by Weerts et al. (2007) for more details regarding these species differences. Second, physiological advantages of nonhuman primates related to a longer life span and larger diameter veins for double-lumen catheter implantation have provided opportunities to conduct sophisticated drug self-administration procedures (Czoty 2015; Huskinson et al. 2016; Maguire et al. 2013; Wade-Galuska et al. 2011) and to study chronic treatment of candidate pharmacotherapies (Banks 2016; Banks et al. 2015; Mello and Negus 1996). Third, phylogenetic similarities between humans and nonhuman primates, such as brain organization and function, have afforded opportunities to coordinate noninvasive brain imaging or neurochemistry techniques with behavioral procedures (Bradberry 2011; Gould et al. 2012; Howell and Murnane 2008). Phylogenetic similarities in immune function between humans and nonhuman primates have also facilitated the development and evaluation of candidate immunopharmacotherapies for substance use disorders (Bonese et al. 1974; Collins et al. 2012; Desai and Bergman 2015; Evans et al. 2016; Howell et al. 2014). Lastly, behavioral phenotypic similarities between nonhuman primates and humans have provided opportunities to elucidate how environmental variables, such as social behavior and early life stress, modulate the abuse-related effects of drugs (Ewing Corcoran and Howell 2010; Morgan et al. 2002; Nader et al. 2012b). The aim of this review article is to discuss the last three of these issues as contributors to the rationale for using nonhuman primates in drug abuse research.
Advantages Related to Technical Considerations for Preclinical Research
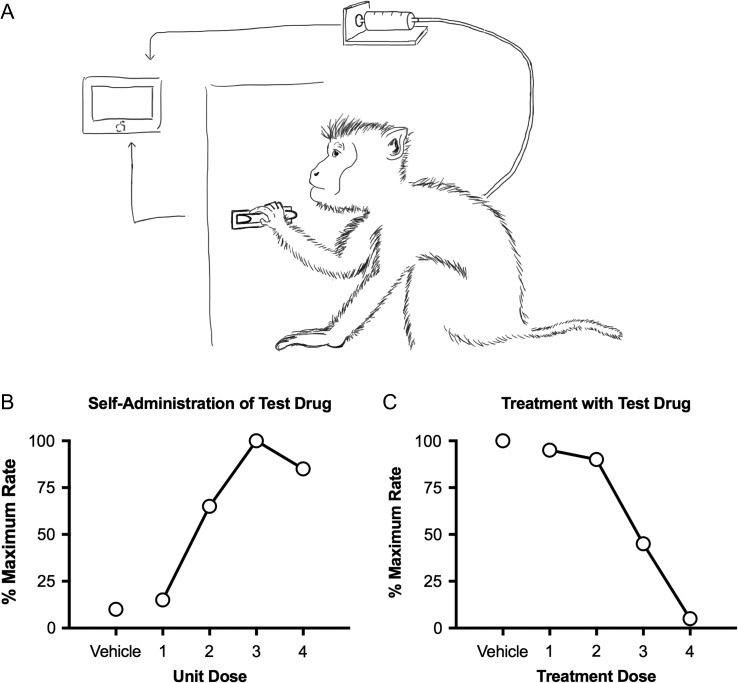
Drug addiction is a behavioral disorder, and addiction research should rely heavily on experimental procedures that measure behavior. The most important family of these procedures is called “drug self-administration,” in which experimental subjects can emit a behavior that results in drug delivery. For example, a human or laboratory animal subject can be placed in an environment that includes a response lever, and contingencies can be programmed so that pressing the lever results in delivery of drug (Figure 1A). Decades of research have established that drugs that are abused by humans (e.g., cocaine) reliably maintain drug taking in laboratory animals (Griffiths and Balster 1979; Griffiths et al. 1979). Two general experimental designs have emerged. First, a novel drug can be made available for i.v. infusion to assess whether subjects will self-administer the drug (Carter and Griffiths 2009). In this type of study, the behavior of interest (e.g., pressing the lever) might produce vehicle administration during some experimental sessions and one of several drug doses during other sessions. Rates of behavior maintained by vehicle and the different drug doses can be measured and compared (Figure 1B). A drug is considered to produce “reinforcing” effects predictive of abuse potential if some dose of drug maintains significantly higher rates of behavior than vehicle. The second type of research design begins by establishing abused drug (e.g., cocaine or heroin) self-administration and assessing whether treatment with a test drug will alter self-administration of the abused drug (Mello and Negus 1996). Specifically, subjects are treated with different test drug doses before experimental sessions during which the abused drug can be self-administered (Figure 1C). Test drugs that decrease self-administration of an abused drug provide insights on mechanisms that underlie abuse of that drug or have therapeutic potential as targets for candidate medications to treat abuse of that drug.
Figure 1.
The top panel shows a schematic of a drug self-administration set-up for nonhuman primates. Subjects are implanted with a chronic indwelling i.v. catheter connected to a drug pump and placed into in environment that contains a response manipulandum, such as a response lever. A computer monitors responding and operates the drug pump to deliver i.v. infusions. Intravenous catheters in nonhuman primates can have either one or two lumens. The bottom left panel shows hypothetical data for an experiment to evaluate whether a test drug can maintain self-administration. In this design, responding produces i.v. infusions of the self-administered drug. If some drug dose maintains higher response rates that drug vehicle, then the drug is considered to produce reinforcing effects suggestive of abuse potential. The bottom right panel shows hypothetical data for an experiment to evaluate whether a test drug can reduce self-administration of a known drug of abuse. In this design, responding produces i.v. infusions of the abused drug (e.g., cocaine), and changes in drug self-administration are evaluated during treatment with the text drug. If the test drug selectively decreases self-administration of the abused drug, then the test drug may warrant further translational study as a candidate medication to treat substance use disorders.
Although drug self-administration procedures are the most predictive behavioral model of drug addiction, they are also the most technically demanding. Of particular importance, drug self-administration is most robust when drug delivery and onset of drug effects occur as soon as possible after self-administration behavior has been expressed (Beardsley and Balster 1993; Woolverton and Anderso 2006). This is often achieved, in both humans (Comer et al. 1998; Foltin and Fischman 1992) and laboratory animals (Johanson and Balster 1978; Thomsen and Caine 2005), by automated i.v. delivery of the self-administered drug. For example, in a typical experimental arrangement, subjects are implanted with an i.v. catheter connected to an infusion pump and drug reservoir (Figure 1A). A computer is used to monitor the behavior of interest (e.g., lever pressing) and to operate the infusion pump and deliver an i.v. infusion as soon as the behavior has occurred. In humans, i.v. catheters are often implanted for relatively short periods of time (hours to days). In laboratory animals, though, more sustained catheterization is preferred to permit behavioral training and repeated testing; the most common approach is to surgically implant a chronic indwelling i.v. catheter. Consequently, the success of preclinical drug self-administration experiments depends in large part on maintaining the viability of chronic indwelling i.v. catheters. The techniques and equipment for implantation and maintenance of i.v. catheters have improved dramatically since the first i.v. drug self-administration studies were published more than 50 years ago (Deneau et al. 1969; Weeks 1962). Intravenous drug self-administration studies are now routinely conducted in a range of species including rodents (rats, mice) and nonhuman primates (macaques, squirrel monkeys, baboons). By virtue of their larger body size, nonhuman primates have larger veins that confer two advantages to their role as research subjects for preclinical drug self-administration studies: longer catheter life, and the possibility of implanting larger, double-lumen catheters.
First, the relatively large veins in monkeys can accommodate larger-diameter catheters, which increases catheter life. Intravenous catheters are long tubes composed of flexible and biocompatible material such as silicone, and they are defined in part by their inner diameter (the “lumen” through with which fluid flows) and outer diameter (the diameter of the lumen plus the walls of the catheter) (Figure 1A). The maximum outer diameter is constrained by the size of the veins in the experimental subject, and the associated inner diameter of the lumen is constrained by the catheter material. For example, illustrative outer-/inner-diameter dimensions for catheters in rhesus monkeys, squirrel monkeys, rats, and mice are 2.51/0.84, 0.76/0.38, 0.64/0.31, and 0.41/0.18 mm, respectively (Czoty et al. 2007; Kimmel et al. 2007; Thomsen and Caine 2005). Larger inner-diameter catheters are more resistant to blockage and retain patency longer than smaller catheters, and consequently, the average life of any given catheter in any nonhuman primate species exceeds that in rats or mice. Additionally, monkeys have more veins than rodents that are sufficiently large to accommodate a chronic i.v. catheter. For example, in both adult rhesus and squirrel monkeys, the internal jugular, external jugular, and femoral veins are all large enough to be routinely used for i.v. catheterization in drug self-administration studies. Furthermore, the brachial veins of rhesus monkeys can also sometimes be used. Conversely, in rats and mice, only the external jugular veins are routinely used. The longer individual catheter life coupled with the larger number of usable veins results in longer overall i.v. catheter life in monkeys than rodents. For example, the mean ± standard deviation i.v. catheter life in a group of 9 adult male rhesus monkeys at one of the author’s laboratory was 35 ± 10 months, whereas the total catheter life in rats and mice reported in a separate study averaged 5 to 6 months (Thomsen and Caine 2005). This longer catheter life in monkeys facilitates studies that involve long-term experimental manipulations such as repeated-measures within-subject testing, use of relatively complex behavioral tasks that require extensive training, or evaluation of long-term dosing regimens with test compounds (Banks 2016; Goodwin 2016; Negus and Banks 2013).
Second, the larger veins in monkeys also permit use of double-lumen catheters, which increases flexibility in experimental design. Single- and double-lumen catheters have one or two lumens, respectively, within the overall catheter structure (Figure 1A). Although single-lumen catheters are commonly used for drug self-administration in monkeys, the relatively large outer diameter can also accommodate two adjacent lumens. For example, two of the authors use double-lumen catheters with an outer diameter of 2.36 mm and two lumens with inner diameters of 0.76 mm each. Double-lumen catheters function as two independent catheters and enable the independent delivery of two different i.v. solutions. For example, one lumen can be used for i.v. infusions of the self-administered drug (e.g., cocaine), and the second lumen can be used for self-administration of a second drug or for chronic infusion of a treatment drug (Huskinson et al. 2016; Negus 2005; Negus and Mello 2003).
Impact on Translational Research
These technical advantages inherent to nonhuman primates as experimental subjects may be especially important in translational research on candidate medications to treat substance use disorders. As noted above, this type of study involves an initial training phase, in which subjects are trained to self-administer a drug of abuse (e.g., cocaine), followed by a subsequent testing phase, in which cocaine self-administration is evaluated during treatment with the candidate medication. The preclinical-to-clinical predictive validity of these procedures is influenced by two key variables (Banks and Negus 2012; Czoty et al. 2016; Haney and Spealman 2008; Weerts et al. 2007).
First, medications used clinically to treat drug addiction (e.g., methadone maintenance to treat opioid addiction) are typically administered chronically for periods of weeks to years. Thus, the predictive validity of preclinical studies is enhanced in experimental designs that use chronic dosing regimens of candidate medications for periods of a week or more. Experimental subjects that support a long i.v. catheter life facilitate these types of experimental designs by assuring reliable i.v. access during both training and testing periods required for sequential exposure to multiple chronic medication doses. Moreover, computer-controlled and continuous medication infusion through one lumen of a double-lumen catheter provides a convenient and reliable method for precisely controlled test medication delivery while allowing simultaneous self-administration of the abused drug through the other catheter lumen.
Second, the predictive validity of preclinical medication assessment can also be enhanced by using relatively complex behavioral procedures that permit evaluation of the behavioral selectivity of medication effects on drug self-administration. Specifically, medications can reduce drug self-administration either by producing a selective decrease in reinforcing effects of the abused drug (the desired outcome) or by producing more general effects that disrupt many behaviors (e.g., sedation or paralysis; undesirable outcomes for a putative medication). Behavioral selectivity can be assessed in preclinical studies by comparing medication effects on drug self-administration with effects on responding maintained by some other reinforcer, such as food (Mello and Negus 1996). Optimal candidate medications will produce sustained decreases in drug self-administration at doses that produce lesser or transient effects on food-maintained responding. Drug self-administration and food-maintained responding can be evaluated in the same subject during alternating behavioral sessions during which only drug or only food is available, and more recently developed “choice” procedures allow subjects to choose between drug and food options that are simultaneously available (Banks 2016; Banks et al. 2015). In choice procedures, optimal medications produce not only a decrease in drug choice, but also a reciprocal increase in food choice as subjects reallocate their behavior away from the drug option and toward the food option. Regardless of the approach used (either alternating sessions of drug and food availability or “choice” sessions of concurrent drug and food availability), behavioral training can take a substantial amount of time. Overall, the long catheter life and the feasibility of using double-lumen catheters in monkeys facilitates experimental designs that assess chronic medication treatment effects on relatively complex behavioral tasks incorporating both drug self-administration and food-maintained responding.
An Example of Translational Drug Self-Administration Research
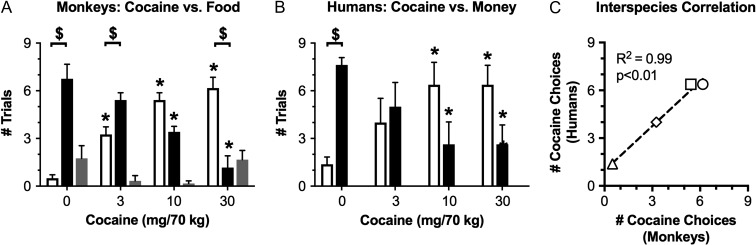
Translational research in any field is generally optimized by the use of homologous procedures in laboratory animal and human studies (Rasakham and Liu-Chen 2011). Use of homologous procedures minimizes discrepancies in variables other than the species of the experimental subject and maximizes potential for direct comparison of results across species. We (Johnson et al. 2016; Lile et al. 2016) and others (Foltin et al. 2015) have recently capitalized on the technical advantages described above to develop homologous procedures in monkeys and humans for translational research on candidate medications to treat cocaine use disorder. In our studies, both monkeys and humans were implanted with i.v. catheters and tested in an environment with two response keys that could be used to earn either i.v. cocaine injections or a species-specific, non-drug alternative reinforcer (food pellets in monkeys, money in humans). Each daily session began with a 30-min “sampling” trial, during which subjects received the i.v. cocaine dose and non-drug reinforcer available on that day. This was followed by nine 30-min “choice” trials, during which subjects could press one key to earn additional i.v. cocaine doses or the other key to earn the non-drug alternative. In both species, the cocaine dose and the magnitude of the alternative were systematically varied. The primary dependent measures were the numbers of cocaine choices, food choices, and trial omissions (i.e., neither option chosen) during each behavioral session. Results in monkeys and humans were highly correlated. For example, Figure 2 shows cocaine-choice dose-effect curves in monkeys and humans when identical ranges of i.v. cocaine doses were available as one choice, and either 10 food pellets (in monkeys) or $3.00 (in humans) was available as the non-drug alternative choice. In both species, increasing cocaine doses maintained dose-dependent increases in cocaine choice associated with reciprocal dose-dependent decreases in choice of the non-drug alternative, and omissions were rare. Moreover, the numbers of cocaine choices earned by monkeys and humans at each cocaine dose were significantly correlated. Notably, the details of these two drug self-administration procedures were initially established in human studies, and the human procedure was then back-translated with minor modifications into monkeys. Moreover, these homologous procedures are now poised for use in studies with chronically administered candidate medications, and a double lumen catheter can be used for medication delivery in monkeys. Development of this translational research platform benefitted greatly from the technical advantages described above.
Figure 2.
Cocaine maintains a dose-dependent increase in cocaine choice in monkeys and humans responding under homologous cocaine choice procedures. Panels A (monkeys; N = 4) and B (humans; N = 8) show the mean ± SEM number of trials completed for cocaine (open bars), trials completed for the non-drug alternative reinforcer (closed bars; food pellets in monkeys and money in humans), or omitted trials (i.e., choice of neither cocaine or the alternative; gray bars) as a function of cocaine dose on the X-axis. Data were analyzed by 2-way ANOVA, and significant ANOVAs were followed by the Holm-Sidak post hoc test. Asterisks indicate statistical significance (p < 0.05) within a trial outcome (cocaine choice, food choice, omission) compared to the 0 dose cocaine data. Dollar signs indicate statistical significance (p < 0.05) with a cocaine dose between the numbers of cocaine vs food trials completed. Panel C shows a significant correlation in cocaine choice data for monkeys and humans. Triangle denotes 0 cocaine dose condition. Diamond denotes 3-mg/70-kg cocaine dose condition. Square denotes 10 mg/70 kg cocaine dose condition. Circle denotes 30-mg/70-kg cocaine dose condition. Adapted from (Johnson et al. 2016; Lile et al. 2016).
Advantages Related to Coordinated Behavioral and Noninvasive Imaging Preclinical Research
Overview of Noninvasive Imaging Techniques
The ability to characterize brain structure and function in living human patients has proven to be an invaluable experimental and diagnostic and tool in understanding and treating human disease. The homology in brain anatomy and function between nonhuman primates and humans, particularly in areas such as the medial prefrontal cortex (e.g., Preuss 1995), provides unique opportunities to elucidate the neurobiological consequences of chronic drug exposure. Many brain imaging methods used in humans have been adapted for use in nonhuman primate. These include radiological techniques such as positron emission tomography (PET) imaging and magnetic resonance imaging (MRI)-based approaches. The methodologies and experimental considerations involved in applying each of these approaches to nonhuman primate have been previously reviewed in detail (Gould et al. 2014; Howell and Murnane 2008; Murnane and Howell 2011; Nader and Czoty 2008). Thus, we will highlight how these techniques can be utilized in areas of substance abuse research in which nonhuman primate offer unique and valuable research opportunities.
Advantages Related to Biological Variables
Biological variables, and in particular sex, have gained greater attention in biomedical research due to recent NIH policy changes (Clayton and Collins 2014), and several review articles have been written regarding sex as a biological variable in substance use disorder research (see Becker and Koob 2016; Carroll and Lynch 2016; Sanchis-Segura and Becker 2016). However, the review articles cited above have focused mainly on sex differences in substance abuse research using rodent models. This is perhaps surprising given that another critical biological variable, research subject species, has emerged from the substance abuse literature as an important determinant of abused drug pharmacokinetic and pharmacodynamic effects (reviewed in Phillips et al. 2014; Weerts et al. 2007). Brain imaging techniques have been used extensively to characterize sex differences in human brain structure and function (reviewed in Andersen et al. 2012; Cosgrove et al. 2007). However, most human substance abuse studies have not been designed to examine sex as an independent biological variable. Sex differences in humans have been reported for both the dopamine transporter, the primary biological target of cocaine and amphetamines, and dopamine D2 and D3 receptors (D2/D3R), critical mediators of the abuse-related effects of many classes of abused drugs (Kaasinen et al. 2001; Lavalaye et al. 2000; Mozley et al. 2001; Pohjalainen et al. 1998). However, other human data do not support a sex difference at these biological targets (Farde et al. 1995; Munro et al. 2006). Discrepancies may arise due to variation in parameters related to subjects’ drug use (e.g., amount and duration of prior drug use, time abstinent, extent of polysubstance use), comorbid psychiatric disease, and other limitations inherent to human studies. Neuroimaging studies in nonhuman primates, in which such potentially confounding variables can be controlled, have shed light on potential biological differences, particularly in D2/D3R function.
In a series of studies in drug-naïve male and female cynomolgus monkeys, D2/D3R were studied using PET imaging and the radiotracer [18F]fluoroclebopride (FCP). The primary dependent variable in PET imaging studies is receptor availability quantified as a distribution volume ratio, which is a ratio of the amount of radiotracer binding in a region of interest (ROI) divided by a comparator region relatively devoid of receptors. It is important to point out that distribution volume ratio is influenced not only by the number of receptors present within a given ROI, but also by the amount of extracellular neurotransmitter (in this case, dopamine) that competes with the radiotracer for binding at the receptor. Early studies used a PET camera with 9-mm resolution and measured D2/D3R availability in a relatively large “basal ganglia” ROI composed of the caudate nucleus and putamen. In these studies, D2/D3R availability was similar across sexes (Grant et al. 1998; Morgan et al. 2002). Subsequent studies used smaller, separate ROIs for caudate and putamen ROIs and, in females, employed a microPET camera with significantly higher spatial resolution (~2 mm). In these studies, D2/D3R availability was higher in both brain regions in females (Czoty et al. 2009; Nader et al. 2012b). Considering the equivocal human data, the nonhuman primate findings suggest that other factors may have obscured detection of sex differences in D2/D3R availability in some human studies. For example, with respect to human studies in cocaine abusers, it is possible that sex differences may dissipate after long-term cocaine use. Although this question has not been directly addressed in nonhuman primates, chronic cocaine self-administration decreases D2/D3R availability in basal ganglia regions in both males (Nader et al. 2002) and females (M Nader, S Nader, A Duke, H Gage, M Goodman, R Voll, K Sai, A Mintz, L Howell, unpublished observations). Moreover, a previous study reported no differences in [18F]FCP binding in young adult male and female rhesus monkeys who had been exposed to cocaine throughout gestation (Hamilton et al. 2010).
In addition to characterizing differences between males and females, nonhuman primates are an ideal animal model in which to study the effects of menstrual cycle in substance abuse research. In contrast to rodents, which have an estrous cycle that lasts only 4 days, the macaque menstrual cycle has a similar duration (~28 days) and similar fluctuations in ovarian hormones compared to humans (Appt 2004; Goodman et al. 1977; Jewett and Dukelow 1972). Regarding the effect of menstrual cycle phase on D2/D3R availability, human studies have yielded equivocal results. Wong et al. (1988) reported lower caudate nucleus D2/D3R availability in the follicular vs luteal phases, but a more recent study using a different radiotracer (Munro et al. 2006) reported higher putamen D2/D3R availability in the follicular phase. A third study reported no differences in the putamen as a function of menstrual cycle phase (Nordstrom et al. 1998). We addressed this question by performing [18F]FCP PET scans in adult female cynomolgus monkeys in the follicular and luteal phases of their menstrual cycle (Czoty et al. 2009). Consistent with the Wong et al. (1988) study, we found that D2/D3R availability was significantly lower in the follicular vs luteal phase of the menstrual cycle in both caudate and putamen. Certainly, there are both procedural and subject-related factors that differ among these studies. It is important to note, however, that in the Wong et al. (1988) and Czoty et al. (2009) studies each subject was scanned twice, once in each phase. In the other studies, fewer subjects were scanned under both conditions (four in the Nordstrom et al. study, none in the Munro et al. study). Moreover, the lower follicular-phase values for caudate D2/D3R availability (2.85 ± 0.11) were much closer to those observed in males (2.42 ± 0.39) than during the luteal phase (3.18 ± 0.14); the same relationship was observed in the putamen. Taken together these nonhuman primate data document sex differences in D2/D3R availability and indicate that menstrual cycle phase can influence these measures. Furthermore, these results suggest that one reason that previous human studies may not have observed sex differences in PET measures may have been a failure to consider the menstrual cycle phase of female subjects.
MRI has been used to provide detailed structural images of the brain without exposure to ionizing radiation. More recently, functional MRI techniques have been developed to provide information about brain function with superior spatial resolution compared to PET imaging and other modalities. Functional MRI has greatly advanced our understanding of brain reward circuitry and the neuropharmacological effects of abused drugs (reviewed in Breiter and Rosen 1999; Volkow et al. 2004; Zahr 2014). One recent application is the study of functional connectivity: temporally correlated activity in spatially distinct brain areas that define integrated networks (Biswal et al. 1995). Application of network theory to the brain has gained popularity because these methods can evaluate the whole brain and identify relationships that may be missed when a ROI-centered analysis is used. One such network, termed the default mode network (DMN; Buckner et al. 2008), is a set of brain regions that displays correlated activity when the brain is not involved in explicit goal-directed activity (Raichle et al. 2001). Studies examining functional connectivity have demonstrated that both acute and chronic drug use can disrupt brain networks, including the DMN in humans (Chanraud et al. 2011; Liang et al. 2015; Ma et al. 2011; Müller-Oehring et al. 2015). For example, older adults with a history of moderate-to-heavy drinking exhibited decreased connectivity in the central executive network while performing a cognitive task (Mayhugh et al. 2016). In the past decade, studies examining functional connectivity have been extended to nonhuman primates, which show a high degree of similarity to humans in identified brain networks including the DMN (Figure 3). More recently, these types of noninvasive imaging studies have been applied to nonhuman primate models of substance abuse (Brevard et al. 2006; Murnane et al. 2015; Telesford et al. 2015). For example, in vervet monkeys who self-administered ethanol daily for over 15 months, monkeys characterized as heavy drinkers showed differences in brain network organization compared to light and non-drinkers (Telesford et al. 2015). As the ability to measure and interpret alterations in global brain activity increases, studies of functional connectivity in nonhuman primates will play a critical translational role.
Figure 3.
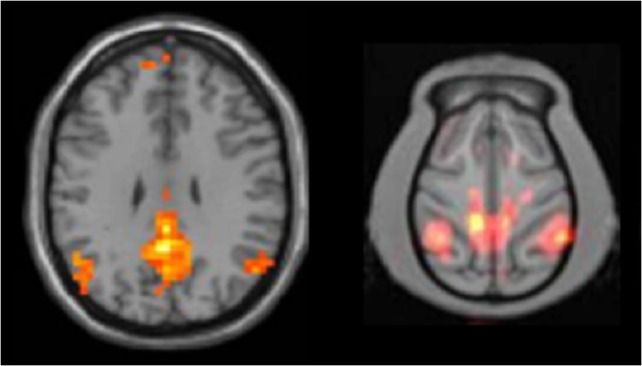
Unpublished demonstration of default mode network in human subjects (left, n = 28) and cynomolgus monkeys (right, n = 4). Images courtesy of Dr. Christopher T. Whitlow, Wake Forest School of Medicine.
Advantages Related to Environmental Variables
Only a subset of those who are exposed to drugs and alcohol will develop substance abuse disorders. Although little is known about the factors that influence vulnerability and resilience to the abuse-related effects of drugs, it is clear that these characteristics can be influenced by long-term exposure to environmental stress and enrichment in both humans and laboratory animals. This highlights an important experimental advantage of nonhuman primate subjects: the ability to conduct longitudinal studies. Brain imaging studies have contributed to our understanding of the effects of chronic exposure to environmental variables that confer vulnerability to drug abuse.
For decades, nonhuman primate social groups have proven useful as models of human susceptibility and resistance to disease. In captivity, group-housed monkeys form dominance hierarchies in which a monkey’s position is determined by the outcomes of social interactions (Kaplan et al. 2009). Biological differences among nonhuman primates in different positions of the social hierarchy have been linked to predictable variation in physiology, neurobiology, and behavior (Cohen et al. 1997; Sapolsky 2005; Shively and Day 2014). Importantly, this social rank-related variation extends to the abuse-related effects of monoamine transporter ligands (i.e. cocaine or amphetamine) and alcohol; studies have consistently reported an inverse relationship between social status and drug effects (Czoty et al. 2005; Ervin et al. 1990; Helms et al. 2012; McKenzie-Quirk and Miczek 2008; Morgan et al. 2002; Peretti and Lewis 1969; Smith and Byrd 1985). One explanation of the physiological and neurobiological differences between high- (dominant) and low-ranking (subordinate) monkeys involves a greater amount of stress experienced by subordinates, as encompassed by the concept of allostatic load (Goymann and Wingfield 2004). Thus, we have conceptualized the linear social dominance hierarchy as representing chronic environmental enrichment at one end (dominance) and chronic social stress (subordination) at the other (Nader et al. 2012a).
PET studies in socially housed monkeys have shed light on neurobiological differences between monkeys of different ranks. The first assessment of the relationship between social status and D2/D3R availability occurred in a well-established group of female cynomolgus monkeys. On average, D2/D3R availability was significantly lower in subordinate monkeys, which was hypothesized to be an effect of chronic social stress (Grant et al. 1998). To investigate this question more directly, groups of male and female cynomolgus monkeys underwent PET scans while individually housed and again after 3 months of social housing, by which time hierarchies had stabilized (Morgan et al. 2002; Nader et al. 2012b). In both sexes, the direction of the effect was the same as in the Grant et al. (1998) study: D2/D3R availability was higher in dominant monkeys. However, contrary to the prevailing hypothesis, the difference arose because D2/D3R availability increased in those monkeys who became socially dominant, while measures in monkeys that became subordinate did not change from their baseline values. Importantly, when male monkeys were permitted to self-administer i.v. cocaine injections, socially dominant monkeys were significantly less sensitive to the reinforcing effects of cocaine (Czoty et al. 2005; Morgan et al. 2002). In this way, the observed relationship between brain D2/D3R and sensitivity to cocaine in male monkeys was consistent with observations of lower D2/D3R in cocaine-abusing humans vs controls (Volkow et al. 1993). The nonhuman primate data extend findings in humans by suggesting that D2/D3R availability is a contributing factor to sensitivity to cocaine rather than an effect of long-term cocaine use. Interestingly, the relationship between social status and sensitivity to cocaine in females was opposite to that found in males: dominant females were found to be more sensitive to cocaine during early exposure (Nader et al. 2012b). Thus, these studies identified sex-by-environment interactions that influence sensitivity to cocaine.
Once male monkeys had self-administered cocaine over several years, rank-related differences in D2/D3R availability had dissipated (Czoty et al. 2004). However, when monkeys, still living in social groups, were scanned after 8 months of abstinence from cocaine, D2/D3R availability was again significantly higher in the caudate nucleus in dominant versus subordinate monkeys. Taken together, these PET imaging studies indicated that the environment exerts powerful effects on the brain dopamine systems. Although these effects can be overridden by chronic drug use, they have the potential to reemerge during abstinence. An important point in the context of this review is that these conclusions would not have been possible without the ability to conduct longitudinal studies in nonhuman primates.
Advantages Related to Interpretations of Preclinical Research
The National Institute on Drug Abuse website operationally defines drug addiction, the most severe stage of the substance use disorder diagnosis, as “a chronic, relapsing brain disease that is characterized by compulsive drug seeking and use, despite harmful consequences” (National Institute on Drug Abuse). Accordingly, there has been significant scientific interest in developing predictive preclinical models to empirically improve our understanding of the neurobiological mechanisms mediating drug addiction and, ultimately, to develop effective treatment strategies. Given the good translational and predictive validity of preclinical drug self-administration procedures to human substance use disorders (Carter and Griffiths 2009; Czoty et al. 2016; Griffiths et al. 1979; Huskinson et al. 2014), most of the scientific focus has been on developing innovative preclinical drug self-administration procedures.
One hypothesized construct of drug addiction is that drug use progresses from controlled to uncontrolled use over time with repeated drug exposure (Koob 1996; Leshner 1997; Volkow et al. 2016). Evidence supporting this hypothesis comes from preclinical drug self-administration studies in which rats are implanted with an indwelling i.v. catheter and afforded the opportunity to self-administer cocaine under either 1-h “short-access” or 6-h “long-access” or “extended-access” conditions (Ahmed and Koob 1998). Rats allowed to self-administer cocaine during 1-h sessions displayed stable rates of cocaine-maintained behavior over the 22-day experimental period. In contrast, rats responding during the 6-h session displayed a steady increase in cocaine-maintained behavior and corresponding cocaine intake over the same 22-day experimental period. Although recent evidence suggests that escalated rates of drug self-administration in rats might occur through discrimination learning processes (Beckmann et al. 2012), the escalated rates of drug self-administration in rats over time have been replicated across numerous abused substances including opioids (Ahmed et al. 2000; Wade et al. 2015), nicotine (Cohen et al. 2012), alcohol (Priddy et al. 2017), and methamphetamine (Kitamura et al. 2006). Overall, the consistency and broad range of abused substances that maintained this “escalated” pattern of drug self-administration in rats provided empirical evidence supporting both continued use of this preclinical model of drug addiction in rats and further studies in other species of laboratory animals, such as nonhuman primates.
When patterns of drug self-administration have been examined in nonhuman primates under similar “extended-access” conditions to those described above, the results have not reproduced those reported above in rats. For example, rhesus monkeys allowed to self-administer cocaine during 6-h “long-access” behavioral sessions failed to display a steady increase or escalation in cocaine-maintained responding and corresponding cocaine intake over a 60-day experimental period (Henry and Howell 2009; Henry et al. 2009). In fact, cocaine-maintained responding was quite stable over the entire experimental period. Moreover, an escalated pattern of cocaine self-administration in rhesus monkeys was not necessary to produce significant alterations in the responsiveness of the mesolimbic dopamine system (Henry et al. 2009). This neurochemical result of chronic cocaine self-administration was significant because it confirms and extends previous results in cocaine-dependent humans (Volkow et al. 1997), thus supporting the predictive validity of intravenous drug self-administration procedures in nonhuman primates as models of the human substance abuse disorder.
Other variants of extended-access drug self-administration conditions have also been examined in nonhuman primates, and the results of these studies also did not reproduce patterns of escalated drug self-administration behavior. For example, when monkeys were afforded the opportunity to self-administer cocaine, d-amphetamine, or d-methamphetamine 23 h/d under unlimited access conditions, there was no consistent pattern of increased rates of drug self-administration over time and pattern of responding was best characterized as a “binge-crash” pattern (Deneau et al. 1969; Johanson et al. 1976). In a recent series of cocaine self-administration studies in nonhuman primates, patterns of cocaine self-administration under 20-h/d cocaine access conditions were also best characterized as a “binge-crash” pattern (Banks and Negus 2010; Banks et al. 2013; Hutsell et al. 2016a, 2016b). Moreover, escalated drug self-administration in rats is typically interpreted as evidence for increased reinforcing efficacy of the self-administered drug. However, studies in nonhuman primates do not always support this interpretation. For example, neither exposure to nor withdrawal from extended cocaine access increased cocaine self-administration in rhesus monkeys responding under a progressive-ratio schedule (Czoty et al. 2006) or choice of cocaine over food in rhesus monkeys responding under a cocaine-vs-food choice procedure (Banks and Negus 2010). Similarly, neither exposure to nor withdrawal from extended methamphetamine access increased methamphetamine vs food choice in rhesus monkeys (M Banks, unpublished observations). Although the exact mechanisms of this species difference remain to be fully elucidated, these results have implications for both understanding the neurobiological mechanisms of substance use disorders and the development of candidate medications.
The clinical implications of this species difference in patterns of drug self-administration has recently emerged in the evaluation of candidate pharmacotherapies targeting the kappa-opioid receptor (KOR)/dynorphin system (for review, see Negus and Banks, 2017). For example, acute administration of the long-acting KOR antagonist nor-binaltorphimine (nor-BNI) or mixtures of buprenorphine + naltrexone (to produce a KOR antagonist effect) attenuated escalated cocaine (Wee et al. 2009, 2012) and heroin (Schlosburg et al. 2013) self-administration in rats under extended drug access conditions. These results were interpreted as support for the clinical utility of KOR antagonists as candidate pharmacotherapies for cocaine or heroin use disorder. However, when acute nor-BNI was evaluated under extended cocaine access conditions, it failed to attenuate either cocaine-vs-food choice or rates of cocaine self-administration under extended cocaine access conditions in rhesus monkeys (Hutsell et al. 2016a). Furthermore, acute treatment with another KOR antagonist 5′-guanidonaltrindole also failed to block withdrawal-associated increases in heroin-vs-food choice in rhesus monkeys (Negus and Rice 2009). Recently, chronic buprenorphine + naloxone plus naltrexone (to produce a KOR antagonist effect) was evaluated in a double-blind, placebo-controlled clinical trial for the treatment of cocaine use disorder, and there was no significant decrease in cocaine use compared to placebo treatment (Ling et al. 2016). Overall, these results highlight a recent and specific example of translational concordance between candidate pharmacotherapy treatment results in nonhuman primates and humans, and support the utility of nonhuman primates in the drug development process for substance use disorder treatments.
Future Directions
One potential direction where nonhuman primate substance abuse research may expand is elucidating the neuropharmacological mechanisms of novel psychoactive substances. The explosion of novel psychoactive substances that target receptors for monoamines (e.g., 3,4-methylenedioxypyrovalerone), opioids (e.g., trans-3,4-dichloro-N-(2-(dimethylamino)cyclohexyl)-N-methylbenzamide; U-47700), or cannabinoids (e.g., 1-pentyl-3-(1-naphthoyl)indole; JWH-018) have emerged as significant public health problems over the past decade (Baumann 2014; Baumann and Volkow 2016). However, there is a paucity of preclinical studies that have determined the pharmacodynamic or pharmacokinetic effects of these novel psychoactive compounds in nonhuman primates.
A recent series of studies has examined the behavioral pharmacology of synthetic cathinone analogs using a drug discrimination procedure in nonhuman primates (Smith et al. 2016, 2017). In a drug discrimination procedure, animals (Lelas et al. 2000; Solinas et al. 2006) or humans (Bolin et al. 2016) are trained to respond on one lever to earn a reinforcer (e.g., a food pellet) after training drug (e.g., cocaine) administration and respond on another lever to earn the same reinforcer after vehicle administration. A test drug can then be administered to determine whether it produces responding on the training drug-associated lever suggestive of shared pharmacological mechanisms with the training drug. Drug discrimination procedures serve as a valuable tool for evaluation of novel psychoactive compounds, because subjects can be trained to discriminate a known drug of abuse, and novel compounds can then be tested for their effectiveness to produce responding similar to that training drug. Discriminative stimulus effects of drugs are often similar across species, but important species differences have been observed. For example, a prominent difference in drug effects between nonhuman primates and rats trained to discriminate cocaine from saline was with the novel psychoactive substance 4-methylmethcathinone (mephedrone). Mephedrone produced partial cocaine-like discriminative stimulus effects in rhesus monkeys (Smith et al. 2016) compared to full cocaine-like effects in rats (Gatch et al. 2015). Moreover, the mephedrone results in rhesus monkeys were consistent with reports of subjective effects of mephedrone in human mephedrone users (Carhart-Harris et al. 2011; Kapitány-Fövény et al. 2013).
Nonhuman primates have also demonstrated utility as research subjects for the evaluation of novel psychoactive substances targeting other receptor systems, such as the opioid or cannabinoid systems. For example, nonhuman primates have served as research subjects evaluating the behavioral effects of novel mu-opioid agonists, such as fentanyl derivatives (France et al. 1995), and novel nonopioid compounds, such as nociceptin/orphanin FQ agonists (Ding et al. 2016; Ko et al. 2009; Saccone et al. 2016). Regarding the cannabinoid system, squirrel monkeys are the only species of research animal for which Δ9-tetrahydrocannabinol and other novel cannabinoid compounds are robustly self-administered (Justinova et al. 2003; Justinová et al. 2011; Schindler et al. 2016). However, reinforcing effects of Δ9-tetrahydrocannabinol and another cannabinoid receptor agonist, CP55,940, was recently demonstrated in a subset of rhesus and cynomolgus monkeys (John et al. 2017). As clandestine laboratories continue to synthesize novel psychoactive substances to circumvent regulatory authorities, nonhuman primates will serve as a valuable resource to elucidate underlying pharmacological, pharmacokinetic, and toxicological mechanisms.
Another future direction for nonhuman primate substance abuse research could be the increased utilization of sophisticated behavioral procedures that assess aspects of cognitive function. Because nonhuman primates have more similar cortical brain structures and functions with humans compared to rodents (Haber and Knutson 2009; Haber et al. 1990), nonhuman primates provide unique opportunities to assess the behavioral and neurobiological consequences of acute and chronic abused drug exposure. Furthermore, these cognitive behavioral procedures could then be coupled with other biological measurements, such as hormone levels or brain activity, to conduct parallel monkey and human experiments as described above. For example, a recent study in female cynomolgus monkeys reported that estradiol and progesterone level changes as part of the menstrual cycle differentially impacted cognitive behavioral procedure performance (Kromrey et al. 2015). Furthermore, recent studies have combined brain imaging and drug self-administration in nonhuman primates to assess the effects of abused drug exposure on cortical brain function (Howell et al. 2010; Porrino et al. 2016; Porter et al. 2014). Overall, the combination of technical and neurobiological advantages support the utility of nonhuman primates to elucidate the cognitive consequences of chronic abused drug exposure.
Conclusions
The continued use of nonhuman primates as models in substance abuse research for over 70 years has provided valuable insights regarding the biological, environmental, and pharmacological determinants of substance use disorders. Here we reviewed three particular associated with use of nonhuman primates as research subjects. First, nonhuman primates afford technical advantages over rats or mice in preclinical substance abuse research with regard to i.v. catheter size, type (i.e., double-lumen), and catheter longevity. These technical advantages have provided researchers the ability to conduct coordinated and translational research in parallel with human laboratory drug self-administration studies. This use of coordinated behavioral procedures in nonhuman primates and humans has the potential to facilitate the drug development process for candidate pharmacotherapies to treat substance use disorders. Second, these technical advantages have also provided opportunities to improve our mechanistic understanding of the biological determinants and consequences of substance use disorders by pairing chronic i.v. drug self-administration with noninvasive imaging techniques, such as PET and MRI. Finally, patterns of abused drug self-administration in nonhuman primates have supported alternative interpretations of results from rat drug self-administration studies. Moreover, these nonhuman primate studies have provided more concordant results with both human laboratory drug self-administration studies and clinical trials evaluating candidate pharmacotherapies for substance use disorders. In summary, the examples described in this review article provide empirical data supporting the continued use of nonhuman primates in substance abuse research. Nonhuman primate research subjects will be invaluable in addressing the emerging public health crisis of novel psychoactive substance abuse and addiction.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgements
The authors have been supported by the National Institute on Drug Abuse of the National Institutes of Health under award numbers R01DA039953, R21DA036683, R01DA033364, R01DA031718, and R01DA026946. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We also acknowledge the artistic assistance of Dr. Megan Jo Moerke for drawing the illustration in Figure 1A.
References
- Ahmed SH, Koob GF. 1998. Transition from moderate to excessive drug intake: Change in hedonic set point. Science 282(5387):298–300. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Walker JR, Koob GF. 2000. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology 22(4):413–421. [DOI] [PubMed] [Google Scholar]
- Andersen ML, Sawyer EK, Howell LL. 2012. Contributions of neuroimaging to understanding sex differences in cocaine abuse. Exp Clin Psychopharmacol 20(1):2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appt SE. 2004. Usefulness of the monkey model to investigate the role of soy in postmenopausal women’s health. ILAR J 45(2):200–211. [DOI] [PubMed] [Google Scholar]
- American Psychological Association 2013. Diagnositc and Statistical Manual of Mental Disorders, 5th ed Arlington, VA: American Psychiatric Association. [Google Scholar]
- Banks ML. 2016. Utility of preclincial drug vs. food choice procedures to evaluate candidate medications for methampehtamine addiction. Ann N Y Acad Sci. doi:10.1111/nyas.13276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Fennell TR, Snyder RW, Negus SS. 2013. Effects of phendimetrazine treatment on cocaine vs food choice and extended-access cocaine consumption in rhesus monkeys. Neuropsychopharmacology 38(13):2698–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Hutsell BA, Schwienteck KL, Negus SS. 2015. Use of preclinical drug vs. food choice procedures to evaluate candidate medications for cocaine addiction. Curr Treat Options Psychiatry 2(2):136–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Negus SS. 2010. Effects of extended cocaine access and cocaine withdrawal on choice between cocaine and food in rhesus monkeys. Neuropsychopharmacology 35(2):493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Negus SS. 2012. Preclinical determinants of drug choice under concurrent schedules of drug self-administration. Adv Pharmacol Sci 2012:281768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Sprague JE, Kisor DF, Czoty PW, Nichols DE, Nader MA. 2007. Ambient temperature effects on 3,4-methylenedioxymethamphetamine-induced thermodysregulation and pharmacokinetics in male monkeys. Drug Metab Dispos 35(10):1840–1845. [DOI] [PubMed] [Google Scholar]
- Baumann MH. 2014. Awash in a sea of ‘bath salts’: Implications for biomedical research and public health. Addiction 109(10):1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Volkow ND. 2016. Abuse of new psychoactive substances: Threats and solutions. Neuropsychopharmacology 41(3):663–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Zolkowska D, Kim I, Scheidweiler KB, Rothman RB, Huestis MA. 2009. Effects of dose and route of administration on pharmacokinetics of (±)-3,4-methylenedioxymethamphetamine in the rat. Drug Metab Dispos 37(11):2163–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Balster RL. 1993. The effects of delay of reinforcement and dose on the self-administration of cocaine and procaine in rhesus monkeys. Drug Alcohol Depend 34(1):37–43. [DOI] [PubMed] [Google Scholar]
- Becker JB, Koob GF. 2016. Sex differences in animal models: Focus on addiction. Pharmacol Rev 68(2):242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann JS, Gipson CD, Marusich JA, Bardo MT. 2012. Escalation of cocaine intake with extended access in rats: Dysregulated addiction or regulated acquisition? Psychopharmacology (Berl) 222(2):257–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. 1995. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34(4):537–541. [DOI] [PubMed] [Google Scholar]
- Bolin BL, Lile JA, Marks KR, Beckmann JS, Rush CR, Stoops WW. 2016. Buspirone reduces sexual risk-taking intent but not cocaine self-administration. Exp Clin Psychopharmacol 24(3):162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonese KF, Wainer BH, Fitch FW, Rothberg RM, Schuster CR. 1974. Changes in heroin self-administration by a rhesus monkey after morphine immunisation. Nature 252(5485):708–710. [DOI] [PubMed] [Google Scholar]
- Bradberry CW. 2011. Cortical and sub-cortical effects in primate models of cocaine use: implications for addiction and the increased risk of psychiatric illness. Neurotox Res 19(2):235–242. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Rosen BR. 1999. Functional magnetic resonance imaging of brain reward circuitry in the human. Ann N Y Acad Sci 877(1):523–547. [DOI] [PubMed] [Google Scholar]
- Brevard ME, Meyer JS, Harder JA, Ferris CF. 2006. Imaging brain activity in conscious monkeys following oral MDMA (“ecstasy”). Magn Reson Imaging 24(6):707–714. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. 2008. The brain’s default network: anatomy, function, and relevance to disease. Ann NY Acad Sci 1124(1):1–38. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, King LA, Nutt DJ. 2011. A web-based survey on mephedrone. Drug Alcohol Depend 118(1):19–22. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ. 2016. How to study sex differences in addiction using animal models. Addict Biol 21(5):1007–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LP, Griffiths RR. 2009. Principles of laboratory assessment of drug abuse liability and implications for clinical development. Drug Alcohol Depend 105(Supplement 1):S14–S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Drug Intelligence Center 2011. The economic impact of illicit drug use on american society. Department of Justice, Washington, DC. [Google Scholar]
- Chanraud S, Pitel A-L, Pfefferbaum A, Sullivan EV. 2011. Disruption of functional connectivity of the default-mode network in alcoholism. Cereb Cortex 21(10):2272–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton JA, Collins FS. 2014. Policy: NIH to balance sex in cell and animal studies. Nature 509(7500):282–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A, Koob GF, George O. 2012. Robust escalation of nicotine intake with extended access to nicotine self-administration and intermittent periods of abstinence. Neuropsychopharmacology 37(9):2153–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Line S, Manuck SB, Rabin BS, Heise ER, Kaplan JR. 1997. Chronic social stress, social status, and susceptibility to upper respiratory infections in nonhuman primates. Psychosom Med 59(3):213–221. [DOI] [PubMed] [Google Scholar]
- Collins GT, Brim RL, Noon KR, Narasimhan D, Lukacs NW, Sunahara RK, Woods JH, Ko M-C. 2012. Repeated administration of a mutant cocaine esterase: effects on plasma cocaine levels, cocaine-induced cardiovascular activity, and immune responses in rhesus monkeys. J Pharmacol Exp Ther 342(1):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Wilson ST, Donovan MR, Foltin RW, Fischman MW. 1998. Effects of an alternative reinforcer on intravenous heroin self-administration by humans. Eur J PHarmacol 345(1):13–26. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Mazure CM, Staley JK. 2007. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry 62(8):847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW. 2015. Effects of chronic binge-like ethanol consumption on cocaine self-administration in rhesus monkeys. Drug Alcohol Depend 153:278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Martelle JL, Nader MA. 2006. Influence of abstinence and conditions of cocaine access on the reinforcing strength of cocaine in nonhuman primates. Drug Alcohol Depend 85(3):213–220. [DOI] [PubMed] [Google Scholar]
- Czoty PW, McCabe C, Nader MA. 2005. Assessment of the relative reinforcing strength of cocaine in socially housed monkeys using a choice procedure. J Pharmacol Exp Ther 312(1):96–102. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Morgan D, Shannon EE, Gage HD, Nader MA. 2004. Characterization of dopamine D1 and D2 receptor function in socially housed cynomolgus monkeys self-administering cocaine. Psychopharmacology (Berl) 174(3):381–388. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Reboussin BA, Calhoun TL, Nader SH, Nader MA. 2007. Long-term cocaine self-administration under fixed–ratio and second-order schedules in monkeys. Psychopharmacology (Berl) 191(2):287–295. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Riddick NV, Gage HD, Sandridge M, Nader SH, Garg S, Bounds M, Garg PK, Nader MA. 2009. Effect of menstrual cycle phase on dopamine D2 receptor availability in female cynomolgus monkeys. Neuropsychopharmacology 34(3):548–554. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Stoops WW, Rush CR. 2016. Evaluation of the “pipeline” for development of medications for cocaine use disorder: A review of translational preclinical, human laboratory, and clinical trial research. Pharmacol Rev 68(3):533–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneau G, Yanagita T, Seevers MH. 1969. Self-administration of psychoactive substances by the monkey. Psychopharmacologia 16(1):30–48. [DOI] [PubMed] [Google Scholar]
- Desai RI, Bergman J. 2015. Effects of the nanoparticle-based vaccine, SEL-068, on nicotine discrimination in squirrel monkeys. Neuropsychopharmacology 40(9):2207–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Czoty PW, Kiguchi N, Cami-Kobeci G, Sukhtankar DD, Nader MA, Husbands SM, Ko M-C. 2016. A novel orvinol analog, BU08028, as a safe opioid analgesic without abuse liability in primates. Proc Natl Acad Sci USA 113(37):E5511–E5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervin FR, Palmour RM, Young SN, Guzman-Flores C, Juarez J. 1990. Voluntary consumption of beverage alcohol by vervet monkeys: Population screening, descriptive behavior and biochemical measures. Pharmacol Biochem Behav 36(2):367–373. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW, Hicks MJ, Rosenberg JB, De BP, Janda KD, Kaminsky SM, Crystal RG. 2016. Efficacy of an adenovirus-based anti-cocaine vaccine to reduce cocaine self-administration and reacqusition using a choice procedure in rhesus macaques. Pharmacol Biochem Behav 150–151:76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing Corcoran SB, Howell LL. 2010. Impact of early life stress on the reinforcing and behavioral-stimulant effects of psychostimulants in rhesus monkeys. Behav Pharmacol 21(1):69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farde L, Hall H, Pauli S, Halldin C. 1995. Variability in D2-dopamine receptor density and affinity: A PET study with [11C]raclopride in man. Synapse 20(3):200–208. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. 1992. Self-administration of cocaine by humans: Choice between smoked and intravenous cocaine. J Pharmacol Exp Ther 261(3):841–849. [PubMed] [Google Scholar]
- Foltin RW, Haney M, Rubin E, Reed SC, Vadhan N, Balter R, Evans SM. 2015. Development of translational preclinical models in substance abuse: Effects of cocaine administration on cocaine choice in humans and non-human primates. Pharmacol Biochem Behav 134:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foundation for Biomedical Research Available online (www.monkeyresearch.org), accessed on January 19, 2017.
- France CP, Gerak LR, Flynn D, Winger GD, Medzihradsky F, Bagley JR, Brockunier LL, Woods JH. 1995. Behavioral effects and receptor binding affinities of fentanyl derivatives in rhesus monkeys. J Pharmacol Exp Ther 274(1):17–28. [PubMed] [Google Scholar]
- Gatch MB, Rutledge MA, Forster MJ. 2015. Discriminative and locomotor effects of five synthetic cathinones in rats and mice. Psychopharmacology (Berl) 232(7):1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman AL, Descalzi CD, Johnson DK, Hodgen GD. 1977. Composite pattern of circulating LH, FSH, estradiol, and progesterone during the menstrual cycle in cynomolgus monkeys. Proc Soc Exp Biol Med 155(4):479–481. [DOI] [PubMed] [Google Scholar]
- Goodwin AK. 2016. An intravenous self-administration procedure for assessing the reinforcing effects of hallucinogens in nonhuman primates. J Pharmacol Toxicol Methods 82:31–36. [DOI] [PubMed] [Google Scholar]
- Goodwin AK, Mueller M, Shell CD, Ricaurte GA, Ator NA. 2013. Behavioral effects and pharmacokinetics of (±)-3,4-methylenedioxymethamphetamine (MDMA, ecstasy) after intragastric administration to baboons. J Pharmacol Exp Ther 345(3):342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould RW, Duke AN, Nader MA. 2014. PET studies in nonhuman primate models of cocaine abuse: Translational research related to vulnerability and neuroadaptations. Neuropharmacology 84:138–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould RW, Porrino LJ, Nader MA. 2012. Nonhuman primate models of addiction and PET imaging: Dopamine system dysregulation In: Carter CS, Dalley JW, eds. Brain Imaging in Behavioral Neuroscience. Berlin, Heidelberg: Springer Berlin Heidelberg; p 25–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goymann W, Wingfield J. 2004. Allostatic load, social status, and stress hormones—the costs of social status matter. Anim Behav 67:591–602. [Google Scholar]
- Grant KA, Shively CA, Nader MA, Ehrenkaufer RL, Line SW, Morton TE, Gage HD, Mach RH. 1998. Effect of social status on striatal dopamine D2 receptor binding characteristics in cynomolgus monkeys assessed with positron emission tomography. Synapse 29(1):80–83. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Balster RL. 1979. Opioids: Similarity between evaluations of subjective effects and animal self-administration results. Clin Pharmacol Ther 25(5 Pt 1):611–617. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Brady JV, Bradford LD. 1979. Predicting the abuse liability of drugs with animal drug self-administration procedures: psychomotor stimulants and hallucinogens In: Thompson T, Dews PB, eds. Advances in Behavioral Pharmacology. New York: Academic Press; p 164–208. [Google Scholar]
- Haber SN, Knutson B. 2009. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35(1):4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Lynd E, Klein C, Groenewegen HJ. 1990. Topographic organization of the ventral striatal efferent projections in the rhesus monkey: An anterograde tracing study. J Comp Neurol 293(2):282–298. [DOI] [PubMed] [Google Scholar]
- Hamilton LR, Czoty PW, Gage HD, Nader MA. 2010. Characterization of the dopamine receptor system in adult rhesus monkeys exposed to cocaine throughout gestation. Psychopharmacology (Berl) 210(4):481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Spealman R. 2008. Controversies in translational research: Drug self-administration. Psychopharmacology (Berl) 199(3):403–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, McClintick MN, Grant KA. 2012. Social rank, chronic ethanol self-administration, and diurnal pituitary–adrenal activity in cynomolgus monkeys. Psychopharmacology (Berl) 224(1):133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry PK, Davis M, Howell LL. 2009. Effects of cocaine self-administration history under limited and extended access conditions on in vivo striatal dopamine neurochemistry and acoustic startle in rhesus monkeys. Psychopharmacology (Berl) 205(2):237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry PK, Howell LL. 2009. Cocaine-induced reinstatement during limited and extended drug access conditions in rhesus monkeys. Psychopharmacology (Berl) 204(3):523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Murnane KS. 2008. Nonhuman primate neuroimaging and the neurobiology of psychostimulant addiction. Ann N Y Acad Sci 1141(1):176–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Nye JA, Stehouwer JS, Voll RJ, Mun J, Narasimhan D, Nichols J, Sunahara R, Goodman MM, Carroll FI, Woods JH. 2014. A thermostable bacterial cocaine esterase rapidly eliminates cocaine from brain in nonhuman primates. Transl Psychiatry 4:e407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Votaw JR, Goodman MM, Lindsey KP. 2010. Cortical activation during cocaine use and extinction in rhesus monkeys. Psychopharmacology (Berl) 208(2):191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huskinson SL, Myerson J, Green L, Rowlett JK, Woolverton WL, Freeman KB. 2016. Shallow discounting of delayed cocaine by male rhesus monkeys when immediate food is the choice alternative. Exp Clin Psychopharmacol 24(6):456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huskinson SL, Naylor JE, Rowlett JK, Freeman KB. 2014. Predicting abuse potential of stimulants and other dopaminergic drugs: Overview and recommendations. Neuropharmacology 87:66–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutsell BA, Cheng K, Rice KC, Negus SS, Banks ML. 2016. a. Effects of the kappa opioid receptor antagonist nor-binaltorphimine (nor-BNI) on cocaine versus food choice and extended-access cocaine intake in rhesus monkeys. Addict Biol 21(2):360–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutsell BA, Negus SS, Banks ML. 2016. b. Effects of 21-day d-amphetamine and risperidone treatment on cocaine vs food choice and extended-access cocaine intake in male rhesus monkeys. Drug Alcohol Depend 168:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett DA, Dukelow WR. 1972. Cyclicity and gestation length ofMacaca fascicularis. Primates 13(3):327–332. [Google Scholar]
- Johanson CE, Balster RL. 1978. A summary of the results of a drug self-administration study using substitution procedures in rhesus monkeys. Bull Narc 30(3):43–54. [PubMed] [Google Scholar]
- Johanson CE, Balster RL, Bonese K. 1976. Self-administration of psychomotor stimulant drugs: The effects of unlimited access. Pharmacol Biochem Behav 4(1):45–51. [DOI] [PubMed] [Google Scholar]
- John WS, Martin TJ, Nader MA. 2017. Behavioral determinants of cannabinoid self-administration in old-world monkeys. Neuropsychopharmacology. doi:10.1038/npp.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AR, Banks ML, Blough BE, Lile JA, Nicholson KL, Negus SS. 2016. Development of a translational model to screen medications for cocaine use disorder I: Choice between cocaine and food in rhesus monkeys. Drug Alcohol Depend 165:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Tanda G, Redhi GH, Goldberg SR. 2003. Self-administration of Δ9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology (Berl) 169(2):135–140. [DOI] [PubMed] [Google Scholar]
- Justinová Z, Yasar S, Redhi GH, Goldberg SR. 2011. The endogenous cannabinoid 2-arachidonoylglycerol is intravenously self-administered by squirrel monkeys. J Neurosci 31(19):7043–7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaasinen V, Någren K, Hietala J, Farde L, Rinne J. 2001. Sex differences in extrastriatal dopamine D2-like receptors in the human brain. Am J Psychiatry 158(2):308–311. [DOI] [PubMed] [Google Scholar]
- Kapitány-Fövény M, Kertész M, Winstock A, Deluca P, Corazza O, Farkas J, Zacher G, Urbán R, Demetrovics Z. 2013. Substitutional potential of mephedrone: An analysis of the subjective effects. Hum Psychopharmacol 28(4):308–316. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Chen H, Manuck SB. 2009. The relationship between social status and atherosclerosis in male and female monkeys as revealed by meta-analysis. Am J Primatol 71(9):732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel HL, O’Connor JA, Carroll FI, Howell LL. 2007. Faster onset and dopamine transporter selectivity predict stimulant and reinforcing effects of cocaine analogs in squirrel monkeys. Pharmacol Biochem Behav 86(1):45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio S, Koob G, Pulvirenti L. 2006. Escalation of methamphetamine self-administration in rats: A dose–effect function. Psychopharmacology (Berl) 186(1):48–53. [DOI] [PubMed] [Google Scholar]
- Ko M-C, Woods JH, Fantegrossi WE, Galuska CM, Wichmann J, Prinssen EP. 2009. Behavioral effects of a synthetic agonist selective for nociceptin/orphanin FQ peptide receptors in monkeys. Neuropsychopharmacology 34(9):2088–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. 1996. Drug addiction: The yin and yang of hedonic homeostasis. Neuron 16(5):893–896. [DOI] [PubMed] [Google Scholar]
- Kromrey SA, Czoty PW, Nader MA. 2015. Relationship between estradiol and progesterone concentrations and cognitive performance in normally cycling female cynomolgus monkeys. Horm Behav 72:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavalaye J, Booij J, Reneman L, Habraken JB, van Royen EA. 2000. Effect of age and gender on dopamine transporter imaging with [123I]FP-CIT SPET in healthy volunteers. Eur J Nucl Med 27(7):867–869. [DOI] [PubMed] [Google Scholar]
- Lelas S, Spealman RD, Rowlett JK. 2000. Using behavior to elucidate receptor mechanisms: A review of the discriminative stimulus effects of benzodiazepines. Exp Clin Psychopharmacol 8(3):294–311. [DOI] [PubMed] [Google Scholar]
- Leshner AI. 1997. Addiction is a brain disease, and it matters. Science 278(5335):45–47. [DOI] [PubMed] [Google Scholar]
- Liang X, He Y, Salmeron BJ, Gu H, Stein EA, Yang Y. 2015. Interactions between the salience and default-mode networks are disrupted in cocaine addiction. J Neurosci 35(21):8081–8090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Stoops WW, Rush CR, Negus SS, Glaser PEA, Hatton KW, Hays LR. 2016. Development of a translational model to screen medications for cocaine use disorder II: Choice between intravenous cocaine and money in humans. Drug Alcohol Depend 165:111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Hillhouse MP, Saxon AJ, Mooney LJ, Thomas CM, Ang A, Matthews AG, Hasson A, Annon J, Sparenborg S, Liu DS, McCormack J, Church S, Swafford W, Drexler K, Schuman C, Ross S, Wiest K, Korthuis PT, Lawson W, Brigham GS, Knox PC, Dawes M, Rotrosen J. 2016. Buprenorphine + naloxone plus naltrexone for the treatment of cocaine dependence: the Cocaine Use Reduction with Buprenorphine (CURB) study. Addiction 111(8):1416–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Liu Y, Fu X-M, Li N, Wang C-X, Zhang H, Qian R-B, Xu H-S, Hu X, Zhang D-R. 2011. Abnormal brain default-mode network functional connectivity in drug addicts. PLoS One 6(1):e16560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, Gerak LR, France CP. 2013. Effect of delay on self-administration of remifentanil under a drug versus drug choice procedure in rhesus monkeys. J Pharmacol Exp Ther 347(3):557–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markert LE, Roberts DCS. 1991. 3,4-methylenedioxyamphetamine (MDA) self-administration and neurotoxicity. Pharmacol Biochem Behav 39(3):569–574. [DOI] [PubMed] [Google Scholar]
- Mayhugh RE, Moussa MN, Simpson SL, Lyday RG, Burdette JH, Porrino LJ, Laurienti PJ. 2016. Moderate-heavy alcohol consumption lifestyle in older adults is associated with altered central executive network community structure during cognitive task. PLoS One 11(8):e0160214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie-Quirk SD, Miczek KA. 2008. Social rank and social separation as determinants of alcohol drinking in squirrel monkeys. Psychopharmacology (Berl) 201(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Negus SS. 1996. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology 14(6):375–424. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. 2002. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci 5(2):169–174. [DOI] [PubMed] [Google Scholar]
- Mozley L, Gur R, Mozley P, Gur R. 2001. Striatal dopamine transporters and cognitive functioning in healthy men and women. Am J Psychiatry 158(9):1492–1499. [DOI] [PubMed] [Google Scholar]
- Müller-Oehring EM, Jung Y-C, Pfefferbaum A, Sullivan EV, Schulte T. 2015. The resting brain of alcoholics. Cereb Cortex 25(11):4155–4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro CA, McCaul ME, Wong DF, Oswald LM, Zhou Y, Brasic J, Kuwabara H, Kumar A, Alexander M, Ye W, Wand WS. 2006. Sex differences in striatal dopamine release in healthy adults. Biol Psychiatry 59(10):966–974. [DOI] [PubMed] [Google Scholar]
- Murnane KS, Gopinath KS, Maltbie E, Daunais JB, Telesford QK, Howell LL. 2015. Functional connectivity in frontal-striatal brain networks and cocaine self-administration in female rhesus monkeys. Psychopharmacology (Berl) 232(4):745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnane KS, Howell LL. 2011. Neuroimaging and drug taking in primates. Psychopharmacology (Berl) 216(2):153–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Czoty PW. 2008. Brain imaging in nonhuman primates: Insights into drug addiction. ILAR J 49(1):89–102. [DOI] [PubMed] [Google Scholar]
- Nader MA, Czoty PW, Nader SH, Morgan D. 2012. a. Nonhuman primate models of social behavior and cocaine abuse. Psychopharmacology (Berl) 224(1):57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Daunais JB, Moore T, Nader SH, Moore RJ, Smith HR, Friedman DP, Porrino LJ. 2002. Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: Initial and chronic exposure. Neuropsychopharmacology 27(1):35–46. [DOI] [PubMed] [Google Scholar]
- Nader MA, Nader SH, Czoty PW, Riddick NV, Gage HD, Gould RW, Blaylock BL, Kaplan JR, Garg PK, Davies HML, Morton D, Garg S, Reboussin BA. 2012. b. Social dominance in female monkeys: Dopamine receptor function and cocaine reinforcement. Biol Psychiatry 72(5):414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Drug Abuse Available online (https://www.drugabuse.gov/publications/media-guide/science-drug-abuse-addiction-basics), accessed on January 19, 2017.
- Negus SS. 2005. Effects of punishment on choice between cocaine and food in rhesus monkeys. Psychopharmacology (Berl) 181(2):244–252. [DOI] [PubMed] [Google Scholar]
- Negus SS, Banks ML. 2013. Medications development for opioid abuse, 1:a012104. In: Pierce RC, Kenny PJ, eds. Addiction. New York: Cold Springs Harbor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Banks ML. 2017. Modulation of drug choice by extended drug access and withdrawal in rhesus monkeys: Implications for negative reinforcement as a driver of addiction and target for medications development. Pharmacol Biochem Behav. doi:10.1016/j.pbb.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Mello NK. 2003. Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a second-order schedule in rhesus monkeys. Drug Alcohol Depend 70(1):39–52. [DOI] [PubMed] [Google Scholar]
- Negus SS, Rice KC. 2009. Mechanisms of withdrawal-associated increases in heroin self-administration: Pharmacologic modulation of heroin vs food choice in heroin-dependent rhesus monkeys. Neuropsychopharmacology 34(4):899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom AL, Olsson H, Halldin C. 1998. A PET study of D2 dopamine receptor density at different phases of the menstrual cycle. Psychiatry Res 83(1):1–6. [DOI] [PubMed] [Google Scholar]
- Pardo-Lozano R, Farré M, Yubero-Lahoz S, O’Mathúna B, Torrens M, Mustata C, Pérez-Mañá C, Langohr K, Cuyàs E, Carbó Ml, de la torre R. 2012. Clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”): the influence of gender and genetics (CYP2D6, COMT, 5-HTT). PLoS One 7(10):e47599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretti PO, Lewis BR. 1969. Affects of alcoholic consumption on the activity patterns of individual rhesus monkeys and their behavior in a social group. Primates 10(2):181–188. [Google Scholar]
- Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, t Hart BA, Hopkins WD, Hu SL, Miller LA, Nader MA, Nathanielsz PW, Rogers J, Shively CA, Voytko ML. 2014. Why primate models matter. Am J Primatol 76(9):801–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohjalainen T, Rinne J, Någren K, SyvÄlahti E, Hietala J. 1998. Sex differences in the striatal dopamine D2 receptor binding characteristics in vivo. Am J Psychiatry 155(6):768–773. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Miller MD, Smith HR, Nader SH, Nader MA. 2016. Neural correlates of exposure to cocaine cues in rhesus monkeys: Modulation by the dopamine transporter. Biol Psychiatry 80(9):702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JN, Minhas D, Lopresti BJ, Price JC, Bradberry CW. 2014. Altered cerebellar and prefrontal cortex function in rhesus monkeys that previously self-administered cocaine. Psychopharmacology (Berl) 231(21):4211–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss TM. 1995. Do rats have prefrontal cortex? The Rose-Woolsey-Akert Program reconsidered. J Cogn Neurosci 7(1):1–24. [DOI] [PubMed] [Google Scholar]
- Priddy BM, Carmack SA, Thomas LC, Vendruscolo JCM, Koob GF, Vendruscolo LF. 2017. Sex, strain, and estrous cycle influences on alcohol drinking in rats. Pharmacol Biochem Behav 152:61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. 2001. A default mode of brain function. Proc Natl Acad Sci USA 98(2):676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasakham K, Liu-Chen L-Y. 2011. Sex differences in kappa opioid pharmacology. Life Sci 88(1–2):2–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone PA, Zelenock KA, Lindsey AM, Sulima A, Rice KC, Prinssen EP, Wichmann J, Woods JH. 2016. Characterization of the discriminative stimulus effects of a NOP receptor agonist ro 64-6198 in rhesus monkeys. J Pharmacol Exp Ther 357(1):17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis-Segura C, Becker JB. 2016. Why we should consider sex (and study sex differences) in addiction research. Addict Biol 21(5):995–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. 2005. The influence of social hierarchy on primate health. Science 308(5722):648–652. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Scherma M, Redhi GH, Vadivel SK, Makriyannis A, Goldberg SR, Justinova Z. 2016. Self-administration of the anandamide transport inhibitor AM404 by squirrel monkeys. Psychopharmacology (Berl) 233(10):1867–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler CW, Thorndike EB, Blough BE, Tella SR, Goldberg SR, Baumann MH. 2014. Effects of 3,4-methylenedioxymethamphetamine (MDMA) and its main metabolites on cardiovascular function in conscious rats. Br J Pharmacol 171(1):83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Whitfield TW, Park PE, Crawford EF, George O, Vendruscolo LF, Koob GF. 2013. Long-term antagonism of κ opioid receptors prevents escalation of and increased motivation for heroin intake. J Neurosci 33(49):19384–19392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Day SM. 2014. Social inequalities in health in nonhuman primates. Neurobiol Stress 1:156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DA, Blough BE, Banks ML. 2017. Cocaine-like discriminative stimulus effects of amphetamine, cathinone, methamphetamine, and their 3,4-methylenedioxy analogs in male rhesus monkeys. Psychopharmacology (Berl) 234:117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DA, Negus SS, Poklis JL, Blough BE, Banks ML. 2016. Cocaine-like discriminative stimulus effects of alpha-pyrrolidinovalerophenone, methcathinone and their 3,4-methylenedioxy or 4-methyl analogs in rhesus monkeys. Addict Biol. doi:10.1111/adb.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EO, Byrd LD. 1985. d-Amphetamine induced changes in social interaction patterns. Pharmacol Biochem Behav 22(1):135–139. [DOI] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Justinova Z, Yasar S, Goldberg SR. 2006. Using drug-discrimination techniques to study the abuse-related effects of psychoactive drugs in rats. Nat Protoc 1(3):1194–1206. [DOI] [PubMed] [Google Scholar]
- Spragg SDS. 1940. Morphine Addiction in Chimpanzees. Comparative Psychology Monographs. Baltimore, MD: The John Hopkins Press; p 1–132. [Google Scholar]
- Telesford QK, Laurienti PJ, Davenport AT, Friedman DP, Kraft RA, Daunais JB. 2015. The effects of chronic alcohol self-administration in nonhuman primate brain networks. Alcohol Clin Exp Res 39(4):659–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. 2005. Chronic intravenous drug self-administration in rats and mice. Curr Protoc Neurosci. 32:9.20:9.20.1–9.20.40. [DOI] [PubMed] [Google Scholar]
- UNODC 2015. World Drug Report 2015. In: Crime UNOoDa, ed. Vienna: United Nations Office on Drugs and Crime. [Google Scholar]
- Volkow ND, Fowler JS, Wang G-J. 2004. The addicted human brain viewed in the light of imaging studies: Brain circuits and treatment strategies. Neuropharmacology 47(Supplement 1):3–13. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. 1993. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse 14(2):169–177. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, McLellan AT. 2016. Neurobiologic advances from the brain disease model of addiction. N Engl J Med 374(4):363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. 1997. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature 386(6627):830–833. [DOI] [PubMed] [Google Scholar]
- Wade CL, Vendruscolo LF, Schlosburg JE, Hernandez DO, Koob GF. 2015. Compulsive-like responding for opioid analgesics in rats with extended access. Neuropsychopharmacology 40(2):421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade-Galuska T, Galuska CM, Winger G. 2011. Effects of daily morphine administration and deprivation on choice and demand for remifentanil and cocaine in rhesus monkeys. J Exp Anal Behav 95(1):75–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Orio L, Ghirmai S, Cashman J, Koob G. 2009. Inhibition of kappa opioid receptors attenuated increased cocaine intake in rats with extended access to cocaine. Psychopharmacology (Berl) 205(4):565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Vendruscolo LF, Misra KK, Schlosburg JE, Koob GF. 2012. A combination of buprenorphine and naltrexone blocks compulsive cocaine intake in rodents without producing dependence. Sci Transl Med 4(146):146ra110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks JR. 1962. Experimental morphine addiction: Method for automatic intravenous injections in unrestrained rats. Science 138(3537):143–144. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Fantegrossi WE, Goodwin AK. 2007. The value of nonhuman primates in drug abuse research. Exp Clin Psychopharmacol 15(4):309–327. [DOI] [PubMed] [Google Scholar]
- Wong DF, Broussolle EP, Wand G, Villemagne V, Dannals RF, Links JM, Zacur HA, Harris J, Naidu S, Braestrup C, Wagner HN Jr, Gjedde A. 1988. In vivo measurement of dopamine receptors in human brain by positron emission tomography age and sex differencesa. Ann N Y Acad Sci 515(1):203–214. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Anderso KG. 2006. Effects of delay to reinforcement on the choice between cocaine and food in rhesus monkeys. Psychopharmacology (Berl) 186(1):99–106. [DOI] [PubMed] [Google Scholar]
- Zahr NM. 2014. Chapter 17—Structural and microstructral imaging of the brain in alcohol use disorders In: Edith VS, Adolf P, eds. Handbook of Clinical Neurology. UK: Elsevier; p 275–290. [DOI] [PMC free article] [PubMed] [Google Scholar]