Abstract
The Zebrafish Model Organism Database (ZFIN; https://zfin.org) is the central resource for genetic, genomic, and phenotypic data for zebrafish (Danio rerio) research. ZFIN continuously assesses trends in zebrafish research, adding new data types and providing data repositories and tools that members of the research community can use to navigate data. The many research advantages and flexibility of manipulation of zebrafish have made them an increasingly attractive animal to model and study human disease.
To facilitate disease-related research, ZFIN developed support to provide human disease information as well as annotation of zebrafish models of human disease. Human disease term pages at ZFIN provide information about disease names, synonyms, and references to other databases as well as a list of publications reporting studies of human diseases in which zebrafish were used. Zebrafish orthologs of human genes that are implicated in human disease etiology are routinely studied to provide an understanding of the molecular basis of disease. Therefore, a list of human genes involved in the disease with their corresponding zebrafish ortholog is displayed on the disease page, with links to additional information regarding the genes and existing mutations. Studying human disease often requires the use of models that recapitulate some or all of the pathologies observed in human diseases. Access to information regarding existing and published models can be critical, because they provide a tractable way to gain insight into the phenotypic outcomes of the disease. ZFIN annotates zebrafish models of human disease and supports retrieval of these published models by listing zebrafish models on the disease term page as well as by providing search interfaces and data download files to access the data. The improvements ZFIN has made to annotate, display, and search data related to human disease, especially zebrafish models for disease and disease-associated gene information, should be helpful to researchers and clinicians considering the use of zebrafish to study human disease.
Keywords: Danio rerio, database, disease, disease models, translational research, zebrafish, ZFIN
Introduction
Danio rerio (zebrafish) have been used as research organisms since the 1960s, but it was not until breakthrough work done by George Streisinger in the early 1980s developing techniques to facilitate genetic analysis in zebrafish that the full potential of zebrafish as a model organism was brought to light (Streisinger et al. 1981). Since then, zebrafish have become a popular laboratory model to study genetics, developmental biology, and gene function due in part to their high fecundity, optical clarity of embryos, extrauterine development, quick maturation reaching larval stage by 72 hours postfertilization, and ease of genetic manipulation. The teleost lineage, which zebrafish are a member of, had a whole genome duplication event in the late Devonian after the divergence of the lob finned and ray finned fish, which resulted in the teleost genome containing multiple orthologs for some mammalian genes (Meyer and Schartl 1999; Taylor et al. 2003). These duplicated genes may be redundant in function, one of the duplicated genes may have lost the original function and acquired new function, or the duplicated genes may have undergone subfunctionalization (Force et al. 1999; Postlethwait 2006). Even though the zebrafish genome has been duplicated, it shares a high degree of genetic similarity with humans and provides a venue to understand subfunctionalization of gene functions related to the evolution of development and the molecular mechanisms that contribute to phenotype (Amores et al. 1998; Clemens et al. 2013; Meyer and Schartl 1999; Teng et al. 2011). At least 70% of human genes have at least one orthologous zebrafish gene, and 82% of the genes that have morbidity descriptions listed in Online Mendelian Inheritance in Man have at least one zebrafish ortholog (K Howe et al. 2013). Due to the high level of orthology between the zebrafish and human genome and the many advantages related to laboratory manipulation, zebrafish have become an important research model to understand human disease-related genes (Lieschke and Currie 2007; Phillips and Westerfield 2014; White 2015). In addition, zebrafish have been recognized to be unique vertebrate models amenable to high throughput drug screening and discovery, which cannot readily be done in other vertebrate models and is especially valuable when studying human disease and associated therapeutics (Deveau et al. 2016; Liu et al. 2016; Williams and Hong 2016).
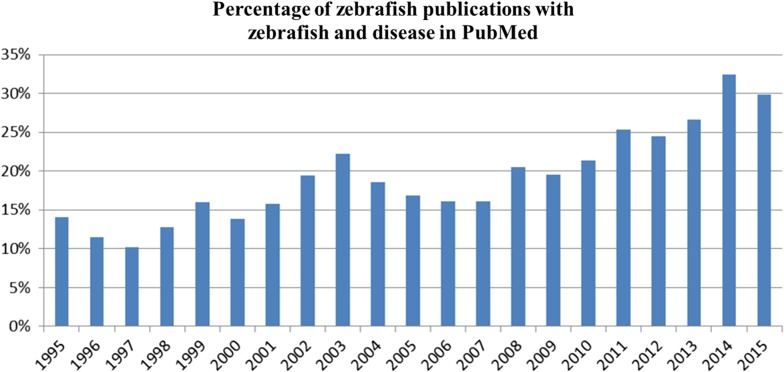
Analysis of the usage trends for the words “zebrafish” and “disease” in PubMed abstracts and text over the years indicates that zebrafish and disease words co-occur in an ever increasing number of publications (Figure 1). This plethora of zebrafish-associated research data is collected and curated by the Zebrafish Model Organism Database (ZFIN, https://zfin.org), which serves as a central repository for zebrafish developmental, genetic, genomic, and phenotypic information (DG Howe et al. 2013). To support the increasing use of zebrafish as a translational model of human disease, ZFIN has enhanced support for human disease information and zebrafish models of human disease.
Figure 1.
‘zebrafish’ and ‘disease/disorder/syndrome/cancer’ words co-occur increasingly in PubMed publications. Percentage of zebrafish publications per year with the word ‘zebrafish’ and ‘disease’ is shown. The number of zebrafish publications was determined by using the words ‘zebrafish’ OR ‘zebra fish’ OR ‘Danio rerio’ in the PubMed advanced search. The amount of publications containing both zebrafish and disease words was determined by using the words ‘zebrafish’ OR ‘zebra fish’ OR ‘Danio rerio’ AND ‘disease’ OR ‘syndrome’ OR ‘disorder’ OR ‘cancer’ in the PubMed advanced search. PubMed searches were conducted September 28, 2016.
ZFIN as a Resource for Zebrafish Translational Research
Accessing Human Disease Information
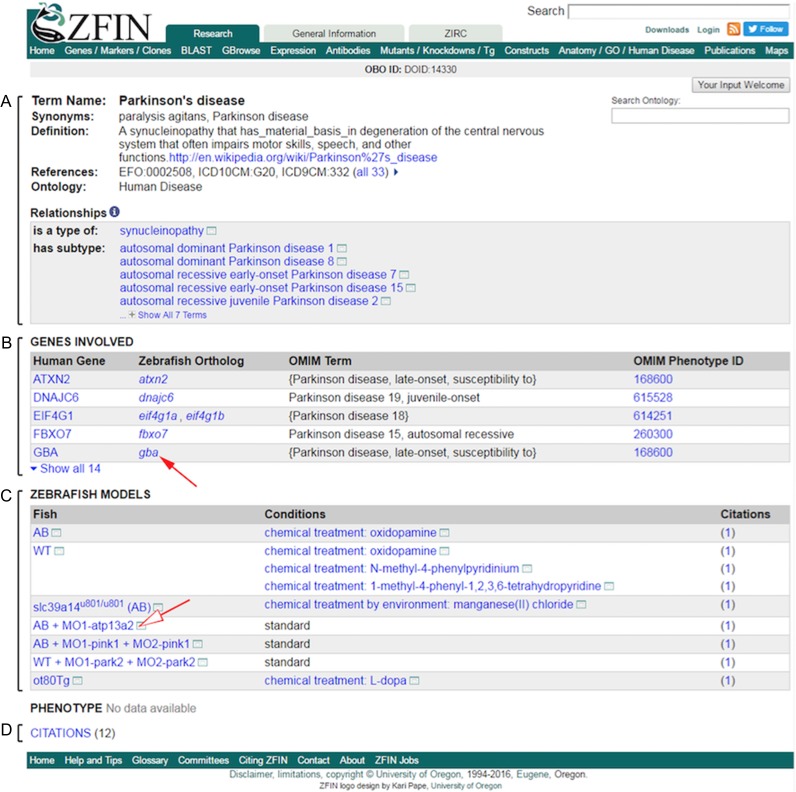
Researchers and clinicians who utilize zebrafish to perform translational research often consult the literature to identify established zebrafish models of human disease and whether disease-associated genes have been studied in zebrafish. In addition, researchers will also determine whether there are mutations or gene knockdown reagents available for disease-associated genes and whether the phenotypes resulting from alteration of these genes resemble ones observed in human patients. To make information relevant to human disease more accessible via a single interface, ZFIN developed a new disease term page (Figure 2). The disease term page utilizes information from the Disease Ontology (DO, Schriml and Mitraka 2015) to provide the disease name, alternate names or synonyms, a definition for the disease, and cross references to other disease related databases such as International Classification of Diseases, Medical Subject Headings, National Cancer Institute Thesaurus, Online Mendelian Inheritance in Man (OMIM), Systematized Nomenclature of Medicine Clinical Terms, and Unified Medical Language System (Figure 2A). DO has grouped some OMIM terms under one DO term, and there is active development to make parent child classes to have a grouping term with OMIM subtypes as children (S. Bello, personal communication). It is worth noting that DO is under active development, and not all subclasses of a disease are currently available. Publications reporting disease studies involving the use of zebrafish are listed at the bottom of the disease page. These publications can also be retrieved via the ZFIN search (Figure 5A).
Figure 2.
The ZFIN disease page displays information about the disease, human genes associated to the disease and their zebrafish orthologs, and a list of zebrafish models for this disease. Snapshot of the ZFIN disease term page for PD. (A) The information about the disease such as synonyms, definition, and references to other human disease databases come from the DO. Relationships with other terms of the ontology are also shown. (B) “Genes Involved” section shows the human genes associated to the disease based on information from OMIM. The zebrafish orthologs to these genes are displayed based on the ZFIN curated orthology. Clicking on these zebrafish genes (arrow) links to the ZFIN gene page. (C) A list of zebrafish models, defined as “Fish” in “Experimental Conditions”, is displayed in the “Zebrafish Models” section. These models are associated with citations in which they were used and/or created. Phenotype of the models can be found on the Fish page which can be reached by clicking on the Fish (open arrow). (D) Publications discussing or studying the disease are listed in the “Citations” section.
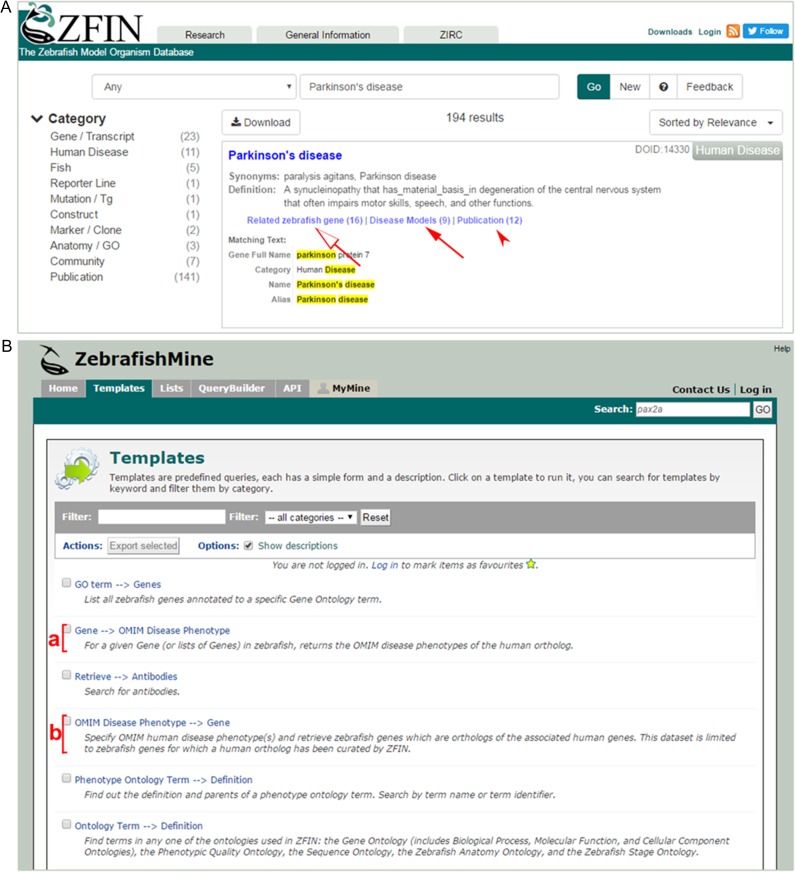
Figure 5.
Human disease information can be retrieved and searched using the ZFIN search box and ZebrafishMine. (A) Single search box in ZFIN retrieves results giving information linking to the disease pages, including zebrafish models for this disease (arrow), zebrafish orthologs to the human gene involved in the disease (open arrow), and disease associated publications (arrow head). (B) ZebrafishMine search interface contains templates supporting searches of disease related information. The ZebrafishMine has predefined queries to provide human disease related data. The Gene-→OMIM Disease Phenotype search (bracket a) returns a list of OMIM disease phenotypes of the human orthologue for a given zebrafish. The OMIM Disease Phenotype → Gene search (bracket b) returns a list of zebrafish genes that are orthologous to disease related genes for a given OMIM disease.
Accessing Disease-Associated Genes and Orthologs
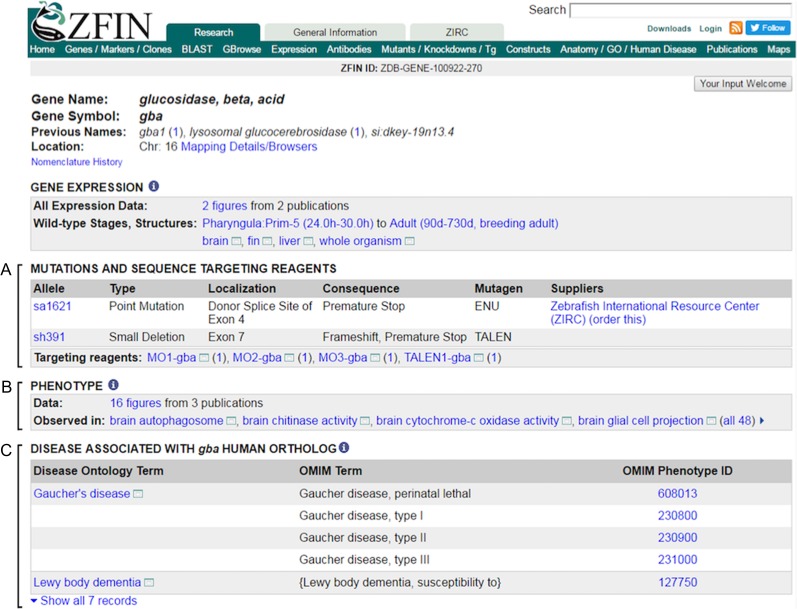
A core concept of translational research is to study orthologous human disease-associated genes in animal models to understand candidate gene function, gain insights about phenotypic outcomes, and conduct preclinical studies of potential therapies. OMIM has curated human clinical reports and publications to provide information about the genetic basis of disease (Amberger et al. 2015). The human disease-associated genes and the corresponding zebrafish orthologous genes were made more accessible in ZFIN through the “Genes Involved” table displayed on the Disease term page (Figure 2B). The information provided in this table is produced by utilizing the cross references in DO to OMIM, in conjunction with human gene associations made to diseases by OMIM (genemap.txt, https://omim.org/downloads/ and https://omim.org/static/omim/data/mim2gene.txt, last accessed 1/6/2017) to populate the “Genes Involved” table computationally with links to human disease-associated genes as well as zebrafish orthologs and OMIM phenotype pages. In cases where there is a defined DO parent disease term with disease subtypes, genes that are associated with the subclass DO terms are not currently pulled forward to the parent DO term page. The listed zebrafish orthologs are identified based on orthology relationships curated by ZFIN. It is worth noting that genes involved in the disease are solely based on disease-associated genes reported by OMIM and are never inferred from information obtained in zebrafish. For example, the “Genes Involved” section on the Parkinson’s disease (PD) page indicates OMIM reported human genes associated with PD and provides the corresponding orthologous zebrafish genes (Figure 2B). Clicking on a zebrafish gene in the table, for example gba, links to the ZFIN gene page that provides information related to gene expression and function (Figure 3). Notably, mutations and reagents that have been reported to disrupt gene function (called sequence targeting reagents, STR, in ZFIN) are listed on the gene page (Figure 3A) as well as the associated phenotypes (Figure 3B). Navigation back to the disease term page can be accomplished by utilizing the disease links displayed in the “Disease Associated with Human Ortholog” table, which lists diseases associated with the human ortholog (Figure 3C). The information in this table is derived computationally based on ZFIN curated orthology data and OMIM gene to disease associations.
Figure 3.
The ZFIN gene page displays information about available mutations and sequence targeting reagents and the phenotype associated and provides a list of diseases associated with the human ortholog. Snapshot of the gba gene page. (A,B) Mutations and sequence targeting reagents reported to alter the gene function are listed, as well as the associated phenotypes. (C) Human diseases associated to the human ortholog are also displayed, and link to the ZFIN disease term page, and the OMIM record.
Zebrafish Models of Human Disease
Studying human disease frequently requires the use of models that recapitulate some or all of the observed pathologies. The use of disease models not only facilitates the understanding of the genetic basis of disease and the phenotypic outcomes, but it also provides a system in which to test potential treatment approaches. Information regarding models for disease is therefore highly valuable; to facilitate retrieval of this data, ZFIN developed methods of annotating zebrafish models of human disease and providing information for these reported models on the disease term page (Figure 2C). ZFIN curates zebrafish models of human diseases either when authors have stated them as such or when the intent of a publication is to report an aspect of a disease using zebrafish and there is sufficient evidence that the phenotypes observed are similar to the human patients. Consequently, mutants of disease-associated genes might not always be listed as disease models.
Annotating and recording zebrafish models of human disease in ZFIN requires identifying how researchers create and report zebrafish disease models. The most common disease models are those in which the disease-associated gene is altered resulting in gene product knockdown or misexpression. Additionally, examination of the zebrafish literature revealed that models are also generated by recreating the physiological and environmental conditions triggering the human disease or by recapitulating phenotypes observed in human patients. These different methods were incorporated in the concept of “zebrafish model” of human disease.
Annotation of Zebrafish Models of Human Disease in ZFIN
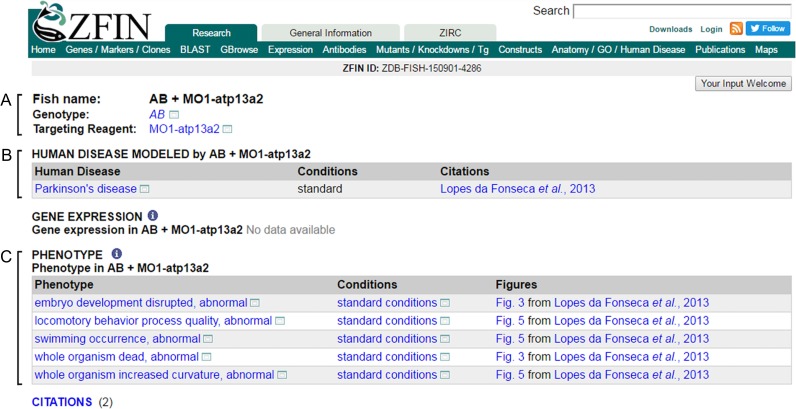
When developing support for zebrafish models of disease in ZFIN, it was imperative to use a single concept to represent the different model types reported in the literature: mutants, fish injected with gene-altering agents, transgenic lines, and fish treated with specific experimental conditions. We adopted a data model in which the “is a disease model” annotation is made to an entity encompassing the genetic and knockdown reagent information, a “Fish,” and the “Experimental Conditions” to which the animals were exposed. The “Fish” concept includes information about the genetic background, the zygosity of the transmittable genomic alterations that are present as a result of mutation or transgene integration (genotype), and any transient STR that have been applied, such as morpholinos or clustered regularly interspaced short palindromic repeats (CRISPRs) that knock down gene expression (Figure 4A). A Fish is therefore defined as containing background + genotype + STR. A Fish requires that a background and/or a genotype be specified, whereas an STR is optional and indicated only when applicable. The background can be representative of a particular strain, such as the AB strain or the Tubingen strain or simply wild type for unspecified backgrounds or strains. When a zebrafish has been injected with a morpholino and does not have a mutant or transgenic allele present, the background is required to be specified in the Fish even if it is wild type. All data pertinent to Fish are displayed on the Fish page, including annotations to human disease, gene expression, associated phenotypes, and citations (Figure 4).
Figure 4.
The concept of ‘Fish’ includes information about background, genotype, and STR. Snapshot of the AB + MO1-atp13a2 Fish page. (A) Background, genotype, and STR constituting ‘Fish’ are shown at the top of page. (B) Human disease modeled by this fish is shown with the ‘Experimental Conditions’ (Conditions) in which this fish would create the model. If there are gene expression annotations they are displayed under ‘Gene Expression’. (C) In the ‘Phenotype’ section, a list of phenotype annotations for the Fish and model are listed, with the corresponding citations that reported these phenotypes.
In addition to the genetic information about zebrafish models of human disease, the conditions in which the experimental disease models were established are also included in the is a disease model annotation. ZFIN uses the concept of Experimental Conditions to represent this information and records it with terms from the newly created Zebrafish Experimental Condition Ontology (ZECO, publication in progress). Experimental conditions encompass a wide variety of treatments and can include information about diet, temperature, chemicals applied, etc. When no specific experimental conditions are applied, Standard Conditions (ZECO:0000103), reflecting the standard husbandry conditions for zebrafish (Westerfield 2007), or Generic Control (ZECO:0000102), reflecting nonstandard conditions used as experimental control, is used in the model annotation.
A zebrafish model of disease annotation in ZFIN is therefore represented as an is a model association between a Fish and Experimental Conditions, and a human disease term from the Disease Ontology (Figure 2C). Examples of the various types of annotations are shown in Table 1. Each annotation has citations to the publication that noted generating or using the model. In addition, each model is also associated with an evidence code of either Traceable Author Statement for annotations that reflect a direct author statement or Inferred by Curator when an annotation is created based on evidence presented in the publication when there is no explicit author statement declaring a disease model. The evidence codes come from the Evidence and Conclusion Ontology (Chibucos et al. 2014), which is used to describe the scientific evidence of an annotation. In the sections below, we will explain the different types of zebrafish disease models and provide the corresponding annotation in Table 1.
Table 1.
Examples of zebrafish models for human diseases
| Zebrafish models | Human disease | |
|---|---|---|
| Fish | Experimental conditions | |
| A. Zebrafish models based on alteration of disease associated orthologous genes | ||
| dmdta222a/ta222a [ZDB-FISH-150901-14025] | Generic control [ZECO:0000102] | Muscular dystrophy (Li and Arner 2015) [DOID:9884] |
| rpl11hi3820bTg/hi3820bTg [ZDB-FISH-150901-8506] | Standard conditions [ZECO:0000103] | Diamond-Blackfan anemia (Danilova et al. 2014; Y Zhang et al. 2014; Jia et al. 2013) [DOID:1339] |
| AB + MO1-rps19 [ZDB-FISH-150901-25995] | Standard conditions [ZECO:0000103] | |
| WT + CRISPR1-mecp2 [ZDB-FISH-151014-40] | Standard conditions [ZECO:0000103] | Rett syndrome (Gao et al. 2015) [DOID:1206] |
| col6a1ama605003/ama605003 (TU) [ZDB-FISH-151110-7] | Standard conditions [ZECO:0000103] | Bethlem myopathy (Radev et al. 2015) [DOID:0050663] |
| cfap53hu10478/hu10478 [ZDB-FISH-160504-2] | Standard conditions [ZECO:0000103] | Visceral heterotaxy (Noël et al. 2015) [DOID:0050545] |
| slc16a2biu4/biu4 [ZDB-FISH-150901-10550] | Standard conditions [ZECO:0000103] | Allan-Herndon-Dudley syndrome (Zada et al. 2014) [DOID:0050631] |
| B. Zebrafish models based on gene misexpression | ||
| tp53zdf1/zdf1; Tg(mitfa:Hsa.BRAF_V600E)czt13Tg [ZDB-FISH-150901-14943] | Standard conditions [ZECO:0000103] | Melanoma (Yen et al. 2013) [DOID:1909] |
| Tg(mitfa:mitfa), Tg(rag2:Hsa.MYC-ERT2)zdf14Tg; Tg(lck:lck-EGFP)cz2Tg [ZDB-FISH-150901-17819] | Standard conditions [ZECO:0000103] | Acute T cell leukemia (Ridges et al. 2012) [DOID:5603] |
| Tg(spi1b:LOXP-EGFP-LOXP-Hsa.NUP98-Hsa.HOXA9)hsi1Tg; Tg(hsp70l:Cre)zdf13Tg [ZDB-FISH-150901-25078] | Heat shock [ZECO:0000166] | Myeloid leukemia (Forrester et al. 2011) [DOID:8692] |
| Tg(fabp10a:edn1,myl7:EGFP)nn1005Tg [ZDB-FISH-150901-2358] | Standard conditions [ZECO:0000103] | Hepatocellular carcinoma (Lu et al. 2014) [DOID:684] |
| C. Zebrafish models based on exposure to disease causing conditions | ||
| AB [ZDB-GENO-960809-7] | Chemical treatment: ethanol [ZECO:0000111: CHEBI:16236] | Fetal alcohol spectrum disorders (Fernandes et al. 2015) [DOID:0050696] |
| WT [ZDB-GENO-030619-2] | Increased food availability [ZECO:0000247] | Obesity (Montalbano et al. 2015) [DOID:9970] |
| WT [ZDB-GENO-030619-2] | Bacterial treatment: Mycobacterium marinum [ZECO:0000106: NCBITaxon:1781] | Tuberculosis (Sridevi et al. 2014) [DOID:399] |
| mitfaw2/w2; roya9/a9 [ZDB-FISH-150901-6638] | Viral treatment: influenza virus [ZECO:0000110: NCBITaxon:11309] | Influenza (Gabor et al. 2014) [DOID:8469] |
| D. Zebrafish models based on disease phenotype similarities | ||
| TU [ZDB-GENO-990623-3] | Chemical treatment: pentetrazol [ZECO:0000111: CHEBI:34910] | Epilepsy (Siebel et al. 2015) [DOID:1826] |
| WT [ZDB-GENO-030619-2] | Chemical treatment: oxidopamine [ZECO:0000111: CHEBI:78741] | Parkinson’s disease (Panula et al. 2006) [DOID:14330] |
| WT [ZDB-GENO-030619-2] | Chemical treatment: N-methyl-4-phenylpyridinium [ZECO:0000111: CHEBI:641] | |
| Tg(ins:CFP-NTR)s892Tg [ZDB-FISH-150901-27537] | Chemical ablation: insulin secreting cell [ZECO:0000169: ZFA:0009101], chemical treatment: metronidazole [ZECO:0000111: CHEBI:6909] | Type 1 diabetes mellitus (Tsuji et al. 2014) [DOID:9744] |
Zebrafish models are defined as ‘Fish’ (genotype, background, and STR) and ‘Experimental Conditions’. They are associated with a disease term from the Disease Ontology (DO).
Zebrafish Models Based on Alteration of Disease-Associated Genes
Altering the function of the orthologous disease-associated gene either by mutations or knock down of the gene product is a common approach to create a disease model. Mutations in zebrafish orthologs of human disease-associated genes and loci can be discovered via forward genetic screens or created by targeted mutagenesis.
Forward genetic screens based on the alkylating agent ethylnitrosourea or retroviral insertional mutagenesis to produce zebrafish mutants have been extensively used (Amsterdam et al. 1999; Driever et al. 1996; Grunwald and Streisinger 1992; Haffter et al. 1996; Kettleborough et al. 2013; Mullins et al. 1994; Solnica-Krezel et al. 1994; Varshney et al. 2013). Although these approaches are not directed at specific genes, they produce a large number of mutants and have led to the identification of zebrafish genes that are orthologous to disease-associated human loci (Amsterdam and Hopkins 2006). For example, a large ethylnitrosourea forward genetic screen for motility and locomotion mutants isolated dmdta222a, and fish homozygous for this mutation raised in generic controlled conditions have been identified as models of muscular dystrophy (Table 1A; Bassett and Currie 2003; Granato et al. 1996). Fish homozygous for rpl11hi3820bTg, which was identified in a retroviral insertion mutagenesis screen, kept in standard conditions have been identified as models of Diamond-Blackfan anemia (Table 1A; Amsterdam et al. 2004; Danilova et al. 2014; Ear et al. 2015; Y Zhang et al. 2014). The improvement of reverse genetic techniques such as the use of zinc finger nucleases (Leong et al. 2011; Sander et al. 2011a, 2011b) CRISPRs (Hruscha and Schmid 2015; Hwang et al. 2013; Hwang et al. 2015) and transcription activator-like effector nucleases (TALENs; Huang et al. 2011, 2016; Ma et al. 2016; Sander et al. 2011a, 2011b) have allowed for the targeted mutation of disease-specific genes. These techniques are becoming the method of choice in zebrafish for creating genetic models of human disease, such as models for visceral heterotaxy, Bethlem myopathy, and Allan-Herndon-Dudley syndrome (Table 1A; Noël et al. 2015; Radev et al. 2015; Zada et al. 2014).
Reverse genetic techniques can also be used in a transient manner to downregulate zebrafish orthologs of human disease-associated genes and create disease models. For example, CRISPRs or TALENs have been injected into the zygote and phenotypes have been analyzed as soon as the embryos have reached the pertinent developmental stage, without waiting for a stable mutant line to be generated. This method has been used to create a zebrafish model of Rett syndrome (Table 1A; Gao et al. 2015). However, the most common transient knock down of specific gene product utilizes a technique where embryos are injected with antisense morpholino oligonucleotides to inhibit gene translation or proper splicing, resulting in the transient knock down of specific gene products (Nasevicius and Ekker 2000; https://www.ncbi.nlm.nih.gov/probe/docs/techmorpholino/, last accessed 1/9/2017). Morpholinos against rpl5a, rps19, and rpl11 have been used to create zebrafish models of Diamond Blackfan anemia (Table 1A; Ear et al. 2015; Jia et al. 2013; Wan et al. 2016; Z Zhang et al. 2013, 2014). Taken together, zebrafish models of human disease created by altering the functions of orthologous disease-associated genes can be represented as genetic mutants or fish injected with gene knockdown reagents raised in generic control or standard conditions.
Zebrafish Models Based on Gene Misexpression
Mutations that produce a knockdown of gene function often cause disease, although mutations that induce misexpression or overexpression of gene products can also lead to human disease (Shastry 1995; Santarius et al. 2010; F Zhang et al. 2009). An alternative and parallel approach to understanding disease-associated gene function is to utilize tools to overexpress or misexpress genes. Oncogene expression, gene overexpression, or genomic context reported in human patients can be reproduced in zebrafish by creating transgenic lines. For example, transgenic lines expressing human oncogenes have been published as zebrafish models for cancers such as melanoma (Table 1B; Yen et al. 2013) and acute T-cell leukemia (Table 1B; Ridges et al. 2012). A human chromosomal translocation causing the production of NUP98-HOXA9 fusion oncogene has been shown to contribute to myeloid leukemia. The expression of this fusion protein in transgenic zebrafish provided new insight into the mechanisms of myeloid leukaemogenesis (Table 1B; Forrester et al. 2011). Endothelin1 has been identified as a gene upregulated in a mouse model of hepatocarcinoma. To analyze whether overexpression of this gene could trigger hepatocarcinogenesis, the nn1005Tg transgenic zebrafish line was generated and aspects of the disease were analyzed using this model (Table 1B; Lu et al. 2014). Additional methods, such as injection of mRNA, can be utilized to recreate gene misexpression observed in human patients. Currently, however, ZFIN does not curate data using these methods, and only transgenic lines recreating gene misexpression raised in standard/generic control conditions or in conditions enabling the transgene expression (such as ‘heat shock’ [ZECO:0000166]) are reported as disease models in ZFIN.
Zebrafish Models Based on Exposure to Disease-Causing Conditions
Modeling human diseases that are triggered by nongenetic factors can be achieved by recreating the environmental and/or physiological conditions reported in human patients. For example, bacterial or viral infectious diseases, like tuberculosis or influenza, can be modeled in zebrafish that have been infected with the disease-causing bacteria or viruses (Table 1C; Gabor et al. 2014; Sridevi et al. 2014). Zebrafish embryos exposed to ethanol have been used as models of fetal alcohol spectrum disorders, which result from maternal alcohol consumption during pregnancy (Table 1C). Zebrafish fetal alcohol spectrum disorders models have been used to understand the effects of prenatal alcohol exposure on behavior (Bailey et al. 2015; Fernandes et al. 2015), to gain an understanding of affected signaling pathways (Pappalardo-Carter et al. 2013; C Zhang et al. 2014; Zhang et al. 2015), and to understand associated retinal tissue defects (Muralidharan et al. 2014). Zebrafish have also been used to understand human obesity, which can have its cause rooted in multiple factors, including genetics and lifestyle. To understand obesity that occurs by an imbalance in the calories consumed versus the calories expended, zebrafish models of obesity induced by diet alterations have been created by modifying the fish diet conditions (Table 1C). These obesity models have very similar pathophysiological conditions to those observed in human obesity (Oka et al. 2010) and have been used to understand the associated changes in gene expression in the brain and gut (Montalbano et al. 2015). Diet-induced models are useful to help understand contributing factors and develop ways to overcome the obesity epidemic. Zebrafish models created by mimicking the conditions triggering human disease are therefore represented in ZFIN as a fish subjected to specific experimental conditions.
Zebrafish Models Based on Similarities to Disease Phenotypes
In situations where it is difficult or inconvenient to reproduce the genetic and/or environmental conditions leading to human pathologies, or when the genetic cause of the disease is unknown, models have been established based solely on phenotypic similarities to the human disease phenotypes. Such models are valuable, as they have the potential to lead to the discovery of novel human disease-associated genes. Most often, such phenocopy models are induced to represent the human disease state by chemical application, such as the models for epilepsy and PD. To facilitate a better understanding of epilepsy, zebrafish models of epilepsy have been created by administering pentetrazol to induce seizures (Table 1D). Studies that utilize this method have investigated potential therapeutic compounds to alleviate seizure states as well as to understand changes in gene expression due to seizure events (Barbalho et al. 2016; Li et al. 2015; Siebel et al. 2015). PD is a neurodegenerative disease that results from the loss of dopaminergic cells in the brain (Beitz 2014; Pienaar et al. 2010). To understand PD better and develop potential therapies, zebrafish models of PD have been created by treating zebrafish with various neurotoxins like 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, rotenone, paraquat, oxidopamine, or N-methyl-4-phenylpyridinium (Table 1D; Bretaud et al. 2004; Lam et al. 2005). The neurotoxin 6-hydroxydopamine was shown by Parng et al. (2007) to reduce the numbers of dopaminergic neurons selectively in zebrafish and has been utilized to generate zebrafish models of PD to understand molecular markers of PD better and to develop therapeutic treatments (Feng et al. 2014; Z Zhang et al. 2012). The loss of insulin-producing cells observed in patients with type 1 diabetes mellitus was recapitulated in zebrafish by targeting these cells for death in a transgenic line expressing nitroreductase. Upon application of metronidazole, nitroreductase enzyme expressed specifically in insulin-producing cells converts this prodrug to a cytotoxic product, resulting in the death of beta cells. This model (Table 1D) allowed Tsuji et al. (2014) to screen compounds that stimulate beta-cell proliferation and regeneration. Zebrafish models based on phenocopying human patients are represented in ZFIN as fish or transgenic lines in specific experimental conditions that induce similar phenotypic disease states.
Finding Human Disease Information in ZFIN
Human disease information in ZFIN can be found by using the single box search on the ZFIN home page (https://zfin.org). Querying for a disease name retrieves results linking to the disease pages, including zebrafish models for this disease, as well as a list of genes involved in the disease, which is based on orthology with human genes and publications related to the disease (Figure 5A). Additionally, disease model and related disease information can be accessed or browsed from the Fish, Gene, Human Disease, and Publication categories available in the search interface. The results of a search query can be downloaded as a csv file. A list of zebrafish models with associated diseases and publications recorded in ZFIN can also be downloaded from the ZFIN downloads files (https://zfin.org/downloads/fish_model_disease.txt).
Zebrafish information related to disease and disease models is also available in ZFIN’s data mining resource, ZebrafishMine (http://www.zebrafishmine.org/begin.do). ZebrafishMine is based on the InterMine data warehousing system and offers options for customizable searches and downloads (Lyne et al. 2015; Ruzicka et al. 2015). Within the ZebrafishMine interface, human disease information can be accessed using a series of predefined search templates listed in the Templates tab on the ZebrafishMine home page (Figure 5B). For each search, a sample disease or gene name is displayed in the search box. The “Human Disease → Zebrafish Models” search template returns a list of zebrafish models for the human disease Rett Syndrome (http://zebrafishmine.org/template.do?name=Disease_Model&scope=all).
Search results for this template include the name of the disease, the fish used to model the disease, the environmental conditions, and the ZFIN database identifiers for each. The search template sample term can be replaced with another search term or with a “wild card” (*) character. For the “Human Disease → Zebrafish Models” search template, a search with the wild card (*) character will return a list of all human diseases for which a zebrafish model has been curated in ZFIN.
Another set of templates allows exploration of the OMIM disease annotations of human orthologs of zebrafish genes. The “Gene → OMIM Disease Phenotype” search template returns a list of OMIM disease phenotypes for PTEN, the ortholog of the zebrafish ptena gene (http://zebrafishmine.org/template.do?name=Gene_OMIMDis&scope=all).
Search results for this template include the zebrafish gene symbol and the zebrafish gene name, the OMIM disease phenotype, and the OMIM identifier. Searches can also be performed with lists of genes or diseases.
ZebrafishMine provides an interface supporting customizable queries of ZFIN data. ZebrafishMine search templates can be edited using the Edit Query button. In the Edit Query mode, additional search parameters can be added to the template. The order of search result columns can also be changed in this mode. Search results can be downloaded in several different formats (such as CSV, TSV, and XML). Web services support programmatic access to ZebrafishMine, with client library support for Perl, Python, Ruby, and Java.
Summary and Future Directions
ZFIN has responded to the increased use of zebrafish for translational research and the need to access human disease-relevant data by annotating zebrafish models of human disease as well as by providing dedicated user interfaces and search platforms to access human disease information. Zebrafish models of human disease have been created using both genetic and experimental condition approaches to understand gene pathways involved and phenotypic outcomes and to aid in the development of therapeutic strategies. ZFIN annotates validated human disease models reported in the primary literature as well as associated phenotypes, making this information more digitally accessible and facilitating advances in translational research. In addition, ZFIN provides disease information for zebrafish genes that are orthologous to human disease-associated genes, supporting directed identification of candidate genes that can be directly mutated to develop new disease models. ZFIN incorporated additional support for annotation of zebrafish models of disease in the summer of 2015, and as of September 2016 ZFIN has annotated 411 zebrafish disease models, corresponding to 175 human diseases. Not all publications that have models have been curated and because all these annotations are made manually, it is likely that some reported models are missing from the database. We always encourage users to contact us with missing or incomplete information by contacting zfinadmin@zfin.org or clicking the Your Input Welcome button at the top right of every web page in ZFIN.
To further improve support for translational research, ZFIN is investigating ways to better display and retrieve phenotypes associated to disease models as well as comparison of reported zebrafish mutant phenotypes with human disease phenotypes. Comparison of related phenotypes can aid in the discovery of the genetic basis of disease. For example, Horstick et al. (2013) utilized the similarities in the myopathic phenotype of zebrafish stac3mi34 mutants with the myopathic phenotypes of Native American Myopathy, which had had been mapped to a region of 12q13 through population single nucleotide polymorphism analysis but still had an unresolved genetic basis (Stamm et al. 2008) and thus identified STAC3 as a causative gene in congenital myopathies. The Monarch Initiative has efforts underway to compare annotated human disease phenotypes computationally with those of mouse and zebrafish mutant phenotypes. The recently developed phenotype widget graphically displays phenotype comparisons between human diseases and model organisms and facilitates the identification of genes and diseases that have similar phenotypes (Haendel et al. 2015; Mungall et al. 2015). ZFIN is investigating the potential to provide access to the Monarch Initiative phenotype widget from the disease term page as a tool to aid in the discovery of novel disease-causing genes. In addition, ZFIN is continually evaluating how to represent phenotypic data better and make it more accessible for use in biomedical investigations, providing better search interfaces to query phenotype data as well as data viewing pages to access reported phenotypic outcomes.
By providing access to information relevant to human disease and zebrafish models of human disease, as well as tools to compare phenotypes, ZFIN is the bioinformatics resource for zebrafish translational research.
Acknowledgments
The authors want to thank the entire ZFIN curatorial and software development team for their help in implementing this project and ongoing data curation. Authors also thank Ceri Van Slyke, curator at ZFIN, for critical review of the manuscript. This work was supported by the National Human Genome Research Institute of the National Institutes of Health [HG002659].
Author Contributions
Y.B., S.T., and S.R. were the lead curators on this project; A.E., P.K., S.T.M., and K.S. were on the software development team; Y.B. and S.T. wrote the manuscript, with contributions from S.R. and L.R.
References
- Amberger JS, Bocchini CA, Schiettecatte F, Scott AF, Hamosh A. 2015. OMIM.org: Online Mendelian Inheritance in Man (OMIM®), an online catalog of human genes and genetic disorders. Nucleic Acids Res 43(Database issue):D789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amores A, Force A, Yan YL, Joly L, Amemiya C, Fritz A, Ho RK, Langeland J, Prince V, Wang YL, Westerfield M, Ekker M, Postlethwait JH. 1998. Zebrafish hox clusters and vertebrate genome evolution. Science 282(5394):1711–4. [DOI] [PubMed] [Google Scholar]
- Amsterdam A, Burgess S, Golling G, Chen W, Sun Z, Townsend K, Farrington S, Haldi M, Hopkins N. 1999. A large-scale insertional mutagenesis screen in zebrafish. Genes Dev 13:2713–2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam A, Nissen RM, Sun Z, Swindell E, Farrington S, Hopkins N. 2004. Identification of 315 genes essential for early zebrafish development. Proc Natl Acad Sci USA 101(35):12792–12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam A, Hopkins N. 2006. Mutagenesis strategies in zebrafish for identifying genes involved in development and disease. Trends Genet 22(9):473–478. [DOI] [PubMed] [Google Scholar]
- Bailey JM, Oliveri AN, Zhang C, Frazier JM, Mackinnon S, Cole GJ, Levin ED. 2015. Long-term behavioral impairment following acute embryonic ethanol exposure in zebrafish. Neurotoxicol Teratol 48:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbalho PG, Lopes-Cendes I, Maurer-Morelli CV. 2016. Indomethacin treatment prior to pentylenetetrazole-induced seizures downregulates the expression of il1b and cox2 and decreases seizure-like behavior in zebrafish larvae. BMC Neurosci 17:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DI, Currie PD. 2003. The zebrafish as a model for muscular dystrophy and congenital myopathy. Hum Mol Genet 12(Suppl. 2):R265–R270. [DOI] [PubMed] [Google Scholar]
- Beitz JM. 2014. Parkinson’s disease: a review Front Biosci (Schol. Ed) 6:65–74. [DOI] [PubMed] [Google Scholar]
- Bretaud S, Lee S, Guo S. 2004. Sensitivity of zebrafish to environmental toxins implicated in Parkinson’s disease. Neurotoxicol Teratol 26(6):857–864. [DOI] [PubMed] [Google Scholar]
- Chibucos MC, Mungall CJ, Balakrishnan R, Christie KR, Huntley RP, White O, Blake JA, Lewis SE, Giglio M. 2014. Standardized description of scientific evidence using the Evidence Ontology (ECO). Database 2014:bau066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens DM, Németh-Cahalan KL, Trinh L, Zhang T, Schilling TF, Hall JE. 2013. In vivo analysis of aquaporin 0 function in zebrafish: permeability regulation is required for lens transparency. Invest Ophthalmol Vis Sci 54(7):5136–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilova N, Bibikova E, Covey TM, Nathanson D, Dimitrova E, Konto Y, Lindgren A, Glader B, Radu CG, Sakamoto KM, Lin S. 2014. The role of DNA damage response in zebrafish and cellular models of Diamond Blackfan Anemia. Dis Model Mech 7(7):895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveau AP, Bentley VL, Berman JN 2017. Using zebrafish models of leukemia to streamline drug screening and discovery. Exp Hematol 45:1–9, ISSN 0301-472X. [DOI] [PubMed] [Google Scholar]
- Driever W, Solnica-Krezel L, Schier AF, Neuhauss SC, Malicki J, Stemple DL, Stainier DY, Zwartkruis F, Abdelilah S, Rangini Z, Belak J, Boggs C. 1996. A genetic screen for mutations affecting embryogenesis in zebrafish. Development 123:37–46. [DOI] [PubMed] [Google Scholar]
- Ear J, Huang H, Wilson T, Tehrani Z, Lindgren A, Sung V, Laadem A, Daniel TO, Chopra R, Lin S. 2015. RAP-011 improves erythropoiesis in zebrafish model of Diamond-Blackfan anemia through antagonizing lefty1. Blood 126(7):880–890. [DOI] [PubMed] [Google Scholar]
- Feng CW, Wen ZH, Huang SY, Hung HC, Chen CH, Yang SN, Chen NF, Wang HM, Hsiao CD, Chen WF. 2014. Effects of 6-hydroxydopamine exposure on motor activity and biochemical expression in zebrafish (Danio rerio) larvae. Zebrafish 11(3):227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes Y, Rampersad M, Gerlai R. 2015. Impairment of social behaviour persists two years after embryonic alcohol exposure in zebrafish: A model of fetal alcohol spectrum disorders. Behav Brain Res 292:102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Force A, Lynch M, Pickett FB, Amores A, Yan YL, Postlethwait J. 1999. Preservation of duplicate genes by complementary, degenerative mutations. Genetics 151(4):1531–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester AM, Grabher C, McBride ER, Boyd ER, Vigerstad MH, Edgar A, Kai FB, Da’as SI, Payne E, Look AT, Berman JN. 2011. NUP98-HOXA9-transgenic zebrafish develop a myeloproliferative neoplasm and provide new insight into mechanisms of myeloid leukaemogenesis. Br J Haematol 155(2):167–181. [DOI] [PubMed] [Google Scholar]
- Gabor KA, Goody MF, Mowel WK, Breitbach ME, Gratacap RL, Witten PE, Kim CH. 2014. Influenza A virus infection in zebrafish recapitulates mammalian infection and sensitivity to anti-influenza drug treatment. Dis Model Mech 7(11):1227–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Bu Y, Wu Q, Wang X, Chang N, Lei L, Chen S, Liu D, Zhu X, Hu K, Xiong JW. 2015. Mecp2 regulates neural cell differentiation by suppressing the Id1-Her2/Hes5 axis in zebrafish. J Cell Sci 128(12):2340–2350. [DOI] [PubMed] [Google Scholar]
- Granato M, van Eeden FJ, Schach U, Trowe T, Brand M, Furutani-Seiki M, Haffter P, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Odenthal J, Nüsslein-Volhard C (1996). Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Development 123:399–413. [DOI] [PubMed] [Google Scholar]
- Grunwald DJ, Streisinger G. 1992. Induction of recessive lethal and specific locus mutations in the zebrafish with ethyl nitrosourea. Genet Res 59:103–116. [DOI] [PubMed] [Google Scholar]
- Haendel MA, Vasilevsky N, Brush M, Hochheiser HS, Jacobsen J, Oellrich A, Mungall CJ, Washington N, Köhler S, Lewis SE, Robinson PN, Smedley D. 2015. Disease insights through cross-species phenotype comparisons. Mamm Genome 26(9–10):548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffter P, Granato M, Brand M, Mullins MC, Hammerschmidt M, Kane DA, Odenthal J, van Eeden FJ, Jiang YJ, Heisenberg CP, Kelsh RN, Furutani-Seiki M, Vogelsang E, Beuchle D, Schach U, Fabian C, Nüsslein-Volhard C. 1996. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development 123:1–36. [DOI] [PubMed] [Google Scholar]
- Horstick EJ, Linsley JW, Dowling JJ, Hauser MA, McDonald KK, Ashley-Koch A, Saint-Amant L, Satish A, Cui WW, Zhou W, Sprague SM, Stamm DS, Powell CM, Speer MC, Franzini-Armstrong C, Hirata H, Kuwada JY. 2013. Stac3 is a component of the excitation-contraction coupling machinery and mutated in Native American myopathy. Nat Commun 4:1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe DG, Bradford YM, Conlin T, Eagle AE, Fashena D, Frazer K, Knight J, Mani P, Martin R, Moxon SA, Paddock H, Pich C, Ramachandran S, Ruef BJ, Ruzicka L, Schaper K, Shao X, Singer A, Sprunger B, Van Slyke CE, Westerfield M. 2013. ZFIN, the Zebrafish Model Organism Database: increased support for mutants and transgenics. Nucleic Acids Res 41(Database issue):D854–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, McLaren S, Sealy I, Caccamo M, Churcher C, Scott C, Barrett JC, Koch R, Rauch GJ, White S, Chow W, Kilian B, Quintais LT, Guerra-Assunção JA, Zhou Y, Gu Y, Yen J, Vogel JH, Eyre T, Redmond S, Banerjee R, Chi J, Fu B, Langley E, Maguire SF, Laird GK, Lloyd D, Kenyon E, Donaldson S, Sehra H, Almeida-King J, Loveland J, Trevanion S, Jones M, Quail M, Willey D, Hunt A, Burton J, Sims S, McLay K, Plumb B, Davis J, Clee C, Oliver K, Clark R, Riddle C, Eliott D, Threadgold G, Harden G, Ware D, Mortimer B, Kerry G, Heath P, Phillimore B, Tracey A, Corby N, Dunn M, Johnson C, Wood J, Clark S, Pelan S, Griffiths G, Smith M, Glithero R, Howden P, Barker N, Stevens C, Harley J, Holt K, Panagiotidis G, Lovell J, Beasley H, Henderson C, Gordon D, Auger K, Wright D, Collins J, Raisen C, Dyer L, Leung K, Robertson L, Ambridge K, Leongamornlert D, McGuire S, Gilderthorp R, Griffiths C, Manthravadi D, Nichol S, Barker G, Whitehead S, Kay M, Brown J, Murnane C, Gray E, Humphries M, Sycamore N, Barker D, Saunders D, Wallis J, Babbage A, Hammond S, Mashreghi-Mohammadi M, Barr L, Martin S, Wray P, Ellington A, Matthews N, Ellwood M, Woodmansey R, Clark G, Cooper J, Tromans A, Grafham D, Skuce C, Pandian R, Andrews R, Harrison E, Kimberley A, Garnett J, Fosker N, Hall R, Garner P, Kelly D, Bird C, Palmer S, Gehring I, Berger A, Dooley CM, Ersan-Ürün Z, Eser C, Geiger H, Geisler M, Karotki L, Kirn A, Konantz J, Konantz M, Oberländer M, Rudolph-Geiger S, Teucke M, Osoegawa K, Zhu B, Rapp A, Widaa S, Langford C, Yang F, Carter NP, Harrow J, Ning Z, Herrero J, Searle SM, Enright A, Geisler R, Plasterk RH, Lee C, Westerfield M, de Jong PJ, Zon LI, Postlethwait JH, Nüsslein-Volhard C, Hubbard TJ, Crollius HR, Rogers J, Stemple DL. 2013. The zebrafish reference genome sequence and its relationship to the human genome. Nature 496(7446):498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruscha A, Schmid B. 2015. Generation of Zebrafish Models by CRISPR/Cas9 Genome Editing. Methods Mol Biol 1254:341–350. [DOI] [PubMed] [Google Scholar]
- Huang P, Xiao A, Zhou M, Zhu Z, Lin S, Zhang B. 2011. Heritable gene targeting in zebrafish using customized TALENs. Nat. Biotechnol. 29(8):699–700. [DOI] [PubMed] [Google Scholar]
- Huang P, Xiao A, Tong X, Lin S, Zhang B. 2016. Targeted mutagenesis in zebrafish by TALENs. Methods Mol Biol 1338:191–206. [DOI] [PubMed] [Google Scholar]
- Hwang WY, Fu Y., Reyon D, Maeder ML, Tsai SQ, Sander JD, Peterson RT, Yeh JR, Joung JK. 2013. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol 31(3):227–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang WY, Fu Y, Reyon D, Gonzales AP, Joung JK, Yeh JR. 2015. Targeted mutagenesis in zebrafish using CRISPR RNA-guided nucleases. Methods Mol Biol 1311: 317–334. [DOI] [PubMed] [Google Scholar]
- Jia Q, Zhang Q, Zhang Z, Wang Y, Zhang W, Zhou Y, Wan Y, Cheng T, Zhu X, Fang X, Yuan W, Jia H. 2013. Transcriptome analysis of the zebrafish model of diamond-blackfan anemia from RPS19 deficiency via p53-dependent and -independent pathways. PLoS One 8(8):e71782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettleborough RN, Busch-Nentwich EM, Harvey SA, Dooley CM, de Bruijn E, van Eeden F, Sealy I, White RJ, Herd C, Nijman IJ, Fényes F, Mehroke S, Scahill C, Gibbons R, Wali N, Carruthers S, Hall A, Yen J, Cuppen E, Stemple DL. 2013. A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature 496(7446):494–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam CS, Korzh V, Strähle U. 2005. Zebrafish embryos are susceptible to the dopaminergic neurotoxin MPTP. Eur J Neurosci 21(6):1758–1762. [DOI] [PubMed] [Google Scholar]
- Leong IU, Lai D, Lan CC, Johnson R, Love DR, Johnson R, Love DR. 2011. Targeted mutagenesis of zebrafish: Use of zinc finger nucleases. Birth Defects Res C Embryo Today 93(3):249–255. [DOI] [PubMed] [Google Scholar]
- Li JL, Zhou J, Chen ZH, Guo SY, Li CQ, Zhao WM. 2015. Bioactive C21 steroidal glycosides from the roots of cynanchum otophyllum that suppress the seizure-like locomotor activity of zebrafish caused by pentylenetetrazole. J Nat Prod 78(7):1548–1555. [DOI] [PubMed] [Google Scholar]
- Li M, Arner A. 2015. Immobilization of dystrophin and laminin α2-chain deficient zebrafish larvae in vivo prevents the development of muscular dystrophy. PLoS One 10:e0139483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieschke GJ, Currie PD. 2007. Animal models of human disease: Zebrafish swim into view. Nat Rev Genet 8(5):353–367. [DOI] [PubMed] [Google Scholar]
- Liu H, Chen S, Huang K, Kim J, Mo H, Iovine R, Gendre J, Pascal P, Li Q, Sun Y, Dong Z, Arkin M, Guo S, Huang B. 2016. A high-content larval zebrafish brain imaging method for small molecule drug discovery. PLoS One 11(10):e0164645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu JW, Liao CY, Yang WY, Lin YM, Jin SL, Wang HD, Yuh CH. 2014. Overexpression of endothelin 1 triggers hepatocarcinogenesis in zebrafish and promotes cell proliferation and migration through the AKT pathway. PLoS One 9(1):e85318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyne R, Sullivan J, Butano D, Contrino S, Heimbach J, Hu F, Kalderimis A, Lyne M, Smith RN, Štěpán R, Balakrishnan R, Binkley G, Harris T, Karra K, Moxon SA, Motenko H, Neuhauser S, Ruzicka L, Cherry M, Richardson J, Stein L, Westerfield M, Worthey E, Micklem G. 2015. Cross-organism analysis using InterMine. Genesis 53(8):547–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma AC, Chen Y, Blackburn PR, Ekker SC. 2016. TALEN-mediated mutagenesis and genome editing. Methods Mol Biol 1451:17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Schartl M. 1999. Gene and genome duplications in vertebrates: The one-to-four (-to-eight in fish) rule and the evolution of novel gene functions. Curr Opin Cell Biol 11:699–704. [DOI] [PubMed] [Google Scholar]
- Montalbano G, Mania M, Guerrera MC, Abbate F, Laurà R, Navarra M, Vega JA, Ciriaco E, Germanà A. 2015. Morphological differences in adipose tissue and changes in BDNF/Trkb expression in brain and gut of a diet induced obese zebrafish model. Ann Anat 204:36–44. [DOI] [PubMed] [Google Scholar]
- Mullins MC, Hammerschmidt M, Haffter P, Nüsslein-Volhard C. 1994. Large-scale mutagenesis in the zebrafish: in search of genes controlling development in a vertebrate. Curr Biol 4:189–202 [DOI] [PubMed] [Google Scholar]
- Mungall CJ, Washington NL, Nguyen-Xuan J, Condit C, Smedley D, Köhler S, Groza T, Shefchek K, Hochheiser H, Robinson PN, Lewis SE, Haendel MA. 2015. Use of model organism and disease databases to support matchmaking for human disease gene discovery. Hum Mutat 36(10):979–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidharan P, Sarmah S, Marrs JA. 2014. Zebrafish retinal defects induced by ethanol exposure are rescued by retinoic acid and folic acid supplement. Alcohol 49(2):149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. 2000. Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet 26:216–220. [DOI] [PubMed] [Google Scholar]
- Noël ES, Momenah TS, Al-Dagriri K, Al-Suwaid A, Al-Shahrani S, Jiang H, Willekers S, Oostveen YY, Chocron S, Postma AV, Bhuiyan ZA, Bakkers J. 2015. A zebrafish loss-of-function model for human CFAP53 mutations reveals its specific role in laterality organ function. Hum Mutat 37(2):194–200. [DOI] [PubMed] [Google Scholar]
- Oka T, Nishimura Y, Zang L, Hirano M, Shimada Y, Wang Z, Umemoto N, Kuroyanagi J, Nishimura N, Tanaka T. 2010. Diet-induced obesity in zebrafish shares common pathophysiological pathways with mammalian obesity. BMC Physiol 10:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panula P, Sallinen V, Sundvik M, Kolehmainen J, Torkko V, Tiittula A, Moshnyakov M, Podlasz P. 2006. Modulatory neurotransmitter systems and behavior: Towards zebrafish models of neurodegenerative diseases. Zebrafish 3(2):235–247. [DOI] [PubMed] [Google Scholar]
- Pappalardo-Carter DL, Balaraman S, Sathyan P, Carter ES, Chen WJ, Miranda RC. 2013. Suppression and epigenetic regulation of MiR-9 contributes to ethanol teratology: Evidence from zebrafish and murine fetal neural stem cell models. Alcoholism Clin Exp Res 37(10):1657–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parng C, Roy NM, Ton C, Lin Y, McGrath P. 2007. Neurotoxicity assessment using zebrafish. J Pharmacol Toxicol Methods 55(1):103–112. [DOI] [PubMed] [Google Scholar]
- Phillips JB, Westerfield M. 2014. Zebrafish models in translational research: tipping the scales toward advancements in human health. Dis Model Mech 7(7):739–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pienaar IS, Götz J, Feany MB. 2010. Parkinson’s disease: Insights from non-traditional model organisms. Prog Neurobiol 92(4):558–571. [DOI] [PubMed] [Google Scholar]
- Postlethwait JH. 2006. The zebrafish genome: A review and msx gene case study. Genome Dyn 2:183–197. [DOI] [PubMed] [Google Scholar]
- Radev Z, Hermel JM, Elipot Y, Bretaud S, Arnould S, Duchateau P, Ruggiero F, Joly JS, Sohm F. 2015. A TALEN-exon skipping design for a bethlem myopathy model in zebrafish. PLoS One 10:e0133986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridges S, Heaton WL, Joshi D, Choi H, Eiring A, Batchelor L, Choudhry P, Manos EJ, Sofla H, Sanati A, Welborn S, Agarwal A, Spangrude GJ, Miles RR, Cox JE, Frazer JK, Deininger M, Balan K, Sigman M, Müschen M, Perova T, Johnson R, Montpellier B, Guidos CJ, Jones DA, Trede NS. 2012. Zebrafish screen identifies novel compound with selective toxicity against leukemia. Blood 119(24):5621–5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzicka L, Bradford YM, Frazer K, Howe DG, Paddock H, Ramachandran S, Singer A, Toro S, Van Slyke CE, Eagle AE, Fashena D, Kalita P, Knight J, Mani P, Martin R, Moxon SA, Pich C, Schaper K, Shao X, Westerfield M. 2015. ZFIN, the Zebrafish Model Organism Database: Updates and new directions. Genesis 53(8):498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander JD, Yeh JR, Peterson RT, Joung JK. 2011. a. Engineering zinc finger nucleases for targeted mutagenesis of zebrafish. Meth Cell Biol 104:51–58. [DOI] [PubMed] [Google Scholar]
- Sander JD, Cade L, Khayter C, Reyon D, Peterson RT, Joung JK, Yeh JR. 2011. b. Targeted gene disruption in somatic zebrafish cells using engineered TALENs. Nat Biotechnol 29(8): 697–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarius T, Shipley J, Brewer D, Stratton MR, Cooper CS. 2010. A census of amplified and overexpressed human cancer genes. Nat Rev Cancer 10:59–64. [DOI] [PubMed] [Google Scholar]
- Schriml LM, Mitraka E. 2015. The Disease Ontology: Fostering interoperability between biological and clinical human disease-related data. Mamm Genome 26:584–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shastry BS. 1995. Overexpression of genes in health and sickness. A bird’s eye view. Comp Biochem Physiol B Biochem Mol Biol 112:1–13. [DOI] [PubMed] [Google Scholar]
- Siebel AM, Menezes FP, Capiotti KM, Kist LW, Schaefer ID, Frantz JZ, Bogo MR, Da Silva RS, Bonan CD. 2015. Role of adenosine signaling on pentylenetetrazole-induced seizures in zebrafish. Zebrafish 12(2):127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solnica-Krezel L, Schier AF, Driever W. 1994. Efficient recovery of ENU-induced mutations from the zebrafish germline. Genetics 136:1401–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridevi JP, Anantaraju HS, Kulkarni P, Yogeeswari P, Sriram D. 2014. Optimization and validation of Mycobacterium marinum-induced adult zebrafish model for evaluation of oral anti-tuberculosis drugs. Int J Mycobacteriol 3:259–267. [DOI] [PubMed] [Google Scholar]
- Stamm DS, Powell CM, Stajich JM, Zismann VL, Stephan DA, Chesnut B, Aylsworth AS, Kahler SG, Deak KL, Gilbert JR, Speer MC. 2008. Novel congenital myopathy locus identified in Native American Indians at 12q13.13-14.1. Neurology 71(22):1764–1769. [DOI] [PubMed] [Google Scholar]
- Streisinger G, Walker C, Dower N, Knauber D, Singer F. 1981. Production of clones of homozygous diploid zebra fish (Brachydanio rerio). Nature 291(5813):293–296. [DOI] [PubMed] [Google Scholar]
- Taylor J, Braasch I, Frickey T, Meyer A, Van De Peer Y. 2003. Genome duplication, a trait shared by 22,000 species of ray-finned fish. Genome Res 13:382–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng Y, Xie X, Walker S, Saxena M, Kozlowski DJ, Mumm JS, Cowell JK. 2011. Loss of zebrafish lgi1b leads to hydrocephalus and sensitization to pentylenetetrazol induced seizure-like behavior. PLoS One 6(9):e24596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji N, Ninov N, Delawary M, Osman S, Roh AS, Gut P, Stainier DY. 2014. Whole organism high content screening identifies stimulators of pancreatic beta-cell proliferation. PLoS One 9(8):e104112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney GK, Lu J, Gildea D, Huang H, Pei W, Yang Z, Huang SC, Schoenfeld DS, Pho N, Casero D, Hirase T, Mosbrook-Davis DM, Zhang S, Jao LE, Zhang B, Woods IG, Zimmerman S, Schier AF, Wolfsberg T, Pellegrini M, Burgess SM, Lin S. 2013. A large-scale zebrafish gene knockout resource for the genome-wide study of gene function. Genome Res 23(4):727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y, Zhang Q, Zhang Z, Song B, Wang X, Zhang Y, Jia Q, Cheng T, Zhu X, Leung AY, Yuan W, Jia H, Fang X. 2016. Transcriptome analysis reveals a ribosome constituents disorder involved in the RPL5 downregulated zebrafish model of Diamond-Blackfan anemia. BMC Med Genomics 9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. 2007. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio), Ed 5 Eugene: University of Oregon Press. [Google Scholar]
- White RM. 2015. Cross-species oncogenomics using zebrafish models of cancer. Curr Opin Genet Dev 30:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CH, Hong CC. 2016. Zebrafish small molecule screens: Taking the phenotypic plunge. Comput Struct Biotechnol J 14:350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen J, White RM, Wedge DC, Van Loo P, de Ridder J, Capper A, Richardson J, Jones D, Raine K, Watson IR, Wu CJ, Cheng J, Martincorena I, Nik-Zainal S, Mudie L, Moreau Y, Marshall J, Ramakrishna M, Tarpey P, Shlien A, Whitmore I, Gamble S, Latimer C, Langdon E, Kaufman C, Dovey M, Taylor A, Menzies A, McLaren S, O Meara S, Butler A, Teague J, Lister J, Chin L, Campbell P, Adams DJ, Zon LI, Patton EE, Stemple DL, Futreal PA. 2013. The genetic heterogeneity and mutational burden of engineered melanomas in zebrafish models. Genome Biol 14(10):R113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zada D, Tovin A, Lerer-Goldshtein T, Vatine GD, Appelbaum L. 2014. Altered behavioral performance and live imaging of circuit-specific neural deficiencies in a zebrafish model for psychomotor retardation. PLoS Genet 10:e1004615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Anderson A, Cole GJ. 2015. Analysis of crosstalk between retinoic acid and sonic hedgehog pathways following ethanol exposure in embryonic zebrafish. Birth Defects Res Part A Clin Mol Teratol 103(12):1046–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Frazier JM, Chen H, Liu Y, Lee JA, Cole GJ. 2014. Molecular and morphological changes in zebrafish following transient ethanol exposure during defined developmental stages. Neurotoxicol Teratol 44:70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Gu W, Hurles ME, Lupski JR. 2009. Copy number variation in human health, disease, and evolution. Annu Rev Genomics Hum Genet 10:451–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ear J, Yang Z, Morimoto K, Zhang B, Lin S. 2014. Defects of protein production in erythroid cells revealed in a zebrafish Diamond-Blackfan anemia model for mutation in RPS19. Cell Death Dis 5:e1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Jia H, Zhang Q, Wan Y, Song B, Jia Q, Liu H, Zhu X, Fang X. 2014. Transcriptome analysis of Rpl11-deficient zebrafish model of Diamond-Blackfan Anemia. Genom Data 2:173–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Jia H, Zhang Q, Wan Y, Zhou Y, Jia Q, Zhang W, Yuan W, Cheng T, Zhu X, Fang X. 2013. Assessment of hematopoietic failure due to Rpl11 deficiency in a zebrafish model of Diamond-Blackfan anemia by deep sequencing. BMC Genomics 14:896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZJ, Cheang LC, Wang MW, Li GH, Chu IK, Lin ZX, Lee SM. 2012. Ethanolic extract of fructus alpinia oxyphylla protects against 6-hydroxydopamine-induced damage of PC12 cells in vitro and dopaminergic neurons in zebrafish. Cell Mol Neurobiol 32(1):27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]