Synopsis
As organisms age the environment fluctuates, exerting differential selection across ontogeny. In particular, highly seasonal environments expose life stages to often drastically different thermal environments. This developmental variation is particularly striking in organisms with complex life cycles, wherein life history stages also exhibit distinct morphologies, physiologies, and behaviors. Genes acting pleiotropically on thermal responses may produce genetic correlations across ontogeny, constraining the independent evolution of each life stage to their respective thermal environments. To investigate whether developmental genetic correlations constrain the evolution thermal hardiness of the fly Drosophila melanogaster, we applied quantitative genetic analyses to cold hardiness measured in both larvae and adults from isogenic lines of the Drosophila Genetic Reference Panel (DGRP), using survival at stressful low temperatures as the phenotypic metric. Using full genome resequencing data for the DGRP, we also implemented genome-wide association (GWA) analysis using Bayesian Sparse Linear Mixed Models (BSLMMs) to estimate associations between naturally segregating variation and cold hardiness for both larvae and adults. Quantitative genetic analyses revealed no significant genetic correlation for cold hardiness between life stages, suggesting complete genetic decoupling of thermal hardiness across the metamorphic boundary. Both quantitative genetic and GWA analyses suggested that polygenic variation underlies cold hardiness in both stages, and that associated loci largely affected one stage or the other, but not both. However, reciprocal enrichment tests and correlations between BSLMM parameters for each life stage support some shared physiological mechanisms that may reflect common cellular thermal response pathways. Overall, these results suggest no developmental genetic constraints on cold hardiness across metamorphosis in D. melanogaster, an important consideration in evolutionary models of responses to changing climates. Genetic correlations for environmental sensitivity across ontogeny remains largely unexplored in other organisms, thus assessing the generality of genetic decoupling will require further quantitative or population genetic analysis in additional species.
Introduction
As organisms progress through development, life history stages experience different environmental conditions that exert differential natural selection across the life cycle (Krebs and Loeschcke 1995; Ragland and Kingsolver 2008; Woods 2013). Moreover, different life stages typically exhibit different relationships between environmental factors and performance or fitness (Coyne et al. 1983). This is particularly important for organisms with complex life cycles, wherein different life history stages often inhabit completely different ecological niches (Kingsolver et al. 2011). Ecophysiological models for, range limits or responses to changing climates are increasingly incorporating such stage-specific performance, increasing accuracy over models assuming a single, “one size fits all” environment versus performance relationship (Levy et al. 2015; Sinclair et al. 2016). Thus, considering the environmental sensitivity of the entire life cycle in the appropriate seasonal context is critical for accurate models and predictions. Yet, empirical studies typically focus on the environmental sensitivity of adult, reproductive stages, whereas juvenile life history stages have received comparatively little attention (Bowler and Terblanche 2008).
In particular, we have comparatively few data on the genetic architecture of juvenile relative to adult environmental sensitivity. The genetics of thermal sensitivity and thermal “hardiness” (ability to maintain fitness despite acute exposure to temperature extremes) has been especially well described in insects, but studies have focused largely on the adult stage. Numerous surveys of geographically variable populations have identified loci varying among populations or species experiencing different environmental temperatures (Bettencourt et al. 2002; Garbuz et al. 2003; Sgrò et al. 2008; Dunning et al. 2014; Lancaster et al. 2016), and some of these loci have been directly linked to thermal hardiness (Rako et al. 2007). In addition, QTL and association mapping have identified candidate loci that may segregate in natural populations (Norry et al. 2004; Morgan and Mackay 2006; Duun Rohde et al. 2016). Studies that have explored thermal hardiness in both adult and juvenile stages suggest that loci affecting adult thermal hardiness may have no effect on juvenile thermal hardiness, that is, there is no developmental pleiotropy. For example, laboratory selection on thermal performance in one life history stage (e.g., adults) leads to no correlated response to selection in other life stages (Tucić 1979; Loeschcke and Krebs 1996; Gilchrist et al. 1997; Dierks et al. 2012). Similarly, comparisons of hardiness among populations and species do not support cross-stage genetic correlations (Hercus et al. 2000).
The above studies all suggest that thermal hardiness may evolve independently across life stages, though they do not reveal whether this lack of correlation is underlain by different mechanisms of hardiness in different stages. To bridge this knowledge gap, we applied a combined quantitative genetic and population genomic study to estimate (1) the genetic correlation between larval and adult cold hardiness in a temperate, natural population of Drosophila melanogaster, and (2) genome-wide associations (GWAs) between naturally segregating variation and thermal hardiness for both larvae and adults. This study is the first genomic analysis of juvenile insect thermal hardiness and provides a genomic and gene-specific perspective on the remarkable genetic independence of thermal traits across a complex life cycle. We performed all analyses using the Drosophila Genetic Reference Panel (DGRP), a collection of fully re-sequenced isogenic lines of D. melanogaster derived from a natural population in Raleigh, NC (Mackay et al. 2012; Huang et al. 2014). Substantial genetic variation exists among the DGRP lines that capture a substantial portion of naturally occurring variation. In nature, larvae and adults experience different thermal environments because of their different habitats (feeding inside rotting fruits vs. foraging and mate finding through flight). Moreover, D. melanogaster overwinters primarily in the adult stage (Saunders et al. 1989), so adults of the overwintering generation in temperate environments experience more chronic and acute exposure to low temperatures compared with larvae. We focused on 139 DGRP lines, measuring larval and adult cold hardiness in each line and estimating broad sense heritabilities, a genetic correlation, and genotype-to-phenotype associations for each trait. Our results suggest (1) a complete quantitative genetic decoupling of thermal hardiness across metamorphosis, (2) that as observed in adults, larval thermal hardiness is polygenic, influenced by many loci of moderate or small effect, and (3) that individual genes with clear effects on thermal hardiness in one stage may have no detectable, pleiotropic effect on another stage. Despite apparent evolutionary independence, we found evidence for shared physiological mechanisms of cold hardiness across ontogeny. We discuss these results with respect to common cellular responses to temperature, and unique aspects of cold survival in juvenile versus adult stages.
Methods
DGRP lines
The DGRP are 205 isogenic lines derived from a natural population in Raleigh, NC (dgrp2.gnets.ncsu.edu) (Mackay et al. 2012; Huang et al. 2014). This design maintains naturally segregating variation as genetic variance among isogenic lines. The among-line genetic variance does not capture dominance variance because each line is homozygous (Falconer and Mackay 1996). Some of these lines achieve low survival and reproductive output in the laboratory, thus we selected 139 vigorous lines maintained under standard rearing conditions at Kansas State University for several years that exhibit no obvious fitness problems under standard rearing conditions.
Line rearing and maintenance
All experimental animals were reared on cornmeal, molasses, and yeast media with propionic acid and benzoic acid added as anti-fungal and anti-bacterial agents. To encourage egg laying, vials were sprinkled with dry active yeast. All rearing was performed under a 12 h/12 h light–dark cycle at 25 °C. Experimental flies from 139 isogenic DGRP lines were reared in vials started from five males and five females. The parents were sorted under light CO2 anesthesia and then placed on fresh media for oviposition. Every 24 h, the parents were transferred to fresh media over 6 days. The first set of replicate vials (Day 1) was discarded to limit the effects of anesthesia on egg-laying. Progeny (experimental individuals) were collected from the vials on Days 2–6, creating replicates 1–5. Parents were discarded 24 h after their final transfer on Day 6. A maximum of five serial replicates for each line and each life stage was created using this design. Experimental individuals were collected for larval and adult assays at predetermined developmental time points (see below).
Survival assays
Larval assays were performed at 120 h (5 days) post-oviposition, using third instar feeding larvae that were extracted from replicate vials using a 20% w/v sucrose solution following the protocol of Nöthiger (1970). The solution was poured into the vial and the media was disturbed to allow larvae to float to the top of the liquid. Larvae were then extracted from the solution with a soft brush, briefly washed in ddH2O, and then transferred to a paper towel to dry for approximately 1 min. After drying, 20 larvae were transferred, using a soft brush, to a vial with fresh media. To determine if this extraction method had any effect on larval survival, we tested cold shock survival for a subset of 10 lines (minimum of four replicates per line) extracted mechanically (using just a soft brush) versus extraction using the sucrose solution (Supplementary File S1). We found that survival of flies extracted using the sucrose solution was slightly higher (6.7%; P = 0.017) than that of larvae extracted mechanically. This result may be due to mechanical injury of forcibly removing the larvae from the food. Additionally, there was no significant interaction between line and extraction method (P = 0.10), suggesting that the differences among lines are unlikely to be caused by different responses to the sucrose solution. After successful extraction, we poked holes into the media to facilitate larval burrowing and feeding. Experimental vials were then immersed in a recirculating bath (ECO RE 2025, Lauda Corporation) containing a 50/50 mixture of distilled water and propylene glycol held at the test temperature for a 1 h exposure time. Thermocouples placed in empty food vials confirmed that the food did not freeze during any of the experimental exposures. Empty vials with adults reached the test temperature only slightly faster than vials containing food and larvae (∼3 min faster averaged across temperatures) representing a difference of only 5% of the 1 h exposure. After exposure, vials were removed from the bath and returned to standard rearing conditions. After 9 days (216 h), larval survivorship was assessed as the proportion of successfully eclosed individuals (Bing et al. 2012); proportion of successful pupation yielded qualitatively similar results.
For the adult assays, adults (12 days post-oviposition) were extracted from replicate vials under light CO2 anesthesia. A total of 10 male and 10 female adults per replicate and per line were then sorted, sexed, and transferred to fresh media. Males and females were kept in separate vials and placed under standard rearing conditions for 5 more days to recover from any effects of anesthesia. During recovery, adults were transferred to new food after 3 days to avoid mortality associated with old, rotting food. After the 5 days had elapsed, 5–7 day post-eclosion (408 h post-oviposition) adults were transferred to empty vials with sexes kept separate and exposed to the test temperatures as described above. After exposure, the adults were transferred to fresh media (sexes separated) and placed under standard rearing conditions for 24 h to recover. The proportion that had survived was assessed by counting the number of adults capable of coordinated movement and flight (Kelty and Lee 2001; Overgaard et al. 2005; Colinet and Hoffmann 2012).
Experimental design
Organisms generally exhibit a roughly logistic relationship between survival and temperature at extreme (stressful) temperatures (Lee and Denlinger 1991). We wished to initially describe this curve over a set of temperatures for a subset of lines in order to determine whether survival at a single temperature near the LD50 (temperature producing 50% survival) could accurately predict the shape of the full curve. For larvae, a subset of 28 lines was randomly selected and for adults, a subset of 36 lines was randomly selected. Larval survival curves were generated from survival data after 1 h exposures at −8°C, −7°C, −6°C, −5°C, −4°C, −3°C, and −2°C (Supplementary File S2-A). Adult survival curves were generated after exposures at −7°C, −6°C, −5°C, −4°C, −3°C, −2°C, and −1°C. (Supplementary File S2-B). These temperature ranges were previously determined to span the range from 0% to 100% survival in most tested lines. For each life stage, survival at each of the seven test temperatures was regressed against cumulative survival summed across all test temperatures. Based on these results, we selected −5°C as the appropriate, single test temperature exhibiting the strongest, highly significant correlation with cumulative survival across temperatures (Adults: R2 = 0.91; Larvae, R2 = 0.95; Supplementary File S3).
Subsequently, we tested survival at −5°C for all 139 lines using the assays described above. The factorial experimental design was as follows: 139 lines × 2 life stages × 5 replicate vials per life stage. Each vial contained 20 larvae and 20 adults (10 males, 10 females), respectively.
Quantitative genetics
Broad sense heritability (H2) of cold hardiness in larvae and adults was estimated as the proportion of variance among lines divided by the total variance (sum of the variance among and within lines) (Lynch and Walsh 1998). Variance components were estimated using generalized linear mixed models (GLMMs) with a logit link function implemented in lme4 (glmer function; Bates et al. 2015) in R Core Team (2017). Line and replicate were modeled as random effects. Sex was only determined for adults. Thus, in the adult model we included a fixed effect of sex (estimates within sexes were qualitatively similar). We estimated 95% confidence intervals (CIs) on the heritability estimates by implementing 500 bootstrap replicates (resampling with replacement from the 139 DGRP lines) of the GLMMs and calculating H2 for each replicate. We estimated the genetic correlation between larval and adult hardiness as:
where is the covariance between adult and larval cold hardiness and and are the variance components among lines for adults and larvae, respectively. These values were estimated using GLMMs with logit link functions implemented in glmer, as above. The and values were estimated as the among line variance from the separate adult and larval models used for the heritability calculations above. We estimated as the among-line variance from a GLMM of the combined larval and adult data including a fixed effect of developmental stage and random effects of line, line-by-stage interaction, and replicate nested within line (Harbison et al. 2013). As above, we estimated CIs using 500 bootstrap replicates.
Genome-wide associations
We performed a GWA analysis to estimate the effects of alleles at individual loci and across the genome on both larval and adult cold hardiness. The analysis included single nucleotide polymorphisms (SNP) and insertions and/or deletions (INDEL) genotypes at 6,149,882 chromosomal positions across the genome identified by Mackay et al. (2012) and Huang et al. (2014) using full genome resequencing. We applied a Bayesian Sparse Linear Mixed Model (BSLMM) separately to raw larval data (survival) and to the residuals of a generalized linear model with logit link function fitting adult survival data and including a fixed effect of sex (to remove the effects of sex prior to GWA; implemented in glmer (lme4 in R). The BSLMM model accounts for linkage among markers and relatedness among individuals (Zhou et al. 2013). We implemented the BSLMM in gemma (Zhou et al. 2013), which uses a Bayesian, Markov Chain Monte Carlo approach to fit the following model:
where is the vector of genetic marker effects, the matrix of genotypes, the vector of random effects, and the vector of errors. The parameter can be decomposed into , where is a vector modeling the infinitesimal genetic effects of each locus, whereas models the additional effects of moderate or large-effect loci. The BSLMM includes an additional hyperparameter gamma which estimates the inclusion probability of each locus, that is, the probability that each locus has a non-zero effect on the phenotype while accounting for all effects at all other loci. Manhattan plots of inclusion probabilities for SNPs higher than 0.00275 in adults and higher than 0.00375 in larvae (top 0.01% of SNPs) were produced in R using the package, qqman (Turner 2014). Finally, gemma also estimates the posterior distribution for the total percent variance explained (PVE) by genotype (across all loci), and the number of loci with measurable effects on the genotype. For sets of loci identified as “candidates” based on values and inclusion probabilities, we applied enrichment analysis using DAVID (Huang et al. 2009a, 2009b) to test for overrepresentation of functional categories among loci that mapped to gene models predicted in the D. melanogaster genome release version 5.36.
We also characterized the correlated effects of loci, focusing on genome-wide effects. We estimated the spearman (non-parametric) correlation between individual locus effect size estimates for larval and adult cold hardiness produced by the BSLMM above, including only loci with non-zero inclusion probabilities. We performed these correlations between the larval and adult values (measureable effects) and values (infinitesimal effects). We also performed this same correlation for values of all loci regardless of inclusion probability. Inclusion probabilities modify the way that the coefficients are modeled, but coefficients contribute to PVE regardless of inclusion probability. Although linkage disequilibrium (LD) decays over short genomic distances in D. melanogaster (Long et al. 1998), LD among loci will still violate the assumption of independence for the spearman rank test. To mitigate non-independence, we estimated the 95% CI of the Spearman correlation from 1000 bootstrap replicates randomly resampling loci across the genome. And, we performed two-tailed permutation tests to assess statistical significance, estimating the correlation for 1000 data sets where larval values were randomly shuffled relative to adult values.
Results
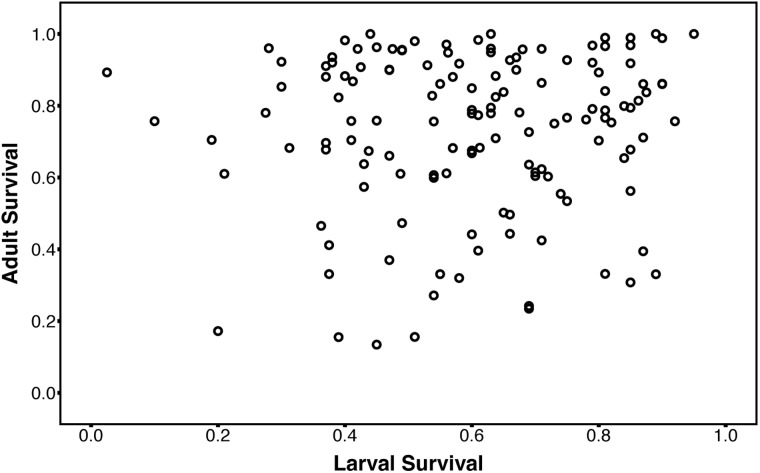
Mean survival after a 1-h exposure to −5°C was significantly higher in adults compared with larvae (proportion surviving ± standard error; Adults = 0.73 ± 0.052; Larvae = 0.60 ± 0.064; P < 0.001, Supplementary File S4). Likewise, broad sense heritability was higher in adults (model including sex as a fixed effect) compared with larvae (adult H2 = 0.44, 95% CI 0.38–0.50; larvae H2 = 0.22, 95% CI 0.16–0.27), though both were significantly greater than zero (1000 permutations produced no values as extreme as the point estimates). Treating sexes separately, estimates of adult broad sense H2 for males and females were 0.48 (95% CI 0.41–0.54) and 0.57 (95% CI 0.50–0.63), respectively. There was no significant genetic correlation between survival at −5°C in larvae and adults (Fig. 1; n = 139, rg = 0.085, 95% CI −0.053 to –0.18, P = 0.12).
Fig. 1.
Scatterplot of average larval survival (proportion surviving) versus average adult survival after a 1 h exposure to − 5 °C. Each circle represents 1 of the 139 DGRP lines. Both the phenotypic (r2 = 0.00525, P = 0.3966) and genetic correlation (rg = 0.085, P = 0.12) are not significantly different from zero.
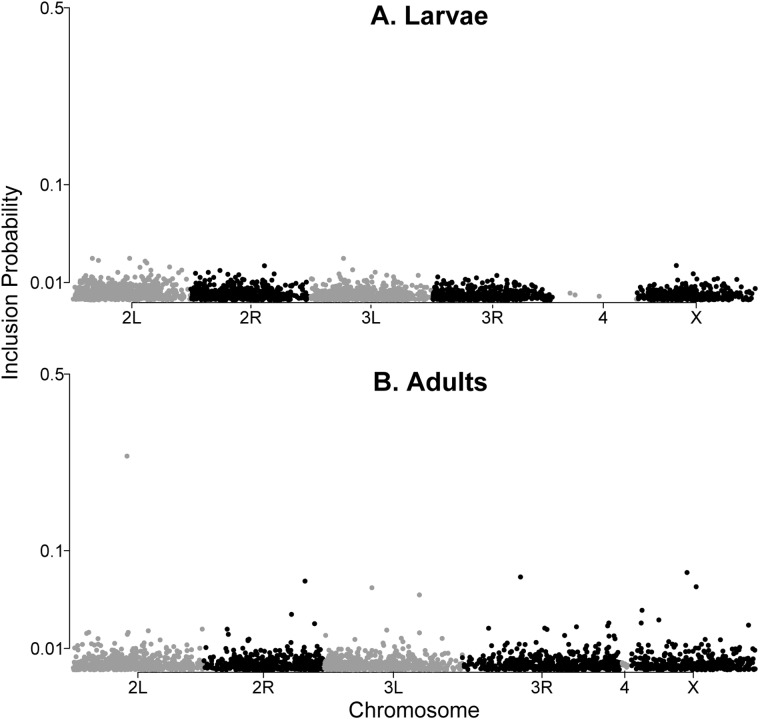
From the posterior inclusion probabilities estimated in the BSLMMs, we inferred that 1–146 (95% CI, median = 57) and 0–234 (95% CI, median = 90) loci had measurable effects on larval and adult cold hardiness, respectively. However, most inclusion probabilities for individual SNPs were low (only 1 and 13 loci with gamma >0.025 in larvae and adults, respectively), whereas point estimates of PVE were relatively high (median = 41, 95% CI 3–91, larvae; median = 53, 95% CI 3–99, adults). The top 100 loci associated with each trait are listed in Supplementary File S5 along with the per-locus estimates of (genetic effect) and (inclusion probability), ranked according to effect size weighted by inclusion probability (). As with any statistical cutoff, the choice of 100 loci is somewhat arbitrary, but falls within the range of the number of loci with measurable effects as inferred from the BSLMM. Most loci had the highest inclusion probabilities in either larvae or adults, but not both (Fig. 2). Only 1803 loci had non-zero inclusion probabilities for both larval and adult models, no more than would be expected by chance alone (P = 0.38, Fisher’s exact test). However, the “top 100” lists for adults and larvae shared three gene ids in common, significantly more than would be expected by chance alone (P = 0.014, Fisher’s exact test). To further explore this overlap, we tested whether gene models harboring SNPs with high inclusion probabilities in adults also tended to harbor SNPs with high inclusion probabilities in larvae. Reciprocal enrichment tests supported this hypothesis; inclusion probabilities in larvae for the top 100 gene models with the highest inclusion probabilities in adults were substantially greater than expected by chance alone (none of 10,000 random permutations generated a median inclusion probability as high as the observed value; the reciprocal test yielded the same result; Supplementary File S6). The “top 100” list for adults was enriched for several functional categories including Uniprot keyword “alternate splicing” and the INTERPRO domain “low-density lipoprotein receptor” (Supplementary File S7), while there was no significant enrichment of any category in the “top 100” list for larvae.
Fig. 2.
Manhattan plots of inclusion probabilities for the top 0.01% of SNPs (highest inclusion probabilities) in larvae (A) and adults (B). Inclusion probabilities (the probability that each locus has a non-zero effect on the phenotype while accounting for all effects at all other loci) for SNPs are plotted against genomic position. The y-axis (inclusion probability) is square-root scaled to compress the range.
Correlation analysis of the estimated larval and adult BSLMM parameters revealed limited evidence for genetic correlation between larval and adult cold hardiness. In particular, there was a non-significant correlation between the values, or large genetic effects for the larval and adult models (median r = −0.004, 95% CI − 0.005 to –0.002, P = 0.15). However, there was a slight but highly significant positive correlation between the values, or infinitesimal effects for the larval and adult models. This was true for only the subset of loci with non-zero inclusion probabilities in either model (median r = 0.090, 95% CI 0.082–0.094) and for all loci (median r = 0.059, 95% CI 0.058–0.061). In both cases no value as extreme as the point estimate (median) were produced in 1000 permutations.
Discussion
Genetic constraint and evolution across ontogeny
Both larval and adult cold hardiness were highly heritable under laboratory conditions, suggesting that the Raleigh, NC population of origin for the DGRP harbors substantial segregating genetic variation for cold hardiness visible to natural selection. Heritability estimates in this study very likely overestimate heritability in the field because (1) they are broad-sense estimates that include non-additive effects, and (2) environmental variance is greater in the field (Fisher 2000; Merilä and Sheldon 2000; McCleery et al. 2004). However, inter-population variation in thermal hardiness has been demonstrated many times in D. melanogaster and is likely underlain by heritable, genetic variation within populations (Krebs and Feder 1997; Hoffmann et al. 2002, 2003). The results of the BSLMMs of genetic association further suggest that both larval and adult cold hardiness behave largely as polygenic traits, with generally low inclusion probabilities and moderate effect sizes for associated genetic variants.
Despite apparently ample genetic variation for larval and adult cold hardiness, we found no evidence for a genetic correlation between the two traits. The estimate for rg was low, and not significantly different from zero. These results align with the results of previous studies that have inferred a lack of correlation through measurements of correlated response to selection (Tucić 1979; Loeschcke and Krebs 1996; Gilchrist et al. 1997; Dierks et al. 2012), and implies that different sets of segregating variants must influence thermal hardiness at different life stages. Here we have directly tested this hypothesis, and indeed, there was little overlap between the sets of variants most strongly associated with each trait, and no correlation in effect sizes for loci with non-zero inclusion probabilities in the BSLMMs. Tucić (1979) found similar results on the whole-chromosome level in the sense that chromosomal variants accounted for different amounts of cold hardiness variation in different stages. But, the most proximal life stages (e.g., consecutive larval instars) did tend to share similar patterns of chromosome-hardiness associations, suggesting that at a small enough developmental increment, genetic correlations may exist.
The above results suggest that cold hardiness is relatively free to evolve independently across ontogeny, an important consideration for evolutionary models of seasonality and response to changing climates (Kingsolver et al. 2011). In contrast, other studies have identified ontogenetic correlations that more severely constrain the evolution of, for example, growth trajectories (Kirkpatrick et al. 1990; Kingsolver et al. 2001) and sexual dimorphism (Chippindale et al. 2001; Badyaev 2002). Induced physiological responses or composite metrics of performance (e.g., growth rate) may generally be less developmentally constrained compared with traits related to morphology and body size or condition (Kingsolver et al. 2015). Different life stages often occur in distinct seasons, and without genetic constraint natural selection should simultaneously optimize (i.e., select for highest fitness) thermal performance/fitness in each life stage (Ragland and Kingsolver 2008). There are no studies that clearly illustrate differential selection and evolutionary responses of different life stages in nature. But, there is at least one appropriate data set that measures evolutionary differences in both larval and adult fly cold hardiness across 16 Drosophilid species spanning millions of years of evolution and a variety of temperate- and tropical-origin species (Mitchell et al. 2013). Mitchell et al. (2013) focused on the effect of acclimation, which appears to be phylogenetically unconstrained. We applied an additional phylogenetically independent contrast to their published cold hardiness values (see Supplementary File S8) and found a relatively high correlation between larval and adult cold hardiness after correcting for phylogeny (r = 0.70, P = 0.004). Given the evidence for lack of correlation within populations of D. melanogaster, this result is consistent with two hypotheses: (1) evolved differences among species do not reflect standing genetic variation segregating in natural populations, and (2) correlational natural selection favors either increased or decreased hardiness across ontogeny (i.e., in all life stages). The first would be difficult to test, though there is evidence that standing variation contributes to physiological differences among species (Barrett and Schluter 2008). The second hypothesis is plausible because most, if not all of the species included in Mitchell et al. (2013) are likely multivoltine with overlapping generations, which will tend to decrease heterogeneity of the thermal environment across life history stages. Further phylogenetic analysis and comparisons of closely related sister species may further elucidate the relative roles of correlational selection and standing genetic variation in shaping thermal hardiness across ontogeny.
Stage-specificity and common genetic effects
Although estimates of genetic correlations and the bulk of genetic variants with measurable effects identified via GWA support the capacity for independent evolution in larvae and adults, there was also a background signal of shared causal genetic variants. In particular, there was a small but significant correlation between the values, or infinitesimal effects of the BSLMM in larvae and adults. In addition, there was small, but statistically significant overlap between sets of genes harboring the top 100 SNP variants most strongly associated with larval and adult cold hardiness. Finally, gene models harboring SNPs with the highest inclusion probabilities (higher values represent greater statistical confidence in a SNP effect) in adults also exhibited significantly higher inclusion probabilities in larvae than would be expected by chance alone (and vice versa). We performed an additional enrichment test to identify functional categories (GO and KEGG) that might be associated with cold hardiness across stages, but no individual categories were notably enriched or statistically significant (Supplementary Material S9).
Evidence for common allelic effects across development is not unexpected, given that cold injury is often mediated at the cellular level (Teets and Denlinger 2013), which should have some common effects in larvae and adults. For example, modifications to cell membrane ratios of fatty acid types (Bennett and Lee 1997; Tomcala et al. 2006; Michaud and Denlinger 2007) and phospholipids (Overgaard et al. 2005; Koštál 2010) are associated with maintenance of membrane fluidity in cold tolerant insects. Increased expression of heat shock proteins, aquaporins, and antioxidant enzymes also associate with various metrics of cold hardiness in insects (Yocum 2001; Colinet et al. 2010; Philip and Lee 2010). And recently, the maintenance of ion homeostasis during cold exposure has been linked to cold hardiness (MacMillan et al. 2015a, 2015b).
The observation that most genetic variants had measureable effects on only one life stage may be largely because of the very different tissue composition and developmental status of adults versus larvae, a hypothesis suggested by Bowler and Terblanche (2008). The ability of adults to survive cold shocks in our assays should be mainly due to resistance to cold injury, whereas larval survival to pupation is influenced by resistance to cold injury and subsequent, successful development to pupation. Indeed, many of the genes harboring variants most strongly associated with larval cold hardiness have known functions in developmental processes (functional annotations for the top 100 list in Supplementary File S5a). Yet, variants affecting cellular cold injury resistance through the mechanisms described above ought to affect both larval and adult cold hardiness. So, why are so few of these variants present in the DGRP, which captures segregating variation from the source population in Raleigh, NC?
Our observations of mainly stage-specific genetic effects are consistent with an alternative hypothesis explaining the lack of genetic correlation between larval and adult thermal hardiness; natural selection against variants with stage-specific effects may be weaker than natural selection against variants that affect hardiness across the life cycle. All else being equal, a variant exposed to more bouts of selection (e.g., a variant that affects cold hardiness across ontogeny) will have greater effects on fitness than a variant exposed to fewer bouts of selection (e.g., a stage-specific variant). Thus, selection should more efficiently cull “cross-ontogeny” causal variants compared with stage-specific variants. This hypothesis assumes that the average strength of selection is relatively homogenous across ontogeny, which would be more likely to apply to multivoltine species with overlapping generations such as D. melanogaster. In principal, laboratory selection on DGRP-derived base populations coupled with pooled resequencing of populations pre- and post-selection could be applied to test this hypothesis.
Genetics of cold hardiness
Both the quantitative genetic and GWA analyses suggest that cold hardiness as measured in this study is a complex, polygenic trait in both adults and larvae. Several studies have identified QTL with moderate to major effect on thermal hardiness phenotypes in D. melanogaster (Norry et al. 2004; Morgan and Mackay 2006; Norry et al. 2007; Rako et al. 2007; Rand et al. 2010). However, most of these studies relied to some extent on comparisons of laboratory selected lines or a small set of inbred lines. In contrast, variants in the DGRP reflect genetic variation segregating in natural populations (Mackay et al. 2012) and variation maintained in the relatively large number of lines is less influenced by chance sampling and fixation compared with studies that compare many fewer selected or laboratory lines. Moreover, previous studies compare lines or populations likely to have fixed alternate QTL alleles in different thermal environments, whereas large effect QTLs are probably transient in a panmictic population with large effective population size such as would be expected for D. melanogaster (Agrawal et al. 2001). Thus, the polygenic nature of cold hardiness in this study is probably a reasonable representation of variation available to respond to selection in a given geographic population.
No variants exceeded a 3% inclusion probability for association with larval cold hardiness, but there was a single apparent outlier SNP with a 28% inclusion probability for adult cold hardiness (Fig. 2A). This SNP occurs in the gene shawl, a voltage-gated potassium channel. The shawl gene also harbored a different SNP variant whose non-zero inclusion probability, while lower (4%), fell within the upper 90th percentile of inclusion probabilities for all variants in the larval analysis. Ion balance is increasingly recognized as an important determinant of thermal hardiness and performance in insects (MacMillan et al. 2015a, 2015b). However, most previous work has focused on ion balance in the gut and Malpighian tubules, whereas shawl appears to be expressed primarily in anatomical regions associated with the central nervous system (see modEncode anatomy expression data in Flybase) and like other voltage-gated potassium channels, may function primarily in action potential propagation. In relation to other genetic studies of cold hardiness we also note a SNP with a moderate inclusion probability (3.4%, but in the top 10 for weighted effect size; Supplementary File S5a) for adult cold hardiness within the gene shaggy, a likely ortholog of human glycogen synthase 3 kinase that has previously been associated with an adult cold hardiness QTL in D. melanogaster (Rand et al. 2010).
Whether the variants identified in this study contribute to cold adaptations in nature depends on either the ecological relevance of the test conditions, or the genetic correlation between the measured phenotype and a field-relevant phenotype such as survival of a transient cold snap. We did not apply thermal ramping, which tends to better represent ecologically relevant conditions, and ramping rates in nature may vary substantially among life history stages that exploit different microclimates (Sinclair 2001; Terblanche et al. 2011). The slightly slower ramping rate for larvae compared with adults (see “Methods” section) in this study was relatively minor, but likely does reflect real difference in nature, for example, the internal temperature of a partially liquefied, rotting apple versus ambient air temperatures.
Beyond better characterization of the phenotypic targets of natural selection, identification of associations between variants and environmental conditions among populations could also strengthen the case for the adaptive value of a given variant. Of two pooled sequencing studies examining genetic variation among high and low latitude populations of D. melanogaster in North America (Fabian et al. 2012) and Australia (Kolaczkowski et al. 2011), the North American study identified both shawl and shaggy as highly differentiated among populations from Florida and Pennsylvania (as quantified using outlier analysis of Fst values; see Supplementary Table S5 in Fabian et al. (2012)).
Summary
Our results suggest that there is no detectable genetic correlation for thermal hardiness between larvae and adults. Furthermore, allelic variants associated with cold hardiness mostly affected the phenotype in only one of the two life stages. However, there was a weak signal of common allelic effects across both life stages that may be consistent with cellular mechanisms universally affected by cold exposure across development. Overall, the results suggest that a single genome can provide independent evolutionary solutions to selection pressures that may vary across development, allowing for thermal strategies to evolve freely in distinct life stages. This is an important consideration as the world’s climate, and particularly seasonality, rapidly changes. Evolutionary responses in thermal hardiness may be relatively unconstrained by cross-stage, or developmental pleiotropy in species with similar life histories to D. melanogaster, though further empirical work will be necessary to evaluate the generality of this genetic architecture.
Supplementary Material
Acknowledgments
We would like to thank Mary Post, Colin Bailey, Adam Schieferecke, Saadia Cleve, Oshadhi Athukorala Arachchige, Ashley Wedin, Nicholas Heter, Mariah Brown, and Jackson Alex for their help with this project. Stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were used in this study.
Funding
This work was supported by NSF IOS 1700773 to GJR and the Kansas State Agricultural Research and Extension.
Supplementary data
Supplementary data are available at ICB online.
References
- Agrawal AF, Brodie ED 3rd, Rieseberg LH.. 2001. Possible consequences of genes of major effect: transient changes in the G-matrix In: Hendry AP, Kinnison MT, editors. Microevolution rate, pattern, process. Dordrecht, Netherlands: Kluwer Academic Publishers; p. 33–43. [Google Scholar]
- Badyaev AV. 2002. Growing apart: an ontogenetic perspective on the evolution of sexual size dimorphism. Trends Ecol Evol 17:369–78. [Google Scholar]
- Barrett RDH, Schluter D.. 2008. Adaptation from standing genetic variation. Trends Ecol Evol 23:38–44. [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S.. 2015. Fitting linear mixed-effects models using lme4. J Stat Softw 67. Available at: http://www.jstatsoft.org/v67/i01/ (accessed 17/09/16). [Google Scholar]
- Bennett V, Lee R.. 1997. Modeling seasonal changes in intracellular freeze-tolerance of fat body cells of the gall fly Eurosta solidaginis (Diptera, Tephritidae). J Exp Biol 200:185–92. [DOI] [PubMed] [Google Scholar]
- Bettencourt BR, Kim I, Hoffmann AA, Feder ME.. 2002. Response to natural and laboratory selection at the Drosophila hsp70 genes. Evolution 56:1796–801. [DOI] [PubMed] [Google Scholar]
- Bing X, Zhang J, Sinclair BJ.. 2012. A comparison of Frost expression among species and life stages of Drosophila: Frost expression in Drosophila. Insect Mol Biol 21:31–9. [DOI] [PubMed] [Google Scholar]
- Bowler K, Terblanche JS.. 2008. Insect thermal tolerance: what is the role of ontogeny, ageing and senescence? Biol Rev Camb Philos Soc 83:339–55. [DOI] [PubMed] [Google Scholar]
- Chippindale AK, Gibson JR, Rice WR.. 2001. Negative genetic correlation for adult fitness between sexes reveals ontogenetic conflict in Drosophila. Proc Natl Acad Sci U S A 98:1671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colinet H, Hoffmann AA.. 2012. Comparing phenotypic effects and molecular correlates of developmental, gradual and rapid cold acclimation responses in Drosophila melanogaster: cold acclimation and stress response. Funct Ecol 26:84–93. [Google Scholar]
- Colinet H, Lee SF, Hoffmann A.. 2010. Knocking down expression of Hsp22 and Hsp23 by RNA interference affects recovery from chill coma in Drosophila melanogaster. J Exp Biol 213:4146–50. [DOI] [PubMed] [Google Scholar]
- Coyne JA, Bundgaard J, Prout T.. 1983. Geographic variation of tolerance to environmental stress in Drosophila pseudoobscura. Am Nat 122:474–88. [Google Scholar]
- Dierks A, Kölzow N, Franke K, Fischer K.. 2012. Does selection on increased cold tolerance in the adult stage confer resistance throughout development? J Evol Biol 25:1650–7. [DOI] [PubMed] [Google Scholar]
- Dunning LT, Dennis AB, Sinclair BJ, Newcomb RD, Buckley TR.. 2014. Divergent transcriptional responses to low temperature among populations of alpine and lowland species of New Zealand stick insects (Micrarchus). Mol Ecol 23:2712–26. [DOI] [PubMed] [Google Scholar]
- Duun Rohde P, Krag K, Loeschcke V, Overgaard J, Sørensen P, Nygaard Kristensen T, 2016. A quantitative genomic approach for analysis of fitness and stress related traits in a Drosophila melanogaster model population. Int J Genomics 2016:e2157494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian DK, Kapun M, Nolte V, Kofler R, Schmidt PS, Schlotterer C, Flatt T.. 2012. Genome-wide patterns of latitudinal differentiation among populations of Drosophila melanogaster from North America. Mol Ecol 21:4748–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer DS, Mackay TFC.. 1996. Introduction to quantitative genetics. 4th ed Harlow: Pearson. [Google Scholar]
- Fisher RA. 2000. The genetical theory of natural selection. 1st ed Oxford: Oxford University Press. [Google Scholar]
- Garbuz D, Evgenev MB, Feder ME, Zatsepina OG.. 2003. Evolution of thermotolerance and the heat-shock response: evidence from inter/intraspecific comparison and interspecific hybridization in the virilis species group of Drosophila. I. Thermal phenotype. J Exp Biol 206:2399–408. [DOI] [PubMed] [Google Scholar]
- Gilchrist GW, Huey RB, Partridge L.. 1997. Thermal sensitivity of Drosophila melanogaster: evolutionary responses of adults and eggs to laboratory natural selection at different temperatures. Physiol Zool 70:403–14. [DOI] [PubMed] [Google Scholar]
- Harbison ST, McCoy LJ, Mackay TF.. 2013. Genome-wide association study of sleep in Drosophila melanogaster. BMC Genomics 14:281.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercus MJ, Berrigan D, Blows MW, Magiafoglou A, Hoffmann AA.. 2000. Resistance to temperature extremes between and within life cycle stages in Drosophila serrata, D. birchii and their hybrids: intraspecific and interspecific comparisons. Biol J Linn Soc 71:403–16. [Google Scholar]
- Hoffmann AA, Anderson A, Hallas R.. 2002. Opposing clines for high and low temperature resistance in Drosophila melanogaster. Ecol Lett 5:614–8. [Google Scholar]
- Hoffmann AA, Scott M, Partridge L, Hallas R.. 2003. Overwintering in Drosophila melanogaster: outdoor field cage experiments on clinical and laboratory selected populations help to elucidate traits under selection. J Evol Biol 16:614–23. [DOI] [PubMed] [Google Scholar]
- Huang DW, Sherman BT, Lempicki RA.. 2009a. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57. [DOI] [PubMed] [Google Scholar]
- Huang DW, Sherman BT, Lempicki RA.. 2009b. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Massouras A, Inoue Y, Peiffer J, Ràmia M, Tarone AM, Turlapati L, Zichner T, Zhu D, Lyman RF, et al. 2014. Natural variation in genome architecture among 205 Drosophila melanogaster genetic reference panel lines. Genome Res 24:1193–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelty JD, Lee RE.. 2001. Rapid cold-hardening of Drosophila melanogaster (Diptera: Drosophiladae) during ecologically based thermoperiodic cycles. J Exp Biol 204:1659–66. [DOI] [PubMed] [Google Scholar]
- Kingsolver JG, Gomulkiewicz R, Carter PA.. 2001. Variation, selection and evolution of function-valued traits. Genetica 112:87–104. [PubMed] [Google Scholar]
- Kingsolver JG, Woods HA, Buckley LB, Potter KA, MacLean HJ, Higgins JK.. 2011. Complex life cycles and the responses of insects to climate change. Integr Comp Biol 51:719–32. [DOI] [PubMed] [Google Scholar]
- Kingsolver JG, Heckman N, Zhang J, Carter PA, Knies JL, Stinchcombe JR, Meyer K.. 2015. Genetic variation, simplicity, and evolutionary constraints for function-valued traits. Am Nat 185:E166–81. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick M, Lofsvold D, Bulmer M.. 1990. Analysis of the inheritance, selection and evolution of growth trajectories. Genetics 124:979–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaczkowski B, Kern AD, Holloway AK, Begun DJ.. 2011. Genomic differentiation between temperate and tropical Australian populations of Drosophila melanogaster. Genetics 187:245–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koštál V. 2010. Cell structural modifications in insects at low temperatures In: Denlinger DL, Lee RE Jr, editors. Low temperature biology of insects. Cambridge: Cambridge University Press; p. 116–40. [Google Scholar]
- Krebs RA, Feder ME.. 1997. Natural variation in the expression of the heat-shock protein HSP70 in a population of Drosophila melanogaster and its correlation with tolerance of ecologically relevant thermal stress. Evolution 51:173.. [DOI] [PubMed] [Google Scholar]
- Krebs RA, Loeschcke V.. 1995. Resistance to thermal stress in preadult Drosophila buzzatii: variation among populations and changes in relative resistance across life stages. Biol J Linn Soc 56:517–31. [Google Scholar]
- Lancaster LT, Dudaniec RY, Chauhan P, Wellenreuther M, Svensson EI, Hansson B.. 2016. Gene expression under thermal stress varies across a geographical range expansion front. Mol Ecol 25:1141–56. [DOI] [PubMed] [Google Scholar]
- Lee RE Jr, Denlinger DL.. 1991. Insects at Low Temperature. New York and London: Chapman and Hall. [Google Scholar]
- Levy O, Buckley LB, Keitt TH, Smith CD, Boateng KO, Kumar DS, Angilletta MJ.. 2015. Resolving the life cycle alters expected impacts of climate change. Proc R Soc Lond B Biol Sci 282:20150837.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeschcke V, Krebs RA.. 1996. Selection for heat-shock resistance in larval and in adult Drosophila buzzatii: comparing direct and indirect responses. Evolution 50:2354–9. [DOI] [PubMed] [Google Scholar]
- Long AD, Lyman RF, Langley CH, Mackay TFC.. 1998. Two sites in the Delta gene region contribute to naturally occurring variation in bristle number in Drosophila melanogaster. Genetics 149:999–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M, Walsh B.. 1998. Genetics and analysis of quantitative traits. 1st ed Sunderland (MA: ): Sinauer Associates. [Google Scholar]
- Mackay TFC, Richards S, Stone EA, Barbadilla A, Ayroles JF, Zhu D, Casillas S, Han Y, Magwire MM, Cridland JM, et al. 2012. The Drosophila melanogaster genetic reference panel. Nature 482:173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan HA, Andersen JL, Davies SA, Overgaard J.. 2015a. The capacity to maintain ion and water homeostasis underlies interspecific variation in Drosophila cold tolerance. Sci Rep 5:18607.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan HA, Ferguson LV, Nicolai A, Donini A, Staples JF, Sinclair BJ.. 2015b. Parallel ionoregulatory adjustments underlie phenotypic plasticity and evolution of Drosophila cold tolerance. J Exp Biol 218:423–32. [DOI] [PubMed] [Google Scholar]
- McCleery RH, Pettifor RA, Armbruster P, Meyer K, Sheldon BC, Perrins CM.. 2004. Components of variance underlying fitness in a natural population of the great tit Parus major. Am Nat 164:E62–72. [DOI] [PubMed] [Google Scholar]
- Merilä J, Sheldon BC.. 2000. Lifetime reproductive success and heritability in nature. Am Nat 155:301–10. [DOI] [PubMed] [Google Scholar]
- Michaud MR, Denlinger DL.. 2007. Shifts in the carbohydrate, polyol, and amino acid pools during rapid cold-hardening and diapause-associated cold-hardening in flesh flies (Sarcophaga crassipalpis): a metabolomic comparison. J Comp Physiol B 177:753–63. [DOI] [PubMed] [Google Scholar]
- Mitchell KA, Sinclair BJ, Terblanche JS.. 2013. Ontogenetic variation in cold tolerance plasticity in Drosophila: is the Bogert effect bogus? Die Naturwissenschaften 100:281–4. [DOI] [PubMed] [Google Scholar]
- Morgan TJ, Mackay TFC.. 2006. Quantitative trait loci for thermotolerance phenotypes in Drosophila melanogaster. Heredity 96:232–42. [DOI] [PubMed] [Google Scholar]
- Norry FM, Dahlgaard J, Loeschcke V.. 2004. Quantitative trait loci affecting knockdown resistance to high temperature in Drosophila melanogaster. Mol Ecol 13:3585–94. [DOI] [PubMed] [Google Scholar]
- Norry FM, Sambucetti P, Scannapieco AC, Gomez FH, Loeschcke V.. 2007. X-linked QTL for knockdown resistance to high temperature in Drosophila melanogaster. Insect Mol Biol 16:509–13. [DOI] [PubMed] [Google Scholar]
- Nöthiger R. 1970. Sucrose density separation: a method for collecting large numbers of Drosophila larvae. Dros Inf Serv 45:177. [Google Scholar]
- Overgaard J, Sørensen JG, Petersen SO, Loeschcke V, Holmstrup M.. 2005. Changes in membrane lipid composition following rapid cold hardening in Drosophila melanogaster. J Insect Physiol 51:1173–82. [DOI] [PubMed] [Google Scholar]
- Philip BN, Lee RE.. 2010. Changes in abundance of aquaporin-like proteins occurs concomitantly with seasonal acquisition of freeze tolerance in the goldenrod gall fly, Eurosta solidaginis. J Insect Physiol 56:679–85. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing (http://www.R-project.org/).
- Ragland GJ, Kingsolver JG.. 2008. Evolution of thermotolerance in seasonal environments: the effects of annual temperature variation and life-history timing in Wyeomyia smithii. Evolution 62:1345–57. [DOI] [PubMed] [Google Scholar]
- Rako L, Blacket MJ, McKechnie SW, Hoffmann AA.. 2007. Candidate genes and thermal phenotypes: identifying ecologically important genetic variation for thermotolerance in the Australian Drosophila melanogaster cline. Mol Ecol 16:2948–57. [DOI] [PubMed] [Google Scholar]
- Rand DM, Weinreich DM, Lerman D, Folk D, Gilchrist GW.. 2010. Three selections are better than one: clinical variation of thermal QTL from independent selection experiments in Drosophila. Evolution 64:2921–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders DS, Henrich VC, Gilbert LI.. 1989. Induction of diapause in Drosophila melanogaster: photoperiodic regulation and the impact of arrhythmic clock mutations on time measurement. Proc Natl Acad Sci U S A 86:3748–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgrò CM, Milton CC, Jensen LT, Frydenberg J, Loeschcke V, Batterham P, Hoffmann AA.. 2008. Nucleotide diversity in the Hsp90 gene in natural populations of Drosophila melanogaster from Australia. Insect Mol Biol 17:685–97. [DOI] [PubMed] [Google Scholar]
- Sinclair BJ. 2001. Field ecology of freeze tolerance: interannual variation in cooling rates, freeze-thaw and thermal stress in the microhabitat of the alpine cockroach Celatoblatta quinquemaculata. Oikos 93:286–93. [Google Scholar]
- Sinclair BJ, Marshall KE, Sewell MA, Levesque DL, Willett CS, Slotsbo S, Dong Y, Harley CDG, Marshall DJ, Helmuth BS, et al. 2016. Can we predict ectotherm responses to climate change using thermal performance curves and body temperatures? Ecol Lett 19:1372–85. [DOI] [PubMed] [Google Scholar]
- Teets NM, Denlinger DL.. 2013. Physiological mechanisms of seasonal and rapid cold-hardening in insects: seasonal and rapid cold-hardening in insects. Physiol Entomol 38:105–16. [Google Scholar]
- Terblanche JS, Hoffmann AA, Mitchell KA, Rako L, le Roux PC, Chown SL.. 2011. Ecologically relevant measures of tolerance to potentially lethal temperatures. J Exp Biol 214:3713–25. [DOI] [PubMed] [Google Scholar]
- Tomcala A, Tollarova M, Overgaard J, Simek P, Kostal V.. 2006. Seasonal acquisition of chill tolerance and restructuring of membrane glycerophospholipids in an overwintering insect: triggering by low temperature, desiccation and diapause progression. J Exp Biol 209:4102–14. [DOI] [PubMed] [Google Scholar]
- Tucić N. 1979. Genetic capacity for adaptation to cold resistance at different developmental stages of Drosophila melanogaster. Evolution 33:350–8. [DOI] [PubMed] [Google Scholar]
- Turner SD. 2014. qqman: an R package for visualizing GWAS results using Q-Q and manhattan plots. biorXiv (doi:10.1101/005165).
- Woods HA. 2013. Ontogenetic changes in the body temperature of an insect herbivore. Funct Ecol 27:1322–31. [Google Scholar]
- Yocum GD. 2001. Differential expression of two HSP70 transcripts in response to cold shock, thermoperiod, and adult diapause in the Colorado potato beetle. J Insect Physiol 47:1139–45. [DOI] [PubMed] [Google Scholar]
- Zhou X, Carbonetto P, Stephens M.. 2013. Polygenic modeling with Bayesian sparse linear mixed models. PLoS Genet 9:e1003264.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.