Synopsis
Sickness is typically characterized by fever, anorexia, cachexia, and reductions in social, pleasurable, and sexual behaviors. These responses can be displayed at varying intensities both within and among individuals, and the adaptive nature of sickness responses can be demonstrated by the context-dependent nature of their expression. The study of sickness has become an important area of investigation for researchers in a wide range of areas, including psychoneuroimmunology (PNI) and ecoimmunology (EI). The general goal of PNI is to identify key interactions among the nervous, endocrine and immune systems and behavior, and how disruptions in these processes might contribute to disease states. EI, in turn, has been established more recently within the perspectives of ecology and evolutionary biology, and is aimed more at understanding natural variation in immune function and sickness responses within a broadly integrative, organismal, and evolutionary context. The goal of this review is to examine the literature on sickness from both basic and biomedical perspectives within PNI and EI and to demonstrate how the integrative study of sickness behavior can serve as an integrating agent to connect ecological and translational approaches to the study of disease. By focusing on a set of specific exemplars, including the energetics of sickness, social context, and environmental influences on sickness, we hope to accomplish the larger goal of developing a common synthetic framework to understand sickness from multiple levels of analysis and varying perspectives across the fields of PNI and EI. By applying this integrative approach to sickness, we will be able to develop a more comprehensive view of sickness as a suite of adaptive responses rather than the simply deleterious consequences of illness.
Introduction
“And as for sickness, are we not almost tempted to ask whether we could get along without it?”
Friedrich Nietzsche
Sickness in vertebrates manifests itself in a wide range of responses, including fever (or hypothermia in some seasonally breeding mammals), lethargy, anorexia, anhedonia as well as decreases in social behavior, motivation, and libido (reviewed in Aubert 1999 and Ashley and Wingfield 2012). And as the quote above by the noted 19th century German philosopher Friedrich Nietzsche suggests, it is perhaps easiest to view most if not all symptoms of sickness as deleterious or debilitating responses, or at best the annoying and unnecessary byproducts of disease. In reality, however, sickness responses play an important adaptive functional role in facilitating defense against pathogens by conserving energy for use in other immune responses and by limiting parasites’ access to energy and nutrients. As such, sickness should be considered more broadly as one among many critical homeostatic mechanisms that have evolved within individuals of virtually all species to combat infections and return an animal to healthy state (Hart 1988; Adelman and Martin 2009).
It was the influential American physiologist Walter Cannon who in the early 1930s developed the concept we now know as “homeostasis.” Cannon’s idea of homeostasis was based largely on previous ideas proffered by French physiologist Claude Bernard who considered the body’s physiology a “milieu interior,” whose role is to integrate and respond to environmental perturbations (i.e., milieu exterior) (Bernard 1957). According to Cannon, “the organism which with the aid of increased adrenal secretion can best muster its energies, call forth sugar to supply the labouring muscles, lessen fatigue, and send blood to the parts essential in the run or the fight for life” (Cannon 1932). Cannon noted that the body is exquisitely adapted to respond to even the subtlest changes in the environment in order to maintain homeostasis. In fact, he eloquently referred to this precise balancing act as the “wisdom of the body” in his 1932 treatise by the same name (Cannon 1932).
This idea of “constancy through change,” and the notion that the body is “wise,” that is to say exquisitely prepared to handle environmental disruption with appropriate and adaptive responses, continues to be an extremely important concept in biology today. It is also a concept that has particular relevance to the study of sickness. For example, one of the hallmark symptoms displayed by a sick animal is a robust decrease, or even complete cessation, of food intake during the period of peak sickness. If one assumes that sickness responses require substantial energy (i.e., “feed a cold”), then these findings make little sense from an adaptive perspective. An initial solution to this apparent paradox was proffered in the 1960s and 1970s when Eugene Weinberg and others demonstrated the concept of iron withholding, whereby sick organisms avoid ingesting foods rich in iron (e.g., Weinberg 1974). Iron is an essential nutrient for many bacteria and parasites to grow and survive, and as such, avoiding iron is an adaptive response by an organism to prevent “feeding the pathogen” (i.e., “starve a fever”). Subsequent research has confirmed these findings more broadly. For example, infected mice who are force-fed to match the level of food intake seen in healthy mice are more likely to die from illness than those allowed to display sickness-induced anorexia (Murray and Murray 1979). In contrast, mice that are acutely starved demonstrate higher survival following bacterial infection than mice fed ad libitum (Wing and Young 1980). Similar findings have been reported for invertebrates as well (e.g., caterpillars, Manduca sexta; Adamo et al. 2007). Research has also demonstrated that macronutrients, in addition to micronutrients like iron, can affect the sickness response and thus the ability of an individual to clear an infection (reviewed in Ashley and Wingfield 2012). For example, dietary intake of protein is reduced in animals with experimentally induced sickness relative to fats or carbohydrates, presumably due to the relatively higher iron content in protein (Aubert et al. 1995). Further, recent studies have also suggested that the amount of dietary fat can also affect sickness responses in both vertebrates (Pohl et al. 2009, 2014) and invertebrates (Adamo et al. 2010).
One aspect of sickness behavior more often discussed in translational research and less so in basic science is decreased motivation, suggestive of a “depressed cognitive state” as thoroughly examined by Aubert and colleagues in a number of studies (reviewed in Aubert 1999). Building on the work of Neal Miller suggesting that sickness behaviors are motivational (Miller 1964), Aubert and colleagues investigated whether sickness behaviors in dams could be modulated according to ambient temperature. Their studies demonstrate that the expression of sickness behaviors may depend on the motivation to perform such behaviors, and therefore, its modulation across different environmental and social contexts seems highly probable (Aubert et al. 1997).
A series of elegant and groundbreaking studies by Matthew Kluger and colleagues demonstrated the adaptive value of a fever response (Kluger et al. 1975; Vaughn et al. 1974). His lab demonstrated that ectothermic lizards that are unable to mount a physiological fever response will voluntarily leave a previously thermoneutral position and move towards a warmer portion of a thermocline when injected with bacteria (or the bacterial mimetic lipopolysaccharide [LPS]), thus inducing “behavioral fever.” Sick lizards return to the original cooler location once sickness has passed. Further, bacterially-treated lizards prevented from inducing behavioral fever results in near complete mortality in these individuals. This response is by no means unique to “cold-blooded” animals. A similar phenomenon can be observed in endothermic mammals, with rabbits (Oryctolagus cuniculus) that were experimentally infected with live bacteria being more likely to die when treated with antipyretics, which inhibit the fever response, when compared with untreated controls (Kluger and Vaughn 1978). In fact, it is now well-established that fever plays multiple adaptive roles in facilitating the ability of a host to fight infection, enhancing both innate and acquired immune responses through temperature-dependent mechanisms and also via direct inhibition of bacterial proliferation by changing core body temperature above or below the pathogen’s optimal temperature for growth and replication (Kluger 1979, 1991; Ashley and Wingfield 2012; Carlton and Demas 2015a).
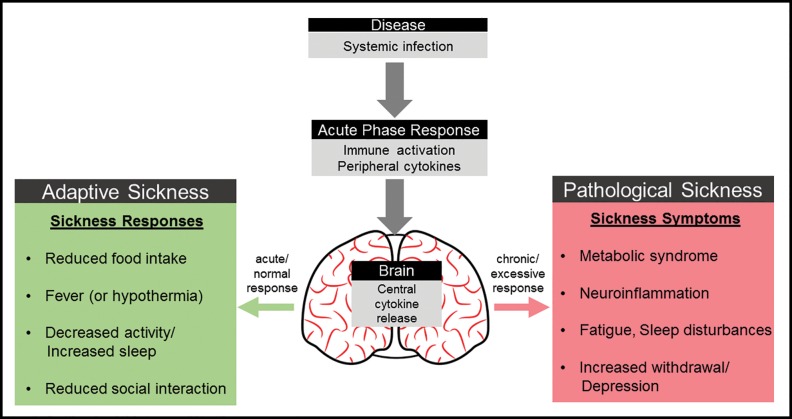
Based on the findings from these and similar studies, Benjamin Hart published a now classic paper (Hart 1988) re-framing the idea of sickness behavior from that of a collection of non-specific, maladaptive byproducts of disease to one of a coordinated set of adaptive responses that aid an organism to effectively fight infections. According to Hart, the behavioral patterns commonly seen in sick animals, including humans, can and should be viewed as a means of enhancing the acute phase and fever responses by conserving energy and thus potentiating the individual’s immune responses targeted towards fighting the infections (Hart 1988). Whereas we believe that his interpretation of sickness is largely correct, we do not deny the existence of a large and perhaps growing number of diseases that are characterized by excessive or prolonged immune responses (McEwen 1998). In fact, as Fig. 1 suggests, sickness responses are best characterized as a precise balance between optimal levels of sickness, which are highly adaptive, and excessive or chronic responses that can lead to pathological conditions. The central thesis of this paper is that by understanding both the adaptive and maladaptive aspects, the study of sickness has the potential to serve as an “integrating agent” to bridge basic and translational research within an ecologically relevant context. Despite this perspective, there has been an increasing emphasis on translational approaches to disease biology in the life sciences within the last several decades, which has led to a decreased appreciation of the adaptive aspects of sickness. In the remainder of this review, we provide several specific examples of sickness research where an appreciation of the basic (adaptive) and translational aspects of sickness have provided a more comprehensive understanding of both the “how” and “why” of sickness and may also contribute to the development of strategies to treat excessive or chronic sickness. Further, although we suggest that while sickness behaviors can in fact aid in recovery, they are not cost-free; organisms must mediate when and to what degree they elicit particular sickness behaviors to increase their chances of survival. Consideration of context (as depicted in Fig. 2), from both proximate and ultimate perspectives, will allow us to develop a common theoretical framework for understanding the mechanisms of sickness within a larger ecological and evolutionary context.
Fig. 1.
Effects of adaptive versus pathological sickness responses on behavior. In normal individuals who become transiently infected with disease, the systemic infection triggers an acute phase response (APR) and the release of peripheral cytokines, which leads to concomitant changes in brain cytokine production. Proinflammatory cytokines coordinate a wide range of adaptive behaviors (e.g., sickness behaviors) that help fight infection, including reduced feeding, fever (or hypothermia), decreased activity/increased sleep and reduced social interactions. When sickness responses become chronic or excessive, however, they can lead to pathological conditions, which may include metabolic syndrome, neuroinflammation, fatigue, and increases in withdrawal and depression. These symptoms of chronic sickness are consistent with symptoms typically associated with a range of neuropsychiatric and affective disorders. Figure modified from Bilbo and Schwarz (2012)
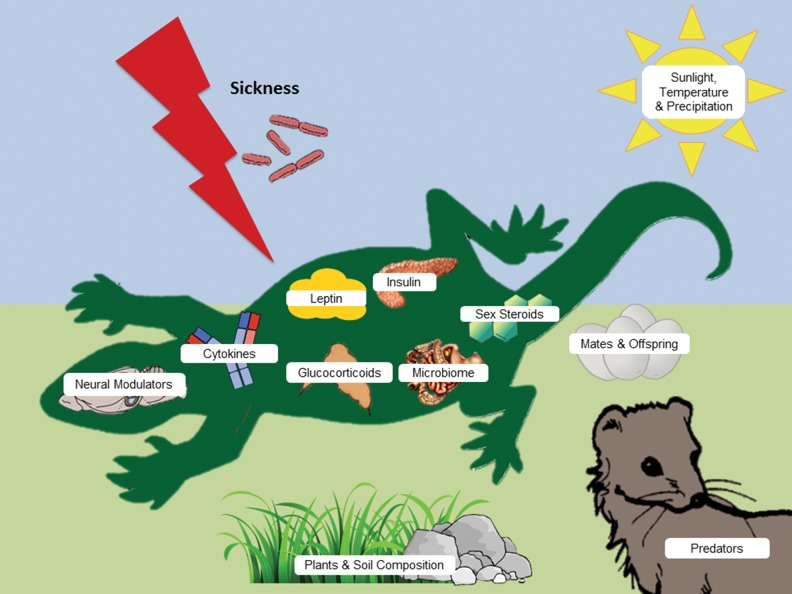
Fig. 2.
Graphical model representing the various internal physiological mechanisms and external factors regulating sickness behavior. Both psychoneuroimmunology (PNI) and ecoimmunology (EI) have provided unique yet complementary perspectives and methodologies to study sickness behavior, allowing researchers to develop a more comprehensive view of sickness as a suite of adaptive responses rather than deleterious consequences, though sickness behavior can be costly in and of itself. Sickness behavior (fever/hypothermia, anorexia, cachexia, reductions in social, pleasurable, and sexual behaviors) can be displayed at varying intensities, and the adaptive nature of sickness responses can be demonstrated by the context-dependent nature of their expression. Here, we present the varying internal physiological mechanisms regulating sickness behavior (e.g., leptin, glucocorticoids, insulin), alongside the environmental (e.g., daylight, temperature, soil composition) and social contexts (e.g., predators, mates, young) that modulate the expression of these behaviors to varying degrees as well. Individuals incorporate complex cues from many different abiotic and biotic factors to appropriately respond to immune challenge. The body must maintain a state of homeostasis by providing energy to the most essential systems in diverse energetic, social, and environmental contexts, and as researchers, we must integrate what we learn from all of these frameworks to understand the function and adaptive capacity of sickness behaviors.
Studying sickness
The study of sickness, and of immune function more broadly, has become an important area of investigation for researchers in a wide range of areas outside the traditional discipline of immunology. Since the early 1980s, psychoneuroimmunology (PNI) has strived to identify key interactions among the nervous, endocrine, and immune systems and behavior. In fact, the establishment of PNI as a field was based on a foundational study by Robert Ader and Nicholas Cohen which utilized a classical conditioning paradigm to demonstrate conditioned immunosuppression in response to a sickness causing agent (cyclophosphamide; Ader and Cohen 1975). More recently, the field of ecological immunology, or ecoimmunology (EI), has been established within the perspectives of ecology and evolutionary biology and aims to understand immune function within a broadly integrative, organismal context, typically from an ultimate, evolutionary perspective (Sheldon and Verhulst 1996; Demas and Nelson 2012). As suggested by Sheldon and Verhulst, it is often useful to consider an individual’s immune response as something subject to optimization in the presence of other likely competing demands to provide more comprehensive insights into mechanisms of life history trade-offs and parasite-mediated selection (Sheldon and Verhulst 1996). Both PNI and EI, with their unique yet complementary perspectives and methodologies, have much to offer to the study of sickness. In fact, by doing so, we will be able to develop a more comprehensive view of sickness as a suite of adaptive responses rather than deleterious consequences (although not denying the potential for disease).
Energetics of sickness
While sickness can be considered as a collection of adaptive physiological and behavioral changes, these changes need not be static. The magnitude and intensity of the underlying immune responses can and do vary largely depending on context. For example, diet-induced obese (DIO) male rats show a greater immune response (e.g., increased fever response and cytokine release) to exogenous LPS when compared to lean controls (Pohl et al. 2009), suggesting that increased body mass results in shifts in the immune response, with the potential to produce long-term damage to the immune system and health. Other studies support this idea that sickness, obesity, and metabolic syndrome are overtly linked and that the elevated inflammation seen in these individuals is maladaptive. Further work by Pohl and colleagues showed a strong association between a range of body masses and sickness behaviors, specifically, as weight increased, the severity and duration of sickness behaviors increased as well (Pohl et al. 2014).
Although the DIO model suggests using sickness as means of measuring disease, these responses can, in contrast, be seen as adaptive measures, especially in ecologically relevant models. For example, in several seasonally breeding animals (e.g., Siberian hamsters [Phodopus sungorus], song sparrows [Melospiza melodia morphna], white-crowned sparrows [Zonotrichia leucophrys gambelii]), changes in body mass are strongly associated with changes in an individual’s display of sickness behaviors in response to immune challenge (Bilbo et al. 2002; Owen-Ashley and Wingfield 2006; Owen-Ashley et al. 2006; Carlton and Demas 2014). Specifically, Siberian hamsters attenuate sickness responses in short “winter-like” days, when reproductive physiology is regressed and body mass is decreased, presumably to better optimize energetically costly immune responses with reduced energy stores (due to lower body mass) thus increasing their chances of survival. (Bilbo et al. 2002; Baillie and Prendergast 2008). In contrast, sparrows attenuate sickness responses in long “spring-like” days during the breeding season, when body mass is lower (Owen-Ashley and Wingfield 2006). Importantly, when comparing across studies, there is no consistent season in which these seasonal breeders display a particular response to sickness, providing evidence that photoperiod and reproductive cues may not be the only signals modulating these changes. Instead, these studies support the idea that sickness responses are attenuated in the season in which animals have the lowest body mass and the magnitude of sickness response correlates with individual weight. This idea is similar to that of DIO rats, which have increased immune responses compared to lean controls potentially because these obese rats have the available energy to invest in such a response. Although these studies demonstrate that sickness responses are more intense in certain seasons, patterns suggest that animals may modulate their sickness response based on their energy limitations. Specifically, the magnitude of a sickness display is constrained by a minimum body mass that an animal can reach before it no longer has the resources to recover and survive (Ashley et al. 2012).
Ecological studies like those above provide evidence that the sickness response seen in more biomedically-relevant models, such as DIO rats, may actually be functioning in the same manner as seasonally breeding animals. By using these comparative approaches, we can also examine individual variation in response to pathogens, which aid in our understanding of the mechanisms mediating sickness behavior. For example, to determine whether the reduced sickness intensity displayed by short-day hamsters (Bilbo et al. 2002; Baillie and Prendergast 2008) is a product of seasonal changes in body mass, Carlton and colleagues food restricted long-day hamsters so that they showed the same body mass loss of animals in a short-day photoperiod, and compared responses to experimentally induced sickness. They found that long-day food-restricted animals showed loss of body mass and hypothermia in response to LPS (comparable to short-day ad libitum animals), but their anorexic response, anhedonia, and nest-building behaviors did not reach those of a short-day ad libitum animal (Carlton and Demas 2015a).
Further, Carlton and colleagues investigated the energetic tradeoffs between reproduction and immunity by experimentally limiting energy availability with 2-deoxy-D-glucose (2-DG), a compound that disrupts cellular utilization of glucose, and treating individuals with leptin, providing a direct signal of available fat stores. They found that varying components of these two systems were suppressed under different levels of glucoprivation, and they suggest that the severity of the tradeoffs depend greatly on the intensity and the context of the stressor (Carlton et al. 2014). Other studies have sought to determine the precise physiological signals by which animals (and particularly seasonally breeding animals) modulate sickness responses, including glucocorticoids, leptin, ghrelin, and insulin (Coelho et al. 1992; Baatar et al. 2011; Carlton and Demas 2014, 2015a, 2015b). What is most important when animals mount an immune response is that they do so in a manner in which they use enough energy to fight off the pathogen, but maintain enough energy to subsist. The intensity of a sickness display must not require more energy than the individual has to perform the processes necessary to survive (Adelman and Martin 2009; Ashley et al. 2012).
Sickness and social context
Resource availability, interactions with conspecifics, and dominance and social status can also influence the intensity of sickness behavior in a range of different contexts (Fairbanks and Hawley 2012). In particular, these circumstances may suppress sickness at times when it is most adaptive, even if it may be damaging to short-term health. Instead of showing signs of sickness during infection, some animals may exhibit fewer and less intense sickness behaviors in a social situation because they may invest more in activities such as searching for mates or fighting for territory. For example, Lopes and colleagues hypothesized that animals in a social (group-housed) environment will display less intense (and fewer) sickness behaviors when immune challenged and will exhibit more intense sickness behavior in response to the same challenge in an isolated environment. To test this idea, they placed zebra finches (Taeniopygia guttata) into two separate social treatments (group housing or isolation), exposed them to exogenous LPS or control treatment, and measured changes in activity between social groups as well as determined physiological measures of the immune response (i.e., interleukin-6 [IL-6]). They found that following treatment, socially-isolated zebra finches showed reduced activity, but those kept in a group-housed environment did not show a reduction in activity; though, all finches exhibited an increase in proinflammatory cytokine, IL-6, suggesting that there may be a tradeoff between exhibiting sickness behaviors in response to an infection and taking advantage of being in a social environment (Lopes et al. 2012). Further work sought to determine whether a weakened immune response was associated with reduced sickness behaviors. To accomplish this, they socially housed zebra finches to weaken their behavioral response to an immune challenge, recorded their behavior via a telemetry system that was attached to the birds as they flew freely around the aviary, and collected blood samples for immunological measures. They found that immune responses (e.g., bacterial killing capacity, change in body temperature) were directly associated with behavior. As the immune defenses increased, the time spent resting also increased (Lopes et al. 2014).
Long after an immune challenge, sickness behaviors and concomitant immune responses can influence behavior and physiology as well. Work from our own lab suggests that female Siberian hamsters (Phodopus sungorus) are more robustly affected by an early-life immune challenge (i.e., smaller ovaries and abnormal estrous cycles), but when faced with the opportunity to reproduce in adulthood, they show normal reproductive behavior, can successfully reproduce, and show no significant changes in fecundity (K. E. Sylvia et al., unpublished data). Female hamsters do, however, require more information about male conspecifics (via chemoinvestigation) in order to successfully mate (K. E. Sylvia et al., manuscript in review). These findings suggest that sickness early in life does in fact produce long-term effects, but that sickness-induced changes in physiology may not always result in long-term consequences on fitness and reproduction.
Furthermore, when animals are faced with external threats, they will often suppress sickness behaviors in order to defend a territory or their young. For example, Weil and colleagues tested whether infections affected nest defense in female CD-1 mice. To do so, they injected dams with varying doses of LPS and measured nest defense behaviors. They found that although LPS affected body mass and food intake in dams, nest defense behavior was not affected by sickness. Specifically, the amount of maternal aggression towards a male intruder was not affected by LPS treatment, with the exception of the highest dose of LPS. Interestingly, LPS treatment affected non-aggressive behaviors, including investigation, suggesting that the mothers were in fact affected by treatment, but were able to selectively behave in a way that was most important for survival of their young (Weil et al. 2006).
Further, animals in particular social and environmental contexts may avoid sick individuals if doing so will increase their chances of survival, or they may avoid exhibiting sickness behaviors to put themselves in a better position for territory defense, mating, or offspring survival. For example, house finches given a choice between a “healthy” partner and a “sick” partner choose to avoid the sick partner more often, but this is not consistent across individuals. Interestingly, those individuals that avoid sick conspecifics more often show lower natural antibody levels than non-avoiders, suggesting that they may be more susceptible to a pathogen themselves upon exposure (Zylberberg et al. 2012). Further, Bouwman and Hawley investigated whether there was a sex difference in the response to foraging near same-sex healthy individuals versus same-sex individuals infected with Mycoplasma gallisepticum, and in contrast to the previous study, they found that male house finches preferred to feed by infected male conspecifics (who were often less aggressive), however, female finches showed no preference (Bouwman and Hawley 2010). These data may suggest that because there were lower levels of aggression in the diseased males, healthy males preferred to feed by those that they could defeat; however, although healthy males could feed more easily around these sick males, exposing themselves to this pathogen may also pose an immediate harm to their health. There is a continuous give-and-take between allocating energy to mount a physiological and behavioral immune response and allocating energy to social behaviors important in survival of oneself or one’s young. The body must maintain a state of homeostasis by providing energy to the most essential system in different social contexts, and as researchers, we must integrate what we learn from all of these social contexts to truly understand the adaptive nature of sickness behaviors.
Environmental influences on sickness
Scientists in the area of EI, as well as those in PNI, have studied sickness behavior across a number of different environmental contexts, including both field and laboratory settings. What is important to note is that the same tests performed in a controlled laboratory setting versus a natural field setting may lead to different results. For instance, to test the hypothesis that energy used for reproduction may trade off with that needed for immune function, French et al. subjected male and female tree lizards (Urosaurus ornatus) at different reproductive stages to small wounds in either a laboratory setting or at a field site. They then tracked wound healing and assessed measures of reproductive function and physiology. It was hypothesized that immune function would be the lowest when energy needed for reproduction was the highest (e.g., reproductive males and females). They found that vitellogenic (reproductive) females had a slower healing rate when compared to non-reproductive females in the field, but that in the laboratory, reproductive stage made no difference in wound healing. Further, males of all reproductive stages in both environments showed no change in wound healing (French and Moore 2008). This work suggests that there may be a tradeoff in energy used to elicit reproduction at the cost of eliciting an immune response in the wild, when resources may not be as readily available (and when reproduction is a necessity for fitness). To determine whether resource availability was in fact mediating wound healing in the previous study, in a subsequent study, French et al. manipulated food resources in a controlled laboratory setting. They found that with limited food availability, wound healing was slowed in reproductive females; however, there was no change in wound healing between reproductive and non-reproductive females that were given ad libitum food (French et al. 2007). These data suggest that under limited resources (in any environmental context), females are unable to elicit both an immune response and reproduce, and therefore, the most energy is allocated towards reproduction in order to improve fitness. If resources are readily available, however, then females can support both reproduction and immune function and do not tradeoff energy for either one or the other.
Sickness responses can be influenced not only by external factors (abiotic and biotic), but also internal factors that are introduced to the body as well, including neuromodulators, synthetic compounds (e.g., antibiotics, endocrine disruptors), and even competing pathogens themselves. One factor that has become of recent interest in the study of animal physiology and behavior is the microbiome, which is a complex ecological community that consists of commensal, symbiotic, and pathogenic microorganisms, as well as fungi and viruses (Ursell et al. 2012; Williamson et al. 2015). Microbes in the environment can enter the gastrointestinal (GI) tract and can influence brain and behavior (Clarke et al. 2014; Dinan and Cryan 2015). Specifically, the bidirectional signaling between the GI tract and the brain (termed the gut-brain axis) helps to maintain homeostasis in the body and is regulated at multiple levels, including the nervous system, the endocrine system, and the immune system (Cryan and O’Mahony 2011).
Many studies suggest that microbiome disruption can lead to psychological disorders, such as anxiety and depression (reviewed in Foster and McVey Neufeld 2013). For example, recent work has shown that mice with disrupted gut microbiota increase exploration in the light/dark preference test, and this increased exploration is proposed to contribute to psychiatric disorders (Bercik et al. 2011). Further, clinical research has determined that Major Depressive Disorder (MDD) patients exhibit significantly different gut microbiota than healthy controls, and transplantation of “depressed microbiota” into germ free mice (mice without microbes in or on them) results in symptoms of depression in these otherwise healthy individuals (Zheng et al. 2016). Because the gut microbiome is hypothesized to influence (and be influenced by) the immune system (e.g., via cytokines), it may be possible that the gut microbiome is an active contributor to the immune response, and therefore, the environmental setting in which an animal is placed in may influence sickness behavior by way of the gut-brain axis, just as these contexts can influence the response to an immune challenge directly. Work in our lab suggests that changes in behavior are specific in nature, and that gut microbiome disruption via antibiotics affects aggressive behaviors in both males and females, but that all other social behaviors are not affected by this disruption (Sylvia et al. 2016). Our work suggests that the response to gut microbial perturbations, and subsequently the immune system’s response to challenge (either directly or indirectly), may trade off with particular behaviors in varying environmental contexts. It is possible that in an experimentally controlled environment (as ours was), animals may decrease aggressive behavior (to conserve energy), but in a natural setting where aggression is necessary for survival, we may see a different result. It is critical to interpret the results of a particular study based on the environmental context in which the organism resides, as changes in context are likely to influence the physiological and behavioral response to experimental manipulations on the immune system.
Summary and conclusions
We hope this review has convinced readers that there is considerable variation in the magnitude of sickness responses and this variation is driven largely by energetic, social, and environmental influences. Mounting an appropriate immune response requires a balance between maintaining optimal levels of sickness while avoiding excessive or chronic responses that can cause prolonged impairment. To understand both the adaptive and maladaptive aspects of the immune response, the study of sickness has the potential to serve as an “integrating agent” to bridge basic and translational research within an ecologically relevant context. Consideration of the context by which animals mount immune and sickness responses will allow us to develop a common approach for understanding the mechanisms of sickness within a larger ecological and evolutionary context. Further, by cultivating this type of approach to these behaviors, we will be able to develop a more comprehensive view of sickness as a suite of adaptive responses rather than deleterious (and unwanted) costs. More broadly, Walter Canon’s “wisdom of the body,” the idea that the normal day-to-day responses of our bodies to environmental challenges, including sickness, are healthy, adaptive responses that only occasionally lead to disease has been largely diminished in the modern translational era. Thus as the first part of our title suggests, we call for a “return to wisdom.” That is, by understanding the basic biology of naturally occurring fluctuations in sickness we have the potential to make real and substantial contributions to our understanding of the role of environmental influences on the immune system. A basic approach to studying sickness will continue to be an extremely useful strategy with which to complement more applied, biomedical approaches to sickness employing disease models.
Acknowledgments
We would like to thank Dr. Jill Schneider and Dr. Pierre Deviche for organizing the symposium on “Molecular and neuroendocrine approaches to the study of evolutionary tradeoffs: food, sex, stress, and longevity” and inviting us to submit this review, and Melissa (Misty) Proffitt for kindly providing the lizard graphic used in Fig. 2.
Funding
This work was supported by the National Science Foundation [IOS-1209564 to G.E.D.]; the National Institute of Child Health and Human Development [T32HD49336 to K.E.S.]; and Indiana University (IU).
References
- Adamo SA, Bartlett A, Le J, Spencer N, Sullivan K.. 2010. Illness-induced anorexia may reduce trade-offs between digestion and immune function. Anim Behav 79:3–10. [Google Scholar]
- Adamo SA, Fidler TL, Forestell CA.. 2007. Illness-induced anorexia and its possible function in the caterpillar, Manduca sexta. Brain Behav Immun 21:292–300. [DOI] [PubMed] [Google Scholar]
- Adelman JS, Martin LB.. 2009. Vertebrate sickness behaviors: adaptive and integrated neuroendocrine immune responses. Integr Comp Biol 49:202–14. [DOI] [PubMed] [Google Scholar]
- Ader R, Cohen N.. 1975. Behaviorally conditioned immunosuppression. Psychosom Med 37:333–40. [DOI] [PubMed] [Google Scholar]
- Ashley NT, Weil ZM, Nelson RJ.. 2012. Inflammation: mechanisms, costs, and natural variation. Annu Rev Ecol Evol Syst 43:385–406. [Google Scholar]
- Ashley NT, Wingfield JC. (2012). Sickness behavior in vertebrates: allostasis, life-history modulation, and hormonal regulation In: Demas GE, Nelson R, editors. Ecoimmunology. New York (NY): Oxford University Press: p. 45–91. [Google Scholar]
- Aubert A. 1999. Sickness and behaviour in animals: a motivational perspective. Neurosci Biobehav Rev 23:1029–36. [DOI] [PubMed] [Google Scholar]
- Aubert A, Goodall G, Dantzer R.. 1995. Compared effects of cold ambient temperature and cytokines on macronutrient intake in rats. Physiol Behav 57:869–73. [DOI] [PubMed] [Google Scholar]
- Aubert A, Goodall G, Dantzer R, Gheusi G.. 1997. Differential effects of lipopolysaccharide on pup retrieving and nest building in lactating mice. Brain Behav Immun 11:107–18. [DOI] [PubMed] [Google Scholar]
- Baatar D, Patel K, Taub DD.. 2011. The effects of ghrelin on inflammation and the immune system. Mol Cell Endocrinol 340:44–58. [DOI] [PubMed] [Google Scholar]
- Baillie SR, Prendergast BJ.. 2008. Photoperiodic regulation of behavioral responses to bacterial and viral mimetics: a test of the winter immunoenhancement hypothesis. J Biol Rhyth 23:81–90. [DOI] [PubMed] [Google Scholar]
- Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, et al. 2011. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 141:599–609. [DOI] [PubMed] [Google Scholar]
- Bernard C. (1957). An introduction to the study of experimental medicine. New York (NY: ): Dover Publications, Inc. [Google Scholar]
- Bilbo SD, Drazen DL, Quan N, He L, Nelson RJ.. 2002. Short day lengths attenuate the symptoms of infection in Siberian hamsters. Proc R Soc B Biol Sci 269:447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM.. 2012. The immune system and developmental programming of brain and behavior. Front Neuroendocrinol 33:267–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwman KM, Hawley DM.. 2010. Sickness behaviour acting as an evolutionary trap? Male house finches preferentially feed near diseased conspecifics. Biol Lett 6:462–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon WB. (1932). The wisdom of the body. New York (NY: ): W W Norton and Co. [Google Scholar]
- Carlton ED, Cooper CL, Demas GE.. 2014. Metabolic stressors and signals differentially affect energy allocation between reproduction and immune function. Gen Comp Endocrinol 208:21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton ED, Demas GE.. 2014. Leptin mediates seasonal variation in some but not all symptoms of sickness in Siberian hamsters. Hormon Behav 66:802–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton ED, Demas GE.. 2015a. Body mass affects seasonal variation in sickness intensity in a seasonally-breeding rodent. J Exp Biol 218:1667–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton ED, Demas GE.. 2015b. Glucose and insulin modulate sickness responses in male Siberian hamsters. Gen Comp Endocrinol 242:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G, O’Mahony SM, Dinan TG, Cryan JF.. 2014. Priming for health: Gut microbiota acquired in early life regulates physiology, brain and behaviour. Acta Paediatr 103:812–9. [DOI] [PubMed] [Google Scholar]
- Coelho MM, Souza GE, Pela IR.. 1992. Endotoxin-induced fever is modulated by endogenous glucocorticoids in rats. Am J Physiol 263:R423–7. [DOI] [PubMed] [Google Scholar]
- Cryan JF, O’Mahony SM.. 2011. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil 23: 187–92. [DOI] [PubMed] [Google Scholar]
- Demas GE, Nelson RJ, (eds.). 2012. Ecoimmunology. New York (NY: ): Oxford University Press. [Google Scholar]
- Dinan TG, Cryan JF.. 2015. The impact of gut microbiota on brain and behaviour: implications for psychiatry. Curr Opin Clin Nutr Metab Care 18:552–8. [DOI] [PubMed] [Google Scholar]
- Fairbanks B, Hawley DM. (2012). Interactions between host social behavior, physiology, and disease susceptibility: the role of dominance status and social context In: Demas GE, Nelson R, editors. Ecoimmunology. New York (NY: ): Oxford University Press; p. 440. [Google Scholar]
- Foster JA, McVey Neufeld KA.. 2013. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci 36:305–12. [DOI] [PubMed] [Google Scholar]
- French SS, Denardo DF, Moore MC.. 2007. Trade‐offs between the reproductive and immune systems: facultative responses to resources or obligate responses to reproduction? Am Nat 170:79–89. [DOI] [PubMed] [Google Scholar]
- French SS, Moore MC.. 2008. Immune function varies with reproductive stage and context in female and male tree lizards, Urosaurus ornatus. Gen Comp Endocrinol 155:148–56. [DOI] [PubMed] [Google Scholar]
- Hart BL. 1988. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev 12:123–37. [DOI] [PubMed] [Google Scholar]
- Kluger MJ. (1979). Fever: its biology, evolution, and function. Princeton (NJ: ): Princeton University Press. [Google Scholar]
- Kluger MJ. 1991. Fever: role of pyrogens and cryogens. Physiol Rev 71:93–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluger MJ, Ringler DH, Anver MR.. 1975. Fever and survival. Science 188:166–8. [PubMed] [Google Scholar]
- Kluger MJ, Vaughn LK.. 1978. Fever and survival in rabbits infected with Pasteurella multocida. J Physiol 282:243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes PC, Adelman J, Wingfield JC, Bentley GE.. 2012. Social context modulates sickness behavior. Behav Ecol Sociobiol 66:1421–8. [Google Scholar]
- Lopes PC, Springthorpe D, Bentley GE.. 2014. Increased activity correlates with reduced ability to mount immune defenses to endotoxin in zebra finches. J Exp Zool A Ecol Genet Physiol 321:422–31. [DOI] [PubMed] [Google Scholar]
- McEwen BS. 1998. Stress, adaptation, and disease: allostasis and allostatic load. Ann N Y Acad Sci 840:33–44. [DOI] [PubMed] [Google Scholar]
- Miller NE. 1964. Some psychophysiological studies of motivation and of the behavioral-effects of illness. Bull Br Psychol Soc 17:1–20. [Google Scholar]
- Murray MJ, Murray AB.. 1979. Anorexia of infection of host defense as a mechanism. Am J Clin Nutr 32:593–6. [DOI] [PubMed] [Google Scholar]
- Owen-Ashley NT, Turner M, Hahn TP, Wingfield JC.. 2006. Hormonal, behavioral, and thermoregulatory responses to bacterial lipopolysaccharide in captive and free-living white-crowned sparrows (Zonotrichia leucophrys gambelii). Horm Behav 49:15–29. [DOI] [PubMed] [Google Scholar]
- Owen-Ashley NT, Wingfield JC.. 2006. Seasonal modulation of sickness behavior in free-living northwestern song sparrows (Melospiza melodia morphna). J Exp Biol 209:3062–70. [DOI] [PubMed] [Google Scholar]
- Pohl J, Sheppard M, Luheshi GN, Woodside B.. 2014. Diet-induced weight gain produces a graded increase in behavioral responses to an acute immune challenge. Brain Behav Immun 35:43–50. [DOI] [PubMed] [Google Scholar]
- Pohl J, Woodside B, Luheshi GN.. 2009. Changes in hypothalamically mediated acute-phase inflammatory responses to lipopolysaccharide in diet-induced obese rats. Endocrinology 150:4901–10. [DOI] [PubMed] [Google Scholar]
- Sheldon BC, Verhulst S.. 1996. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol Evol 11:317–21. [DOI] [PubMed] [Google Scholar]
- Sylvia KE, Jewell CP, Rendon NM, St. John EA, Demas GE.. 2016. Sex-specific modulation of the gut microbiome and behavior in Siberian hamsters. Brain Behav Immun 60:51–62. [DOI] [PubMed] [Google Scholar]
- Ursell LK, Metcalf JL, Laura WP, Knight R.. 2012. Defining the human microbiome. Nutr Rev 70(Suppl 1):S38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn LK, Bernheim HA, Kluger MJ.. 1974. Fever in the lizard Dipsosaurus dorsalis. Nature 248:81–2. [DOI] [PubMed] [Google Scholar]
- Weil ZM, Bowers SL, Dow ER, Nelson RJ.. 2006. Maternal aggression persists following lipopolysaccharide-induced activation of the immune system. Physiol Behav 87:694–9. [DOI] [PubMed] [Google Scholar]
- Weinberg ED. 1974. Iron and susceptibility to infectious disease. Science 184:952–6. [DOI] [PubMed] [Google Scholar]
- Williamson LL, Mckenney EA, Holzknecht ZE, Belliveau C, Rawls JF, Poulton S, Parker W, Bilbo SD.. 2015. Got worms? Perinatal exposure to helminths prevents persistent immune sensitization and cognitive dysfunction induced by early-life infection. Brain Behav Immun 51:14–28. [DOI] [PubMed] [Google Scholar]
- Wing EJ, Young JB.. 1980. Acute starvation protects mice against Listeria monocytogenes. Infect Immun 28:771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, Zeng L, Chen J, Fan S, Du X, et al. 2016. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry 21:786–96. [DOI] [PubMed] [Google Scholar]
- Zylberberg M, Klasing KC, Hahn TP. (2012). House finches (Carpodacus mexicanus) balance investment in behavioural and immunological defences against pathogens. Biol Lett 9:20120856. [DOI] [PMC free article] [PubMed] [Google Scholar]