Abstract
OBJECTIVES
Ischaemic brain injury is a major complication in patients undergoing surgery for congenital heart disease, with the hippocampus being a particularly vulnerable region. We hypothesized that neuronal injury resulting from cardiopulmonary bypass and associated circulatory arrest is ameliorated by pretreatment with granulocyte colony-stimulating factor (G-CSF), a cytokine and an anti-apoptotic neurotrophic factor.
METHODS
In a model of ischaemic brain injury, 4 male newborn piglets were anaesthetized and subjected to deep hypothermic circulatory arrest (DHCA) (cooled to 18°C, DHCA maintained for 60 min, rewarmed and recovered for 8–9 h), while 4 animals received G-CSF (34 µg/kg, intravenously) 2 h prior to the DHCA procedure. At the end of each experiment, the animals were perfused with a fixative, the hippocampus was extracted, cryoprotected, cut and the brain sections were immunoprocessed for activated caspase 3, a pro-apoptotic factor. Immunopositive neuronal nuclei were counted in multiple counting boxes (440 × 330 µm) centred on the CA1 or CA3 hippocampal regions and their mean numbers compared between the different treatment groups and regions.
RESULTS
G-CSF pretreatment resulted in significantly lower counts of caspase 3-positive nuclei per counting box in both the CA1 [52.2 ± 9.3 (SD) vs 61.6 ± 8.4, P < 0.001] and CA3 (41.2 ± 6.9 vs 60.4 ± 16.4, P < 0.00002) regions of the hippocampus as compared to DHCA groups. The effects of G-CSF were significant for pyramidal cells of both regions and for interneurons in the CA3 region.
CONCLUSIONS
In an animal model of ischaemic brain injury, G-CSF reduces neuronal injury in the hippocampus, thus potentially having beneficial effect on neurologic outcomes.
Keywords: Apoptosis, Cardiac surgery, Congenital heart disease, Developing brain, Hippocampus, Caspase 3
INTRODUCTION
Brain injury leading to neuropsychological dysfunction is a significant long-term complication in neonates undergoing surgery for congenital heart disease (CHD). Long-term follow-up studies in these patients revealed distinct patterns of neurodevelopment dysfunction characterized by cognitive impairment, compromised executive function, decline in consolidating information from short-term memory into long-term memory, expressive speech and language abnormalities, impaired visuospatial and visuomotor skills, attention-deficit/hyperactivity disorder, motor delays and other learning disabilities. These impairments are associated with the brain ischaemia that may be a component of corrective surgery for CHD. One of the brain regions that is most vulnerable to ischaemic injury and involved in these cognitive functions is the hippocampus [1–7]. Understanding the pathophysiology and developing effective strategies to protect vulnerable regions of the brain from ischaemic/hypoxic injury are fundamental in improving outcomes in neonates and infants undergoing surgery for CHD. To date, strategies aimed at reducing neurological injury during cardiac surgery have focused, for the most part, on the technical aspects of cardiopulmonary bypass (CPB). Substantial additional brain protection may be achieved by administering neuroprotective agents and/or modulating the conditions during and after cardiac surgery, before the neurological injury becomes irreversible. We propose that granulocyte colony-stimulating factor (G-CSF) injected prior to DHCA can diminish ischaemia-dependent brain injury in the newborn piglet.
G-CSF, a member of the cytokine family of growth factors, is a glycoprotein broadly present within the central nervous system and has been studied in a variety of brain injury models with highly promising results. Exogenous administration of G-CSF has been shown to be neuroprotective in a variety of stroke models [8–10], where induction of neurogenesis near the damaged area leads to neurological and functional recovery [8, 11]. Our earlier studies have shown that injection of G-CSF prior to DHCA decreases apoptotic and inflammatory activity in the striatum and hippocampus of newborn piglets and increases anti-apoptotic and anti-inflammatory activity, thus suggesting that it may have a protective effect in hypoxia-related brain injury [12, 13]. The present study focuses on amelioration of this injury in the hippocampus, as determined by measurements of immunoreactivity for activated caspase 3.
Caspase 3 is a protease that, in the young brain, has been implicated as an ‘effector’ enzyme associated with the initiation of the ‘death cascade’. Therefore, it is an important marker for the cell’s entry point into the apoptotic signalling pathway. Activation of the caspase 3 pathway is a hallmark of apoptosis and has been used to quantify activation or inhibition of the ‘death cascade’.
MATERIALS AND METHODS
Animal model
Experiments were conducted on newborn piglets from multiple litters obtained from Meck Swine, LLC (Refton, PA, USA). When acquired, the animals were 3–4 days old. They were utilized for the experiment in the next 3 days. The piglets were anaesthetized with 4% isoflurane, intubated and mechanically ventilated with air/30% oxygen mixture with a goal to maintain normocapnia. Anaesthesia was then maintained with 1.5% isoflurane supplemented as needed with fentanyl (30 µg/kg bolus), and neuromuscular paralysis was induced with pancuronium bromide (0.1 mg/kg bolus repeated, as needed). A femoral arterial catheter was placed to monitor mean arterial blood pressure, blood gases [partial pressure of carbon dioxide (CO2) and partial pressure of oxygen], pH, haemoglobin, electrolytes and glucose concentrations using i-STAT blood gas machine (Abbot Point of Care Inc., Princeton, NJ, USA). Electrocardiogram and nasopharyngeal and rectal temperatures were continuously monitored throughout the study.
All animal procedures were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Experimental groups
Anaesthetized animals were either subjected to the CPB-DHCA protocol that included cooling to 18°C, followed by circulatory arrest for 60 min and then rewarming and recovery with artificial ventilation for 8–9 h (the DHCA group) or the same protocol with intravenous injection of G-CSF (34 µg/kg) recombinant human protein (Thermo Fisher Sci. Inc., catalog # PHC 2031) 2 h prior to initiation of bypass (G-CSF group). Based on power analysis derived from outcomes of our prior similar studies [12, 13], and in order to keep the numbers of animals used down to a necessary minimum, each group was comprised of 4 animals. G-CSF administration 2 h before the beginning of bypass procedures was considered appropriate for allowing the G-CSF to distribute to all tissues, including the brain. This timing is based on reports that a significant fraction of G-CSF crosses the blood–brain barrier within an hour and the concentration in the extravascular space continues to increase during the subsequent several hours [8].
The concentration of G-CSF use in our study is well within the range used by other investigators. None of the previous animal studies reported significant negative side effects even at the highest doses used. Clinical doses of G-CSF vary from 5 to 10 µg/kg/day, to much higher amounts if given as single injection. In different animal studies, intravenous dose of G-CSF used ranged from 5 to 60 µg/kg body weight.
We’ve elected to use a dose of 34 µg/kg (vs 17 µg/kg, as used in our early study) in order to be certain that any effects were maximized, and therefore, more likely to be statistically significant with the relatively small groups of animals.
Cardiopulmonary bypass technique
The CPB circuit consisted of a Cobe Roller Pump (Cobe, Lakewood, CO, USA), a membrane oxygenator (Lilliput 1, Dideco, Mirandola, Italy), arterial filter (Terumo Cardiovascular System Corp., Ann Arbor, MI, USA) and Sarns Heater-Cooler System (Terumo Cardiovascular System Corp.). The circuit was primed with Plasmalyte-A (Baxter Healthcare Corp., Deerfield, IL, USA) and 25% albumin. Donor whole blood was added to maintain a CPB haematocrit value of at least 30%. Heparin (2000 units), fentanyl (50 µg), pancuronium bromide (1 mg), calcium chloride (500 mg), dexamethasone (30 mg), cefazolin (500 mg), furosemide (3 mg) and sodium bicarbonate (25 mEq) were then added to the pump prime.
For the CPB procedure, median sternotomy was performed, 500 U heparin was administered intravenously, and the ascending aorta and the right atrial appendage were cannulated. CPB flow rate was maintained at ∼150 ml/kg/min. The piglets were then cooled over a 20- to 30-min period with pH-stat blood gas management to a nasopharyngeal/brain temperature of 18°C. The temperature-corrected arterial blood pH was maintained at 7.4 by the addition of CO2 (typically 50 ml/min of CO2 was added to the gas flow of 1.5 l/min through the blood oxygenator). During cooling, the temperature was adjusted to keep the oxygenator/body temperature gradient no greater than 10°C. Once the target temperature was achieved, the piglets were subjected to 60 min of DHCA. CPB was then resumed at 150 ml/kg/min and the piglets were rewarmed to 37 ± 1°C for a 30-min period. CPB was discontinued when body temperature reached 37°C and ventilation was reinitiated 5 min before weaning from CPB. All animals received analgesia, paralysis, mechanical ventilation and were continuously monitored throughout the recovery period of 8–9 h after weaning from CPB. No inotropes were used and the post-CPB arterial blood pressure was maintained with an infusion of saline/packed red blood cells to keep mean arterial blood pressure above 50 mmHg. At the end of each experiment, piglets were first perfused with heparinized saline and then with 4% phosphate-buffered paraformaldehyde. The forebrain was extracted, post-fixed, cryoprotected in 30% sucrose and then cut into 10 µm transverse sections. Every fifth section through the hippocampus was mounted.
Caspase 3 immunohistochemistry
Formalin-fixed, 10-µm thick, transverse sections through the hippocampus were mounted and incubated with cleaved caspase 3 (Asp175) primary antibodies (Catalog #9661, Cell Signaling Technology, Beverly, MA, USA; 1:100), followed by tetramethyl rhodamine isothiocyanate (TRITC)-tagged secondary antibodies (Catalog #111-025-003, Jackson ImmunoResearch Labs, West Grove, PA, USA). Control staining procedures in which primary antibodies were omitted did not yield any caspase 3-like immunoreactivity. DNA in neuronal nuclei were counterstained with 4ʹ,6-diamidino-2-phenylindole dihydrochloride (Catalog #S7113, Millipore, Temecula, CA, USA). Digital images of the CA1 and CA3 regions of the hippocampus were taken using appropriate filters for tetramethyl rhodamine isothiocyanate and 4ʹ,6-diamidino-2-phenylindole dihydrochloride. The corresponding frames were then processed using Photoshop CS4 software (v. 11.0.2, Adobe Systems, San Jose, CA, USA) to enhance the red staining of caspase 3 and blue nuclear staining and superimposed (merged). Between 4 and 10 non-overlapping photographs (counting boxes) measuring 440 × 330 µm were taken from 2 to 4 brain sections per animal. In total, 73 frames were acquired, with a mean ± standard deviation (SD) of 4.9 ± 2.1 per hippocampal region and animal. Caspase 3-positive cells with distinctly large nuclei and aggregating in a characteristic manner were distinguished from smaller and less orderly distributed cells also present in the counting boxes. The former were deemed to represent hippocampal pyramidal neurons and the latter were deemed to be interneurons (Fig. 1). Caspase 3-containing cells of each type and the total numbers of cells of both types were counted within each counting box.
Figure 1.
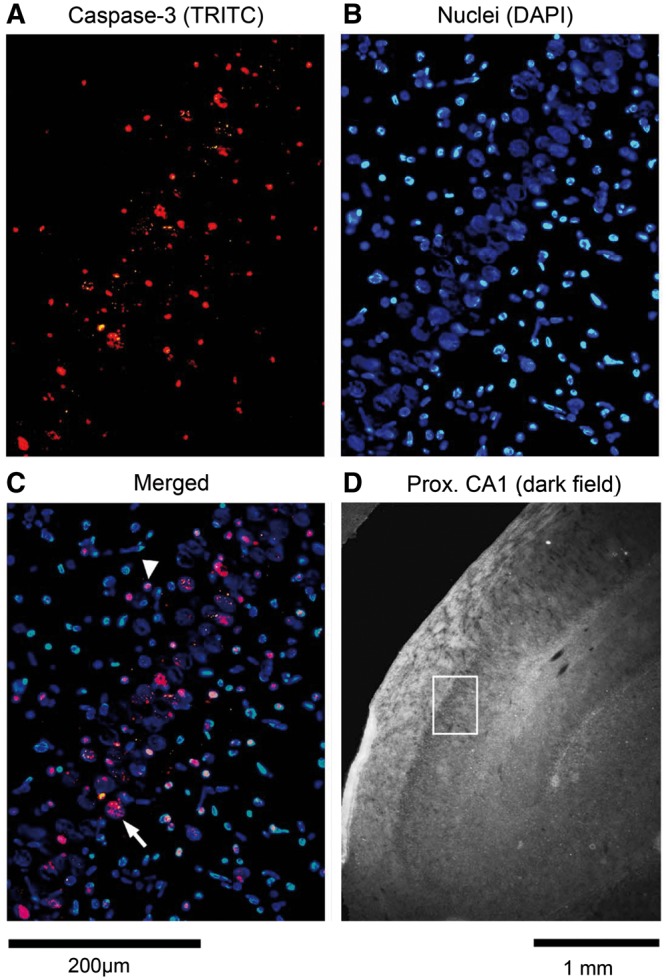
Caspase 3 immunoreactivity in the proximal CA1 region of the hippocampus of a piglet subjected to cardiopulmonary bypass with deep hypothermic cardiac arrest. (A) Image of caspase 3 immunostaining with tetramethyl rhodamine isothiocyanate (TRITC). (B) Same frame as in (A) but with blue nuclear staining (DAPI). (C) Merged frames shown in (A) and (B) demonstrate co-localization of caspase 3 in large (pyramidal) neurons and interneurons. Arrow indicates example of a caspase 3-positive pyramidal neuron; arrowhead indicates example of a caspase 3-positive interneuron. (D) Low-magnification, dark field image of a portion of the hippocampus with the white rectangle marking the location of the frame shown in panels (A–C).
Statistical analysis
The numbers of apoptotic cells were determined per counting box within each of the 2 hippocampal regions of interest. Following the determination that the data sets met the criteria for normality (Shapiro–Wilk test), 2-way analysis of variance (ANOVA) with interactions was used to determine whether counts in individual counting boxes depended on the type of treatment (DHCA or DHCA preceded with G-CSF) and/or the hippocampal region (CA1 or CA3) (Sigma Plot v.12.3, San Jose, CA, USA). The analysis was separately applied to data sets for all caspase 3-positive cells, caspase 3-positive pyramidal cells only or caspase 3-positive interneurons only. The results are presented as means ± SD. The reported P-values derived from 2-way ANOVA with interactions include correction for multiple comparisons (Holm–Sidak method) and, where relevant, are accompanied by F statistics values for the corresponding factor. The latter include 3 indices showing the degrees of freedom, with the first and second corresponding to the 2 factors (treatment and region) each having 2 levels and the third referring to residuals. Hence, in F1,1,69, 1 and 1 describe degrees of freedom for the 2 factors, and 69 represents degrees of freedom for residuals when 2-way ANOVA included interactions and was conducted on a data set containing 73 separate measurements.
RESULTS
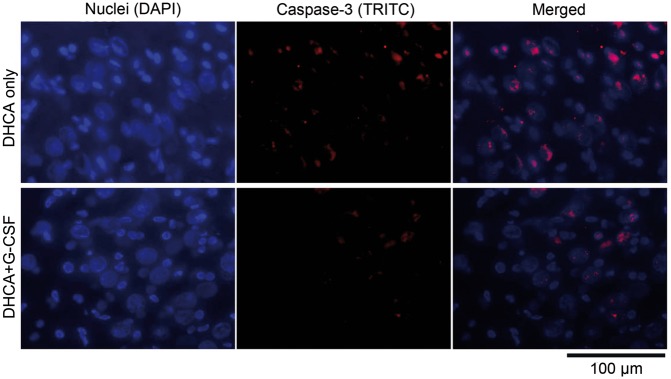
For the CA1 region of the hippocampus, caspase 3-positive cells were counted in 22 non-overlapping hippocampal counting boxes (images) from 4 piglets exposed to DHCA and 23 images from 4 piglets treated with G-CSF prior to DHCA. Figure 1 shows representative individual fluorescent images with the cells positive for caspase 3 (red) and all cells present within the counting box (blue) obtained from the CA1 region of the hippocampus of a piglet subjected to DHCA without G-CSF pretreatment. The merged image demonstrates that caspase 3 was present in both some pyramidal cells (large nuclei) and some interneurons (small nuclei). For the CA3 region, cells were counted in 16 images of brain sections from the animals exposed to DHCA only and 12 images from piglets pretreated with G-CSF.
For the entire data set comprising all individual counting boxes from both treatment groups and both hippocampal regions (n = 73), there was a highly significant effect of G-CSF pretreatment on the total counts of caspase 3-positive cells in both regions (F1,1,69 = 30.2, P = 0.6 × 10−6; 2-way ANOVA with interactions). There was also a relatively strong effect of the region (F1,1,69=5.5, P = 0.022). This was related to slightly lower counts of caspase 3-positive cells in the CA3 than CA1 region, independent of the treatment (see below). There was no significant correlation between the treatment and region (P = 0.064), indicating that the highly significant effect of G-CSF had a similar magnitude and direction in both regions. Overall, the key quantitative outcome from this analysis was that the mean counts of all types of caspase 3-positive cells were significantly lower in the animal group pretreated with G-CSF than in the DHCA group for both the CA1 region (52.2 ± 9.3 vs 61.6 ± 8.4, P < 0.005) and the CA3 (41.2 ± 6.9 vs 60.4 ± 16.4, P < 0.0002) region. Figure 2 shows 2 sets of photographic frames obtained with nuclear DNA staining only (blue), caspase 3 staining only (red) and merged images from the CA3 region from an animal subjected to DHCA (top) and another animal that was pre-treated with G-CSF prior to DHCA (bottom).
Figure 2.
Comparison of caspase 3 immunoreactivity in the distal CA3 region of the hippocampus of a piglet subjected to cardiopulmonary bypass with deep hypothermic cardiac arrest (DHCA) (top) and a piglet pretreated with G-CSF prior to DHCA (bottom). The 3 panels for each case successively show blue nuclear DNA staining (DAPI), red caspase 3 immunostaining (TRITC), and a superimposition of both images (merged). In the animal pretreated with G-CSF (bottom), caspase 3 immunostaining is less extensive and occurs in fewer neurons than in the animal subjected to DHCA without pretreatment (top).
A similar picture of G-CSF having an ameliorating effect on caspase 3 expression also emerged when 2-way ANOVA was applied separately to individual counts of pyramidal cells or to separately counted interneurons, with only minor exceptions. The first one was that no regional differences were revealed for pyramidal cell counts, whereas they persisted for interneuronal counts. Thus, the significant effect of the region found for all cells was related to the counts of interneurons and not the pyramidal neurons. The second difference was that the effect of G-CSF was significant in both regions for pyramidal neurons, whereas for interneurons this effect was significant only in the CA3 region.
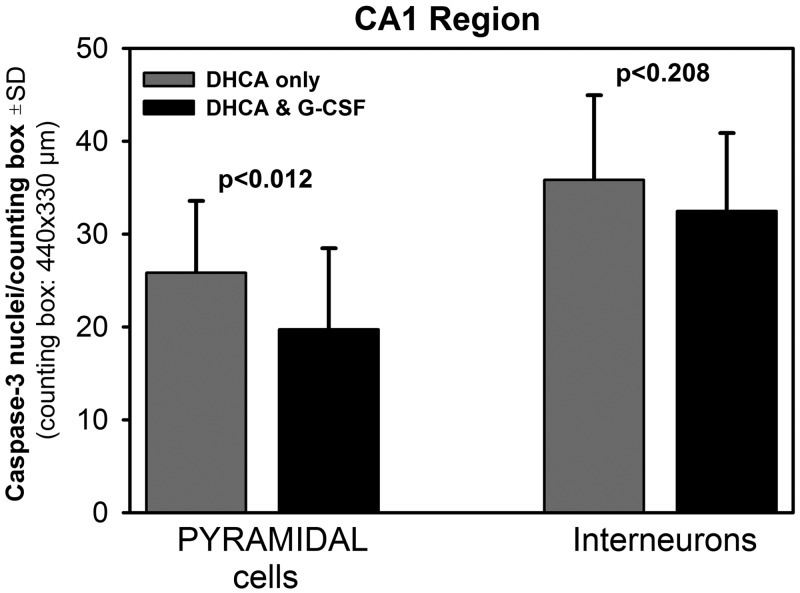
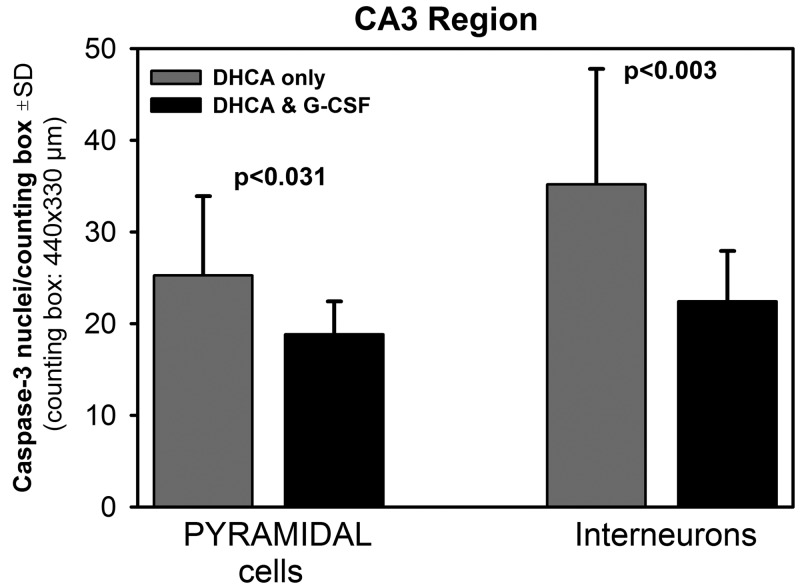
The average data for pyramidal cells and interneurons are shown in Fig. 3 for the CA1 region and in Fig. 4 for the CA3 region. In the CA1 region, there were significantly fewer caspase 3-positive pyramidal neurons in the G-CSF group than in the DHCA group (19.7 ± 8.7 vs 25.8 ± 7.7), and there was also a trend for lower interneuronal counts in the G-CSF group (Fig. 3). In the CA3 region, the counts of both cell types were significantly lower in the G-CSF group than in the DHCA group (18.8 ± 3.6 vs 25.2 ± 8.6 for pyramidal neurons and 22.4 ± 5.5 vs 35.2 ± 12.6 for interneurons, respectively; Fig. 4).
Figure 3.
Mean counts of caspase 3-positive pyramidal cells and interneurons in the hippocampal CA1 region in the deep hypothermic circulatory arrest (DHCA)-only animals and the DHCA animals pretreated with G-CSF. There were 5.6 ± 2.5 (SD) cell counting boxes per animal and 4 animals per group. Significance levels were obtained using independent samples t-tests.
Figure 4.
Mean counts of caspase 3-positive pyramidal cells and interneurons in the hippocampal CA3 region in the deep hypothermic circulatory arrest (DHCA)-only animals and the DHCA animals pretreated with G-CSF. There were 4.0 ± 1.3 (SD) cell counting boxes per animal and 4 animals per group. Significance levels were obtained using independent samples t-tests.
DISCUSSION
The purpose of this study was to determine whether, in a model of DHCA-related brain ischaemia, pretreatment with G-CSF could decrease apoptotic hippocampal injury, as defined by an increase in immunostaining for active caspase 3. Caspase 3 is one of the key mediators of apoptosis in animals (see [14, 15] for reviews) and human brain after ischaemia [16]. Caspase-dependent apoptotic cell death is particularly prominent in the neonatal brain [17–19]. Numerous studies have reported that inhibition of caspases cascade [20] can protect the brain from ischaemic injury. When some of them focused on a particularly hypoxia-sensitive area of the hippocampus (CA1), increase of active caspase 3 following ischaemia was shown to be related mostly to degenerating pyramidal neurons (DNA fragmentation), with inhibition of this enzyme significantly diminishing ischaemia-related cell death [21].
The results of our present study show that DHCA-related ischaemia increases the number of caspase 3-positive nuclei in both CA1 and CA3 regions of the hippocampus as compared to sham-operated (control) animals. Measurements in sham-operated animals did not show any, or an only very insignificant number, of the caspase 3 active cells in CA1 and CA3 regions of the hippocampus (data not shown).
The increase in caspase 3 activity in both regions of hippocampus after DHCA contrasts with studies done by others, who reported that distinct populations of hippocampal neurons demonstrate different vulnerability to ischaemia, with CA1 pyramidal neurons being more sensitive than the neurons of CA3 region as well as most of the interneurons [22].
Lack of difference in the caspase 3 activity between CA1 and CA3 in our study may be due to the fact that our injury model is significantly different from most models of hypoxic/ischaemic injury reported in the literature. In this model, during period of ischaemia, the animals are in deep hypothermia, with the brain temperature far below normal. Hypothermia is a well-known protective mechanism in hypoxic–ischaemic injury. This makes it difficult to compare the response of hippocampal regions in our model to those under normothermic conditions. It is clear that the reaction of hippocampal tissue depends on the type and duration of cerebral ischaemia [23], with temperature playing an important role. If the period of ischaemia is relatively short, then cell death tends to be delayed and selective for the neurons. Longer episodes of ischaemia cause broader and more destructive changes (see ref. [22] for review).
Another factor that is different in our study versus others is the relatively short time of recovery. Han et al. reported that, in a model with unilateral carotid ligation and exposure to hypoxia in 7-day-old rats, active caspase 3 begins to accumulate by 6 h and peaks at 24 h after the insult [17]. Hippocampal CA1 neuronal death occurs 3–4 days after the ischaemic insult [24]. The degeneration of pyramidal cell bodies increases progressively leading to death of 79.5% of CA1 neurons within 7 days [22]. Therefore, it is possible that we would see a more pronounced nuclear accumulation of active caspase 3 in the CA1 and CA3 regions in our DHCA model after longer recovery times than 8–9 h.
Our data also show that DHCA increased the number of caspase 3-positive nuclei among the hippocampal interneurons. It has been suggested that hippocampal interneurons are generally more resistant than pyramidal cells to excitotoxic insults, such as ischaemia, possibly because of differences in N-methyl-d-aspartate receptor expression [22]. It has, however, also been reported that some interneurons can be more sensitive to ischaemia and excitotoxicity than others [22]. Our study did not distinguish between the different types of interneurons, so the early injury observed in this study could be due to an injury of a particular, more sensitive to ischaemia, type of interneurons.
The observed number of caspase 3-positive nuclei in pyramidal cells of CA1 and CA3, as well as the interneurons in CA3 region, was significantly diminished by the injection of G-CSF prior to bypass. This neuroprotection can at least partly be caused by the anti-inflammatory and anti-apoptotic properties of G-CSF. We have previously shown that treatment with G-CSF prior to CPB in newborn piglet diminished pro-apoptotic (increase in Bax) and increased anti-apoptotic signaling (increase in pAkt and Bcl-2) in the hippocampus and striatum [12, 13]. This was accompanied by decreased neuronal injury, as determined by TUNEL, in both of these brain regions [25].
The exact mechanism of G-CSF neuroprotection in different regions of the hippocampus requires further investigation. Hippocampus is a heterogeneous structure containing different types of neurons and non-neuronal cells, with the principal cell type being the pyramidal neurons of CA1 area [2]. These cells represent the main output of the hippocampus. They send projections to many brain areas including the entorhinal cortex, amygdalar complex, hypothalamus, prefrontal cortex, nucleus accumbens, olfactory regions, auditory cortex and visual cortex [1]. They are some of the most studied cells in the mammalian brain [2]. One proposed mechanism of injury in pyramidal neurons of CA1 following ischaemia is excitotoxicity [24]. The CA1 region has a high concentration of glutamate as well as N-methyl-d-aspartate receptor, which play an important role in neuronal injury [26–28]. However, some studies have also reported that the number of N-methyl-d-aspartate receptors and the amount of messenger RNA for the receptor are not very different between the CA1 and CA3 regions [24]. Therefore, it may be proposed that suppression of the excitotoxic mechanisms is the major mechanism of G-CSF neuroprotection in CA1 and possibly the CA3 region of hippocampus during early recovery from ischaemic insult. This is consistent with the finding by Schäbitz et al., who reported that G-CSF has excitoprotective properties, which are involved in neuroprotection [9]. Further studies need to be done in order to determine whether the excitoprotective effects of GCSF also suppress the delayed neuronal death of CA1 neurons. Hippocampal CA1 neuronal death usually occurs 3 to 4 days after an initial ischaemic insult [24]. It has been proposed that delayed and rapid neuronal death follow a similar sequence of cellular events [22]. Therefore, decreasing apoptotic cell injury following treatment with G-SCF may be of major importance in protecting all hippocampal output to various targets throughout the brain. As stated above, the hippocampus is the site for signal processing responsible for cognition and is critically involved in the formation, organization and retrieval of new memories. The principal type of cells in this region are the excitatory pyramidal neurons that integrate spatial, contextual and emotional information and transmits all hippocampal output to various targets throughout the brain [2].
The exact mechanisms of G-CSF neuroprotection require further investigation. However, with no reported adverse effects of G-CSF at the doses used in this study, our findings indicate that treatment with G-CSF is a very promising approach to ameliorating ischaemic brain injury and thus potentially improving neurologic outcomes in neonates undergoing surgery for CHD.
Funding
This work was supported by the National Institutes of Health [grant number HL-58669] and Children’s Mercy Hospital and Clinics, Kansas City, MO, USA.
Conflict of interest: none declared.
REFERENCES
- 1. Arszovszki A, Borhegyi Z, Klausberger T.. Three axonal projection routes of individual pyramidal cells in the ventral CA1 hippocampus. Front Neuroanat 2014;8:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Graves AR, Moore SJ, Bloss EB, Mensh BD, Kath WL, Spruston N.. Hippocampal pyramidal neurons comprise two distinct cell types that are countermodulated by metabotropic receptors. Neuron 2012;76:776–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barth AMI, Mody I.. Changes in hippocampal neuronal activity during and after unilateral selective hippocampal ischemia in vivo. J Neurosci 2011;31:851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schmidt-Kastner R, Freund TF.. Selective vulnerability of the hippocampus in brain ischemia. Neuroscience 1991;40:599–636. [DOI] [PubMed] [Google Scholar]
- 5. Akai F, Yanagihara T.. Identity of the dorsal hippocampal region most vulnerable to cerebral ischemia. Brain Res 1993;603:87–95. [DOI] [PubMed] [Google Scholar]
- 6. Bliss TV, Collingridge GL.. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 1993;361:31–9. [DOI] [PubMed] [Google Scholar]
- 7. Squire LR. Memory systems: a biological concept In: Roediger R, Dudai Y, Fitzpatrick S (eds). Science of Memory: Concepts. Oxford: Oxford University Press, 2007, 339–43. [Google Scholar]
- 8. Schneider A, Krüger C, Steigleder T, Weber D, Pitzer C, Laage R. et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest 2005;115:2083–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schäbitz WR, Kollmar R, Schwaninger M, Juettler E, Bardutzky J, Scholzke MN. et al. Neuroprotective effect of granulocyte colony-stimulating factor after focal cerebral ischemia. Stroke 2003;34:745–51. [DOI] [PubMed] [Google Scholar]
- 10. Gibson CL, Bath PM, Murphy SP.. G-CSF reduces infarct volume and improves functional outcome after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab 2005;25:431–511. [DOI] [PubMed] [Google Scholar]
- 11. Shyu WC, Lin SZ, Yang HI, Tzeng YS, Pang CY, Yen PS. et al. Functional recovery of stroke rats induced by granulocyte colony-stimulating factor-stimulated stem cells. Circulation 2004;110:1847–54. [DOI] [PubMed] [Google Scholar]
- 12. Pastuszko P, Schears GJ, Pirzadeh A, Kubin J, Greeley WJ, Wilson DF. et al. Effect of granulocyte-colony stimulating factor (G-CSF) on expression of select proteins involved in apoptosis in a neonatal piglet brain following cardiopulmonary bypass (CPB) and deep hypothermic circulatory arrest (DHCA). J Thorac Cardiovasc Surg 2012;143:1436–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pastuszko P, Schears GJ, Kubin J, Greeley WJ, Nadkarni V, Wilson DF. et al. Granulocytecolony stimulating factor suppresses early inflammatory response of striatum in a cardiopulmonary bypass-circulatory arrest model of ischemic brain injury in newborn piglets. World J Cardiovasc Dis 2013;3:197–205. [Google Scholar]
- 14. McIlwain DR, Berger T, Mak TW.. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol 2013;5:a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Broughton BRS, Reutens DC, Sobey CG.. Apoptotic mechanisms after cerebral ischemia. Stroke 2009;40:e331–9. [DOI] [PubMed] [Google Scholar]
- 16. Rami A, Sims J, Botez G, Winckler J.. Spatial resolution of phospholipid scramblase 1 (PLSCR1), caspase-3 activation and DNA-fragmentation in the human hippocampus after cerebral ischemia. Neurochem Int 2003;43:79–87. [DOI] [PubMed] [Google Scholar]
- 17. Han BH, D’Costa A, Back SA, Parsadanian M, Patel S, Shah AR. et al. BDNF blocks Caspase-3 activation in neonatal hypoxia-ischemia. Neurobiol Dis 2000;7:38–53. [DOI] [PubMed] [Google Scholar]
- 18. Cheng Y, Deshmukh M, D’Costa A, Demaro JA, Gidday J, Shah A. et al. Caspase inhibitor affords neuroprotection with delayed administration in a rat model of neonatal hypoxic ischemic brain injury. J Clin Invest 1998;101:1992–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pulera MR, Adams LM, Liu HT, Santos DG, Nishimura RN, Yang FS. et al. Apoptosis in a neonatal rat model of cerebral hypoxia-ischemia. Stroke 1998;29:2622–9. [DOI] [PubMed] [Google Scholar]
- 20. Endres M, Namura S, Shimizu-Sasamata M, Waeber C, Zhang L, Gomez-Isla T. et al. Attenuation of delayed neuronal death after mild focal ischemia in mice by inhibition of the caspase family. J Cereb Blood Flow Metab 1998;18:238–47. [DOI] [PubMed] [Google Scholar]
- 21. Chen J, Nagayama T, Jin K, Stetler RA, Zhu RL, Graham SH. et al. Induction of caspase-3 like protease may mediate delayed neuronal death in the hippocampus after transient cerebral ischemia. J Neurosci 1998;18:4914–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nikonenko AG, Radenovic L, Andjus PR, Skibo GG.. Structural features of ischemic damage in the hippocampus. Anat Rec 2009;292:1914–21. [DOI] [PubMed] [Google Scholar]
- 23. Rosenblum WI. Histopathologic clues to the pathways of neuronal death following ischemia/hypoxia. J Neurotrauma 1997;14:313–26. [DOI] [PubMed] [Google Scholar]
- 24. Abe K, Aoki M, Kawagoe J, Yoshida T, Hattori A, Kogure K. et al. Ischemic delayed neuronal death a mitochondrial hypothesis. Stroke 1995;26:1478–89. [DOI] [PubMed] [Google Scholar]
- 25. Pastuszko P, Schears GJ, Greeley WJ, Kubin J, Wilson DF, Pastuszko A.. Granulocyte colony stimulating factor reduces brain injury in a cardiopulmonary bypass-circulatory arrest model of ischemia in a newborn piglet. Neurochem Res 2014;39:2085–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Monaghan DT, Cotman CW.. Distribution of N-methyl-o-aspartate-sensitive L-(3H)glutamatebinding sites in rat brain. J Neurosci 1985;5:2909–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jafari-Sabet M. Involvement of dorsal hippocampal muscarinic cholinergic receptors on muscimol state-dependent memory of passive avoidance in mice. Life Sci 2011;88:1136–41. [DOI] [PubMed] [Google Scholar]
- 28. Watson DJ, Stanton ME.. Intrahippocampal administration of an NMDA-receptor antagonist impairs spatial discrimination reversal learning in weanling rats. Neurobiol Learn Mem 2009;92:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]