Abstract
The comparative biology of reproduction and development in mammalian species is remarkable. Hence, because of similarities in environmental and neuroendocrine control of the reproductive axis, the cyclic function of the ovary and reproductive tract, establishment and control of the maternal-fetal-placental unit during pregnancy, and reproductive aging from puberty through menopause, nonhuman primates (NHPs) are valuable models for research related to women's reproductive health and its disorders. This chapter provides examples of research over the past 10+ years using Old World monkeys (notably macaque species), baboons, and to a lesser extent New World monkeys (especially marmosets) that contributed to our understanding of the etiology and therapies or prevention of: (1) ovarian disorders, e.g., polycystic ovary syndrome, mitochondrial DNA-based diseases from the oocyte; (2) uterine disorders, for example, endometriosis and uterine transplantation; and (3) pregnancy disorders, for example, preterm labor and delivery, environmental factors. Also, emerging opportunities such as viral (e.g., Zika) induced fetal defects and germline genomic editing to generate valuable primate models of human diseases (e.g., Huntington and muscular dystrophy) are addressed. Although the high costs, specialized resources, and ethical debate challenge the use of primates in biomedical research, their inclusion in fertility and infertility research is vital for continued improvements in women's reproductive health.
Keywords: contraception, endometriosis, germline genome editing, infertility, mitochondrial DNA-based diseases, polycystic ovary syndrome, preterm labor and delivery, uterine transplantation, Zika virus-induced fetal defects
Introduction
Significant advances in women's reproductive health have occurred since 2004, when Jay Kaplan organized an entire issue of the ILAR Journal (Vol 45, No 2, 2004; Kaplan 2004) focused on the value and contributions made from the use of nonhuman primate (NHP) models. Yet the need for basic and applied research related to women's health remains as great as ever. The world's human population continues to increase, with over 7.5 billion people inhabiting the earth (United Nations Population Database) and consuming its limited resources. New approaches are needed to eliminate the unmet need for low-cost, effective contraceptives that are acceptable to women throughout the societies of the world, and reduce the number of unintended pregnancies in countries where contraceptives are available by reducing side effects and increasing compliance (Binette et al. 2017). On the other end of the spectrum is infertility (Hanson et al. 2016); approximately 10% of couples are infertile, a disease typically diagnosed when they fail to conceive and thus fail to fulfill their desire to have children and family. As discussed later, knowledge of the causes and specific treatments to prevent or cure various types of infertility remains elusive. The major trend of affluent women/couples to use artificial reproductive technologies (ARTs, e.g., in vitro fertilization) has led to over five million babies worldwide, but there are limitations including at best a 30% to 40% success rate/protocol in reproductive-prime women (Toner et al. 2016). Moreover, evidence is increasing that environmental factors, such as diet or contaminants (often manmade) that we ingest or breathe, can detrimentally affect reproduction. Health care for various diseases can also affect fertility. An emerging area of research and health care, termed “oncofertility” (Ataman et al. 2016), addresses how to prevent the loss of or restore gametes (i.e., oocytes in young girls or reproductive-age women) lost during radiation or chemotherapy to treat cancer. To improve efficacy in these reproductive interventions, animal models are necessary, and the more they recapitulate the biology of human reproduction, the more likely the advances will be integrated into clinical practice (Duncan et al. 2016).
A number of animal species, but particularly laboratory rodent (rat, now superseded by mice) and agricultural (e.g., sheep) models, have contributed greatly to our understanding of the function and regulation of female reproduction. But a remarkable feature of this field is its comparative biology (Chaffin and VandeVoort 2013; Jiang et al. 2015). Consequently, it is only their closest relatives, the NHPs, that share with women many of the key aspects of hormonal, neural, and local control of reproduction through fetal development (Phillips et al. 2014). These include neuroendocrine hypothalamic-pituitary activity (Plant 2012), cyclic function of the ovary (Chaffin and VandeVoort 2013) and reproductive tract (oviduct, uterus, and vagina), establishment and control of the maternal-fetal-placental unit during pregnancy, and reproductive aging culminating in menopause. Recent advances continue to demonstrate aspects of basic reproductive biology that differ from nonprimates and are similar to women. For example, molecular analyses of the transcriptome (mRNAs) in the ovulatory follicle of rhesus macaques (Macaca mulatta) discovered activities that are not evident in the mouse model, including the critical role of leukemia inhibiting factor in ovulation (Murphy et al. 2016). Elegant studies by Golos and colleagues (Golos et al. 2010) on the maternal-fetal immune response in NHPs identified molecules of the nonclassical major histocompatibility complex class I genes and decidual immune cells (natural killer cells and macrophages) that are comparable to those in women during pregnancy. This information is leading to manipulative (e.g., passive immunization) studies, which could not be performed in women, to identify their roles in the initiation and maintenance of pregnancy. Such efforts illustrate how NHP research contributes to the framework of basic reproductive biology on which manipulations or treatments can be added to consider the causes and novel treatments of infertility disorders or to develop novel contraceptives.
Thus, despite trends to reduce or eliminate research on our “closest relatives,” especially chimpanzees (Pan troglodytes; Bennett and Panicker 2016), there remains a strong rationale and value for using NHP models when optimal for research on women's reproductive health and disease. This selective rationale appears actively embraced by researchers (Carlsson et al. 2004), with considerable attention to the best NHP species employed in experiments. While Old World macaque species, especially the rhesus macaque, have been the mainstay for reproductive research for decades (Story and Kennedy 2004), the advantages of the cynomolgus macaque (Macaca fascicularis), for example, its docile nature and lack of summer anovulatory cycles, are being recognized for contraceptive trials (Peluffo et al. 2014). Since the rhesus macaque has a tortuous cervical canal, the baboon (genus Papio) was chosen for studies requiring transcervical delivery of agents into the uterine lumen (Jensen et al. 2016) because of similarity to the human cervix. Finally, although New World monkeys, such as common marmosets (Callithrix jacchus), differ markedly from macaques or baboons (e.g., lack of menstrual cycles), their smaller size and shorter developmental interval offer advantages for some studies (Fereydouni et al. 2016; Worley et al. 2014).
The following sections provide examples of recent advances using NHP models that added to our understanding of the causes and treatment of women's reproductive health disorders in the area of infertility, as well as emerging opportunities related to ARTs/genome editing for generating novel disease models and preventing developmental defects. For necessary brevity, the focus is on the authors’ areas of interest and expertise in reproductive tract biology and disorders. The use of NHPs in other areas, such as the neuroendocrine aspects of reproduction and aging (Bethea et al. 2016; Kohama et al. 2016), are no less important. The authors also acknowledge the use of recent reviews in each area and recommend their perusal for more details.
Recent Advances
Ovarian Disorders
Polycystic Ovary Syndrome
Polycystic ovary syndrome (PCOS) is an endocrine disorder accompanied by infertility in reproductive-age women. The phenotype of PCOS is remarkably heterogeneous, leading to international meetings attempting to offer guidelines for diagnosis and clinical care (for review, see Dumesic et al. 2015). In general, patients must exhibit at least two of three major characteristics: clinical or biochemical signs of hyperandrogenism, oligo- or an-ovulation, and abnormal number of small-to-medium antral follicles in the ovaries, (mistermed as “polycystic” ovaries). Accompanying symptoms may be elevated levels of luteinizing hormone (LH) and follicle-derived anti-mullerian hormone, plus metabolic changes, notably insulin resistance and obesity. It is now generally accepted that PCOS defects extend beyond an ovarian disorder to encompass hypothalamic dysfunction and aspects of metabolic disease.
Unfortunately, the etiology of this relatively common disorder (up to 15% in women; March et al. 2010), which is clinically diagnosed after puberty, is unclear. Clearly, there is a genetic component; observations of first-generation female relatives of mothers with PCOS and of Dutch Twins suggest that over 70% of PCOS pathogenesis has a genetic cause (Vink et al. 2006). Recent genome-wide association studies identified mutations or polymorphisms in areas associated with over 20 genes (Puttabyatappa et al. 2016), but to date the functionality of only one gene, DENND1a, has been documented (McAllister et al. 2014). It is estimated that identified gene changes account for only a small fraction of the heritability of PCOS. Since PCOS appears to have a complex, polygenic inheritance, much larger population sizes similar to those required to analyze type 2 diabetes (Horikoshi et al. 2016) may be needed for genome-wide association studies. Also, epigenetic changes within the mother or offspring, for example, due to environmental factors, could contribute to the PCOS phenotype. One such factor is likely nutritional status and subsequent obesity. Puttabyatappa and colleagues (Puttabyatappa et al. 2016) propose a model whereby genetic susceptibility “organized” in early fetal life combines with environmental influences and lifestyle choices in later (through adolescence) life to “activate” PCOS and influence its severity.
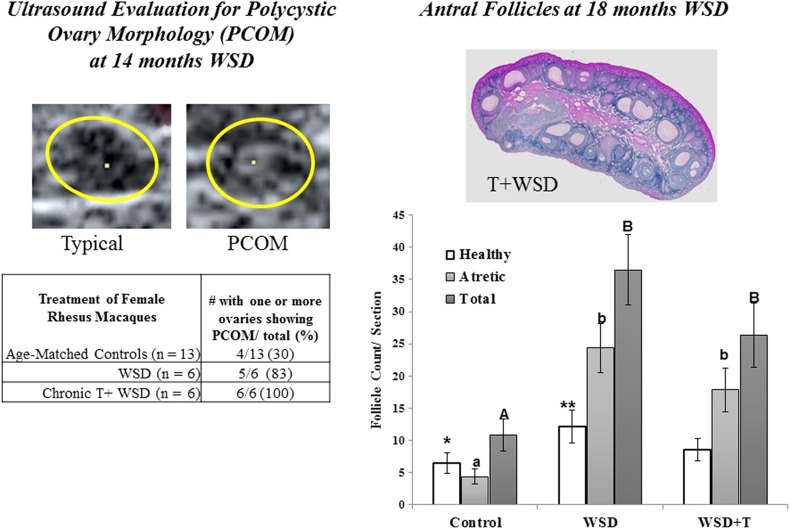
PCOS is typically diagnosed in young adults due to features of androgen excess (e.g., hirsutism) menstrual irregularity and infertility or obesity, but it is difficult to study children or fetal development. Thus, there is little known about the etiology of this syndrome in women. Likewise, the lack of naturally occurring PCOS in any animal model limits research. Nonprimate and NHP species are used in PCOS research, including typical laboratory rodents, sheep, and rhesus macaques, with each model having advantages and disadvantages. Rhesus macaques are valuable because of their genetic makeup, plus the regulation and function of the menstrual cycle is similar to humans. Notably, these macaques are monovular and the kinetics of follicle development is believed similar to that in women; hence, appreciable numbers of growing (small to medium) antral follicles are not present in the ovaries in the presence of the dominant follicle or the corpus luteum (Bishop et al. 2009). Thus “polycystic ovaries” can be identified by ultra-sonography (Bishop et al. 2016).
Over the past decade, Abbott and Dumesic (see review, Abbott et al. 2016) characterized the effects of exposing adult female rhesus macaques and their fetuses to excess androgen (testosterone, T) during early-to-mid or late gestation. Although initial protocols were designed to study the role of sex steroids in brain organization for reproductive behaviors (Goy and Phoenix 1972), follow-up led to the discovery that early-to-mid gestation exposure to T led to PCOS-like characteristics in two-thirds of the female offspring in adulthood. When clinical criteria were applied, 42% exhibited “clinical” PCOS (hyperandrogenism + oligo- or an-ovulation + PCO morphology or hyperandrogenism, + oligo- or an-ovulation). Another 25% displayed “milder” PCOS (with either ovulatory cycles or normal androgen levels). The heterogeneity of features is similar to that in PCOS-diagnosed women. The authors (Abbott et al. 2016) propose that this phenotypic diversity reflects the effects of multiple “hits,” that is, genetic changes, onto which epigenetic changes due to environmental influences, such as elevated T levels during pregnancy or metabolic perturbations during fetal-to-adult life promote various features of PCOS.
This research on the rhesus macaque as well as that using sheep by Padmanabhan (see review, Puttabyatappa et al. 2016) provide evidence supporting the concept of fetal/prenatal influences (fetal T exposure) and programming that result in adult-onset diseases, such as PCOS. The direct translation of these data to clinical PCOS is not currently possible; it would require blood/tissue samples from human fetuses during early-to-mid gestation and positive correlation of elevated T levels in those that ultimately develop PCOS postpubertally. There may be some indirect assessments made postnatally that reflect fetal exposure to excess T, such as anogenital distance, clitoral volume, or increased ratio of 2D:4D finger length (Abbott et al. 2016). T levels measured in umbilical cord blood of newborns are not helpful and too late to reflect earlier events, but androgens in scalp hair of newborns may be informative (Kapoor et al. 2014).
The rhesus macaque (and sheep) models of inducible PCOS offer opportunities to study the effects of fetal T exposure during fetal, childhood, adolescence, and adult life. For example, after exogenous T treatment in early-to-mid gestation (in which large doses are given to the mother due to active metabolism by the placenta and neutralization by sex hormone binding globulin; however, T levels in the female fetus approximate those observed in the male), endogenous hyperandrogenemia does not appear in natal life. However, following delivery, these female infants are hyperandrogenemic (Abbott et al. 2016) without a polycystic ovary phenotype. Subsequently, ovarian function is quiescent until puberty; however, fetal T exposure delays menarche and initial menstrual cycles display luteal phase deficiency compared to controls. The latter may be due to elevated LH, but not FSH, levels, perhaps resulting from impaired negative feedback by steroids as described in adolescent girls at risk for PCOS (Burt Solorzano et al. 2012). A number of genetic studies in PCOS women (Puttabyatappa et al. 2016) identified altered genes associated with LH signaling (including the LH receptor). The importance of neuroendocrine (hypothalamic-pituitary) changes in etiology of PCOS and as a site of therapy (George et al. 2016) warrants further attention.
It is also becoming clear that fetal T exposure resulting in PCOS-like features in rhesus macaques causes marked changes in metabolism from fetal development to adulthood (Abbott et al. 2016). Fetal T exposure resulted in transient hyperglycemia at mid gestation, a modest elevation in circulating insulin levels through late gestation, abnormal fetal lipid patterns, and increased fetal size. Many of the newborns were hyper-insulinemic, plus their pancreas exhibited a higher beta:alpha cell ratio. By adulthood, the fetal T-exposed females exhibiting a PCOS-like phenotype displayed preferential accumulation of visceral, as opposed to subcutaneous, fat. This adipose feature included epigenetic (DNA methylation) changes in genes of visceral fat (Xu et al. 2011) as well as a decline in subcutaneous abdominal adipogenesis. The findings suggest that fetal T excess results in early (gestational and child hood) changes in metabolic processes that are important components in the PCOS phenotype and its heterogeneity during reproductive maturation and onset of obesity.
Further studies are needed in NHP models to study the prenatal and prepubertal events following T exposure, their role(s) in developing the reproductive and metabolic features of subsequent PCOS, and whether their manipulation could prevent this syndrome. Recent studies on fetal T-exposed sheep (Puttabyatappa et al. 2016) indicate that prenatal antiandrogen treatment prevents many, but not all (Cardoso et al. 2016), of the reproductive features, but not the metabolic abnormalities. In contrast, prenatal treatment with an insulin sensitizer ameliorated the insulin resistance and normalized the onset of puberty. However, antiandrogen or insulin sensitizer administration to women at risk for having PCOS offspring is unlikely without extensive efficacy and safety trials in NHPs that can be translated to human pregnancy and development.
As noted by Abbott and colleagues (Abbott et al. 2016), a safer approach likely involves pre- or peripubertal intervention to prevent PCOS in adolescent girls at risk for the syndrome or to reverse PCOS in young adults. Since patients typically present at the clinic as young adults when the symptoms of hyperandrogemia (hirsutism) and diet (obesity) become a concern. Cameron, Stouffer, and colleagues (McGee et al. 2012, 2014) initiated studies on young female rhesus macaques to determine the effects of chronic T exposure beginning at 1 year of age, with added high-fat diet at 5 years of age. Although T exposure caused some neuroendocrine changes (e.g., increased LH pulse frequency) during puberty, there was only a hint of altered follicular dynamics (absence of an antral follicle of the expected 2 mm diameter in the early follicular phase). However, the marked increase in percent body fat after 1.5 years of combined Western-style diet and T was associated with a marked increase in the number of small-to-medium antral follicles in the ovaries, plus a PCOS-like morphology evident by ultrasound (Figure 1; Bishop et al. 2016). While these features were also evident in animals receiving the WSD alone, it was only with the combined T + WSD that insulin sensitivity declined significantly and further alterations in the menstrual cycle (e.g., reduced size of the dominant follicle in late follicular phase; suppressed progesterone levels in the luteal phase) were noted. Given the limitations of this sequential treatment regimen, further studies are ongoing with greater numbers of rhesus macaques per treatment (control diet, Western-style diet, T alone, T plus WSD) to evaluate the chronic (up to 5 years) effects on neuroendocrine, reproductive (including fertility), and metabolic parameters. Initial results after 3 years of treatment are discovering effects of T or diet alone, but also confirming more significant effects of combined T + WSD, for example, on percent increase in body fat, decreased insulin sensitivity (True et al. 2017), and ovarian features (e.g., decreased progesterone levels; True et al. 2016). Whether these PCOS-traits will expand (e.g., from the presence of abnormal ovarian menstrual cycles to absence of cycles) with further treatment or result in infertility awaits further testing. Whether therapies that prevent T action or offer diet intervention will restore characteristics to those in controls also warrants study.
Figure 1.
Ultrasound evaluation of polycystic ovary morphology (PCOM); (left panels) and histologic estimation of the numbers of antral follicles (right panels) in adult female rhesus macaques after chronic exposure to exogenous androgen (testosterone, T) from 1 year of age, with a high-fat/-fructose diet (western-style diet, WSD), compared to age-matched colony controls or WSD alone. PCOM was prevalent in both treatment groups by 14 months on WSD, and the ovaries of WSD and T + WSD--treated animals contained greater numbers of small antral follicles, many undergoing atresia at 18 months on WSD. Adapted from (Bishop et al. 2016).
Mitochondrial DNA-based Diseases from the Oocyte
Mitochondria are cytoplasmic organelles, likely derived from bacteria, which play a vital role in cellular energy generation by oxidative phosphorylation as well as number of other activities such as steroidogenesis (Wolf et al. 2015). Mitochondrial (mt) activity involves the products of a limited number of genes (n = 37 in humans) residing in the organelle (i.e., mtDNA) that interact with those of nuclear genes. Diseases involving mtDNA have been recognized for nearly two decades; currently over 700 mutations are known and associated with myopathies, neurodegeneration, diabetes, cancer, and infertility. There are large numbers of mitochondria in highly energetic cells, including the oocyte. The latter is particularly important, since offspring exclusively inherit mtDNA from their mother (i.e., the egg). Germline mtDNA mutations from the mother (i.e., maternal transmission) can potentially cause disease at a frequency of 1 in 200 newborns. Symptoms can begin at any age, and disease severity depends on a number of factors, including the percent mtDNA within the cell that has the mutation (i.e., the level of heteroplasmy). Typically a cell/oocyte contains only one mtDNA genome (homoplasmy); however, if it contains two or more types of mtDNA (heteroplasmy), the mutation can persist and be passed to the next generation.
Current methods for assisting couples at risk for germline transmission of mtDNA-based disorders include preimplantation genetic diagnosis. While useful in low heteroplasmic scenarios, there are risks involved, and it is not useful for women with a mutation incidence of 100%. Recently, Mitalipov and colleagues (Tachibana et al. 2009) used an NHP model to develop a novel method of mitochondrial replacement therapy (MRT) called spindle transfer, or ST. To validate the technique, the meiotic spindle with a very small amount of cytoplasm was extracted from the mature (metaphase II, fertilizable) oocyte of one rhesus macaque and combined with an enucleated oocyte from another. The spindle was collected from the oocyte of a Chinese-origin rhesus macaque, whereas the enucleated oocyte was from an Indian-origin rhesus macaque, and vice versa, thereby allowing analyses of mtDNA origin. Fertilization and blastocyst development were comparable to controls. When 15 ST-derived blastocysts were transferred to the reproductive tract of nine recipient females, three pregnancies resulted in four live births (Figure 2). Moreover, the percent carryover of mtDNA from oocytes used for spindle collection was minimal at <2%. The offspring are healthy and exhibit normal developmental parameters, now through puberty and young adulthood. Moreover, embryonic stem cell lines were generated from eight ST-derived blastocysts for detailed studies.
Figure 2.
Spindle transfer from one oocyte to an enucleated oocyte (left panel), and two of the resulting rhesus macaques, Mito and Tracker, soon after birth (middle panel) and at two years of age (right panel). They are now both healthy adults. Provided by S. Mitalipov. For details of process, see Tachibana et al. (2009).
Clinical studies suggest that it is possible to retain a patient's nuclear genome by collecting the spindle-chromosomal complex, while leaving behind the maternal cohort of mitochondria with mutant mtDNA, and transferring the nuclear genome into an enucleated egg containing normal mtDNA donated by a healthy compatible female (Adashi and Cohen 2016). After fertilization with the sperm of the patient's partner, a resulting child would be free of the risk from the patient's mutated mtDNA, while having all the usual nuclear genetic features of the female patient and male partner. The child would contain the mitochondrial genome from the egg donor, leading the general press to coin the term “three-parent babies” from such therapy.
There are ethical and regulatory issues related to ST or other methods of MRT such as pro-nuclear transfer. Moreover, there are biological issues remaining due to the small sample size and lack of next inter-generation data. Nevertheless, after considerable due process, the Human Fertilization and Embryology Authority and Parliament of the United Kingdom provided support for human studies on MRT, which are proceeding. While such research in the United States is limited by regulation and cannot use federal funding, Zhang and colleagues (Zhang 2016) reported in 2016 the first live birth using a human oocyte reconstituted by ST to eliminate a mtDNA mutation causing Leigh Syndrome. Despite early applications to human patients and their oocytes, further studies on NHPs are needed into the efficacy and safety of MRT, including the developmental features of first and subsequent generations of MRT-derived offspring.
Uterine Disorders
Endometriosis
Endometriosis is a gynecologic disease affecting reproductive-age women (for review, see Braundmeier and Fazleabas 2009; Story and Kennedy 2004) and characterized by the presence of endometrial tissue outside its typical site in the uterus (bordering the lumen). The most common symptom is chronic pelvic pain, but it is also associated with painful intercourse and menstrual periods, plus it is associated with sub- or infertility. Endometriosis is estimated to affect up to 10% of reproductive-age women, but this is probably an underestimate due to the multi-year delay between symptom onset and its threshold to clinical diagnosis. There is significantly decreased quality of life, loss of work productivity, and economic burden associated with endometriosis. Simoens and colleagues (Klein et al. 2014) estimate the total health care costs for endometriosis are similar to those of other chronic diseases such as diabetes and rheumatoid arthritis. To date, there is no curative or preventative therapies for endometriosis. Surgery, typically involving laparoscopic removal of lesions from the pelvic area and clearance of adhesions is palliative, but symptoms reportedly recur in up to 40% of women. Pharmacologic approaches involve hormonal manipulation, for example, the oral contraceptive pill with progestin, or GnRH analogs to eliminate estrogen with or without added progestin. These treatments may relieve pain but eliminate fertility. Current drug therapies do not eliminate lesions and have side effects that reduce their acceptability for chronic use.
There have been three international workshops to develop and highlight the research priorities in the field of endometriosis, the most recent held in 2014 (Rogers et al. 2017). Despite significant research activity, it was noted “somewhat disappointingly” that only 2 of the 54 previous recommendations were adequately addressed. The etiology of endometriosis is difficult to study in women, because the disease is well established by the time of presentation at clinic. The workshop continued to call for “appropriate animal and in vitro models for preclinical studies” to increase our understanding of the causes, diagnoses, and treatment plus prevention of this disease. Both nonprimate and NHP models have been used to study endometriosis for many years (Story and Kennedy 2004), but NHP species offer some advantages. For example, Old World monkeys and baboons have monthly cycles in ovarian and uterine structure-function that are comparable to those in women; these animals menstruate. Moreover, hormonal control of endometrial proliferation followed by differentiation and then sloughing, in response to sequential estrogen and progestin exposure and withdrawal, also occurs in these species. Several species of NHPs, but not nonprimates, spontaneously develop endometriosis (Story and Kennedy 2004). Nevertheless, there are limitations to general use of NHP models for endometriosis in that their availability is limited and their use is very expensive. The population size required to secure an adequate cohort of NHPs spontaneously developing endometriosis severely limits study (D'Hooghe et al. 2009). However, considerable progress has occurred with an NHP model of experimental induced endometriosis that allowed investigators to: (1) characterize events in the pathogenesis of endometriosis, (2) study the molecular changes in the ectopic and eutopic endometrium that cause pelvic pain and infertility, respectively, and (3) perform preclinical trials of new drug therapies for endometriosis.
Several macaque species have been utilized for developing experimental endometriosis, but most success occurred from the baboon model championed by the groups of Fazleabas (Braundmeier and Fazleabas 2009) and D'Hooghe (D'Hooghe et al. 2009). The procedure, wherein menstrual tissue is collected and immediately transferred intra-abdominally, provides substantial evidence supporting the Sampson Hypothesis (Slayden 2013) that endometriosis arises from retrograde menstruation of sloughed endometrial fragments through the oviducts, after which they attach to sites in the pelvic cavity, such as the ovary and peritoneum. Sequential laparoscopies for up to 15 months after inoculation (Harirchian et al. 2012), plus removal of endometriotic lesions at 6 months, provided macroscopic (color, type), microscopic (e.g., vascularization, immune cells), and molecular (enzymes, hormone receptors, local factors) indices of the events during the development of endometriotic lesions. Braundmeier and Fazleabas (2009) summarized their findings of gene alterations in baboon ectopic endometrium during the attachment, invasion, and maintenance (including angiogenesis) of endometrial lesions and compared their results to human lesions. Notably, the dysregulation of steroidogenic enzymes and steroid hormone receptors promotes lesion development by maintaining cell proliferation. Also, the increased numbers of immune cells and cytokines likely promote an inflammatory response, plus invasion by ectopic lesions through activation of enzymes (e.g., matrix metalloproteinases) for tissue remodeling. Elevated expression of angiogenic factors (e.g., vascular endothelial growth factor) may also facilitate lesion development and maintenance.
In addition, by comparing the characteristics of the eutopic endometrium to those of endometriotic lesions over time, insight into the likely causes of infertility was acquired (Braundmeier and Fazleabas 2009). Data suggest that the development of ectopic lesions leads to early (<3 months; e.g., miRNA-451, YWHAZ mRNA, Joshi et al. 2015; regulatory T cells; Braundmeier et al. 2012) and later (>6 months, e.g., PGR and PGR-regulated gene activity, Fazleabas 2010) changes in the eutopic endometrium. These changes cause resistance to progesterone action and altered expression of a number of genes required for decidualization and embryo implantation (Braundmeier and Fazleabas 2009). However, the fertility potential of baboons with chronic spontaneous or experimental endometriosis has yet to be tested.
The baboon with experimental endometriosis is also proving useful for evaluating the efficacy of novel drug therapies. Recently, Langoi and colleagues (Langoi et al. 2013) reported that 6 months of treatment with the aromatase inhibitor letrazole reduced the volume of endometriotic lesions in baboons, presumably by suppressing the conversion of androgen to estrogen in endometriotic tissue (which expresses high levels of aromatase) as well as the ovary, thereby reducing the trophic and proliferative action of estradiol. Thus letrazole therapy can decrease the disease burden as well as reduce pelvic pain in women (Langoi et al. 2013), but it does not cure the disease. D'Hooghe and colleagues (Falconer et al. 2006; Lebovic et al. 2010) also report that nonendocrine therapies designed to reduce inflammation have potential to reduce endometriosis. Initial evidence suggested that: (1) addition of a binding protein to neutralize tumor necrosis factor-α (recombinant human TBP-1), prior to or after endometriosis induction, and (2) the ligand to the peroxisome proliferator-activated receptor PPAR-γ reduced the size of endometriotic lesions, with the former reducing pelvic adhesion formation. Hussein et al. (Hussein et al. 2016) also report that 60 days of treatment with an inhibitor of c-Jun NH2-terminal kinase (JNK), bentamapimod, reduced the surface area and volume of endometriotic lesions compared to placebo controls. The combination of JNK inhibitor and progestin (medroxyprogesterone acetate) did not increase these effects but increased remodeling of active (red) lesions to white scar lesions. Importantly, no severe side effects or changes in cyclic ovarian function were observed. Therefore, disruptors of immune cell function and inflammatory pathways offer promise in controlling endometriosis; early phase clinical trials are under way to evaluate the safety and efficacy of a JNK inhibitor AS602801 in reproductive-age women (Hussein et al. 2016).
Uterine Transplantation
Despite some success in therapies for certain causes of infertility in women, uterine-factor infertility (UFI) remains untreatable (for review, see Kisu et al. 2013). UFI describes patients that are unable to conceive and maintain an intra-uterine pregnancy because their uterus is absent or nonfunctional. The most common cause of UFI is the presence of leiomyoma (fibroids) in the uterus, which leads to infertility if a hysterectomy is indicated. Any condition that results in hysterectomy, for example, 50% of patients with cervical cancer, results in UFI. But the prevalence of uterine malformations (up to 7% of women) and intrauterine adhesions also provides a patient population. These patients may perceive a decline in quality of life and loss of female identity, their only option for motherhood being adoption or, in a limited number of countries, gestational surrogacy.
However, with advances in organ transplantation and prevention of allogenic tissue rejection, ranging from vital organs such as heart and lung to “nonvital” “quality of life” organs such as limbs and face (Siemionow et al. 2010), clinician teams are considering uterine transplantation as a cure for UFI. Uterine transplant research has been performed on a number of species, most recently in NHPs such as macaque species (Kisu et al. 2013) and baboons (Brännström et al. 2012). The value and need for further studies on uterine transplantation in NHPs was recognized, based on similarities in anatomy and physiology to women, and the challenges that can be addressed including: (1) optimization of surgical procedures and minimization of organ ischemia-reperfusion injury, (2) evaluation of transplanted uterus viability, including blood flow, (3) assessment of immunosuppression and tissue rejection, and (4) pregnancy and fetal development, including teratogenic effects of immunosuppressive drugs (Kisu et al. 2013). Nevertheless, there are limitations to use of NHPs for uterine transplantation research. However, (Kisu et al. 2013) report a successful pregnancy and delivery after an autologous uterine autograft in the cynomolgus macaque. Also, (Brännström et al. 2012) performed detailed studies in sheep and baboon to provide insight towards uterine transplantation in women. After studies improved the surgical method for vascular anastomoses, autologous uterine transplantation in a cohort of female baboons resulted in the resumption of menstruation in 60% of the animals. However, pregnancies did not occur during mating trials, possibly due to occluded oviducts (Johannesson et al. 2012). There is less success to date on allogenic uterine transplantation in NHPs (Tryphonopoulos et al. 2014). Tissue rejection and failure to recover function (e.g., menstruation) likely occurred in baboons because of initial difficulties in defining and maintain adequate circulating levels of immunosuppressive agents. Nevertheless, information gathered from such studies was deemed sufficient to begin clinical trials. As of 2016, 11 attempts at uterine transplantation have been reported, with two live births achieved, the most recent after transplanting the uterus from a mother to her daughter (Brännström et al. 2016). The uterus was removed 3 months after delivery due to extensive adhesions. Further studies on uterine transplantation are warranted in NHP models, which should influence its successful application to women with UFI.
Pregnancy Disorders
Placental Dysfunction: Environmental Influences
There is very limited information on the normal trajectory of maternal-embryo/fetal interaction from implantation through placental development and function in women. Studies are difficult due to ethical issues to avoid risk to mother and fetus. Also, there are limitations to study of human tissues from pathologic situations collected at late-stage disease or at term delivery when placental structure-function is already degenerating. (Grigsby 2016) recently provided an overview of the advantages and disadvantages of various animal models for the study of pregnancy disorders. Higher order primates are valuable, because many aspects of maternal recognition and control pregnancy and placentation are similar to women. For example, the role of chorionic gonadotropin in prolongation of corpus luteum structure-function in early pregnancy and the decidualization of the uterus (Banerjee and Fazleabas 2011), the regulation of placental steroidogenesis, and lack of serum progesterone withdrawal at term (Haluska et al. 2002), and the prolonged length of gestation are exclusive features of such primates. Most importantly, these NHPs have a hemochorial placenta with a villous structure within regional cotyledons. Thus, unlike in most species, there is direct contact of maternal blood with fetal tissue; invasion of the trophoblast into the uterine endometrium after implantation leads to erosion of maternal vessels, such that the syncytio-trophoblast layer is directly bathed by maternal blood from the spiral arteries entering each cotyledon. Although the depth of trophoblast invasion is less in NHPs (e.g., rhesus macaques), the features of the hemochorial wall and the pattern of intervillous circulation is comparable to that of women (Grigsby 2016).
While it is possible to manipulate parameters and assess changes at specific times during NHP gestation, the development of novel techniques that noninvasively monitor placental structure-function and permit chronologic analysis over the course of pregnancy would be very valuable. This is one of the overall goals of the National Institutes of Health (NICHD RFA-HD-15–030 and 034) recently announced “Human Placenta Project” for real-time assessment of placental development and function in normal and diseased pregnancies. Preclinical studies in NHP models are already contributing major advances. For example, Slayden and colleagues (Keator et al. 2011) recently used “contrast-enhanced” ultrasonography to quantitate blood flow and volume in uterine compartments and implantation site of rhesus macaques around pregnancy initiation.
Also, Frias and colleagues (Frias et al. 2015) used dynamic contrast-enhanced magnetic resonance imaging to spatially and quantitatively characterize maternal blood perfusion in the intervillous space of the macaque placenta during the third trimester (gestation day 133; term day 165). Sixteen perfusion domains were identified that corresponded to individual cotyledon units observed after delivery. Remarkably, the blood flow within each domain/cotyledon varied greatly from 9 to 45 mL/s. This technique will greatly increase opportunities for evaluation of the characteristics of placental development and vascular perfusion during normal gestation as well as conditions of environmental changes or varied embryo quality. For example, (Frias et al. 2011) reported that a maternal high-fat diet (HFD) resulted in marked (up to 56%) reduction in uterine and placental blood flow, regardless of whether the animals remained lean or became obese. However, histologic features of uteroplacental insufficiency were more frequent in the HFD-obese animals, with decreased vascular flow evident on both the maternal and fetal sides of the placenta. Notably, an appreciable rate of fetal death was associated with the HFD-obese group (7 of 20 pregnancies), compared controls, or HFD-lean animals (1 per group). Additional studies in NHPs using such emerging, noninvasive techniques (Figure 3; Schabel et al. 2016) will be valuable in studies on the effects of dietary (e.g., alcohol; Lo et al. 2017) and environmental factors (e.g., over- and undernutrition) and other agents on placental structure-function and fetal development. Frias and colleagues (Lo et al. 2015) recently reported that female rhesus macaques receiving chronic nicotine (to approximate levels in pregnancy women smokers) displayed reduced placental blood flow, as measured near term, with abnormal villous histology. However, co-administration of ascorbic acid mitigated these changes. These NHP studies underscore the presumed harmful effects of prenatal nicotine exposure on placental structure-function and support emerging evidence that vitamin C supplementation can limit some adverse effects of smoking during pregnancy.
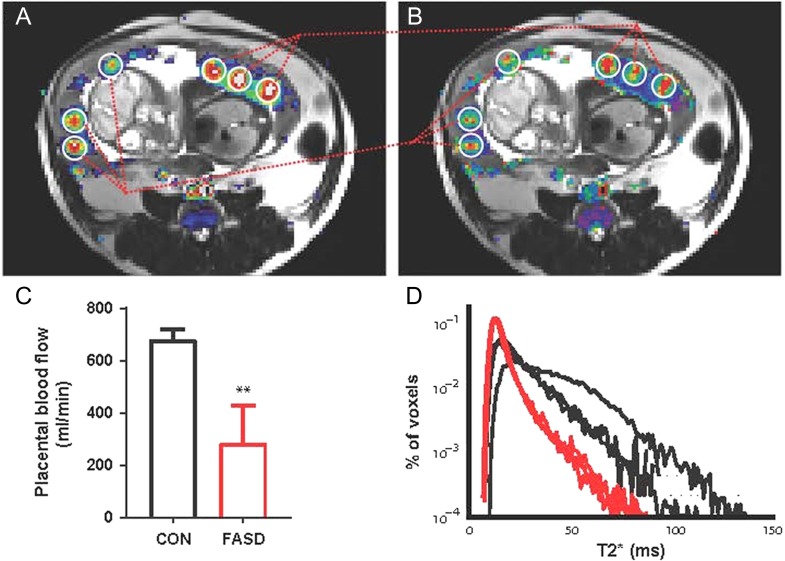
Figure 3.
Dynamic contrast-enhanced (DCE)- and T2*-MRI of the macaque placenta. Correlation between blood flow (A) and blood oxygen level dependent (BOLD, B; Schabel et al. 2016) contrast is demonstrated with images from a rhesus macaque on gestation day 110 (GD 110) during a single MRI session. Blood flow data from DCE-MRI are super-imposed on the T2w image in A. C summarizes the decreased total placental blood flow in animals consuming 1.5 mg ethanol/kg/d during the first 60 days of gestation (FASD) compared to controls (CON, P < .01). Ethanol-treated macaques also had a smaller fraction of large T2* values than controls, demonstrating decreased fetal oxygen availability at GD60. Adapted from (Lo et al. 2017).
Preterm Labor and Delivery
Intrauterine infections are a well-recognized feature of preterm labor and delivery, which results in the majority of very-low birth weight babies before 30 weeks of gestation (Waites et al. 2009). These babies have the highest rates of serious neonatal complications and death, generating a large share of perinatal health care costs as well as health problems into adulthood. Ureaplasma species are the bacteria most commonly isolated from amniotic fluid and placenta of women who deliver prematurely and in the cord blood, respiratory tract, and cerebrospinal fluid of their premature infants. These bacteria are detectable in amniotic fluid as early as 16 weeks of gestation but result in a “clinically silent” but progressive infection until labor and delivery occurs several weeks later. However, a direct causal role of these organisms in preterm labor has not been established in women, in part because of the polymicrobial population in the reproductive tract and the difficulty of performing controlled experiments in human pregnancy.
Novy and colleagues (Novy et al. 2009) employed an NHP model, the rhesus macaque, to determine if intra-amniotic inoculation of either U. parvum or Mycoplasma hominis caused preterm labor and delivery. Indeed, either bacteria caused a sequential upregulation of leukocytes, proinflammatory cytokines, prostaglandins, and select matrix metalloproteinases in the amnion, which was associated with increased numbers of bacteria and progressive uterine contractions, culminating in preterm labor and delivery. The histologic appearance of fetal membranes was similar after infection with either bacteria, but the inflammatory changes were more pronounced with U. parvum. This study also provided direct evidence that these bacterial infections can cause problems in the fetus that become evident at birth, including lung and brain disease. Macaque fetuses infected in utero with either U. parvum or M. hominis displayed inflammatory changes in the respiratory tract which, with chronic infection, included aggregates of peribronchiolar lymphoid tissue and hyperplastic changes in the overlying epithelium. These results support those of human studies to generate a model whereby Ureaplasma spp. infection causes an inflammatory cascade in the lung and subsequently impairs alveolar development directly or in conjunction in side-effects of assisted ventilation (O2 toxicity, ventilator trauma) of premature infants (Waites et al. 2009). Also, while there was no evidence of hemorrhage or leukomalacia in the fetal brain after intra-amniotic infection with U. parvum, there were areas of microgliosis and astrocytosis as well as foci of neural injury in the white-matter of the periventricular region (Coksaygan et al. 2010). Additional studies are ongoing to determine if such neural changes result in behavioral or cognitive defects during postnatal development (Roberts et al. 2012).
Subsequently, Grigsby and colleagues (Grigsby et al. 2012) used the intra-amniotic U. parvum inoculation model to determine if maternal administration of the antibiotic azithromycin (AZI) with or without antiinflammatory agents would prevent preterm birth and fetal lung inflammation. AZI treatment (intravenous, twice a day for 10 days) eliminated U. parvum from the amniotic fluid within 4 days. Also, placental and fetal tissues were typically negative (90%, by bacterial culture) at delivery. AZI treatment significantly delayed the onset of labor and delivery by a week compared to controls; addition of dexamethasone (DEX) and indomethacin (INDO) did not further delay labor/delivery. AZI therapy also prevented many of the inflammatory features observed in the fetal lung after bacterial inoculation in the amniotic fluid. Preliminary evidence also suggests that maternal AZI treatment alone or with DEX/INDO reduces the development of brain inflammation and neuronal injury (Grigsby et al. 2012). Notably, the combination of AZI with DEX/INDO markedly reduced the extent and severity of the chorioamnionitis compared to AZI alone.
These detailed NHP studies definitively establish that intrauterine infections can cause premature labor and delivery as well as deleterious effects on the fetus that portend respiratory and neural deficits after birth. Moreover, the findings offer feasibility for clinical trials with optimal antibiotic/antiinflammatory regimens to delay, if not prevent, premature labor and delivery, plus reduce fetal lung and CNS injury in pregnant women, thereby improving perinatal outcomes following intrauterine bacterial infections.
Emerging Opportunities
Fetal Development and Birth Defects
Because of the increasing controversy over the use of human fetal tissue for research in the United States as well as restrictions on abortions in many countries, there is concern regarding the ability to study factors or agents that may impair fetal development and to design therapies that prevent defects (Hayden 2016). The emergence of Zika virus (ZikV; F. Flavivirus) is a recent example (Cugola et al. 2016) and demonstrates the value of NHP models to help fill this research void. This arbovirus, first detected in 1947 from blood samples of rhesus macaques, is spread by Aedes aegypti mosquitoes and caused its first epidemic in the South Pacific in 2007. By 2015, the Asian-lineage virus spread to countries in Central and South America; in Brazil, the virus was linked to congenital defects during pregnancy, including neurological diseases and microcephaly. Since macaques are widely used in both infectious disease and obstetrical research, this NHP model was recently employed to study ZikV infection.
(Dudley et al. 2016) report that following ZikV inoculation, all rhesus macaques (n = 8, including 2 pregnant females) tested positive for ZikV RNA in saliva, urine, and cerebral spinal fluid within 1 day and remained viremic for 3 weeks in nonpregnant animals. Neutralizing antibodies were detected by 21 days postinoculation. Also, re-inoculation 10 weeks after the initial challenge did not elicit a second round of viral replication, suggesting that protective immunity developed rapidly to homologous strains. However, pregnant animals remained viremic as long as the study continued; at pregnancy termination, the fetal brain contained 108 viral copies/mL tissue. In a follow-up report (Nguyen et al. 2017), maternal viremia persisted in three of four pregnancies, with ZikV RNA detected in all four fetuses at term delivery. The viremia persisted in pregnant macaques despite activation of immune (NK and T) cells and development of neutralizing antibodies (Dudley et al. 2016). The macaque should prove a valuable NHP model for studying the mechanisms of maternal-fetal infection by ZikV, the processes whereby the viruses causes disease in both the mother and fetus, and therapies to prevent infection or postinfection disease. Notably, Aliota and colleagues (Aliota et al. 2016) reported that the immune response elicited by an East African strain of ZikV completely protected rhesus macaques from viremia when subsequently challenged by an Asian strain. This should portend successful development of a vaccine that is protective against diverse ZikV strains.
Another recent report by Golos and colleagues (Wolfe et al. 2017) highlights the unexpected and unique insights that can be gained from a NHP model. These investigators used the cynomolgus macaque as a model to study pregnancy complications arising from maternal infection with the bacterium Listeria monocytogenes. Listeriosis, often caused by ingestion of contaminated food, typically does not occur in healthy individuals but is a significant risk under certain conditions, including pregnancy. Adverse outcomes are generally associated with the third trimester, including miscarriage, preterm labor, stillbirth, and fetal infection. However, in establishing the macaque model, it was discovered that first trimester inoculation resulted in the greatest bacterial burden in the maternal decidua, the placenta, and fetus, with fetal death occurring with 1 to 2 weeks postinoculation. This NHP model will permit detailed studies on the mechanisms of host-pathogen interaction at the maternal placental-fetal interface and broaden clinical attention to L. monocytogenes infections in early pregnancy (Wolfe et al. 2017).
Another example of the value of NHPs is in research on the etiology and consequences of fetal growth restriction or FGR (Tang et al. 2017), which occurs in 5% to 10% of human pregnancies. It is a major cause of perinatal mortality and morbidity and, as a disruptor of developmental programming, results in children with increased risks for neurologic, cardiovascular, and metabolic disease later in life. FGR is a heterogeneous disease with a variety of clinical subgroups. In one scenario, Nathanielsz and colleagues used a baboon model (Cox et al. 2013) to investigate how even moderate nutrient insufficiency during pregnancy, via a 30% reduction in maternal nutrition, results in suboptimal development of structure-function in multiple organ systems, including the placenta (Kavitha et al. 2014) and fetal kidney (Pereira et al. 2015), liver (Abu Shehab et al. 2014), heart (Kuo et al. 2017), and brain (Franke et al. 2017). The later study using a novel noninvasive biomarker revealed premature “aging” of the brain when the fetus is exposed to maternal undernutrition. The studies demonstrated differential effects between males and female fetuses as well as remarkable effects in the absence of FGR or marked maternal weight reduction at term gestation (Franke et al. 2017). These important findings provide impetus for more clinical oversight during conditions of moderate nutritional reduction in pregnancy (e.g., dieting to control weight gain) as well as insight into the molecular and cellular events leading to fetal and ultimately postnatal deficits of malnutrition in pregnant women.
New Disease Models by NHP Genome Editing
Since the creation of the first transgenic mice in the mid-1970s, genome editing (for review, see Chen et al. 2016; Porteus 2015) that either over- or underexpresses specific gene products has been vital to increasing our knowledge of the role(s) of genes in physiologic and pathologic conditions. Moreover, application of the Cre-lox technique to conditionally target gene modifications to specific cell types/tissues further advanced the field and generated disease models, especially in mice. However, mouse models with gene modifications do not necessarily recapitulate the phenotype observed in some human diseases, especially in neurological syndromes (Yang et al. 2008). NHPs could provide better models for various human diseases due to similarities in genetics, developmental biology (as noted earlier), neural structure-function, and other organ features. Nevertheless, the application of genome editing to NHPs has been remarkably limited; the first transgenic monkey (a rhesus macaque expressing the green fluorescent protein gene) was not reported until 2001 (Chan et al. 2001). Moreover, the use of this technology to generate a disease model for Huntington's disease in rhesus macaques was not reported until 2008 (Yang et al. 2008), with no known application to other diseases in almost a decade. The lack of progress can be attributed to several limitations, including the relatively low numbers of mature oocytes available for microinjection and the low efficiency of transgenesis. Nevertheless, the technique demonstrates the potential value of NHP disease models, as the rhesus macaque offspring expressing the expanded exon1 of the human HTT gene displayed brain histologic changes and behavioral features of a dystonia and chorea observed in HD patients, but not in rodent models. More recently, (Sasaki et al. 2009) generated the first transgenic marmosets and demonstrated germline transmission following IVF with wild-type oocytes and transgenic (EGFP-expressing) sperm to yield healthy offspring.
A major advance, especially pertinent to genome editing in larger animals such as NHPs, occurred in 2012 with the demonstration that CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) RNA-Cas 9 protein complexes from streptococcus bacteria could function as RNA-guided endonucleases and cause specific double-stranded breaks in the DNA with insertions or deletions during nonhomologous DNA repair (for review, see details, Peng et al. 2016). While the so-called CRISPR/Cas 9 system continues to be optimized, it is now used successfully to cause loss-of-function mutations in a number of species, including plants, animals, and cell lines of human origin. Moreover, the technique can be applied to successfully target multiple genes. Likewise, the transcription activator-like endonucleases (TALENs) are “programmable” site-specific enzymes that activate DNA repair processes that can be used to knockout or knockin specific genes (Porteus 2015). These methods are revolutionizing genome editing and, while not without limitations, are being applied to NHPs to generate potential disease models (see review, Chen et al. 2016).
Liu and colleagues (Liu et al. 2014) successfully created macaques with a loss-of-function mutation in the MECP2 gene using the TALENs technique. Methyl-CpG-binding protein 2 (MECP2) deficiency is associated with Rett syndrome, a neurodevelopmental disorder in the autism spectrum. When three TALEN targets to multiple sites in exon 3 of MECP2 were injected into one-cell zygotes, the mutation rate was 81.3% (13 of 16; Chen et al. 2016). Most fetuses were lost but live offspring carried to the mutations.
Other groups are using the CRISPR/Cas 9 approach to modify genes in NHPs, in part because compared to TALENs it is easier to use, highly specific and efficient, more economical, and can potentially target multiple genes during one procedure. Niu and colleagues (Niu et al. 2014) simultaneously disrupted two (PPAR-γ and Rag 1) genes following co-injection into one-cell zygotes from cynomolgus macaques. Subsequently, there were three reports whereby the CRISPR/Cas 9 system was used to knockout the dystrophin (Chen et al. 2015b), Dax1 (Kang et al. 2015), and p53 (Wan et al. 2015) genes in macaques. The presence of degenerated muscle cells (with depleted dystrophin) in newborns suggests that the dystrophin-mutated macaque will be a valuable model for studying early-stage Duchenne muscular dystrophy and designing novel therapies. The Dax1-mutated macaque displayed defects in adrenal and testes development that mimic those seen in patients with adrenal hypoplasia and hypogonadotropic hypogonadism, thus confirming the critical role for the Dax1 in adrenal and gonadal development and function. In the p53 study, biallelic mutant macaques were generated, which can be bred to yield homozygous offspring.
While challenges remain (e.g., identifying and eliminating off-target mutations), the opportunities to explore the role of individual or multiple genes in normal physiological events in NHPs and to generate disease models for studying the etiology and treatment of human disorders is now a reality (Kishi et al. 2014). Moreover, with the generation of embryonic and induced pluripotent stem cells in NHPs (Liu et al. 2008; Thomson et al. 1995; Wolf et al. 2017), it is possible to apply genome editing and study the effects in vitro or in vivo (e.g., through chimeras, Chen et al. 2015a) as a prelude to efforts to repair or replace diseased or aged tissues.
Final Thoughts
Although challenges exist in the high costs and specialized resources required to include nonhuman primates in biomedical research, their inclusion has advanced fertility and infertility research in ways that directly improved women's reproductive health. While these advances have been vital, ethical debate on the appropriate use and limits in experimental manipulation of our NHP “relatives” has been equally robust and important. Indeed, this chapter is based in part on a presentation at the NIH Workshop “Ensuring the Continued Responsible Oversight of Research with Nonhuman Primates” (Woodruff 2016). Vigilance by the scientific community and public on behalf of all organisms is a critical part of biomedical research and was active in the breakthrough studies documented in this chapter. Future research is expected to follow a similar paradigm, with ethical reflection assisting in the next generation of fundamental discoveries and clinical applications in women's reproductive health (Phillips et al. 2014).
Acknowledgments
Special thanks to scientists that contributed to the NIH presentation, Drs. Charles Roberts (Oregon National Primate Research Center), Jon Levine and David Abbott (Wisconsin National Primate Research Center), Kyle Orwig (University of Pittsburgh), and Asgi Fazeleabas (Michigan State University). The authors’ research is funded primarily through the NIH, especially the Eunice Kennedy Shriver National Institute of Child Health and Human Development, including the National Centers for Translational Research in Reproduction and Infertility (P50 HD076188, TKW; P50 HD071836, RLS) and Contraceptive Development and Research Center (U54 HD055744, RLS). Dr. Stouffer's research is supported by the resources and infrastructure of the NIH Primate Centers program, Office of the Director (P51 OD011092).
References
- Abbott DH, Levine JE, Dumesic DA. 2016. Translational insight into polycystic ovary syndrome (PCOS) from female monkeys with PCOS-like traits. Curr Pharm Des 22:5625–5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu Shehab M, Damerill I, Shen T, Rosario FJ, Nijland M, Nathanielsz PW, Kamat A, Jansson T, Gupta MB. 2014. Liver mTOR controls IGF-1 bioavailability by regulation of protein kinase CK2 and IGFBP-1 phosphorylation in fetal growth restriction. Endocrinology 155:1327–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adashi EY, Cohen IG. 2016. Going germline: Mitochondrial replacement as a guide to genome editing. Cell 164:832–835. [DOI] [PubMed] [Google Scholar]
- Aliota MT, Dudley DM, Newman CM, Mohr EL, Gellerup DD, Breitbach ME, Buechler CR, Rasheed MN, Mohns MS, Weiler AM. 2016. Heterologous protection against asian Zika virus challenge in rhesus macaques. PLoS Negl Trop Dis 10:e0005168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataman LM, Rodrigues JK, Marinho RM, Caetano JP, Chehin MB, Alves da Motta EL, Serafini P, Suzuki N, Furui T, Takae S. 2016. Creating a global community of practice for oncofertility. J Glob Oncol 2:83–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee P, Fazleabas AT. 2011. Extragonadal actions of chorionic gonadotropin. Rev Endocr Metab Disord 12:323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett AJ, Panicker S. 2016. Broader impacts: International implications and integrative ethical consideration of policy decisions about US chimpanzee research. Am J Primatol 78:1282–1303. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Kohama SG, Reddy AP, Urbanski HF. 2016. Ovarian steroids regulate gene expression in the dorsal raphe of old female macaques. Neurobiol Aging 37:179–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binette A, Howatt K, Waddington A, Reid RL. 2017. Ten challenges in contraception. J Womens Health 26:44–49. [DOI] [PubMed] [Google Scholar]
- Bishop CV, Sparman ML, Stanley JE, Bahar A, Zelinski MB, Stouffer RL. 2009. Evaluation of antral follicle growth in the macaque ovary during the menstrual cycle and controlled ovarian stimulation by high-resolution ultrasonography. Am J Primatol 71:384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop CV, Xu F, Xu J, Ting AY, Galbreath E, McGee WK, Zelinski MB, Hennebold JD, Cameron JL, Stouffer RL. 2016. Western-style diet, with and without chronic androgen treatment, alters the number, structure, and function of small antral follicles in ovaries of young adult monkeys. Fertil Steril 105:1023–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brännström M, Bokstrom H, Dahm-Kahler P, Diaz-Garcia C, Ekberg J, Enskog A, Hagberg H, Johannesson L, Kvarnstrom N, Molne J. 2016. One uterus bridging three generations: First live birth after mother-to-daughter uterus transplantation. Fertil Steril 106:261–266. [DOI] [PubMed] [Google Scholar]
- Brännström M, Diaz-Garcia C, Hanafy A, Olausson M, Tzakis A. 2012. Uterus transplantation: Animal research and human possibilities. Fertil Steril 97:1269–1276. [DOI] [PubMed] [Google Scholar]
- Braundmeier A, Jackson K, Hastings J, Koehler J, Nowak R, Fazleabas A. 2012. Induction of endometriosis alters the peripheral and endometrial regulatory T cell population in the non-human primate. Hum Reprod 27:1712–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braundmeier AG, Fazleabas AT. 2009. The non-human primate model of endometriosis: Research and implications for fecundity. Mol Hum Reprod 15:577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt Solorzano CM, Beller JP, Abshire MY, Collins JS, McCartney CR, Marshall JC. 2012. Neuroendocrine dysfunction in polycystic ovary syndrome. Steroids 77:332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso RC, Burns A, Moeller J, Skinner DC, Padmanabhan V. 2016. Developmental programming: Insulin sensitizer prevents the GnRH-stimulated LH hypersecretion in a sheep model of PCOS. Endocrinology 157:4641–4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson HE, Schapiro SJ, Farah I, Hau J. 2004. Use of primates in research: A global overview. Am J Primatol 63:225–237. [DOI] [PubMed] [Google Scholar]
- Chaffin CL, VandeVoort CA. 2013. Follicle growth, ovulation, and luteal formation in primates and rodents: A comparative perspective. Exp Biol Med 238:539–548. [DOI] [PubMed] [Google Scholar]
- Chan AW, Chong KY, Martinovich C, Simerly C, Schatten G. 2001. Transgenic monkeys produced by retroviral gene transfer into mature oocytes. Science 291:309–312. [DOI] [PubMed] [Google Scholar]
- Chen Y, Niu Y, Ji W. 2016. Genome editing in nonhuman primates: Approach to generating human disease models. J Intern Med 280:246–251. [DOI] [PubMed] [Google Scholar]
- Chen Y, Niu Y, Li Y, Ai Z, Kang Y, Shi H, Xiang Z, Yang Z, Tan T, Si W. 2015a. Generation of cynomolgus monkey chimeric fetuses using embryonic stem cells. Cell Stem Cell 17:116–124. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zheng Y, Kang Y, Yang W, Niu Y, Guo X, Tu Z, Si C, Wang H, Xing R. 2015b. Functional disruption of the dystrophin gene in rhesus monkey using CRISPR/Cas9. Hum Mol Genet 24:3764–3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coksaygan T, Viscardi RM, Waites KB, Robwerts VHJ, Novy MJ, Girigsby PL. 2010. Periventricular neuronal damage associated with Ureaplasma intra-amniotic infection is diminished by maternal Azithromycin (AZI) treatment. Reprod Sci 17:178A. [Google Scholar]
- Cox LA, Comuzzie AG, Havill LM, Karere GM, Spradling KD, Mahaney MC, Nathanielsz PW, Nicolella DP, Shade RE, Voruganti S. 2013. Baboons as a model to study genetics and epigenetics of human disease. ILAR J 54:106–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JL, Guimaraes KP, Benazzato C, Almeida N, Pignatari GC, Romero S. 2016. The Brazilian Zika virus strain causes birth defects in experimental models. Nature 534:267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Hooghe TM, Kyama CM, Chai D, Fassbender A, Vodolazkaia A, Bokor A, Mwenda JM. 2009. Nonhuman primate models for translational research in endometriosis. Reprod Sci 16:152–161. [DOI] [PubMed] [Google Scholar]
- Dudley DM, Aliota MT, Mohr EL, Weiler AM, Lehrer-Brey G, Weisgrau KL, Mohns MS, Breitbach ME, Rasheed MN, Newman CM Gellerup DD, Moncla LH, Post J, Schultz-Darken N, Schotzko ML, Hayes JM, Eudailey JA, Moody MA, Permar SR, O'Connor SL, Rakasz EG, Simmons HA, Capuano S, Golos TG, Osorio JE, Friedrich TC, O'Connor DH. 2016. A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun 7:12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. 2015. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev 36:487–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan FE, Zelinski M, Gunn AH, Pahnke JE, O'Neill CL, Songsasen N, Woodruff RI, Woodruff TK. 2016. Ovarian tissue transport to expand access to fertility preservation: From animals to clinical practice. Reproduction 152:R201–R210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer H, Mwenda JM, Chai DC, Wagner C, Song XY, Mihalyi A, Simsa P, Kyama C, Cornillie FJ, Bergqvist A, Fried G, D'Hooghe TM. 2006. Treatment with anti-TNF monoclonal antibody (c5N) reduces the extent of induced endometriosis in the baboon. Hum Reprod 21:1856–1862. [DOI] [PubMed] [Google Scholar]
- Fazleabas AT. 2010. Progesterone resistance in a baboon model of endometriosis. Semin Reprod Med 28:75–80. [DOI] [PubMed] [Google Scholar]
- Fereydouni B, Salinas-Riester G, Heistermann M, Dressel R, Lewerich L, Drummer C, Behr R. 2016. Long-term oocyte-like cell development in cultures derived from neonatal marmoset monkey ovary. Stem Cells Int 2016:2480298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke K, Clarke GD, Dahnke R, Gaser C, Kuo AH, Li C, Schwab M, Nathanielsz PW. 2017. Premature brain aging in baboons resulting from moderate fetal undernutrition. Front Aging Neurosci 9:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frias AE, Morgan TK, Evans AE, Rasanen J, Oh KY, Thornburg KL, Grove KL. 2011. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology 152:2456–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frias AE, Schabel MC, Roberts VH, Tudorica A, Grigsby PL, Oh KY, Kroenke CD. 2015. Using dynamic contrast-enhanced MRI to quantitatively characterize maternal vascular organization in the primate placenta. Magn Reson Med 73:1570–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George JT, Kakkar R, Marshall J, Scott ML, Finkelman RD, Ho TW, Veldhuis J, Skorupskaite K, Anderson RA, McIntosh S, Webber L. 2016. Neurokinin B receptor antagonism in women with polycystic ovary syndrome: A randomized, placebo-controlled trial. J Clin Endocrinol Metab 101:4313–4321. [DOI] [PubMed] [Google Scholar]
- Golos TG, Bondarenko GI, Dambaeva SV, Breburda EE, Durning M. 2010. On the role of placental major histocompatibility complex and decidual leukocytes in implantation and pregnancy success using non-human primate models. Int J Dev Biol 54:431–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goy RW, Phoenix CH. 1972. The effects of testosterone propionate administered before birth on the development of behavior in genetic female rhesus monkeys. UCLA Forum Med Sci 15:193–201. [PubMed] [Google Scholar]
- Grigsby PL. 2016. Animal models to study placental development and function throughout normal and dysfunctional human pregnancy. Semin Reprod Med 34:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigsby PL, Novy MJ, Sadowsky DW, Morgan TK, Long M, Acosta E, Duffy LB, Waites KB. 2012. Maternal azithromycin therapy for Ureaplasma intraamniotic infection delays preterm delivery and reduces fetal lung injury in a primate model. Am J Obstet Gynecol 207:475 e471–475 e414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haluska GJ, Wells TR, Hirst JJ, Brenner RM, Sadowsky DW, Novy MJ. 2002. Progesterone receptor localization and isoforms in myometrium, decidua, and fetal membranes from rhesus macaques: Evidence for functional progesterone withdrawal at parturition. J Soc Gynecol Investig 9:125–136. [PubMed] [Google Scholar]
- Hanson B, Johnstone E, Dorais J, Silver B, Peterson CM, Hotaling J. 2016. Female infertility, infertility-associated diagnoses, and comorbidities: A review. J Assist Reprod Genet 34:167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harirchian P, Gashaw I, Lipskind ST, Braundmeier AG, Hastings JM, Olson MR, Fazleabas AT. 2012. Lesion kinetics in a non-human primate model of endometriosis. Hum Reprod 27:2341–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden EC. 2016. Zika highlights role of fetal-tissue research. Nature 532:16. [DOI] [PubMed] [Google Scholar]
- Horikoshi M, Beaumont RN, Day FR, Warrington NM, Kooijman MN, Fernandez-Tajes J, Feenstra B, van Zuydam NR, Gaulton KJ, Grarup N, Bradfield JP, Strachan DP, Li-Gao R, Ahluwalia TS, Kreiner E, Rueedi R, Lyytikäinen LP, Cousminer DL, Wu Y, Thiering E, Wang CA, Have CT, Hottenga JJ, Vilor-Tejedor N, Joshi PK, Boh ET, Ntalla I, Pitkänen N, Mahajan A, van Leeuwen EM, Joro R, Lagou V, Nodzenski M, Diver LA, Zondervan KT, Bustamante M, Marques-Vidal P, Mercader JM, Bennett AJ, Rahmioglu N, Nyholt DR, Ma RC, Tam CH, Tam WH CHARGE Consortium Hematology Working Group , Ganesh SK, van Rooij FJ, Jones SE, Loh PR, Ruth KS, Tuke MA, Tyrrell J, Wood AR, Yaghootkar H, Scholtens DM, Paternoster L, Prokopenko I, Kovacs P, Atalay M, Willems SM, Panoutsopoulou K, Wang X, Carstensen L, Geller F, Schraut KE, Murcia M, van Beijsterveldt CE, Willemsen G, Appel EV, Fonvig CE, Trier C, Tiesler CM, Standl M, Kutalik Z, Bonàs-Guarch S, Hougaard DM, Sánchez F, Torrents D, Waage J, Hollegaard MV, de Haan HG, Rosendaal FR, Medina-Gomez C, Ring SM, Hemani G, McMahon G, Robertson NR, Groves CJ, Langenberg C, Luan J, Scott RA, Zhao JH, Mentch FD, MacKenzie SM, Reynolds RM; Early Growth Genetics (EGG) Consortium , Lowe WL, Tönjes A, Stumvoll M, Lindi V, Lakka TA, van Duijn CM, Kiess W, Körner A, Sørensen TI, Niinikoski H, Pahkala K, Raitakari OT, Zeggini E, Dedoussis GV, Teo YY, Saw SM, Melbye M, Campbell H, Wilson JF, Vrijheid M, de Geus EJ, Boomsma DI, Kadarmideen HN, Holm JC, Hansen T, Sebert S, Hattersley AT, Beilin LJ, Newnham JP, Pennell CE, Heinrich J, Adair LS, Borja JB, Mohlke KL, Eriksson JG, Widén E, Kähönen M, Viikari JS, Lehtimäki T, Vollenweider P, Bønnelykke K, Bisgaard H, Mook-Kanamori DO, Hofman A, Rivadeneira F, Uitterlinden AG, Pisinger C, Pedersen O, Power C, Hyppönen E, Wareham NJ, Hakonarson H, Davies E, Walker BR, Jaddoe VW, Järvelin MR, Grant SF, Vaag AA, Lawlor DA, Frayling TM, Smith GD, Morris AP, Ong KK, Felix JF, Timpson NJ, Perry JR, Evans DM, McCarthy MI, Freathy RM. 2016. Genome-wide associations for birth weight and correlations with adult disease. Nature 538:248–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein M, Chai DC, Kyama CM, Mwenda JM, Palmer SS, Gotteland JP, D'Hooghe TM. 2016. c-Jun NH2-terminal kinase inhibitor bentamapimod reduces induced endometriosis in baboons: An assessor-blind placebo-controlled randomized study. Fertil Steril 105:815–824 e815. [DOI] [PubMed] [Google Scholar]
- Jensen JT, Hanna C, Yao S, Thompson E, Bauer C, Slayden OD. 2016. Transcervical administration of polidocanol foam prevents pregnancy in female baboons. Contraception 94:527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Dong H, Zheng X, Marjani SL, Donovan DM, Chen J, Tian XC. 2015. mRNA levels of imprinted genes in bovine in vivo oocytes, embryos and cross species comparisons with humans, mice and pigs. Sci Rep 5:17898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannesson L, Enskog A, Dahm-Kahler P, Hanafy A, Chai DC, Mwenda JM, Diaz-Garcia C, Olausson M, Brännström M. 2012. Uterus transplantation in a non-human primate: Long-term follow-up after autologous transplantation. Hum Reprod 27:1640–1648. [DOI] [PubMed] [Google Scholar]
- Joshi NR, Su RW, Chandramouli GV, Khoo SK, Jeong JW, Young SL, Lessey BA, Fazleabas AT. 2015. Altered expression of microRNA-451 in eutopic endometrium of baboons (Papio anubis) with endometriosis. Hum Reprod 30:2881–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Zheng B, Shen B, Chen Y, Wang L, Wang J, Niu Y, Cui Y, Zhou J, Wang H, Guo X, Hu B, Zhou Q, Sha J, Ji W, Huang X. 2015. CRISPR/Cas9-mediated Dax1 knockout in the monkey recapitulates human AHC-HH. Hum Mol Genet 24:7255–7264. [DOI] [PubMed] [Google Scholar]
- Kaplan JR. 2004. Modeling women's health with nonhuman primates and other animals. ILAR J 45:83–88. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Lubach G, Hedman C, Ziegler TE, Coe CL. 2014. Hormones in infant rhesus monkeys’ (Macaca mulatta) hair at birth provide a window into the fetal environment. Pediatr Res 75:476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavitha JV, Rosario FJ, Nijland MJ, McDonald TJ, Wu G, Kanai Y, Powell TL, Nathanielsz PW, Jansson T. 2014. Down-regulation of placental mTOR, insulin/IGF-1 signaling, and nutrient transporters in response to maternal nutrient restriction in the baboon. FASEB J 28:1294–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keator CS, Lindner JR, Belcik JT, Bishop CV, Slayden OD. 2011. Contrast-enhanced ultrasound reveals real-time spatial changes in vascular perfusion during early implantation in the macaque uterus. Fertil Steril 95:1316–1321 e1311-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi N, Sato K, Sasaki E, Okano H. 2014. Common marmoset as a new model animal for neuroscience research and genome editing technology. Dev Growth Differ 56:53–62. [DOI] [PubMed] [Google Scholar]
- Kisu I, Banno K, Mihara M, Suganuma N, Aoki D. 2013. Current status of uterus transplantation in primates and issues for clinical application. Fertil Steril 100:280–294. [DOI] [PubMed] [Google Scholar]
- Klein S, D'Hooghe T, Meuleman C, Dirksen C, Dunselman G, Simoens S. 2014. What is the societal burden of endometriosis-associated symptoms? A prospective Belgian study. Reprod Biomed Online 28:116–124. [DOI] [PubMed] [Google Scholar]
- Kohama SG, Renner L, Landauer N, Weiss AR, Urbanski HF, Park B, Voytko ML, Neuringer M. 2016. Effect of ovarian hormone therapy on cognition in the aged female rhesus macaque. J Neurosci 36:10416–10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo AH, Li C, Li J, Huber HF, Nathanielsz PW, Clarke GD. 2017. Cardiac remodelling in a baboon model of intrauterine growth restriction mimics accelerated ageing. J Physiol 595:1093–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langoi D, Pavone ME, Gurates B, Chai D, Fazleabas A, Bulun SE. 2013. Aromatase inhibitor treatment limits progression of peritoneal endometriosis in baboons. Fertil Steril 99:656–662 e653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebovic DI, Mwenda JM, Chai DC, Santi A, Xu X, D'Hooghe T. 2010. Peroxisome proliferator-activated receptor-(gamma) receptor ligand partially prevents the development of endometrial explants in baboons: A prospective, randomized, placebo-controlled study. Endocrinology 151:1846–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Chen Y, Niu Y, Zhang K, Kang Y, Ge W, Liu X, Zhao E, Wang C, Lin S, Jing B, Si C, Lin Q, Chen X, Lin H, Pu X, Wang Y, Qin B, Wang F, Wang H, Si W, Zhou J, Tan T, Li T, Ji S, Xue Z, Luo Y, Cheng L, Zhou Q, Li S, Sun YE, Ji W. 2014. TALEN-mediated gene mutagenesis in rhesus and cynomolgus monkeys. Cell Stem Cell 14:323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Zhu F, Yong J, Zhang P, Hou P, Li H, Jiang W, Cai J, Liu M, Cui K, Qu X, Xiang T, Lu D, Chi X, Gao G, Ji W, Ding M, Deng H. 2008. Generation of induced pluripotent stem cells from adult rhesus monkey fibroblasts. Cell Stem Cell 3:587–590. [DOI] [PubMed] [Google Scholar]
- Lo JO, Schabel MC, Roberts VH, Morgan TK, Rasanen JP, Kroenke CD, Shoemaker SR, Spindel ER, Frias AE. 2015. Vitamin C supplementation ameliorates the adverse effects of nicotine on placental hemodynamics and histology in nonhuman primates. Am J Obstet Gynecol 212:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo JO, Schabel MC, Roberts VH, Wang X, Lewandowski KS, Grant KA, Frias AE, Kroenke CD. 2017. First trimester alcohol exposure alters placental perfusion and fetal oxygen availability affecting fetal growth and development in a non-human primate model. Am J Obstet Gynecol 216:302.e1–302.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. 2010. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod 25:544–551. [DOI] [PubMed] [Google Scholar]
- McAllister JM, Modi B, Miller BA, Biegler J, Bruggeman R, Legro RS, Strauss JF 3rd. 2014. Overexpression of a DENND1A isoform produces a polycystic ovary syndrome theca phenotype. Proc Natl Acad Sci USA 111:E1519–E1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee WK, Bishop CV, Bahar A, Pohl CR, Chang RJ, Marshall JC, Pau FK, Stouffer RL, Cameron JL. 2012. Elevated androgens during puberty in female rhesus monkeys lead to increased neuronal drive to the reproductive axis: A possible component of polycystic ovary syndrome. Hum Reprod 27:531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee WK, Bishop CV, Pohl CR, Chang RJ, Marshall JC, Pau FK, Stouffer RL, Cameron JL. 2014. Effects of hyperandrogenemia and increased adiposity on reproductive and metabolic parameters in young adult female monkeys. Am J Physiol Endocrinol Metab 306:E1292–E1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MJ, Halow NG, Royer PA, Hennebold JD. 2016. Leukemia inhibitory factor is necessary for ovulation in female rhesus macaques. Endocrinology 157:4378–4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Y, Shen B, Cui Y, Chen Y, Wang J, Wang L, Kang Y, Zhao X, Si W, Li W Xiang AP, Zhou J, Guo X, Bi Y, Si C, Hu B, Dong G, Wang H, Zhou Z, Li T, Tan T, Pu X, Wang F, Ji S, Zhou Q, Huang X, Ji W, Sha J. 2014. Generation of gene-modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell 156:836–843. [DOI] [PubMed] [Google Scholar]
- Nguyen SM, Antony KM, Dudley DM, Kohn S, Simmons HA, Wolfe B, Salamat MS, Teixeira LBC, Wiepz GJ, Thoong TH et al. 2017. Highly efficient maternal-fetal zika virus transmission in pregnant rhesus macaques. PLoS Pathog 13:e1006378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novy MJ, Duffy L, Axthelm MK, Sadowsky DW, Witkin SS, Gravett MG, Cassell GH, Waites KB. 2009. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci 16:56–70. [DOI] [PubMed] [Google Scholar]
- Peluffo MC, Stanley J, Braeuer N, Rotgeri A, Fritzemeier KH, Fuhrmann U, Buchmann B, Adevai T, Murphy MJ, Zelinski MB, Lindenthal B, Hennebold JH, Stouffer RL. 2014. A prostaglandin E2 receptor antagonist prevents pregnancies during a preclinical contraceptive trial with female macaques. Hum Reprod 29:1400–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng R, Lin G, Li J. 2016. Potential pitfalls of CRISPR/Cas9-mediated genome editing. FEBS J 283:1218–1231. [DOI] [PubMed] [Google Scholar]
- Pereira SP, Oliveira PJ, Tavares LC, Moreno AJ, Cox LA, Nathanielsz PW, Nijland MJ. 2015. Effects of moderate global maternal nutrient reduction on fetal baboon renal mitochondrial gene expression at 0.9 gestation. Am J Physiol Renal Physiol 308:F1217–F1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, ‘t Hart BA, Hopkins WD, Hu SL, Miller LA, Nader MA, Nathanielsz PW, Rogers J, Shively CA, Voytko ML. 2014. Why primate models matter. Am J Primatol 76:801–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant TM. 2012. A comparison of the neuroendocrine mechanisms underlying the initiation of the preovulatory LH surge in the human, Old World monkey and rodent. Front Neuroendocrinol 33:160–168. [DOI] [PubMed] [Google Scholar]
- Porteus MH. 2015. Towards a new era in medicine: Therapeutic genome editing. Genome Biol 16:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puttabyatappa M, Cardoso RC, Padmanabhan V. 2016. Effect of maternal PCOS and PCOS-like phenotype on the offspring's health. Mol Cell Endocrinol 435:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts VHJ, Robertson ND, McEvoy C, Schelonka RL, Grigsby PL. 2012. Serial assessments of neurobehavioral and cognitive development following prenatal maternal Azithromycin (AZI) therapy for Ureaplasma Intra-amniotic Infection (IAI) in neonatal rhesus monkeys. Reprod Sci 19:128A. [Google Scholar]
- Rogers PA, Adamson GD, Al-Jefout M, Becker CM, D'Hooghe TM, Dunselman GA, Fazleabas A, Giudice LC, Horne AW, Hull ML, Hummelshoj L, Missmer SA, Montgomery GW, Stratton P, Taylor RN, Rombauts L, Saunders PT, Vincent K, Zondervan KT. 2017. Research priorities for endometriosis. Reprod Sci 24:202–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki E, Suemizu H, Shimada A, Hanazawa K, Oiwa R, Kamioka M, Tomioka I, Sotomaru Y, Hirakawa R, Eto T. 2009. Generation of transgenic non-human primates with germline transmission. Nature 459:523–527. [DOI] [PubMed] [Google Scholar]
- Schabel MC, Roberts VH, Lo JO, Platt S, Grant KA, Frias AE, Kroenke CD. 2016. Functional imaging of the nonhuman primate placenta with endogenous blood oxygen level-dependent contrast. Magn Reson Med 76:1551–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemionow MZ, Zor F, Gordon CR. 2010. Face, upper extremity, and concomitant transplantation: Potential concerns and challenges ahead. Plast Reconstr Surg 126:308–315. [DOI] [PubMed] [Google Scholar]
- Slayden OD. 2013. Induced endometriosis in nonhuman primates. Biol Reprod 88:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story L, Kennedy S. 2004. Animal studies in endometriosis: A review. ILAR J 45:132–138. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Sparman M, Sritanaudomchai H, Ma H, Clepper L, Woodward J, Li Y, Ramsey C, Kolotushkina O, Mitalipov S. 2009. Mitochondrial gene replacement in primate offspring and embryonic stem cells. Nature 461:367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L, He G, Liu X, Xu W. 2017. Progress in the understanding of the etiology and predictability of fetal growth restriction. Reproduction 153:R227–R240. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Kalishman J, Golos TG, Durning M, Harris CP, Becker RA, Hearn JP. 1995. Isolation of a primate embryonic stem cell line. Proc Natl Acad Sci USA 92:7844–7848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toner JP, Coddington CC, Doody K, Van Voorhis B, Seifer DB, Ball GD, Luke B, Wantman E. 2016. Society for Assisted Reproductive Technology and assisted reproductive technology in the United States: a 2016 update. Fertil Steril 106:541–546. [DOI] [PubMed] [Google Scholar]
- True C, Bishop CV, Varlamov O, Takahashi D, Thompson E, Bond K, Wilcox C, Burns S, Ryan N, Handu J. 2016. Combined effects of hyperandrogenemia and western-style diet on metabolic and reproductive function in young adult, female monkeys. Endocrinocrine Society's 98th Annual Meeting and Expo held April 1–4, 2016, in Boston, MA.
- True C, Takahashi DL, Burns SE, Mishler EC, Bond KR, Wilcox MC, Calhoun AR, Bader LA, Dean TA, Ryan ND, Slayden OV, Cameron JL, Stouffer RL. 2017. Chronic combined hyperandrogenemia and western-style diet in young female rhesus macaques causes greater metabolic impairments compared to either treatment alone. Hum Reprod In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryphonopoulos P, Tzakis AG, Tekin A, Johannesson L, Rivas K, Morales PR, Wagner J, Molne J, Enskog A, Diaz-Garcia C, Dahm-Kähler P, Berho M, Zimberg S, Falcone T, Ruiz P, Olausson M, Brännström M. 2014. Allogeneic uterus transplantation in baboons: Surgical technique and challenges to long-term graft survival. Transplantation 98:e51–e56. [DOI] [PubMed] [Google Scholar]
- Vink JM, Sadrzadeh S, Lambalk CB, Boomsma DI. 2006. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J Clin Endocrinol Metab 91:2100–2104. [DOI] [PubMed] [Google Scholar]
- Waites KB, Schelonka RL, Xiao L, Grigsby PL, Novy MJ. 2009. Congenital and opportunistic infections: Ureaplasma species and Mycoplasma hominis. Semin Fetal Neonatal Med 14:190–199. [DOI] [PubMed] [Google Scholar]
- Wan H, Feng C, Teng F, Yang S, Hu B, Niu Y, Xiang AP, Fang W, Ji W, Li W, Zhao X, Zhou Q. 2015. One-step generation of p53 gene biallelic mutant Cynomolgus monkey via the CRISPR/Cas system. Cell Res 25:258–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf DP, Mitalipov N, Mitalipov S. 2015. Mitochondrial replacement therapy in reproductive medicine. Trends Mol Med 21:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf DP, Morey R, Kang E, Ma H, Hayama T, Laurent LC, Mitalipov S. 2017. Concise Review: Embryonic stem cells derived by somatic cell nuclear transfer: A horse in the race? Stem Cells 35:26–34. [DOI] [PubMed] [Google Scholar]
- Wolfe B, Wiepz GJ, Schotzko M, Bondarenko GI, Durning M, Simmons HA, Mejia A, Faith NG, Sampene E, Suresh M, Kathariou S, Czuprynski CJ, Golos TG. 2017. Acute fetal demise with first trimester maternal infection resulting from listeria monocytogenes in a nonhuman primate model. MBio 8:e01938-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff T. 2016. Nonhuman priamtes: critical models that advance fertility and infertility research. Available online (https://videocast.nih.gov/summary.asp?Live=19663&bhcp=1), accessed on August 9, 2017.
- Worley KC, Warren WC, Rogers J, Locke D, Muzny DM, Mardis ER, Weinstock GM, Tardif SD, Aagaard KM, Archidiacono N, Rayan NA, Batzer MA, Beal K, Brejova B, Capozzi O, Capuano SB, Casola C, Chandrabose MM, Cree A, Dao MD, de Jong PJ, Del Rosario RC, Delehaunty KD, Dinh HH, Eichler EE, Fitzgerald S, Flicek P, Fontenot CC, Fowler RG, Fronick C, Fulton LA, Fulton RS, Gabisi RA, Gerlach D, Graves TA, Gunaratne PH, Hahn MW, Haig D, Han Y, Harris RA, Herrero J, Hillier LW, Hubley R, Hughes JF, Hume J, Jhangiani SN, Jorde LB, Joshi V, Karakor E, Konkel MK, Kosiol C, Kovar CL, Kriventseva EV, Lee SL, Lewis LR, Liu YS, Lopez J, Lopez-Otin C, Lorente-Galdos B, Mansfield KG, Marques-Bonet T, Minx P, Misceo D, Moncrieff JS, Morgan MB, Nazareth LV, Newsham I, Nguyen NB, Okwuonu GO, Prabhakar S, Perales L, Pu LL, Puente XS, Quesada V, Ranck MC, Raney BJ, Raveendran M, Deiros DR, Rocchi M, Rodriguez D, Ross C, Ruffier M, Ruiz SJ, Sajjadian S, Santibanez J, Schrider DR, Searle S, Skaletsky H, Soibam B, Smit AF, Tennakoon JB, Tomaska L, Ullmer B, Vejnar CE, Ventura M, Vilella AJ, Vinar T, Vogel JH, Walker JA, Wang Q, Warner CM, Wildman DE, Witherspoon DJ, Wright RA, Wu Y, Xiao W, Xing J, Zdobnov EM, Zhu B, Gibbs RA, Wilson RK. 2014. The common marmoset genome provides insight into primate biology and evolution. Nat Genet 46:850–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N, Kwon S, Abbott DH, Geller DH, Dumesic DA, Azziz R, Guo X, Goodarzi MO. 2011. Epigenetic mechanism underlying the development of polycystic ovary syndrome (PCOS)-like phenotypes in prenatally androgenized rhesus monkeys. PLoS One 6:e27286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Cheng PH, Banta H, Piotrowska-Nitsche K, Yang JJ, Cheng EC, Snyder B, Larkin K, Liu J, Orkin J, Fang ZH, Smith Y, Bachevalier J, Zola SM, Li SH, Li XJ, Chan AW. 2008. Towards a transgenic model of Huntington's disease in a non-human primate. Nature 453:921–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. 2016. First live birth using human oocytes reconstituted by spindle nuclear transfer for mitochondrial DNA mutation causing Leigh Syndrome. Annual Meeting of the American Society for Reproductive Medicine, held October 15–19, Salt Lake City, UT.