Abstract
Background
Hydroxyapatite (calcium and phosphate) spherules have been implicated in the initiation and establishment of age-related macular degeneration. Bisphosphonates could increase the dissolution of hydroxyapatite crystals and when they are used in elderly patients with osteoporosis they could reduce the risk of developing age-related macular degeneration.
Objective
To determine if oral bisphosphonate (BP) use is associated with the incidence of age-related macular degeneration in a large “real-world” population-based cohort of incident hip fracture patients.
Design
A cohort of 13,974 hip fracture patients (1999 to 2013) were used to conduct a: (a) propensity score matched cohort analysis and (b) nested case-control analysis.
Setting
A population-based study using electronic health records from UK primary care (Clinical Practice Research Datalink).
Participants
Hip fracture patients aged ≥50 years without age-related macular degeneration diagnosis prior to hip fracture date or in the first year of follow-up. Prior BP-users and those who died, transferred out or with last data collected before hip fracture were excluded.
Exposures
Incident BP use following hip fracture. BP use was further categorised according to medication possession ratio in quartiles.
Main outcome measures
Primary outcome was a diagnosis of age-related macular degeneration after the first year from index date. Propensity scores were used to match 1:1 BP-users to non-BP users. Covariates in the propensity score were index year, age, gender, body mass index, smoking, alcohol drinking, region, drug confounders and comorbidities. Subhazard ratios and their 95% confidence intervals were calculated including death as a competing risk. A nested 1:20 case-control analysis using conditional logistic regression provided risk estimates according to medication possession ratio.
Results
Among 6,208 matched patients and during 22,142 person-years of follow-up, 57 (1.8%) and 42 (1.4%) age-related macular degeneration cases occurred in BP-users and non-BP users, respectively. The survival analysis model did not provide significant evidence of a higher risk of AMD in BP-users (subhazard ratio: 1.60; CI: 0.95-2.72; P=0.08) although there was a significant increased risk among BP-users with high medication possession ratio (top quartile) relative to non-BP users (odds ratio: 5.08, 3.11-8.30; P <0.001, respectively).
Conclusions
Overall, oral BP use was not associated with an increased risk of age-related macular degeneration in this cohort of hip fracture patients, although the risk increased significantly with higher medication possession ratio. More data are needed to confirm these findings.
Keywords: Oral bisphosphonates, age-related macular degeneration, hip fracture, propensity score matching, nested case-control study
Introduction
Age-related macular degeneration (AMD) is irreversible central vision loss which is involved in 8.7% of all blindness worldwide1. It is the leading cause of permanent vision impairment and the most prevalent condition of ocular deterioration in the population aged over 502. In the UK there are around an estimated 71,000 new cases of late AMD per year3, contributing to the total £1.6 billion 4 annual cost of AMD. AMD is subdivided into dry (non-neovascular, non-exudative) and wet (neovascular, exudative) forms and is characterized by distortion of central vision, black or grey patches affecting the central field of vision, and difficulty in reading, driving, or seeing fine detail2, 5.
Osteoporosis is a musculoskeletal disease defined by low bone mass and degradation of bone microarchitecture, culminating in an escalation in bone frailty and susceptibility to fracture. It is estimated to affect approximately one-tenth of women aged 60 and up to two-thirds of women aged 906, with approximately 200 million women affected worldwide. Several parts of the skeleton are affected with the hip being a common site sustaining a fracture, reported in the region of 65,000 cases each year across England, Wales and Northern Ireland7.
The molecular events and their sequence leading to AMD are poorly understood. Hydroxyapatite (calcium and phosphate) spherules, has now been identified as a key component in the development of the disease8. Bisphosphonates, the first line of treatment for osteoporosis, could increase the dissolution of hydroxyapatite crystals and thus they could reduce the risk of AMD. Our aim in this study was to investigate whether the risk of developing AMD is affected by the oral BP use in a “real-world” population-based cohort of incident hip fracture patients.
Methods
Data source and sample size
This study was carried out using data from the Clinical Practice Research Datalink (CPRD). CPRD contains primary care data from 674 UK practices and includes 11.3 million patients, covering 7% of the UK population9. Many aspects of CPRD have been well validated and it has been applied for a variety of safety studies comprising BPs10–12. A cohort of patients with an incident hip fracture was retrieved for the period 1999 to 2013. Mortality data were matched to the Office for National Statistics database.
Study design
Baseline characteristics were collected closest (during the previous 12 months) to the hip fracture event. We used a cohort study design among patients followed forward from hip fracture until the earliest date of either being lost to follow-up, death, or 31 July 2013. A nested case-control study was also conducted to estimate the association between BP dose and risk of AMD.
Exposure
Post index-date prescription records were used to categorise patients as either oral BP-users or non-users. Oral BP use was further classified according to defined daily dose (DDD) and medication possession ratio (MPR). MPR was calculated as DDD between date of first BP prescription and outcome date (or last prescription). DDDs and MPRs were categorised into quartiles.
Immortal time bias is a common issue in pharmaco-epidemiological studies. In the time from index date until receipt of BP, those in the ‘BP-user group’ cannot have the outcome by design as otherwise they would have been classified as a non-BP user. To address this issue we used time varying exposures in the survival model, where the time period previous to becoming a BP-user was reclassified as non-use for those in the oral BP-user group.
Outcome
Our defined outcome was AMD recorded at least one year after follow-up initiation. Cases were identified using standard UK clinical terminology “Read” codes: F425100 (“dry senile macular degeneration”), F425200 (“wet senile macular degeneration”), F425z00 (“degeneration of macula or posterior pole, not otherwise specified”), F425.00 (“degeneration of macula and posterior pole”), F425.11 (“senile macular degeneration”) and F425000 (“unspecified senile macular degeneration”).
Exclusion criteria
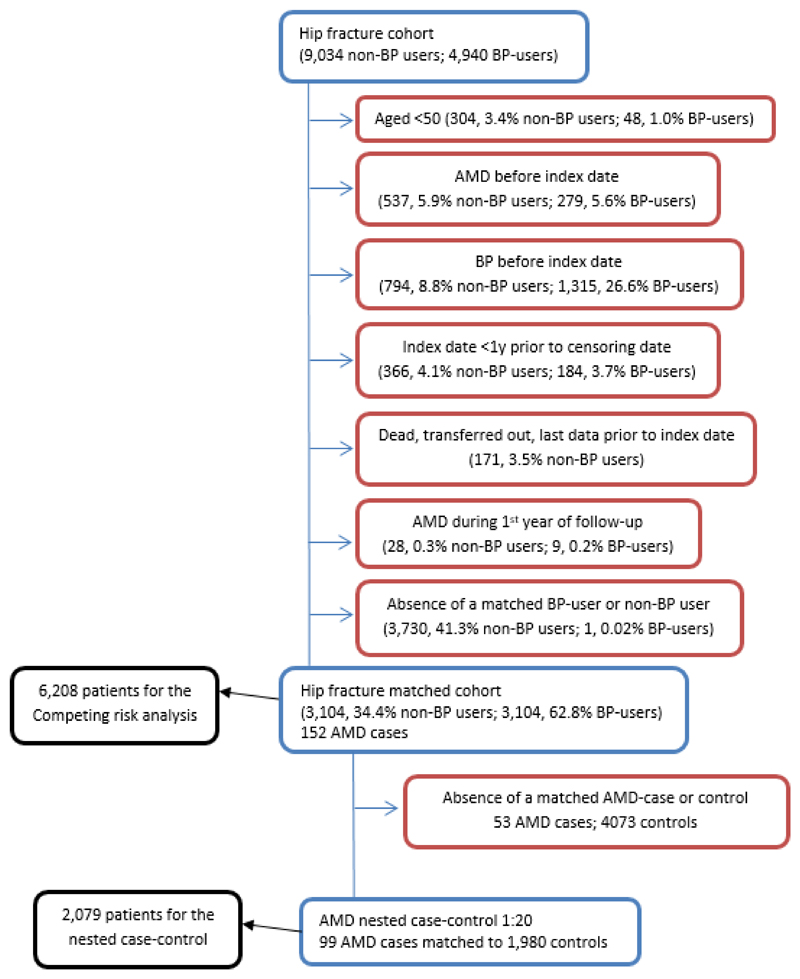
Patients younger than 50 years at hip fracture were excluded (Figure 1) given their low risk of AMD 5 and that bisphosphonates are infrequently prescribed in younger individuals. Patients were excluded if they had a diagnosis of AMD either before index date or during the first year of follow-up. Patients with a BP prescription, or who transferred out of CPRD prior to index hip fracture date were also excluded, as were patients with an index hip fracture occurring after 31 July 2012 given that they had insufficient follow-up time (less than one year) to experience the outcome. Finally, patients on non-nitrogen containing BPs (etidronate), parenteral antiresorptives (zoledronic acid, pamidronate and denosumab) together with those on teriparatide (a bone forming agent) were not included in the study, because the number of those patients was negligible.
Fig. 1.
Flow of included patients. Proportions of excluded observations at each point of exclusion. BP indicates oral bisphosphonates.
Confounders
Factors considered potential confounders were calendar year of hip fracture, age, gender, body mass index (BMI), smoking (no, ex-smoker, smoker), alcohol consumption (no, ex-drinker, drinker) and region (North England [North East, North West, Yorkshire & The Humber, East Midlands, West Midlands, East of England], South England [South West, South Central, London, South East Coast], Northern Ireland, Scotland and Wales).
Drug confounders were antiarrhythmics, antidepressants, antiepileptics, antiparkinsonians, anxiolytics, proton pump inhibitors (PPI), non-steroidal anti-inflammatory drugs (NSAID), hormone replacement therapy (HRT), selective estrogen receptor modulators (SERMs), strontium ranelate, calcium, non-ocular corticoids, ocular corticoids and insulin. Denosumab and teriparatide were not prescribed to our study population and were not included in the analysis. Comorbidity confounders were Charlson comorbidity index (none, mild (1 to 2), moderate (3 to 4), or severe (≥5)), asthma, inflammatory bowel disease, hypertension, hyperlipidemia, ischemic heart disease, cerebrovascular disease (CVD), chronic obstructive pulmonary disease (COPD), chronic renal failure, cancer, cataract surgery and type 1/type 2 diabetes.
Missing data
Confounders with missing values after applying exclusion criteria and prior to using propensity score matching were BMI (101, 21.1%), smoking (900, 9.1%) and drinking (1,729, 17.4%). They were imputed to avoid the exclusion of a large number of patients from the original sample. We generated a single imputed dataset using a chained equation across 50 iterations to reach a stationary distribution. We included all confounders, BP exposure and the AMD diagnosis in the imputation process.
Statistics
Standard descriptive statistics were used to describe the characteristics and follow-up time of our sample. A propensity score for BP-use was calculated using a logistic regression model13, 14, which included all confounders. BP-users were matched to non-BP users on the logit of the propensity score using a caliper width of 0.2 of the standard deviation of the logit of the propensity score15. A matching ratio of 1:1 was used16. We assessed standardised differences between the two groups, with 10% or more considered as suggestive of imbalance17. Occurrence of AMD between BP-users and non-BP users were evaluated after matching using two-tailed Fisher's exact tests. Kaplan-Meier survival curves were drawn for AMD incidence and differences among BP exposures were compared using the log rank test18.
A multivariable competing-risk regression (death as competing event) was fitted to estimate the effect of BPs on AMD occurrence19, 20, the output of which was a sub-distributional hazard ratio (sHR) and 95% CI, adjusted for the pairing of patients in the matching process. The confidence interval was informed using robust variance estimators, assuming that observations were independent across groups (pairs of matched patients) but not necessarily within groups21. A time-to-exposure variable was calculated splitting follow-up time of BP-users into non-BP users and BP user (when the patient became a user), so as to account for any lag between index date and BP initiation. Effect of BP dose was assessed within a nested case-control study using DDDs and MPR. Each AMD case was matched to 20 controls using the propensity scores obtained previously. A caliper width of 0.2 was again applied. A conditional logistic regression model was fitted to ascertain how the risk of AMD was associated with the degree of exposure to BP in terms of quartiles of prescribed daily dose and MPR. This yielded odds ratios (OR) and 95% CI, adjusted for matched cases and controls.
Analyses were conducted using the Stata version 13.1 statistical software (StataCorp, College Station, Texas). Matching on BP was performed using the R Package “MatchIt”22. While matching nested cases with controls used “Matching”23.
Results
Descriptive statistics
Of the 13,974 hip fracture patients, 4,035 (28.9%) were excluded prior to matching (Figure 1). Of the 9,939 patients meeting the inclusion criteria, 3,105 (31.2%) were BP users. AMD developed in 152 patients. Of them, 57 (37.5%) were on BP. The median time on BP until AMD event was 2.9 years (0.5 to 8.9 years).
For the competing risk analysis 3,104 BP users were matched 1:1 to 3,104 non-BP users, and of these matched patients 99 developed AMD.
For the nested case-control study, all 99 patients that developed AMD were matched 1:20 to 1980 controls that did not develop AMD. Of the 99 AMD cases 57 (57.6%) were BP users compared to 951 (48.0%) in the 1980 matched controls.
Matching on BP
3,104 BP-users were matched 1:1 to a non-BP user (Table 1). Covariate balance was improved after matching compared to before matching, the standardised differences for all the covariates were less than 10%, indicating acceptable balance. E.g., before matching difference between non-BP and BP-users for women was imbalanced (SMD=0.31) and after matching that difference achieved an appropriate balance smaller than 10% (SMD=0.04). After matching, there were 42 (1.4%) vs. 57 (1.8%) AMD cases among non-BP vs. BP-users. The number of AMD cases according to patient characteristics, drug confounders and co-morbidity confounders are shown in Table 2. There were more AMD cases in women, non-smokers, current drinkers, and individuals using antidepressants, PPIs or NSAIDs. There was also (independent of BP consumption) more AMD cases in those with hypertension. BP-use was associated with more cases among men, former drinkers, antiepileptic-users, PPI-users, those with chronic renal failure and Type 2 diabetes among BP-users. However, the only statistically significant differences found were for drinking (P=0.02) and NSAID consumption (P=0.04). Person-years of follow-up were 11,793 vs. 10,350 among non-BP vs. BP-users. At year five there were 772 and 926 patients at risk for AMD, and 25 and 32 had developed AMD, respectively.
Table 1.
Baseline characteristics among non-BP and BP-users: before and after matching.
| before matching | after matching 1 to 1 | |||||
|---|---|---|---|---|---|---|
| Non-BP | BP-users | SMD | Non-BP | BP-users | SMD | |
| (n=6834) | (n=3105) | (n=3104) | (n=3104) | |||
| Age, mean (SD), years | 81.04 (10.15) | 79.93 (9.34) | 0.11 | 80.28 (10.78) | 79.94 (9.34) | 0.03 |
| Women | 4730 (69.2%) | 2552 (82.2%) | 0.31 | 2500 (80.5%) | 2551 (82.2%) | 0.04 |
| BMI, mean (SD), kg/m2 | 24.30 (4.68) | 24.50 (4.64) | 0.04 | 24.59 (4.81) | 24.50 (4.64) | 0.02 |
| Smoking | 0.07 | 0.02 | ||||
| Never | 4184 (61.2%) | 1864 (60.0%) | 1867 (60.1%) | 1864 (60.1%) | ||
| Former | 1531 (22.4%) | 783 (25.2%) | 795 (25.6%) | 782 (25.2%) | ||
| Current | 1119 (16.4%) | 458 (14.8%) | 442 (14.2%) | 458 (14.8%) | ||
| Drinking | 0.06 | 0.02 | ||||
| Never | 2073 (30.3%) | 912 (29.4%) | 922 (29.7%) | 912 (29.4%) | ||
| Former | 223 (3.3%) | 74 (2.4%) | 81 (2.6%) | 74 (2.4%) | ||
| Current | 4538 (66.4%) | 2119 (68.2%) | 2101 (67.7%) | 2118 (68.2%) | ||
| Index year | 0.53 | 0.06 | ||||
| 1999 | 733 (10.7%) | 153 (4.9%) | 138 (4.4%) | 153 (4.9%) | ||
| 2000 | 684 (10.0%) | 135 (4.3%) | 121 (3.9%) | 135 (4.3%) | ||
| 2001 | 646 (9.5%) | 149 (4.8%) | 156 (5.0%) | 149 (4.8%) | ||
| 2002 | 632 (9.2%) | 187 (6.0%) | 198 (6.4%) | 187 (6.0%) | ||
| 2003 | 626 (9.2%) | 205 (6.6%) | 218 (7.0%) | 205 (6.6%) | ||
| 2004 | 574 (8.4%) | 194 (6.2%) | 202 (6.5%) | 194 (6.2%) | ||
| 2005 | 460 (6.7%) | 259 (8.3%) | 268 (8.6%) | 259 (8.3%) | ||
| 2006 | 460 (6.7%) | 289 (9.3%) | 309 (10.0%) | 289 (9.3%) | ||
| 2007 | 435 (6.4%) | 286 (9.2%) | 288 (9.3%) | 286 (9.2%) | ||
| 2008 | 403 (5.9%) | 269 (8.7%) | 282 (9.1%) | 269 (8.7%) | ||
| 2009 | 347 (5.1%) | 287 (9.2%) | 272 (8.8%) | 287 (9.2%) | ||
| 2010 | 346 (5.1%) | 270 (8.7%) | 259 (8.3%) | 269 (8.7%) | ||
| 2011 | 302 (4.4%) | 272 (8.8%) | 250 (8.1%) | 272 (8.8%) | ||
| 2012 | 186 (2.7%) | 150 (4.8%) | 143 (4.6%) | 150 (4.8%) | ||
| Region | 0.09 | 0.02 | ||||
| North England | 3310 (48.4%) | 1469 (47.3%) | 1477 (47.6%) | 1468 (47.3%) | ||
| South England | 2413 (35.3%) | 1053 (33.9%) | 1055 (34.0%) | 1053 (33.9%) | ||
| Northern Ireland | 303 (4.4%) | 199 (6.4%) | 190 (6.1%) | 199 (6.4%) | ||
| Scotland | 334 (4.9%) | 157 (5.1%) | 148 (4.8%) | 157 (5.1%) | ||
| Wales | 474 (6.9%) | 227 (7.3%) | 234 (7.5%) | 227 (7.3%) | ||
| Drug confounders | ||||||
| Antiarrhythmics | 483 (7.1%) | 209 (6.7%) | 0.01 | 206 (6.6%) | 209 (6.7%) | <0.01 |
| Antidepressants | 2446 (35.8%) | 1139 (36.7%) | 0.02 | 1169 (37.7%) | 1139 (36.7%) | 0.02 |
| Antiepileptics | 429 (6.3%) | 212 (6.8%) | 0.02 | 220 (7.1%) | 212 (6.8%) | 0.01 |
| Antiparkinson | 366 (5.4%) | 118 (3.8%) | 0.07 | 124 (4.0%) | 118 (3.8%) | 0.01 |
| Anxiolytics | 1325 (19.4%) | 601 (19.4%) | <0.01 | 610 (19.7%) | 601 (19.4%) | 0.01 |
| PPI | 2207 (32.3%) | 1166 (37.6%) | 0.11 | 1176 (37.9%) | 1165 (37.5%) | 0.01 |
| NSAIDS | 5585 (81.7%) | 2671 (86.0%) | 0.12 | 2652 (85.4%) | 2671 (86.1%) | 0.02 |
| Systemic HRT | 374 (5.5%) | 344 (11.1%) | 0.21 | 285 (9.2%) | 344 (11.1%) | 0.06 |
| SERMS | 2 (0.0%) | 4 (0.1%) | 0.04 | 1 (0.0%) | 3 (0.1%) | 0.03 |
| Strontium | 10 (0.1%) | 2 (0.1%) | 0.03 | 3 (0.1%) | 2 (0.1%) | 0.01 |
| Calcium | 718 (10.5%) | 438 (14.1%) | 0.11 | 443 (14.3%) | 437 (14.1%) | 0.01 |
| Non-ocular corticoids | 440 (6.4%) | 265 (8.5%) | 0.08 | 245 (7.9%) | 265 (8.5%) | 0.02 |
| Ocular corticoids | 160 (2.3%) | 85 (2.7%) | 0.03 | 90 (2.9%) | 85 (2.7%) | 0.01 |
| Insulin | 100 (1.5%) | 28 (0.9%) | 0.05 | 33 (1.1%) | 28 (0.9%) | 0.02 |
| Co-morbidity confounders | ||||||
| Charlson Comorbidity in previous 5-years | 0.11 | 0.03 | ||||
| None | 3614 (52.9%) | 1808 (58.2%) | 1774 (57.2%) | 1807 (58.2%) | ||
| Mild (1 to 2) | 2160 (31.6%) | 884 (28.5%) | 899 (29.0%) | 884 (28.5%) | ||
| Moderate (3 to 4) | 707 (10.3%) | 272 (8.8%) | 275 (8.9%) | 272 (8.8%) | ||
| Severe (5+) | 353 (5.2%) | 141 (4.5%) | 156 (5.0%) | 141 (4.5%) | ||
| Asthma | 825 (12.1%) | 456 (14.7%) | 0.08 | 440 (14.2%) | 456 (14.7%) | 0.02 |
| Inflammatory bowel disease | 81 (1.2%) | 41 (1.3%) | 0.01 | 39 (1.3%) | 41 (1.3%) | 0.01 |
| Hypertension | 3075 (45.0%) | 1619 (52.1%) | 0.14 | 1619 (52.2%) | 1619 (52.2%) | <0.01 |
| Hyperlipidaemia | 762 (11.2%) | 527 (17.0%) | 0.17 | 503 (16.2%) | 527 (17.0%) | 0.02 |
| Ischemic heart disease | 1393 (20.4%) | 582 (18.7%) | 0.04 | 586 (18.9%) | 582 (18.8%) | <0.01 |
| CVD | 789 (11.5%) | 276 (8.9%) | 0.09 | 274 (8.8%) | 276 (8.9%) | <0.01 |
| COPD | 565 (8.3%) | 252 (8.1%) | 0.01 | 247 (8.0%) | 252 (8.1%) | 0.01 |
| Chronic renal failure | 294 (4.3%) | 131 (4.2%) | <0.01 | 148 (4.8%) | 131 (4.2%) | 0.03 |
| Cancers | 1324 (19.4%) | 534 (17.2%) | 0.06 | 579 (18.7%) | 534 (17.2%) | 0.04 |
| Cataract surgery | 903 (13.2%) | 307 (9.9%) | 0.10 | 322 (10.4%) | 307 (9.9%) | 0.02 |
| Type 1 diabetes | 34 (0.5%) | 17 (0.5%) | 0.01 | 17 (0.5%) | 17 (0.5%) | <0.01 |
| Type 2 diabetes | 739 (10.8%) | 329 (10.6%) | 0.01 | 321 (10.3%) | 329 (10.6%) | 0.01 |
Standardised mean difference, SMD; standard deviation, SD; body mass index, BMI; proton pump inhibitor, PPI; non-steroidal anti-inflammatory drug, NSAID; hormone replacement therapy, HRT; selective estrogen receptor modulator, SERM; cardio vascular disease, CVD; chronic obstructive pulmonary disease, COPD. SMD values <0.10 are indicators of good balance and suitability for survival analysis of the matched cohort.
Table 2.
AMD events among non-BP and BP-users after matching.
| Non-BP | BP-users | P-value | |
|---|---|---|---|
| (n=42) | (n=57) | ||
| Sex | |||
| Women | 39 (92.9%) | 49 (86.0%) | 0.35 |
| Men | 3 (7.1%) | 8 (14.0%) | |
| Smoking | 1.00 | ||
| Never | 25 (59.5%) | 35 (61.4%) | |
| Former | 12 (28.6%) | 16 (28.1%) | |
| Current | 5 (11.9%) | 6 (10.5%) | |
| Drinking | 0.02 | ||
| Never | 9 (21.4%) | 11 (19.3%) | |
| Former | 0 (0.0%) | 3 (5.3%) | |
| Current | 33 (78.6%) | 43 (75.4%) | |
| Index year | 1.00 | ||
| 1999 | 2 (4.8%) | 5 (8.8%) | |
| 2000 | 2 (4.8%) | 1 (1.8%) | |
| 2001 | 3 (7.1%) | 4 (7.0%) | |
| 2002 | 4 (9.5%) | 6 (10.5%) | |
| 2003 | 5 (11.9%) | 7 (12.3%) | |
| 2004 | 4 (9.5%) | 4 (7.0%) | |
| 2005 | 4 (9.5%) | 6 (10.5%) | |
| 2006 | 6 (14.3%) | 6 (10.5%) | |
| 2007 | 4 (9.5%) | 5 (8.8%) | |
| 2008 | 3 (7.1%) | 6 (10.5%) | |
| 2009 | 2 (4.8%) | 4 (7.0%) | |
| 2010 | 3 (7.1%) | 1 (1.8%) | |
| 2011 | 0 (0.0%) | 2 (3.5%) | |
| 2012 | ─ | ─ | |
| Region | 0.24 | ||
| North England | 21 (50.0%) | 30 (52.6%) | |
| South England | 14 (33.3%) | 19 (33.3%) | |
| Northern Ireland | 0 (0.0%) | 2 (3.5%) | |
| Scotland | 1 (2.4%) | 4 (7.0%) | |
| Wales | 6 (14.3%) | 2 (3.5%) | |
| Drug confounders | |||
| Antiarrhythmics | 2 (4.8%) | 5 (8.8%) | 0.70 |
| Antidepressants | 13 (31.0%) | 17 (29.8%) | 1.00 |
| Antiepileptics | 0 (0.0%) | 5 (8.8%) | 0.07 |
| Antiparkinson | 2 (4.8%) | 3 (5.3%) | 1.00 |
| Anxiolytics | 8 (19.1%) | 6 (10.5%) | 0.26 |
| PPI | 16 (38.1%) | 27 (47.4%) | 0.42 |
| NSAIDS | 41 (97.6%) | 48 (84.2%) | 0.04 |
| Systemic HRT | 2 (4.8%) | 4 (7.0%) | 1.00 |
| SERMS | ─ | ─ | |
| Strontium | ─ | ─ | |
| Calcium | 4 (9.5%) | 4 (7.0%) | 0.72 |
| Non-ocular corticoids | 5 (11.9%) | 3 (5.3%) | 0.28 |
| Ocular corticoids | 2 (4.8%) | 1 (1.8%) | 0.57 |
| Insulin | 1 (2.4%) | 0 (0.0%) | 0.42 |
| Co-morbidity confounders | |||
| Charlson Comorbidity in previous 5-years | 0.86 | ||
| None | 24 (57.1%) | 29 (50.9%) | |
| Mild (1 to 2) | 15 (35.7%) | 23 (40.3%) | |
| Moderate (3 to 4) | 2 (4.8%) | 2 (3.5%) | |
| Severe (5+) | 1 (2.4%) | 0 (0.0%) | |
| Asthma | 9 (21.4%) | 6 (10.5%) | 0.16 |
| Inflammatory bowel disease | 1 (2.4%) | 1 (1.8%) | 1.00 |
| Hypertension | 21 (50.0%) | 32 (56.1%) | 0.68 |
| Hyperlipidaemia | 6 (14.3%) | 14 (24.6%) | 0.31 |
| Ischemic heart disease | 5 (11.9%) | 11 (19.3%) | 0.41 |
| CVD | 5 (11.9%) | 2 (3.5%) | 0.13 |
| COPD | 5 (11.9%) | 3 (5.3%) | 0.28 |
| Chronic renal failure | 0 (0.0%) | 4 (7.0%) | 0.14 |
| Cancers | 6 (14.3%) | 8 (14.0%) | 1.00 |
| Cataract surgery | 4 (9.5%) | 6 (10.5%) | 1.00 |
| Type 1 diabetes | 1 (2.4%) | 0 (0.0%) | 0.42 |
| Type 2 diabetes | 3 (7.1%) | 8 (14.0%) | 0.35 |
Proton pump inhibitor, PPI; non-steroidal anti-inflammatory drug, NSAID; hormone replacement therapy, HRT; selective estrogen receptor modulator, SERM; cardio vascular disease, CVD; chronic obstructive pulmonary disease, COPD. P-values from Fisher's exact of 2 tails.
Survival probability of AMD
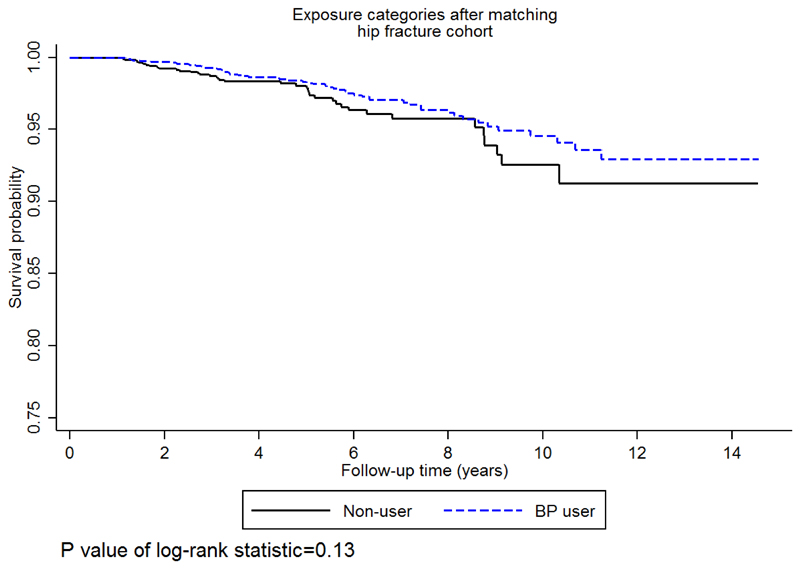
The Kaplan-Meier probability of AMD was not significantly different between non-BP and BP-users (P=0.13) (Figure 2). According to the Kaplan-Meier estimator, one in 100 hip-fracture patients would develop AMD in 5 years for non-BP, while this was 2 in100 for BP-users. Therefore, the probability of not experiencing AMD, some time after 5 years, was 0.986 (95% CI: 0.979 to 0.991) for non-BP and 0.978 (95% CI: 0.968 to 0.984) for BP-users. For 10 years of follow up these probabilities increased to 6 out of 100, 0.942 (95% CI: 0.910 to 0.963) and 0.936 (95% CI: 0.913 to 0.953), for non-BP and BP-users respectively.
Fig. 2.
Kaplan-Meier survival curves. Survival curves showing probability of age-related macular degeneration for bisphosphonate (BP)-users Vs. non-BP users. P values were calculated using the log-rank test.
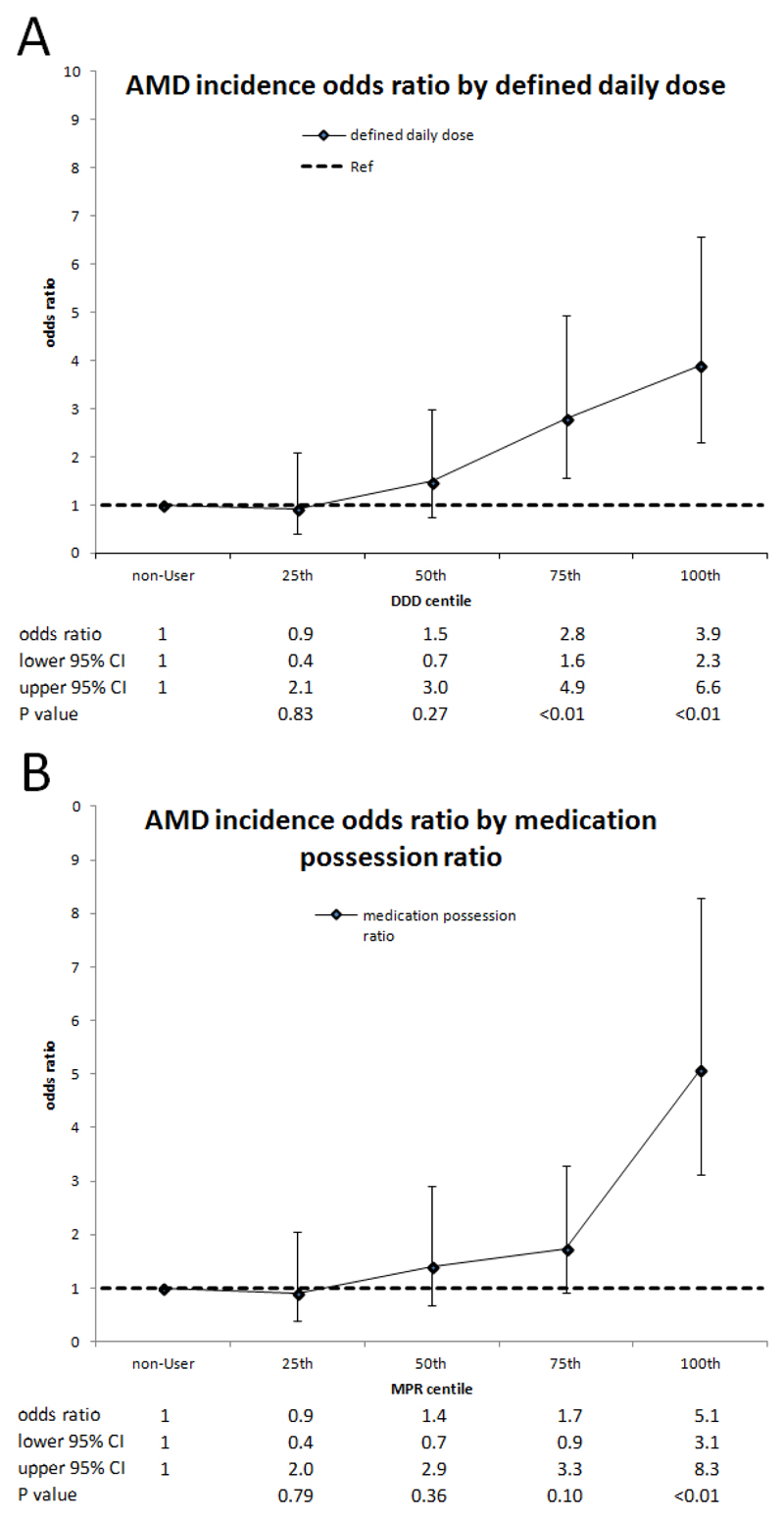
Using multivariable competing risk survival models, there was weak evidence of an increased risk of AMD development among BP-users (sHR: 1.6; CI: 0.9-2.7; P=0.08). Conditional logistic regression analysis on DDDs showed a trend increasing across the quartiles, which become significant for the 3rd and 4th quartiles (OR75th:2.8, CI: 1.6-4.9, P<0.01; OR100th: 3.9, CI: 2.3-6.6, P<0.01) (Figure 3). A similar ascending trend was observed for the quartiles of MPR, achieving significance for the highest quartile (OR100th: 5.1, 3.1-8.3, P<0.01) (Figure 3).
Fig. 3.
Age-related macular degeneration incidence according to (A) bisphosphonate defined daily dose (DDD) and (B) medication possession ratio (MPR)*.
* Odds ratios for AMD incidence (black marker) were obtained from conditional logistic regression. They are presented with their 95% confidence intervals (CI) (error bars) and their P values. Odds ratios are presented for bisphosphonate (BP) use according to quartile of DDD and MPR compared to non-BP use. Markers or error bars lying into the dotted horizontal line denoted statistically non-significant association.
Discussion
We found that in an incident hip fracture cohort, oral BP use was not overall significantly associated with increased risk of AMD. After matching, there were 42 (1.4%) vs. 57 (1.8%) AMD cases among non-BP users vs. BP-users, equating to a low and non-significant absolute risk difference of 0.49%, CI: -0.13% to 1.11%; P=0.06) (6 more cases per 1000). However, the increased risk became somewhat greater and statistically significant among BP-users who had a high MPR. According to our results, one AMD case at five years would be prevented if 12 hip fracture patients in the top quartile of MPR avoided BP treatment longer than one year.
To our knowledge, there is only one previous study analysing the risk of AMD in patients taking oral BP24. This study used data from the Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) database in the USA, which captures spontaneous adverse drug reactions. Two patient cohorts from Canada were also included, covering the period 2009-2013 for the cases and 2000-2007 for the controls. This study found a higher risk of developing wet AMD in BP-users, although a caveat to these findings has to be that the selection of patients was limited to reported cases to FAERS without a clear definition of AMD for a disproportionality analysis. In addition, although this prior study conducted a case-control and a self-controlled case series analysis, this was limited to patients with wet AMD or controls without any type of AMD (dry, wet or unspecified) selected among those visiting the ophthalmologist, and as such was not population-based or inclusive of dry and unspecified AMD cases. Additionally, some concerns can arise when using different time periods for cases vs. controls, and despite the fact that their results reached significance, the magnitude of the effect was marginal25.
Several risk factors have been previously identified as being associated with the development of AMD, with age being the strongest one. The pathogenesis however is not well understood. Inflammation may play a significant role and most recently, hydroxyapatite (calcium and phosphate) spherules, which is commonly found in bones and teeth, has been identified as a key player in the development of the disease8.
In terms of a potential mechanism of effect, ocular inflammatory reactions have been described in patients on bisphosphonate treatment. Inflammatory proteins such as interleukins have also been linked to AMD development26–31 and BPs could cause systemic release of cytokines and other acute phase proteins. Moreover, a causative link between bisphosphonates and inflammatory ocular events is likely and is based on reports of inflammatory eye reaction relapses after affected patients were re-challenged with the same or another bisphosphonate32.
If indeed inflammation is the cause, it begs the question why AMD is not a common complication in patients with inflammatory diseases such as rheumatoid arthritis, inflammatory bowel disease, or sarcoidosis? The explanation could be that the inflammation alone is not sufficient by itself but requires the presence of hydroxyapatite (calcium and phosphate) spherules, which could be the critical factor in the development of AMD. Interestingly, BPs could not only retard the growth but also increase the dissolution of hydroxyapatite crystals33, in which case they would be expected to provide some protective effect. Our results did not show any beneficial effects of nitrogen containing oral bisphosphonates on the incidence of AMD. On the other hand, there is no convincing explanation for the increased risk with higher DDDs and MPR. Therefore, if there is an association between their use and AMD, this association may not be a causal one.
Another plausible mechanism, and opposed to our results (increased risk with higher DDDs and MPR), could relate bisphosphonate use to a reduced risk of AMD is their potential antiangiogenic effect especially in the wet form of AMD where anti-vascular endothelial growth factor (VEGF) intravitreal agents are the first line of treatment. In vitro, administration of BPs (alendronate and etidronate) on cultured retinal pigment epithelial cells reduced the expression of a number of angiogenic factors34. Also, in two pilot, non-randomized studies of relatively short duration (6 and 24 months), administration of 5 mg alendronate daily in patients with wet AMD showed promising results35, 36.
BPs have been demonstrated to stop bone loss and reduce significantly the occurrence of fractures in individuals with osteoporosis, and consequently, they are the most extensively prescribed antiresorptive drugs37. Although they have been associated with some rare and severe adverse effects (atypical femur fractures and osteonecrosis of the jaw), the “real-world” anti-fracture benefit to patients at high risk of fracture far exceeds the potential risks38.
Strengths and limitations
There are multiple strengths to our current study. Firstly, we conducted the analysis in a population of hip fracture patients for which BP treatment is recommended for the secondary prevention of fragility fractures. Secondly, we have used an incident user design to minimise biases associated with the inclusion of prevalent BP-users. Thirdly, despite the fact that our study was observational, we show an effect of BP after adjusting for a large number of confounders, including comorbidity, co-medication and other important factors using propensity matching. Furthermore, we used a time dependent exposure variable and conducted a subsequent nested case-control analysis for assessing dose-response. Using a nested case-control analysis gave us an unbiased assessment like the sHR obtained from the time to event analysis in the matched cohort, almost free of immortal time bias39.
Included in the limitations is the fact that prescriptions in the CPRD may have been misclassified in terms of BP exposure due to data entry errors by GPs and other potential sources of error in the use of electronic systems40. Nevertheless, given that we only included patients into the BP-user cohort if they were newly treated, misclassification is probably low given that CPRD is nowadays well known and established in the routine of health personnel. Another limitation is that the study is observational in nature and unmeasured or unknown factors may have resulted in residual confounding, e.g. genetic risk factors41. Furthermore, deprivation index, ethnicity42 and measurements of the marker of inflammation C reactive protein were in the dataset but were not used due to high level of missingness and/or inaccuracy. Misclassification of AMD cannot be ruled out, as well as the further subdivision between wet and dry categories with many patients included in the category of unspecific. For this reason we have not made distinctions between types of AMD. Furthermore, we may have potentially overestimated BP use as the analyses were based only on prescription data given that information on adherence is not captured in CPRD. However, the nested case-control design did allow us to estimate the effect of BP dose in AMD occurrence. Finally, there is the possibility of detection bias given that BP use is associated with one eye condition, therefore giving rise to the possibility that BP-users were more likely than non-BP users to have an eye exam where early AMD could be diagnosed, although this is unlikely given that eye examination is not routine for patients on BPs or in those with a hip fracture.
Conclusion
Our study of incident hip fracture patients offers an external validation showing that overall, oral BP use was not associated with increased risk of AMD in a cohort of hip fracture patients. However we found increased risk with higher DDDs and MPR. More data are needed to confirm these findings.
Acknowledgements
We would like to acknowledge and thank Dr Sanni Ali, Maria T. Sanchez Santos, Dr Sara Khalid and Ed Burn for their rich input provided. We also thank Dr Rohini Mathur for her help with the ethnicity variable gathered in the CPRD.
Funding: No funding.
Footnotes
Contributors: MP had the original idea for this study. AJ contributed to the development of the idea and the study design. CG reviewed the literature, contributed to the study design, undertook the primary analysis and the first interpretation, and wrote the first draft of the paper. MP, CC, DPA, AD, SH and AJ critically reviewed the paper. All authors approved the submitted version.
Competing interests: All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any additional organisation for the submitted work.
CG and MP have no conflicts of interest.
D.P.A. has received unrestricted research and educational grants from AMGEN and Bioiberics S.A.
C.C. has received consultancy, lecture fees and honoraria from AMGEN, GSK, Alliance for Better Bone Health, MSD, Eli Lilly, Pfizer, Novartis, Merck, Servier, Medtronic and Roche.
A.J. has received consultancy, lecture fees and honoraria from Servier, UK Renal Registry, Oxford Craniofacial Unit, IDIAP Jordi Gol, Freshfields Bruckhaus Deringer, is a member of the Data Safety and Monitoring Board (which involved receipt of fees) from Anthera Pharmaceuticals, INC., and received consortium research grants from ROCHE.
Ethical approval: For CPRD data analysis, the protocol was approved by independent scientific advisory committee (reference No 13_069RA2).
Data sharing: None.
Transparency declaration: The corresponding author affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Patient involvement: Patients were not involved in the production of this article.
Copyright: The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, a worldwide licence to the Publishers and its licensees in perpetuity, in all forms, formats and media (whether known now or created in the future), to i) publish, reproduce, distribute, display and store the Contribution, ii) translate the Contribution into other languages, create adaptations, reprints, include within collections and create summaries, extracts and/or, abstracts of the Contribution, iii) create any other derivative work(s) based on the Contribution, iv) to exploit all subsidiary rights in the Contribution, v) the inclusion of electronic links from the Contribution to third party material where-ever it may be located; and, vi) licence any third party to do any or all of the above.
References
- 1.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. The Lancet Global Health. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 2.Gehrs KM, Anderson DH, Johnson LV, Hageman GS. Age-related macular degeneration--emerging pathogenetic and therapeutic concepts. Annals of medicine. 2006;38:450–471. doi: 10.1080/07853890600946724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Owen CG, Jarrar Z, Wormald R, Cook DG, Fletcher AE, Rudnicka AR. The estimated prevalence and incidence of late stage age related macular degeneration in the UK. British Journal of Ophthalmology. 2012 doi: 10.1136/bjophthalmol-2011-301109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minassian D, Reidy A. Future Sight Loss in the decade 2010 to 2020: an Epidemiological and Economic Model. London: Royal National Institute of Blind People; 2009. p. 130. [Google Scholar]
- 5.Rees A, Zekite A, Bunce C, Patel PJ. How many people in England and Wales are registered partially sighted or blind because of age-related macular degeneration? Eye. 2014;28:832–837. doi: 10.1038/eye.2014.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanis JA. Assessment of osteoporosis at the primary health-care level. Sheffield, UK: World Health Organization Collaborating Centre for metabolic bone diseases, University of Sheffield; 2007. p. 339. [Google Scholar]
- 7.National Hip Fracture Database annual report 2015. Vol. 2015. London: Royal College of Physicians; p. 99. [Google Scholar]
- 8.Thompson RB, Reffatto V, Bundy JG, et al. Identification of hydroxyapatite spherules provides new insight into subretinal pigment epithelial deposit formation in the aging eye. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:1565–1570. doi: 10.1073/pnas.1413347112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herrett E, Gallagher AM, Bhaskaran K, et al. Data Resource Profile: Clinical Practice Research Datalink (CPRD) International journal of epidemiology. 2015;44:827–836. doi: 10.1093/ije/dyv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green J, Czanner G, Reeves G, Watson J, Wise L, Beral V. Oral bisphosphonates and risk of cancer of oesophagus, stomach, and colorectum: case-control analysis within a UK primary care cohort. BMJ : British Medical Journal. 2010;341:c4444. doi: 10.1136/bmj.c4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jick SS, Kaye JA, Vasilakis-Scaramozza C, et al. Validity of the General Practice Research Database. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2003;23:686–689. doi: 10.1592/phco.23.5.686.32205. [DOI] [PubMed] [Google Scholar]
- 12.Vinogradova Y, Coupland C, Hippisley-Cox J. Exposure to bisphosphonates and risk of gastrointestinal cancers: series of nested case-control studies with QResearch and CPRD data. BMJ : British Medical Journal. 2013;346:f114. doi: 10.1136/bmj.f114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Austin PC. A Tutorial and Case Study in Propensity Score Analysis: An Application to Estimating the Effect of In-Hospital Smoking Cessation Counseling on Mortality. Multivariate Behavioral Research. 2011;46:119–151. doi: 10.1080/00273171.2011.540480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Statistics in medicine. 2014;33:1242–1258. doi: 10.1002/sim.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharmaceutical Statistics. 2011;10:150–161. doi: 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Austin PC. Comparing paired vs non-paired statistical methods of analyses when making inferences about absolute risk reductions in propensity-score matched samples. Statistics in medicine. 2011;30:1292–1301. doi: 10.1002/sim.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Statistics in medicine. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kleinbaum DG, Klein M. Survival analysis : a self-learning text. New York: Springer; 2012. [Google Scholar]
- 19.Andersen PK, Geskus RB, de Witte T, Putter H. Competing risks in epidemiology: possibilities and pitfalls. International journal of epidemiology. 2012;41:861–870. doi: 10.1093/ije/dyr213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 21.McCullagh P, Nelder JA. Generalized linear models. CRC press; 1989. [Google Scholar]
- 22.Ho D, Imai K, King G, Stuart EA. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. J Stat Softw. 2011;42:28. [Google Scholar]
- 23.Sekhon JS. Multivariate and Propensity Score Matching Software with Automated Balance Optimization: The Matching package for R. J Stat Softw. 2011;42:52. [Google Scholar]
- 24.Mammo Z, Guo M, Maberley D, Matsubara J, Etminan M. Oral Bisphosphonates and Risk of Wet Age-Related Macular Degeneration. American journal of ophthalmology. 2016 doi: 10.1016/j.ajo.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 25.Grzybowski A, Iribarren R, Iribarren G, Honda S. Oral Bisphosphonates and Risk of Wet Age-Related Macular Degeneration. American journal of ophthalmology. 176:255. doi: 10.1016/j.ajo.2016.12.028. [DOI] [PubMed] [Google Scholar]
- 26.Chan CC, Ardeljan D. Molecular pathology of macrophages and interleukin-17 in age-related macular degeneration. Advances in experimental medicine and biology. 2014;801:193–198. doi: 10.1007/978-1-4614-3209-8_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goverdhan SV, Ennis S, Hannan SR, et al. Interleukin-8 promoter polymorphism -251A/T is a risk factor for age-related macular degeneration. Br J Ophthalmol. 2008;92:537–540. doi: 10.1136/bjo.2007.123190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seddon JM, George S, Rosner B, Rifai N. Progression of age-related macular degeneration: prospective assessment of C-reactive protein, interleukin 6, and other cardiovascular biomarkers. Archives of ophthalmology (Chicago, Ill : 1960) 2005;123:774–782. doi: 10.1001/archopht.123.6.774. [DOI] [PubMed] [Google Scholar]
- 29.Theodoropoulou S, Copland DA, Liu J, Dick AD. Role of interleukin 33/ST2 axis in the immune-mediated pathogenesis of age-related macular degeneration. Lancet (London, England) 2015;385(Suppl 1):S97. doi: 10.1016/S0140-6736(15)60412-3. [DOI] [PubMed] [Google Scholar]
- 30.Tsai YY, Lin JM, Wan L, et al. Interleukin gene polymorphisms in age-related macular degeneration. Investigative ophthalmology & visual science. 2008;49:693–698. doi: 10.1167/iovs.07-0125. [DOI] [PubMed] [Google Scholar]
- 31.Zhang S, Liu Y, Lu S, Cai X. Genetic variants of interleukin 17A are functionally associated with increased risk of age-related macular degeneration. Inflammation. 2015;38:658–663. doi: 10.1007/s10753-014-9973-3. [DOI] [PubMed] [Google Scholar]
- 32.Pazianas M, Clark EM, Eiken PA, Brixen K, Abrahamsen B. Inflammatory eye reactions in patients treated with bisphosphonates and other osteoporosis medications: Cohort analysis using a national prescription database. Journal of Bone and Mineral Research. 2013;28:455–463. doi: 10.1002/jbmr.1783. [DOI] [PubMed] [Google Scholar]
- 33.Russell RG. Bisphosphonates: the first 40 years. Bone. 2011;49:2–19. doi: 10.1016/j.bone.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 34.Or C, Cui J, Matsubara J, Forooghian F. Pro-inflammatory and anti-angiogenic effects of bisphosphonates on human cultured retinal pigment epithelial cells. British Journal of Ophthalmology. 2013;97:1074–1078. doi: 10.1136/bjophthalmol-2013-303355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Honda S, Nagai T, Kondo N, et al. Therapeutic effect of oral bisphosphonates on choroidal neovascularization in the human eye. Journal of ophthalmology. 2010;2010:7. doi: 10.1155/2010/206837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miki A, Honda S, Nagai T, Tsukahara Y, Negi A. Effects of oral bisphosphonates on myopic choroidal neovascularisation over 2 years of follow-up: comparison with anti-VEGF therapy and photodynamic therapy. A pilot study. Br J Ophthalmol. 2013;97:770–774. doi: 10.1136/bjophthalmol-2012-303007. [DOI] [PubMed] [Google Scholar]
- 37.Maraka S, Kennel KA. Bisphosphonates for the prevention and treatment of osteoporosis. BMJ : British Medical Journal. 2015;351 doi: 10.1136/bmj.h3783. [DOI] [PubMed] [Google Scholar]
- 38.Hawley S, Leal J, Delmestri A, et al. Anti-Osteoporosis Medication Prescriptions and Incidence of Subsequent Fracture Among Primary Hip Fracture Patients in England and Wales: An Interrupted Time-Series Analysis. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2016;31:2008–2015. doi: 10.1002/jbmr.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suissa S. Pharmacoepidemiology. 4th Edition. 2005. Novel Approaches to Pharmacoepidemiology Study Design and Statistical Analysis; pp. 811–829. [Google Scholar]
- 40.Brown CL, Mulcaster HL, Triffitt KL, et al. A systematic review of the types and causes of prescribing errors generated from using computerized provider order entry systems in primary and secondary care. Journal of the American Medical Informatics Association : JAMIA. 2017;24:432–440. doi: 10.1093/jamia/ocw119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. The Lancet. 379:1728–1738. doi: 10.1016/S0140-6736(12)60282-7. [DOI] [PubMed] [Google Scholar]
- 42.Mathur R, Bhaskaran K, Chaturvedi N, et al. Completeness and usability of ethnicity data in UK-based primary care and hospital databases. Journal of Public Health. 2013;36:684–692. doi: 10.1093/pubmed/fdt116. [DOI] [PMC free article] [PubMed] [Google Scholar]