Abstract
Background
Static and dynamic functional connectivity are being increasingly used to measure the effects of disease and a range of different interventions on brain networks. Whilst preliminary evidence suggests that static connectivity can be modulated by chronic antidepressants administration in healthy individuals and in major depression, much less is known about the acute effects of antidepressants especially on dynamic functional connectivity changes. Here we examine acute effects of antidepressants on dynamic functional connectivity within the default mode network. The default mode network is a well described network with many functions in which the role of serotonin is not clear.
Methods
In this work we measured acute pharmacological effects of an infusion of the selective serotonin reuptake inhibitor (SSRI) citalopram 10 mg in a sample of thirteen healthy volunteers randomised to receive on two occasions the active compound or placebo in a cross over dosing.
Results
Acute citalopram administration relative to placebo increased static connectivity between the medial prefrontal cortex and right dorsolateral prefrontal cortex and posterior cingulate cortex. The SSRI also induced a reduction in variability of connectivity with the medial prefrontal cortex in the precuneus and posterior cingulate cortex.
Discussion
The measured changes are compatible with modified serotonin cortical availability.
Keywords: Serotonin, MRI, functional MRI, Citalopram, SSRI
1. Introduction
The medial prefrontal cortex, a pivotal component of the default mode network (DMN) involved in decision-making, attention, and self-referential processes (Perkins et al., 2015), shares extensive connectivity with cortical and limbic regions involved in affective modulation.
Connectivity strength between these regions and other overlapping areas including the ‘dorsal nexus’ has been shown to be increased in major depression (Sheline et al., 2010) and reduced in affected individuals by antidepressants such as selective serotonin reuptake inhibitors (SSRIs) (Wang et al., 2015), indicating that it plays a pivotal role in affective dysregulation and that serotonergic dysfunction might underpin aberrant function in this network. A similar effect has been shown in healthy individuals with chronic antidepressant administration, an effect primarily directed towards subcortical limbic nodes such as the hippocampus and amygdala (McCabe and Mishor, 2011)(McCabe et al., 2011)(Wang et al., 2015) functionally related to medial prefrontal regions.
Recent interest has focused on investigating the variability of functional connectivity to complement a more conventional measure of the strength of the functional interdependence within brain networks. Whilst a measure of connectivity strength assumes that the neural signal within the network is constant, the proposed measure of functional variability explores the variability of the signal within the very same network which reflects the dynamic nature of intra and inter-region neural processes (Hutchison and Morton, 2016)(Calhoun et al., 2014). Understanding connectivity variations over time might provide further information on the biological mechanisms underlying affective disorders in conjunction with measures of connectivity strength.
For example, a recent experiment in major depression explored differences in the variability of functional connectivity originating from the medial prefrontal cortex vs. healthy individuals and indicated an increased variability in functional connectivity with dorsolateral prefrontal cortex and insula, possibly an indication of a self-directed ruminative cognitive style, and decreased variability with the parahippocampal gyrus (Kaiser et al., 2016). This indicates that abnormalities in connectivity dynamics may be an important pathological feature of the disorder. Despite their effectiveness and widespread use as first line treatment for affective disorders, the mechanism of action of selective serotonin reuptake inhibitors (SSRIs) is not fully elucidated (Cleare et al., 2015). Studying the effects of SSRIs in healthy volunteers provides an opportunity for improving our knowledge and to perfect future pharmacological interventions. To our knowledge, there is no study to date investigating pharmacological effects on both static and dynamic functional connectivity following acute administration of an SSRI in healthy individuals.
In this study we set out to evaluate the acute pharmacological effects of serotonergic modulation using an SSRI on strength and stability of resting state functional connectivity in the medial prefrontal cortex in healthy individuals. We chose to focus on the default mode network, as it is a well-described network with many functions relevant to depression and in which the role of serotonin is not clear. As such, our method used independent component analysis (ICA) to identify this network, and further analyses were performed within the identified default mode network component. In view of the wide distribution in the brain of serotonergic receptors (Klaassens et al., 2015) and the known interaction between the DMN and the central executive and salience networks relevant to depressive disorders (Manoliu et al., 2013) we also performed exploratory analyses in these two resting state networks.
A number of lines of evidence indicate that the immediate effect of acute SSRI administration is to reduce serotonin neuronal firing and hence postsynaptic serotonin cortical availability in animals (Gartside et al., 1995) and humans (Selvaraj et al., 2012), likely mediated by the dorsal raphe nuclei via inhibitory 5HT1A autoreceptors (Selvaraj et al., 2012). In view of this we hypothesized that acute SSRI administration would result in heightened connectivity strength as shown in untreated major depression in the medial prefrontal cortex. We also postulated that the SSRI infusion would result in altered stability in the medial prefrontal cortex depending on the regions involved in line with findings in major depression.
2. Methods
2.1. Sample
Inclusion criteria were male and female healthy subjects aged between 35 and 65 years (Selvaraj et al., 2012) (Selvaraj et al., 2017). Thirteen healthy male participants agreed to take part and were screened using DSM-IV (SCID) criteria to exclude any significant current or past psychiatric history prior to participating in this study. Exclusion criteria included any significant psychopathology, any relevant medical history or contraindication to MRI scanning, present and past (previous 3 months) use of psychotropic medication, current significant illicit substance/alcohol misuse. Drug use was excluded with a urine drug screen prior to each scan. Sinus rhythm and absence of any QT prolongation were confirmed electrocardiographically prior to study inclusion.
The study involved two sessions separated by at least a one-week interval between them. Each session consisted of a 30 minutes intravenous infusion of either normal saline or 10 mg of citalopram followed by a functional MRI resting state scan. The scan took place approximately 15-30 minutes after the infusion when circulating citalopram was at its peak concentration (Selvaraj et al., 2012). Hence each participant received infusions with a single-blind (study subject), random order, cross-over design. Blood samples were collected in both experiments for citalopram levels prior to the infusion (t=0min) and before the MRI scan (t=45min). The Hamilton Depression Rating Scale 17 items (HAMD-17), the Beck Depression Inventory (BDI) and the State-Trait Anxiety Inventory were administered to investigate potential pharmacological emotional effects on the subjects and the Visual Analogue Scales (VAS) to ascertain subjective affective responses on emotions (including anxiety, sadness, happiness, anger, and irritability) across sessions. Potential side effects were systematically monitored.
The National Research Ethics Service, West London Committee, approved the study and all the subjects consented to their participation. Participants were given a small compensation for taking part in the study. MRI scans were carried out at the Hammersmith Hospital in London, Institute of Clinical Studies, MRC Centre.
2.2. Design
2.2.1. Functional MRI Acquisition
MRI was performed on a 3T scanner (3T Intera Philips Medical Systems (Best, The Netherlands) to acquire 32 T2*-weighted transverse echoplanar images (EPI) per volume with blood oxygenation level dependent (BOLD) contrast. EPIs were acquired with 1.95 mm*1.95 mm voxel dimensions in-plane, repetition time=2s, echo time=30 ms, phase encoding direction=anterior-posterior, field of view=576 mm2, matrix size 112x112. These comprised 3.25 mm-thick axial slices with no between slice gap. A total of 192 EPI volumes were collected with slices acquired in an even-odd interleaved in a descending direction. Real-time reconstruction, z-shimming correction and a slice tilt of −30° to the anterior commissure–posterior commissure line were used to minimize orbitofrontal and temporal signal dropout as a result of magnetic field inhomogeneities due to air tissue susceptibility differences in these regions (Weiskopf et al., 2006)(Weiskopf et al., 2007). A whole-brain 3D-MPRAGE scan was acquired (TR=9.6 ms, TE=4.5 ms, flip angle=8°, slice thickness = 1.2 mm, 0.94 mm × 0.94 mm in plane resolution, 150 slices) after EPI scans.
2.2.2. Functional MRI Preprocessing
Data were pre-processed with custom Nipype (http://nipy.org/nipype/) scripts, using tools from SPM12 (http://www.fil.ion.ucl.ac.uk/spm) and FSL 5.0.9 (http://fsl.fmrib.ox.ac.uk/) in addition to custom code. Data were first realigned to the first functional image before slice timing correction was performed. These images were then co-registered to the T1 scan and normalised to MNI space using the forward deformation fields generated from segmentation of the structural image. Time series from white matter and CSF regions, plus six motion parameters (three translation plus three rotation) were regressed from the data, before temporally filtered from 0.009 to 0.08hz. These images were finally demeaned, detrended, and smoothed with a 6mm FWHM kernel.
2.2.3. Motion scrubbing
Head motion is an important source of noise in resting state data, and can lead to spurious estimates of resting state connectivity (Power et al., 2012). To minimise the influence of head motion on our results, we performed motion scrubbing. Time points demonstrating excessive motion were identified using FSL motion outliers tools based on RMS intensity differences between volumes and DVARS (Power et al., 2012), with default settings. In traditional connectivity analyses these time points are typically removed from the time series, however this would affect the length of the time series and hence dynamic functional connectivity estimates. We therefore interpolated the scrubbed time points using 3rd order b-spline interpolation. To further ensure that differences between drug conditions were not influenced by motion, we compared motion parameters (total distance travelled, mean frame-wise displacement, and maximum frame-wise displacement) between drug and placebo conditions.
2.2.4. Seed region of interest definition
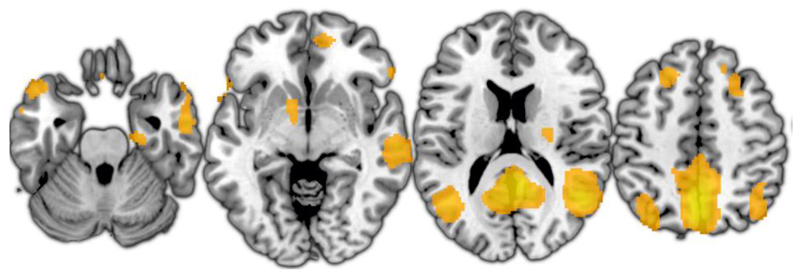
Default mode network regions were identified using group Canonical ICA (Varoquaux et al., 2010) with 20 clusters. The default mode network component is illustrated in Figure 3, and peak coordinates are provided in Table 1. A 3D map of the component is available to download from ‘Figshare’ at the following address: https://figshare.com/articles/Citalopram_connectivity_DMN_component/4309439. A spherical region of interest with a diameter of 10mm was generated at the peak coordinates of the mPFC cluster identified as described above, and the mean time series was extracted from this region for further analysis. The entire default mode network component was used to provide regions of interest for subsequent voxelwise analyses.
Figure 3.
Default mode network component derived using independent component analysis.
Table 1.
Results of voxelwise analysis of connectivity strength between medial prefrontal cortex and other default mode network regions. X, Y, and Z values represent MNI coordinates. Clusters defined at a voxelwise threshold of .001 uncorrected with a cluster-level threshold of .05 FDR corrected.
| Region | Brodmann area | Cluster extent (voxels) | F | FDR-corrected p | X | Y | Z |
|---|---|---|---|---|---|---|---|
| Effect of drug condition | |||||||
| Right dorsolateral prefrontal cortex | 8 | 18 | 48.06 | 0.020 | 28 | 12 | 54 |
| Posterior cingulate cortex | 39 | 17 | 61.56 | 0.002 | 0 | -24 | 26 |
| Left dorsolateral prefrontal cortex | 8 | 13 | 94.04 | 0.033 | -22 | 22 | 52 |
| Effect of order | |||||||
| No significant clusters | |||||||
| Drug X order interaction | |||||||
| No significant clusters | |||||||
We also performed exploratory analyses in two further resting state networks (the central executive network and salience network) to examine whether the effects of citalopram extended beyond our hypothesised default mode regions. We used a similar process to identify seed regions and regions of interest, extracting signal from a 10mm sphere at the peak of a dorsolateral prefrontal cluster (MNI coordinates = 8, 30, 62) for the central executive network, and an anterior cingulate cortex cluster (0, -2, 56) for the salience network. These entire networks were then used for small volume correction when assessing effects of citalopram within these networks.
2.2.5. Static functional connectivity analysis
We also computed static connectivity using the Fisher Z-transformed Pearson correlation between the entire mPFC time series and the time series of all other voxels in the brain to produce whole brain maps of connectivity with the mPFC seed. These maps were entered into a two-way ANOVA with drug condition and order of administration as factors, allowing both main effects of drug and order to be explored in addition to the drug X order interaction. To control for multiple comparisons, statistical maps were thresholded with a cluster-defining voxelwise threshold of .001 and a cluster-wise threshold of .05 FDR corrected using small volume correction within the DMN, central executive network, or salience network regions identified previously using ICA.
2.2.6. Dynamic functional connectivity analysis
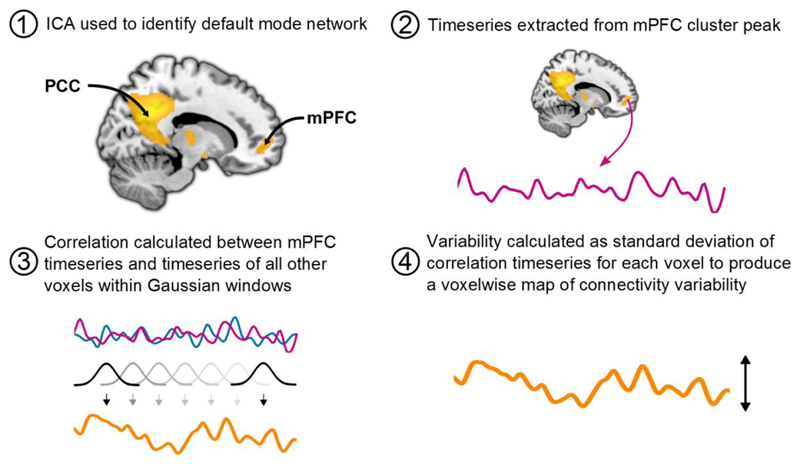
Whole-brain sliding window analysis was performed using custom Python (https://www.python.org/) scripts. Pearson correlations between the mPFC time series and time series of all other voxels were calculated using a series of 40 second Gaussian windows with a standard deviation of 8 seconds, sliding by 1 TR. This window length was chosen to provide optimal sensitivity to fluctuations on connectivity strength (see Wise et al., in press for further discussion on the choice of window lengths and related preprocessing steps). The correlation values for each window were then transformed to Fisher’s Z-scores. Variability of these correlation values across the brain was measured using their standard deviation to create whole brain variability maps. Placebo and citalopram variability maps were compared using the same method as the static analyses. The dynamic functional connectivity analysis procedure is illustrated in Figure 1.
Figure 1.
Illustration of the dynamic functional connectivity analysis procedure. mPFC region of interest MNI coordinates: 14, 56, 2. mPFC: Medial prefrontal cortex, PCC: Posterior cingulate cortex, ICA: Independent component analysis.
3. Results
3.1. Demographic details and mood ratings
Thirteen male participants (mean age of 48 +/-9 years) completed the study with 30 days mean interval between visits. Average rating scales score and standard deviation (SD) were HAMD-17 = 0.82 (3.1), BDI = 1.46 (1.8), State Anxiety Inventory = 42.62 (3.4) and Trait Anxiety Inventory = 28.20 (6.7). There were no reported significant side effects following citalopram infusion and affective measures did not report significant differences between sessions on subjective VAS affective state measures (paired t tests, all Ps>0.1).
3.2. Static and dynamic connectivity analyses
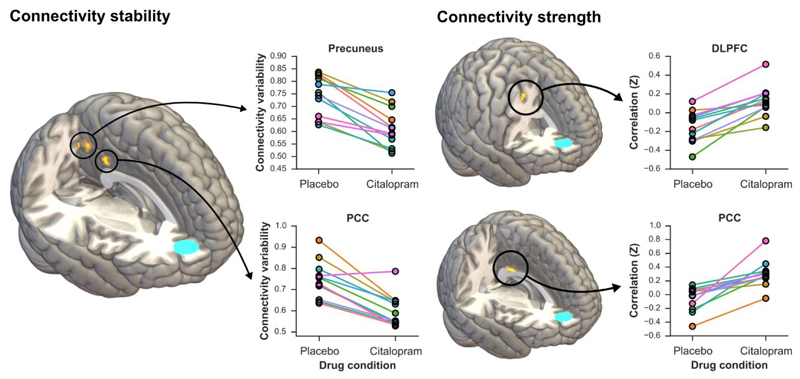
When comparing citalopram with placebo, static connectivity with the medial prefrontal cortex was increased under citalopram in the bilateral dorsolateral prefrontal cortex and posterior cingulate cortex (Table 1 and Figure 2). This finding suggests that neural communication between the medial prefrontal cortex and these two regions is strengthened following acute citalopram infusion. Dynamic functional connectivity analysis demonstrated that in the citalopram vs. placebo comparison the variability of the connectivity between medial prefrontal cortex, precuneus and posterior cingulate cortex was significantly reduced (Table 2, Figure 2 and Supplementary Table 1), indicating that connectivity within between these areas had become more stable.
Figure 2.
Strength and stability of connectivity within the default mode network under placebo and citalopram conditions. The medial prefrontal cortex seed is represented in blue on the 3D images. PCC: Posterior cingulate cortex, DLPFC: Dorsolateral prefrontal cortex.
Table 2.
Results of voxelwise analysis of connectivity variability between medial prefrontal cortex and other default mode network regions. C: citalopram, P: placebo, PC: placebo first, citalopram second, CP: citalopram first, placebo second. X, Y, and Z values represent MNI coordinates. Clusters defined at a voxelwise threshold of .001 uncorrected with a cluster-level threshold of .05 FDR corrected.
| Region | Brodmann area | Cluster extent (voxels) | F | FDR-corrected p | X | Y | Z | Direction |
|---|---|---|---|---|---|---|---|---|
| Effect of drug condition | ||||||||
| Precuneus | 31 | 21 | 70.35 | < .001 | -2 | -56 | 38 | C > P |
| Posterior cingulate cortex | 23 | 17 | 45.68 | < .001 | -2 | -38 | 30 | C > P |
| Effect of order | ||||||||
| Angular gyrus | 39 | 20 | 66.25 | 0.001 | -46 | -72 | 30 | PC > CP |
| Precuneus | 23 | 7 | 45.65 | 0.041 | -4 | -64 | 28 | PC > CP |
| Angular gyrus | 39 | 9 | 35.24 | 0.032 | -40 | -56 | 24 | PC > CP |
| Drug X order interaction | ||||||||
| Middle temporal gyrus | 21 | 7 | 36.95 | 0.044 | 48 | -50 | 16 | - |
We found a main effect of drug administration order on static connectivity strength in the left angular gyrus and the left precuneus, and a significant drug X order effect in the middle temporal gyrus (Table 1). There were no effects of order or interactions with drug condition in the dynamic functional connectivity analysis.
Analysis of motion parameters demonstrated no significant differences between drug and placebo conditions in total distance moved (t(11) = -0.70, p = .42), mean frame-wise displacement (t(11) = -0.54, p = .60), or maximum frame-wise displacement (t(11) = 0.73, p = .48). There were also no differences in the number of interpolated time points (t(11) = -0.51, p = .62) suggesting that the measured differences were induced by the infusion of citalopram.
We also performed exploratory analyses of the effects of citalopram on static and dynamic functional connectivity in the central executive network and salience network. However these analyses did not reveal any significant effects of citalopram or drug administration order, and did not show any effects of a drug X administration order interaction when looking at either dynamic or static connectivity.
4. Discussion
We set out to investigate the effect of the SSRI citalopram on static and dynamic resting state connectivity of the medial prefrontal cortex in a sample of healthy individuals. Acute citalopram administration increased static connectivity between the medial prefrontal cortex and right dorsolateral prefrontal cortex and posterior cingulate cortex relative to placebo. The SSRI also induced a reduction in variability of connectivity with the medial prefrontal cortex in the precuneus and posterior cingulate cortex. Together these findings indicate that citalopram has effects on both connectivity strength and stability. The medial prefrontal cortex is a central component of the DMN and shares connectivity with the ‘dorsal nexus’, posterior cingulate cortex, the dorsolateral prefrontal cortex and precuneus relevant in affective regulation (Sheline et al., 2010).
Acutely, citalopram blocks the reuptake of serotonin leading to decreased cortical synaptic serotonin via initial inhibitory mechanisms based on raphe 5HT1A autoreceptor activation which is believed to dominate the initial phase of antidepressant treatment (Selvaraj et al., 2012)(Nord et al., 2013)(Arnone et al., 2002). Studies in healthy participants indicate that a more prolonged period of treatment (7 days) with citalopram might actually decrease static connectivity between prefrontal cortex and limbic regions including the amygdala and hippocampus (McCabe and Mishor, 2011). This would seem in keeping with the belief that chronic or sub-chronic SSRI administration leads to adaptive synaptic changes compatible with an optimal level of serotonergic synaptic availability. The acute SSRI challenge in this experiment resulted in increased static connectivity between the medial prefrontal cortex and right dorsolateral prefrontal cortex and posterior cingulate cortex relative to placebo complemented by a reduction in connectivity variability in the medial prefrontal cortex with precuneus and posterior cingulate cortex. These findings add to Schaefer and others’ work investigating the effect of a single oral dose of escitalopram on brain functional connectivity. The authors reported a general decrease in intrinsic connectivity at whole brain level in most cortical and sub-cortical areas with localised increases in cerebellum and thalamus although their methodology did not allow exploration of static and dynamic connectivity (Schaefer et al., 2014). Our approach expands on their work and indicates a localised increase in static connectivity in the network comprising the medial prefrontal cortex, right dorsolateral prefrontal cortex and posterior cingulate cortex. This alteration in functional synchronisation might speculatively reflect functional ‘rigidity’ or an exaggerated state of over coupling in this network as postulated by Voytek and Knight to explain disruption in neural communication occurring in neuropsychiatric disorders (Voytek and Knight, 2015) and possibly reflecting a state of hyperconnectivity noted in similar regions in the depressed state (Sheline et al., 2010). There is no clear consensus with regard to the interpretation of functional dynamic changes in the context of a pharmacological challenge. The most striking element is that dynamic connectivity provides a window to capture neural communication compatible with fluctuations in level of consciousness, which in the context of this experiment might reflect synaptic changes associated with an increase in the level of serotonin. A reduction in connectivity variability in the identified network in line with variation associated by sleep, sedation, and pharmacological compounds capable of inducing anaesthesia (Deng et al., 2016) (Hutchison and Morton, 2016), suggests a decrease in spontaneous ‘mind wandering’ and ‘self generated thoughts (Kucyi, 2017), potentially related to a ‘ruminative cognitive style’ linked with conditions such as major depression and anxiety disorders (Perkins et al., 2015). Interestingly we have recently demonstrated greater connectivity variability in unmedicated individuals with major depression vs. healthy controls in the network which includes the medial prefrontal cortex and the posterior cingulate cortex significantly correlating with measures of a ‘ruminative response style’ in the presence of more pronounced anxiety symptoms (Wise et al., 2017) suggesting the possibility that SSRIs might be able to modify brain responses in these disorders.
Pharmacological manipulation of serotoninergic neurotransmission resulting in reduction of functional connectivity in the anterior and posterior cingulate cortices, hippocampal complex and lateral parietal regions in healthy volunteers has been demonstrated with the SSRIs escitalopram (van de Ven et al., 2013) and sertraline (Klaassens et al., 2015) and generic decreased in functional resting state connectivity within the DMN have been demonstrated with the serotonin and noradrenaline reuptake inhibitor duloxetine (van de Ven et al., 2013) and with psilocybin, a 5-HT2A/2C receptor agonist (Carhart-Harris et al., 2012). Studies inducing a reduction in 5-HT brain availability by administering a mixture of amino acids without the 5-HT precursor tryptophan (acute tryptophan depletion), have reported conflicting results. Helmbold and others in a sample of women demonstrated enhanced resting state connectivity in the left inferior and middle frontal cortices (including the DLPFC), and reduced functional connectivity in the left precuneus (Helmbold et al., 2016). These findings are opposite to what we would expect based on our results following an acute reduction in 5-HT brain availability. Our data are more consistent with Kunisato and colleagues reporting a decrease in the middle orbitofrontal cortex and precuneus and an increase in the superior parietal lobule, paracentral lobule and precentral gyrus following tryptophan depletion (Kunisato et al., 2011). Discrepancies in the results can be explained by study design and methodological differences including citalopram dosing and potential variations in the techniques used to obtain tryptophan depletion which could contribute to pharmacokinetics variations. Further studies which take into consideration these parameters appear necessary to ensure replication.
The dorsolateral prefrontal cortex (DLPFC) is more commonly associated with the executive control network than the DMN. However the DLPFC is a large region composed of multiple sub-regions with varying patterns of anatomical and functional connectivity, and sub-regions of the DLPFC do often appear in network parcellations. For example the lateral areas of Brodmann area 8 appear in the default mode network component of the Yeo parcellations (Yeo et al., 2011), which represent standard templates for resting state networks derived from a large sample, and this region of the DLPFC was shown in some of the first studies of the default mode network (Fransson, 2005; Greicius et al., 2003). As such, the appearance of the DLPFC in our ICA component is expected. Additionally, the exact location of the default mode network will always vary from sample to sample due to individual anatomical and functional variability. This was in fact the reasoning behind our use of an ICA-derived default mode network template rather than an existing network template. As a result, although the precise location of default mode clusters may differ from study to study, we can be confident that our DLPFC cluster represents the same component of the default mode network as the DLPFC cluster shown in many previous studies of the DMN.
Notably, we found no effect of citalopram on either static or dynamic functional connectivity in the central executive network or salience network in exploratory analyses. Although this may suggest that citalopram affects functional connectivity primary in the default mode network, it is important to interpret this result in the context of our relatively small sample size which may have limited our ability to detect weaker effects in these networks. Another possibility is that acute citalopram administration affects the DMF because this is the major active network during resting state, contrary to the central executive or salience networks which are not functionally active. The acute strengthening of the DMN resting state functional connectivity (by increasing functional connectivity and decreasing connectivity variability) between MPFC and PCC, does not preclude subsequent changes in central executive or salience networks which were not measured in the time frame of this study. Based on the results from this study and in un-medicated patients with major depression (Wise et al., 2017), SSRIs might act by stabilizing the DMN.
The neural networks under investigation in this study, part of the DMN, are involved in a wide range of cognitive and behavioural functions and include neural circuits responsible for social behaviour, executive functions, motivation, vigilance, self-awareness and autonomic control. These functions are mediated by cortical and subcortical centres, including the limbic system and medial prefrontal cortex which show alterations in both structure and function in mood disorders (Wise et al., 2014)(Arnone et al., 2016)(Wise et al., 2016). Similarly to acute tryptophan depletion studies (Cowen and Browning, 2015), in the absence of significant perceived mood changes or ‘state effects’, the acute administration of an SSRI in healthy volunteers could induce detectable changes in the availability of serotonin at neural level.
This study shows that changes in serotonergic tone can be measured at brain level as variation in strength and stability of functional connectivity within the DMN. According to conventional theories this might speculatively represent the neural equivalent of decreased cortical serotonin availability mediated by inhibitory 5HT1A autoreceptor agonism. It is however becoming evident that the molecular and cellular mechanisms, which constitute the theoretical basis for SSRI acute and chronic synaptic adaptation, are likely to be incomplete (Harmer et al., 2017). As an example 5HT1A-blocking agents do not accelerate clinical response (Scorza et al., 2012)(Harmer et al., 2017), and the onset of clinical effects have been shown to supervene sooner than previously believed (Taylor et al., 2006). Hence, other mechanisms may also contribute to the observed neural effects and rapid adaptive process to explain the acute SSRI action shown in this work. These might include fast acting synaptic changes medicated by Ca2+ channels, activation of tropomyosin related kinase signalling pathways, changes in brain derived neurotrophic factor (Zetterström et al., 1999)(Schaefer et al., 2014) and in glutamatergic neurotransmission (Arnone et al., 2015).
Further work is necessary to fully understand circuit level functioning at regional level responsible for the measured changes in connectivity strength and stability. This might be achieved by using complementary techniques such as electroencephalography (EEG) to investigate electric neuronal activity concomitant to BOLD signal changes (Hutchison and Morton, 2016). Furthermore it would be important to study temporal variability of functional connectivity in major depression in response to treatment and clinical improvement as dynamic changes measured in healthy volunteers may not reflect baseline functional connectivity variability in affected samples.
Study limitations include current technical capabilities in capturing dynamic variability in functional connectivity, differences in bioavailability between participants for the compound of choice, and the relatively small sample size. In relation to sample size 12 subjects is generally considered the minimum number for functional neuroimaging studies (Desmond and Glover, 2002) although the within-subjects design and the use of an experimental intervention are likely to increase the power to detect a meaningful signal. We did not detect any significant order or drug X order interactions on static connectivity. A drug X order interaction was detected in one of the connectivity stability significant clusters which although not necessarily relevant in view of the counter-balanced design, it actually increased the strength of our main effects (likely due to accounting for additional order-related variance in the model). Further studies are needed to compare functional connectivity strength and variability and its relationship with serotonin function and major depression in men and women.
There is also no clear consensus on what constitutes the optimal time window for dynamic functional connectivity analysis and data interpretation (Hutchison and Morton, 2016), and there is some debate over temporal filtering of the BOLD signal (Leonardi and Van De Ville, 2015). The combination of a voxelwise cluster-defining threshold of <.001 and cluster-level threshold of <.05 FDR corrected used in this work, has been shown to provide an acceptable false-positive rate (Eklund et al., 2016)(Flandin and Friston, 2017). As a result, we believe that our current method (using cluster-level correction within the network masks) is the most appropriate way to control for multiple comparisons in our analyses. Different methods are however available including using peak-level correction. Using this approach within our large volumes would have been overly conservative and cluster-level correction provides a more powerful way to correct for multiple comparisons in this case. Peak-level correction allows inferences to be made about the specific location of activations, while this specificity is lost with cluster-level correction. Hence, we cannot make any strong claims beyond stating that the cluster as a whole shows an effect, as making more precise statements about the location of these effects would be inappropriate based on cluster-level thresholding.
Additionally, the 6-½ minute resting state scan used here may not have been of sufficient duration to capture less rapid fluctuations in connectivity, and may limit the reliability of our connectivity estimates. However, it is not clear from the published literature what constitutes an optimal scan duration for dynamic functional connectivity analysis, and to what extent the scan duration used here limits our estimates of dynamic functional connectivity. Finally, there has been some debate in the literature regarding methods of hypothesis testing in dynamic functional connectivity research. This is typically with testing for the presence of dynamic functional connectivity and the need to set a null model where fluctuations in connectivity are absent (Hindriks et al., 2016a)(Hindriks et al., 2016b). The issue of testing for effects of experimental interventions on connectivity dynamics has received little attention and future work should focus on optimising methods for detecting these effects. Finally, the clusters observed in the posterior cingulate cortex and angular gyrus in our dynamic connectivity analysis were small and by no means indicate that the entirety of these default mode regions exhibited different patterns of connectivity depending on drug administration order. Additionally, it is not clear from the current study why such an effect of order may be present.
Nevertheless, this study shows for the first time that acute SSRI administration can affect both static and dynamic functional connectivity within relevant brain networks and although the use of acutely administered citalopram is of limited clinical relevance to longer-term citalopram administration or to later antidepressant efficacy, individual differences in the brain’s response to citalopram administration may be used as a useful tool for stratification.
Supplementary Material
Acknowledgements
This study was supported by Academy of Medical Sciences UK grant to Dr Selvaraj (no. AMS-SGCL6) and by Medical Research Council-UK (no. MC-A656-5QD30), Brain and Behavior Research Foundation, and Wellcome Trust (no. 094849/Z/10/Z) grants to Dr Howes and the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. Dr Arnone is supported by the Academy of Medical Sciences, UK (grant no. AMS-SGCL8). The authors are grateful to all the participants and the staff at the Hammersmith Imanet (Andrew Blyth, Hope McDevitt, Andreanna Williams, Safiye Osman and Noora Ali) and MRI unit Hammersmith Hospital (Robert Steiner) for the technical expertise and support they provided. Dr Arnone and Dr Wise would like to thank Professor AJ Cleare for his support.
All procedures were approved by the National Research Ethics Service, West London Committee. Dr Arnone has received travel grants from Jansenn-Cilag and Servier. Dr Wise was supported by a PhD studentship from the National Institute of Health Research. Dr Cowen has been a member of advisory boards of Servier and Lundbeck and has been a paid lecturer for Servier and Lundbeck. Dr Howes has received investigator-initiated research funding from and/or participated in advisory/speaker meetings organised by Astra-Zeneca, Autifony, BMS, Eli Lilly, Heptares, Jansenn, Lundbeck, Lyden-Delta, Otsuka, Servier, Sunovion, Rand and Roche. Neither Dr Howes or his family have been employed by or have holdings/a financial stake in any biomedical company. Intravenous citalopram was kindly provided by Lundbeck, UK.
Footnotes
There were no other disclosures or conflicts of interest reported by the authors.
References
- Arnone D, Hansen L, Kerr JS. Acute dystonic reaction in an elderly patient with mood disorder after titration of paroxetine: possible mechanisms and implications for clinical care. J Psychopharmacol Oxf Engl. 2002;16:395–397. doi: 10.1177/026988110201600418. [DOI] [PubMed] [Google Scholar]
- Arnone D, Job D, Selvaraj S, Abe O, Amico F, Cheng Y, Colloby SJ, O’brien JT, Frodl T, Gotlib IH, Ham B-J, et al. Computational meta-analysis of statistical parametric maps in major depression. Hum Brain Mapp. 2016 doi: 10.1002/hbm.23108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone D, Mumuni AN, Jauhar S, Condon B, Cavanagh J. Indirect evidence of selective glial involvement in glutamate-based mechanisms of mood regulation in depression: meta-analysis of absolute prefrontal neuro-metabolic concentrations. Eur Neuropsychopharmacol J Eur Coll Neuropsychopharmacol. 2015;25:1109–1117. doi: 10.1016/j.euroneuro.2015.04.016. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Miller R, Pearlson G, Adalı T. The Chronnectome: Time-Varying Connectivity Networks as the Next Frontier in fMRI Data Discovery. Neuron. 2014;84:262–274. doi: 10.1016/j.neuron.2014.10.015.10.1016/j.neuron.2014.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Erritzoe D, Williams T, Stone JM, Reed LJ, Colasanti A, Tyacke RJ, Leech R, Malizia AL, Murphy K, Hobden P, et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci U S A. 2012;109:2138–2143. doi: 10.1073/pnas.1119598109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleare A, Pariante CM, Young AH, Anderson IM, Christmas D, Cowen PJ, Dickens C, Ferrier IN, Geddes J, Gilbody S, Haddad PM, et al. Evidence-based guidelines for treating depressive disorders with antidepressants: A revision of the 2008 British Association for Psychopharmacology guidelines. J Psychopharmacol Oxf Engl. 2015;29:459–525. doi: 10.1177/0269881115581093. [DOI] [PubMed] [Google Scholar]
- Cowen PJ, Browning M. What has serotonin to do with depression? World Psychiatry Off J World Psychiatr Assoc WPA. 2015;14:158–160. doi: 10.1002/wps.20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Sun J, Cheng L, Tong S. Characterizing dynamic local functional connectivity in the human brain. Sci Rep. 2016;6:26976. doi: 10.1038/srep26976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond JE, Glover GH. Estimating sample size in functional MRI (fMRI) neuroimaging studies: statistical power analyses. J Neurosci Methods. 2002;118:115–128. doi: 10.1016/s0165-0270(02)00121-8. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 2016;113:7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flandin G, Friston KJ. Analysis of family-wise error rates in statistical parametric mapping using random field theory. Hum Brain Mapp. 2017 doi: 10.1002/hbm.23839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: An fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartside SE, Umbers V, Hajós M, Sharp T. Interaction between a selective 5-HT1A receptor antagonist and an SSRI in vivo: effects on 5-HT cell firing and extracellular 5-HT. Br J Pharmacol. 1995;115:1064–1070. doi: 10.1111/j.1476-5381.1995.tb15919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer CJ, Duman RS, Cowen PJ. How do antidepressants work? New perspectives for refining future treatment approaches. Lancet Psychiatry. 2017;4:409–418. doi: 10.1016/S2215-0366(17)30015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmbold K, Zvyagintsev M, Dahmen B, Biskup CS, Bubenzer-Busch S, Gaber TJ, Klasen M, Eisert A, Konrad K, Habel U, Herpertz-Dahlmann B, et al. Serotonergic modulation of resting state default mode network connectivity in healthy women. Amino Acids. 2016;48:1109–1120. doi: 10.1007/s00726-015-2137-4. [DOI] [PubMed] [Google Scholar]
- Hindriks R, Adhikari MH, Murayama Y, Ganzetti M, Mantini D, Logothetis NK, Deco G. Can sliding-window correlations reveal dynamic functional connectivity in resting-state fMRI? NeuroImage. 2016a;127:242–256. doi: 10.1016/j.neuroimage.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindriks R, Adhikari MH, Murayama Y, Ganzetti M, Mantini D, Logothetis NK, Deco G. Corrigendum to “Can sliding-window correlations reveal dynamic functional connectivity in resting-state fMRI?” [NeuroImage 127 (2016) 242-256] NeuroImage. 2016b;132:115. doi: 10.1016/j.neuroimage.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Morton JB. It’s a matter of time: Reframing the development of cognitive control as a modification of the brain’s temporal dynamics. Dev Cogn Neurosci. 2016;18:70–77. doi: 10.1016/j.dcn.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RH, Whitfield-Gabrieli S, Dillon DG, Goer F, Beltzer M, Minkel J, Smoski M, Dichter G, Pizzagalli DA. Dynamic Resting-State Functional Connectivity in Major Depression. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2016;41:1822–1830. doi: 10.1038/npp.2015.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassens BL, van Gorsel HC, Khalili-Mahani N, van der Grond J, Wyman BT, Whitcher B, Rombouts SARB, van Gerven JMA. Single-dose serotonergic stimulation shows widespread effects on functional brain connectivity. NeuroImage. 2015;122:440–450. doi: 10.1016/j.neuroimage.2015.08.012. [DOI] [PubMed] [Google Scholar]
- Kucyi A. Just a thought: How mind-wandering is represented in dynamic brain connectivity. NeuroImage. 2017 doi: 10.1016/j.neuroimage.2017.07.001. [DOI] [PubMed] [Google Scholar]
- Kunisato Y, Okamoto Y, Okada G, Aoyama S, Demoto Y, Munakata A, Nomura M, Onoda K, Yamawaki S. Modulation of default-mode network activity by acute tryptophan depletion is associated with mood change: a resting state functional magnetic resonance imaging study. Neurosci Res. 2011;69:129–134. doi: 10.1016/j.neures.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Leonardi N, Van De Ville D. On spurious and real fluctuations of dynamic functional connectivity during rest. NeuroImage. 2015;104:430–436. doi: 10.1016/j.neuroimage.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Manoliu A, Meng C, Brandl F, Doll A, Tahmasian M, Scherr M, Schwerthöffer D, Zimmer C, Förstl H, Bäuml J, Riedl V, et al. Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Front Hum Neurosci. 2013;7:930. doi: 10.3389/fnhum.2013.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C, Mishor Z. Antidepressant medications reduce subcortical-cortical resting-state functional connectivity in healthy volunteers. NeuroImage. 2011;57:1317–1323. doi: 10.1016/j.neuroimage.2011.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C, Mishor Z, Filippini N, Cowen PJ, Taylor MJ, Harmer CJ. SSRI administration reduces resting state functional connectivity in dorso-medial prefrontal cortex. Mol Psychiatry. 2011;16:592–594. doi: 10.1038/mp.2010.138. [DOI] [PubMed] [Google Scholar]
- Nord M, Finnema SJ, Halldin C, Farde L. Effect of a single dose of escitalopram on serotonin concentration in the non-human and human primate brain. Int J Neuropsychopharmacol Off Sci J Coll Int Neuropsychopharmacol CINP. 2013;16:1577–1586. doi: 10.1017/S1461145712001617. [DOI] [PubMed] [Google Scholar]
- Perkins AM, Arnone D, Smallwood J, Mobbs D. Thinking too much: self-generated thought as the engine of neuroticism. Trends Cogn Sci. 2015;19:492–498. doi: 10.1016/j.tics.2015.07.003. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A, Burmann I, Regenthal R, Arélin K, Barth C, Pampel A, Villringer A, Margulies DS, Sacher J. Serotonergic modulation of intrinsic functional connectivity. Curr Biol CB. 2014;24:2314–2318. doi: 10.1016/j.cub.2014.08.024. [DOI] [PubMed] [Google Scholar]
- Scorza MC, Lladó-Pelfort L, Oller S, Cortés R, Puigdemont D, Portella MJ, Pérez-Egea R, Alvarez E, Celada P, Pérez V, Artigas F. Preclinical and clinical characterization of the selective 5-HT(1A) receptor antagonist DU-125530 for antidepressant treatment. Br J Pharmacol. 2012;167:1021–1034. doi: 10.1111/j.1476-5381.2011.01770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj S, Turkheimer F, Rosso L, Faulkner P, Mouchlianitis E, Roiser JP, McGuire P, Cowen PJ, Howes O. Measuring endogenous changes in serotonergic neurotransmission in humans: a [11C]CUMI-101 PET challenge study. Mol Psychiatry. 2012;17:1254–1260. doi: 10.1038/mp.2012.78. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MJ, Freemantle N, Geddes JR, Bhagwagar Z. Early onset of selective serotonin reuptake inhibitor antidepressant action: systematic review and meta-analysis. Arch Gen Psychiatry. 2006;63:1217–1223. doi: 10.1001/archpsyc.63.11.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Ven V, Wingen M, Kuypers KPC, Ramaekers JG, Formisano E. Escitalopram Decreases Cross-Regional Functional Connectivity within the Default-Mode Network. PloS One. 2013;8:e68355. doi: 10.1371/journal.pone.0068355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoquaux G, Sadaghiani S, Pinel P, Kleinschmidt A, Poline JB, Thirion B. A group model for stable multi-subject ICA on fMRI datasets. NeuroImage. 2010;51:288–299. doi: 10.1016/j.neuroimage.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Voytek B, Knight RT. Dynamic network communication as a unifying neural basis for cognition, development, aging, and disease. Biol Psychiatry. 2015;77:1089–1097. doi: 10.1016/j.biopsych.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Xia M, Li K, Zeng Y, Su Y, Dai W, Zhang Q, Jin Z, Mitchell PB, Yu X, He Y, et al. The effects of antidepressant treatment on resting-state functional brain networks in patients with major depressive disorder. Hum Brain Mapp. 2015;36:768–778. doi: 10.1002/hbm.22663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskopf N, Hutton C, Josephs O, Deichmann R. Optimal EPI parameters for reduction of susceptibility-induced BOLD sensitivity losses: a whole-brain analysis at 3 T and 1.5 T. NeuroImage. 2006;33:493–504. doi: 10.1016/j.neuroimage.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Weiskopf N, Hutton C, Josephs O, Turner R, Deichmann R. Optimized EPI for fMRI studies of the orbitofrontal cortex: compensation of susceptibility-induced gradients in the readout direction. Magma N Y N. 2007;20:39–49. doi: 10.1007/s10334-006-0067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise T, Cleare AJ, Herane A, Young AH, Arnone D. Diagnostic and therapeutic utility of neuroimaging in depression: an overview. Neuropsychiatr Dis Treat. 2014;10:1509–1522. doi: 10.2147/NDT.S50156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise T, Marwood L, Perkins AM, Herane-Vives A, Joules R, Lythgoe DJ, Luh W-M, Williams SCR, Young AH, Cleare AJ, Arnone D. Instability of default mode network connectivity in major depression: a two-sample confirmation study. Transl Psychiatry. 2017;7:e1105. doi: 10.1038/tp.2017.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise T, Radua J, Via E, Cardoner N, Abe O, Adams T, Cheng Y, Cleare AJ, Arnone D. Common and Distinct Patterns of Grey Matter Volume Alteration in Major Depression and Bipolar Disorder: Evidence from Voxel-Based Meta-Analysis. Mol Psychiatry. 2016 doi: 10.1038/mp.2016.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, Fischl B, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterström TS, Pei Q, Madhav TR, Coppell AL, Lewis L, Grahame-Smith DG. Manipulations of brain 5-HT levels affect gene expression for BDNF in rat brain. Neuropharmacology. 1999;38:1063–1073. doi: 10.1016/s0028-3908(99)00022-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.