Abstract
Polymorphisms in genes involved in IL-4 responses segregate with allergic disease risk and correlate with IgE levels in humans, and IL-4 promotes IgE and IgG1 antibody production against allergens in mice. We report that mice having only one intact Il4 gene copy are significantly impaired in their ability to make specific IgE responses against allergens, while IgG1 responses to allergens remain unaffected. Il4-hemizygosity also resulted in a modest but detectable drop in IL-4 production by CD4+ T cells isolated from lymph nodes and prevented IgE-dependent oral allergen-induced diarrhoea. We conclude that a state of haploinsufficiency for the Il4 gene locus is specifically relevant for IL-4-dependent IgE responses to allergens with the amount of IL-4 produced in the hemizygous condition falling close to the threshold required for switching to IgE production. These results may be relevant for how polymorphisms in genes affecting IL-4 responses influence the risk of IgE mediated allergic disease in humans.
Introduction
IL-4 is an immunoregulatory cytokine produced by CD4+ Th2 cells and TFH cells, and influences numerous cellular and humoral aspects of type 2 immune responses mounted against parasites and allergens. Typically, IL-4 promotes tissue mastocytosis and eosinophilia, alters intestinal fluid flux, enhances smooth muscle cell hypercontractility, and increases vascular permeability in response to the vasoactive mediators of allergies (1-6). IL-4 also regulates B cell activation and class-switch recombination. Most notably, IL-4 is essential for IgE production (7-9), but IL-4 additionally increases naïve B cell MHC-II and CD23 (FcεRII) expression (10), augments germinal centre B cell expansion and promotes IgG1 antibody production (8, 9, 11, 12). IL-4 may also contribute to antibody affinity maturation (12). The influences of IL-4 and an additional Th2 cell-produced cytokine, IL-13, on asthma pathogenesis have led to the development of clinical IL-4-receptor antagonists that are shown to diminish exacerbation frequencies in specific subgroups of asthma sufferers (13-16).
The importance of IL-4 in type 2 immune responses has generated much interest in defining its cellular sources in vivo. Tissue-localized CD4+ effector Th2 cells, lymphoid CD4+ CXCR5+PD-1hi TFH cells, invariant NK T cells, basophils and eosinophils have been identified as IL-4 producing cells (10, 12, 17-23). Many of these populations were first identified as IL-4 producers through evaluations of Il4 mRNA transcript frequencies, or IL-4 production after ex vivo restimulation; however, more sensitive studies utilizing IL-4-reporter mice were integral in validating these results. Reporter mice have further permitted sensitive probing of the comparative importance of distinct IL-4-producing populations during type 2 immune responses (21, 24). The two most widely used IL-4 reporter mice are the KN2 and G4 IL-4-substituting reporters (22, 25). Both reporters faithfully reflect commitment to IL-4 production in vivo, with the KN2 reporter expressing modified human CD2 (hCD2), and the G4 reporter expressing enhanced GFP, both in place of the native IL-4 protein. A third, more recently developed IL-4 reporter, the BAC transgenic 4C13R IL-4 reporter mouse (17, 26, 27) expresses AmCyan fluorescent protein under control of the Il4 gene regulatory elements without manipulation of the endogenous Il4 gene locus, which permits evaluation of commitment to IL-4 production without ex vivo restimulation, and leaves the endogenous Il4 gene locus unmodified.
IL-4-substituting reporter mice provide insight into complex, difficult to monitor features of IL-4 production. However, these mice carrying a single IL-4-producing allele present the challenge of determining whether this accurately reflects normal Th2 immune response biology, and thus the conditions under which it is appropriate to apply these tools. In a previous study in IL-4G4/+ hemizygous mice, IgE responses seemed much lower than expected (3), which led us to consider the impact individual Il4 alleles have on the type 2 immune response to allergens. Here, we use protein/alum immunisations and reporter mice to show that Il4-hemizygous mice exhibit substantially diminished IgE levels and curtailed IgE-dependent allergic disease sequelae without significant changes to the IgG responses. Our findings imply that the serum IgE isotype response is disproportionately affected by the loss of one IL-4 producing allele, while other features of the allergic immune response are more resistant to changes in the availability of IL-4.
Materials and Methods
Mice
IL-4KN2/KN2 (22) backcrossed for 10 generations to BALB/c background were crossed to BALB/c and BALB/cByJ mice to generate IL-4KN2/+ mice. IL-4G4/G4 (25) mice crossed to BALB/c and C57BL/6 backgrounds were respectively crossed to BALB/c and BALB/cByJ or C57BL/6J mice to generate IL-4G4/+ mice. 4C13R (27) reporter mice were bred and maintained on a C57BL/6 background in the Malaghan Institute of Medical Research Biomedical Research Unit. 4C13R mice were crossed with IL-4G4/G4 mice to generate 4C13R x IL-4G4/+ mice and 4C13R x IL-4G4/+ mice were crossed with IL-4G4/G4 mice to generate 4C13R x IL-4G4/G4 mice. C57BL/6J background IL-4+/+ and IL-4KN2/+ Basoph8 (28) mice were maintained in facilities at the University of California, San Francisco, and were used for ASC, basophil and mast cell analysis experiments. All procedures were performed in accordance with institutional guidelines.
Systemic and intestinal anaphylaxis
Mice were i.p. injected on days 0 and 14 with 50 μg grade V OVA. (Sigma, St Louis, MO) plus 1 mg Alu-Gel-S (Serva Electrophoresis GmbH, Heidelberg, Germany). To induce active systemic anaphylaxis, on day 28 following i.p. immunisation (C57BL/6 background) mice were i.v. injected with 100 μg OVA and monitored for temperature changes intrarectally. To induce intestinal anaphylaxis(29), mice on a BALB/c genetic background were i.p. immunised on days 0, 14, 28, and 42 prior to receiving 50 mg OVA intragastrically (i.g.) after a 4 h fast, three times a week, every second day, beginning on day 56. Diarrhoea was monitored 60-90 minutes after challenge.
OVA- and NP-specific ELISAs
For OVA-specific IgE ELISAs, wells of Immunosorp Maxi plates (Nunc) were coated with 100 uL 5 μg/mL anti-mouse IgE (6HD5) overnight at 4oC in pH=9.6 0.1 M carbonate coating buffer. For OVA-specific IgG2α and IgG1, wells were coated with 100 μL 10 μg/mL OVA. For all OVA-specific ELISAs, the next day, wells were washed and blocked with 200 μL 10% FBS in PBS (blocking buffer) for 2 h at room temperature. Blocking buffer was replaced with 100 μL sample, serially diluted in blocking buffer. After 2h at room temperature, or overnight at 4°C, wells were washed and incubated with 100 μL 20 μg/mL biotinylated OVA (for OVA-specific IgE), 1 μg/mL biotinylated anti-mouse IgG2α (for OVA-specific IgG2α; R19-15) or 1 μg/mL biotinylated anti-mouse IgG1 (for OVA-specific IgG1; A85.1) for 60-90 minutes at room temperature. Wells were washed and incubated with 100 μL 1/1000 streptavidin-conjugated horse radish peroxidase (GE Healthcare, Auckland, New Zealand) for 1 h. Colorimetry was performed with TMB substrate (BD Pharmingen) and stopped with 1 M H2SO4. Absorbances were read at 450 nm using a Versamax plate reader. IgG1 titre was the inverse serum dilution at the OD50. NP-specific IgE and IgG1 ELISAs were performed as described (30).
Chloroacetate esterase (CAE) staining for mast cells
Excised 1.5 cm jejunal sections were placed in 10% formalin solution (4% weight/volume; Sigma) and stored at 4ºC until embedded in paraffin. Tissues were processed into 4 μm sections using standard histological techniques. Sections were stained with chloroacetate esterase and lightly counterstained with hematoxylin. Mast cells were easily identified as bright red cells as described (31).
Murine mast cell protease-1 (mMCP-1) ELISA
mMCP-1 ELISA was performed according to the manufacturer's instructions (eBiosciences).
Ear immunisations
Mice were anaesthetised using xylazine and ketamine (Phoenix, New Zealand). For T cell analyses, 30 μL of a solution containing 50 μg OVA or NP16OVA (Biosearch technologies, Petaluma, CA, USA) with 1 mg Imject Alum (Pierce) was injected into the ear pinnae as described (32). After euthanasia, auricular lymph nodes were isolated and stored in IMDM until cell harvest and isolation. For antibody-secreting cell (ASC) analyses, 25 μL containing 50 μg NP16OVA in a 1:1 PBS:Alhydrogel (Accurate Chemical & Scientific Corporation, Westbury, NY, USA) solution was injected into the ear pinnae as described (32). After euthanasia, auricular lymph nodes were isolated and stored in handling medium (10% FBS, 1% Penicillin-streptomycin-L-glutamine solution (Gibco Life Technologies), in Dulbeco's modification of Eagle's Medium (Corning Mediatech)) until cell isolation.
Cell isolation
For T cell analyses, lymph nodes were disrupted using the rubber bung of a 1 mL tuberculin syringe (BD) and liberated cells were washed through 70 μm cell strainers into complete IMDM (5% FBS (Sigma), 100 U/mL penicillin/100 μg/mL streptomycin (Invitrogen) and 55 μM β-mercaptoethanol (Invitrogen) in IMDM). CD4+ T cells were purified by positive selection using FlowComp Mouse CD4 Dynabeads (Invitrogen). Live cell counts were determined using a haemocytometer and trypan blue (Invitrogen) exclusion. For ASC and basophil analyses, processing was as for T cells, except tissues were harvested and cells resuspended in handling medium and counts determined with a Z-series Coulter Particle Counter after treatment with ZAP-OGLOBIN II (Beckman Coulter, Hialeah, FL, USA). Mast cells were isolated by peritoneal lavage with PBS.
FACS analysis
For T cell analyses, cells were resuspended in 0.01% NaN3 (Sigma) and 2% FBS in PBS then incubated with anti-CD16/32 antibody (clone 2.4G2) before staining with cocktails of fluorophore-conjugated antibodies. For ASC, basophil and mast cell analyses, the staining buffer contained 0.1% NaN3 (Andwin Scientific), 2% FBS and 2 mM EDTA (Life Technologies), and cells were incubated with anti-CD16/32 (clone 93; Biolegend) before staining. For T cell analyses, lymphoid cells were stained with antibodies against the following molecules (clone, conjugate; source): B220 (RA3-6B2, Horizon V450; BD), CD4 (RM4-5, Qdot605; BD), PD-1 (RMP1-30, PE/Cy7; Biolegend), CXCR5 (2G8, biotin; BD); streptavidin-PE-Texas Red® (Life Technologies) or streptavidin-allophycocyanin (Invitrogen) were also used. Splenic basophils and peritoneal mast cells were stained with antibodies against IgE (R35-118, biotinylated; BD) cKit (2B8, PE/Cy7; BD) and IgD (1126c.2a, BV510; BD) or CD49b (DX5, PE; BD), FceR1α (MAR-1, APC; Biolegend) and cKit (2B8, APC/Cy7; Biolegend). Also used were streptavidin Qdot605 (Life Technologies) and NP-allophycocyanin (prepared in house as described(30, 33)). ASC staining was performed as described (30) using purified antibodies against IgE (RME-1 Biolegend), and fluorophore labelled antibodies against IgE (RME-1, Fluorescein; BD), IgG1 (A85.1, Horizon V450; BD), IgD (1126c.2a, BV510; BD), B220 (RA3-6B2, Qdot 655; Life Technologies), CD138 (281-2, BV711; BD), IgM (II/41, PE/Cy7; eBioscience) and CD38 (90, AF700; eBioscience); additional staining reagents were biotinylated peanut agglutinin (Vector laboratories, Burlingame, CA, USA), Qdot605 streptavidin conjugate (Invitrogen), Fixable Viability Dye eFluor780 (eBioscience) and NP-allophycocyanin. T cell data were acquired on a BD LSRII SORP flow cytometer (Becton Dickinson, San Jose, CA); ASC, basophil and mast cell data were acquired on an LSR Fortessa (Becton Dickinson). Gates excluded doublets and DAPI+, propidium iodide+ or eFluor780+ events. CD4+ T cells were gated as CD4+B220- events; TFH were CXCR5+PD-1hi CD4+ T cells. NP-binding ASC were gated as CD138+B220mid/loIgD-IgM-CD38-NP(surface+intracellular)+ cells. Basophils were identified in Basoph8 mice as Yellow Fluorescent Protein (YFP)+cKit-IgD- and peritoneal mast cells as side-scatterhi YFP-cKit+. Splenic basophils from C57BL/6 background IL-4+/+ and IL-4G4/+ mice were identified as DX5+FceR1α+cKit- and peritoneal mast cells as DX5+FceR1α+cKit+. Flow data were analysed using FlowJo software (Treestar).
IL-4 ELISA
Wells of Immunosorp Maxi plates (Nunc) were coated with 100 uL 2 μg/mL anti-mouse IL-4 (11B11) overnight at 4oC in pH=9.6 0.1 M carbonate coating buffer. Wells were washed and blocked with 200 μL 1% BSA in PBS (blocking buffer) for 1 h at room temperature. Blocking buffer was replaced with 100 μL sample or IL-4 standard, serially diluted in blocking buffer. After 2h at room temperature wells were washed and incubated with 100 μL 2 μg/mL biotinylated anti-mouse IL-4 (Pharmingen) for 2 h at room temperature. Wells were washed and incubated with 100 μL 1/1000 streptavidin-conjugated horse radish peroxidase (Biolegend) for 1 h. Colorimetry was performed with BD OptEIA substrate reagent set and stopped with 1 M H2SO4. Absorbances were read at 450 nm using a Versamax plate reader.
Statistical analysis and graphics
Statistical analyses were performed using Graphpad Prism software (Graphpad Software Inc., San Diego, CA, USA). Statistical comparisons used are specified in figure legends. Data shown on log scales were log-transformed prior to analysis. p≤0.05 was considered as significant.
Results
Il4-hemizygosity differentially affected OVA-specific IgG1 and IgE antibody production
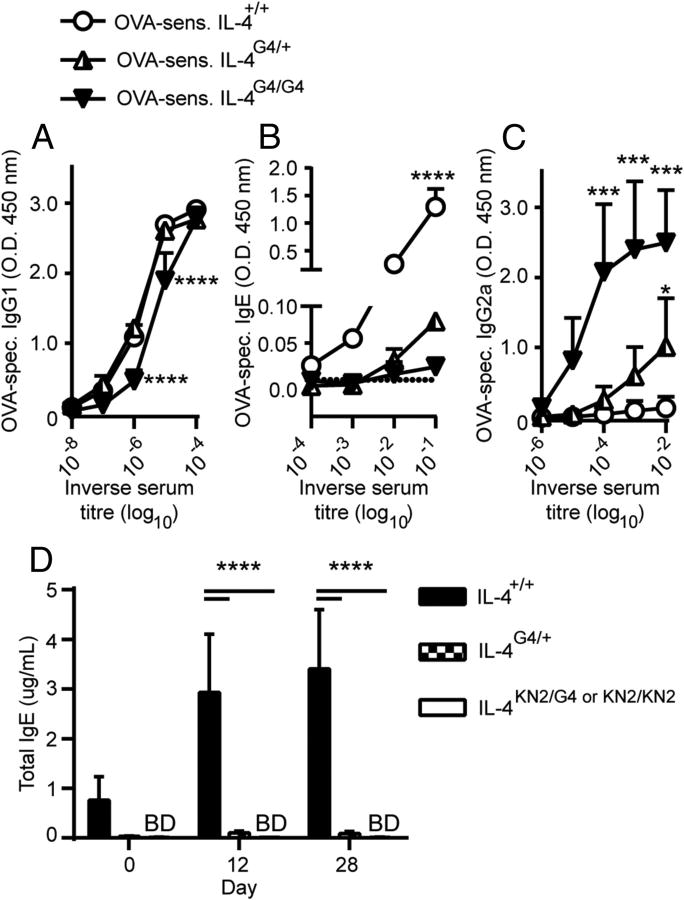
We sought to determine whether the IL-4 haploinsufficiency resulting from the Il4-hemizygous state affected antigen specific antibody production. Mice that were either IL-4+/+, IL-4G4/+, or IL-4G4/G4 were immunised with OVA/Alum and the OVA-specific IgG1, IgE and IgG2α serum antibody titres evaluated by ELISA. Equivalent OVA-specific IgG1 titres of up to 106 were detected in IL-4+/+ and IL-4G4/+ mice following allergen challenge while the IL-4 deficient, IL-4G4/G4 mice had a 4-fold reduced serum titre (Figure 1A). OVA-specific IgE responses were also significantly induced in IL-4+/+ mice, reaching serum titres of 103 (Figure 1B). However, OVA/Alum immunised IL-4G4/+ hemizygous mice had 100-fold lower OVA-specific IgE responses. No OVA-specific IgE could be detected in immunised IL-4G4/G4 mice, although these mice had elevated serum titres of OVA-specific IgG2α antibody compared with both IL-4+/+ and IL-4G4/+ mice (Figure 1C). Total IgE levels in mice before and after immunisation of IL-4G4/+ mice were also significantly lower than in IL-4+/+ mice (Figure 1D).
Figure 1. OVA-specific antibody responses in Il4-hemizygous mice.
Balb/cByJ background mice were immunised twice i.p. with OVA/Alum and two weeks later ELISAs were performed for serum (A) OVA-specific IgG1, (B) OVA-specific IgE and (C) OVA-specific IgG2α. (D) Kinetic analysis of Total IgE production after one (day 12) or two (day 28) fortnightly i.p. immunisations with OVA/Alum. Data shown represent groups of (A-C) n=4 mice/group (IL4+/+ and IL-4G4/+) or n=3 mice/group (IL-4G4/G4) from one of two similar experiments or (D) groups of n=3 mice (IL-4+/+ and IL-4G4/+) or n=6 mice (IL-4KN2/G4 or KN2/KN2) from one of three similar experiments. Data show mean + SD. *p≤0.05, ***p≤0.001, ****p≤0.0001 vs. both other groups by two-way ANOVA with Tukey's post-test. BD = below detection.
The reduced IgE levels in Il4-hemizygous mice were not caused by the abundance of serum OVA-specific IgG antibodies competing for allergen binding, because the ELISA we employed first captures total IgE and then uses an OVA-biotin conjugate to reveal the OVA-specific IgE serum immunoglobulin.
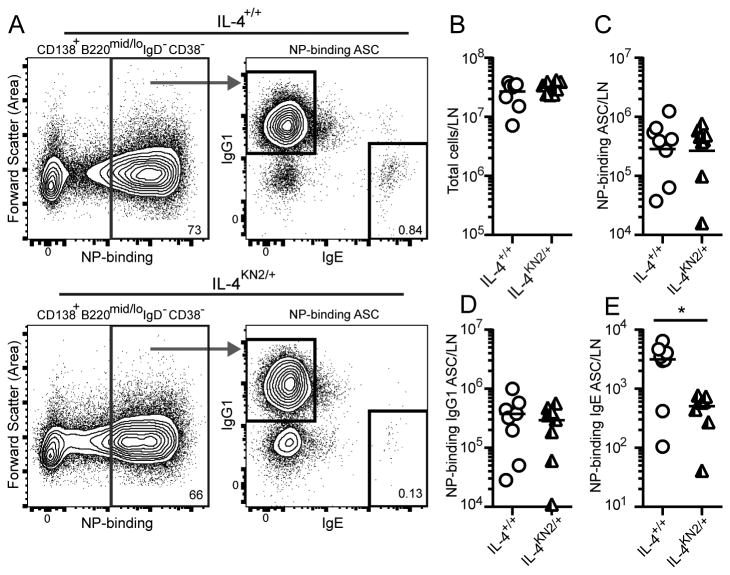
We next examined hapten-specific antibody responses at the level of responding B cells using NP conjugated OVA (NP-OVA) as the immunising antigen. Seven days after immunisation in the ear with NP-OVA/Alum, draining lymph nodes were excised and numbers of ASC binding the dominant hapten, NP, were evaluated by FACS (Figure 2A). Similar total cell, NP-binding ASC and NP-binding IgG1 ASC numbers were detected in draining auricular lymph nodes of IL-4+/+ and Il4-hemizygous IL-4KN2/+ mice (Figure 2B-2D). In contrast, NP-binding IgE ASC were around six-fold less abundant in IL-4KN2/+ mice (Figure 2E). These data demonstrate that the genesis of antigen-specific IgE ASC specifically was impaired in the Il4-hemizygous mice.
Figure 2. IgE ASC development in Il4-hemizygous mice.
Basoph8 IL-4+/+ and IL-4KN2/+ mice were immunised in the ear with NP-OVA/Alum and seven days later auricular lymph nodes were analysed for ASC responses. (A) Total NP-binding ASC (CD138+B220mid/loIgD-IgM-CD38- NP-binding cells (left) were examined for total IgG1 versus intracellular IgE expression (right). (B) Total cells, (C) total NP-binding ASC, (D) IgG1+ NP-binding ASC and (E) IgE+ NP-binding ASC were enumerated. Data points show individual mice combined from two similar experiments (n=3-5 mice/group); lines show geometric means. Numbers on FACS plots show percent of parent gate. *p≤0.05, two-tailed Student's t-test after log-transformation.
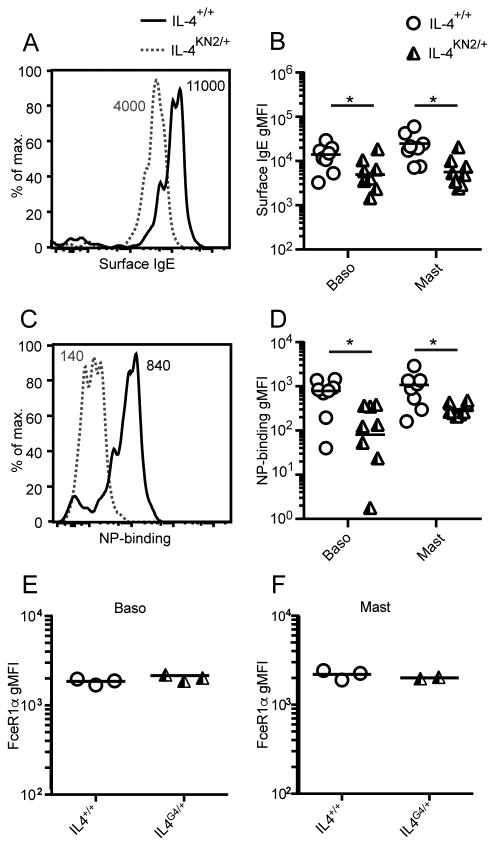
To determine whether the IgE production deficiency affected the amount of IgE bound by high affinity IgE receptor (FcεRI)-expressing cells, we examined surface IgE expression and NP-binding levels on basophils from NP-OVA immunised IL-4+/+ and IL-4KN2/+ hemizygous Basoph8 reporter mice, in which basophils can be identified through the expression of YFP(28). As expected, YFP+ splenic basophils from IL-4KN2/+ Il4-hemizygous mice had lower surface IgE levels and bound less NP-allophycocyanin than did basophils from the IL-4+/+ strain (Figure 3A-D). The geometric mean fluorescence intensity (gMFI) of IgE- and NP-binding on basophils from the Il4-hemizygous mice were 3-10-fold lower than in the IL-4+/+ Basoph8 reporter mice (Figure 3B, 3D), similar to the fold reduction in the number of NP-specific IgE ASC that were generated in these mice (Figure 2E). Surface-bound IgE and NP staining were similarly affected on peritoneal mast cells (Figure 3B, 3D). The reduced levels of surface IgE and NP-binding in Il4-hemizygous mice was not due to reduced levels of FcεR1 receptor on the surface of these cells, as the gMFI of FcεRIα on basophils (Figure 3E) and mast cells (Figure 3F) was comparable in IL-4+/+ and IL-4G4/+ mice. These data demonstrate that basophils and mast cells from Il4-hemizygous mice captured less NP-specific IgE than those from IL-4+/+ mice.
Figure 3. Basophil and mast cell sensitisation in Il4-hemizygous mice.
Basophils and mast cells were isolated from the immunised Basoph8 mice from Figure 2. A & C show representative flow analysis of basophils, B & D respectively show the geometric mean fluorescence intensity (gMFI) of IgE and NP-APC on both basophils and mast cells. C57BL/6J IL-4+/+ and IL-4G4/+ mice were immunised i.d. in the ear pinnae with NP-OVA/Alum and the gMFI of FceR1α on splenic basophils (E) and peritoneal mast cells (F) measured 7 days later. Data points show individual mice combined from two similar experiments (n=3-5 mice/group) in B and D and a single experiment (n=2-3 mice/group) in E and F, while lines show geometric means. Numbers on FACS histograms indicate sample gMFI. *p≤0.05 two-tailed Student's t-test after log transformation.
These data indicate that immunised Il4-hemizygous mice generate fewer IgE ASC than IL-4+/+ mice, resulting in lower levels of IgE both in circulation and bound to the surface of basophils and mast cells.
Il4-hemizygosity affected the outcome of antibody-mediated allergic immune responses
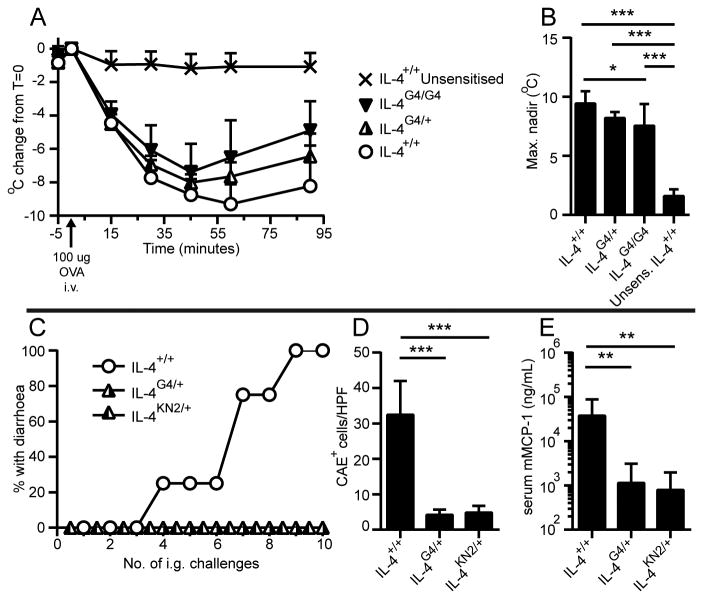
We next examined the impact of Il4-hemizygosity on antibody-mediated allergic immune responses. To characterize antibody-mediated active systemic anaphylaxis, mice were immunised i.p. with OVA/Alum and then challenged i.v. with OVA. Upon i.v. challenge, IL-4+/+, IL-4G4/+ and IL-4G4/G4 mice became hypothermic, a characteristic feature of systemic anaphylaxis (Figure 4A). Allergen challenge-induced hypothermia was more severe in IL-4+/+ than IL-4G4/G4 mice, while IL-4G4/+ mice presented intermediate decreases in temperature (Figure 4B). These data indicate that active systemic anaphylaxis was affected by Il4-hemizygosity in relative proportion with Il4 allele dose.
Figure 4. Impact of Il4-hemizygosity on systemic versus intestinal allergic immune responses.
OVA/Alum immunised or naïve (unsensitised) C57BL/6J background mice were i.v. challenged with OVA then monitored for (A) rectal temperature, then (B) the maximum temperature change was determined. (C) BALB/c background mice were immunised i.p. with OVA/Alum four times then i.g. OVA challenged and monitored for diarrhoea. After ten i.g. challenges, (D) jejunal mast cell numbers and (E) serum mMCP-1 levels were determined. Data symbols show (A, B, D) mean + SD (C) group incidence or (E) geometric mean + geometric SD. Data shown (A, B) are combined from 2 experiments (n=4-10 mice/group) or (C-E) are from one experiment (n=3-4 mice/group). In (A, B) one IL-4G4/G4 mouse died at T=35. *p≤0.05, **p≤0.01, ***p≤0.001 by One-Way ANOVA with Tukey's post-test. Data in E were log-transformed prior to statistical analysis.
We also investigated whether Il4-hemizygosity affected disease development in a mouse model of IgE dependent oral allergen-induced intestinal anaphylaxis (29). In this model, OVA-sensitised mice exhibit diarrhoea after successive i.g. OVA challenges. Protection from oral allergen-induced diarrhoea occurred in two independently developed strains of Il4-hemizygous mice, as 0/4 IL-4G4/+ and 0/3 IL-4KN2/+ hemizygous mice exhibited diarrhoea when primed i.p. four times prior to i.g. challenge compared with 4/4 IL-4+/+ mice (Figure 4C). Similar observations were made in two additional experiments with mice primed i.p. twice prior to i.g. challenge as is standard in this model (unpublished data). Protection from diarrhoea was associated with reduced frequencies of mast cells in intestinal jejuna and ≈30-fold lower serum mMCP-1 levels in the IL-4G4/+ hemizygous mice compared with IL-4+/+ mice after ten i.g. challenges (Figure 4D and 4E). These data confirm that Il4-hemizygosity impaired disease development in an IgE dependent model of allergic disease.
Il4-hemizygosity did not affect the frequency of CD4+ T cells committed to IL-4 production but impacted on the amount of IL-4 available in allergen immunised mice
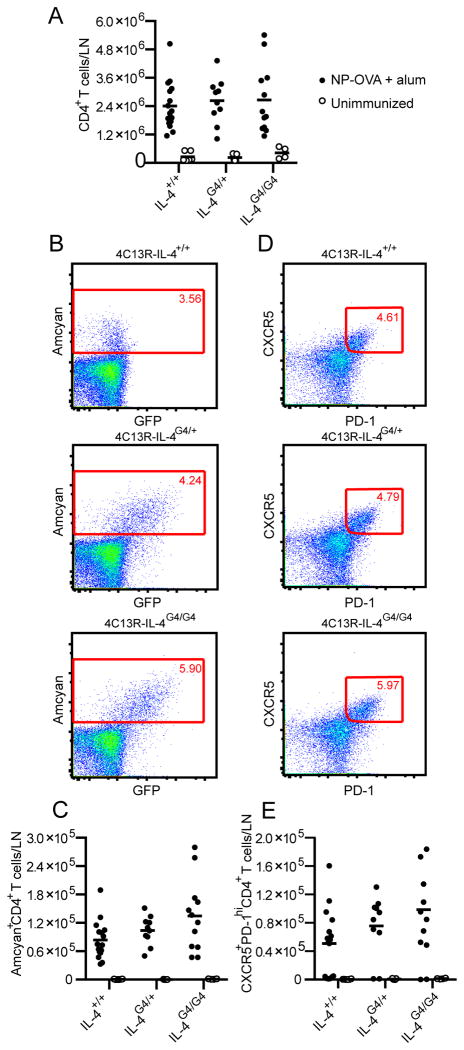
Allergen specific IgE production is dependent on the allergen induced expansion of IL-4-producing CD4+ T cells in the draining lymph node (11, 34). We therefore sought to establish the extent to which Il4-hemizygosity affected the frequency and quality of IL-4 producing CD4+ T cells in the draining lymph nodes of allergen immunised mice. To determine the effect of Il4-hemizygosity on the in vivo genesis of IL-4 producing CD4+ T cells in the draining lymph nodes of immunised mice we used BAC transgenic 4C13R reporter mice (17, 27). In these mice, the developing CD4+ Th cell that commits to IL-4 production can simultaneously produce IL-4 from its endogenous Il4 gene locus and the AmCyan fluorophore located in the Il4 locus of the BAC transgene. Using this system we were able to evaluate the impact of Il4-hemizygosity on CD4+ T cell IL-4 production by manipulating the number of intact wild type Il4 alleles and using the expression of AmCyan from the BAC transgene to identify those T cells that had become committed to IL-4 production. This was determined by comparing the frequencies of AmCyan+ CD4+ T cells in immunised 4C13R-IL-4+/+, 4C13R-IL-4G4/+ and 4C13R-IL-4G4/G4 strains of mice.
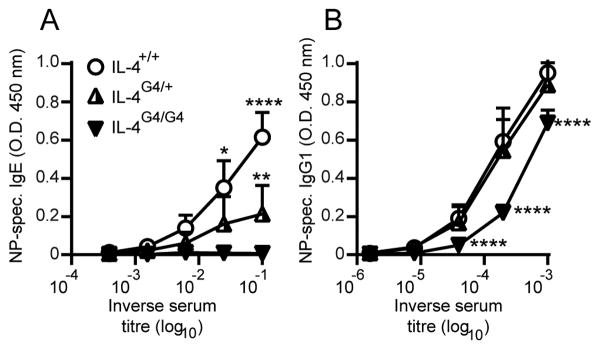
Firstly, the previously observed effect of Il4-hemizygosity on OVA induced IgE responses was confirmed in the 4C13R mouse strain using NP-OVA as the immunising allergen. Serum levels of NP-specific IgE antibody were reduced 10-fold in 4C13R-IL-4G4/+ mice compared to IL-4 sufficient 4C13R-IL-4+/+ mice (Figure 5A). Serum NP-specific IgG1 titres were similar in 4C13R-IL-4+/+ and 4C13R-IL-4G4/+ mice, but reduced 3-4 fold in IL-4 deficient 4C13R-IL-4G4/G4 mice (Figure 5B). When draining lymph node CD4+ T cell responses were evaluated in these mice, all three strains (4C13R-IL-4+/+, 4C13R-IL-4G4/+ and 4C13R-IL-4G4/G4 mice) generated similar numbers of CD4+ T cells, AmCyan+ CD4+ T cells and CXCR5+PD-1hi TFH cells (Figure 6A - 6E), indicating that even though Il4-hemizygosity impaired IgE production, similar numbers of CD4+ T cells were genetically committed to the production of IL-4. These AmCyan+ CD4+ T cells in the lymph node likely represent TFH cells (10, 12). This finding suggests that rather than Il4-hemizygosity impairing the development of IL-4 producing CD4+ TFH cells, it may be the reduced production of IL-4 by these TFH cells that affects IgE levels in the Il4-hemizygous mice.
Figure 5. Effect of Il4-hemizygosity on antibody responses in 4C13R IL-4 reporter mice.
Seven days after i.d. ear immunisation with NP-OVA/Alum, serum (A) NP-specific IgE and (B) NP-specific IgG1 levels were determined by ELISA. Data are shown from one experiment (n=3 mice/group). Data show mean + SD. *p≤0.05, **p≤0.01, ****p≤0.0001 vs. both other groups by Two-Way ANOVA with Tukey's post-test.
Figure 6. Effect of Il4-hemizygosity on the lymphoid CD4+ B helper T cell response.
Six to seven days after i.d. ear immunisation of C57BL/6J 4C13R mice with NP-OVA/Alum, auricular lymph nodes were analysed by FACS for (A) CD4+ T cells, (B, C) AmCyan+ CD4+ T cells and (D, E) CXCR5+PD-1hiCD4+ TFH cells. FACS plot show representative staining. Data points represent individual mice and lines show means from n=4-16 lymph nodes combined from three experiments.
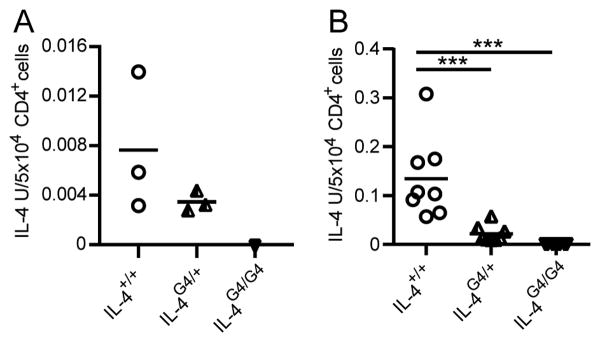
To evaluate the ability of lymph node CD4+ Th cells to produce IL-4, we examined IL-4 production in NP-OVA/Alum immunised C57BL/6J IL-4+/+, IL-4G4/+ and IL-4G4/G4 mice (Figure 7A) and OVA/Alum immunised BALB/c-ByJ IL-4+/+, IL-4G4/+ and IL-4G4/G4 mice (Figure 7B). CD4+ T cells were purified by positive selection and cultured on anti-CD3 coated plates, supernatants were harvested and examined for IL-4 production by ELISA. Cultures of CD4+ T cells from either NP-OVA/Alum or OVA/Alum immunised IL-4G4/+ mice produced between 2 and 6-fold less IL-4 than CD4+ T cell cultures from IL-4+/+ mice. Since similar numbers of CD4+ T cells were shown to become committed to Il4 gene expression in IL-4+/+ and IL-4G4/+ mice, it would appear that the two fold reduction in the amount of IL-4 produced by Il4-hemizygous CD4+ T cells is sufficient to profoundly affect the subsequent serum IgE response.
Figure 7. Effect of Il4-hemizygosity on CD4+ T cell IL-4 production.
Seven days after i.d. ear immunisation of C57BL/6J background mice with NP-OVA/Alum (A), or of BALB/c-ByJ background mice with OVA/alum (B), auricular lymph node CD4+ T cells were purified by positive selection and restimulated on anti-CD3-coated 96-well plates for IL-4 ELISA analysis. Data points show responses from individual mice from a single experiment (n=3 mice/group) in A and pooled from three experiments (n=2-3 mice/group per experiment) in B. ***p<0.01 by One-way ANOVA with Tukey's post-test.
Discussion
We report that Il4-hemizygous mice exhibited severely compromised IgE responses but not IgG responses against OVA or hapten in OVA/Alum or hapten-OVA/Alum immunisation models. Serum OVA or hapten-specific IgE was diminished by 10-100 fold in Il4-hemizygous mice, six-fold fewer hapten-specific IgE ASC were generated, and hapten-specific IgE levels on mast cells and basophils were reduced 3-10 fold following immunisation of Il4-hemizygous mice. The reduced levels of IL-4 produced by in vitro restimulated IL-4G4/+ CD4+ T cells from allergen immunised Il4-hemizygous mice supports the notion that it is the reduced amount of IL-4 produced by the lymph node CD4+ T cell compartment that leads to the IgE deficiency.
In addition to reduced IgE production, Il4-hemizygous mice were also protected from IgE-dependent oral allergen-induced diarrhoea, but remained susceptible to i.v.-induced antibody-mediated active systemic anaphylaxis. Given that both the production of IgE and mastocytosis is a necessary step for diarrhoea development in this model(1, 2, 35), it was not surprising that the attenuated mastocytosis combined with the deficiency in OVA specific IgE production resulted in the Il4-hemizygous mice being protected from disease(31, 36-38). In the i.v. allergen model, Il4-hemizygous mice exhibited some signs of active systemic anaphylaxis when challenged i.v. with OVA. However, the allergen induced hypothermia was generally less severe in Il4 deficient and hemizygous mice. In addition to influences of IgE on these responses, the reduced hypothermia may reflect an influence of Il4-hemizygosity on the IL-4 dependent sensitivity of vascular smooth muscle to mediators of anaphylaxis, including platelet-activating factor (6), which plays an important role in systemic anaphylaxis mediated by IgG (40).
The observation that normal levels of activation and development of IgG1 producing ASC occurred in Il4-hemizygous mice, while the development of IgE ASC was profoundly reduced, indicated that each response had different requirements for IL-4 and that a lower amount of IL-4 is sufficient for normal IgG1 production to protein/alum immunisation, while IgE responses are very sensitive to reduced levels of IL-4. Consistent with this, anti-IL-4 antibody treatment can ablate the in vivo IgE response in mice without significantly impacting the IgG antibody response (7). Further, IgG1 antibody production is also unaffected by hemizygosity for the genes encoding the IL-4 signalling components IL4Rα and STAT6, whereas—similar to our findings for IL-4+/- mice—haploinsufficiencies have been described for IgE responses in both Il4ra+/- and Stat6+/- mice (42, 43). Taken together, these data indicate that there are critical threshold IL-4 and IL-4 signalling requirements for IgE synthesis that cannot be achieved when IL-4 and its signalling pathways are reduced by half.
The IgE-restricted haploinsufficiency we observed implies that some IL-4 dependent features of the immune response against allergens are compromised more significantly than would be predicted for the Il4-hemizygous state. Therefore, we sought to determine whether either the commitment of CD4+ T cells to IL-4 production or the expansion of TFH was affected by Il4-hemizygosity. In our analysis of IL-4 producing CD4+ T cell development in immunised Il4-hemizygous mice, we observed that there was a normal commitment of CD4+ T cells to Il4 gene expression, suggesting that the IgE deficiency was not caused by the impaired expansion of lymphoid CD4+ TFH cells. Instead, it seems likely that the amount of IL-4 that the lymphoid CD4+ T cells produce does not reach the threshold required for stimulating the IL-4 dependent signals required for IgE class-switching.
How a threshold IL-4 signal for IgE switching could be so sensitive to the 2-6 fold reduction in IL-4 is not entirely clear. However, as IL-4 is reportedly secreted synaptically (44), the IL-4 concentration to which cognate B cells are exposed may be many times higher than the concentration to which non-cognate B cells in distal parts of the follicle are exposed; high level IL-4 exposure resulting from focused synaptic secretion may be critical for the induction of IgE class-switch recombination. Such “super Th2 cells” that could contribute high levels of IL-4 to intercellular synaptic events, have been demonstrated to occur as part of the normal distribution of T cells expressing the Il4 gene either biallelically or monoallelically when activated. Although the majority of activated Th2 cells have been reported to follow a monoallelic expression pattern, a variable proportion (10-50%) of cells express IL-4 biallelically (25, 45-47). These biallelic Th2 cells would have the potential for very high IL-4 production, however they would require expression from both Il4 alleles, and as such, would not be able to occur in Il4-hemizygous mice, perhaps explaining the profound loss of IgE production.
Our finding that IgE production is disproportionately affected by the genetic loss of one IL-4 producing Il4 gene copy may be relevant for why some, but not all individuals become sensitised to allergens. In humans, numerous polymorphisms and chromatin modifications surrounding IL-4 pathway genes correlate with serum IgE levels and allergic disease risk (for examples, see references (50-55)). It may be that some of the polymorphisms enhance or prolong IL-4/STAT-6 signalling to increase allergic disease risk, as has been suggested previously (56, 57). Minor changes in IL-4 signaling events may also be relevant for the associations observed between some IL-4Rα polymorphisms and IL-4Rα antagonist efficacy (13, 58). It also follows that more potent Th2 immunogens, such as parasitic hookworms, would generate significantly more IL-4 and make type 2 immune responses against these parasites more stable in the face of minor changes to IL-4 signaling thresholds.
In conclusion our findings suggest that Il4-hemizygous mice exhibit significantly attenuated IgE responses. Such observations indicate that agents able to reduce IL-4 to such levels have the potential to significantly attenuate allergen induced allergic disease.
Footnotes
Author contributions: MR, HM & GLG hypothesized research. GLG and CDCA supervised research. MR, MP, HM & GLG designed research. MR, MP, RK, HM, EEF & MC performed research; MR, MP, RK & GLG analysed data. WEP provided critical feedback and discussion. MR, CDCA and GLG wrote the paper. This paper is submitted post-humously for WEP.
The authors declare no conflict of interest.
References
- 1.Brandt EB, Munitz A, Orekov T, Mingler MK, McBride M, Finkelman FD, Rothenberg ME. Targeting IL-4/IL-13 signaling to alleviate oral allergen-induced diarrhea. The Journal of allergy and clinical immunology. 2009;123:53–58. doi: 10.1016/j.jaci.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burton OT, Darling AR, Zhou JS, Noval-Rivas M, Jones TG, Gurish MF, Chatila TA, Oettgen HC. Direct effects of IL-4 on mast cells drive their intestinal expansion and increase susceptibility to anaphylaxis in a murine model of food allergy. Mucosal immunology. 2013;6:740–750. doi: 10.1038/mi.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Panhuys N, Tang SC, Prout M, Camberis M, Scarlett D, Roberts J, Hu-Li J, Paul WE, Le Gros G. In vivo studies fail to reveal a role for IL-4 or STAT6 signaling in Th2 lymphocyte differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12423–12428. doi: 10.1073/pnas.0806372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao A, McDermott J, Urban JF, Jr, Gause W, Madden KB, Yeung KA, Morris SC, Finkelman FD, Shea-Donohue T. Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves. Journal of immunology. 2003;171:948–954. doi: 10.4049/jimmunol.171.2.948. [DOI] [PubMed] [Google Scholar]
- 5.Finkelman FD, Shea-Donohue T, Morris SC, Gildea L, Strait R, Madden KB, Schopf L, Urban JF., Jr Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunological reviews. 2004;201:139–155. doi: 10.1111/j.0105-2896.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- 6.Strait RT, Morris SC, Smiley K, Urban JF, Jr, Finkelman FD. IL-4 exacerbates anaphylaxis. Journal of immunology. 2003;170:3835–3842. doi: 10.4049/jimmunol.170.7.3835. [DOI] [PubMed] [Google Scholar]
- 7.Finkelman FD, Holmes J, Katona IM, Urban JF, Jr, Beckmann MP, Park LS, Schooley KA, Coffman RL, Mosmann TR, Paul WE. Lymphokine control of in vivo immunoglobulin isotype selection. Annual review of immunology. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 8.Kopf M, Le Gros G, Bachmann M, Lamers MC, Bluethmann H, Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362:245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 9.Kuhn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 10.King IL, Mohrs M. IL-4-producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. The Journal of experimental medicine. 2009;206:1001–1007. doi: 10.1084/jem.20090313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cunningham AF, Serre K, Toellner KM, Khan M, Alexander J, Brombacher F, MacLennan IC. Pinpointing IL-4-independent acquisition and IL-4-influenced maintenance of Th2 activity by CD4 T cells. European journal of immunology. 2004;34:686–694. doi: 10.1002/eji.200324510. [DOI] [PubMed] [Google Scholar]
- 12.Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol. 2009;10:385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slager RE, Otulana BA, Hawkins GA, Yen YP, Peters SP, Wenzel SE, Meyers DA, Bleecker ER. IL-4 receptor polymorphisms predict reduction in asthma exacerbations during response to an anti-IL-4 receptor alpha antagonist. The Journal of allergy and clinical immunology. 2012;130:516–522. doi: 10.1016/j.jaci.2012.03.030. e514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Apter AJ. Advances in adult asthma diagnosis and treatment in 2013. The Journal of allergy and clinical immunology. 2014;133:49–56. doi: 10.1016/j.jaci.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, Wang L, Kirkesseli S, Rocklin R, Bock B, Hamilton J, Ming JE, Radin A, Stahl N, Yancopoulos GD, Graham N, Pirozzi G. Dupilumab in persistent asthma with elevated eosinophil levels. The New England journal of medicine. 2013;368:2455–2466. doi: 10.1056/NEJMoa1304048. [DOI] [PubMed] [Google Scholar]
- 16.Gandhi NA, Bennett BL, Graham NM, Pirozzi G, Stahl N, Yancopoulos GD. Targeting key proximal drivers of type 2 inflammation in disease. Nature reviews Drug discovery. 2015 doi: 10.1038/nrd4624. [DOI] [PubMed] [Google Scholar]
- 17.Guo L, Huang Y, Chen X, Hu-Li J, Urban JF, Jr, Paul WE. Innate immunological function of TH2 cells in vivo. Nat Immunol. 2015;16:1051–1059. doi: 10.1038/ni.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dickgreber N, Farrand KJ, van Panhuys N, Knight DA, McKee SJ, Chong ML, Miranda-Hernandez S, Baxter AG, Locksley RM, Le Gros G, Hermans IF. Immature murine NKT cells pass through a stage of developmentally programmed innate IL-4 secretion. Journal of leukocyte biology. 2012;92:999–1009. doi: 10.1189/jlb.0512242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glatman Zaretsky A, Taylor JJ, King IL, Marshall FA, Mohrs M, Pearce EJ. T follicular helper cells differentiate from Th2 cells in response to helminth antigens. The Journal of experimental medicine. 2009;206:991–999. doi: 10.1084/jem.20090303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Min B, Prout M, Hu-Li J, Zhu J, Jankovic D, Morgan ES, Urban JF, Jr, Dvorak AM, Finkelman FD, LeGros G, Paul WE. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. The Journal of experimental medicine. 2004;200:507–517. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Panhuys N, Prout M, Forbes E, Min B, Paul WE, Le Gros G. Basophils are the major producers of IL-4 during primary helminth infection. Journal of immunology. 2011;186:2719–2728. doi: 10.4049/jimmunol.1000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohrs K, Wakil AE, Killeen N, Locksley RM, Mohrs M. A two-step process for cytokine production revealed by IL-4 dual-reporter mice. Immunity. 2005;23:419–429. doi: 10.1016/j.immuni.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang HE, Reinhardt RL, Bando JK, Sullivan BM, Ho IC, Locksley RM. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat Immunol. 2012;13:58–66. doi: 10.1038/ni.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Voehringer D, Shinkai K, Locksley RM. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity. 2004;20:267–277. doi: 10.1016/s1074-7613(04)00026-3. [DOI] [PubMed] [Google Scholar]
- 25.Hu-Li J, Pannetier C, Guo L, Lohning M, Gu H, Watson C, Assenmacher M, Radbruch A, Paul WE. Regulation of expression of IL-4 alleles: analysis using a chimeric GFP/IL-4 gene. Immunity. 2001;14:1–11. doi: 10.1016/s1074-7613(01)00084-x. [DOI] [PubMed] [Google Scholar]
- 26.Roediger B, Kyle R, Tay SS, Mitchell AJ, Bolton HA, Guy TV, Tan SY, Forbes-Blom E, Tong PL, Koller Y, Shklovskaya E, Iwashima M, McCoy KD, Le Gros G, Fazekas de St Groth B, Weninger W. IL-2 is a critical regulator of group 2 innate lymphoid cell function during pulmonary inflammation. The Journal of allergy and clinical immunology. 2015 doi: 10.1016/j.jaci.2015.03.043. [DOI] [PubMed] [Google Scholar]
- 27.Roediger B, Kyle R, Yip KH, Sumaria N, Guy TV, Kim BS, Mitchell AJ, Tay SS, Jain R, Forbes-Blom E, Chen X, Tong PL, Bolton HA, Artis D, Paul WE, Fazekas de St Groth B, Grimbaldeston MA, Le Gros G, Weninger W. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol. 2013;14:564–573. doi: 10.1038/ni.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sullivan BM, Liang HE, Bando JK, Wu D, Cheng LE, McKerrow JK, Allen CD, Locksley RM. Genetic analysis of basophil function in vivo. Nat Immunol. 2011;12:527–535. doi: 10.1038/ni.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brandt EB, Strait RT, Hershko D, Wang Q, Muntel EE, Scribner TA, Zimmermann N, Finkelman FD, Rothenberg ME. Mast cells are required for experimental oral allergen-induced diarrhea. The Journal of clinical investigation. 2003;112:1666–1677. doi: 10.1172/JCI19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Z, Sullivan BM, Allen CD. Fluorescent in vivo detection reveals that IgE(+) B cells are restrained by an intrinsic cell fate predisposition. Immunity. 2012;36:857–872. doi: 10.1016/j.immuni.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Forbes EE, Groschwitz K, Abonia JP, Brandt EB, Cohen E, Blanchard C, Ahrens R, Seidu L, McKenzie A, Strait R, Finkelman FD, Foster PS, Matthaei KI, Rothenberg ME, Hogan SP. IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. The Journal of experimental medicine. 2008;205:897–913. doi: 10.1084/jem.20071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Camberis M, Prout M, Tang SC, Forbes-Blom E, Robinson M, Kyle R, Belkaid Y, Paul W, Le Gros G. Evaluating the in vivo Th2 priming potential among common allergens. Journal of immunological methods. 2013;394:62–72. doi: 10.1016/j.jim.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 33.McHeyzer-Williams LJ, McHeyzer-Williams MG. Analysis of antigen-specific B-cell memory directly ex vivo. Methods in molecular biology. 2004;271:173–188. doi: 10.1385/1-59259-796-3:173. [DOI] [PubMed] [Google Scholar]
- 34.Oeser K, Maxeiner J, Symowski C, Stassen M, Voehringer D. T cells are the critical source of IL-4/IL-13 in a mouse model of allergic asthma. Allergy. 2015;70:1440–1449. doi: 10.1111/all.12705. [DOI] [PubMed] [Google Scholar]
- 35.Kucuk ZY, Strait R, Khodoun MV, Mahler A, Hogan S, Finkelman FD. Induction and suppression of allergic diarrhea and systemic anaphylaxis in a murine model of food allergy. The Journal of allergy and clinical immunology. 2012;129:1343–1348. doi: 10.1016/j.jaci.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knight AK, Blazquez AB, Zhang S, Mayer L, Sampson HA, Berin MC. CD4 T cells activated in the mesenteric lymph node mediate gastrointestinal food allergy in mice. American journal of physiology Gastrointestinal and liver physiology. 2007;293:G1234–1243. doi: 10.1152/ajpgi.00323.2007. [DOI] [PubMed] [Google Scholar]
- 37.Mathias CB, Hobson SA, Garcia-Lloret M, Lawson G, Poddighe D, Freyschmidt EJ, Xing W, Gurish MF, Chatila TA, Oettgen HC. IgE-mediated systemic anaphylaxis and impaired tolerance to food antigens in mice with enhanced IL-4 receptor signaling. The Journal of allergy and clinical immunology. 2011;127:795–805. doi: 10.1016/j.jaci.2010.11.009. e791-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noti M, Kim BS, Siracusa MC, Rak GD, Kubo M, Moghaddam AE, Sattentau QA, Comeau MR, Spergel JM, Artis D. Exposure to food allergens through inflamed skin promotes intestinal food allergy through the thymic stromal lymphopoietin-basophil axis. The Journal of allergy and clinical immunology. 2014;133:1390–1399. doi: 10.1016/j.jaci.2014.01.021. e1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oettgen HC, Martin TR, Wynshaw-Boris A, Deng C, Drazen JM, Leder P. Active anaphylaxis in IgE-deficient mice. Nature. 1994;370:367–370. doi: 10.1038/370367a0. [DOI] [PubMed] [Google Scholar]
- 40.Jonsson F, Mancardi DA, Kita Y, Karasuyama H, Iannascoli B, Van Rooijen N, Shimizu T, Daeron M, Bruhns P. Mouse and human neutrophils induce anaphylaxis. The Journal of clinical investigation. 2011;121:1484–1496. doi: 10.1172/JCI45232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feyerabend TB, Weiser A, Tietz A, Stassen M, Harris N, Kopf M, Radermacher P, Moller P, Benoist C, Mathis D, Fehling HJ, Rodewald HR. Cre-mediated cell ablation contests mast cell contribution in models of antibody- and T cell-mediated autoimmunity. Immunity. 2011;35:832–844. doi: 10.1016/j.immuni.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 42.Muller U, Stenzel W, Kohler G, Polte T, Blessing M, Mann A, Piehler D, Brombacher F, Alber G. A gene-dosage effect for interleukin-4 receptor alpha-chain expression has an impact on Th2-mediated allergic inflammation during bronchopulmonary mycosis. The Journal of infectious diseases. 2008;198:1714–1721. doi: 10.1086/593068. [DOI] [PubMed] [Google Scholar]
- 43.Burgis S, Gessner A. Unexpected phenotype of STAT6 heterozygous mice implies distinct STAT6 dosage requirements for different IL-4 functions. International archives of allergy and immunology. 2007;143:263–268. doi: 10.1159/000100571. [DOI] [PubMed] [Google Scholar]
- 44.Poo WJ, Conrad L, Janeway CA., Jr Receptor-directed focusing of lymphokine release by helper T cells. Nature. 1988;332:378–380. doi: 10.1038/332378a0. [DOI] [PubMed] [Google Scholar]
- 45.Mariani L, Schulz EG, Lexberg MH, Helmstetter C, Radbruch A, Lohning M, Hofer T. Short-term memory in gene induction reveals the regulatory principle behind stochastic IL-4 expression. Molecular systems biology. 2010;6:359. doi: 10.1038/msb.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo L, Hu-Li J, Paul WE. Probabilistic regulation in TH2 cells accounts for monoallelic expression of IL-4 and IL-13. Immunity. 2005;23:89–99. doi: 10.1016/j.immuni.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 47.Bix M, Locksley RM. Independent and epigenetic regulation of the interleukin-4 alleles in CD4+ T cells. Science. 1998;281:1352–1354. doi: 10.1126/science.281.5381.1352. [DOI] [PubMed] [Google Scholar]
- 48.Suto A, Nakajima H, Hirose K, Suzuki K, Kagami S, Seto Y, Hoshimoto A, Saito Y, Foster DC, Iwamoto I. Interleukin 21 prevents antigen-induced IgE production by inhibiting germ line C(epsilon) transcription of IL-4-stimulated B cells. Blood. 2002;100:4565–4573. doi: 10.1182/blood-2002-04-1115. [DOI] [PubMed] [Google Scholar]
- 49.Yang Z, Robinson MJ, Allen CD. Regulatory constraints in the generation and differentiation of IgE-expressing B cells. Current opinion in immunology. 2014;28C:64–70. doi: 10.1016/j.coi.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soto-Ramirez N, Arshad SH, Holloway JW, Zhang H, Schauberger E, Ewart S, Patil V, Karmaus W. The interaction of genetic variants and DNA methylation of the interleukin-4 receptor gene increase the risk of asthma at age 18 years. Clinical epigenetics. 2013;5:1. doi: 10.1186/1868-7083-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang H, Tong X, Holloway JW, Rezwan FI, Lockett GA, Patil V, Ray M, Everson TM, Soto-Ramirez N, Arshad SH, Ewart S, Karmaus W. The interplay of DNA methylation over time with Th2 pathway genetic variants on asthma risk and temporal asthma transition. Clinical epigenetics. 2014;6:8. doi: 10.1186/1868-7083-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Basehore MJ, Howard TD, Lange LA, Moore WC, Hawkins GA, Marshik PL, Harkins MS, Meyers DA, Bleecker ER. A comprehensive evaluation of IL4 variants in ethnically diverse populations: association of total serum IgE levels and asthma in white subjects. The Journal of allergy and clinical immunology. 2004;114:80–87. doi: 10.1016/j.jaci.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 53.Loza MJ, Chang BL. Association between Q551R IL4R genetic variants and atopic asthma risk demonstrated by meta-analysis. The Journal of allergy and clinical immunology. 2007;120:578–585. doi: 10.1016/j.jaci.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 54.Kabesch M, Schedel M, Carr D, Woitsch B, Fritzsch C, Weiland SK, von Mutius E. IL-4/IL-13 pathway genetics strongly influence serum IgE levels and childhood asthma. The Journal of allergy and clinical immunology. 2006;117:269–274. doi: 10.1016/j.jaci.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 55.Weidinger S, Gieger C, Rodriguez E, Baurecht H, Mempel M, Klopp N, Gohlke H, Wagenpfeil S, Ollert M, Ring J, Behrendt H, Heinrich J, Novak N, Bieber T, Kramer U, Berdel D, von Berg A, Bauer CP, Herbarth O, Koletzko S, Prokisch H, Mehta D, Meitinger T, Depner M, von Mutius E, Liang L, Moffatt M, Cookson W, Kabesch M, Wichmann HE, Illig T. Genome-wide scan on total serum IgE levels identifies FCER1A as novel susceptibility locus. PLoS genetics. 2008;4:e1000166. doi: 10.1371/journal.pgen.1000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tachdjian R, Al Khatib S, Schwinglshackl A, Kim HS, Chen A, Blasioli J, Mathias C, Kim HY, Umetsu DT, Oettgen HC, Chatila TA. In vivo regulation of the allergic response by the IL-4 receptor alpha chain immunoreceptor tyrosine-based inhibitory motif. The Journal of allergy and clinical immunology. 2010;125:1128–1136. doi: 10.1016/j.jaci.2010.01.054. e1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ford AQ, Heller NM, Stephenson L, Boothby MR, Keegan AD. An atopy-associated polymorphism in the ectodomain of the IL-4R(alpha) chain (V50) regulates the persistence of STAT6 phosphorylation. Journal of immunology. 2009;183:1607–1616. doi: 10.4049/jimmunol.0803266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Slager RE, Hawkins GA, Ampleford EJ, Bowden A, Stevens LE, Morton MT, Tomkinson A, Wenzel SE, Longphre M, Bleecker ER, Meyers DA. IL-4 receptor alpha polymorphisms are predictors of a pharmacogenetic response to a novel IL-4/IL-13 antagonist. The Journal of allergy and clinical immunology. 2010;126:875–878. doi: 10.1016/j.jaci.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]