Abstract
Macrophages (Ma) loaded with gold-based nanoparticles, which convert near infrared light to heat, have been studied as targeted transport vectors for photothermal therapy (PTT) of tumors. The purpose of the experiments reported here was to compare the efficacy of gold-silica nanoshells (AuNS) and gold nanorods (AuNR) in macrophage-mediated PTT. Photothermal therapy efficacy was evaluated in hybrid glioma spheroids consisting of human glioma cells and either AuNS- or AuNR-loaded Ma, designated MaNS and MaNR, respectivly. Spheroids were irradiated for 10 minutes with light from an 810-nm diode laser at irradiances ranging from 0 to 28 W/cm2. Photothermal therapy efficacy was determined from spheroid growth over a 14-day period. The uptake by Ma of pegylated AuNR (3.9 ± 0.9 %) was twice that of pegylated AuNS (7.9 ± 0.7%). Hybrid spheroids consisting of a 5:1 ratio of glioma cells to loaded Ma exhibited significant growth inhibition with MaNS when subjected to irradiances of 7 W/cm2 or greater. In contrast, no significant growth inhibition was observed for the MaNR hybrid spheroids at this 5:1 ratio, even at the highest irradiance investigated (28 W/cm2). Although AuNR were taken up by Ma in larger numbers than AuNS, MaNS were shown to have greater PTT efficacy compared to MaNR for equivalent numbers of loaded Ma.
Keywords: glioma, photothermal therapy, gold-silica nanoshells, gold nanorods, murine macrophages
I. INTRODUCTION
The ability of gold nanoparticles to convert near-infrared (NIR) light to heat is highly efficient; thus, they are well suited for hyperthermia applications such as photothermal therapy (PTT).1–3 The most studied nanoparticles for PTT applications are gold nanoshells (AuNS) and gold nanorods (AuNR). Both of these nanoparticles have strong absorption in the NIR, which makes them ideally suited for biological applications because NIR wavelengths have relatively deep penetration in tissues. The absorption peak of AuNS can be tuned by varying the ratio of gold shell to silica core thickness,4 while peak absorption wavelengths for AuNR are dependent on their aspect ratio (length to width).5,6
A fundamental characteristic for the success of nanoparticle-mediated therapy is the ability of these constructs to penetrate the therapeutic site at sufficient concentrations while minimizing accumulation at undesired sites such as the reticulo-endothelial system. Nanoparticle delivery via passive approaches, such as those employing the enhanced permeability and retention (EPR) effect, is unlikely to achieve sufficient target specificity.7 In contrast, macrophages (Ma) employed as nanoparticle delivery vehicles have the ability to leave the circulation and migrate to and infiltrate the tumor interstitium by active processes (e.g., by following gradients of chemokines, cytokines, and growth factors).8–10
AuNS-loaded macrophages for PTT have been found to be effective in a number of studies.11–14 The overall objective of the this study was to compare the PTT efficacy of Ma loaded with either AuNS (designated MaNS) or Ma loaded with AuNR (designated MaNR). The MaNS or MaNR were incorporated into multicell hybrid spheroids formed by a mixture of loaded Ma and human tumor cells. The relative PTT efficacies of the two types of nanoparticles were evaluated based on inhibition of spheroid growth following exposure to NIR laser irradiation.
II. MATERIALS AND METHODS
A. Cell Lines
Murine macrophages (P388D1; ATCC# CCL-46) and human grade IV GBM cells (ACBT; G. Granger, University of California, Irvine) were used in this study.
B. Nanoparticles
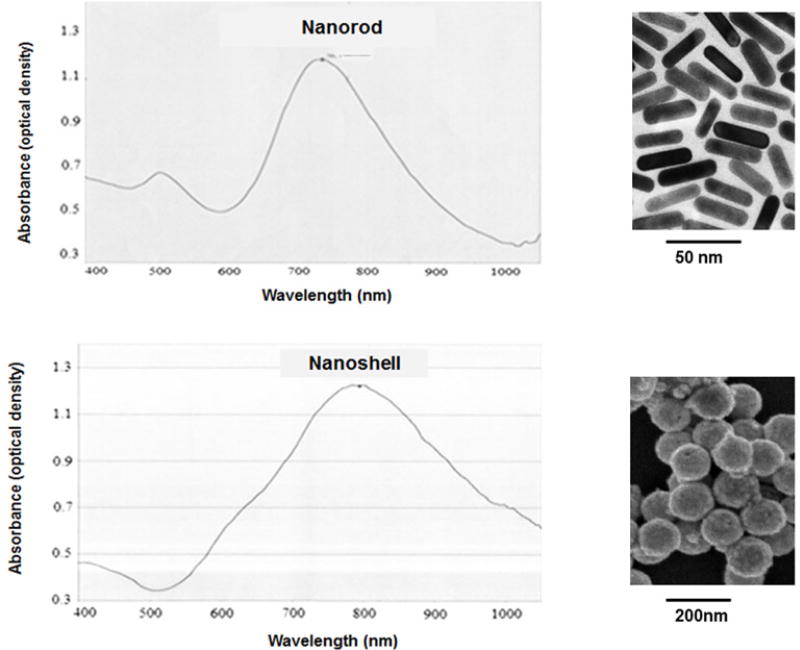
Polyethylene glycol (PEG)-coated AuNS (AuroShell™) and AuNR were purchased from Nanospectra Biosciences (Houston, TX, USA). AuNS were supplied at a concentration of 2.82 ×1011 particles/mL, and consisted of a 120-nm diameter silica core encased in a gold shell of 15-nm thickness, giving a total particle diameter of 150 nm. The peak absorption was found at a wavelength of 819 nm (optical density = 105). AuNR were supplied at a concentration of 2.0 × 1011 particles/mL and had a length of 45 nm and a width of 15 nm. The peak absorption occurred at a wavelength of 765 nm (optical density = 1.09). The absorption and electron micrographs for both types of nanoparticles (supplied by the manufacturer) are shown in Figure 1.
FIG. 1.
Vis-NIR absorption spectra and electron micrographs of pegylated AuNR (top) and pegylated AuNS (bottom)
C. Nanoparticle Uptake
AuNS or AuNR uptake by Ma was studied using UV-Vis-NIR spectrophotometry. Ma (5.0 × 106) were incubated with either AuNS (4.29 × 109 per mL) or an equivalent concentration of AuNR for 24 h. Following incubation, Ma were centrifuged at 600 rpm for 7 min and then washed twice with phosphate-buffered saline (PBS) to remove excess particles, and the cells then resuspended. The absorbance of the resultant cellular suspension was measured with a Varian UV-Vis-NIR spectrophotometer (Cary 6000i, Varian, Palo Alto, CA). The percentage uptake of nanoparticles was calculated by applying the following formula:
| (1) |
where AM+ N is the absorbance of endocytosed nano-shells at λ = 819 nm (or nanorods at 765 nm), and AN is the absorbance of the reference nanoshell or nanorod solution at the same wavelength.
D. Hybrid Spheroid Generation
Hybrid tumor/Ma spheroids were formed as previously described.12 Prior to spheroid formation, both nanoparticle-loaded and empty Ma were incubated for 1 hour in 20 µg/mL mitomycin C (Sigma-Aldrich, St. Louis, MO, USA) to inhibit cell division and their subsequent contribution to spheroid growth. Hybrid spheroids using either 5 × 103 ACBT cells and 1 × 103 or 2 × 103 loaded Ma (designated MaNS or MaNR) were generated. The cell combinations were alloquated into the wells of ultra-low attachment 96-well round-bottom plates (Corning, Corning, NY, USA) in 200 µL culture medium. The plates were centrifuged at 1,000 ×g for 10 min. The plates were incubated for 48 h to allow the spheroids to stabilize. Previous experiments employing two-photon microscopy have shown an even distribution of the Ma throughout the spheroids.12
E. External Hyperthermia Treatment
Forty-eight hours after initiation, hybrid spheroids containing empty Ma were incubated at temperatures ranging from 37 to 46°C for 45 minutes at each temperature. The spheroids were followed for 14 days, and their volume was calculated on the basis of of their diameters as determined from light microscopy measurements.
F. Photothermal Therapy of Spheroids
Individual spheroids in each well of a 96-well round-bottom plate were irradiated with 810 nm light from a laser diode (Intense, New Brunswick, NJ, USA) at irradiances ranging from 0 to 28 W/cm2 with a beam diameter of approximately 3 mm and an irradiation time of 10 min. The maximum power output, corresponding to an irradiance of 28 W/cm2, was 1.98 W. Treatment efficacy was monitored by spheroid growth following 14 days of incubation. Spheroid diameters were measured using an ordinary light microscope with a calibrated eyepiece and spheroid volumes were estimated assuming a perfect sphere.
G. Statistical Analysis
Microsoft Excel (Redmond, WA, USA) was used to determine the mean, standard deviation and standard error. Data were analyzed using one-way ANOVA at the significance level of P < 0.05 and presented as mean with standard error unless otherwise noted.
H. Experimental Protocol
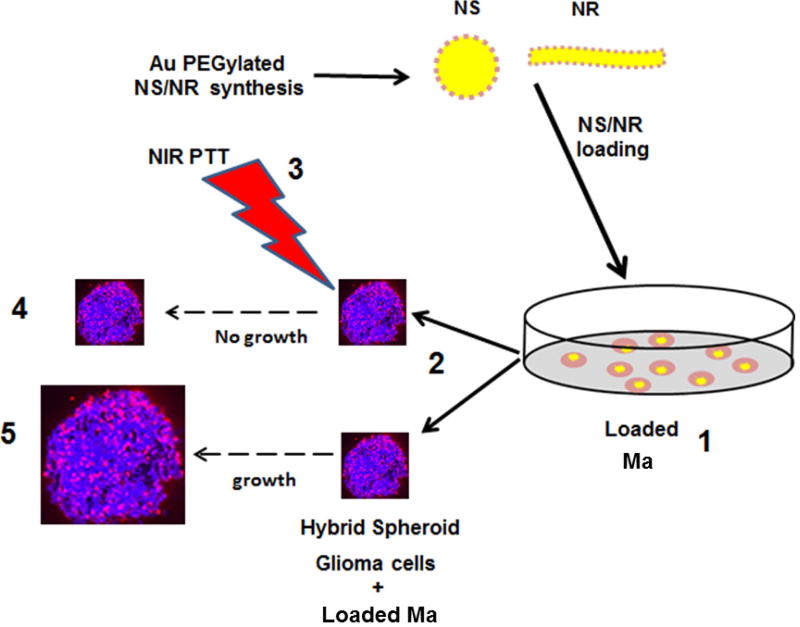
The basic experimental protocol used in this study is shown in Figure 2. As seen in the figure, Ma loaded with either AuNS or AuNR were used to form hybrid spheroids consisting of ACBT glioma cells and a variable number of MaNS or MaNR. The experimental groups were subjected to NIR laser light at irradiances of 2, 7, 14, or 28 W/cm2, while control groups received no irradiation. Spheroid growth in both groups were monitored for 14 days.
FIG. 2.
Basic experimental setup, (1) Ma loaded with AuNS or AuNR by 24 h co-incubation; (2) Hybrid spheroid consisting of ACBT glioma cells and a variable number of MaNS or MaNR; (3) One group of hybrid spheroids irradiated with variable levels of NIR laser light; Control groups received no irradiation; (4) Growth of experimental group followed for 14 days; (5) Growth of control group followed for 14 days.
III. RESULTS AND DISCUSSION
A. Nanoparticle Uptake by Macrophages
Equal concentrations of gold nanoshells and nanorods were incubated with macrophages. Following 24 hours of coincubation, spectrophotometric analysis revealed uptakes of 3.9 ± 0.9 and 7.9 ± 0.7% for AuNS and AuNR, respectively. Based on the initial nanoparticle concentrations, these uptakes corresponded to 337 AuNS and 680 AuNR per macrophage, respectively. The difference in uptake was statistically significant (P < 0.05). Macrophage phagocytosis is influenced by size, shape, and surface chemistry of the nanoparticle.15–17 The increased ability of Ma to take up more AuNR compared to AuNS might be due to the smaller size of the AuNR and their rod-like shape, which is similar to many pathogens.
B. Toxic Effects of Hyperthermia
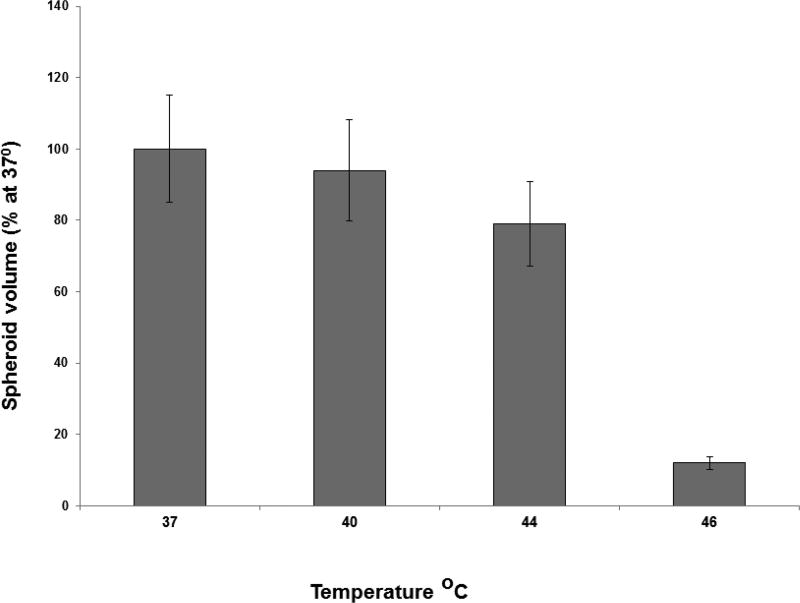
The direct effect of hyperthermia on hybrid spheroid growth was examined in the wells of 96-well plates. Forty-eight hours after spheroid generation, the plates were incubated at 37, 40, 44 or 46°C for 45 minutes at each temperature. Following hyperyhermia, the plates were incubated at 37°C and monitored for growth for an additional 14 days. A temperature of 46°C for 45 minutes resulted in total spheroid death, while the majority of spheroids subjected to 44°C or below for the same interval, survived (Fig. 3). The toxic threshold for hyperthermia was estimated to be between 44 and 46°C and could therefore be used to infer the temperature obtained during PTT.
FIG. 3.
Effects of temperature on ACBT spheroid growth. Spheroids 48 h after initiation were incubated at temperatures ranging from 37 to 46°C, for 45 minutes at each temperature. Each data point represents spheroid volume after two weeks in culture as a % of 37°C controls and is the mean of three experiments. Error bars denote standard errors.
C. Photothermal Therapy of Nanoparticle-Loaded Hybrid Spheroids
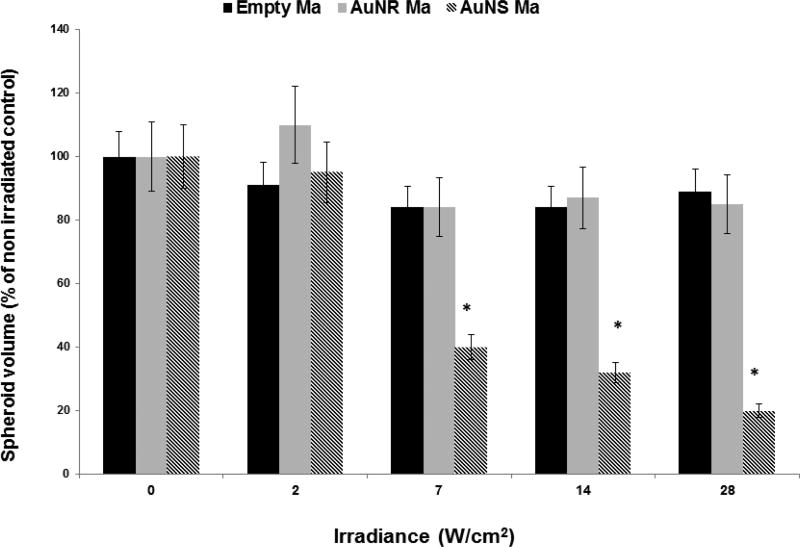
Survival of ACBT/loaded Ma hybrid spheroids exposed to a range of laser irradiances is illustrated in Figure 4. Control spheroids consisting of ACBT cells and “empty” macrophages (MaE) were irradiated over a range of 0–28 W/cm2. Minimal growth inhibition at all irradiances compared to nonirradiated controls was demonstrated.
FIG. 4.
Growth of hybrid spheroids as a function of 808-nm laser irradiance. MaE denotes hybrid control spheroids consisting of empty macrophages and Tc denotes tumor cells. The ratio of Tc: MaNS or Tc: MaNR was 5:1. Each data point corresponds to the mean of three trials as a % of controls on day 14. Error bars denote standard deviations. * denotes significant difference from nonirradiated controls (P < 0.05).
MaNS mediated PTT significantly inhibited spheroid growth at irradiances of 7 W/cm2 and higher (P < 0.05). Spheroid survival was approximately 40%, 30%, and 18% of controls at irradiances of 7, 14 and 28 W/cm2, respectively. In contrast, MaNR hybrid spheroids showed no significant growth inhibition compared to MaE controls at any of the irradiances investigated. Increasing the number of MaNR in each spheroid to 2 × 103 resulted in a modest inhibition of spheroid growth but only at an irradiance of 28 W/cm2. A 30% reduction of spheroid volume compared to controls was achieved (data not shown). Although Ma ingested approximately twice as many AuNR compared to AuNS, AuNS have a much larger cross-sectional area compared to AuNR, resulting in a larger photothermal transduction cross-section.18 Calculations show that the absorption efficiency of AuNS similar to the ones used in this study is approximately ten times greater than that of the AuNR used in this work.19 The significantly increased conversion efficiency of NIR light to heat demonstrated by MaNS compared to MaNR is the likely explanation for the results shown in Figure 4.
IV. CONCLUSIONS
Although AuNR were taken up by Ma in larger numbers than AuNS, MaNS were shown to have greater PTT efficacy than MaNR for equivalent numbers of loaded Ma in the tumor cell spheroids. This finding was most probably due to the considerably larger cross-sectional area of the AuNS compared to the AuNR used in this study, resulting in a larger photothermal transduction cross-section. Theoretical calculations suggest the ratio of nanorods to nanoshells required, for equivalent PTT effect, is approximately 10:1
Acknowledgments
The authors are grateful for support from the Norwegian Radium Hospital Research Foundation. Portions of this work were made possible through access to the LAMMP Program NIBIB P41EB015890. Steen Madsen was supported, in part, by the Tony and Renee Marlon Charitable Foundation.
References
- 1.Hirsch LR, Gobin AM, Lowery AR, Tam F, Jalas NJ. Metal nanoshells. Ann Biomed Eng. 2006;34(1):15–22. doi: 10.1007/s10439-005-9001-8. [DOI] [PubMed] [Google Scholar]
- 2.Everts M. Thermal scalpel to target cancer. Expert Rev Med Devices. 2007;4(2):131–6. doi: 10.1586/17434440.4.2.131. [DOI] [PubMed] [Google Scholar]
- 3.Cole JR, Mirin NA, Knight MW, Goodrich GP, Halas NJ. Photothermal efficiencies of nanoshells and nanorods for clinical therapeutic applications. J Phys Chem C. 2009;113(28):12090–4. [Google Scholar]
- 4.Loo C, Lin A, Hirsch L, Lee MH, Halas N, West J, Drezek R. Nanoshells-enabled photonics-based imaging and therapy of cancer. Technol Cancer Res Treat. 2004;3(1):33–40. doi: 10.1177/153303460400300104. [DOI] [PubMed] [Google Scholar]
- 5.Huang X, El-Sayed IH, Qian W, El-Sayed MA. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc. 2006;128(6):2115–20. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- 6.Huang X, Jain PK, El-Sayed IH, El-Sayed MA. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med Sci. 2008;23:217–8. doi: 10.1007/s10103-007-0470-x. [DOI] [PubMed] [Google Scholar]
- 7.Barua S, Mitragotri S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: a review of current status and future prospects. Nano Today. 2014;9(2):223–43. doi: 10.1016/j.nantod.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones GE. Cellular signaling in macrophage migration and chemotaxis. J Leukoc Biol. 2008;68(5):593–602. [PubMed] [Google Scholar]
- 9.Basel MT, Shrestha TB, Bossmann SH, Troyer DL. Cells as delivery vehicles for cancer therapeutics. Ther Deliv. 2014;5(5):555–67. doi: 10.4155/tde.14.24. [DOI] [PubMed] [Google Scholar]
- 10.Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 2014;6(3):1670–90. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi MR, Stanton-Maxey KJ, Stanley JK, Levin CS, Bardhan R, Akin D, Badve S, Sturgis J, Robinson JP, Bashir R, Halas NJ, Clare SE. A cellular Trojan horse for delivery of therapeutic nanoparticles into tumors. Nano Lett. 2007;7(12):3759–65. doi: 10.1021/nl072209h. [DOI] [PubMed] [Google Scholar]
- 12.Baek S-K, Makkouk AR, Krasieva T, Sun C-H, Madsen SJ, Hirschberg H. Photothermal treatment of glioma: an in vitro study of macrophage-mediated delivery of gold nanoshells. J Neurooncol. 2011;104(2):439–48. doi: 10.1007/s11060-010-0511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trinidad A, Hong SJ, Peng Q, Madsen SJ, Hirschberg H. Combined concurrent photodynamic and gold nanoshell loaded macrophage-mediated photothermal therapies: an in vitro study on squamous cell head and neck carcinoma. Lasers Surg Med. 2014;46(4):310–8. doi: 10.1002/lsm.22235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christie C, Madsen SJ, Peng Q, Hirschberg H. Macrophages as nanoparticle delivery vectors for photothermal therapy of brain tumors. Ther Deliv. 2015;6(3):371–84. doi: 10.4155/tde.14.121. [DOI] [PubMed] [Google Scholar]
- 15.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci USA. 2006;103:4930–4. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahsan FL, Rivas IP, Khan MA, Suarez AI. Targeting to macrophages: Role of physicochemical properties of particulate carriers—liposomes and microspheres—on the phagocytosis by macrophages. J Control Release. 2002;79:29–40. doi: 10.1016/s0168-3659(01)00549-1. [DOI] [PubMed] [Google Scholar]
- 17.Beningo KA, Wang YL. Fc-receptor-mediated phagocytosis is regulated by mechanical properties of the target. J Cell Sci. 2002;115(4):849–56. doi: 10.1242/jcs.115.4.849. [DOI] [PubMed] [Google Scholar]
- 18.Young JK, Figueroa ER, Drezek RA. Tunable nanostructures as photothermal theranostic agents. Ann Biomed Eng. 2012;40:438–59. doi: 10.1007/s10439-011-0472-5. [DOI] [PubMed] [Google Scholar]
- 19.Jain PK, Lee KS, El-Sayed I. Calculated absorption and scattering properties of gold nanoparticles of different sizes. J Phys Chem B. 2006;110(14):7238–48. doi: 10.1021/jp057170o. [DOI] [PubMed] [Google Scholar]