Abstract
Study Objectives:
Obstructive sleep apnea (OSA) is an independent risk factor for stroke. The objective of this study was to assess the effect of continuous positive airway pressure (CPAP) treatment on prevention of new vascular events among patients with stroke and OSA.
Methods:
Consecutive conscious patients presenting with first imaging-confirmed arterial stroke were included, 6 weeks or more after ictus. All patients underwent clinical and polysomnography (PSG) testing. Patients with an apnea-hypopnea index (AHI) of > 15 events/h were randomized to posttitration nightly CPAP treatment and non-CPAP (received best medical treatment) groups. On follow-up at 3, 6, and 12 months from randomization, evaluation was carried out for any new vascular events as the primary outcome measure, and for clinical stroke outcomes (using the Barthel Index and modified Rankin scale) and neuropsychological parameters as the secondary outcome measures.
Results:
Among the 679 patients with stroke who were screened, 116 reported for PSG, 83 had AHI > 15 events/h, and 70 (34 in CPAP and 36 in non-CPAP) were randomized. Thirteen patients could not be randomized because of a lack of CPAP devices. Four patients crossed over from the CPAP to the non-CPAP group. Age (mean age 53.41 ± 9.85 in CPAP versus 52.69 ± 13.23 years in non-CPAP, P = .81) and sex distribution (24 males in CPAP versus 33 males in non-CPAP, P = .79) were similar in both groups. At 12-month follow-up, there was 1 vascular event (3.33%) in the CPAP group and 6 events (15%) in the non-CPAP group (P = .23). Modified Rankin scale score improvement by ≥ 1 at 12-month follow-up was found in significantly more patients in the CPAP group than in the non-CPAP group (53% versus 27%).
Conclusions:
These findings suggest significantly better stroke outcomes and statistically nonsignificant favorable outcomes in terms of recurrence of vascular events for patients with stroke and OSA who use CPAP treatment.
Clinical Trial Registration:
Registry: Clinical Trials Registry - India, CTRI Registration No: CTRI/2016/07.007104, Title: Sleep Disordered Breathing in stroke patients: Effect of treatment trial, URL: http://ctri.nic.in/Clinicaltrials/showallp.php?mid1=8682&EncHid=&userName=sleep%20disordered%20breathing
Citation:
Gupta A, Shukla G, Afsar M, Poornima S, Pandey RM, Goyal V, Srivastava A, Vibha D, Behari M. Role of positive airway pressure therapy for obstructive sleep apnea in patients with stroke: a randomized controlled trial. J Clin Sleep Med. 2018;14(4):511–521.
Keywords: cerebrovascular disease, clinical trial, CPAP treatment, obstructive sleep apnea, secondary prevention, sleep apnea treatment, stroke, vascular events
BRIEF SUMMARY
Current Knowledge/Study Rationale: Stroke is a common cause of death and disability and obstructive sleep apnea (OSA) is an independent risk factor for stroke. The objective of this study was to assess the effect of continuous positive airway pressure (CPAP) treatment in prevention of new vascular events among patients with stroke and OSA. We included patients presenting with their first-ever stroke after 6 weeks of ictus. Patients with stroke and OSA were randomized to CPAP and non-CPAP treatment groups.
Study Impact: At 1 year from randomization, a nonsignificantly higher number of new vascular events occurred in the non-CPAP group and significantly better stroke outcomes were observed in the CPAP group.
INTRODUCTION
Stroke is common, the second leading cause of death and disability, and is the cause of high health care costs.1,2 Important risk factors for stroke are hypertension, atrial fibrillation, diabetes, and smoking. These are well-established intervention targets for stroke's primary and secondary prevention.3 Despite adequate treatment of these recognized risk factors, there is variation in stroke incidence and outcomes even in apparently homogenous populations. Obstructive sleep apnea (OSA) is characterized by periodic upper airway obstruction that leads to oxygen desaturation and sleep fragmentation. This further leads to an increased risk of cardiovascular disease, stroke, and death.4–6 It has been established that OSA is an independent risk factor for stroke1,7 and also aggravates other risk factors (hypertension, coronary artery disease, diabetes, and atrial fibrillation).1 The prevalence of OSA among patients with stroke is around 50% to 80%. Even with neurological improvement in patients with stroke, their OSA does not remit or improve.8–10 The high prevalence of OSA among patients with stroke is associated with an unfavorable clinical course in terms of early neurological worsening, delirium, depressed mood, impaired functional capacity and cognition, longer period of hospitalization and rehabilitation, and increased mortality.11–15 In addition to these conditions, OSA not only increases the risk of stroke recurrence but also that of sudden death during sleep.16–19
OSA leads to intermittent oxygen desaturation which causes oxidative stress that further leads to cardiovascular (hypertension), metabolic (diabetes, insulin resistance, dyslipidemia, and obesity), and neurocognitive complications (impaired memory, attention, and executive dysfunction).20 Sleep fragmentation is another possible mechanism responsible for cardiovascular, metabolic, and neurocognitive complications.21,22
There are few studies reporting the effect of continuous positive airway pressure (CPAP) therapy on the recurrence of vascular events in stabilized patients with stroke. The aim of this study was to assess the effect of CPAP treatment in prevention of new vascular events among stroke patients with OSA at least 6 weeks after the ictus.
METHODS
Consecutive eligible patients with their first-ever arterial stroke, presenting to Neurology inpatient or outpatient services, were the target population of this study. This study was a single-blind randomized control trial (RCT), approved by the Ethics Committee of the All India Institute of Medical Sciences, New Delhi. Outcome assessment investigators (AM and SP) were blinded for OSA treatment status of patients. The study was initiated in September 2009 and recruitment was completed in November 2013. The last follow-up was conducted and completed in November 2014, and analysis was conducted over the next 6 months.
Patient Population
Inclusion Criteria
All patients presenting to Neurology services, with history suggestive of a first-ever arterial stroke that took place less than 6 months prior to enrollment; with CT scan or MRI evidence of the same; with normal consciousness (Glasgow Coma Scale eye and motor scores of 10); with or without cognitive impairment; and not requiring life support, were included.
Exclusion Criteria
All eligible patients with previous history of stroke; those with history of other neurological illnesses such as Parkinson disease, neuroinfections, neuromuscular disorders; and patients with known primary sleep disorders other than OSA were excluded.
Consecutive patients were interviewed for history suggestive of OSA and other stroke risk factors, and screening was carried out exclusively by a single investigator (AG) using a standardized format based on all inclusion and exclusion criteria. Detailed informed, written consent was obtained from all patients (or close family members in case of aphasic or cognitively impaired patients).
Polysomnographic Evaluation
All patients who provided consent underwent polysomnography (PSG) at least 6 weeks after ictus. PSG was conducted on a 40-channel Nicolet One Sleep system (Natus Medical Incorporated, Middleton, Wisconsin, United States) with analysis conducted in accordance with the AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.2 (2012). All studies were carried out at the usual sleep times of the patients and were acquired by trained technologists. Scoring and analysis were carried out live by the technologist during the PSG acquisition, then checked and revised (after concealment of patient identity) by the sleep fellow (AG) and sleep physician (GS).
For scoring hypopneas, the following criteria were used: peak signal excursions drop by ≥ 30% of preevent baseline using nasal pressure transducer; the duration of the ≥ 30% drop being ≥ 10 seconds; and ≥ 3% oxygen desaturation from pre-event baseline or association of the event with an arousal.23
Randomization, Titration, and Initiation of CPAP Therapy
All patients with an apnea-hypopnea index (AHI) greater than 15 events/h were randomized by computer-generated random numbers and the sheets (with numbers printed) were sealed in opaque envelopes. One envelope was opened by a clinical assistant each time a patient was to be randomized and information about the category mentioned in the sheet was then communicated to the sleep technologists in person or by telephone. The CPAP group consisted of patients put on nightly CPAP treatment following a titration PSG, and the non-CPAP group was formed by those not on any treatment for OSA. Those in the non-CPAP group received the “best medical treatment.” This included oral enteric coated aspirin (150 mg), oral atorvastatin (10–20 mg), folic acid (5 mg), and antihypertensive or antidiabetic treatment as indicated for the patients with ischemic stroke. Similarly, for the patients with hemorrhagic stroke, only anti-hypertensive drugs were part of the standard best medical treatment. Edema-reducing measures were used in the acute phase if necessary. Titration was manually carried out according to guidelines published by the American Academy of Sleep Medicine.23 All patients were titrated on CPAP and were prescribed auto-adjusting CPAP devices. The devices were set to a narrow range of titrated pressure ± 2 cm H2O. All patients used a nasal mask except for one patient who used an oronasal mask because he had difficulty with the nasal mask and dryness of the mouth. The purchase of the CPAP devices was sponsored by the funding agency (Department of Science and Technology, Government of India) and devices were purchased from Tyco Healthcare (GoodKnight 420E, Puritan Benett, Pleasantom, California, United States) and Phillips Respironics (REMstar Auto Aflex, Koninklijke Philips Electronics N.V., Murrysville, Pennsylvania, United States). Using CPAP, sleep apnea improved in all patients to an AHI < 5 events/h, except for one who responded only to bilevel positive airway pressure. He had to be reported as a crossover to the non-CPAP arm, because he was unable to afford the cost to procure a bilevel device (not provided through the research funding).
Initialization on CPAP therapy was carried out by the sleep technologists after successful titration. Troubleshooting as far as the equipment and/or masks was concerned was also handled by the sleep technologists. CPAP adherence data were collected by sleep technologists, from the memory cards of the CPAP devices, after a minimum of 3 months and maximum of 6 months of CPAP use.
Follow-Up and Outcome Assessment
Measurement of outcome parameters was standardized and assessors were blinded. Patients as well as their caregivers were instructed in detail, in advance, to maintain confidentiality about their OSA treatment status while being assessed by AM, SP, or AG.
Primary Outcome Measure Assessment
Primary outcome measure was assessed solely by a single investigator (GS), by confirming prospectively the occurrence of any vascular event (stroke, death, acute myocardial infarction, unstable angina, subclinical coronary events) through history, clinical examination, electrocardiogram, cardiac enzymes (for suspected acute coronary events), and brain imaging (for suspected stroke recurrence). An electrocardiogram was obtained at enrollment, at 6-week follow-up, and at 3, 6, and 12 months from randomization and treatment initiation.
Secondary Outcome Measures Assessment
Stroke outcomes: Stroke outcomes were assessed by a single investigator (GS) at baseline. Assessment at all three follow-up visits was carried out by AG (blinded to information about treatment arms). Patients were assessed for their clinical status in detail. Their independence in activities of daily living was evaluated using the Barthel Index.24 Clinical stroke outcomes were categorized using the modified Rankin scale (mRS)25—a scale most widely used for disability and dependence outcome assessment among patients with stroke.
Neuropsychological assessment scores: Detailed neuropsychological assessment was carried out using the Mini-Mental Status Examination (for overall cognitive status), PGI Memory Scale26 (for evaluation of memory) and the Indian aphasia battery27 (to evaluate poststroke language dysfunction, specifically in the Hindi-speaking North Indian population). Detailed sleep history was also obtained. For neuropsychological testing, investigators AM and SP were the sole evaluators during the first half and second half of the study period, respectively. Their interrater agreement was checked at the time of transition between first and second halves of the study period on 10 consecutive subjects, and was confirmed to be high (Cohen Kappa = 0.89). They remained completely blinded to treatment being received by the patients.
Statistical Analysis
Statistical analysis was carried out by using the statistical software STATA version 12.0 (StataCorp, College Station, Texas, United States). All categorical variables summarized as frequency (%). Continuous variables following normal distribution were summarized as mean ± standard deviation, and non-normal variables were summarized as median (range). The Student t test was used to compare continuous variables when data distribution was normal and the Wilcoxon rank-sum test was used for skewed data. The chi-square test was used to compare categorical variables and Fisher exact statistics were used if cell frequency was less than 5. We also calculated the number of patients who showed improvement on the mRS ≥ 1 point, at each follow-up.
Kaplan-Meier survival estimates analysis and multifactorial Cox proportional hazards regression analysis of factors associated with cardiovascular events or mortality (age sex, body mass index, history of hypertension, ischemic heart disease, smoking, dyslipidemia, Epworth Sleepiness Scale, Barthel Index, mRS, AHI) were carried out.
RESULTS
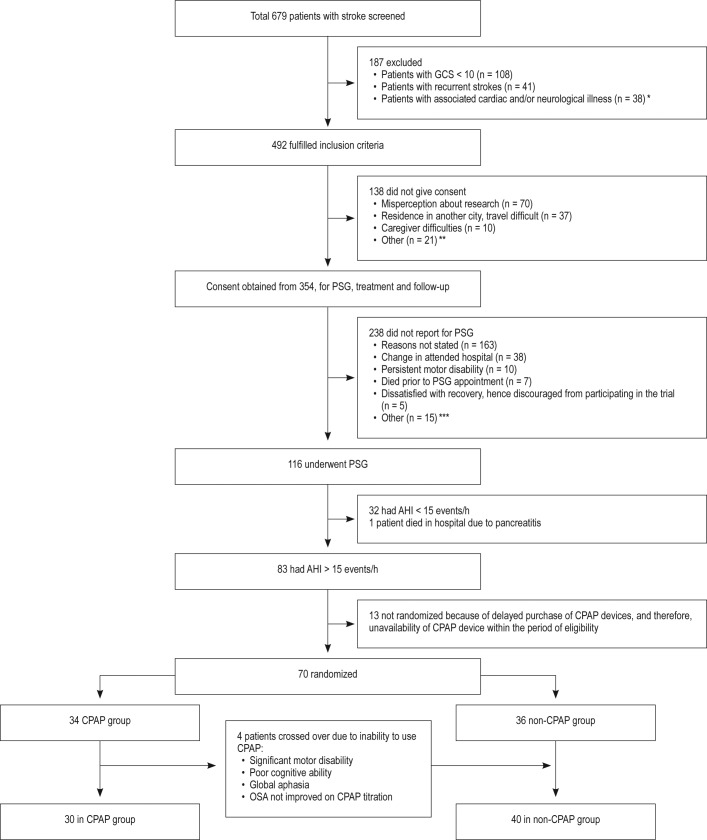
A total of 679 consecutive patients with stroke were screened for enrollment into this study. We excluded 187 patients because of low Glasgow Coma Scale scores < 10 (n = 108), history of prior stroke (n = 41), and other associated neurological illnesses (n = 38). The remaining 492 patients fulfilled inclusion criteria and were informed about the trial design and objectives. Of these, 138 did not consent to the study for a variety of reasons.
A total of 354 patients gave consent to participate in the study and were scheduled to undergo PSG. Of these 354 patients, 116 underwent PSG. Seven patients died before their scheduled PSG, and 231 patients did not report for their scheduled PSG. The reasons for the large number of patients not participating in the study after initial consent included: refusal due to change in hospital chosen for follow-up, disinterest in pursuing secondary prevention in the face of severe disability, lack of availability of caregivers who could accompany them, new comorbidities, and despondence resulting from dealing with poor stroke outcome. The patient flow for the study is presented in Figure 1.
Figure 1. Flow chart demonstrating details of various steps of the randomized controlled study.
Symbols defined as follows: * = cardiac valve endocarditis, congestive heart failure, atrial fibrillation, Parkinson disease, ** = had to consult their primary care physician or already decided to consult another hospital or physician for their treatment, *** = bad weather conditions, distance, other family commitments necessitating withdrawal from study. AHI = apnea-hypopnea index, CPAP = continuous positive airway pressure, GCS = Glasgow Coma Scale, PSG = polysomnography.
Demographic and Clinical Details
Patients Who Qualified for Inclusion (n = 492)
The mean age of patients who met inclusion criteria was 57.05 ± 13.69 years and 360 (73.17%) were male. A total of 300 patients had ischemic and 192 had hemorrhagic stroke. Patients who did not give consent were significantly older than those who gave consent (64.31 ± 15.04 versus 54, 22 ± 12.01).
Patients Who Consented to Participate (n = 354)
The mean age of patients who gave consent was 54.22 ± 12.01 years and 278 (78.53%) were male. The mean Barthel Index of patients at the time of recruitment (6 weeks to 6 months) was 65 ± 41 and median was 75 years (range 35–100 years). See Table S1 in the supplemental material.
Patients Who Underwent PSG (n = 116)
Mean age of patients who reported for and underwent PSG, was 54.58 ± 12.73 years and 92 (79.31%) were male. The mean Barthel Index of patients at the time of PSG evaluation (> 6 weeks from stroke ictus) was 65 ± 41 (range 35–100). See Table S1.
Stroke was confirmed by MRI in 13 patients and by CT scanning in 57 patients.
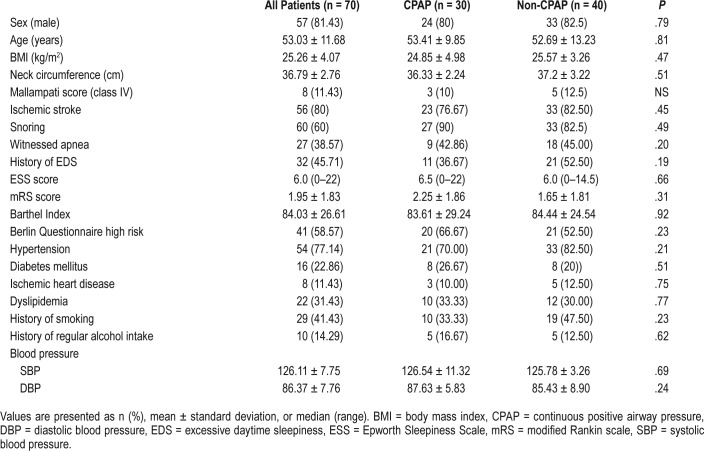
A total of 70 patients were randomized, with a mean age of 53.03 ± 11.68 years and 57 (81.43%) were males. Fifty-six patients (80%) had ischemic stroke, out of which 41 (73.21%) had large vessel and 15 (26.79%) had small vessel involvement. Two patients had infratentorial stroke located in the pons and medulla, respectively, and the remaining patients had supratentorial strokes. All patients with hemorrhagic stroke (n = 14) had supratentorial bleeds. The mean body mass index was 25.3 ± 4.07 kg/m2. Other clinical characteristics (Table 1) as well as scores on neuropsychological assessment (Table 2) were similar among patients in both treatment arms.
Table 1.
Baseline clinical and demographic details of patients randomized to the CPAP and non-CPAP treatment arms.
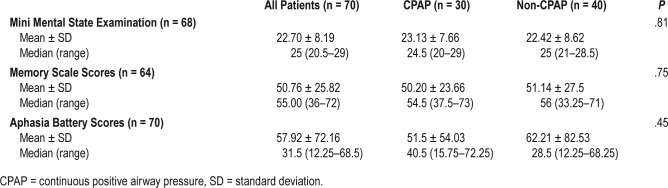
Table 2.
Baseline neuropsychological assessment.
Observations From PSG
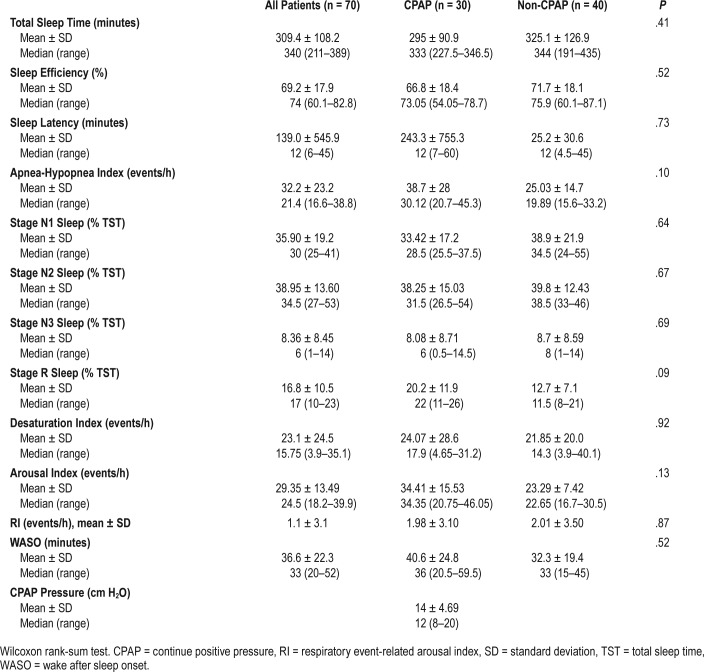
A total of 116 patients underwent PSG. Of these, 83 (72.42%) had an AHI > 15 events/h (moderate to severe OSA) and were eligible for randomization (Table 3).
Table 3.
Baseline polysomnography parameters (at least 6 weeks from stroke ictus) among patients randomized to receive CPAP treatment (CPAP group) or best medical treatment (non-CPAP group).
Randomization
Twelve patients withdrew consent and one patient died of acute pancreatitis after PSG but before randomization. Hence, 70 patients were randomized according to a computerized random number table. Thirty-four patients were randomized to the CPAP arm and 36 to the non-CPAP arm. Three of 33 patients (9%) were nonadherent and refused to use the CPAP device, because of various reasons such as motor disability, poor cognitive ability, and aphasia. Three patients crossed over during the study, at 1 month, 3.5 months, and 15 days from randomization, respectively. One patient was randomized to the CPAP arm but did not show any significant improvement on CPAP, but showed 50% reduction in AHI at higher pressures (25/21) on treatment using a bilevel positive airway pressure device. Therefore, these four patients were considered to have treatment arm crossover and were followed up in the non-CPAP arm. Final analysis was carried out for 70 patients: 30 in the CPAP arm and 40 in the non-CPAP arm. Crossover analysis was not carried out because the sample size was small. Three patients were observed to have one or more central apneas during their diagnostic studies. The mean central apnea index was 0.02 ± 0.11 events/h during titration studies, and no patients were found to have treatment-emergent central apnea.
CPAP Adherence
To ensure CPAP adherence, patients enrolled in the CPAP group as well as their caregivers were counseled about OSA and its consequences. Sessions were also held for training in mask application, ramp function, and cleaning of the device. Close follow-up was kept for each patient who was on the CPAP arm by telephone conversation by sleep technologists and support staff at least once per month. Patients were advised or counseled by the sleep physician if they reported any concerns or queries. Apart from this, immediate family members or caregivers were asked to keep a close watch on CPAP use and periodically report about adherence. The most common problem reported was inability to use CPAP because of independent handling of the device and lack of learning capability due to cognition, which improved gradually with improvement in the patient's condition. The median time taken for CPAP acceptability and acclimation from CPAP prescription was 34 days (range: 0–150). Some patients did encounter difficulty in tolerating the CPAP device, reporting lack of understanding of the device, dry mouth, anxiety about using the device, involuntary removal of the mask during sleep, and aerophobia. Patients were advised accordingly (Table S3 in the supplemental material). Average number of hours of CPAP use in the CPAP group was 4.2 ± 1.32 h/night and the average percentage of nights CPAP was used was 76 ± 22.8%. The percentage of nights CPAP was used for more than 4 hours was 58.9 ± 19.9%. The CPAP efficacy was good, with a mean AHI of 1.6 ± 0.9 events/h during use. See Table S2 in the supplemental material.
Outcomes in the CPAP Versus Non-CPAP Groups
New Vascular Events
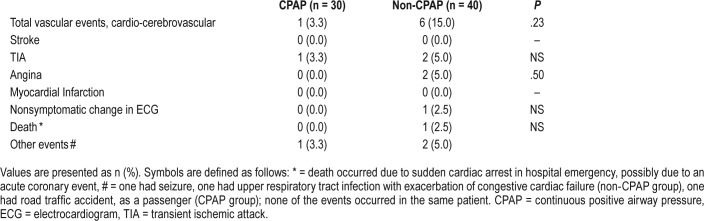
There was a statistically nonsignificant difference between the number of vascular events (6 [15%]) in the non-CPAP group at 12-month follow-up, as compared to a single event (3.3%) noted in the CPAP group. See Table 4.
Table 4.
Incidence of new vascular events in patients randomized to CPAP and non-CPAP groups at 12-month follow-up.
Stroke
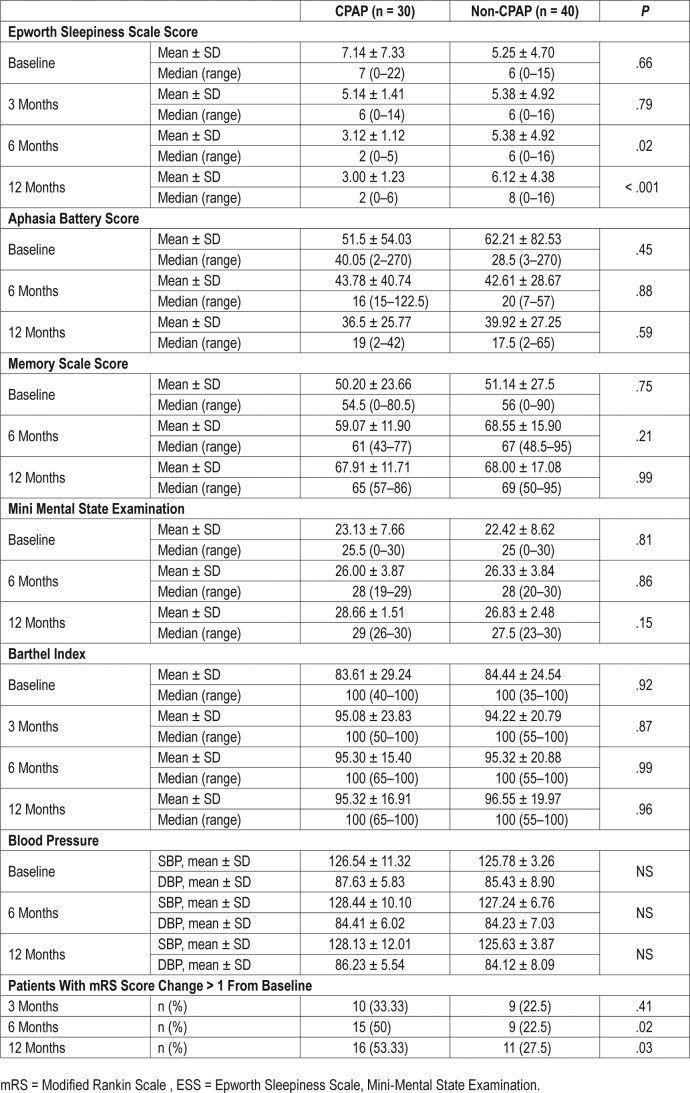
The baseline Barthel Index scores were improved in both groups. No significant differences were observed between the mean Barthel Index scores in both groups.
At 6-month follow-up, 15 patients (50%) in the CPAP arm compared with 9 patients (22%) in the non-CPAP arm showed improvement on the mRS by ≥ 1 point.28 At the 12-month follow-up, 16 patients (53.3%) in the CPAP arm showed such improvement compared with only 11 patients (27.5%) in the non-CPAP arm. These differences are statistically significant, as well as clinically meaningful and relevant (Table 5).
Table 5.
Secondary outcomes in the CPAP versus non-CPAP groups at follow-up.
Kaplan-Meier survival analysis showed statistically nonsignificant difference favoring the CPAP arm (Figure S1 in the supplemental material).
Cognitive Performance
Patients in both groups did not show any significant difference in their cognitive performance at any of the follow-up evaluations.
Blood Pressure Control
All patients except one had good blood pressure control29 with or without antihypertensive medications.
Other Parameters
No difference was found in other follow-up parameters except in the daytime sleepiness at the 6-month and 12-month follow-up. ESS scores at 6 months: 3.12 ± 1.12 versus 5.38 ± 4.92, P = .02, in the CPAP versus non-CPAP groups respectively. ESS scores at 12 months: 3.00 ± 1.23 versus 6.12 ± 4.38, P < .001, in the CPAP versus non-CPAP groups, respectively.
DISCUSSION
This study is an important addition to the literature on the effect of CPAP treatment on patients with OSA and stroke. Our results indicate that new vascular events may be better prevented—and significantly more patients may make good stroke recovery—with CPAP treatment as compared to only best medical treatment.
CPAP Effect on New Vascular Events
In the current study, we found more frequent occurrence of new vascular events in the group that was not treated with CPAP than the CPAP group, though this difference was not found to be statistically significant. Tomfohr et al. extensively reviewed the effect of CPAP on stroke survivors, and summarized that most published studies were observational.30 The randomized studies evaluating the difference in new vascular event occurrence rate with CPAP after stroke have either included patients with acute transient ischemic attack31 or have been conducted in an acute stroke setting,32 after which much sleep-disordered breathing could subside or evolve. Although the study on patients with transient ischemic attack found a significant difference brought about in the new vascular event occurrence rate, the latter study (on patients with acute stroke) found no significant difference in the occurrence of new vascular events. Martinez-Garcia et al. reported a significant decrease in mortality33 and the number of new vascular events34 through their 5- to 7-year follow-up observational studies. In the observational studies, the numbers included were similar to those in our study; however, they used level 3 PSG studies35 that may have biased them toward the inclusion and randomization of patients with more severe OSA than those in our study. They also did not carry out titration with CPAP prior to the initiation of nightly use by patients. In comparison with the aforementioned studies, ours is the first RCT with the most appropriate poststroke timing of PSG and randomization. We also used level 1 sleep studies and in-laboratory CPAP titration prior to nightly use by patients.
CPAP Effect on Stroke Outcome
In the current study, the stroke outcome was observed to be significantly better in the CPAP arm compared to the non-CPAP arm with the number of patients on CPAP who showed ≥ 1-point improvement on the mRS being significantly greater than the latter group at 6-month and 12-month follow-up. Our findings are similar to those observed by many other studies with different design, methodology, and primary outcome measures. A change in mRS score ≥ 1 was reported to be significantly more frequent among patients using CPAP in a CPAP treatment trial on patients with acute stroke.36 In a randomized open-label trial with 4 weeks of CPAP treatment for OSA, 3 weeks after stroke and after 4 weeks of treatment, significant improvement in stroke-related impairment was observed.37 In another randomized trial on patients with acute stroke, the group treated with auto-adjusting CPAP for OSA showed greater improvement on 30-day National Institutes of Health stroke scale scores.38,39 Wessendorf et al., in an observational intervention study, made similar observations in stroke outcomes, as measured through Barthel Index scores.40 Sandberg et al. observed no difference in Barthel Index scores between CPAP and non-CPAP groups at a 28-day follow-up of CPAP therapy in patients with acute stroke recruited 2 to 4 weeks after ictus.41
CPAP Effect on Neuropsychological and Cognitive Outcomes
We found no significant difference between the CPAP and the non-CPAP arms in the cognitive performance on follow-up. Similar observations about cognitive outcomes were reported by Ryan et al.37; however, they found a significant difference between effective components of depression. In other trials of patients with acute stroke, Sandberg et al. did not observe any significant difference in MMSE scores between the CPAP versus non-CPAP groups.41 In a recently published study, it was shown that cognitive outcome was better among patients with CPAP at 8-week follow-up.42
CPAP Effect on Blood Pressure Control
Although we found no difference in the blood pressure control between the two treatment arms, Wessendorf et al. did observe significant reduction in mean nocturnal blood pressure in patients who were adherent to CPAP. The study by Wessendorf et al. was performed in a rehabilitation center treating OSA, and patients started treatment with an auto-adjusting CPAP device at least 60 days following stroke ictus.40 In our study, most patients already received aggressive blood pressure reduction treatment apart from management of other modifiable risk factors during the immediate poststroke period prior to PSG and randomization. Hence, obtaining the additional benefit of blood pressure control via CPAP use was likely not possible in our study.
CPAP Adherence
We found good CPAP adherence in our study. The rate of acceptable adherence to CPAP—defined as CPAP use ≥ 75% of nights for ≥ 4 h/night—was 62.5% in the study by Bravata et al.38 and 40% in the study by Minnerup et al.39 In a recently published study from initial data from the multicenter SAVE study, adherence was found to be significantly lower during 12-month follow-up, and adherence could be predicted by first experience with the CPAP device.43 Close follow-up, periodic reminders, and counseling based on patients' adherence data were likely the most important factors determining good adherence in our study.
Strengths and Limitations
RCT design, appropriate timing at randomization, and in-laboratory diagnostic and titration PSG with meticulous follow-up and CPAP adherence confirmation were the main strengths of our study.
There are some limitations that remained in this study. The younger age and male dominance of the population included represents the general presenting characteristics of patients from India with stroke44 in our study. It may affect the general-izability of our results to other ethnic populations.
The choice of tests for cognitive performance was based mainly on applicability to an Indian (especially North Indian) population. The memory and language scales used were the only scales validated in a majority Hindi-speaking population at the inception of the study. This may have resulted in lower sensitivity of the cognitive measurement tools. This may also be a potential cause for lack of significant association of CPAP use with change in cognitive performance. Poststroke depression may be highly prevalent and lack of adjustment for depression and educational level also remains a limitation of this study.
There could be a possible sampling bias as a large number of patients did not give consent or report for PSG after consenting. The adherence to CPAP in the CPAP arm is higher than what has recently been reported in other larger clinical trials in stroke populations, so it is possible that subjects may not be representative of larger stroke populations. At the same time, another possible limitation is the smaller sample size than anticipated through an a priori power analysis that had shown 80 subjects in each arm to be the required amount for a power of 80%. Various reasons for this have been stated earlier. Future multicenter studies using this methodology would certainly have a great effect on transforming management of patients with stroke after the acute phase.
The low incidence of new vascular events, a favorable point resulting possibly from aggressive risk factor management, also reduced the strength of the main conclusions of this study.
CONCLUSIONS
The treatment of OSA through the use of CPAP in patients with first-ever arterial stroke, starting more than 6 weeks from the stroke ictus, appears to have a beneficial effect in reduction of incidence of new vascular events and it significantly improves stroke recovery at 6- and 12-month follow-up. Careful selection of patients, manual CPAP titration, and close follow-up assist in good CPAP adherence.
DISCLOSURE STATEMENT
This study was supported by SERB, Department of Science and Technology, Government of India, New Delhi, India. All authors have seen and approved the manuscript. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors acknowledge the valuable help obtained from Prof. Kameshwar Prasad in planning the study design for this work. We sincerely acknowledge the help of Jyoti Katoch in coordinating patient follow-ups, data management, and secretarial support, and Bharat Singh and Umesh Chandra for being the technological backbones, acquiring and scoring polysomnography tests and titrating patients. Dr. Deepak Shrivastava's pre-submission review was helpful and is sincerely acknowledged. Author contributions: Anupama Gupta – Acquisition of data, data analysis, clinical outcome assessment, preparation of manuscript draft; Garima Shukla – Study concept, design, Study supervision, Data interpretation, manuscript editing and finalization; Mohammed Afsar – Acquisition of data – Neuropsychological assessment; Shivani Poornima – Acquisition of data – Neuropsychological assessment, data tabulation; Ravindra M Pandey – Statistical analysis of data; Vinay Goyal – Study methodology planning, selection of patients; Achal Srivastava – Study supervision, recruitment of patients; Deepti Vibha – Recruitment and selection of patients, ECG interpretation on follow up; Madhuri Behari – Study concept and design, important intellectual contribution. Statistical analysis conducted by Prof. Ravindra M Pandey and his team, Department of Biostatistics, All India Institute of Medical Sciences, New Delhi.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- AI
arousal index
- BMI
body mass index
- CPAP
continuous positive airway pressure
- DI
desaturation index
- EDS
excessive daytime sleepiness
- ESS
Epworth Sleepiness Scale
- mRS
modified Rankin scale
- OSA
obstructive Sleep apnea
- PSG
polysomnography
- RCT
randomized control trial
- REM
rapid eye movement
- RERA
respiratory event-related arousal
- RI
RERA index
- TST
total sleep time
- WASO
wake after sleep onset
REFERENCES
- 1.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 2.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2010;182(2):269–277. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arzt M, Young T, Peppard PE, et al. Dissociation of obstructive sleep apnea from hypersomnolence and obesity in patients with stroke. Stroke. 2010;41(3):e129–e134. doi: 10.1161/STROKEAHA.109.566463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peppard PE. Is obstructive sleep apnea a risk factor for hypertension?— differences between the Wisconsin Sleep Cohort and the Sleep Heart Health Study. J Clin Sleep Med. 2009;5(5):404–405. [PMC free article] [PubMed] [Google Scholar]
- 5.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 6.Sahlin C, Sandberg O, Gustafson Y, et al. Obstructive sleep apnea is a risk factor for death in patients with stroke: a 10-year follow-up. Arch Intern Med. 2008;168(3):297–301. doi: 10.1001/archinternmed.2007.70. [DOI] [PubMed] [Google Scholar]
- 7.Loke YK, Brown JW, Kwok CS, Niruban A, Myint PK. Association of obstructive sleep apnea with risk of serious cardiovascular events a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2012;5(5):720–728. doi: 10.1161/CIRCOUTCOMES.111.964783. [DOI] [PubMed] [Google Scholar]
- 8.Mohsenin V, Valor R. Sleep apnea in patients with hemispheric stroke. Arch Phys Med Rehabil. 1995;76(1):71–76. doi: 10.1016/s0003-9993(95)80046-8. [DOI] [PubMed] [Google Scholar]
- 9.Dyken ME, Im KB. Obstructive sleep apnea and stroke. Chest. 2009;136(6):1668–1677. doi: 10.1378/chest.08-1512. [DOI] [PubMed] [Google Scholar]
- 10.Yaggi H, Mohsenin V. Obstructive sleep apnoea and stroke. Lancet Neurol. 2004;3(6):333–342. doi: 10.1016/S1474-4422(04)00766-5. [DOI] [PubMed] [Google Scholar]
- 11.Kaneko Y, Hajek VE, Zivanovic V, Raboud J, Bradley TD. Relationship of sleep apnea to functional capacity and length of hospitalization following stroke. Sleep. 2003;26(3):293–297. doi: 10.1093/sleep/26.3.293. [DOI] [PubMed] [Google Scholar]
- 12.Good DC, Henkle JQ, Gelber D, Welsh J, Verhulst S. Sleep-disordered breathing and poor functional outcome after stroke. Stroke. 1996;27(2):252–259. doi: 10.1161/01.str.27.2.252. [DOI] [PubMed] [Google Scholar]
- 13.Harbison J, Ford GA, James OF, Gibson GJ. Sleep-disordered breathing following acute stroke. QJM. 2002;95(11):741–747. doi: 10.1093/qjmed/95.11.741. [DOI] [PubMed] [Google Scholar]
- 14.Sandberg O, Franklin KA, Bucht G, Gustafson Y. Sleep apnea, delirium, depressed mood, cognition, and ADL ability after stroke. J Am Geriatr Soc. 2001;49(4):391–397. doi: 10.1046/j.1532-5415.2001.49081.x. [DOI] [PubMed] [Google Scholar]
- 15.Turkington PM, Elliott MW. Sleep disordered breathing following stroke. Monaldi Arch Chest Dis. 2004;61(3):157–161. doi: 10.4081/monaldi.2004.695. [DOI] [PubMed] [Google Scholar]
- 16.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 17.Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352(12):1206–1214. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- 18.Gami AS, Olson EJ, Shen WK, et al. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J Am Coll Cardiol. 2013;62(7):610–616. doi: 10.1016/j.jacc.2013.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martínez-García MÁ, Galiano-Blancart R, Román-Sánchez P, Soler-Cataluna JJ, Cabero-Salt L, Salcedo-Maiques E. Continuous positive airway pressure treatment in sleep apnea prevents new vascular events after ischemic stroke. Chest. 2005;128(4):2123–2129. doi: 10.1378/chest.128.4.2123. [DOI] [PubMed] [Google Scholar]
- 20.Sforza E, Roche F. Chronic intermittent hypoxia and obstructive sleep apnea: an experimental and clinical approach. Hypoxia. 2016;4:99–108. doi: 10.2147/HP.S103091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daurat A, Sarhane M, Tiberge M. [Obstructive sleep apnea syndrome and cognition: a review] Neurophysiol Clin. 2016;46(3):201–215. doi: 10.1016/j.neucli.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Tobaldini E, Costantino G, Solbiati M, et al. Sleep, sleep deprivation, autonomic nervous system and cardiovascular diseases. Neurosci Biobehav Rev. 2017;74(Pt B):321–329. doi: 10.1016/j.neubiorev.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4(2):157–171. [PMC free article] [PubMed] [Google Scholar]
- 24.Collin C, Wade DT, Davies S, Horne V. The Barthel ADL Index: a reliability study. Int Disabil Stud. 1988;10(2):61–63. doi: 10.3109/09638288809164103. [DOI] [PubMed] [Google Scholar]
- 25.Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials. Stroke. 2007;38(3):1091–1096. doi: 10.1161/01.STR.0000258355.23810.c6. [DOI] [PubMed] [Google Scholar]
- 26.Pershad D, Wig NN. P.G.I. Memory Scale: a normative study on elderly subjects [abstract] Indian J Clin Psychol. 1977;4(1):6–8. [Google Scholar]
- 27.Nehra A, Pershad D, Sreenivas V. Indian Aphasia battery: tool for specific diagnosis of language disorder post stroke. J Neurol Sci. 2013;333(suppl 1):e165. [Google Scholar]
- 28.Lai SM, Duncan PW. Stroke recovery profile and the Modified Rankin assessment. Neuroepidemiology. 2001;20(1):26–30. doi: 10.1159/000054754. [DOI] [PubMed] [Google Scholar]
- 29.Macaskill P, Glasziou PP, Irwig L, Aronson J. Control charts and control limits in long-term monitoring. In: Glasziou PP, Irwing L, Aronson JK, editors. Evidence-Based Medical Monitoring: From Principles to Practice. Boston, MA: BMJ Books; 2008. [Google Scholar]
- 30.Tomfohr LM, Hemmen T, Natarajan L, et al. Continuous positive airway pressure for treatment of obstructive sleep apnea in stroke survivors: what do we really know? Stroke. 2012;43(11):3118–3123. doi: 10.1161/STROKEAHA.112.666248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bravata DM, Concato J, Fried T, et al. Auto-titrating continuous positive airway pressure for patients with acute transient ischemic attack: a randomized feasibility trial. Stroke. 2010;41(7):1464–1470. doi: 10.1161/STROKEAHA.109.566745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parra O, Sánchez-Armengol Á, Capote F, et al. Efficacy of continuous positive airway pressure treatment on 5-year survival in patients with ischaemic stroke and obstructive sleep apnea: a randomized controlled trial. J Sleep Res. 2015;24(1):47–53. doi: 10.1111/jsr.12181. [DOI] [PubMed] [Google Scholar]
- 33.Martínez-García MÁ, Soler-Cataluña JJ, Ejarque-Martínez L, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med. 2009;180(1):36–41. doi: 10.1164/rccm.200808-1341OC. [DOI] [PubMed] [Google Scholar]
- 34.Martínez-García MA, Campos-Rodríguez F, Soler-Cataluña JJ, Catalán-Serra P, Román-Sánchez P, Montserrat JM. Increased incidence of nonfatal cardiovascular events in stroke patients with sleep apnoea: effect of CPAP treatment. Eur Respir J. 2012;39(4):906–912. doi: 10.1183/09031936.00011311. [DOI] [PubMed] [Google Scholar]
- 35.Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3(7):737–747. [PMC free article] [PubMed] [Google Scholar]
- 36.Parra O, Sánchez-Armengol A, Bonnin M, et al. Early treatment of obstructive apnoea and stroke outcome: a randomised controlled trial. Eur Respir J. 2011;37(5):1128–1136. doi: 10.1183/09031936.00034410. [DOI] [PubMed] [Google Scholar]
- 37.Ryan CM, Bayley M, Green R, Murray BJ, Bradley TD. Influence of continuous positive airway pressure on outcomes of rehabilitation in stroke patients with obstructive sleep apnea. Stroke. 2011;42(4):1062–1067. doi: 10.1161/STROKEAHA.110.597468. [DOI] [PubMed] [Google Scholar]
- 38.Bravata DM, Concato J, Fried T, et al. Continuous positive airway pressure: evaluation of a novel therapy for patients with acute ischemic stroke. Sleep. 2011;34(9):1271–1277. doi: 10.5665/SLEEP.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minnerup J, Ritter MA, Wersching H, et al. Continuous positive airway pressure ventilation for acute ischemic stroke a randomized feasibility study. Stroke. 2012;43(4):1137–1139. doi: 10.1161/STROKEAHA.111.637611. [DOI] [PubMed] [Google Scholar]
- 40.Wessendorf TE, Wang M, Thilmann AF, Sorgenfrei U, Konietzko N, Teschler H. Treatment of obstructive sleep apnoea with nasal continuous positive airway pressure in stroke. Eur Respir J. 2001;18(4):623–629. doi: 10.1183/09031936.01.00057201. [DOI] [PubMed] [Google Scholar]
- 41.Sandberg O, Franklin KA, Bucht G, Eriksson S, Gustafson Y. Nasal continuous positive airway pressure in stroke patients with sleep apnoea: a randomized treatment study. Eur Respir J. 2001;18(4):630–634. doi: 10.1183/09031936.01.00070301. [DOI] [PubMed] [Google Scholar]
- 42.Aaronson JA, Hofman WF, van Bennekom CA, et al. Effects of continuous positive airway pressure on cognitive and functional outcome of stroke patients with obstructive sleep apnea: a randomized controlled trial. J Clin Sleep Med. 2015;12(4):533–541. doi: 10.5664/jcsm.5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chai-Coetzer CL, Luo YM, Antic NA, et al. Predictors of long-term adherence to continuous positive airway pressure therapy in patients with obstructive sleep apnea and cardiovascular disease in the SAVE study. Sleep. 2013;36(12):1929–1937. doi: 10.5665/sleep.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pandian JD, Sudhan P. Stroke epidemiology and stroke care services in India. J Stroke. 2013;15(3):128–134. doi: 10.5853/jos.2013.15.3.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.