Abstract
Study Objectives:
To measure prevalence and severity of third trimester obstructive sleep apnea and evaluate postpartum resolution. To assess a novel biomarker for screening for obstructive sleep apnea in pregnancy.
Methods:
This prospective observational study was performed at Wake Forest School of Medicine obstetrics clinics between April 2014 and December 2015. Fractional exhaled nitric oxide measurements and sleep studies were obtained and compared at 32 0/7 to 35 6/7 weeks gestation and postpartum. Exhaled nitric oxide and risk factors for the development of gestational sleep apnea were evaluated for predictive ability independently and in screening models.
Results:
Of 76 women enrolled, 73 performed valid sleep studies in pregnancy and 65 had an additional valid study 6 to 15 weeks postpartum. Twenty-four women (37%) had gestational sleep apnea compared with 23 (35%) with postpartum sleep apnea (P > .99). Eight of 11 women (73%) retested 6 to 8 months postpartum had persistent sleep apnea. Exhaled nitric oxide had moderate discrimination screening for sleep apnea in pregnancy (area under the receiver operating characteristic curve = 0.64). A model utilizing exhaled nitric oxide, pregnancy-specific screening, and Mallampati score improved ability to identify women at risk for gestational sleep apnea (sensitivity = 46%, specificity = 91% and likelihood ratio = 5.11, area under receiver operating characteristic curve = 0.75).
Conclusions:
Obstructive sleep apnea is common in the early postpartum period and often persisted at least 6 months. Exhaled nitric oxide as a sole biomarker to screen for sleep apnea in pregnancy has only modest discrimination. Combined with additional parameters sensitivity and specificity improved.
Clinical Trial Registration:
Registry: ClinicalTrials.gov, Identifier: NCT02100943, Title: Exhaled Nitric Oxide as a Biomarker of Gestational Obstructive Sleep Apnea and Persistence Postpartum, URL: https://clinicaltrials.gov/ct2/show/NCT02100943
Citation:
Street LM, Aschenbrenner CA, Houle TT, Pinyan CW, Eisenach JC. Gestational obstructive sleep apnea: biomarker screening models and lack of postpartum resolution. J Clin Sleep Med. 2018;14(4):549–555.
Keywords: exhaled nitric oxide, gestational obstructive sleep apnea, postpartum, pregnancy
BRIEF SUMMARY
Current Knowledge/Study Rationale: Obstructive sleep apnea is common during pregnancy but postpartum persistence and course are unknown. Additionally, current screening methods for gestational sleep apnea are poor.
Study Impact: As knowledge about incidence and ramifications of obstructive sleep apnea in pregnancy for both the mother and infant continues to grow, it becomes increasingly important to identify a feasible screen for gestational sleep apnea. Additionally, knowledge of the expected postpartum course is important for advising women on appropriate postpartum follow-up.
INTRODUCTION
Obstructive sleep apnea (OSA) is estimated to occur in 8.7% of women aged 30 to 49 years.1 Patients with OSA have episodes of upper airway collapse leading to apnea (cessation of airflow for ≥ 10 seconds) or hypopnea (reduction of airflow for ≥ 10 seconds accompanied by an arousal or oxyhemoglobin desatu-ration). Obesity has been long recognized as a risk factor for OSA, but more recently pregnancy has also been recognized as a risk factor, with prevalence of 11% in the first trimester increasing throughout gestation to 27% in the third trimester.2 Gestational OSA has been linked to adverse pregnancy outcomes such as hypertensive disorders of pregnancy and gestational diabetes.3 Whereas other medical complications of pregnancy including gestational diabetes and preeclampsia are associated with development of type 2 diabetes and long-term cardiovascular risk,4,5 whether gestational OSA predisposes to long term OSA and its attendant long-term morbidity is unknown. As continuous positive airway pressure (CPAP) treatment can ameliorate the long-term risks of OSA, knowing the postpartum course of gestational OSA is vital from a public health perspective.
Despite high prevalence in research studies, practical diagnosis of gestational OSA using polysomnography is hampered by expense and lack of availability in many areas. OSA screening tools used routinely outside pregnancy such as the Berlin questionnaire, Epworth Sleepiness Scale, and STOP-BANG questionnaire have only poor predictive value during gestation.6–8 Many positive screens are falsely positive6 because of normal pregnancy symptoms. A sign and symptom scoring method for OSA screening during pregnancy recently described by Facco et al.7 has shown promise, but to date has not been replicated.
OSA is characterized by recurrent collapse of the upper airway, which presumably leads to increased inflammation. The fraction of exhaled nitric oxide (FENO) is an easily obtained marker of upper airway inflammation. As such, FENO has been proposed as a biomarker for OSA in nonpregnant adults, with conflicted reports of utility.9–19 However, to date FENO has not been evaluated as a biomarker for gestational OSA. Because pregnancy involves multiple physiologic changes, a biomarker may perform differently in this state. The primary objective of this study was to determine the prevalence of OSA in the third trimester of pregnancy and subsequently in the postpartum period at a time (6 to 15 weeks) when physiologic and pathophysiologic changes of pregnancy have largely resolved. In addition, we sought to determine the utility of FENO as a biomarker for gestational and postpartum OSA. Finally, we sought to replicate the previously described pregnancy-specific screening tool for OSA by Facco et al. (Facco score).7
METHODS
Study Design and Setting
This is a prospective observational study to evaluate the prevalence of OSA in pregnant women in the third trimester and again postpartum and the utility of FENO as an OSA bio-marker during these times. Women were recruited from prenatal outpatient clinics at the Wake Forest School of Medicine Department of Obstetrics and Gynecology. These clinics include general obstetric and maternal fetal medicine practices. Women were approached between April 2014 and April 2015 for enrollment at a routine visit by study personnel if they were between 32 0/7 weeks and 35 6/7 weeks gestation and anticipated delivery at the Maya Angelou Center for Women's Health and Wellness at Forsyth Medical Center in Winston-Salem, North Carolina. Women were excluded if they had active substance abuse, current treatment for OSA, age younger than 18 years, inability to speak/read English proficiently, or cardiac conditions that excluded them from using the device to measure exhaled nitric oxide. The institutional review board at both Wake Forest School of Medicine and Forsyth Medical Center approved the protocol and all participants provided written informed consent.
Upon enrollment, the women were taught how to use the Watch-PAT200 device (Itamar Medical Ltd., Caesarea, Israel) for overnight, home evaluation of OSA. This device has been previously validated in pregnancy against overnight polysomnography20 and provides a cost-effective option to evaluate for OSA. The use of Watch-PAT200 is also practical for pregnant women, many of whom have other small children to care for at home. The Watch-PAT200 is worn on the wrist to provide accelerometry and utilizes a plethysmographic-based finger-mounted probe, to measure the peripheral arterial tone and pulse oximetry. A proprietary algorithm is applied to identify apnea and hypopnea and periods of sleep. The entire study period can be viewed and the automatically detected events can be revised manually.21 The sleep study was uploaded and analyzed by the proprietary zzzPAT software program (Version 4.3.61, Itamar Medical Ltd., Caesarea, Isreal). The respiratory event index (REI) was calculated by the software and an REI ≥ 5 events/h was considered diagnostic for OSA. Moderate to severe OSA was diagnosed if REI ≥ 15 events/h.
Women completed the Berlin Questionnaire,22 a survey used outside of pregnancy to assess OSA risk that evaluates symptoms such as daytime fatigue and snoring. Participants additionally had their neck circumference measured, and upper airway evaluated using a four-point score developed by Mallampati.23 The next morning prior to 10:00 am the patient returned the Watch-PAT200 and FENO was measured using the NIOX-MINO device (Aerocrine AB, Solna, Sweden). The NIOX-MINO has been validated for clinical use against the gold standard chemiluminescence analyzer.24
The NIOX MINO is a portable device with a filtered input tube into which the subject exhales slowly after taking a breath from residual capacity to total lung capacity. A visual clue provides feedback for the subject to regulate expiratory flow. FENO is measured with a detection limit of 5 ppb and an accuracy of ± 10 ppb or maximum of 10%.
Questionnaires and measurements including sleep study and FENO were repeated at 6 to 15 weeks postpartum. If the woman did not attend her postpartum visit she was contacted by phone three times to attempt to obtain follow-up. Those women who were positive for OSA at the postpartum visit were invited for an additional visit at 6 to 8 months postpartum. Should the patient be identified as having OSA, both the patient and her provider were notified by the primary investigator. They were both notified that CPAP is the current standard treatment for OSA and were provided with the contact information for the Wake Forest School of Medicine facilities that can provide titration.
The patients' medical records were reviewed to obtain and verify antepartum, intrapartum, and postpartum information about the study participant's medical history as well as documentation of a live birth and neonatal outcomes from available medical records. To ensure accuracy, medical records were abstracted by only the principal investigator and co-investigators who were trained personnel with expertise in obstetric and perioperative research. All controversial categorizations were flagged and reviewed by the primary investigator.
The primary outcomes were the prevalence of OSA in this population both during and after pregnancy and FENO values and their relationship to the woman's OSA status at these time points. Predetermined secondary outcomes included evaluating the sensitivity and specificity of the Facco score7 for gestational OSA, risk factors for development of OSA in pregnancy and postpartum persistence, and pregnancy outcomes in the presence of gestational OSA. The variables comprising the Facco score are age, body mass index, preexisting hypertension, and self-report of snoring 3 or more nights per week.
We attempted to limit selection bias by recruiting from both general obstetric and maternal fetal medicine clinics. Women not attending their postpartum visit were contacted to set up the postpartum study evaluation. Women who elected for CPAP treatment after the initial study were asked to refrain from its use the evening of subsequent studies to decrease confounding.
The study was powered to test the hypothesis that the prevalence of gestational OSA was reduced postpartum to that near the population prevalence of nonpregnant women. We anticipated a prevalence of 25% during the third trimester,2,7,25 reducing to 6% postpartum.1,26,27 A sample size of 65 individuals was determined to provide 80% power to detect the hypothesized difference. Because of historical high loss to follow-up in the postpartum period in routine prenatal care, we anticipated the need to recruit up to 90 individuals in order to have 65 with complete datasets. Ultimately 76 patients were enrolled.
Statistical Analysis
Analyses were conducted using IBM SPSS Statistics version 22 (IBM Corp, Armonk, New York, United States). Baseline characteristics were summarized with frequencies and percentages for categorical data and mean ± standard deviation or median plus interquartile ranges for continuous data as appropriate. An exact McNemar test was run to determine if there was a difference in the proportion of participants testing positive for OSA during the third trimester of pregnancy versus postpartum. To account for attrition bias, only women with complete datasets were included in this analysis. A generalized estimating equation was performed to examine the difference in FENO values in the third trimester of pregnancy in women with and without OSA. The effect of time and interaction between time and FENO were evaluated. In order to determine the utility of FENO, the Facco score, and Mallampati score as risk factors for development of OSA in pregnancy, a multivariable logistic regression was conducted. Confounding effects were controlled for in the model. An area under the receiver operating characteristic (ROC) curve was then calculated to assess the discrimination of the model.
Reproducible Science
The full clinical and statistical protocols are available from Dr. Eisenach (jimeisenach@gmail.com).
RESULTS
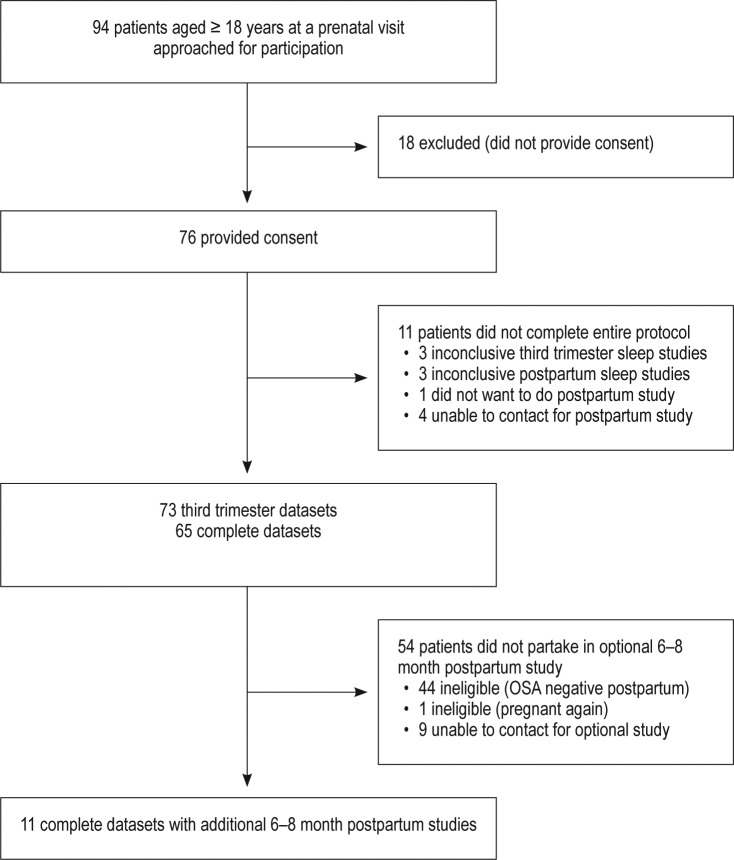
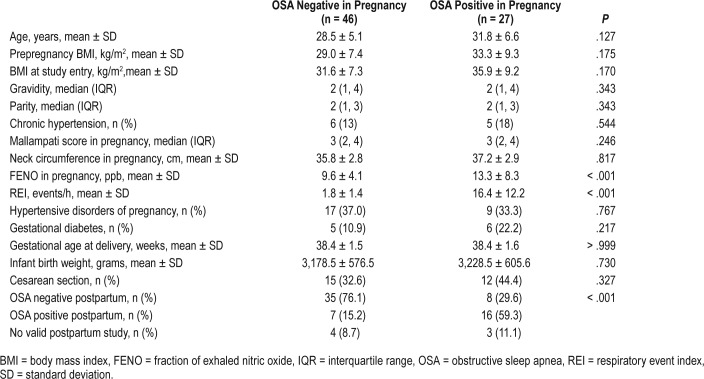
Valid third trimester sleep data and FENO measurements were obtained in 73/76 participants (96%) (Figure 1). Valid sleep data and FENO measurements at both the third trimester and postpartum time points (mean 7.4 ± 2.1 weeks) were obtained in 65/76 participants (86%). Maternal demographics such as age and body mass index were compared and found to be similar between women with and without OSA during the third trimester (Table 1). Additionally, as chronic hypertension is a common risk factor for OSA, it was evaluated and found to be similar between groups (P = .544). Pregnancy complications and neonatal birthweights were similar in women with and without OSA in the third trimester (Table 1). All 21 women with positive postpartum OSA studies were approached for an additional evaluation at 6 to 8 months postpartum and 11 of these agreed (52% of those with positive OSA studies in the initial postpartum period).
Figure 1. Study flow from approached patients to analysis sample.
OSA = obstructive sleep apnea.
Table 1.
Baseline characteristics and outcome of patients stratified by gestational OSA status.
Of the 65 women with valid data at both time points, 24 were positive for OSA during the third trimester of pregnancy (37%), and this proportion did not meaningfully decline at the first postpartum visit (23 subjects, 35%, P > .99). This reflected to some extent, however, a crossover, with eight participants with gestational OSA who tested negative postpartum counter-balanced by seven participants without gestational OSA who tested positive at the postpartum visit (Figure 2A). Of the eight participants whose OSA resolved after delivery, all except one had mild OSA, and of the seven participants in whom OSA developed postpartum, all had mild OSA (Figure 2A). The mean REI of women with gestational OSA was significantly higher both in pregnancy and postpartum in those women who did not experience postpartum resolution (Table 2). Interestingly, the FENO was lower in the women who continued to test positive for OSA postpartum. The demographics of the women who had gestational OSA did not differ between those who continued to test positive for OSA and those who tested negative postpartum (Table 2). Two women sought treatment with CPAP between the pregnancy and postpartum visits. They both refrained from wearing their CPAP device the night of the postpartum study. Of the women who were positive for OSA in the early postpartum period, 8 (73%) of those retested continued to be positive for OSA at 6 to 8 months postpartum (Figure 2B).
Figure 2. Participant-specific trajectory of REI during pregnancy and postpartum.
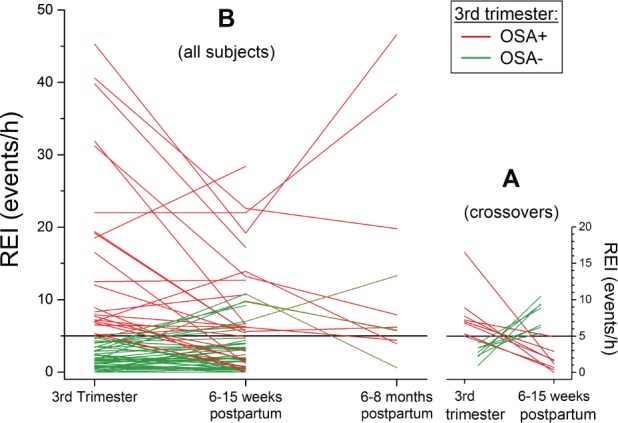
(A) Data from participants who met criteria for OSA at one of the two visits, coded for whether OSA was present during pregnancy (red) or not (green). (B) Data from all participants, including those who elected to return for testing at 6 to 8 months after delivery. OSA = obstructive sleep apnea, REI = respiratory event index.
Table 2.
Baseline characteristics and outcome of patients stratified by postpartum persistence or resolution of OSA.
FENO was associated with OSA only during pregnancy, with the generalized estimating equation showing a significant effect of time (P = .037) and a time versus FENO interaction (P = .023) (Figure 3A). The risk factors of FENO, Facco score, and Mallampati score for OSA during pregnancy were analyzed in the 73 participants with valid data during pregnancy. The logistic regression model was statistically significant, χ2(3) = 12.76, df = 3, P = .005. The model explained 22.7% (Nagelkerke R2) of the variance in OSA. Sensitivity was 46%, specificity was 91%, positive predictive value was 75%, and negative predictive value was 74%.
Figure 3.
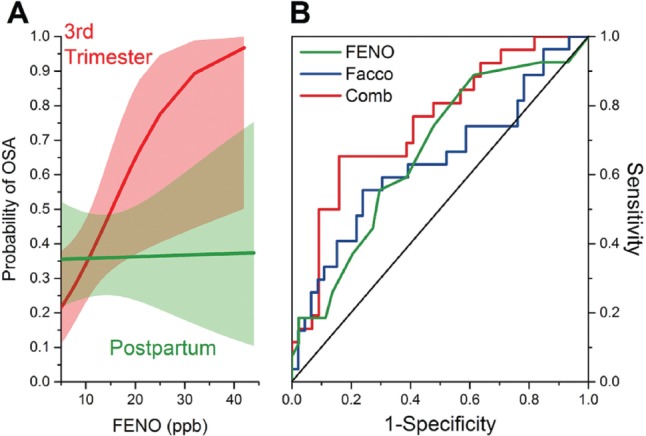
(A) Logistic regression relationship between probability of a diagnosis of obstructive sleep apnea based on FENO value in pregnancy (red) and postpartum (green; medians shown in lines with 95% CI). (B) ROC curves for diagnosis of OSA by FENO (green), a sign and symptom score developed by Facco (blue), and their combination (red). CI = confidence interval, FENO = fractional exhaled nitric oxide, OSA = obstructive sleep apnea, ROC = receiver operating characteristic.
Of the three predictor variables, two were statistically significant: FENO (P = .019) and Facco score (P = .020). In examining a stepwise regression, FENO accounted for 10.8% of the variance in OSA, Facco score 11.5%, and Mallampati score 0.4%. Neck circumference was not entered as a predictor because it was highly correlated with Facco score (Spearman ρ = 0.6). After controlling for confounding effects, the odds of testing positive for OSA increases by 13.5% for each part per breath increase in FENO and 4.3% for each 1-unit increase in Facco score. The area under the curve (AUC) was signifi-cant for FENO 0.638 (95% CI 0.501–0.776) and for Facco score 0.665 (0.501–0.776). The areas under the ROC curve of the model which included Facco score, Mallampati score, and FENO shows an AUC of 0.751 (0.632–0.870) (Figure 3B).
DISCUSSION
Population screening, diagnosis, and treatment of OSA remain difficult. The current data suggest ways to improve women's health through more effective screening during pregnancy, a time of high risk for OSA symptoms that persist for at least many months after delivery. Other diseases of pregnancy, gestational diabetes mellitus and preeclampsia, are routinely screened for in this population. Although these diseases typically resolve in the first weeks after delivery, they represent important predictors for the development of chronic diabetes and cardiovascular risk, respectively. Data from the current study suggest that gestational OSA differs from these diseases in that it may resolve considerably more slowly after delivery, but is potentially similar in that it may serve as a marker or predisposition to chronic OSA. This adds importantly to previous work that has to date focused on acute maternal and fetal risks of OSA during pregnancy. At a minimum, these data demonstrate ongoing OSA pathology for 2 to 3 months after delivery, and suggest that the prevalence of OSA 6 to 8 months after delivery approximates 20%.
Gestational OSA is common in the third trimester of pregnancy and occurred in 37% of our patients at this stage of gestation and was moderate to severe in 11% of study participants. This is slightly higher than previously published prevalence data despite limitations due to excluding women already undergoing treatment for OSA.2,25,28–32 Although there were some hints that OSA disease severity may diminish in the first 6 to 15 weeks after delivery, the overall prevalence of OSA was not reduced at this time. Data at 6 months, which should be considered preliminary, are consistent with a gradually decreasing severity of OSA in most subjects, but a prevalence that remains above the population average for adult women. These data add to increased emphasis in obstetrics to screen for OSA during pregnancy not only to potentially reduce OSA related disorders of pregnancy, but as an opportunity to make an early diagnosis of a chronic and treatable condition.
Increased likelihood of OSA during pregnancy has been speculated to reflect weight gain, upper airway edema due primarily to increased estrogen effects, increased airflow from the progesterone-induced increase in tidal volume, and altered sleeping position from the enlarging uterus. All of these conditions should rapidly resolve following delivery and are unlikely to explain the unchanged prevalence of OSA 7.4 weeks after delivery. It is conceivable that lactation effects, sleep deprivation, and fragmentation from the demands of neonatal care may explain the overall changed prevalence and the new-onset, mild OSA during this time period, but are less likely to explain the persistence of OSA at 6 to 8 months after delivery. Postpartum weight retention was minimal and did not differ between groups, making it unlikely to solely account for such a high rate of persistent OSA.
Screening for OSA in pregnancy is difficult because of the lack of sensitivity and specificity of tools developed in the non-pregnancy population. For example, the STOP-BANG questionnaire relies on advancing age and male sex as two of its eight criteria, and it is not surprising that it performed poorly. Similarly, fatigue is a common symptom in pregnancy, especially during the third trimester, and this and other scales such as the Epworth and Berlin scales that rely in part on this symptom are likely to lose specificity. This is the first replication of a pregnancy-specific screening tool developed by Facco et al.7 and not surprisingly, this tool performed less well in the current replication cohort (ROC AUC of 0.67) compared to that observed in the dataset from which it was derived (ROC AUC of 0.86). The lack of demographic differences such as age and weight in our population between women testing positive and negative both during pregnancy and postpartum further emphasizes the need for a novel screening approach.
The current data suggest that although a single-breath FENO provides only modest discrimination and is not clinically useful as a screen alone, it may be a useful adjunct to clinical characteristics to more effectively screen for OSA during pregnancy with high specificity. When combined with the Facco score and a simple airway assessment, FENO provided good discrimination. At a minimum, the screening model we have proposed may provide a useful adjunct to traditional screening options until a more sensitive screen in pregnancy is identified. FENO was not a useful screen in the postpartum period in our population, similar to the mixed literature on its performance in the general population.
Limitations of this study include a modest sample size from a single institution, lack of OSA sleep testing prior to pregnancy, and systematic follow-up remote from delivery in a small subset as a hypothesis-generating exercise. The study was not powered or intended to replicate the association between gestational OSA and adverse obstetric or neonatal outcomes. Patients in both groups in the current study were obese, reflecting both the high incidence of obesity in women in general and pregnant women in particular, but could limit the generalizability of these results to populations with more nonobese women.
In conclusion, gestational OSA is common, occurring in more than one of three women in the third trimester, fails to resolve in most these women in the first 2 to 3 months after delivery, and, preliminarily, that there is slow if any reduction in OSA prevalence 6 to 8 months after delivery. Women in whom OSA is diagnosed during pregnancy should be reevaluated for persistence of OSA postpartum. A recently described, pregnancy-specific OSA screening tool performed less well in this replication cohort than in the original description, but performed better than tools developed in general populations. Single-breath exhaled nitric oxide alone served as a fair biomarker for OSA during, but not after pregnancy. However, even when FENO was combined with the pregnancy-specific OSA screening tool and a simple airway assessment it remained a modest screening tool at best. Continued effort is needed to develop a simple screening option for OSA during pregnancy, a time of high risk for development of OSA in women, lasting at least months and potentially chronically after delivery.
DISCLOSURE STATEMENT
Work for this study was performed at Forsyth Medical Center and Wake Forest School of Medicine, Winston-Salem, North Carolina. All authors have seen and approved the manuscript. This research was partially supported by grant R37 GM48085 from the National Institutes of Health, Bethesda, Maryland to Dr. Eisenach. Dr. Eisenach has no competing interests related to the current manuscript. In the past 3 years he has consulted to pharmaceutical firms developing opioid and nonopioid analgesics: Adynxx and Tevo Pharmaceuticals. The other authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Regina Curry, RN and Lynne Harris, RN (both of the Department of Anesthesiology, Wake Forest School of Medicine, Winston-Salem, North Carolina) for their assistance in patient enrollment and data collection.
ABBREVIATIONS
- AUC
area under the curve
- CPAP
continuous positive area pressure
- FENO
fraction of exhaled nitric oxide
- OSA
obstructive sleep apnea
- REI
respiratory event index
- ROC
receiver operating characteristic
REFERENCES
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pien GW, Pack AI, Jackson N, Maislin G, Macones GA, Schwab RJ. Risk factors for sleep-disordered breathing in pregnancy. Thorax. 2014;69(4):371–377. doi: 10.1136/thoraxjnl-2012-202718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pamidi S, Pinto LM, Marc I, Benedetti A, Schwartzman K, Kimoff RJ. Maternal sleep-disordered breathing and adverse pregnancy outcomes: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014;210(1):52.e1–52.e14. doi: 10.1016/j.ajog.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 4.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 5.Irgens HU, Reisaeter L, Irgens LM, Lie RT. Long term mortality of mothers and fathers after pre-eclampsia: population based cohort study. BMJ. 2001;323(7323):1213–1217. doi: 10.1136/bmj.323.7323.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antony KM, Agrawal A, Arndt ME, et al. Obstructive sleep apnea in pregnancy: reliability of prevalence and prediction estimates. J Perinatol. 2014;34(8):587–593. doi: 10.1038/jp.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Facco FL, Ouyang DW, Zee PC, Grobman WA. Development of a pregnancy-specific screening tool for sleep apnea. J Clin Sleep Med. 2012;8(4):389–394. doi: 10.5664/jcsm.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tantrakul V, Sirijanchune P, Panburana P, et al. Screening of obstructive sleep apnea during pregnancy: differences in predictive values of questionnaires across trimesters. J Clin Sleep Med. 2015;11(2):157–163. doi: 10.5664/jcsm.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Depalo A, Carpagnano GE, Spanevello A, et al. Exhaled NO and iNOS expression in sputum cells of healthy, obese and OSA subjects. J Intern Med. 2008;263(1):70–78. doi: 10.1111/j.1365-2796.2007.01875.x. [DOI] [PubMed] [Google Scholar]
- 10.Olopade CO, Christon JA, Zakkar M, et al. Exhaled pentane and nitric oxide levels in patients with obstructive sleep apnea. Chest. 1997;111(6):1500–1504. doi: 10.1378/chest.111.6.1500. [DOI] [PubMed] [Google Scholar]
- 11.Chua AP, Aboussouan LS, Minai OA, Paschke K, Laskowski D, Dweik RA. Long-term continuous positive airway pressure therapy normalizes high exhaled nitric oxide levels in obstructive sleep apnea. J Clin Sleep Med. 2013;9(6):529–535. doi: 10.5664/jcsm.2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.JalilMirmohammadi S, Mehrparvar AH, Safaei S, Samimi E, Torab Jahromi M. The association between exhaled nitric oxide and sleep apnea: the role of BMI. Respir Med. 2014;108(8):1229–1233. doi: 10.1016/j.rmed.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Carpagnano GE, Spanevello A, Sabato R, Depalo A, Turchiarelli V, Foschino Barbaro MP. Exhaled pH, exhaled nitric oxide, and induced sputum cellularity in obese patients with obstructive sleep apnea syndrome. Transl Res. 2008;151(1):45–50. doi: 10.1016/j.trsl.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Fortuna AM, Miralda R, Calaf N, Gonzalez M, Casan P, Mayos M. Airway and alveolar nitric oxide measurements in obstructive sleep apnea syndrome. Respir Med. 2011;105(4):630–636. doi: 10.1016/j.rmed.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Culla B, Guida G, Brussino L, et al. Increased oral nitric oxide in obstructive sleep apnoea. Respir Med. 2010;104(2):316–320. doi: 10.1016/j.rmed.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Petrosyan M, Perraki E, Simoes D, et al. Exhaled breath markers in patients with obstructive sleep apnoea. Sleep Breath. 2008;12(3):207–215. doi: 10.1007/s11325-007-0160-8. [DOI] [PubMed] [Google Scholar]
- 17.Agusti AG, Barbe F, Togores B. Exhaled nitric oxide in patients with sleep apnea. Sleep. 1999;22(2):231–235. [PubMed] [Google Scholar]
- 18.Foresi A, Leone C, Olivieri D, Cremona G. Alveolar-derived exhaled nitric oxide is reduced in obstructive sleep apnea syndrome. Chest. 2007;132(3):860–867. doi: 10.1378/chest.06-3124. [DOI] [PubMed] [Google Scholar]
- 19.Hua-Huy T, Le-Dong NN, Duong-Quy S, Luchon L, Rouhani S, Dinh-Xuan AT. Increased alveolar nitric oxide concentration is related to nocturnal oxygen desaturation in obstructive sleep apnoea. Nitric Oxide. 2015;45:27–34. doi: 10.1016/j.niox.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 20.O'Brien LM, Bullough AS, Shelgikar AV, Chames MC, Armitage R, Chervin RD. Validation of Watch-PAT-200 against polysomnography during pregnancy. J Clin Sleep Med. 2012;8(3):287–294. doi: 10.5664/jcsm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Itamar Medical Ltd. Watch-PAT200é Operation Manual. Caesarea, Israel: Itamar Medical Ltd; 2009. [Google Scholar]
- 22.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 23.Mallampati SR, Gatt SP, Gugino LD, et al. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J. 1985;32(4):429–434. doi: 10.1007/BF03011357. [DOI] [PubMed] [Google Scholar]
- 24.Kim SH, Moon JY, Kwak HJ, et al. Comparison of two exhaled nitric oxide analyzers: the NIOX MINO hand-held electrochemical analyzer and the NOA280i stationary chemiluminescence analyzer. Respirology. 2012;17(5):830–834. doi: 10.1111/j.1440-1843.2012.02163.x. [DOI] [PubMed] [Google Scholar]
- 25.Louis J, Auckley D, Miladinovic B, et al. Perinatal outcomes associated with obstructive sleep apnea in obese pregnant women. Obstet Gynecol. 2012;120(5):1085–1092. doi: 10.1097/AOG.0b013e31826eb9d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yaggi HK, Strohl KP. Adult obstructive sleep apnea/hypopnea syndrome: definitions, risk factors, and pathogenesis. Clin Chest Med. 2010;31(2):179–186. doi: 10.1016/j.ccm.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 28.Champagne K, Schwartzman K, Opatrny L, et al. Obstructive sleep apnoea and its association with gestational hypertension. Eur Respir J. 2009;33(3):559–565. doi: 10.1183/09031936.00122607. [DOI] [PubMed] [Google Scholar]
- 29.Chen YH, Kang JH, Lin CC, Wang IT, Keller JJ, Lin HC. Obstructive sleep apnea and the risk of adverse pregnancy outcomes. Am J Obstet Gynecol. 2012;206(2):136.e131–136.e135. doi: 10.1016/j.ajog.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Facco FL, Ouyang DW, Zee PC, Grobman WA. Sleep disordered breathing in a high-risk cohort prevalence and severity across pregnancy. Am J Perinatol. 2014;31(10):899–904. doi: 10.1055/s-0033-1363768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olivarez SA, Maheshwari B, McCarthy M, et al. Prospective trial on obstructive sleep apnea in pregnancy and fetal heart rate monitoring. Am J Obstet Gynecol. 2010;202(6):552.e1–552.e7. doi: 10.1016/j.ajog.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Reid J, Skomro R, Gjevre J, Cotton D, Ward H, Olatunbosun O. Fetal heart rate monitoring during nocturnal polysomnography. Clin Exp Obstet Gynecol. 2011;38(2):123–125. [PubMed] [Google Scholar]