Abstract
Study Objectives:
Epidemiological associations have demonstrated the effects of long-term air pollution to obstructive sleep apnea (OSA) through a physiological mechanism linking particulate matter exposure to OSA. This study aimed to determine the relationship between bedroom environmental conditions, OSA severity, and sleep quality.
Methods:
Sixty-three participants were enrolled for an overnight polysomnography; OSA was diagnosed between May to August 2016. Personal characteristics and sleep quality were obtained by a face-to-face interview. Bedroom environments, including data on particulate matter with an aerodynamic diameter less than 10 μm (PM10), temperature, and relative humidity, were collected by personal air sampling and a HOBO tempt/RH data logger.
Results:
Sixty-eight percent of the participants experienced poor sleep. An elevation in 1-year mean PM10 concentration was significantly associated with an increase in apnea-hypopnea index (beta = 1.04, P = .021) and respiratory disturbance index (beta = 1.07, P = .013). An increase of bedroom temperature during sleep was significantly associated with poorer sleep quality (adjusted odds ratio 1.46, 95% confidence interval 1.01–2.10, P = .044). Associations between PM10 concentration and respiratory disturbance index were observed in the dry season (beta = 0.59, P = .040) but not in the wet season (beta = 0.39, P = .215). PM10 was not associated with subjective sleep quality.
Conclusions:
Elevation of PM10 concentration is significantly associated with increased OSA severity. Our findings suggest that reduction in exposure to particulate matter and suitable bedroom environments may lessen the severity of OSA and promote good sleep.
Citation:
Lappharat S, Taneepanichskul N, Reutrakul S, Chirakalwasan N. Effects of bedroom environmental conditions on the severity of obstructive sleep apnea. J Clin Sleep Med. 2018;14(4):565–573.
Keywords: bedroom environments, obstructive sleep apnea, particulate matter, sleep quality
BRIEF SUMMARY
Current Knowledge/Study Rationale: Particulate matter has been linked to obstructive sleep apnea (OSA), but the influence of indoor environmental conditions, particularly in bedrooms on severity of OSA, has not been well studied. The association of indoor environmental conditions with OSA severity is investigated in this study.
Study Impact: An increase in PM10 concentrations is associated with more severe OSA as measured by an apnea-hypopnea index and respiratory disturbance index. Reduction in exposure to particulate matter may lessen the severity of OSA.
INTRODUCTION
Obstructive sleep apnea (OSA) is a sleep disorder characterized by recurrent collapse of the upper airway during sleep, resulting in intermittent hypoxia, sleep disruption, and daytime sleepiness.1 It is a subtype of sleep-disordered breathing (SDB), which is one of the most common sleep disorders that can occur in people of all ages, although it is most commonly observed in middle-aged and elderly populations.2 OSA has been linked to morbidities including hypertension, cardiovascular disease, and diabetes mellitus, as well as an increase in mortality.2–5 Globally, OSA affects approximately 3% to 7% of male adults and 2% to 5% of female adults.3 It is a pandemic global public health problem in both developed and developing countries.4 In Thailand, OSA affects approximately 4.8% and 1.9% of men and women, respectively,6 with a higher prevalence reported in poorer urban environments.7
Air pollution is one of the major environmental risks to health. Exposure to urban outdoor and indoor pollutants may elevate the incidence and severity of OSA.8 Several studies have demonstrated epidemiological associations between long-term air pollution and OSA through a plausible physiological mechanism linking particulate matter exposure to OSA.9–11 Particulate matter with an aerodynamic diameter of less than 10 μm (PM10) comprise coarse particles, which primarily deposit in the upper airways. They can cause irritation or breathing problems.11 Thus, PM10 may play an essential role to OSA through direct mechanical and inflammatory effects on the upper respiratory system.10,12–15 Additionally, a previous study found that there was an association between long-term black carbon exposure and short sleep duration in men. However, sleep latency was not associated with this exposure.16 Furthermore, a previous study also indicated a strong relation between an increase in temperature and elevation in the severity of OSA.17 Studies conducted by Jokic et al.,18 however, did not find a significant association between humidity and the severity of OSA. The study of Kim and Kum19 reported the best range of air temperature for good sleep is 24°C to 26°C at 50% relative humidity (RH). However, no previous studies have measured indoor environmental conditions in relation to OSA severity, particularly in bedrooms.
Given that there currently are no available data on the effect of indoor air pollution on OSA severity, it is crucial to gain a better understanding of bedroom environmental conditions and its relationship with OSA severity and sleep quality. Our hypothesis was that alterations in bedroom environmental conditions including PM10, temperature, and humidity would affect severity of OSA, as well as sleep quality in patients with OSA. This information may deliver some evidence needed for developing preventive and therapeutic strategies designed to alleviate the burden of these environmental conditions in patients with OSA.
METHODS
Study Design and Participants
This cross-sectional observational study was conducted to monitor changes in bedroom environmental conditions in patients with OSA who resided in the city. Sixty-three patients who were referred for an overnight polysomnography and in whom OSA was diagnosed were enrolled from the Excellence Center for Sleep Disorders at King Chulalongkorn Memorial Hospital, Bangkok, Thailand during the period of May 2016 to August 2016. The exclusion criteria were patients younger than 25 years or older than 75 years, heavy smokers (≥ 15 cigarettes/ day), pregnant women, patients with heart failure, or those with chronic respiratory failure. Data on personal characteristics and sleep quality were obtained from a set of questions carried out by a face-to-face interview. All participants gave their written consent to participate and all research protocols were reviewed and approved by The Ethics Review Committee for Research Involving Human Research Subjects, Health Science Group, Chulalongkorn University (RECCU No. 053/59), and the Faculty of Medicine Chulalongkorn University Institutional Review Board (Med Chula IRB No. 038/59).
Personal information including age (years), sex (male/female), weight (kilograms), height (centimeters), alcohol consumption status (yes/no), smoking status (yes/no), history of secondhand smoke exposure (yes/no), underlying diseases, and air conditioner usage (yes/no) were obtained by a face-to-face interview and medical records.
Pittsburgh Sleep Quality Index
The Pittsburgh Sleep Quality Index (PSQI) was applied to evaluate subjective sleep quality.20 It is a subjective standard questionnaire for estimating overall quality of sleep during the previous month. It contains 19 self-rated items that assess various components including sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, use of sleep medication, daytime dysfunction, and overall sleep quality. Each sleep component yields a score ranging from 0 to 3.20 These sleep component scores are combined to yield a total score, namely a “global score” ranging from 0 to 21. The global score indicates whether the participant's sleep quality is good or poor.20 Based on previous literature,20 participants with a score of 5 or lower were classified as good sleepers, and those with a score of 6 or higher were classified as poor sleepers. For sleep quality component subscales, a dichotomous variable of optimal and suboptimal sleep quality was used. According to the original scale, sleep latency was subdivided into: ≤ 15 minutes, 16–30 minutes, 31–60 minutes, and > 60 minutes. Those in the highest two groups of sleep latency (> 30 minutes) were defined as experiencing long sleep latency. Additionally, sleep duration was evaluated using the PSQI questionnaire, which questioned participants on how many hours of actual sleep nightly they had during the past month. In accordance with the original scale, sleep duration was categorized: < 5 hours, 5.0–5.9 hours, 6–6.9 hours, and ≥ 7 hours. Those in the lowest two groups of sleep duration (< 6 hours) were classified as having short sleep duration. With regard to sleep efficiency, the original scale was grouped: ≥ 85%, 75% to 84%, 65% to 74%, and < 65%. Those in the groups with 85% or less of sleep efficiency were defined as experiencing poor sleep efficiency.
Polysomnography
Overnight polysomnography is the gold-standard diagnostic test for OSA. All participants underwent in-laboratory polysomnography at the beginning of the study during the wet season (May to August 2016). We utilized a standard polysomnography system with related software (Profusion 3, Compumedics, Charlotte, North Carolina, United States). The stages of sleep were scored in 30-second intervals following the standard criteria from The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specification (AASM Scoring Manual).21 Apnea and hypopnea were defined using oral-nasal thermocouple excursion and nasal pressure transducer excursion, respectively. Scoring apnea, hypopnea, and respiratory effort-related arousals (RERAs) were performed following the standard criteria from the AASM Scoring Manual.21 Apnea was defined when dropping in peak signal excursions by ≥ 90% compared to pre-event baseline for ≥ 10 seconds. Hypopnea was defined when peak signal excursions drop by ≥ 30% of pre-event baseline for ≥ 10 seconds, and there was a ≥ 3% oxygen desaturation compared to pre-event baseline or the event was associated with an arousal (1A criteria from the AASM Scoring Manual). RERAs were defined when there was a sequence of breaths lasting ≥ 10 seconds characterized by increasing respiratory effort or by flattening of flow leading to arousal in which the event did not meet criteria for apnea or hypopnea. The apneahypopnea index (AHI) was computed as the ratio of the count of all apneas and hypopneas to the total sleep time, expressed as events per hour. Respiratory disturbance index (RDI) was calculated as the ratio of the count of all respiratory events including RERAs to the total sleep time. Sleep efficiency was computed as the proportion of total sleep time over the total recording time by percentage.21 Parameters of oxygenation, including absolute minimum SpO2 during sleep and mean oxygen saturation during sleep, were measured by pulse oximetry. Based on the polysomnography results, OSA was diagnosed when AHI ≥ 5 events/h, and classified as mild when AHI was 5 to 14.9 events/h, moderate when AHI was 15 to 30 events/h, and severe when AHI > 30 events/h.22
Bedroom Environmental Conditions
The data on bedroom environments including PM10, temperature, and relative humidity were collected in two seasons, namely the wet season from late May to mid-August 2016, and the dry season that began in late December 2016 and continued into mid-March 2017. All mentioned factors were collected in the participants' bedrooms for 3 consecutive nights in each season.23 After the polysomnography was performed, the first bedroom environmental conditions data were collected within 1 week for the wet season and the second data collection was conducted in the dry season. All 63 participants completed data collection of bedroom environmental conditions for both the wet and the dry seasons. PM10 samples were continuously monitored by a SKC personal sampling pump (model: 224-PCXR8, SKC Inc., Pittsburgh, Pennsylvania, United States) using 2.5-L/min aluminum cyclone loaded with 37 mm, 5.0 μm pore size, polyvinyl chloride filters (SKC Inc., Pittsburgh, Pennsylvania, United States) with a support pad. The filters were preweighed and post-weighed in a temperature- and relative humidity (RH)-controlled environment following National Institute for Occupational Safety and Health guidelines.24 The personal air sampling was calibrated, and the start and the end period of data sampling were set. The device was placed in an insulated plastic box (cooler) and sound-absorbing materials were inserted to reduce the noise of the device.25 Therefore, the level of noise from the device was not over an annoyance level (approximately 45 decibels). The methods of preparation, collection, and PM10 calculation followed the national institution's occupational safety and health code 0600 manual.24 Temperature and RH were continually detected and recorded by a HOBO tempt/RH data logger device (Onset Computer Corporation, Bourne, Massachusetts, United States). The device was calibrated and set to sample every 5 minutes.26 It was subsequently attached on the top of the insulated plastic box. Temperature and RH during sleep time were drawn from the entire sampling period. The average temperature and RH were reported. All environmental measuring devices were delivered to participants' homes with a written instruction. The participants received a follow-up phone call by the investigator and were instructed to place the device in their bedroom within 1 meter from the bed at the level of the nose while sleeping at night. Absolute humidity (AH) was calculated using the following formula (equation 1); where T is temperature in degree Celsius, RH is relative humidity in %, and e is the natural logarithm.27
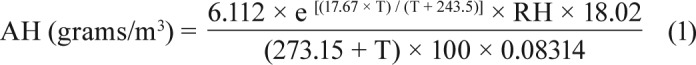 |
Statistical Analysis
All analyses of this study were performed using SPSS version 22.0 (IBM Corp., Armonk, New York, United States). Personal characteristics, sleep quality parameters, polysomnography sleep parameters, and bedroom environmental conditions of participants were reported using means (± standard deviation) for continuous variables and counts (percentages) for categorical variables. For non-normal distributed variables, medians (interquartile range) were provided. Paired t tests were applied to determine whether the mean values of continuous variables were different between two seasons. Multivariable-adjusted logistic regression models were utilized to estimate adjusted odds ratio and a 95% confidence interval (CI) for the associations between bedroom environmental conditions and subjective sleep quality parameters. The models were controlled for age, sex, body mass index (BMI), alcohol consumption, smoking, secondhand smoke, and AHI. The short sleep latency (≤ 30 minutes), longer sleep duration (≥ 6 hours), good sleep efficiency ≥ 85.00%), and good sleep quality (PSQI ≤ 5) were used as the reference group in the analyses. To investigate the associations between the bedroom environmental conditions and polysomnography sleep parameters, multiple linear regression models were applied and adjusted for age, sex, BMI, alcohol consumption, smoking, and secondhand smoke. Values of P of < .05 were considered statistically significant.
RESULTS
Of 63 participants, most were men (73.00%) with a median age of 42 years. Their median BMI was 26.20 kg/m2. Approximately 22.20% reported active alcohol consumption, whereas 6.30% and 9.50% reported active smoking and current secondhand smoke exposure, respectively. Hypertension was reported by 34.90% of the participants. Most of them (92.10%) used an air conditioner at night. According to the PSQI questionnaire, the medians of sleep latency and sleep duration of participants were 20 minutes and 6 hours, respectively. The average habitual sleep efficiency was 89.21 ± 14.16%. Most participants (68.30%) were classified as having poor sleep quality. Their mean RDI and AHI were 47.66 ± 26.06 events/h and 45.83 ± 27.21 events/h, respectively. More than half of them were classified as having severe OSA (Table 1).
Table 1.
Baseline characteristics and PSG parameters of patients with OSA (n = 63).

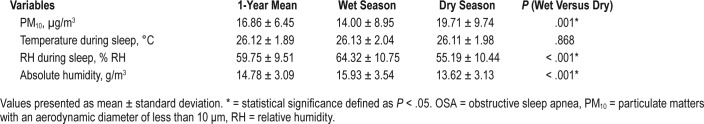
Table 2 shows the summary of bedroom environmental conditions. The mean of PM10 concentration in the dry season (19.71 μg/m3) was significantly greater than that in the wet season (14.00 μg/m3). The average temperature during sleep in the dry season was fairly similar to that of the wet season. The means of both relative humidity during sleep and absolute humidity in the dry season (55.19% RH and 13.62 g/m3) were lower than those in the wet season (64.32% RH and 15.93 g/m3). However, temperature was not significantly different between the seasons.
Table 2.
Comparison between the wet and the dry season of bedroom environmental conditions in patients with OSA (n = 63).
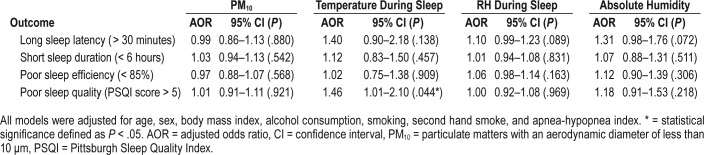
The associations between the 1-year mean bedroom environmental conditions, defined as an average of the bedroom environmental conditions data in the wet and the dry season, and subjective sleep quality, as assessed by PSQI, are shown in Table 3. In the multivariable-adjusted model, patients whose bedroom had a higher temperature reported poorer sleep quality (adjusted odds ratio 1.46, 95% CI 1.01–2.10, P = .044). There were no other significant associations between the 1-year mean bedroom environmental conditions and subjective sleep quality.
Table 3.
Association of 1-year mean bedroom environmental conditions and subjective sleep parameters.
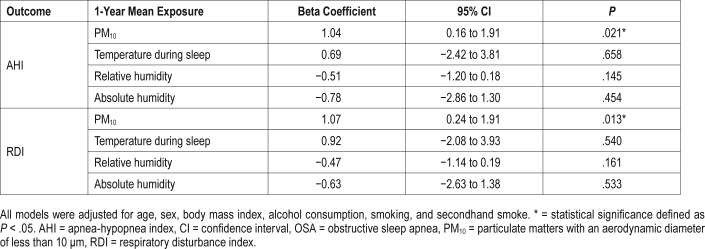
As shown in Table 4, multiple linear regression models demonstrated that an elevation in the 1-year mean PM10 concentration was significantly associated with an increase in AHI (beta = 1.04, P = .021). The average one-year mean exposure to temperature during sleep (beta = 0.69, P = .658), relative humidity (beta = -0.51, P = .145), and absolute humidity (beta = -0.78, P = .454) were not associated with AHI. Higher 1-year mean PM10 concentration was also significantly associated with higher RDI (beta = 1.07, P = .013). The associations between the 1-year mean exposure (temperature, relative humidity and absolute humidity) and RDI were in similar directions as AHI but none were statistically significant.
Table 4.
Association of bedroom environmental conditions and AHI and RDI of patients with OSA.
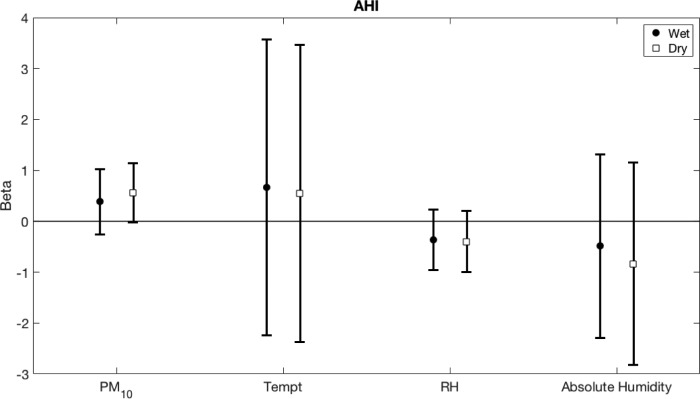
We further explored if the associations between bedroom environmental conditions and OSA severity differed by season (Figure 1). The analysis revealed a trend towards significant association between PM10 concentration during the dry season and AHI (beta = 0.56, 95% CI -0.02 to 1.14, P = .059) but not in the wet season (beta = 0.40, 95% CI -0.25 to 1.05, P = .22). No differences were found in other associations between other bedroom environmental conditions and AHI in the dry and the wet seasons.
Figure 1. Beta coefficients with 95% CI for the association of bedroom environmental conditions (stratified by seasons) and AHI.
AHI = apnea-hypopnea index, CI = confidence interval, PM10 = particulate matters with an aerodynamic diameter of less than 10 μm, RH = relative humidity, Tempt = temperature.
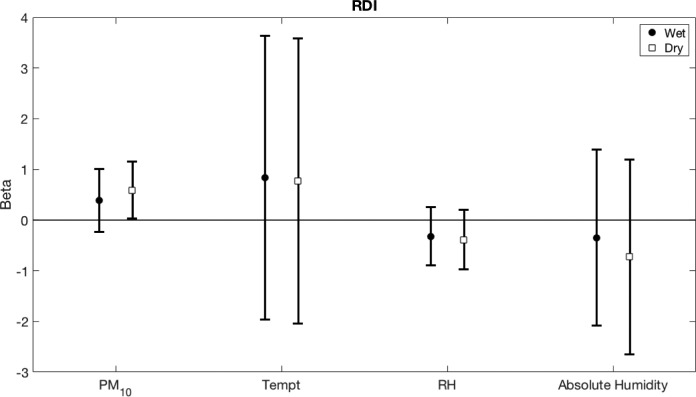
A differentiation of the association between bedroom environmental conditions (the wet and the dry season) and RDI is illustrated in Figure 2. It demonstrates an association of PM10 concentration in the dry season and RDI (beta = 0.59, 95% CI 0.03–1.15, P = .040) but not in the wet season (beta = 0.39, 95% CI, -0.23 to 1.01, P = .215). No differences were found in other associations between other bedroom environmental conditions and RDI in the dry and the wet seasons.
Figure 2. Beta coefficients with 95% CI for the association of bedroom environmental conditions (stratified by seasons) and RDI.
CI = confidence interval, PM10 = particulate matters with an aerodynamic diameter of less than 10 μm, RDI = respiratory disturbance index, RH = relative humidity, Tempt = temperature.
DISCUSSION
In this study, we found that PM10 concentration had an effect on severity of OSA. Elevation of AHI and RDI were significantly associated with an increase in PM10 concentration. The associations were particularly seen in the dry season. We also found a significant association between an increase of bedroom temperature during sleep and poorer sleep quality. Our data are novel as our study is the first to measure participants' bedroom environmental conditions whereas previous studies have mostly utilized outdoor measurements. In agreement with our results, Zanobetti et al. used the data from the Sleep Heart Health Study, a multicenter cohort study in the United States and reported that an interquartile increase in short-term PM10 levels was associated with a 12.9% increase in RDI (95% CI 2.77–24.09) during summer.9 In another study, the outdoor PM10 data was retrieved using information from United States Environmental Protection Agency Air Quality System Technology Transfer Network. A study by Glaser et al.28 also supported the link between outdoor pollution exposure and OSA. This study reported that 81% of at-risk World Trade Center (WTC)-exposed rescue/recovery workers in whom OSA was diagnosed. Severe OSA was associated with WTC exposure that occurred on September 11, 2001 with odds ratio of 1.91 (95% CI 1.15–3.17). However, not all studies were in support of such associations, as the population-based cohort study using outdoor environmental data conducted by Weinreich et al.17 reported no association between PM10 and AHI. There are several potential explanations for inconsistent results of the associations between air pollution and OSA. All previous studies, unlike our study, were conducted using outdoor data, which might not reflect the amount of pollution exposure during sleep. Seasonal variations may also play a role as the study by Zanobetti et al. observed an association between PM10 and RDI exclusively during the summer time.9 During the summer season compared with other seasons, windows are often kept open during the night; therefore, the indoor PM10 concentration may be more affected by the outdoor air. It is possible that in-bedroom and outdoor environments might have different effects on OSA severity, which might explain some differences in the findings of various studies. Though PM10 level, temperature, and humidity of bedroom were controlled during the night by an air conditioner, our results reported a significant difference of PM10 and humidity level between the wet and the dry seasons.
There is no clear literature that explicitly reports duration of changes in the brain or the upper airway systems caused by environmental factors linking to OSA. However, environmental factors had been shown to have cumulative adverse effects over long-term exposure and result in the development of chronic diseases.29 Air pollution, especially particulate matter, potentially affects sleep through the central nervous system and the upper airways.10,30 Pollutants may directly increase nasal or pharyngeal inflammatory responses, which increase an upper airway resistance and reduce airway patency.31,32 These mechanisms may alter ventilation and perfusion, resulting in exacerbation of hypoxia associated with OSA. The results of bedroom environmental conditions in our study showed that the mean 1-year PM10 concentration was 16.86 μg/m3 which was well below the National Ambient Air Quality Standards of outdoor air in Thailand (50 μg/m3)33 and the World Health Organization (20 μg/m3).34 However, even such a low level might have a negative effect on the respiratory health of susceptible individuals, because there is no known threshold limit for pollutants to trigger respiratory problems35 or OSA.9
The average bedroom PM10 concentrations in the dry and the wet season were 19.71 μg/m3 and 14.00 μg/m3, respectively. Overall, seasonal trends indicated significantly higher PM10 concentrations in the dry season compared to the wet season. Srithawirat et al.36 and Jinsart et al.37 reported similar results. Moreover, the data on outdoor PM10 concentrations from the Pollution Control Department of Thailand found higher concentrations in the dry season compared with the wet season in Bangkok.38 Several studies have demonstrated that levels of particulate matter in Asian countries are mainly affected by seasonal variations.39,40 It appears to be possible that weather precipitation or dispersion may influence the levels of particulate matter. The higher level of PM10 observed during the dry season may explain the stronger association of PM10 concentration and RDI in the dry season compared with the wet season. This observation proposes that a decrease in the PM10 concentration below a certain amount does not affect the AHI or RDI.
Our study did not demonstrate associations of PM10 concentration and other subjective sleep parameters. A previous study supported that a decrease in sleep efficiency is related to short-term elevations in PM10 in a cross-sectional study using objective measures of sleep.9 Elder et al.12 and Wang et al.13 reported that particles moved from the nose up to the olfactory nerve into the striatum frontal cortex and cerebellum. This likely induced brain inflammation14 and a change in neurotransmitter levels,15,29 which later influenced sleep quality. Further evidence of particle deposition in the brain was linked to neural inflammation,41,42 which might disrupt sleep-wake cycles.43,44 However, similar to our findings, a study conducted by Fang et al. did not observe an association between long-term black carbon exposures and any sleep parameters in their overall studied participants.16 The inconsistent results could be due to the heterogeneity of the study population.
Our study did not demonstrate an association between humidity level and AHI. Theoretically, drying of upper airway mucosa during the night, through increasing surface tension forces, can contribute to increasing severity of OSA.45 High ambient humidity might lessen OSA severity by moistening the upper airway mucosa. However, similar to our findings, a previous study did not report an association between humidity level and AHI.18 Contrary to a positive finding of topical phosphocholinamin, a long-acting tissue lubricating agent that acts as an application to the upper airway mucosa in reducing AHI,46 an addition of liquid to the airway surface may be less important.
Weinreich et al. demonstrated a significant association between temperature and AHI.17 Upper airway dilator muscle activity, measured by genioglossus electromyograms, has been shown to be greater during cold air breathing compared to warm air breathing. Therefore, reduction in upper airway muscle activity may result in higher AHI in a warmer environment.47 However, our study did not demonstrate an association between temperature and AHI. The discrepancy of the results of the studies may come from difference in observed temperature (mean temperature of 13.1 ± 6.2°C in Weinreich et al. study compared to 26.12 ± 1.89°C in our study).
Our study found a statistically significant association between poorer sleep quality and elevation in bedroom temperatures. One study19 claimed that the best range of air temperature for good sleep using objective measures of sleep quality was 24°C to 26°C at 50% RH, and the upper limit for the best sleep quality was 28.1°C at 50% RH. It appears that suitable room temperature and humidity during sleep can play an essential role in influencing good sleep, especially in patients with OSA. In order to ensure good sleep quality among patients with varied levels of OSA severity, further study should investigate appropriate room temperature, RH, and absolute humidity.
Several limitations of this study should be taken into consideration. First, the sample size of this study is relatively small; thus, it might not have adequate power in detecting some significant relationships. Second, we used a cross-sectional study design, which makes it difficult to draw conclusions regarding causation because we cannot be certain if the exposure preceded the outcomes. Future prospective cohort studies in which environmental conditions and stage of OSA development are recorded in the general population would allow us to clarify the understanding of environmental effect on OSA severity. Third, half of our participants had severe OSA, which may have limited the generalizability of our study findings to a broader general population. In addition, patients in this study had polysomnography performed only in the wet season. However, the significant association between PM10 concentration and RDI was observed only in the dry season when polysomnography was not performed. Moreover, the patients' weight data that may have an effect on the associations of OSA with other parameters in the dry season were not collected after the initial measurement. Fourth, we used self-reported sleep quality (PSQI) to measure sleep quality scores. Actigraphy recordings would be more appropriate for retrieving accurate sleep quality. In addition, the noise of the personal air sampling device may have disrupted the patient's sleep quality during the data collection at night. Lastly, we collected only PM10 in this study, whereas PM2.5 is smaller and more harmful to health than PM10, and it could have a greater effect on OSA severity. More detailed studies could explore this aspect in the future. Additionally, our 1-year mean PM10 concentrations are based on an average concentration of three samplings from two seasons (the wet and the dry season) in the participants' bedrooms; hence, they may not accurately indicate the annual average of indoor PM10 concentrations. More frequent samplings should be considered to obtain a better representative of annual mean indoor PM10 concentrations. Further studies may explore the effect of PM10 on OSA severity in patients with OSA under a certain bedroom environmental condition (with/without air conditioner usage) to study other factors such as therapeutic conditions that may influence the severity. For example, the effect of PM10 on patients with OSA who regularly use continuous positive airway pressure devices may be different from those who do not use these devices.
CONCLUSIONS
Our findings suggest that bedroom environmental conditions can be linked to OSA severity. Although environmental factors might not be a direct cause of OSA, they may play a role in exacerbating the severity of OSA. Despite an increase in public awareness of OSA, most of those affected still remain undiag-nosed and untreated. Along with treatment, these new findings suggest that reduction in exposure to particulate matter might lessen the severity of OSA.
DISCLOSURE STATEMENT
This study was funded by the 90th Anniversary of Chulalongkorn University Fund (Ratchadaphiseksomphot Endowment Fund) and Chulalongkorn Academic Advancement into Its 2nd Century Project (CUAASC). Work for this study was performed at the Excellence Center for Sleep Disorders at King Chulalongkorn Memorial hospital. SL, NT, and NC report no conflicts of interest. SR received a grant from Merck Sharp and Dohme, speaker fees from Novo Nordisk, Medtronic and Sanofi Aventis, and research equipment support from Resmed Thailand.
ACKNOWLEDGMENTS
This study was done in collaboration with the Excellence Center for Sleep Disorders at King Chulalongkorn Memorial hospital; the authors thank members and staff for their assistance and support. Author contributions: SL, NT, SR and NC conceived and designed the study. All authors analyzed the data and drafted the manuscript. All authors interpreted the data, critically revised the draft for important intellectual content, and gave final approval of the manuscript to be published. All authors contributed equally in the preparation of this manuscript.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- OSA
obstructive sleep apnea
- PM10
particulate matters with an aerodynamic diameter of less than 10 μm
- PSQI
Pittsburgh Sleep Quality Index
- RDI
respiratory disturbance index
- RH
relative humidity
REFERENCES
- 1.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: prospective cohort study. PLoS Med. 2009;6(8):e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ancoli-Israel S. Sleep and its disorders in aging populations. Sleep Med. 2009;10(1):7–11. doi: 10.1016/j.sleep.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;22(4):352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182(2):269–277. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neruntarat C, Chantapant S. Prevalence of sleep apnea in HRH Princess Maha Chakri Srinthorn Medical Center, Thailand. Sleep Breath. 2011;15(4):641–648. doi: 10.1007/s11325-010-0412-x. [DOI] [PubMed] [Google Scholar]
- 7.Mirrakhimov AE, Sooronbaev T, Mirrakhimov EM. Prevalence of obstructive sleep apnea in Asian adults: a systematic review of the literature. BMC Pulm Med. 2013;13:10. doi: 10.1186/1471-2466-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tenero L, Piacentini G, Nosetti L, Gasperi E, Piazza M, Zaffanello M. Indoor/ outdoor not-voluptuary-habit pollution and sleep disordered breathing in children: a systematic review. Transl Pediatr. 2017;6(2):104–110. doi: 10.21037/tp.2017.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanobetti A, Redline S, Schwartz J, et al. Associations of PM10 with sleep and sleep-disordered breathing in adults from seven U.S. urban areas. Am J Respir Crit Care Med. 2010;182(6):819–825. doi: 10.1164/rccm.200912-1797OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dockery DW, Pope CA., 3rd Acute respiratory effects of particulate air pollution. Annu Rev Public Health. 1994;15:107–132. doi: 10.1146/annurev.pu.15.050194.000543. [DOI] [PubMed] [Google Scholar]
- 11.Pope CA, 3rd, Dockery DW, Spengler JD, Raizenne ME. Respiratory health and PM10 pollution. A daily time series analysis. Am Rev Respir Dis. 1991;144(3 Pt 1):668–674. doi: 10.1164/ajrccm/144.3_Pt_1.668. [DOI] [PubMed] [Google Scholar]
- 12.Elder A, Gelein R, Silva V, et al. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ Health Perspect. 2006;114(8):1172–1178. doi: 10.1289/ehp.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang B, Feng WY, Wang M, et al. Transport of intranasally instilled fine Fe2O3 particles into the brain: micro-distribution, chemical states, and histopathological observation. Biol Trace Elem Res. 2007;118(3):233–243. doi: 10.1007/s12011-007-0028-6. [DOI] [PubMed] [Google Scholar]
- 14.Campbell A, Oldham M, Becaria A, et al. Particulate matter in polluted air may increase biomarkers of inflammation in mouse brain. Neurotoxicology. 2005;26(1):133–140. doi: 10.1016/j.neuro.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Tin-Tin-Win-Shwe, Mitsushima D, Yamamoto S, et al. Changes in neurotransmitter levels and proinflammatory cytokine mRNA expressions in the mice olfactory bulb following nanoparticle exposure. Toxicol Appl Pharmacol. 2008;226(2):192–198. doi: 10.1016/j.taap.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Fang SC, Schwartz J, Yang M, Yaggi HK, Bliwise DL, Araujo AB. Traffic-related air pollution and sleep in the Boston Area Community Health Survey. J Expo Sci Environ Epidemiol. 2015;25(5):451–456. doi: 10.1038/jes.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinreich G, Wessendorf TE, Pundt N, et al. Association of short-term ozone and temperature with sleep disordered breathing. Eur Respir J. 2015;46(5):1361–1369. doi: 10.1183/13993003.02255-2014. [DOI] [PubMed] [Google Scholar]
- 18.Jokic R, Bhagchandani L, Zintel T, Baetz M, Fitzpatrick MF. Effect of high versus low ambient humidity on the severity of obstructive sleep apnoea. Thorax. 1999;54(8):711–713. doi: 10.1136/thx.54.8.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim DG, Kum JS. Evaluation of thermal comfort during sleeping in summer - part II: about mean skin temperatures and physiological signals. Korean Journal of Air-Conditioning and Refrigeration Engineering. 2006;18(1):1–6. [Google Scholar]
- 20.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 21.Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 22.Ruehland WR, Rochford PD, O'Donoghue FJ, Pierce RJ, Singh P, Thornton AT. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep. 2009;32(2):150–157. doi: 10.1093/sleep/32.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diette GB, Hansel NN, Buckley TJ, et al. Home indoor pollutant exposures among inner-city children with and without asthma. Environ Health Perspect. 2007;115(11):1665–1669. doi: 10.1289/ehp.10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Institution Occupational Safety and Health (NIOSH) Particulates Not Otherwise Regulated, Respirable 0600. NIOSH Manual of Analytical Methods (NMAM) Fourth Edition. https://www.cdc.gov/niosh/docs/81-123/pdfs/0600.pdf. Published January 15, 1998.
- 25.Tsai FC, Smith KR, Vichit-Vadakan N, Ostro BD, Chestnut LG, Kungskulniti N. Indoor/outdoor PM10 and PM2.5 in Bangkok, Thailand. J Expo Anal Environ Epidemiol. 2000;10(1):15–26. doi: 10.1038/sj.jea.7500071. [DOI] [PubMed] [Google Scholar]
- 26.Tunno BJ, Shields K, Cambal L, et al. Indoor air sampling for fine particulate matter and black carbon in industrial communities in Pittsburgh. Sci Total Environ. 2015;536:108–115. doi: 10.1016/j.scitotenv.2015.06.117. [DOI] [PubMed] [Google Scholar]
- 27.Mander P. How to convert relative humidity to absolute humidity. [Accessed January 28, 2017]. https://carnotcycle.wordpress.com/2012/08/04/how-to-convert-relative-humidity-to-absolute-humidity. Published August 4, 2012.
- 28.Glaser MS, Shah N, Webber MP, et al. Obstructive sleep apnea and World Trade Center exposure. J Occup Environ Med. 2014;56(Suppl 10):S30–S34. doi: 10.1097/JOM.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 29.Coogan PF, White LF, Jerrett M, et al. Air pollution and incidence of hypertension and diabetes in African American women living in Los Angeles. Circulation. 2012;125(6):767–772. doi: 10.1161/CIRCULATIONAHA.111.052753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleinman MT, Araujo JA, Nel A, et al. Inhaled ultrafine particulate matter affects CNS inflammatory processes and may act via MAP kinase signaling pathways. Toxicol Lett. 2008;178(2):127–130. doi: 10.1016/j.toxlet.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehra R, Redline S. Sleep apnea: a proinflammatory disorder that coaggregates with obesity. J Allergy Clin Immunol. 2008;121(5):1096–1102. doi: 10.1016/j.jaci.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeMeo DL, Zanobetti A, Litonjua AA, Coull BA, Schwartz J, Gold DR. Ambient air pollution and oxygen saturation. Am J Respir Crit Care Med. 2004;170(4):383–387. doi: 10.1164/rccm.200402-244OC. [DOI] [PubMed] [Google Scholar]
- 33.Simachaya W. Overview of Air Quality Management in Thailand. [Accessed May 1, 2017]. http://infofile.pcd.go.th/air/AIT061109_sec5.pdf.
- 34.World Health Organization. WHO Air quality guidelines for particulate matter, ozone, nitrogen dioxide and sulfur dioxide: global update 2005: summary of risk assessment. [Accessed May 1, 2017]. http://apps.who.int/iris/bitstream/10665/69477/1/WHO_SDE_PHE_OEH_06.02_eng.pdf. Published 2006.
- 35.Zanobetti A, Schwartz J, Gold D. Are there sensitive subgroups for the effects of airborne particles? Environ Health Perspect. 2000;108(9):841–845. doi: 10.1289/ehp.00108841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srithawirat T, Latif MT, Sulaiman FR. Indoor PM10 and its heavy metal composition at a roadside residential environment, Phitsanulok, Thailand. Atmosfera. 2016;29(4):311–322. [Google Scholar]
- 37.Jinsart W, Kaewmanee C, Inoue M, et al. Driver exposure to particulate matter in Bangkok. J Air Waste Manag Assoc. 2012;62(1):64–71. doi: 10.1080/10473289.2011.622854. [DOI] [PubMed] [Google Scholar]
- 38.Pollution Control Department (PCD) Ministry of Natural Resources and Environment. Annual Air Quality [in Thai] [Accessed May 1, 2017]. http://aqnis.pcd.go.th. Published 2010.
- 39.Begum BA, Biswas SK, Hopke PK. Temporal variations and spatial distribution of ambient PM2.2 and PM10 concentrations in Dhaka, Bangladesh. Sci Total Environ. 2006;358(1-3):36–45. doi: 10.1016/j.scitotenv.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 40.Wang H, Zhuang Y, Wang Y, et al. Long-term monitoring and source apportionment of PM2.5/PM10 in Beijing, China. J Environ Sci. 2008;20(11):1323–1327. doi: 10.1016/s1001-0742(08)62228-7. [DOI] [PubMed] [Google Scholar]
- 41.Gerlofs-Nijland ME, Van Berlo D, Cassee FR, Schins RP, Wang K, Campbell A. Effect of prolonged exposure to diesel engine exhaust on proinflammatory markers in different regions of the rat brain. Part Fibre Toxicol. 2010;7:12. doi: 10.1186/1743-8977-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levesque S, Surace MJ, McDonald J, Block ML. Air pollution & the brain: subchronic diesel exhaust exposure causes neuroinflammation and elevates early markers of neurodegenerative disease. J Neuroinflammation. 2011;8:105. doi: 10.1186/1742-2094-8-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bertini G, Colavito V, Tognoli C, Seke Etet PF, Bentivoglio M. The aging brain, neuroinflammatory signaling and sleep-wake regulation. Ital J Anat Embryol. 2010;115(1-2):31–38. [PubMed] [Google Scholar]
- 44.Pan W, Wu X, He Y, et al. Brain interleukin-15 in neuroinflammation and behavior. Neurosci Biobehav Rev. 2013;37(2):184–192. doi: 10.1016/j.neubiorev.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miki H, Hida W, Kikuchi Y, et al. Effects of pharyngeal lubrication on the opening of obstructed upper airway. J Appl Physiol. 1992;72(6):2311–2316. doi: 10.1152/jappl.1992.72.6.2311. [DOI] [PubMed] [Google Scholar]
- 46.Jokic R, Klimaszevski A, Mink J, Fitzpatrick MF. Surface tension forces in sleep apnea: the role of a soft tissue lubricant: a randomized double-blind, placebo-controlled trial. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1522–1525. doi: 10.1164/ajrccm.157.5.9708070. [DOI] [PubMed] [Google Scholar]
- 47.Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90(1):47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]