Abstract
Study Objectives:
To assess the overall clinical effectiveness of a sleep position trainer (SPT) in patients with positional obstructive sleep apnea (POSA) and to evaluate how many patients were willing to continue treatment after a 1-month trial period.
Methods:
Patients in whom POSA was diagnosed underwent a 1-month trial period with the SPT. Home sleep apnea tests were used to measure baseline data and data following the trial period with the SPT.
Results:
The 79 patients who completed the study protocol were 81% male, had a mean age of 52 ± 12 years, and a median baseline respiratory event index (REI) of 11 (8, 16) events/h. A significant reduction in overall REI to 5 (3, 10) events/h was observed with the SPT as compared to baseline (P < .001). The median percentage of sleep time in the supine position decreased significantly from 27 (20, 48) to 7 (2, 20) with the SPT (P < .001). Adherence was found to be 95 ± 8%. Of the 44 patients who decided to continue treatment, 27 were categorized as responders (having a decrease in REI of at least 50%) and 17 were non-responders. The most important reasons for not purchasing the SPT were poor objective results, intolerance to the vibrations, cost of the device, persistent daytime sleepiness, or patient preference for other treatment options.
Conclusions:
Treatment with the SPT came with high adherence rates and was effective in reducing REI and supine sleep position. The trial period is in the patients' best interest, as it may prevent those who will not benefit from positional training from purchasing an SPT.
Citation:
Beyers J, Dieltjens M, Kastoer C, Opdebeeck L, Boudewyns AN, De Volder I, Van Gastel A, Verbraecken JA, De Backer WA, Braem MJ, Van de Heyning PH, Vanderveken OM. Evaluation of a trial period with a sleep position trainer in patients with positional sleep apnea. J Clin Sleep Med. 2018;14(4):575–583.
Keywords: obstructive sleep apnea-hypopnea syndrome, positional therapy, sleep-disordered breathing, supine dependent
BRIEF SUMMARY
Current Knowledge/Study Rationale: In more than 50% of patients with obstructive sleep apnea (OSA), it is possible that the disorder is position dependent, which is called positional OSA (POSA). Positional therapy is a noninvasive treatment option for POSA. In the past, treatment of POSA was hampered by a low adherence rate to devices aimed to reduce sleep time in the supine position.
Study Impact: In the current study, patients with POSA were given a 1-month trial period with a sleep position trainer (SPT). We found that treatment of POSA with the SPT was effective and that patients were highly adherent to this treatment. Furthermore, we stress the importance of the trial period so that patients who will not benefit from SPT treatment can avoid purchasing the device.
INTRODUCTION
Snoring and obstructive sleep apnea (OSA) have a high prevalence in the general population and are associated with age and sex.1 Peppard et al. estimated that 14% of men and 5% of women have an apnea-hypopnea index (AHI) ≥ 5 events/h with additional symptoms of daytime sleepiness.2 When OSA is left untreated, patients are at higher risk for cardiovascular diseases, stroke, motor vehicle accidents, and diminished quality of life, which makes adequate treatment of utmost importance.3,4 Long-term, multidisciplinary management is required consisting of medical, behavioral, and surgical options. Continuous positive airway pressure (CPAP) is the gold standard of treatment for moderate (15 ≤ AHI ≤ 30 events/h) and severe (AHI > 30 events/h) OSA. Alternative treatment of OSA is dependent on disease severity, upper airway anatomy, risk factors, and patient preference.5
Behavioral therapy includes weight loss, exercise, avoidance of alcohol and sedatives before bedtime, and positional therapy. Positional therapy deters sleep in the supine position and is an effective treatment for patients with positional OSA (POSA).6 These patients have a higher AHI when sleeping in the supine position compared to nonsupine positions.5 The percentage of patients with OSA who also have POSA varies according to the definition that is used.7 The first, and still most frequently used, definition of POSA was introduced by Cartwright and states that patients with POSA have an AHI while sleeping in the supine position that is at least twice as high as their AHI in nonsupine positions.8 According to this definition, approximately 56% to 67% of the patient population with OSA has POSA.7,9,10 In general, patients with POSA are younger, have a lower body mass index (BMI), and have less severe OSA.7,9,11,12
The so-called “tennis ball technique” (TBT), in which a tennis ball or other bulky objects are placed in the center of the back, is widely used to prevent sleep in the supine position. This technique has shown a significant decrease of sleep time in the supine position and has led to a reduction in AHI.13 Variations of this technique, such as special pillows, vests, positional alarms, and verbal instructions also have been tested with similar results as the TBT.6,14–19 However, overall long-term adherence to such therapies is low, because the bulky object is uncomfortable for patients and results in disturbed sleep.6,18 A neck-worn device and a chest-worn device that activate a vibration alarm to deter sleep in supine position have also been evaluated in an attempt to overcome the aforementioned adherence problems. These novel techniques showed promising results in reducing OSA severity and increasing short-term and long-term adherence.20–25 Furthermore, the use of the chest-worn sleep position trainer (SPT) was studied as part of combination therapy in patients who have residual POSA after treatment with oral appliances (OA) or after upper airway surgery.26,27 The authors of both studies concluded that additional positional training in a group of patients with residual POSA after standard treatment can increase the overall therapeutic effectiveness.
The aim of the current study was to evaluate the clinical effectiveness of the chest-worn SPT in routine clinical practice after a 1-month trial period and to investigate whether patients would decide to purchase the SPT after the trial period.
METHODS
Patients and Study Design
From March 2014 to August 2016, patients in whom POSA was diagnosed based on polysomnography (PSG), performed between June 2010 and June 2016, were invited to participate in a 1-month trial period with the SPT as part of a standardized clinical pathway. This group of consecutive patients consisted of patients consulting for an in-hospital visit at the ear, nose, and throat (ENT) department after a new diagnosis or after failure of previous treatment. PSG was scored according to the rules published in 1999 by the American Academy of Sleep Medicine28 and POSA was defined as having an overall AHI ≥ 5 events/h, a supine AHI at least twice as high as the nonsupine AHI, and 10% to 90% of the total sleep time (TST) spent in the supine position documented during full-night PSG. Patients were included if they were willing to participate and could understand and use a home sleep apnea test (HSAT) for registering the respiratory and positional events. The study protocol was approved by the institution's ethical committee and informed consent was obtained in each patient.
The clinical pathway consisted of four study visits as shown in Figure 1, which started within 3 months after the in-hospital visit with the ENT specialist. On visit 1, patients received a HSAT (Medibyte, Braebon Medical Corporation, Kanata, Ontario, Canada) and a validated 2-night test at home was conducted to record baseline measurements including body position, oximetry, pulse rate, snoring volume, airflow, and respiratory effort.29 The results were analyzed and discussed with the patient during visit 2. If POSA could be confirmed, patients received an “SPT experience” for a trial period of 28 days (NightBalance, Delft, The Netherlands), with a vibration activity that increases progressively during the first 10 nights. After approximately 3 weeks, the same 2-night HSAT was performed with the SPT to evaluate its efficacy. On the fourth and last visit, results of the baseline HSAT were compared with the results of the HSAT with SPT, together with the readout data of the SPT and subjective improvement. Subjective improvement was asked during the outpatient visit and was reported in questionnaires filled out by the patient. Based on the overall results, patients were invited to purchase the SPT or not.
Figure 1. Timeline of the four study visits in the clinical pathway.
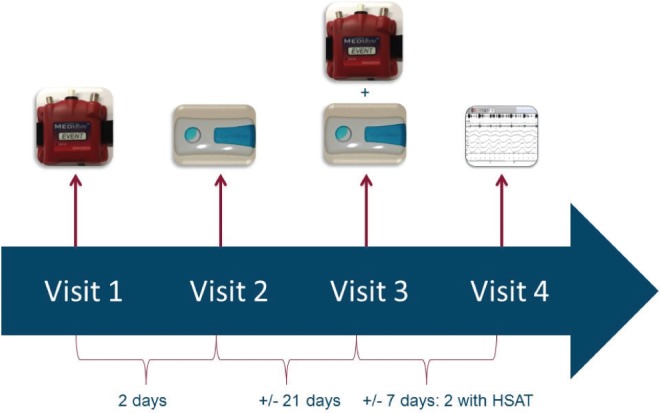
At visit 1, the patients received an HSAT to conduct a validated 2-night test at home to assess baseline measurements. At visit 2, the results were analyzed and discussed with the patients. If POSA was confirmed, the patients received an SPT for a trial period of 28 days. At visit 3, the patients received the same HSAT to perform a 2-night sleep test with the SPT, during the last week of the SPT-trial. At visit 4, the results were discussed and compared to the results of the baseline HSAT. HSAT = home sleep apnea test, POSA = positional obstructive sleep apnea, SPT = sleep position trainer.
Home Sleep Apnea Tests
The validated HSAT device used in this study analyzed both respiration and body position at home.29 The two belts measuring respiratory effort were attached around the chest and abdomen, with the device itself positioned at the sternum. To register the snoring sounds, a microphone was taped to the patient's forehead. A nasal cannula pressure transducer and thermistor to measure airflow were placed under the nose. Finally, finger pulse oximetry sensor measured oxygen saturation and heart rate. Data were read out using Pursuit software (version 8.0, Braebon Medical Corporation, Canada) allowing data analysis to be conducted. Respiratory events were scored following the criteria published by the American Academy of Sleep Medicine in 1999 and 2007.28–30 The respiratory event index (REI) was calculated during the HSAT and was used to judge the success of the treatment.
Sleep Position Trainer
The SPT is a chest-worn device, placed at the sternum. It is a small, lightweight device (72 × 35 × 10 mm, 25 g) that monitors body position. When the supine position is detected, it vibrates so that the patient changes sleeping positions. It allows for body position changes during sleep without any movement restrictions. The frequency of the vibration stimulus is variable and its amplitude and duration increase gradually until the patient shifts to a nonsupine position. The trial period with the SPT was divided into three phases: a baseline/diagnostic phase, a build-up phase, and a treatment phase. During the 2 nights of the baseline phase, the SPT monitored and recorded the body position without generating vibrations. The next phase, the build-up phase, lasted 7 days: the SPT vibrated in an increasing number of episodes while in supine position. During the last phase, the treatment phase, which began at night 10, the SPT vibrated every time the patient slept in supine position for more than 3 seconds to deter this position. When a nonsupine position was detected, the vibrations stopped.
Definitions
Therapeutic efficacy is defined as a reduction in either REI or supine position. Responders were defined as patients with a reduction in REI ≥ 50% compared to baseline, and non-responders were defined as patients with a reduction in REI < 50% from baseline. Treatment success was obtained when an REI below 5 events/h of sleep was achieved.
The percentage of SPT use in hours per night was divided by the TST derived from a routine PSG performed for diagnosis of POSA to calculate the adjusted adherence (mean SPT use per night / TST on baseline PSG). The TST was derived from PSG because the HSAT does not measure sleep. Objective information on adherence was obtained from the data stored by the SPT. Effective adherence was defined as the minimum use of the SPT for at least 4 h/night and 5 nights/wk.31 Finally, the mean disease alleviation (MDA), a measurement of therapeutic effectiveness, was calculated. The MDA is the product of therapeutic efficacy with the adjusted adherence divided by 100 and expressed as a percentage.32
Statistical Analysis
Data were analyzed using SPSS version 23 (Statistical Package for Social Sciences Inc., Chicago, Illinois, United States). Data were expressed as median values or their lower and upper quartiles (quartile 1, quartile 3) when not normally distributed. When data were not normally distributed, an independent samples Mann-Whitney U test was performed. Categorical variables were tested using Pearson chi-square test, and the nonparametric Wilcoxon signed rank test was used to test changes in parameters before and after treatment. A value of P < .05 was considered a statistically significant result.
RESULTS
Patient Screening
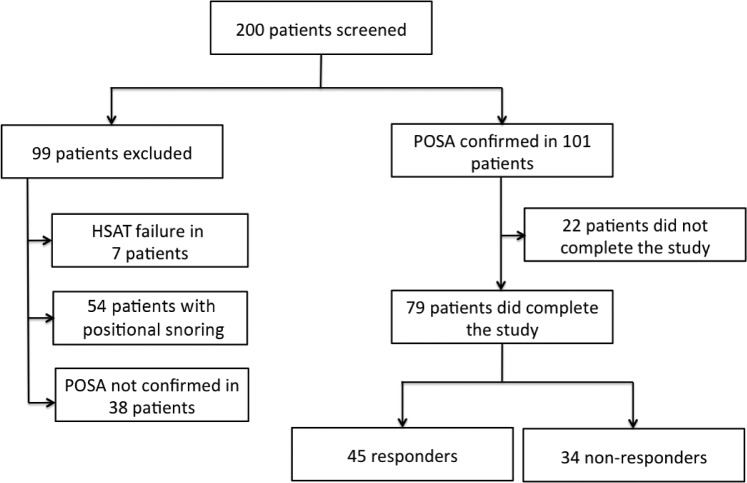
A flowchart of the patient population is shown in Figure 2. A total of 200 patients in whom POSA was diagnosed on full-night attended PSG were screened with the HSAT. Data derived at time of PSG are as follows: mean age 50 ± 12 years, male/female 161/39, overall median AHI 8 (4, 13) events/h, and median body mass index (BMI) 26 (24, 29) kg/m2. In 38 of the 200 patients, the diagnosis of POSA could not be confirmed based on the baseline HSAT measurements, and these patients were therefore excluded. Furthermore, baseline HSAT measurements failed in 7 patients, whereas in 54 patients the diagnosis was positional snoring (REI < 5 events/h). These patients were also excluded from participation in this study. POSA could be confirmed in 101 patients. Of those 101 patients, 22 patients did not complete the study (21.8%): the main reasons for which were patient intolerance to the SPT vibrations or technical failures (eg, HSAT measurement failed).
Figure 2. Flowchart of the patient population.
79 of the 200 included patients completed the study protocol. 45 patients were responders (reduction in REI ≥ 50%), whereas 34 patients did not meet the responder criteria. HSAT = home sleep apnea test, POSA = positional obstructive sleep apnea, REI = respiratory event index.
Therapeutic Efficacy
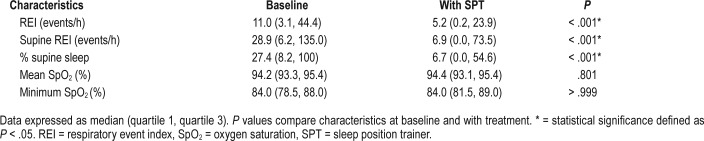
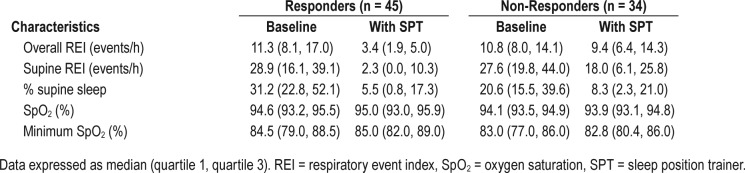
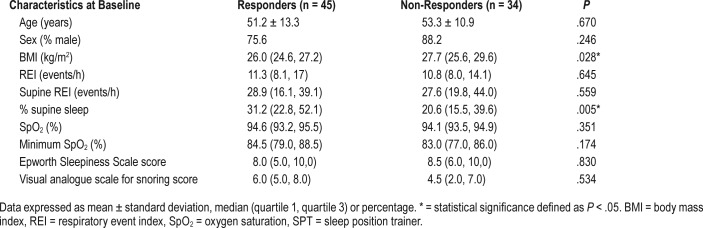
Table 1 shows the parameters of the remaining 79 patients at baseline and after 1 month of SPT use. The overall REI, percentage of supine position and supine REI decreased significantly with SPT treatment (P < .001). The therapeutic efficacy was calculated for each patient, resulting in 45 responders and 34 non-responders (Table 2). In both responders and non-responders, a significant reduction in supine REI (P < .001 in responders and P = .001 in non-responders) and percentage of supine position (P < .001 in both groups) was found while using the SPT. Furthermore, a significant reduction in overall REI was obtained in the responder group (P < .001). When the baseline characteristics of both groups were compared, a significant difference in BMI (P = .028) and percentage of supine position at baseline was found (P = .005). The responder group showed a median BMI of 26 kg/m2 and a median of 31% supine position at baseline, while the non-responder group showed a median BMI of 28 kg/m2 and a median of 21% supine position at baseline. Despite the difference in BMI between the responders and the non-responders, it should be noted that the BMI in both groups was rather low. No differences were seen in age, sex, overall REI, supine REI and oxygen saturation between responders and non-responders. These results are shown in Table 3.
Table 1.
Respiratory parameters at baseline and after 1 month of SPT use in the overall group of 79 patients.
Table 2.
Respiratory parameters at baseline and with SPT in the responder and the non-responder groups.
Table 3.
Comparison of baseline characteristics between responders and non-responders.
Adherence and MDA
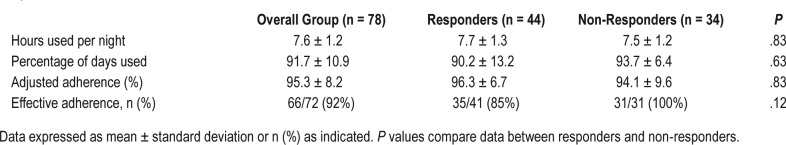
Adherence data for the overall group, and in the responder and non-responder groups are shown in Table 4. Data on the SPT use of one patient could not be recovered due to technical problems. In another 11 patients, data on the percentage supine position and total days used could be read out but TST or other data on SPT use was not available. The adjusted adherence could thus be calculated in 67 patients. The SPT use in hours per night and in percentage of days was not significantly different between the responder and the non-responder groups. Furthermore, 68 of 79 patients (86%) used the SPT more than 80% of the nights.
Table 4.
Adherence for the sleep position trainer during the 1-month trial in the overall group, the responders and the nonresponders.
No differences were found in the adjusted adherence between responders (96 ± 7%) and non-responders (94 ± 10%) (P = .312). In the overall group, an adjusted adherence of 95 ± 8% was achieved.
In the responder group, there was a mean reduction in REI and in supine position of 71% and 74%, respectively. Together with an adjusted adherence of 96%, this resulted in a MDA of 68% for reduction in REI and of 72% for reduction in supine position (Figure 3). In the non-responder group, a mean reduction in REI and in supine position of 21% and 58% was obtained respectively, which combined with an adjusted adherence of 94%, resulted in a MDA of 18% for reduction in REI and a MDA of 57% for reduction in supine position.
Figure 3. Mean disease alleviation.
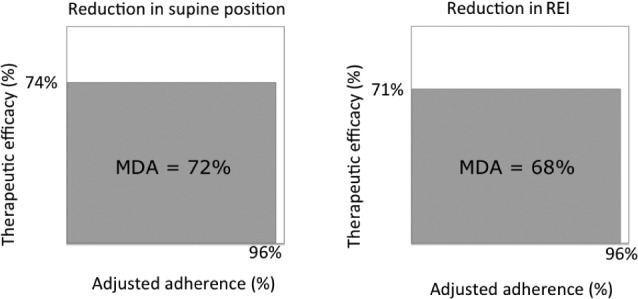
The mean disease alleviation was calculated by multiplying the adjusted adherence and the therapeutic efficacy, divided by 100, to measure reductions in supine position and REI in the responder group of 45 patients. MDA = mean disease alleviation, REI = respiratory event index.
Thirty-two of the 79 patients (41%) were both CPAP and oral appliance (OA) naïve, 23 patients (29%) had used CPAP but no OA in the past, 2 patients (3%) were OA intolerant, 9 patients (11%) were CPAP and OA intolerant and the remaining 13 patients (16%) were still using an OA and did use this OA in combination with SPT in this study. In the latter group, 5 patients had a history of CPAP treatment (6%), meaning that almost half of the patients (39 out of 79, 49%) were intolerant of some form of OSA treatment. No differences in the percentage of days used or adjusted adherence for SPT use were found between the patients who had received other treatment in the past and the patients who were treatment naïve (P = .639 and P = .953, respectively).
Purchase
The cost of the studied SPT in Belgium is 399 EUR without any reimbursement via the national health system. This is one of the reasons why it is interesting to investigate whether patients are willing to purchase the SPT after a 1-month trial period, based on the objective and subjective results.
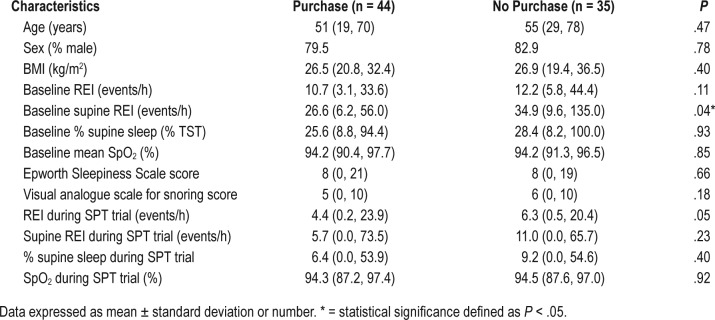
A total of 44 patients purchased the SPT (56%): the other 35 patients did (44%) not. A significant difference in baseline supine REI between these two groups was found (P = .036): the median supine REI of the patients who purchased the SPT was 27 events/h, whereas the median supine REI of the other group was 35 events/h. No differences in age, sex, BMI, overall REI, percentage of supine position, oxygen saturation index, Ep-worth Sleepiness Scale (ESS) score, or the visual analog scale for snoring could be found (Table 5). In the responder group, 27 of 45 patients decided to continue treatment with the SPT (60%) and 18 patients decided to stop treatment after the trial period (40%). Finally, 17 patients (50%) in the non-responder group wanted to purchase the SPT. Even if the objective results did not improve enough to be classified as a responder, there was improvement in objective results and they did feel better with the use of the SPT. The main reasons for not purchasing the SPT are given in Table 6.
Table 5.
Characteristics of patients who purchased the SPT compared with those of patients who did not buy the device.
Table 6.
Reasons for not purchasing the SPT with their prevalence in the total group of patients who did not purchase the device (n = 35).

DISCUSSION
In our study, POSA was defined as having an AHI in supine position that is at least twice as high as the AHI in nonsupine positions. We also required that patients had spent 10% to 90% of measurable TST during PSG in the supine position and had an overall AHI ≥ 5 events/h. During drug-induced sedation endoscopy, it has been illustrated that moving the head from a supine to a lateral position leads to improvement in upper airway collapsibility in patients with POSA.33 This suggest that positional therapy could be a good treatment option for those with POSA.6 In fact, the efficacy of positional therapy has been studied in various clinical trials and was recently summarized by Ravesloot et al.34 In addition, Barnes et al.35 reviewed the positional modification techniques. In this study, the SPT was used in the routine clinical practice for the treatment of POSA, meaning that any patient in whom POSA was diagnosed was offered to try treatment with the SPT.
Our study demonstrated the effectiveness of the SPT therapy in routine clinical practice to deter sleep in the supine position and to reduce REI in well-selected patients with relatively mild OSA and relatively low BMI. The results indicate that the SPT used in this study significantly reduces overall REI, REI in the supine position, and percentage of time spent in the supine position when compared to baseline measurements. Fifty-seven percent of the patients were considered to be responders as defined as a reduction in REI ≥ 50 % according to the literature.36 A mean reduction in overall REI of 71% was shown in the responder group. Treatment success (REI < 5 events/h) was seen in 36 of the 45 responders (80%).
A device treatment for a chronic disease is only truly successful when adherence is high. In the current study, patients used the SPT on average for 7 ± 1 h/night for 92 ± 11% of the study nights. The definition of adherence in this study was in line with that of CPAP adherence (≥ 4 h/night + ≥ 5 nights/ wk).31 An effective adherence of 94% was reached, which is high compared to other treatment options. For example, 29% to 83% of patients are not adherent to CPAP.37 In addition to the high effective adherence, an adjusted adherence of 95 ± 8% and 96 ± 7% was reached in the overall group and the responder group, respectively. No significant differences were found in adherence between responders and non-responders, and between patients who decided to continue treatment and patients who preferred not to continue. This can be explained by the fact that during this trial, the patient was not yet aware if the treatment would be successful. In other words, even if the therapy is not working well, the patient will continue to use it during the trial period and some of the non-responders would purchase the device because they are subjectively feeling better. Therefore, follow-up and monitoring of the effect by a physician is of utmost importance. If the patient would purchase the device online without prescription of a physician, there would be no guidance and evaluation or guarantee that the treatment is effective. In the past, positional therapy had a poor adherence rate due to ineffectiveness, backaches, discomfort, and lack of improvement in sleep quality or daytime alertness.6,18,38 The improvements obtained with the SPT that was used in this study may seem to have had a positive effect on adherence rates, which also was seen in the study of van Maanen et al.21 The improvement in adherence can be explained by the size and weight of the device compared to the TBT used in the past. In addition, the SPT's buildup phase allows patients to get used to the vibrations, thereby gradually decreasing supine position. Although adherence was high, the number of responders was relatively low. Non-responders tended to be more obese and had more severe OSA in supine position. Excess weight is not only a risk factor for developing OSA but also compromises treatment success as demonstrated in other treatments such as OA and hypoglossal nerve stimulation.39,40 In the current study, no differences in adherence were found between patients who received CPAP and/or OA treatment in the past and patients who did not show intolerance for other treatment options.
The MDA is a measure that takes both efficacy and adherence into account and therefore can be used to compare the outcome of different treatment options in terms of their overall clinical effectiveness. The MDA is calculated by multiplying the therapeutic efficacy by the adjusted adherence divided by 100. In the total group, an MDA of 46% and 63% was achieved for the reduction in REI and reduction in supine position, respectively. In the responder group, the MDA was much higher compared to the non-responder group: 68% versus 18% reduction in REI and 72% versus 57% reduction in supine position. The difference in MDA can be explained by the higher therapeutic efficacy in the responder group (71% versus 21% reduction in REI and 74% versus 58% reduction in supine position) as the adjusted adherence was similar in both groups (96% versus 94%). In the literature, a mean MDA of approximately 50% is published for CPAP and OA therapy.32,41 For CPAP in general, the adjusted adherence is lower compared to the SPT or OA therapy. It has been suggested that the comparable MDA for CPAP and OA therapy may be explained by the greater efficacy of CPAP (in terms of a lower residual AHI) being offset by the inferior adherence of CPAP, possibly resulting in equal effectiveness.42,43
At the end of the trial period, 44 of the 79 patients (56%) purchased the SPT. The remaining 35 patients (44%) did not purchase the SPT, citing no objective benefit, intolerance to the vibrations, or the expense of the device as their main reasons. It is interesting that 17 patients who did not meet the criteria of being a responder did purchase the SPT. They were perhaps swayed by what objective improvement was seen in their results, or it could be that the patients' subjective improvement was enough. Subjective improvement may be of great importance because when patients subjectively feel better with treatment, they are then more likely to continue to use it.
The authors stress the importance of the SPT trial period to prevent patients who objectively and/or subjectively would not benefit from the therapy from purchasing the device. Furthermore, patients suffering from residual POSA under a given therapy can be good candidates for combination therapy. Dieltjens et al.26 studied the effect of the combination of OA therapy with SPT, whereas Benoist et al.27 investigated the effect of additional SPT therapy after upper airway surgery. Both studies showed promising results.
Some study limitations need to be addressed. First, a difference in baseline respiratory indices (AHI/REI), percentage of supine sleep, and respiratory indices while in the supine position was noted between the baseline PSG in the hospital and the HSAT measurement at home, with the PSG values being higher. A first explanation for this is the fact that the HSAT used in this study does not measure sleep signals, which may have led to a systematic bias of underreporting. Adjusted adherence was calculated as the use in hours per night as the percentage of TST derived from the PSG. During PSG, the TST is used to calculate AHI, which is not possible with the HSAT. When using an HSAT, the time in bed is used to calculate the REI so there is no correction for sleep onset latency or the time the patient is awake during the night. In addition, it is not possible to score an arousal during the HSAT, meaning that the definition of an arousal is only based on the limitation in flow and the percentage of desaturation. However, the HSAT was tested and found to be a valid method to measure respiration and accurately identify patients without OSA.29
Another explanation for the aforementioned differences in baseline measurements could be night-to-night variability.44 This phenomenon can be explained by both the so-called “first-night effect” and intrinsic variability.45,46 Intrinsic variability can be divided into variables such as behavioral factors, differences in body position, and sleep architecture between recordings. Therefore, an important factor that influences night-to-night variability is the time spent sleeping in the supine position. In our study this was higher on the PSG measurements compared to the HSAT measurements. A possible explanation is that patients slept more in the supine position during the PSG nights because the equipment forced them to do so.
Another potential limitation is that sleep position was measured by a position sensor placed on the trunk. Van Kesteren et al. showed that although measurement at the trunk is important, measurement at the head is also valuable.47 Future studies might consider using sensors at both locations. Furthermore, this study showed that the subjective parameters play a crucial role in whether or not patients are willing to purchase the SPT. Five of the 35 patients (14%) who decided not to purchase the SPT reported persistent snoring or persistent daytime sleepiness as the main reason for not buying the device. In future follow-up with all patients, these factors need more attention and will be considered.
A final limitation is that patients used the SPT for only 28 days; therefore, no results on long-term effectiveness can be reported. This is important because although the SPT shows promising short-term results regarding adherence when compared to the TBT, adherence problems with positional therapy usually occur after a longer period of use.6,18 Because long-term adherence needs to be achieved, further research is needed and currently is ongoing. In addition, Cartwright et al.48 suggested that patients might learn to avoid the supine position following positional therapy. This could indicate that there would be no need to use the SPT every night. It is also possible that some patients only may need positional therapy periodically to reinforce training, whereas other patients may need SPT consistently to ensure sleep in nonsupine positions.48 A study by van Maanen et al. of patients with mild to moderate POSA (5 ≤ AHI < 15 events/h) showed promising results concerning the effectiveness of SPT over a period of 6 months, including improvement to sleep-related quality of life and diminished subjective daytime sleepiness.23 There were no subjective results collected after 1 month of SPT use in this study. Because the subjective experiences of the patients and their bed partners are important factors to determine long-term adherence, these parameters will be collected during further follow-up.
CONCLUSIONS
It can be stated that after a 1-month trial period, the SPT is an effective treatment for 57% of well-selected patients with POSA (45 of 79). We measured reduction in REI from 11 events/h at baseline to 3 events/h with treatment in the responder group (decrease in REI of at least 50% compared to baseline). Responders also showed a reduction in supine position from 31% to 6%. These results come with a high adherence of 96%. At the end of the trial period, 44 patients (27 responders and 17 non-responders) decided to purchase the SPT. However, only 60% of the patients who would potentially benefit from the treatment bought the device after finishing the trial period. The key reasons that responders and non-responders decided not to purchase the SPT were insufficient objective results, intolerance for the vibrations, the cost of the device, persistent daytime sleepiness, or the patient's preference for another treatment.
In conclusion, the results of this prospective trial illustrate that SPT treatment can be a successful short-term treatment option for well-selected patients with mild POSA who are mildly overweight. The SPT treatment came with high adherence rates and was effective in reducing REI and supine sleep position. In addition, a trial period with the SPT may prevent patients who do not benefit from positional training from purchasing an SPT.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. This was not an industry-supported study. Prof. Dr. Vanderveken reports research support from NightBalance, Inspire Medical Systems, ReVent, and Somnomed; grants from Philips Respironics and Somnomed, all outside the submitted work. The other authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Mrs. Adelheidis Hoogewijs for her assistance with this research project.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CPAP
continuous positive airway pressure
- ENT
ear, nose and throat
- ESS
Epworth Sleepiness Scale
- HSAT
home sleep apnea test
- MDA
mean disease alleviation
- OA
oral appliance
- OSA
obstructive sleep apnea
- POSA
positional obstructive sleep apnea
- PSG
polysomnography
- REI
respiratory event index
- SPT
sleep position trainer
- TBT
tennis ball technique
- TST
total sleep time
REFERENCES
- 1.Kryger MH. Diagnosis and management of sleep apnea syndrome. Clin Cornerstone. 2000;2(5):39–47. doi: 10.1016/s1098-3597(00)90039-5. [DOI] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 4.Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90(1):47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–276. [PMC free article] [PubMed] [Google Scholar]
- 6.Oksenberg A, Silverberg D, Offenbach D, Arons E. Positional therapy for obstructive sleep apnea patients: A 6-month follow-up study. Laryngoscope. 2006;116(11):1995–2000. doi: 10.1097/01.mlg.0000237674.66716.a7. [DOI] [PubMed] [Google Scholar]
- 7.Oksenberg A, Silverberg DS, Arons E, Radwan H. Positional vs nonpositional obstructive sleep apnea patients: anthropomorphic, nocturnal polysomnographic, and multiple sleep latency test data. Chest. 1997;112(3):629–639. doi: 10.1378/chest.112.3.629. [DOI] [PubMed] [Google Scholar]
- 8.Cartwright RD. Effect of sleep position on sleep apnea severity. Sleep. 1984;7(2):110–114. doi: 10.1093/sleep/7.2.110. [DOI] [PubMed] [Google Scholar]
- 9.Richard W, Kox D, den Herder C, Laman M, van Tinteren H, de Vries N. The role of sleep position in obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol. 2006;263(10):946–950. doi: 10.1007/s00405-006-0090-2. [DOI] [PubMed] [Google Scholar]
- 10.Teerapraipruk B, Chirakalwasan N, Simon R, et al. Clinical and polysomnographic data of positional sleep apnea and its predictors. Sleep Breath. 2012;16(4):1167–1172. doi: 10.1007/s11325-011-0627-5. [DOI] [PubMed] [Google Scholar]
- 11.Joosten SA, Hamza K, Sands S, Turton A, Berger P, Hamilton G. Phenotypes of patients with mild to moderate obstructive sleep apnoea as confirmed by cluster analysis. Respirology. 2012;17(1):99–107. doi: 10.1111/j.1440-1843.2011.02037.x. [DOI] [PubMed] [Google Scholar]
- 12.Mador MJ, Kufel TJ, Magalang UJ, Rajesh SK, Watwe V, Grant BJ. Prevalence of positional sleep apnea in patients undergoing polysomnography. Chest. 2005;128(4):2130–2137. doi: 10.1378/chest.128.4.2130. [DOI] [PubMed] [Google Scholar]
- 13.Randerath WJ, Verbraecken J, Andreas S, et al. Non-CPAP therapies in obstructive sleep apnoea. Eur Respir J. 2011;37(5):1000–1028. doi: 10.1183/09031936.00099710. [DOI] [PubMed] [Google Scholar]
- 14.Zuberi NA, Rekab K, Nguyen HV. Sleep apnea avoidance pillow effects on obstructive sleep apnea syndrome and snoring. Sleep Breath. 2004;8(4):201–207. doi: 10.1007/s11325-004-0201-5. [DOI] [PubMed] [Google Scholar]
- 15.Wenzel S, Smith E, Leiacker R, Fischer Y. [Efficacy and longterm compliance of the vest preventing the supine position in patients with obstructive sleep apnea] Laryngorhinootologie. 2007;86(8):579–583. doi: 10.1055/s-2007-966179. [DOI] [PubMed] [Google Scholar]
- 16.Loord H, Hultcrantz E. Positioner--a method for preventing sleep apnea. Acta Otolaryngol. 2007;127(8):861–868. doi: 10.1080/00016480601089390. [DOI] [PubMed] [Google Scholar]
- 17.Jokic R, Klimaszewski A, Crossley M, Sridhar G, Fitzpatrick MF. Positional treatment vs continuous positive airway pressure in patients with positional obstructive sleep apnea syndrome. Chest. 1999;115(3):771–781. doi: 10.1378/chest.115.3.771. [DOI] [PubMed] [Google Scholar]
- 18.Bignold JJ, Deans-Costi G, Goldsworthy MR, et al. Poor long-term patient compliance with the tennis ball technique for treating positional obstructive sleep apnea. J Clin Sleep Med. 2009;5(5):428–430. [PMC free article] [PubMed] [Google Scholar]
- 19.Skinner MA, Kingshott RN, Filsell S, Taylor DR. Efficacy of the ‘tennis ball technique’ versus nCPAP in the management of position-dependent obstructive sleep apnoea syndrome. Respirology. 2008;13(5):708–715. doi: 10.1111/j.1440-1843.2008.01328.x. [DOI] [PubMed] [Google Scholar]
- 20.van Maanen JP, Richard W, Van Kesteren ER, et al. Evaluation of a new simple treatment for positional sleep apnoea patients. J Sleep Res. 2012;21(3):322–329. doi: 10.1111/j.1365-2869.2011.00974.x. [DOI] [PubMed] [Google Scholar]
- 21.van Maanen JP, Meester KA, Dun LN, et al. The sleep position trainer: a new treatment for positional obstructive sleep apnoea. Sleep Breath. 2013;17(2):771–779. doi: 10.1007/s11325-012-0764-5. [DOI] [PubMed] [Google Scholar]
- 22.Bignold JJ, Mercer JD, Antic NA, McEvoy RD, Catcheside PG. Accurate position monitoring and improved supine-dependent obstructive sleep apnea with a new position recording and supine avoidance device. J Clin Sleep Med. 2011;7(4):376–383. doi: 10.5664/JCSM.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Maanen JP, de Vries N. Long-term effectiveness and compliance of positional therapy with the sleep position trainer in the treatment of positional obstructive sleep apnea syndrome. Sleep. 2014;37(7):1209–1215. doi: 10.5665/sleep.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eijsvogel MM, Ubbink R, Dekker J, et al. Sleep position trainer versus tennis ball technique in positional obstructive sleep apnea syndrome. J Clin Sleep Med. 2015;11(2):139–147. doi: 10.5664/jcsm.4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levendowski DJ, Seagraves S, Popovic D, Westbrook PR. Assessment of a neck-based treatment and monitoring device for positional obstructive sleep apnea. J Clin Sleep Med. 2014;10(8):863–871. doi: 10.5664/jcsm.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dieltjens M, Vroegop AV, Verbruggen AE, et al. A promising concept of combination therapy for positional obstructive sleep apnea. Sleep Breath. 2015;19(2):637–644. doi: 10.1007/s11325-014-1068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benoist LB, Verhagen M, Torensma B, van Maanen JP, de Vries N. Positional therapy in patients with residual positional obstructive sleep apnea after upper airway surgery. Sleep Breath. 2017;21(2):279–288. doi: 10.1007/s11325-016-1397-x. [DOI] [PubMed] [Google Scholar]
- 28.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22(5):667–689. [PubMed] [Google Scholar]
- 29.Driver HS, Pereira EJ, Bjerring K, et al. Validation of the MediByte(R) type 3 portable monitor compared with polysomnography for screening of obstructive sleep apnea. Can Respir J. 2011;18(3):137–143. doi: 10.1155/2011/760958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 31.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147(4):887–895. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 32.Vanderveken OM, Dieltjens M, Wouters K, De Backer WA, Van de Heyning PH, Braem MJ. Objective measurement of compliance during oral appliance therapy for sleep-disordered breathing. Thorax. 2013;68(1):91–96. doi: 10.1136/thoraxjnl-2012-201900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Safiruddin F, Koutsourelakis I, de Vries N. Analysis of the influence of head rotation during drug-induced sleep endoscopy in obstructive sleep apnea. Laryngoscope. 2014;124(9):2195–2199. doi: 10.1002/lary.24598. [DOI] [PubMed] [Google Scholar]
- 34.Ravesloot MJL, White D, Heinzer R, Oksenberg A, Pepin JL. Efficacy of the new generation of devices for positional therapy for patients with positional obstructive sleep apnea: a systematic review of the literature and meta-analysis. J Clin Sleep Med. 2017;13(6):813–824. doi: 10.5664/jcsm.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barnes H, Edwards BA, Joosten SA, Naughton MT, Hamilton GS, Dabscheck E. Positional modification techniques for supine obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev. 2017;36:107–115. doi: 10.1016/j.smrv.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Ferguson KA, Cartwright R, Rogers R, Schmidt-Nowara W. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep. 2006;29(2):244–262. doi: 10.1093/sleep/29.2.244. [DOI] [PubMed] [Google Scholar]
- 37.de Vries N, Ravesloot MJ, van Maanen PJ, editors. Positional Therapy in Obstructive Sleep Apnea. Switzerland: Springer International Publishing; 2015. [Google Scholar]
- 38.Permut I, Diaz-Abad M, Chatila W, et al. Comparison of positional therapy to CPAP in patients with positional obstructive sleep apnea. J Clin Sleep Med. 2010;6(3):238–243. [PMC free article] [PubMed] [Google Scholar]
- 39.Van de Heyning PH, Badr MS, Baskin JZ, et al. Implanted upper airway stimulation device for obstructive sleep apnea. Laryngoscope. 2012;122(7):1626–1633. doi: 10.1002/lary.23301. [DOI] [PubMed] [Google Scholar]
- 40.Marklund M, Stenlund H, Franklin KA. Mandibular advancement devices in 630 men and women with obstructive sleep apnea and snoring. Chest. 2004;125(4):1270–1278. doi: 10.1378/chest.125.4.1270. [DOI] [PubMed] [Google Scholar]
- 41.Grote L, Hedner J, Grunstein R, Kraiczi H. Therapy with nCPAP: incomplete elimination of sleep related breathing disorder. Eur Respir J. 2000;16(5):921–927. doi: 10.1183/09031936.00.16592100. [DOI] [PubMed] [Google Scholar]
- 42.Phillips CL, Grunstein RR, Darendeliler MA, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2013;187(8):879–887. doi: 10.1164/rccm.201212-2223OC. [DOI] [PubMed] [Google Scholar]
- 43.Vanderveken OM, Braem MJ, Dieltjens M, De Backer WA, Van de Heyning PH. Objective measurement of the therapeutic effectiveness of continuous positive airway pressure versus oral appliance therapy for the treatment of obstructive sleep apnea. Am J Respir Crit Care Med. 2013;188(9):1162. doi: 10.1164/rccm.201305-0809LE. [DOI] [PubMed] [Google Scholar]
- 44.Stoberl AS, Schwarz EI, Haile SR, et al. Night-to-night variability of obstructive sleep apnea. J Sleep Res. 2017;26(6):782–788. doi: 10.1111/jsr.12558. [DOI] [PubMed] [Google Scholar]
- 45.Agnew HW, Jr, Webb WB, Williams RL. The first night effect: an EEG study of sleep. Psychophysiology. 1966;2(3):263–266. doi: 10.1111/j.1469-8986.1966.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 46.Aarab G, Lobbezoo F, Hamburger HL, Naeije M. Variability in the apneahypopnea index and its consequences for diagnosis and therapy evaluation. Respiration. 2009;77(1):32–37. doi: 10.1159/000167790. [DOI] [PubMed] [Google Scholar]
- 47.van Kesteren ER, van Maanen JP, Hilgevoord AA, Laman DM, de Vries N. Quantitative effects of trunk and head position on the apnea hypopnea index in obstructive sleep apnea. Sleep. 2011;34(8):1075–1081. doi: 10.5665/SLEEP.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cartwright RD, Lloyd S, Lilie J, Kravitz H. Sleep position training as treatment for sleep apnea syndrome: a preliminary study. Sleep. 1985;8(2):87–94. doi: 10.1093/sleep/8.2.87. [DOI] [PubMed] [Google Scholar]