Abstract
Study Objectives:
To report the diagnostic and treatment challenges of sighted non–24-hour sleep-wake disorder (N24SWD).
Methods:
We report a series of seven sighted patients with N24SWD clinically evaluated by history and sleep diaries, and when available wrist actigraphy and salivary melatonin levels, and treated with timed melatonin and bright light therapy.
Results:
Most patients had a history of a delayed sleep-wake pattern prior to developing N24SWD. The typical sleep-wake pattern of N24SWD was seen in the sleep diaries (and in actigraphy when available) in all patients with a daily delay in midpoint of sleep ranging 0.8 to 1.8 hours. Salivary dim light melatonin onset (DLMO) was evaluated in four patients but was missed in one. The estimated phase angle from DLMO to sleep onset ranged from 5.25 to 9 hours. All six patients who attempted timed melatonin and bright light therapy were able to entrain their sleep-wake schedules. Entrainment occurred at a late circadian phase, possibly related to the late timing of melatonin administration, though the patients often preferred late sleep times. Most did not continue treatment and continued to have a non–24-hour sleep-wake pattern.
Conclusions:
N24SWD is a chronic debilitating disorder that is often overlooked in sighted people and can be challenging to diagnose and treat. Tools to assess circadian pattern and timing can be effectively applied to aid the diagnosis. The progressive delay of the circadian rhythm poses a challenge for determining the most effective timing for melatonin and bright light therapies. Furthermore, once the circadian sleep-wake rhythm is entrained, long-term effectiveness is limited because of the behavioral and environmental structure that is required to maintain stable entrainment.
Citation:
Malkani RG, Abbott SM, Reid KJ, Zee PC. Diagnostic and treatment challenges of sighted non–24-hour sleep-wake disorder. J Clin Sleep Med. 2018;14(4):603–613.
Keywords: bright light, circadian rhythm, delayed sleep-wake phase disorder, melatonin, non–24-hour sleep-wake disorder
BRIEF SUMMARY
Current Knowledge/Study Rationale: Non–24-hour sleep-wake disorder in sighted people is uncommon, often overlooked, and severely affects daily function and quality of life. We aimed to highlight the challenges in diagnosis and circadian-based treatment of non–24-hour sleep-wake disorder with bright light exposure and melatonin in a clinical setting.
Study Impact: Although timed melatonin and bright light therapy is effective to entrain the sleep-wake rhythm, it requires frequent physician-patient contact, and long-term patient adherence to treatment is low. This study highlights the need for understanding the pathophysiology of this disorder, to develop clinical tools for diagnosis and treatment planning, and to develop and test a targeted and effective long-term therapeutic strategy.
INTRODUCTION
All humans have endogenous circadian rhythms that are regulated by the suprachiasmatic nucleus (SCN), a central clock located in the hypothalamus.1 The endogenous period in humans is usually slightly longer than 24 hours, requiring daily adjustment to entrain to the 24-hour light/dark cycle.2,3 People with non– 24-hour sleep-wake disorder (N24SWD), previously known as “free-running rhythm disorder,” are unable to entrain their endogenous circadian rhythm to the 24-hour environment. As a result, the circadian rhythm of sleep and wake typically delays each day, resulting in symptoms of difficulty sleeping, excessive sleepiness, or both. The recurring circadian misalignment significantly disrupts social function and impairs quality of life.4
N24SWD is observed in up to 50% of blind people but is thought to be rare in sighted individuals.5 The first case report of a sighted individual with N24SWD was published in 1971, describing a male whose “day” was 26 hours long.6 Since then, there have been several case reports; the largest of these consisted of 57 individuals.4
The diagnosis and management of patients with N24SWD are challenging. An adequate duration of sleep diary or actigraphy monitoring must be obtained to detect the non–24-hour pattern. There are limited data on management of sighted N24SWD, and therapies are based on treatment of N24SWD in the blind and on phase response curves (PRCs) for light and melatonin determined in healthy adults.7,8 Bright light exposure in the biological evening results in a delay of the circadian rhythm, and light in the biological morning produces an advance.7,9 Exogenous melatonin given during an individual's biological evening leads to an advance of phase of the circadian rhythm, whereas melatonin in the biological morning produces a delay. The maximum phase advance occurs with melatonin 0.5 mg administered 3 hours before the dim light melatonin onset (DLMO) or 3 mg 5 hours before the DLMO.10,11 Because DLMO typically occurs an average of 2 to 3 hours before sleep onset in healthy adults, melatonin should be given 5 to 6 hours before sleep onset for the 0.5-mg dose or 7 to 8 hours before sleep onset for the 3-mg dose to achieve the maximum phase advance. In blind N24SWD, melatonin at 9:00 pm12 or 1 hour before bedtime13 entrained the sleep-wake schedule and is useful to advance and stabilize the sleep-wake schedule. In addition, a combination of bright light in the biological morning and melatonin in the biological evening leads to greater phase advances than either stimulus alone14; therefore, combination therapy may be more likely to entrain people with N24SWD than either melatonin or bright light therapy alone.
Although earlier reports focus on specific characteristics of the disorder, in this new report we use a case-based approach to illustrate the diagnostic and treatment challenges in sighted N24SWD and highlight the need for a multimodal treatment strategy. For each case we provide detailed self-reported and, in most cases, objective (actigraphic) measures of sleep-wake patterns and response to treatment.
REPORT OF CASES
The following are a series of seven cases of N24SWD who presented to the Northwestern University Sleep Disorders Clinic from 2009 to 2013.
Case 1
History
A 15-year-old male presented with difficulty falling asleep and excessive sleepiness since the first grade that initially occurred intermittently. There was no history of long sleep duration or mood disturbance during the episodes of excessive sleepiness. Over several years, these symptoms became persistent; because his sleep-wake times were typically delayed, the diagnosis of delayed sleep-wake phase disorder (DSWPD) was made. Chronotherapy (gradually delaying the patient's bedtime until he was sleeping at the desired bedtime) helped him fall asleep more easily at night, but the benefit lasted only a few weeks. Despite sleeping for 8 to 9 hours, he continued to feel groggy for 1 to 2 hours after waking. He previously tried melatonin 3 mg at his desired bedtime to stabilize his sleep-wake schedule for 1 week and bright light therapy on a separate occasion (timing unknown) without success. At the time of our evaluation, his bed and wake times were delaying each day.
Medical history included abdominal discomfort, exercise-induced asthma, and mononucleosis. Family history was notable for insomnia in his father.
Evaluation and Treatment
Sleep diaries over 14 days (Figure 1A) showed a mean daily delay in midpoint of sleep of 1.5 hours and an average sleep duration of 9.0 hours, consistent with a diagnosis of N24SWD. Twenty-four-hour salivary melatonin sampling was done hourly under dim light conditions at home. Based on the daily delay in the sleep-wake schedule of approximately 1.5 hours, the predicted sleep onset time was 10:00 pm on the day of sampling. Sampling began on day 6 at 5:00 pm, 5 hours before predicted sleep onset. The patient was instructed to wear dark goggles (Uvex Bandit, espresso tint, Honeywell Int. Morris Plains, New Jersey, United States) starting 30 minutes prior to and during sampling and to keep the lights dim but sufficient to move from room to room. In addition, the patient was instructed not to eat or drink anything within 20 minutes prior to taking a sample and to not brush his teeth or use a breath mint for 2 hours prior to and during sampling. The patient slept during part of the sampling period and was awakened to obtain saliva. The patient collected saliva hourly by placing a cotton swab (Salivette, Sarstedt AG & Co, Nümbrecht, Germany), under the tongue for 2 to 3 minutes. Vials containing the swab were frozen and sent to the laboratory for analysis. Melatonin concentration was measured using a Buhlman direct radioimmunoassay (ALPCO, Windham, New Hampshire, United States).15 The DLMO was defined as the point greater than 2 standard deviations above baseline melatonin concentration.
Figure 1. Circadian phase marker assessment of N24SWD.
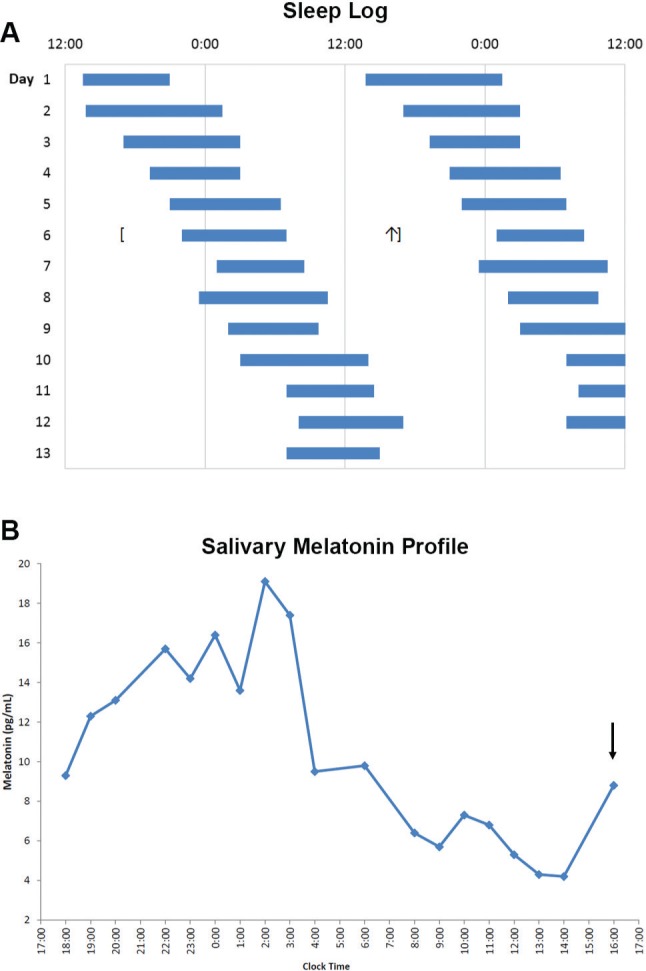
Sleep log (A) and salivary melatonin profile (B) in case 1. (A) Double-plotted sleep diaries from case 1 show daily delays of the sleep-wake schedule typical of N24SWD. (B) Salivary melatonin profile under dim light conditions performed on day 6 of the sleep log (between brackets) revealed a DLMO (defined as exceeding 2 standard deviations from baseline) at 4:00 PM (arrow). The time from DLMO at 4:00 PM to sleep onset at 1:00 AM was 9 hours. DLMO = dim light melatonin onset, N24SWD = non–24-hour sleep-wake disorder.
Melatonin levels appeared to have already risen prior to the start of sampling at 5:00 pm (Figure 1B). The following day, the DLMO occurred at approximately 4:00 pm (last sample taken), and the patient's subsequent sleep onset was at 1:00 am. Therefore, we estimated the phase angle from DLMO to sleep onset to be 7 to 9 hours, compared to a reported average DLMO to sleep onset phase angle of approximately 2 to 3 hours among normal adults, though there can be significant interindividual variability.16,17
When the patient's sleep onset reached the desired time of 10:00 pm, melatonin 3 mg at 9:00 pm and bright light therapy (10,000 lux for 30 minutes) before 11:00 am for 30 to 60 minutes were initiated. He was also instructed to keep lights dim (eg, avoid use of overhead lighting) after 9:00 pm. His sleep-wake rhythm became more predictable though not stable, and his abdominal discomfort improved. After 1 week, 1 mg of melatonin was added at 6:30 pm, and the bright light exposure timing was advanced to 7:30 am and the duration was increased to 120 to 150 minutes. These changes resulted in a stable bedtime of 12:30 am and wake time of 7:30 am and further improvement in abdominal discomfort. The combination of an earlier and later dose of melatonin may have provided both phase shifting (earlier dose, and perhaps later dose) and soporific effects (later dose). At follow-up 4 months later, he had a stable bedtime and wake time of 10:30 pm and 8:00 am, respectively. At that time, his regimen was melatonin 1 mg at 7:00 pm and 3 mg at 10:30 pm and light exposure duration of 120 minutes starting at 8:00 am.
Case 2
History
A 19-year-old male presented with difficulty falling asleep since childhood. During high school, he was unable to fall asleep before 1:00 am and required at least 9 hours of sleep to feel refreshed. At the time of evaluation, his bedtime was 3:30 am and wake time was 9:00–10:30 am with an alarm (3:00–4:00 pm without alarm). On occasion his sleep-wake rhythm progressively delayed, resulting in sleep onset time of 6:00 am or later, which interfered with his college performance. He tried zolpidem but experienced hallucinations and grogginess upon awakening.
Medical history included depression and two concussions with brief amnesia in high school, before his sleep-wake rhythm started progressively delaying. Family history was notable for obstructive sleep apnea (OSA) in his father.
Evaluation and Treatment
Sleep diaries and actigraphy over 14 days showed a N24SW pattern with a mean daily delay in the midpoint of the sleep period of 0.8 hours consistent with the diagnosis of N24SWD. Serial salivary melatonin for DLMO was obtained, similar to case 1, except that saliva was sampled every 30 minutes between 7:00 am and 1:00 pm, with a predicted sleep onset time of 11:00 am based on sleep diaries. However, the melatonin profile remained low throughout sampling. Although timed evening melatonin 1 mg and morning bright light therapy were recommended when his sleep schedule aligned with his goal bedtime of 11:00 pm, he did not attempt treatment, and daily delays of his sleep-wake schedule continued.
Case 3
History
A 15-year-old male presented with difficulty falling asleep and excessive daytime sleepiness. As a child, he was unable to fall asleep before 1:00 am. At age 13 years his sleep onset time delayed further to 5:00–6:00 am. By age 14 years, he had a stable bedtime of 2:00 am and wake time of 11:00 am, but would occasionally sleep for 18 to 20 hours.
Medical history included two concussions with a few seconds of loss of consciousness at ages 10 and 14 years. OSA was diagnosed at age 14 years, but resolved with tonsillectomy. Excessive sleepiness continued and did not improve with modafinil 400 mg daily. Family history was notable for a brother with a delayed but stable bedtime of 5:00 am and wake time of 12:00 pm.
Evaluation and Treatment
Sleep diaries over 21 days and actigraphy over 14 days demonstrated a non–24-hour sleep-wake pattern with a mean daily delay in midpoint of the sleep period of approximately 0.8 hours (Figure 2A). Home salivary melatonin sampling, with methods similar to case 1, was performed every hour beginning at 2:00 am (11 hours before predicted bedtime) until 6:00 am and then every 30 minutes until 4:30 pm (after predicted sleep onset). The DLMO occurred at 6:30 am, and sleep onset occurred at 11:45 am. The phase angle between DLMO and sleep onset was 5.25 hours.
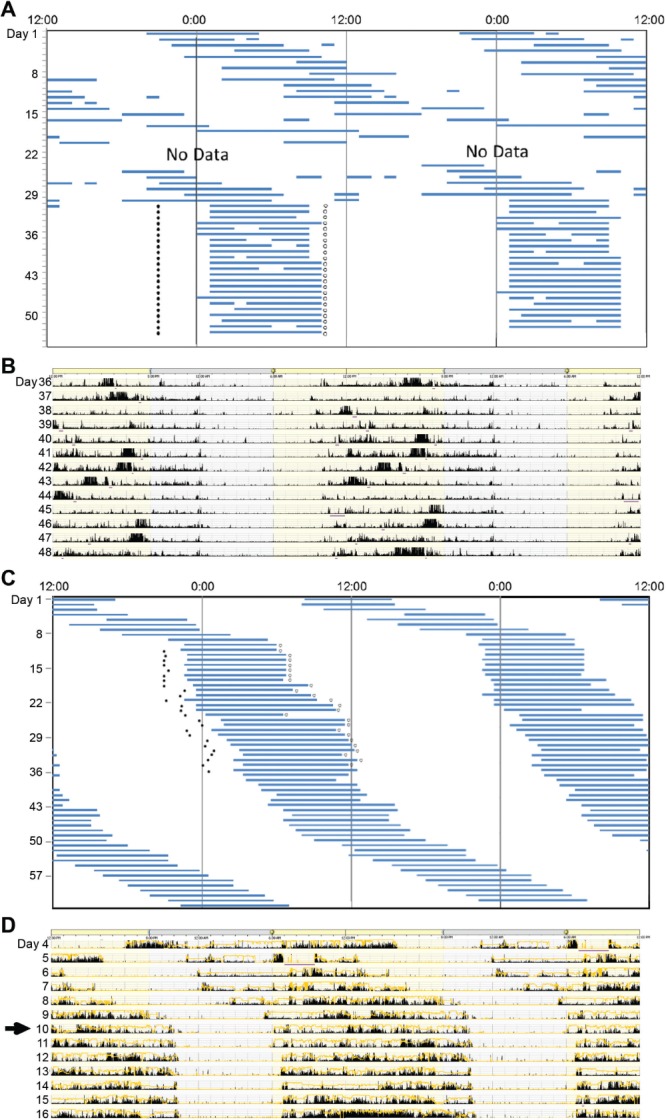
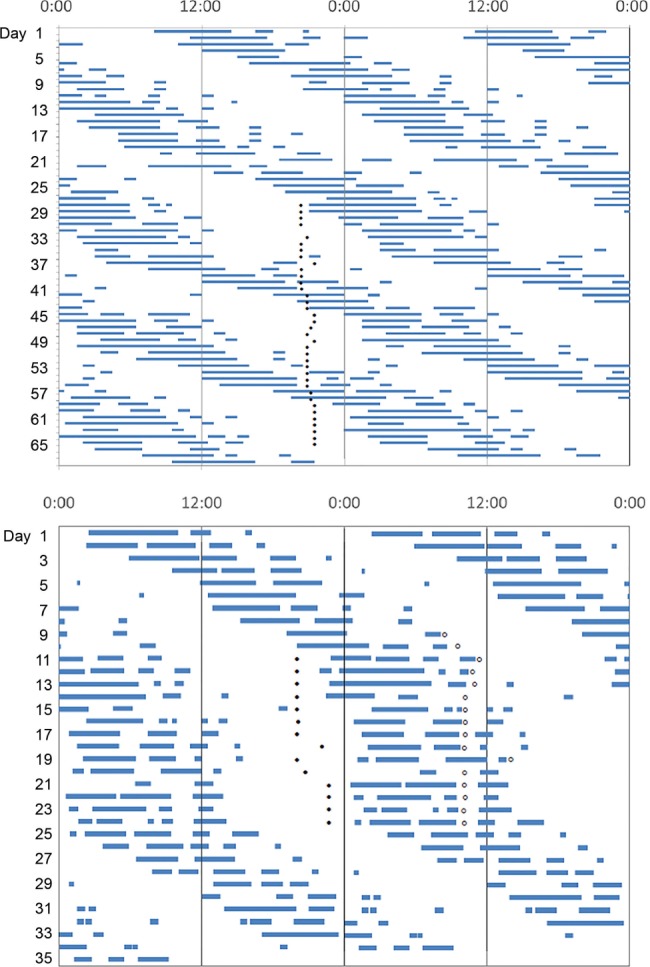
Figure 2. Actigraphic assessment of N24SWD.
Double-plotted rest-activity rhythms in case 3 (A), case 5 (B), case 6 (C), and case 7 (D). Rates of delay can be variable among patients with N24SWD. As seen in (A) and (B), rates of daily delays are slower than in (C) and (D). In those who delay more slowly, the N24SWD pattern may not be apparent in monitoring for 7 days. Therefore, monitoring for at least 14 days is recommended. In addition, the rate of delay can change within an individual (C), with greater delays when the sleep onset time occurs during the day. N24SWD = non–24-hour sleep-wake disorder.
After allowing the patient's sleep-wake times to delay to the desired sleep time of 10:00 pm, treatment was initiated with melatonin 0.5 mg between 8:00 pm and 9:30 pm (1 to 2 hours before desired sleep time). He used a goLITE BLU light box (Koninklijke Philips N.V., Amsterdam, Netherlands), and was instructed to keep it approximately 2 feet away from his face and in his visual field for 30 to 60 minutes between 10:00 am and 12:00 pm. These treatments resulted in a stable sleep-wake rhythm with approximate sleep and wake times of 11:00 pm and 9:30 am, respectively. However, he was unable to maintain treatment after several weeks due to a combination of staying up late to complete homework or socialize and not waking up early enough for bright light therapy. The inability to maintain entrainment may have been due to an inability to adhere to therapy, inappropriate use of the light box, or the timing of melatonin. It is also possible that dosing the melatonin earlier, about 5 hours before desired sleep time, may have been more effective. In addition, if the patient's sleep-wake schedule continued to delay then it would have been increasingly difficult to maintain treatment and entrain at the desired time until his sleep-wake schedule eventually delayed until his desired timing again.
Case 4
History
A 26-year-old male presented with difficulty falling asleep at night and excessive daytime sleepiness. At age 14 years, he was unable to fall asleep until after midnight and had difficulty waking up in the morning for school. On weekends his wake time was 10:00–11:00 am. In college, when his schedule was more flexible, sleep and wake times delayed each day. At age 22 years, he began to experience headaches, associated with increased sleep totaling 14 to 20 hours per day. At presentation, he had a bedtime of 9:00 am and wake time of 7:00 pm, with approximately 1 hour of daily delay in the sleep time. This instability of his schedule led to inability to hold a regular job or schedule social activities.
Medical history included myopia, treated depression and anxiety, and arachnophobia. There was no family history of sleep disorders.
Evaluation and Treatment
Sleep diaries over 19 days confirmed the diagnosis of N24SWD with a mean daily delay in midpoint of the sleep period of approximately 1.8 hours (Figure 3A).
Figure 3. Timed melatonin and bright light therapy for N24SWD.
Double-plotted sleep diaries and wrist actigraphy in case 4 (A,B) and case 5 (C,D) are shown. The days on the actigraphy overlap with those on the sleep log. Prior to treatment, both cases had daily delays of the sleep-wake schedule. Treatment was initiated when the predicted sleep time was at the patient's desired time. In case 4, melatonin 1 mg (closed circles) 3 to 4 hours before the desired sleep time and bright light therapy for 60 minutes (open circles) after waking led to successful entrainment to a 24-hour schedule. In case 5, melatonin (closed circles) 2 to 3 hours before the desired sleep time and bright light therapy for usually 45 minutes (open circles) on awakening initially led to successful entrainment as seen in the first 8 days of treatment. In (D), the arrow indicates the day treatment was initiated. However, the patient gradually delayed the timing of melatonin and light, leading to delays in the sleep-wake schedule. After discontinuing treatment, he continued to delay in a N24SWD pattern. These cases highlight the ability of timed melatonin and bright light therapy to successfully entrain the sleep-wake schedule in patients with N24SWD, but consistent timing and dose is required to maintain the effect. N24SWD = non–24-hour sleep-wake disorder.
When his predicted sleep times delayed to 1:00 am, he began treatment with evening melatonin 1 mg at 9:00 pm. He was also instructed to use a 10,000 lux light box and keep it 2 feet in front of him for up to 2 hours upon awakening. Unfortunately, we do not have a record of what type of light box he used, how he placed it, or how long he used it each day, and these variables can affect the effective light intensity. The melatonin and light therapy resulted in entrainment of his sleep-wake schedule to a 24-hour cycle based on sleep diaries and wrist actigraphy (Figure 3A and Figure 3C). On follow-up 11 months later, he had lost motivation to continue therapy, and his sleep-wake rhythm continued to delay each day.
Case 5
History
A 33-year-old male presented with difficulty falling asleep at night and inability to maintain a stable sleep-wake schedule since age 26. By age 28, his sleep and wake times were delaying each day. He also reported anxiety, fatigue, daytime sleepiness, intermittent gastrointestinal discomfort, and morning headaches. At evaluation, his bedtime was 10:00 pm and wake time was 5:00–6:00 am. He felt unrefreshed even after 10 hours of sleep. He was unable to hold a job or keep social activities. He tried several medications, including ramelteon, aripiprazole, sertraline, trazodone, and dextroamphetamine but they either did not help or caused intolerable side effects.
Medical history included mild untreated OSA and gastroesophageal reflux. Family history was notable for his mother having late sleep and wake times.
Evaluation and Treatment
Sleep diary and actigraphy (Figure 2B) showed a mean daily delay in sleep midpoint of 1.5 hours, consistent with N24SWD; delays were greater when his sleep onset time was during the daytime.
When his sleep time aligned with his goal bedtime of 11:00 pm, the patient was instructed to begin taking melatonin 1 mg at 9:00 pm and use bright light therapy (goLITE BLU) placed 2 feet in front of him upon awakening for 45 minutes. This helped to stabilize his sleep-wake time (Figure 3B and Figure 3D). He continued to report fatigue despite entrainment. After 14 days, he gradually and intentionally delayed the timing of melatonin and bright light therapy to as late as 2:15 am and 12:40 pm, respectively, leading to a slow delay of the sleep-wake times. However, he was unable to adhere to the melatonin or morning light therapy. Approximately 2.5 years later, he resumed treatment. When his predicted bedtime was at 2:30 am, he restarted melatonin 1 mg at 12:30 am and bright light therapy (goLITE BLU) at 10:00 am for 30 minutes, later changed to 10:30 am for 60 minutes. His bed and wake times remained stable at approximately 2:00 am and 10:30 am for more than 1 year while using melatonin 1 mg at 12:30 am and light therapy for 40 to 50 minutes upon awakening.
Case 6
History
A 19-year-old female presented with difficulty maintaining a regular sleep-wake schedule since age 15 years. Initially, she reported being an “owl” and a long sleeper, with a preferred sleep time of midnight to noon. However, over her recent summer break she began to sleep progressively later, with a daily delay of approximately 1 hour. Previous treatment using melatonin 3 to 10 mg with zolpidem 10 mg at bedtime and intermittent light exposure at 10:00 am were not effective.
Medical history included anxiety and treated social phobia. Family history was notable for OSA in her father.
Evaluation and Treatment
Sleep diary and actigraphy over 14 days (Figure 2C) showed a non–24-hour pattern with a mean daily delay of 1 hour. Dim light salivary melatonin sampling at home, with methods similar to case 1, revealed a DLMO at approximately 5:15 pm, when the melatonin level was at 9 pg/mL, or 25% of the maximum of 35 pg/mL (Figure 4). On that day, the sleep onset time was midnight, resulting in phase angle between DLMO and sleep onset of 6.75 hours. Treatment with 0.5 mg of melatonin at 9:00 pm and light therapy using a 10,000 lux light box kept 2 feet in front of her for 1 hour at 8:30 am was recommended. Treatment resulted in entrainment of her sleep-wake schedule to a 24-hour cycle, with sleep onset of 11:30 pm and wake time of 8:30 am, and significant improvement in anxiety. She was since lost to follow-up.
Figure 4. Salivary melatonin profile in case 6.
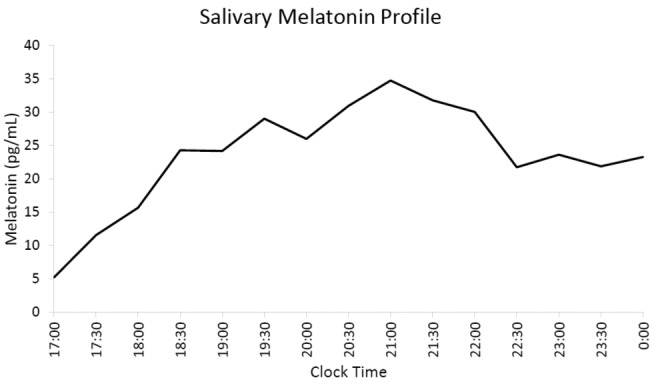
Sampling began at 5:00 PM and continued every 30 minutes until midnight. The DLMO occurred at approximately 5:15 PM, when the melatonin concentration exceeded the 25% of maximum melatonin threshold. DLMO = dim light melatonin onset.
Case 7
History
A 55-year-old man presented with a 30-year history of difficulty maintaining a stable sleep-wake schedule. A non–24-hour schedule first developed in this patient during high school on winter break when he was on his own schedule. He could reentrain to a 24-hour schedule when school restarted but with a preferred bedtime of 2:00 am. In his mid-20s the patient experienced severe depression and was unable to work. He has since followed a non–24-hour schedule, delaying daily by 45 minutes when sleeping at night and by several hours when sleeping during the daytime. Prior to our evaluation, he tried on two occasions to convert to a 24-hour schedule. The first time, he took melatonin 10 to 20 mg at 9:00 pm, but his mood and energy worsened and he did not entrain (Figure 5A). The second time, he used bright light at 7:00 am daily, but his sleep schedule continued to gradually delay.
Figure 5. Effect of high dose melatonin versus low dose melatonin with bright light on N24SWD.
Double-plotted sleep diaries in case 7 are shown before taking melatonin, (top) while taking melatonin 10 or 20 mg (closed circles), and (bottom) while taking melatonin 0.5 mg (closed circles) and using bright light therapy (open circles) for 60 minutes. (Top) This patient had daily delaying of the sleep-wake schedule, which was slower when the sleep onset time occurred during the night. With addition of melatonin 10 to 20 mg, the patient continued to delay each day. Furthermore, the rate of daily delaying increased when the melatonin was taken after or near the end of the primary sleep episode, and it was slower when melatonin was taken within several hours before sleep onset. (Bottom) Combination of low dose melatonin and bright light therapy led to entrainment of the sleep-wake cycle, though sleep remained fragmented. After treatment was discontinued, the sleep-wake scheduled resumed delaying. Low dose melatonin may be more effective than high dose melatonin but the combination with timed bright light therapy may also be necessary for successful entrainment in patients with N24SWD. N24SWD = non–24-hour sleep-wake disorder.
Medical history included severe depression, for which he took fluoxetine, and occasional symptoms of restless legs syndrome occurring before his sleep window. Family history was notable for a nephew with a similar sleep schedule.
Evaluation and Treatment
Sleep diaries and actigraphy demonstrated a non–24-hour pattern, with an average delay of 1.7 hours (Figure 2D).
Treatment with 0.5 mg of melatonin 2 hours before desired bedtime, 1 mg of melatonin 1 hour before desired bedtime, and bright light therapy (using 10,000 lux light box kept 2 feet in front of him) for 1 hour after scheduled awakening entrained him to a 24-hour schedule, with bedtime of 1:30 am and wake time 10:00 am, which he maintained for approximately 1 week; however, he stopped therapy due to difficulty concentrating and low energy (Figure 5B).
DISCUSSION
These cases demonstrate the diagnostic and treatment challenges for sighted patients with N24SWD. A summary of clinically relevant points is provided in Table 1. Due to the varied and intermittent presentation of symptoms and the comorbidity with other sleep disorders, including OSA and DSWPD, the diagnosis of N24SWD is often missed. Most of our patients had symptoms since adolescence, similar to reported findings on sighted N24SWD.4 The most common phenotype is an individual who presents with a delayed sleep-wake schedule and then progresses to a N24SWD pattern. As noted in previous reports, the patients in our series had difficulty maintaining social obligations such as work due to the unstable sleep-wake schedule in N24SWD. This social disruption is different from social jet lag, where there is stable entrainment but social activities result in circadian misalignment and sleep deprivation.18 However, attempts to maintain social obligations in N24SWD can lead to further sleep loss and potentially further disruption of the circadian rhythm from external cues (eg, light exposure) depending on the time of activity relative to the person's circadian phase.
Table 1.
Clinical pearls for the diagnosis and treatment of N24SWD.
Pathophysiology
The potential causes of N24SWD include: a prolonged endogenous circadian period, disruption of the light signaling pathway to the SCN, inappropriate zeitgeber (German for time giver) exposure, and head trauma.4,19
A long circadian period is a risk factor for free-running circadian rhythms in blind people,20 and those with longer periods have more difficulty entraining with orally administered melatonin.13 One report described a patient with a non-entrained melatonin rhythm and a long intrinsic period of 24.6 hours in cultured fibroblasts.21 Long periods in melatonin rhythms have been observed in sighted patients with N24SWD when placed in a forced desynchrony protocol compared to intermediate chronotypes.22 However, the period of the sleep-wake rhythm prior to the forced desynchrony protocol was longer than the period of the melatonin rhythm during forced desynchrony,22 suggesting that the long melatonin period does not entirely explain the presentation of N24SWD.
Disrupted light signaling to the SCN could also contribute to the pathophysiology. N24SWD is most commonly seen in blind people. Although there is no apparent visual impairment in our cases, pathology of the retino-hypothalamic tract, ranging from the melanopsin-containing ganglion cells to the SCN, could still be affected. Two reported N24SWD cases have shown blunted response to melatonin suppression by light,23,24 though a third case did not confirm this finding.21 To our knowledge, the phase shifting responses to light in patients with sighted N24SWD have not been assessed.
Inappropriate timing of light exposure, particularly in those with a preexisting circadian rhythm sleep-wake disorder, may also contribute to the development of N24SWD. Cases 2, 3, and 4 had DSWPD prior to the development of N24SWD. This often occurs as social responsibilities change and patients have more scheduling freedom, as seen during the transition from high school to college. The resulting sleep deprivation, inappropriate light exposure during the phase delay portion of the light PRC, and potentially decreased light exposure in the phase advance portion of the PRC may trigger development of N24SWD. Studies have also shown that those with N24SWD have a shorter phase angle between sleep onset and core body temperature nadir and between sleep onset and melatonin midpoint, and a longer phase angle between core body temperature nadir and sleep offset and between melatonin midpoint and sleep offset.25,26 These phase angles may lead to being awake later into the phase delay portion of the light PRC, resulting in phase delays. Additionally, chronotherapy, the process of deliberately delaying the sleep schedule each day in order to reach the desired sleep window, has been associated with the development of N24SWD.27 One of the earliest case reports of an individual with N24SWD was a 37-year-old man with DSWPD treated with chronotherapy. Rather than re-entraining at the desired schedule, the sleep-wake rhythm continued to delay. By increasing his light exposure during the day and strictly enforcing darkness at night, he was able to re-entrain to a 24-hour schedule.27 Case 1 in the current report experienced N24SWD after attempting chronotherapy. Initially, between trials of chronotherapy, he was able to maintain a stable sleep-wake schedule but only for a few weeks at a time, suggesting that chronotherapy may have triggered development of N24SWD in his case. However, none of our other cases attempted chronotherapy, so it is not required for development of N24SWD.
Head trauma may be an additional trigger for N24SWD. There are several case reports of the development of either DSWPD28,29 or N24SWD19 in patients following head trauma, though the underlying mechanism is unclear. Two of our patients (cases 2 and 3) had a history of concussion. In case 3, head trauma preceded N24SWD by 2 years. In case 2, temporal correlation of head trauma with the onset of N24SWD is unclear. It is possible that the SCN is damaged from the head trauma, as evidenced in a case where in one individual, sighted N24SWD developed following a car accident that resulted in an internal carotid artery aneurysm impinging on the SCN.30 Additionally, head trauma can disrupt the pathways that regulate the secretion of melatonin. Melatonin secretion is normally regulated through a pathway extending from the hypothalamus, through the cervical spinal cord, to the pineal gland. Low-amplitude melatonin rhythms have been reported following brain injury and may alter responses to zeitgebers.31,32
Diagnosis
The diagnosis of N24SWD can be challenging. Although previous criteria for diagnosis included sleep diaries or actigraphy for at least 7 days,33 the N24SWD pattern may not be fully apparent over such a limited time window. For example, case 2 in the current report initially appeared to be entrained to a delayed sleep phase; however, examination of additional sleep diaries demonstrated a nonentrained pattern. Furthermore, some people with N24SWD delay more slowly than others (Figure 2). As such, the criteria for diagnosis of N24SWD were recently revised to include monitoring for at least 14 days.34 Actigraphy can also monitor for naps, sleep fragmentation, and light exposure, which can inform behavioral interventions to improve entrainment.
Evaluation of circadian phase with a single day of melatonin sampling may have limited value because of the delay in circadian and sleep-wake timing. Although salivary melatonin is easier to measure in the outpatient clinical setting than serum melatonin, it can be difficult to determine the appropriate sampling window. First, the phase angles between DLMO and sleep onset in N24SWD may be long. In the general population it is 2 to 3 hours, but despite collecting samples based on predicted sleep onset, the DLMO was missed in several of our patients. For example, in cases 1 and 6, the phase angle between DLMO and sleep onset appears to be 7 to 8 hours (Figure 1); however, because we did not assess DLMO serially in any single case, we cannot comment if the phase angle is consistently long.16 Individuals with N24SWD appear to have a shorter duration between sleep onset and midpoint of melatonin secretion compared to controls, despite no difference in duration of melatonin secretion.26 Therefore, when salivary melatonin sampling is performed, it should be done over a wide time window (ideally longer than 24 hours). In addition, further study is needed to determine whether melatonin secretion occurs with the same period as other behaviors or moves in and out of phase with other body rhythms. Second, the missed DLMO may also be caused by suppression of melatonin levels due to light exposure. Although we instructed patients to wear the provided dark goggles and stay in dim light throughout the collection window, we did not directly control the light exposure during sampling at home. This is a known practical limitation of DLMO assessment in the field.35,36 Third, DLMO assessment at least twice may be clinically helpful to show the moving circa-dian phase indicative of N24SWD, but this has practical limitations. The patient burden can be high; because the DLMO can be missed35,36 without 24-hour sampling, DLMO assessment twice would require sampling for 48 hours. DLMO assessment can also cause high financial burden because salivary melatonin assays are not covered by health insurance. Therefore, repeated DLMO assessment is not a practical clinical strategy at this time.
Examining 6-sulfatoxymelatonin, the urine-excreted metabolite of melatonin, may be a more practical method to determine circadian phase. Specifically, because DLMO is difficult to predict accurately in this population, this technique will allow collection over a 24-hour interval without the disruptions to sleep that would occur with continuous saliva collection.37
Finally, two of our cases did not even complete their recommended diagnostic evaluation, highlighting the high burden required for circadian rhythm disorder assessments. More practical circadian biomarkers are needed if they are to be used as diagnostic tools. Molecular techniques are being developed, with the potential to determine endogenous period using cultured fibroblasts, resulting in far less patient burden.38
Treatment
The goal of treatment of N24SWD is to entrain the individual's rhythm to a stable 24-hour cycle.39 Current available data on the treatment of N24SWD are based on small case reports or series. There are several approaches to using melatonin therapy in N24SWD in the blind. Melatonin given in the biological evening at a fixed interval (10 mg 1 hour before bedtime),13 at a fixed time nightly (5 mg at 9:00 pm),40 or with a dose (20–300 μg) and timing adjusted based on melatonin onset,41 all can lead to entrainment of the sleep-wake cycle. However, there are no specific treatment guidelines for sighted N24SWD.39 In sighted N24SWD, treatment approaches similar to that in N24SWD in the blind have been tried. Melatonin in the biological evening can reduce the delays in circadian rhythms but may not be sufficient to produce stable entrainment due to the longer circa-dian period in sighted versus blind N24SWD (24.9 versus 24.3 hours, respectively),4,42 differences in dose and timing of treatment, or both. Low-dose melatonin (0.5 mg) given at 9:00 pm partially entrained the sleep-wake cycle in one case,23 and high dose (5 mg) given at 8:00 pm completely entrained another with a sleep onset of approximately 10:00–11:00 pm.43 Bright light monotherapy successfully entrained one case with sighted N24SWD.44 In two case reports, bright light upon awakening combined with melatonin 2 mg, 2 to 3 hours before planned bedtime45 or 3 mg, 1 hour before planned bedtime46 successfully entrained the sleep-wake rhythm but with a delayed sleep phase. In another case report, morning bright light therapy at 10,000 lux for 30 minutes and evening melatonin 0.5 to 0.75 mg appeared to stabilize sleep offset but not sleep onset, and melatonin rhythms did not entrain. However, the patient did not tolerate melatonin after 4 days of treatment.21 One series of six cases reported that evening melatonin or the melatonin receptor agonist ramelteon combined with morning bright light therapy successfully entrained three patients.22
Our series demonstrates that a combination of bright light and melatonin can be effective in treating N24SWD (Figure 3). An algorithm of our diagnostic and therapeutic strategy is shown in Figure 6. Once the diagnosis is confirmed using sleep diaries, with or without actigraphy, a target sleep schedule should be established. Timing the melatonin and light therapy can be challenging. Our approach with melatonin was similar to that used in blind N24SWD. When the predicted bedtime aligns with the target bedtime, we initiate melatonin 0.5 to 1 mg 2 hours before predicted bedtime and bright light therapy for 1 hour after predicted wake time. We did not time the melatonin based on DLMO because it was not obtained in each person, and there are no available PRC data in N24SWD. We communicated frequently with the patients and adjusted the timing based on the clinical response. We gave instructions to the patients on how to use light therapy (ie, 10,000 lux light box kept approximately 2 feet away from the face, in the visual field, for the prescribed amount of time). We did not enter the home to confirm actual placement or directly monitor use. Therefore, we did not control the light therapy, including the type and exact positioning of the light box used and the timing and duration of light therapy. However, these factors can be very important for entrainment. We reviewed instructions for light therapy with patients but more frequent follow-up with the patient, standardization of the light box type and use, including setup in the home, may improve success with entrainment. In addition, morning outdoor bright light should be recommended to patients because of the greater light intensity from sunlight.
Figure 6. An algorithm for the evaluation and management of N24SWD.
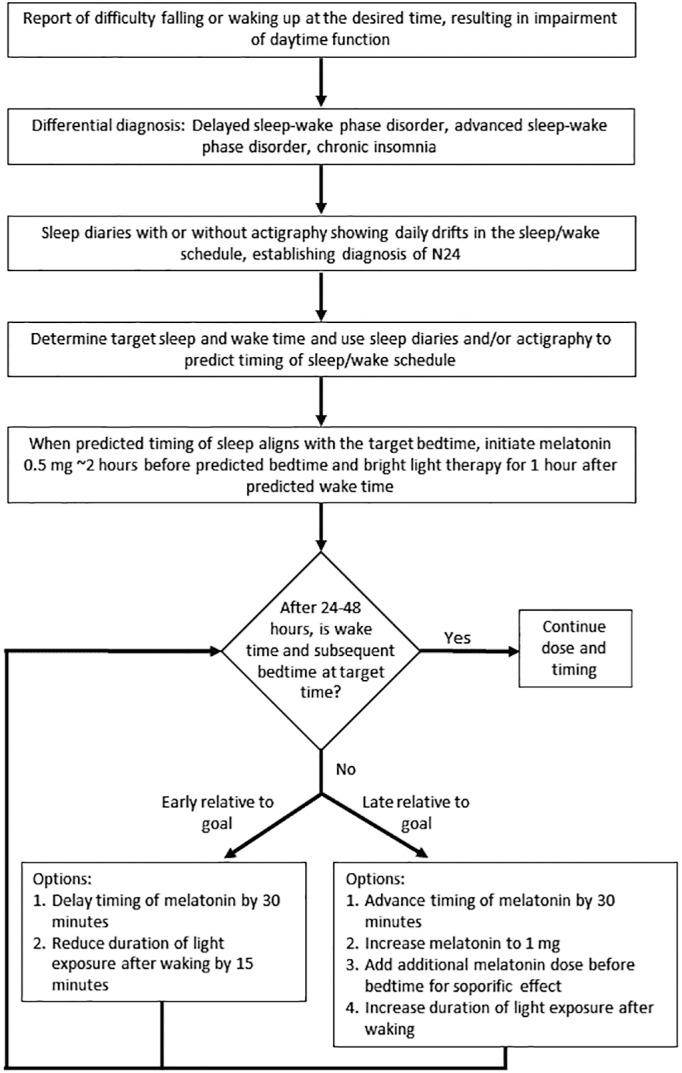
N24SWD = non–24-hour sleep-wake disorder.
Monitoring the individual's sleep and wake timing using actigraphy or diaries throughout treatment is important. Dayto-day adjustments are often needed based on the responses of the day before. Successful adherence requires both a highly motivated patient and frequent communication between the patient and treating physician. Of the subjects who entrained to a 24-hour schedule, most remained slightly delayed with respect to the environment, a finding noted in previous reports of successful entrainment in N24SWD.23,45,46 The delayed timing of entrainment may be due to the timing of melatonin close to bedtime. The time of entrainment depends on achieving an adequate phase advance, which depends on timing of melatonin dosing relative to the person's PRC.47 This is particularly important if the patient has difficulty entraining at the desired time using melatonin and light therapy, which may reduce adherence to treatment. In our cases, the patients desired somewhat late bed and wake times, and they entrained close to their desired time.
The final doses of melatonin in the patients were 0.5 to 4 mg, approximately 1.5 to 4 hours before the desired bedtime. Higher doses (10 to 20 mg) of melatonin are not necessarily more effective,48 as illustrated in case 7. Split dosing of melatonin was effective in case 1, which is likely related to its dual circadian (first dose) and soporific (second dose) effects.49 Although we were able to achieve full or partial entrainment in most of our patients, long-term adherence to treatment is quite challenging. Case 3 found it too difficult to wake up to use light therapy and did not want to maintain a regular bedtime because of school and social activities, case 4 lost motivation to continue treatment, and case 7 discontinued treatment after 1 week because of worsening concentration and energy. In two prior case reports, only one patient was able to maintain long-term entrainment.45,46
The limited adherence with our approach may also be due to the timing of melatonin. Based on the PRC in healthy adults, timing melatonin approximately 7 hours before sleep onset would result in the maximum phase advance,11 and timing it 2 to 3 hours before bedtime, or close to DLMO, results in little to no effect. It is possible that the limited adherence in our cases could have been improved with giving melatonin much earlier, based on the available PRC data in adults without a circadian rhythm sleep-wake disorder. In cases 1, 5, and 6, the patients could sleep within 1.5 to 3.5 hours of taking melatonin. Cases 3 and 4, who ultimately did not continue treatment, were unable to sleep until 3 to 4 hours after taking melatonin; they were entrained but to a delayed sleep phase that may have made it more difficult to wake up at the desired time, so perhaps an earlier timing of melatonin (eg, 7 hours before goal sleep time) could have had greater phase shifting effects and may have improved adherence. Because this approach would result in taking melatonin in the afternoon, the dose of melatonin should be kept low (eg, 0.5 to 1 mg) due to possible soporific effects with higher doses.50 Further studies are needed to systematically compare timing of melatonin to determine optimal approach in the treatment of N24SWD.
To properly treat these patients, knowledge of the individual's biological time is important. Using salivary DLMO as a phase marker to guide treatment has limited utility because salivary melatonin samples often take several days to process, and the patient's internal phase relationship with the 24-hour environment is unstable, often shifting before the salivary melatonin results are obtained. This problem underscores the need to develop accurate circadian markers that can be obtained in real time, with minimal burden to the patient. Until such markers are available, it is important to assess sleep and wake timing with actigraphy and/or diaries to appropriately estimate the timing of treatment.
Deeper understanding of the pathophysiology of N24SWD will be necessary to appropriately tailor treatment. For example, if daily delays are due to a long endogenous period or there is disrupted light signaling or a blunted circadian photic response, light exposure after awakening in the biological morning may be less effective. In such cases, a focus on reducing evening light exposure and/or evening melatonin administration may be more effective.
Underlying psychiatric disorders should be addressed in people with N24SWD, as they are prevalent4,45 and affect quality of life and may affect adherence to treatment. Four of our cases had depression, anxiety, or both. Of these, case 2 did not attempt treatment and cases 4 and 7 discontinued treatment, perhaps due to underlying depression, though all were being treated for depression. Treatment of N24SWD may also improve mood; one (case 6) noted improvement in anxiety with successful entrainment. Additionally, one case of N24SWD showed entrainment and improvement with mood with a low dose valproic acid.51 It is unclear if the effect of valproic acid was due to improvements in mood or if there was a direct circadian effect; that case did not respond to other antidepressants, mood stabilizers, or ramelteon, or bright light therapy.
Finally, it is important to educate patients about behavioral strategies that maintain stable entrainment because decreased or inappropriate zeitgeber exposure can be a trigger. Strategies include maintaining a regular sleep-wake schedule, avoidance of bright light in the evening, increased exposure to natural light in the morning, and regular exercise. Perhaps the greatest challenge is adherence to these strategies, highlighting the need to develop behavioral treatment programs aimed at enhancing long-term adherence.
Summary
This series highlights the significant challenges faced when diagnosing and treating sighted N24SWD disorder. Because DSWPD can overlap with N24SWD in sighted individuals, monitoring of the sleep-wake rhythm using sleep diaries and actigraphy for a minimum of 14 days is necessary to detect the pattern of N24SWD. Timed melatonin at least 2 hours (and perhaps several hours) before bedtime and bright light exposure after waking can be effective to entrain the sleep-wake schedule but can be burdensome for the patient. Furthermore, timing appropriate treatment is challenging in these patients who do not have a stable circadian phase. These treatments also require strict social schedules and avoidance of behaviors that can trigger N24SWD, but this may reduce long-term adherence to treatment. Even if entrainment to a 24-hour schedule is achieved, these patients may not feel better. Future research should focus on improved diagnostic testing, including clinically relevant and practical circadian biomarkers and treatment approaches that not only target entrainment of the central clock, but also innovative methods to enhance alignment of peripheral clocks.
DISCLOSURE STATEMENT
Work for this study was performed at Northwestern University. All authors have seen and approved the manuscript. Financial support was not received for the work presented in this manuscript. The authors have no conflicts of interest related to the work in this manuscript. Roneil Malkani has received grant support from the National Institutes of Health, Illinois Department of Public Health, and the Alzheimer's Association. Sabra Abbott is a site principal investigator for an investigational medication trial (Eisai) and has received grant support from the American Sleep Medicine Foundation and Defense Advanced Research Projects Agency. Kathryn Reid reports no disclosures. Phyllis Zee serves as a consultant for Philips, Merck, Eisai, received grant support from Eisai, Jazz, and Technogel, and has stock ownership in Teva.
ABBREVIATIONS
- DLMO
dim light melatonin onset
- DSWPD
delayed sleep wake disorder
- N24SWD
non–24-hour sleep-wake disorder
- OSA
obstructive sleep apnea
- PRC
phase response curve
- SCN
suprachiasmatic nucleus
REFERENCES
- 1.Saper CB, Lu J, Chou TC, Gooley J. The hypothalamic integrator for circadian rhythms. Trends Neurosci. 2005;28(3):152–157. doi: 10.1016/j.tins.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Scheer FA, Wright KP, Jr, Kronauer RE, Czeisler CA. Plasticity of the intrinsic period of the human circadian timing system. PloS One. 2007;2(8):e721. doi: 10.1371/journal.pone.0000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Czeisler CA, Duffy JF, Shanahan TL, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284(5423):2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 4.Hayakawa T, Uchiyama M, Kamei Y, et al. Clinical analyses of sighted patients with non-24-hour sleep-wake syndrome: a study of 57 consecutively diagnosed cases. Sleep. 2005;28(8):945–952. doi: 10.1093/sleep/28.8.945. [DOI] [PubMed] [Google Scholar]
- 5.Sack RL, Lewy AJ, Blood ML, Keith LD, Nakagawa H. Circadian rhythm abnormalities in totally blind people: incidence and clinical significance. J Clin Endocrinol Metab. 1992;75(1):127–134. doi: 10.1210/jcem.75.1.1619000. [DOI] [PubMed] [Google Scholar]
- 6.Eliott AL, Mills JN, Waterhouse JM. A man with too long a day. J Physiol. 1971;212(2):30P–31P. [PubMed] [Google Scholar]
- 7.Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549(Pt 3):945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewy AJ, Bauer VK, Ahmed S, et al. The human phase response curve (PRC) to melatonin is about 12 hours out of phase with the PRC to light. Chronobiol Int. 1998;15(1):71–83. doi: 10.3109/07420529808998671. [DOI] [PubMed] [Google Scholar]
- 9.Czeisler CA, Allan JS, Strogatz SH, et al. Bright light resets the human circadian pacemaker independent of the timing of the sleep-wake cycle. Science. 1986;233(4764):667–671. doi: 10.1126/science.3726555. [DOI] [PubMed] [Google Scholar]
- 10.Burgess HJ, Revell VL, Eastman CI. A three pulse phase response curve to three milligrams of melatonin in humans. J Physiol. 2008;586(2):639–647. doi: 10.1113/jphysiol.2007.143180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgess HJ, Revell VL, Molina TA, Eastman CI. Human phase response curves to three days of daily melatonin: 0.5 mg versus 3.0 mg. J Clin Endocrinol Metab. 2010;95(7):3325–3331. doi: 10.1210/jc.2009-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hack LM, Lockley SW, Arendt J, Skene DJ. The effects of low-dose 0.5-mg melatonin on the free-running circadian rhythms of blind subjects. J Biol Rhythms. 2003;18(5):420–429. doi: 10.1177/0748730403256796. [DOI] [PubMed] [Google Scholar]
- 13.Sack RL, Brandes RW, Kendall AR, Lewy AJ. Entrainment of free-running circadian rhythms by melatonin in blind people. N Engl J Med. 2000;343(15):1070–1077. doi: 10.1056/NEJM200010123431503. [DOI] [PubMed] [Google Scholar]
- 14.Burke TM, Markwald RR, Chinoy ED, et al. Combination of light and melatonin time cues for phase advancing the human circadian clock. Sleep. 2013;36(11):1617–1624. doi: 10.5665/sleep.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mundey K, Benloucif S, Harsanyi K, Dubocovich ML, Zee PC. Phase-dependent treatment of delayed sleep phase syndrome with melatonin. Sleep. 2005;28(10):1271–1278. doi: 10.1093/sleep/28.10.1271. [DOI] [PubMed] [Google Scholar]
- 16.Sletten TL, Vincenzi S, Redman JR, Lockley SW, Rajaratnam SM. Timing of sleep and its relationship with the endogenous melatonin rhythm. Front Neurol. 2010;1:137. doi: 10.3389/fneur.2010.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burgess HJ, Eastman CI. The dim light melatonin onset following fixed and free sleep schedules. J Sleep Res. 2005;14(3):229–237. doi: 10.1111/j.1365-2869.2005.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006;23(1-2):497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- 19.Boivin DB, James FO, Santo JB, Caliyurt O, Chalk C. Non-24-hour sleep-wake syndrome following a car accident. Neurology. 2003;60(11):1841–1843. doi: 10.1212/01.wnl.0000061482.24750.7c. [DOI] [PubMed] [Google Scholar]
- 20.Lockley SW, Skene DJ, Butler LJ, Arendt J. Sleep and activity rhythms are related to circadian phase in the blind. Sleep. 1999;22(5):616–623. doi: 10.1093/sleep/22.5.616. [DOI] [PubMed] [Google Scholar]
- 21.Garbazza C, Bromundt V, Eckert A, et al. Non-24-hour sleep-wake disorder revisited - a case study. Front Neurol. 2016;7:17. doi: 10.3389/fneur.2016.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitamura S, Hida A, Enomoto M, et al. Intrinsic circadian period of sighted patients with circadian rhythm sleep disorder, free-running type. Biol Psychiatry. 2013;73(1):63–69. doi: 10.1016/j.biopsych.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 23.McArthur AJ, Lewy AJ, Sack RL. Non-24-hour sleep-wake syndrome in a sighted man: circadian rhythm studies and efficacy of melatonin treatment. Sleep. 1996;19(7):544–553. doi: 10.1093/sleep/19.7.544. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura K, Hashimoto S, Honma S, Honma K. Daily melatonin intake resets circadian rhythms of a sighted man with non-24-hour sleep-wake syndrome who lacks the nocturnal melatonin rise. Psychiatry Clin Neurosci. 1997;51(3):121–127. doi: 10.1111/j.1440-1819.1997.tb02373.x. [DOI] [PubMed] [Google Scholar]
- 25.Uchiyama M, Okawa M, Shibui K, et al. Altered phase relation between sleep timing and core body temperature rhythm in delayed sleep phase syndrome and non-24-hour sleep-wake syndrome in humans. Neurosci Lett. 2000;294(2):101–104. doi: 10.1016/s0304-3940(00)01551-2. [DOI] [PubMed] [Google Scholar]
- 26.Uchiyama M, Shibui K, Hayakawa T, et al. Larger phase angle between sleep propensity and melatonin rhythms in sighted humans with non-24-hour sleep-wake syndrome. Sleep. 2002;25(1):83–88. doi: 10.1093/sleep/25.1.83. [DOI] [PubMed] [Google Scholar]
- 27.Oren DA, Giesen HA, Wehr TA. Restoration of detectable melatonin after entrainment to a 24-hour schedule in a ‘free-running’ man. Psychoneuroendocrinology. 1997;22(1):39–52. doi: 10.1016/s0306-4530(96)00038-8. [DOI] [PubMed] [Google Scholar]
- 28.Patten SB, Lauderdale WM. Delayed sleep phase disorder after traumatic brain injury. J Am Acad Child Adolesc Psychiatry. 1992;31(1):100–102. doi: 10.1097/00004583-199201000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Quinto C, Gellido C, Chokroverty S, Masdeu J. Posttraumatic delayed sleep phase syndrome. Neurology. 2000;54(1):250–252. doi: 10.1212/wnl.54.1.250. [DOI] [PubMed] [Google Scholar]
- 30.Bloch KE, Brack T, Wirz-Justice A. Transient short free running circadian rhythm in a case of aneurysm near the suprachiasmatic nuclei. J Neurol Neurosurg Psychiatry. 2005;76(8):1178–1180. doi: 10.1136/jnnp.2004.059295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paparrigopoulos T, Melissaki A, Tsekou H, et al. Melatonin secretion after head injury: a pilot study. Brain Injury. 2006;20(8):873–878. doi: 10.1080/02699050600832114. [DOI] [PubMed] [Google Scholar]
- 32.Seifman MA, Gomes K, Nguyen PN, et al. Measurement of serum melatonin in intensive care unit patients: changes in traumatic brain injury, trauma, and medical conditions. Front Neurol. 2014;5:237. doi: 10.3389/fneur.2014.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American Academy of Sleep Medicine. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. International Classification of Sleep Disorders: Diagnostic and Coding Manual. [Google Scholar]
- 34.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 35.Burgess HJ, Park M, Wyatt JK, Fogg LF. Home dim light melatonin onsets with measures of compliance in delayed sleep phase disorder. J Sleep Res. 2016;25(3):314–317. doi: 10.1111/jsr.12384. [DOI] [PubMed] [Google Scholar]
- 36.Pullman RE, Roepke SE, Duffy JF. Laboratory validation of an in-home method for assessing circadian phase using dim light melatonin onset (DLMO) Sleep Med. 2012;13(6):703–706. doi: 10.1016/j.sleep.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flynn-Evans EE, Tabandeh H, Skene DJ, Lockley SW. Circadian rhythm disorders and melatonin production in 127 blind women with and without light perception. J Biol Rhythms. 2014;29(3):215–224. doi: 10.1177/0748730414536852. [DOI] [PubMed] [Google Scholar]
- 38.Brown SA, Kunz D, Dumas A, et al. Molecular insights into human daily behavior. Proc Natl Acad Sci U S A. 2008;105(5):1602–1607. doi: 10.1073/pnas.0707772105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Auger RR, Burgess HJ, Emens JS, Deriy LV, Thomas SM, Sharkey KM. Clinical practice guideline for the treatment of intrinsic circadian rhythm sleep-wake disorders: advanced sleep-wake phase disorder (ASWPD), delayed sleep-wake phase disorder (DSWPD), non-24-hour sleep-wake rhythm disorder (N24SWD), and irregular sleep-wake rhythm disorder (ISWRD). An update for 2015: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2015;11(10):1199–1236. doi: 10.5664/jcsm.5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lockley SW, Skene DJ, James K, Thapan K, Wright J, Arendt J. Melatonin administration can entrain the free-running circadian system of blind subjects. J Endocrinol. 2000;164(1):R1–R6. doi: 10.1677/joe.0.164r001. [DOI] [PubMed] [Google Scholar]
- 41.Lewy AJ, Emens JS, Lefler BJ, Yuhas K, Jackman AR. Melatonin entrains free-running blind people according to a physiological dose-response curve. Chronobiol Int. 2005;22(6):1093–1106. doi: 10.1080/07420520500398064. [DOI] [PubMed] [Google Scholar]
- 42.Emens J, Lewy AJ, Laurie AL, Songer JB. Rest-activity cycle and melatonin rhythm in blind free-runners have similar periods. J Biol Rhythms. 2010;25(5):381–384. doi: 10.1177/0748730410379080. [DOI] [PubMed] [Google Scholar]
- 43.Tomoda A, Miike T, Uezono K, Kawasaki T. A school refusal case with biological rhythm disturbance and melatonin therapy. Brain Devel. 1994;16(1):71–76. doi: 10.1016/0387-7604(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 44.Eastman C, Anagnopoulos C, Cartwright R. Can bright light entrain a free-runner. Sleep Res. 1988;17:372. [Google Scholar]
- 45.Kuzniar TJ, Kovacevic-Ristanovic R, Nierodzik CL, Smith LC. Free-running (non-entrained to 24-h period) circadian sleep disorder in a patient with obstructive sleep apnea, delayed sleep phase tendency, and lack of social interaction. Sleep Breath. 2012;16(2):313–315. doi: 10.1007/s11325-011-0535-8. [DOI] [PubMed] [Google Scholar]
- 46.Brown MA, Quan SF, Eichling PS. Circadian rhythm sleep disorder, free-running type in a sighted male with severe depression, anxiety, and agoraphobia. J Clin Sleep Med. 2011;7(1):93–94. [PMC free article] [PubMed] [Google Scholar]
- 47.Emens JS, Eastman CI. Diagnosis and treatment of non-24-h sleep-wake disorder in the blind. Drugs. 2017;77(6):637–650. doi: 10.1007/s40265-017-0707-3. [DOI] [PubMed] [Google Scholar]
- 48.Lewy AJ, Emens JS, Sack RL, Hasler BP, Bernert RA. Low, but not high, doses of melatonin entrained a free-running blind person with a long circadian period. Chronobiol Int. 2002;19(3):649–658. doi: 10.1081/cbi-120004546. [DOI] [PubMed] [Google Scholar]
- 49.Brzezinski A, Vangel MG, Wurtman RJ, et al. Effects of exogenous melatonin on sleep: a meta-analysis. Sleep Med Rev. 2005;9(1):41–50. doi: 10.1016/j.smrv.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 50.Revell VL, Burgess HJ, Gazda CJ, Smith MR, Fogg LF, Eastman CI. Advancing human circadian rhythms with afternoon melatonin and morning intermittent bright light. J Clin Endocrinol Metab. 2006;91(1):54–59. doi: 10.1210/jc.2005-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kurita M, Moriya T, Nishino S, et al. Non-24-hour sleep-wake syndrome improved by low-dose valproic acid: a case report. Neuropsychiatr Dis Treat. 2016;12:3199–3203. doi: 10.2147/NDT.S115648. [DOI] [PMC free article] [PubMed] [Google Scholar]