Abstract
Study Objectives:
The aim of this study was to verify the reliability and validity of the Spanish short version of the Functional Outcomes of Sleep Questionnaire (FOSQ-10SV) in Peruvian patients with obstructive sleep apnea (OSA).
Methods:
Participants underwent physical examinations, completed the FOSQ-10SV, and polysomnography tests were carried out.
Results:
A total of 672 patients were analyzed, 75 females (11%), mean age 50.5 ± 13.8 years. A total of 563 patients (84%) had OSA. The mean FOSQ-10SV score was 15.96 ± 3.23. The FOSQ-10SV Cronbach alpha was 0.84 and two significant factors were extracted in the factor analysis—both factors explained a variance of 43% and 14%. A significant correlation was found between the FOSQ-10SV score and the apnea-hypopnea index. Patients with more severe disease have a lower FOSQ-10SV score (P = .003). Ninety-nine patients with OSA who started continuous positive airway pressure treatment were followed, and we observed an improvement in the FOSQ-10SV score from pretreatment to posttreatment (P < .001).
Conclusions:
The FOSQ-10SV has internal consistency, construct validity, and the sensitivity to change in Peruvian patients with OSA who undergo treatment.
Citation:
Rey de Castro J, Rosales-Mayor E, Weaver TE. Reliability and validity of the Functional Outcomes of Sleep Questionnaire – Spanish short version (FOSQ-10SV) in Peruvian patients with obstructive sleep apnea. J Clin Sleep Med. 2018;14(4):615–621.
Keywords: Functional Outcomes of Sleep Questionnaire, FOSQ-10, quality of life, reliability, sleep apnea, validity
BRIEF SUMMARY
Current Knowledge/Study Rationale: Obstructive sleep apnea (OSA) is a chronic disease that affects quality of life (QOL). Thus, we evaluated the reliability and validity of a Spanish short version of the Functional Outcomes of Sleep Questionnaire (FOSQ-10SV) in a clinical population of Peruvian patients suspected to have OSA.
Study Impact: The FOSQ-10SV has an internal validity and construct validity in patients with OSA. The FOSQ-10SV has the sensitivity to change in patients with OSA who started continuous positive airway pressure treatment.
INTRODUCTION
The classification of sleep-related respiratory disorders proposed by the American Academy of Sleep Medicine (AASM) includes obstructive sleep apnea (OSA), central apnea syndrome, and related hypoventilation disorders.1,2 The prevalence of OSA in the general middle-aged population is 2% to 6% and in the population between age 70 to 100 years 15% to 26%.3–6 OSA is caused by a partial or complete obstruction of the upper airway during sleep, leading to an intermittent oxygen desaturation, electroencephalographic microarousals, sleep fragmentation, daytime sleepiness, neurocognitive changes, and poor quality of life (QOL).7 The most frequent symptoms are intense snoring, respiratory pauses with choking during sleep, and daytime sleepiness. Medical information has consistently documented that OSA is a risk factor for high blood pressure, cerebrovascular and heart disease, traffic accidents while driving due to sleepiness, and death due to myocardial infarction or stroke.8–13
Researchers have designed instruments to evaluate the effect of sleep disorders on the multidimensional concept of QOL from the patient's perspective.14 The tools provide complementary information from that obtained by conventional clinical practice methods. Among QOL questionnaires, the best known is the SF-36 or Short Form-36.15,16 Those specifically oriented toward OSA include the Calgary Sleep Apnea Quality of Life Instrument (SAQLI),17 Obstructive Sleep Disorders-6 Survey (OSD-6),18 Functional Outcomes of Sleep Questionnaire (FOSQ),19 and Pediatric Obstructive Sleep Apnea Instrument (OSA-18), used in the pediatric population.20 The purpose of this study was to establish the reliability and validity of a Spanish short version of the Functional Outcomes of Sleep Questionnaire (FOSQ-10SV) in a clinical population of Peruvian patients suspected to have OSA.
METHODS
The population recruited for the study all presented to the Sleep Laboratory, Clínica Anglo Americana, Lima-Perú between 2012 to 2016 for evaluation of suspected OSA. Patient information was prospectively registered in a database program, and all patients completed the FOSQ-10SV before an initial interview as a component of the laboratory's protocol. The medical interviews were conducted by a pulmonologist and breathing sleep disorders specialist. The Institutional Bioethics Committee of the Clínica Anglo Americana approved the study and all patients authorized their participation by signing an informed consent form.
Supervised polysomnography (PSG) was performed between 15 and 30 days after the initial interview using the Easy II and Easy III devices (Caldwell Inc, Kennewick, Washington, United States). The PSG included recording the following channels: electroencephalogram (F4-M1, C4-M1, O2-M1), bilateral electro-oculogram, chin electromyography, flow with thermistor and nasal cannula/pressure sensor, snore microphone placed in the pretracheal area, respiratory effort with thoracic and abdominal bands, pulse oximeter with sampling time of 4 to 5 seconds, electrocardiogram in Lead II, bilateral surface electromyography of the tibialis anterior, and body position sensor. Guidelines published by the American Academy of Sleep Medicine were used to score sleep stages and respiratory events.21
We excluded patients with a previous diagnosis of OSA and patients receiving treatment with continuous or bilevel positive airway pressure or mandibular advancement devices. We also excluded all patients with other primary sleep-related disorders, circadian rhythm disorders, nightshift workers, and those with any physical or psychological condition that could alter the normal sleeping pattern.
The PSG studies were both conventional PSG and split-night PSG (SN-PSG). According to Sanders et al.,22 there is no significant difference in the apnea-hypopnea index (AHI) calculated during the first 2 hours of sleep and the total sleep time. Likewise, there is no significant difference between the maximal desaturation values in rapid eye movement sleep obtained during the first 2 hours of recording and the full-night recording. A paper from Khawaja et al.23 established the correlation of the AHI obtained in the first 2 hours of the study with the total sleep times was consistent (r = .92). The value of the receiver operating characteristic curve in 2 hours using the SN-PSG with AHI ≥ 5 events/h was 0.93. Considering these arguments, the results obtained by conventional PSG tests and SN-PSG were analyzed jointly. OSA diagnosis was established using criteria from the American Academy of Sleep Medicine24: mild, AHI 5 to < 15 events/h, moderate, AHI 15 to < 30 events/h, and severe, AHI ≥ 30 events/h. Patients with primary snoring and/or AHI < 5 events/h were categorized into the non-OSA group.
Functional Outcomes of Sleep Questionnaire
The original Functional Outcomes of Sleep Questionnaire (FOSQ) was developed by Weaver et al. in 1997.19 It is a self-administered tool designed to quantify the effect of disorders of excessive daytime sleepiness on the activities of everyday living. The FOSQ evaluates five dimensions: general productivity, social, vigilance, intimacy, and intimate and sexual relationships in a total of 30 questions. Ferrer et al.25 developed the Spanish version in order to form a preliminary evaluation of the acceptability and reliability of the FOSQ in Barcelona. The reliability of the questionnaire exceeded the standard proposed for the performance of individual comparisons with a Cron-bach alpha > 0.9. The results indicated that the Spanish version is conceptually equivalent to the original, presenting similar reliability and validity. Vidal et al.26 applied this validation in healthy persons and those suspected to have OSA. Significant differences were found in both groups in all subscales except that used to measure social relationships. Moderate correlations were obtained between the FOSQ and the AHI (r = −.54 and P = .05). The authors concluded that the Spanish version of the FOSQ was a good evaluation instrument for the effect of sleepiness on the activities of everyday life when OSA is suspected.
Spanish Short Version of the Functional Outcomes of Sleep Questionnaire (FOSQ-10SV)
A short version of the FOSQ, developed in English (the FOSQ-10) was designed based on the original in order to have a shorter and easier-to-use instrument.27 After selecting 10 questions from the 5 dimensions, the questionnaire was applied in patients receiving continuous positive airway pressure (CPAP) treatment for OSA. The internal consistency was α = 0.87. The pretreatment correlation between the FOSQ-39 and FOSQ-10 was r = .96. After 3 months of treatment with CPAP, the correlation was r = .97 (P < .0001). The authors concluded that the FOSQ-10 is a consistent psychometric instrument that performs in a manner similar to that of the long version.
The Spanish short version (FOSQ-10SV; Table 1) was designed based on the validated version in Spanish.25,26 The following two modifications were made from the English version by Chasens et al.,27 under the supervision of Dr. Weaver:
Question 3: “¿Tiene dificultad para conducir un vehículo de motor durante períodos cortos (menos de una hora) porque se cansa o se duerme?” was changed to “¿Tiene dificultad para conducir un vehículo de motor durante distancias cortas menores de 160 km porque está cansado o con sueño?”
Question 4: “¿Tiene dificultad para conducir un vehículo de motor durante períodos largos (más de 1 hora) porque se cansa o se duerme?” was changed to “¿Tiene dificultad para conducir un vehículo de motor durante distancias largas mayores de 160 km porque está cansado o con sueño?”
The same system proposed by Chasens et al.27 was used to obtain the total score. A mean weighted item score was first computed for those subscales with more than one item. This prevented the distortion of the score resulting from missing responses. The total score was derived by calculating the mean of the subscale scores and multiplying that mean by 5. Dr. Weaver authorized the use of the questionnaire for this study.
Table 1.
Items included in the Spanish short version of the Functional Outcomes of Sleep Questionnaire.
Statistical Analysis
Stata 10.0 (StataCorp, College Station, Texas, United States) was used for statistical analysis. Data were analyzed as n (percent) for categorical variables and mean ± standard deviation for continuous variables. To compare the mean of the two samples, Student t test was used. To compare the means of more than two samples, analysis of variance (ANOVA) was used. The degree of correlation between continuous variables was calculated using the Pearson correlation. The internal consistency was measured with the Cronbach alpha and the construct validity with the factor analysis. A value of P < .05 was considered statistically significant.
RESULTS
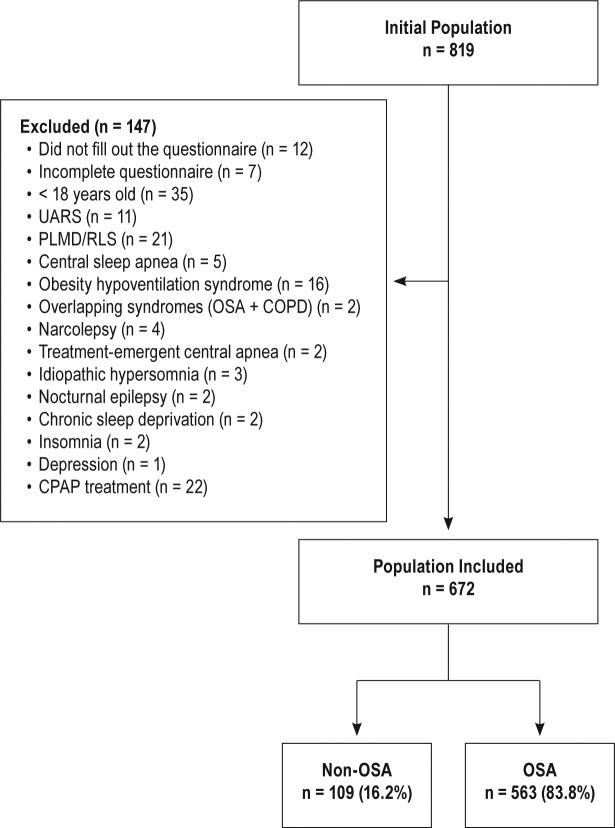
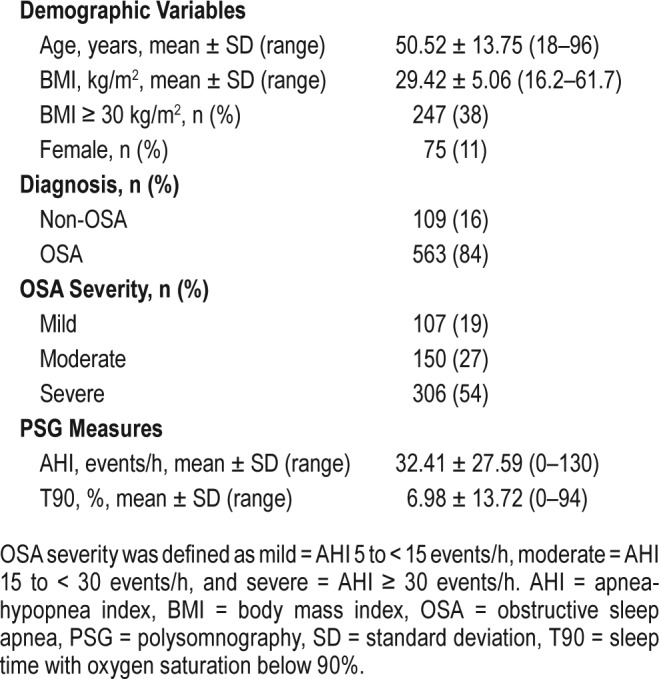
Between 2012 to 2016 a total of 819 adult patients were evaluated for OSA. One hundred forty-seven patients were excluded (Figure 1). All patients underwent PSG. Finally, 672 patients were included in the analysis; 563 (83.8%) had OSA and 75 (11.2%) were women. The population characteristics are presented in Table 2.
Figure 1. Patient flowchart.
COPD = chronic obstructive pulmonary disease, OSA = obstructive sleep apnea, PLMD = periodic limb movement disorder, RLS = restless legs syndrome, UARS = upper airway resistance syndrome.
Table 2.
Population characteristics (n = 672).
Description of FOSQ-10SV
In the correlation analysis between the FOSQ-10SV score and the main PSG variables such as AHI (Figure 2) and sleep time with oxygen saturation below 90% (T90), the Pearson r values obtained were −.135 (P < .001) and −.102 (P = .008), respectively. The mean FOSQ-10SV score of all patients was 15.96 ± 3.23, and in patients with and without OSA was 15.88 ± 3.20 and 16.35 ± 3.39 respectively (t test P = .160). The FOSQ-10SV scores according to OSA severity were: mild 16.39 ± 3.04, moderate 16.36 ± 2.98, and severe 15.45 ± 3.30 (ANOVA P = .003). The Pearson correlations between FOSQ-10SV according to OSA severity were −.1795 (P = .063), −.0347 (P = .673) and −.0415 (P = .4715) in mild, moderate, and severe groups, respectively.
Figure 2. Dispersion graph between the FOSQ-10SV and AHI.
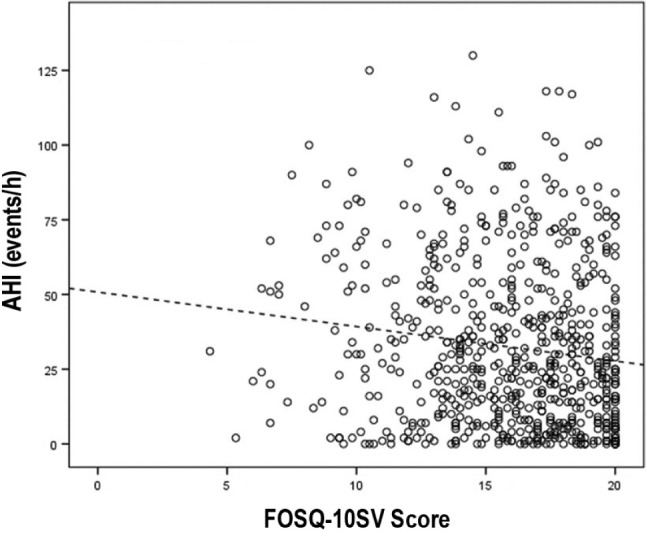
r = −.135, P < .001. AHI = apnea-hypopnea index, FOSQ-SV = Functional Outcomes of Sleep Questionnaire – Spanish short version.
Internal Consistency and Construct Validity
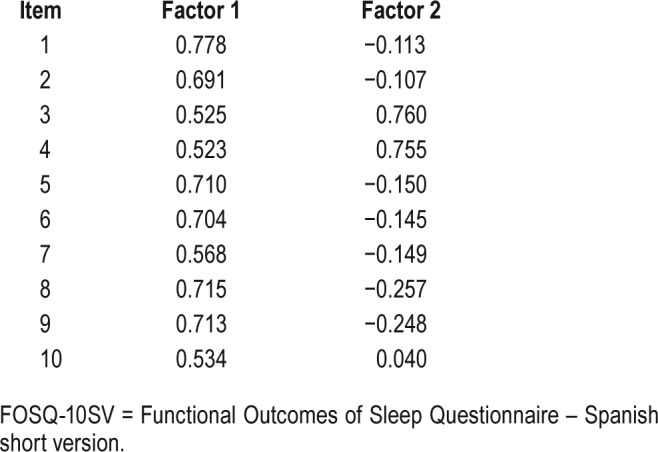
The Cronbach alpha index was 0.836, and did not improve by removing any of the items. The item-total coefficients correlation ranged from 0.437 to 0.659. When we separately analyzed the people with and without OSA, the Cronbach alpha index was 0.832 and 0.855. The Kaiser–Meyer–Olkin value was 0.853, and the Bartlett test of sphericity was significant (P < .001); both results showed that the sample met the criteria for factor analysis. Only two factors were extracted with eigenvalues of 4.258 and 1.366, the others had an eigenvalue below 1. Both factors explained a variance of 42.6% and 13.7%, respectively. Table 3 shows the factor loadings of the eight items to the two factors extracted (see items in Table 1). Only two items had a significant factor loading to Factor 2.
Table 3.
Factor loadings for each item in the FOSQ-10SV.
Sensitivity to Change
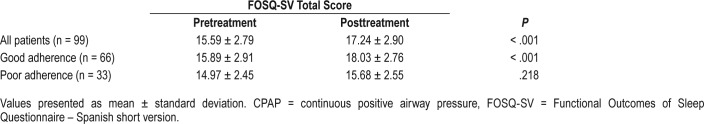
Ninety-nine patients with OSA who started CPAP treatment were followed-up. These patients completed the FOSQ-10SV both before and after treatment. Of these patients, 66 (66.6%) had good adherence defined as documented time using the device for more than 4 h/night on at least 70% of nights. Table 4 shows the mean FOSQ-10SV total scores pretreatment and posttreatment in patients with good and poor adherence.
Table 4.
Mean FOSQ-SV total score before and after CPAP treatment.
DISCUSSION
As defined by Wenger et al., QOL is “…a range of capabilities, limitations, symptoms and psychosocial characteristics that describe an individual's ability to function and derive satisfaction from a variety of roles.”28 Jones stated that “…quality of life may be defined as the gap between that which is desired in life and that which is achieved.”29
Patient experience assessed by QOL tools have been increasing as an important variable of chronic diseases.14 General health questionnaires have the advantage that their reproducibility and validity have been verified in different diseases. This enables comparison not only between subjects but also between different patient populations. Their limitation is low sensitivity to specific diseases, meaning that they may provide inaccurate results for these diseases.30 QOL-specific instruments for assessing patients with OSA have been designed in order to be more sensitive than generic ones, to measure response to treatment, and to quantify subjective clinical results.31 Our group has previously validated and applied the Multicultural Quality of Life Index in Peruvian patients with OSA.32
Patients assessed by the present study were middle-aged, mostly male, and on average, overweight or having obesity. Cases of severe OSA were predominant.
The FOSQ-10SV had an internal validity similar to the English version (Cronbach alpha index 0.87). According to Sánchez et al.,33,34 this homogeneity measure must be between a range of 0.7 and 0.9 to be considered consistent. Rahavi-Ezabadi et al.35 validated the FOSQ-10 in Farsi with Iranian patients with OSA. They found a Cronbach alpha coefficient of 0.85 in the total score and a range from 0.78 to 0.83 in each subscale. They did not find a statistically significant difference between total score and OSA severity according to AHI. The total score before and after CPAP treatment was 12.72 and 16.21 (P < .001). A Mandarin Chinese version was validated in 242 first-trimester Taiwanese pregnant women. The Cronbach alpha was 0.85. A modest but significant difference was found between the Chinese FOSQ-10 total score and daytime sleepiness.36
In the factorial analysis (Table 3) 8 of 10 items showed statistically significant factorial loads (0.55 or more) to Factor 1, although the other two items (3 and 4) had nearly a signifi-cant factorial load. Only items 3 and 4 had significant factorial loads to Factor 2. These two items ask about the same issue: difficulty of driving a motor vehicle because of sleepiness or tiredness, so this factor could be “sleepiness.” Finally, we concluded that the questionnaire measures a single variable or main factor, which theoretically should be QOL.
Significant correlations were found between the FOSQ-10SV score and PSG measures, such as AHI and T90. However, when we categorized OSA severities by AHI, we found no noteworthy correlations with the FOSQ-10SV score. This is not unexpected, as patient perception of QOL includes a constellation of factors beyond physiological processes and does not typically have a strong relationship with physiologic processes.37
The FOSQ-10SV score was lower in patients with an OSA diagnosis and in patients with more severe disease, and the differences were statistically significant. Also, we observed that the FOSQ-10SV score improved in patients with OSA who started treatment with CPAP, especially in those who had good adherence. Two other studies have shown that the FOSQ-10 score changes with interventions. Erman et al.38 used the FOSQ-10 questionnaire in a double-blind, placebo-controlled trial. They evaluated the effect of armodafinil in patients with shift-work disorder. The active drug group compared to the placebo group showed a greater improvement in total FOSQ-10 score from baseline to final visit. Soose et al.39 used the FOSQ-10 to measure outcomes in a surgical intervention for OSA, specifically in hypoglossal cranial nerve upper airway stimulation therapy. Significant improvement in FOSQ-10 scores were seen at 12 and 24 months after the implant.
The limitations of this study include the low number of women, that the study participants were all recruited from one center, and the sample size for the sensitivity to changes with treatment was low. The latter cannot be understated, because one of the most useful applications of this kind of instrument is in its sensitivity to changes with treatment.
In conclusion, this short Spanish version of FOSQ has internal consistency, construct validity, and the sensitivity to change in patients with OSA undergoing treatment. It should be pointed out that the scores obtained in these types of tools have differing results among differing cultural populations.
DISCLOSURE STATEMENT
Work for this study was performed at the Sleep Laboratory, Clínica Anglo Americana, Lima-Perú. All authors have seen and approved the manuscript. This was not an industry-supported study. Dr. Weaver is a site Principal Investigator for a Jazz Pharmaceutical clinical trial; has received royalty fees for corporate use of the FOSQ from Respironics, Inc, ResMed, NightBalance, Jazz Pharmaceuticals, Nyxoah. Dr. Rey de Castro and Dr. Rosales-Mayor report no conflicts of interest.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AIH
apnea-hypopnea index
- ANOVA
analysis of variance
- BMI
body mass index
- CPAP
continuous positive airway pressure
- FOSQ
Functional Outcomes of Sleep Questionnaire
- FOSQ-10SV
Functional Outcomes of Sleep Questionnaire – Spanish short version
- OSA
obstructive sleep apnea
- PSG
polysomnography
- QOL
quality of life
- ROC
receiver operating characteristic
- SN-PSG
split night polysomnography
- T90
sleep time with oxygen saturation below 90%
REFERENCES
- 1.Sateia MJ. International Classification of Sleep Disorders-third edition: highlights and modifications. Chest. 2014;146(5):1387–1394. doi: 10.1378/chest.14-0970. [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 3.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 4.Duran J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163(3 Pt 1):685–689. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez C, Duran-Cantolla J, Lloberes P, Gonzalez M. [Innovations in the epidemiology, natural history, diagnosis and treatment of sleep apneahypopnea syndrome] Arch Bronconeumol. 2009;45(Suppl 1):3–10. doi: 10.1016/S0300-2896(09)70264-9. [DOI] [PubMed] [Google Scholar]
- 6.Duran J, Esnaola S, Rubio R, De la Torre G. Obstructive sleep apnoeahypopnoea in the elderly. A population-based study in the general population aged 71-100. Annu Meet ERS. 2000;16:167S. [Google Scholar]
- 7.Grupo Español de Sueño. Consenso nacional sobre Síndrome de Apneas-Hipopneas del Sueño. Arch Bronconeumol. 2005;41(Suppl 4):1–110. [Google Scholar]
- 8.Hedner J, Bengtsson-Bostrom K, Peker Y, Grote L, Rastam L, Lindblad U. Hypertension prevalence in obstructive sleep apnoea and sex: a population-based case-control study. Eur Respir J. 2006;27(3):564–570. doi: 10.1183/09031936.06.00042105. [DOI] [PubMed] [Google Scholar]
- 9.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283(14):1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 10.Rey de Castro J, Rosales E, Ferreyra J. Relación entre la severidad del sindrome de apneas-hipopneas del sueño y la hipertensión arterial. Análisis en una población clínica. Rev Med Hered. 2009;20(3):123–132. [Google Scholar]
- 11.Kuniyoshi FH, Garcia-Touchard A, Gami AS, et al. Day-night variation of acute myocardial infarction in obstructive sleep apnea. J Am Coll Cardiol. 2008;52(5):343–346. doi: 10.1016/j.jacc.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 13.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 14.Testa MA, Simonson DC. Assessment of quality-of-life outcomes. N Engl J Med. 1996;334(13):835–840. doi: 10.1056/NEJM199603283341306. [DOI] [PubMed] [Google Scholar]
- 15.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 16.Ware JE., Jr SF-36 health survey update. Spine (Phila Pa 1976) 2000;25(24):3130–3139. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 17.Flemons WW, Reimer MA. Measurement properties of the calgary sleep apnea quality of life index. Am J Respir Crit Care Med. 2002;165(2):159–164. doi: 10.1164/ajrccm.165.2.2010008. [DOI] [PubMed] [Google Scholar]
- 18.De Serres LM, Derkay C, Sie K, et al. Impact of adenotonsillectomy on quality of life in children with obstructive sleep disorders. Arch Otolaryngol Head Neck Surg. 2002;128(5):489–496. doi: 10.1001/archotol.128.5.489. [DOI] [PubMed] [Google Scholar]
- 19.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20(10):835–843. [PubMed] [Google Scholar]
- 20.Constantin E, Tewfik TL, Brouillette RT. Can the OSA-18 quality-of-life questionnaire detect obstructive sleep apnea in children? Pediatrics. 2010;125(1):e162–e168. doi: 10.1542/peds.2009-0731. [DOI] [PubMed] [Google Scholar]
- 21.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanders MH, Black J, Costantino JP, Kern N, Studnicki K, Coates J. Diagnosis of sleep-disordered breathing by half-night polysomnography. Am Rev Respir Dis. 1991;144(6):1256–1261. doi: 10.1164/ajrccm/144.6.1256. [DOI] [PubMed] [Google Scholar]
- 23.Khawaja IS, Olson EJ, van der Walt C, et al. Diagnostic accuracy of split-night polysomnograms. J Clin Sleep Med. 2010;6(4):357–362. [PMC free article] [PubMed] [Google Scholar]
- 24.American Academy of Sleep Medicine. International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 25.Ferrer M, Vilagut G, Monasterio C, Montserrat JM, Mayos M, Alonso J. [Measurement of the perceived impact of sleep problems: the Spanish version of the functional outcomes sleep questionnaire and the Epworth sleepiness scale] Med Clin. 1999;113(7):250–255. [PubMed] [Google Scholar]
- 26.Vidal S, Ferrer M, Masuet C, Somoza M, Martinez Ballarin JI, Monasterio C. [Spanish version of the Functional Outcomes of Sleep Questionnaire: scores of healthy individuals and of patients with sleep apnea-hypopnea syndrome] Arch Bronconeumol. 2007;43(5):256–261. doi: 10.1016/s1579-2129(07)60063-9. [DOI] [PubMed] [Google Scholar]
- 27.Chasens ER, Ratcliffe SJ, Weaver TE. Development of the FOSQ-10: a short version of the Functional Outcomes of Sleep Questionnaire. Sleep. 2009;32(7):915–919. doi: 10.1093/sleep/32.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wenger NK, Mattson ME, Furberg CD, Elinson J. Assessment of quality of life in clinical trials of cardiovascular therapies. Am J Cardiol. 1984;54(7):908–913. doi: 10.1016/s0002-9149(84)80232-5. [DOI] [PubMed] [Google Scholar]
- 29.Jones PW. Quality of life measurement: the value of standardization. Eur Respir Rev. 1997;7:46–49. [Google Scholar]
- 30.Guyatt G. Measuring health status in chronic airflow limitation. Eur Respir J. 1988;1(6):560–564. [PubMed] [Google Scholar]
- 31.Flemons WW, Reimer MA. Development of a disease-specific health-related quality of life questionnaire for sleep apnea. Am J Respir Crit Care Med. 1998;158(2):494–503. doi: 10.1164/ajrccm.158.2.9712036. [DOI] [PubMed] [Google Scholar]
- 32.Rey de Castro J, Rosales-Mayor E, Ferreyra-Pereyra J. Using a generic measure of quality of life in patients with obstructive sleep apnea. Sleep Breath. 2011;15(4):729–735. doi: 10.1007/s11325-010-0429-1. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez R, Gómez C. Conceptos básicos sobre validación de escalas. Rev Col Psiquiatr. 1998;27:121–130. [Google Scholar]
- 34.Sanchez R, Echeverry J. Validación de escalas de medición en salud. Rev Salud Pública. 2004;6:302–318. [PubMed] [Google Scholar]
- 35.Rahavi-Ezabadi S, Amali A, Sadeghniiat-Haghighi K, Montazeri A. Adaptation of the 10-Item Functional Outcomes of Sleep Questionnaire to Iranian Patients with Obstructive Sleep Apnea. Qual Life Res. 2016;25(2):337–341. doi: 10.1007/s11136-015-1081-9. [DOI] [PubMed] [Google Scholar]
- 36.Tsai S-Y, Shun S-C, Lee P-L, Lee C-N, Weaver TE. Validation of the Chinese Version of the Functional Outcomes of Sleep Questionnaire-10 in Pregnant Women. Res Nurs Health. 2016;39(6):463–471. doi: 10.1002/nur.21750. [DOI] [PubMed] [Google Scholar]
- 37.Megari K. Quality of life in chronic disease patients. Heal Psychol Res. 2013;1(3):e27. doi: 10.4081/hpr.2013.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Erman MK, Yang R, Seiden DJ. The effect of armodafinil on patient-reported functioning and quality of life in patients with excessive sleepiness associated with shift work disorder: a randomized, double-blind, placebo-controlled trial. Prim Care Companion CNS Disord. 2012;14(4) doi: 10.4088/PCC.12m01345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soose RJ, Woodson BT, Gillespie MB, et al. Upper Airway Stimulation for Obstructive Sleep Apnea: Self-Reported Outcomes at 24 Months. J Clin Sleep Med. 2016;12(1):43–48. doi: 10.5664/jcsm.5390. [DOI] [PMC free article] [PubMed] [Google Scholar]