Abstract
Study Objectives:
The nature of sleep disorders in children with Ehlers-Danlos syndrome (EDS) is unknown. We aimed to describe the type, the management, and the short-term outcome of sleep disorders in children with EDS referred to sleep clinics.
Methods:
This is a retrospective review of medical records and polysomnography tests of children with EDS younger than 18 years who were referred to the sleep clinic. Demographic information and medical history were collected, and polysomnography tests were reviewed. Questionnaires completed during previous clinic visits, including the Pediatrics Sleep Questionnaire (PSQ), Epworth Sleepiness Scale (ESS), and Pediatric Quality of Life Inventory (PedsQL), were also evaluated.
Results:
Sixty-five patients with EDS-hypermobility type were included. The mean age was 13.15 ± 3.9 years. There were 68% of patients who were female, and 91% of patients were Caucasian. The mean follow-up period was 1.14 ± 1.55 years. Common sleep diagnoses included insomnia (n = 14, 22%), obstructive sleep apnea (OSA) (n = 17, 26%), periodic limb movement disorder (PLMD) (n = 11, 17%), and hypersomnia (n = 10, 15%). In addition, 65% required pharmacologic treatment and 29% were referred to behavioral sleep medicine. For OSA, two patients required continuous positive airway pressure. A significant improvement was observed in the PSQ, ESS, and PedsQL scores during follow-up visits after treatment (n = 34; P = .0004, 0.03, and 0.01, respectively).
Conclusions:
There is a high prevalence of sleep disorders, including OSA, insomnia, PLMD, and hypersomnia in children with EDS referred to sleep clinics. Specific management can improve quality of life and questionnaire scores of this patient population. Our study emphasizes the importance of screening for sleep disorders in children with EDS.
Citation:
Domany KA, Hantragool S, Smith DF, Xu Y, Hossain M, Simakajornboon N. Sleep disorders and their management in children with Ehlers-Danlos syndrome referred to sleep clinics. J Clin Sleep Med. 2018;14(4):623–629.
Keywords: Ehlers-Danlos, obstructive, pediatrics, sleep apnea
BRIEF SUMMARY
Current Knowledge/Study Rationale: There is evidence of a high frequency of sleep problems in adults with Ehlers-Danlos syndrome (EDS); specifically, high rates of obstructive sleep apnea (OSA), low sleep quality, and periodic limb movement disorder (PLMD). However, no data exist regarding OSA and other sleep disorders as well as their management in children with EDS.
Study Impact: Our data suggest that a high prevalence of sleep disorders, specifically OSA, insomnia, circadian rhythm disorders, PLMD, and hypersomnia already exist by late childhood in children with EDS referred to sleep clinics, and that specific management can improve quality of life. Hence, a high index of suspicion for sleep disorders is necessary in this population.
INTRODUCTION
Ehlers-Danlos syndrome (EDS) is a clinically and genetically rare heterogeneous group of inherited connective tissue disorders characterized by joint hypermobility, skin hyper-extensibility, and tissue fragility. It is estimated to occur in approximately 1 in every 5,000 births, and symptoms usually present in early childhood.1 According to the Villefranche classification, there are six types of EDS. The hypermobility type is the most common, followed by the classic type, and both account for 90% of cases.2,3
Fatigue is a common symptom of patients with EDS and is associated with poor sleep, greater psychologic distress, and sleep disruption.4–6 Other associated conditions that can interfere with sleep quality are chronic pain,7,8 dysautonomia,9 and psychiatric disorders.10 Moreover, previous research in adults showed a high frequency of distinct sleep disorders in this population. Adult populations with EDS are more likely to have sleep-disordered breathing (SDB) and obstructive sleep apnea (OSA) compared to the general population, and these patients seem to respond well to nasal continuous positive airway pressure (CPAP) therapy.5,11 It was also shown that EDS and OSA in adults were associated with impaired quality of life and excessive daytime sleepiness.11 This predisposition for SDB has been explained by genetic abnormalities in oral-facial growth, which lead to cartilaginous defects.5 Other sleep problems common in these adult patients with EDS, other than OSA, include low sleep quality and periodic limb movement disorder (PLMD).12
The consequence of sleep deprivation or nonrestorative sleep from primary sleep disorders, or EDS itself, might aggravate fatigue, impair physical performance,13 increased pain severity,14 aggravate depression,15–17 and impair quality of life.6
Because symptoms of specific sleep disorders and EDS might overlap and exacerbate the other, the diagnoses and management is more challenging but crucial for appropriate treatment. However, no data exist in children with EDS regarding the presence of sleep disorders, the management of these sleep disorders, or the outcomes of treatment. Hence, we aimed to address these questions by describing the sleep disorders diagnosis, the management, and the short-term outcome for various sleep disorders in children with EDS.
METHODS
Study Participants
Following institutional review board approval at the Cincinnati Children's Hospital Medical Center, we performed a retrospective chart review of patients with EDS younger than 18 years who presented to our sleep clinic from July 2009 to June 2017. Patients who had an indeterminate diagnosis of EDS or incomplete medical records were excluded from the study. Patients were identified through the Cincinnati Children's Hospital Medical Center medical database.
Demographic information and medical history were collected, and diagnostic polysomnography (PSG) tests were reviewed. In addition, we evaluated the following information: (1) chart review for major sleep diagnoses and management through the sleep clinic for each individual encounter, including pharmacological and nonpharmacological treatments, (2) questionnaire scores of the Pediatrics Sleep Questionnaire (PSQ), Epworth Sleepiness Scale (ESS), Pediatric Quality of Life Inventory (PedsQL), and (3) the pediatric pain score that was performed either at the rheumatology or pain clinics within 1 month of the first sleep clinic encounter.
Polysomnography
PSG tests were performed in the sleep laboratory at Cincinnati Children's Sleep Center with the use of a digitized system (Twin Software, Grass Technologies, West Warwick, Rhode Island, United States). The standard pediatric montage was used and the following parameters were simultaneously recorded during the study: electroencephalogram (F3A2, F4A1, O1A2, O2A1, C4A1, C3A2), right and left electro-oculogram (ROC/A1, LOC/A2), submental, tibial and intercostal electro-myogram, electrocardiography, airflow with thermistor and nasal pressure transducer, end-tidal pCO2 (BCI Capnocheck, Smiths Medical, St. Paul, Minnesota, United States), oxygen saturation by pulse oximeter, oximeter pulse waveform, and video monitoring using an infrared video camera and recorded on a videotape. Rib cage and abdominal volume changes were recorded with a computer-assisted respiratory inductance plethysmograph.
PSG Interpretation
All PSG tests were scored according to the American Academy of Sleep Medicine guidelines.18 The severity of OSA was defined by the obstructive apnea-hypopnea index (oAHI). Mild OSA was defined as an oAHI between 1 and < 5 events/h, moderate OSA was defined as an oAHI between 5 and < 10 events/h, and severe OSA was defined as an oAHI ≥ 10 events/h. Periodic limb movement index during sleep (PLMS) was defined as periodic limb movement index more than 5 events/h. PLMD is defined by the presence of PLMS associated with symptoms of insomnia or excessive daytime sleepiness. For subjects who underwent multiple PSG tests, the sleep study with the highest obstructive index was used to confirm and exclude a diagnosis of OSA. Multiple Sleep Latency Test (MSLT) with a mean sleep onset latency < 8 minutes and ≥ 2 sleep onset REM periods (SOREMs) was considered consistent with a diagnosis of narcolepsy. An MSLT with a mean sleep onset latency < 8 minutes and < 2 SOREMs was considered consistent with the diagnosis of idiopathic hypersomnia19
Other Sleep Disorder Diagnoses
Other sleep disorder diagnoses were determined by the diagnoses given during the sleep clinic visits. Subjects who were lost to follow-up and did not undergo PSG were grouped as lost to follow-up. Subjects who had normal PSG and clinical presentation that did not suggest any known sleep disorder were grouped as undetermined diagnosis.
Sleep Questionnaires
As part of the quality improvement initiatives at Cincinnati Children's Hospital Medical Center, all sleep patients or care-givers are requested to complete three sets of questionnaires prior to the physician visit. The first questionnaire, the PSQ, is designed to evaluate sleep-related breathing disorders in pediatric patients.20 It is composed of 22 items with the total score ranging from 0–1. A cutoff value of 0.33 is used to identify pediatric sleep-disordered breathing. The ESS is designed to evaluate patients for average sleep propensity across a wide range of activities in their daily lives. It is composed of 8 items, each range from 0–3. The total score ranges from 0–24, and a score > 10 is considered significant excessive daytime sleepiness.21,22 The PedsQL is composed of a patient self-report and a parent-proxy report form. The PedsQL consists of 23 items addressing 4 core domains: physical, emotional, social, and school functioning. PedsQL is summarized into the following two measures: physical health summary score and psychosocial health summary score. The total score ranges from 0–100, and higher scores indicate a better quality of life.23
Our institution uses the numerical rating scale pain scale from 0 to 10 that is obtained by verbal assessment.24 Pain scores were reported for those subjects who were evaluated within 1 month before or after the sleep clinic visit. The assessment was performed in the rheumatology or pain clinics.
Statistical Analysis
Data distributions were reported as means with standard deviations and as percentages in categorical variables or median and interquartile range for continuous variables. Comparison of questionnaire scores from the first visit to the last visit was performed using a two-tailed t test. Adjustment for fewer than three visits and three visits or more was performed using mixed-model fitting. A value of P < .05 was considered statistically significant.
RESULTS
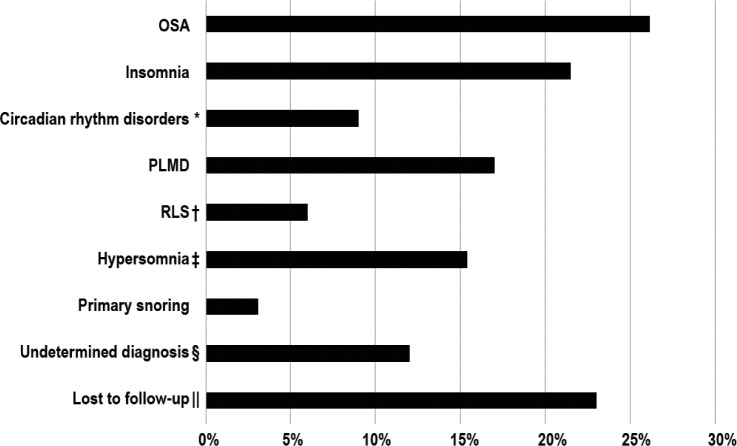
Sixty-five children were included in our study. All 65 patients received a diagnosis of EDS-hypermobility type (type 3). The mean age presenting to the sleep clinic was 13.15 ± 3.9 years. A total of 68% of patients were female, and 91% of patients were Caucasian. The demographic and sleep clinic characteristics are presented in Table 1. Common sleep diagnoses included insomnia (n = 14, 22%), circadian rhythm disorders (n = 6, 9%), OSA (n = 17, 26%), PLMD (n = 11, 17%) and hypersomnia (n = 10, 15%) (Figure 1). Restless legs syndrome (RLS) was diagnosed in four patients (n = 6%), of whom three had a previous diagnosis of PLMD. All subjects had one to two diagnoses, except for a single patient in whom three disorders were diagnosed.
Table 1.
The demographic and clinical characteristics (n = 65).
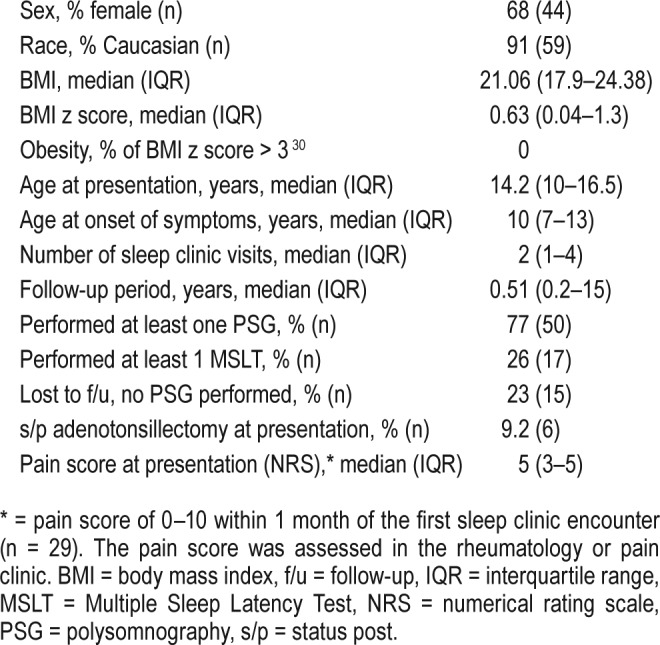
Figure 1. Sleep disorder diagnosis.
* = 6 patients received a diagnosis of circadian rhythm disorders, and all met the criteria for delayed sleep phase syndrome. † = 3 of 4 patients with RLS had a previous diagnosis of PLMD. ‡ = 4 patients received a diagnosis of idiopathic hypersomnia based on the clinical presentation and PSG, 6 received a diagnosis of narcolepsy and of those, 1 was lost to follow-up. § = subjects who presented with either excessive daytime sleepiness or restless sleeper but had normal PSG and no identified clinical sleep disorders. ‖ = subjects who did not complete PSG and were lost to follow-up; hence, they did not reach a final sleep disorder diagnosis. OSA = obstructive sleep apnea, PLMD = periodic limb movement disorder, PSG = polysomnography, RLS = restless legs syndrome.
Sleep Management
Nonpharmacological
Subjects were given recommendations on improving their sleep hygiene in the first visit (42/65, 65%). A total of 19/65 (29%) were referred to behavioral sleep medicine at any visit, whereas 12/61 (18%) were referred in the first visit.
Pharmacological Treatment
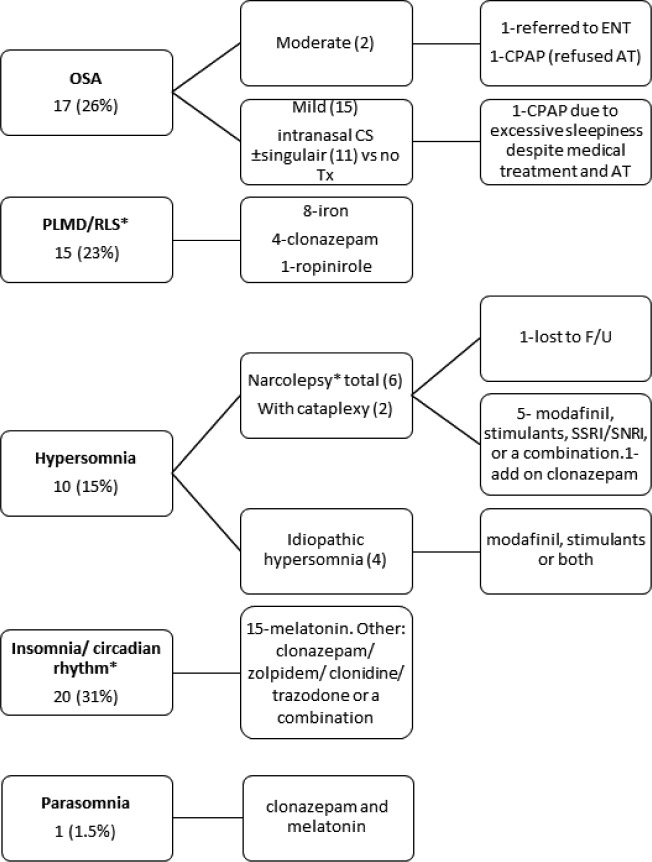
Subjects were prescribed pharmacological treatment (42/65, 65%). Some were treated with 2 medications or more (27/65, 41%). The pharmacological treatment that was prescribed in the sleep clinic is presented in Figure 2. Subjects who were lost to follow-up and subjects with undetermined diagnosis were not included. Twelve patients (20%) were treated with sleep medication by other medical teams prior to the first sleep clinic visit.
Figure 2. Treatment for the most common sleep disorders.
* = PLMD/RLS and insomnia/circadian rhythm were grouped together. Subjects who were lost to follow-up and did not undergo PSG were excluded from the analysis. AT = adenotonsillectomy, CPAP = continuous positive airway pressure, CS = corticosteroids, ENT = ear, nose and throat, F/U = follow-up, PLMD = periodic limb movement disorder, OSA = obstructive sleep apnea, PSG = polysomnography, RLS = restless legs syndrome, SNRI = serotonin norepinephrine reuptake inhibitors, SSRI = selective serotonin reuptake inhibitors, Tx = treatment.
CPAP Treatment
Two patients, one with mild OSA and excessive daytime sleepiness despite medical treatment and one with moderate OSA who refused adenotonsillectomy, were treated with CPAP (Figure 2).
Short-Term Follow-Up
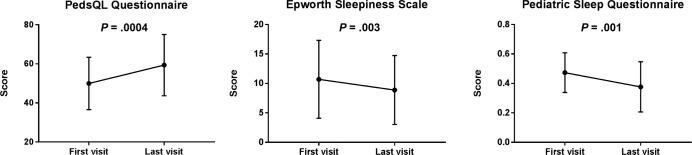
The longitudinal changes in PSQ, ESS, and PedsQL questionnaire scores from the first to the last visit are presented in Figure 3. Only subjects who had more than one encounter with complete questionnaire scores were included in this analysis (n = 34). The PSQ and ESS scores from the last visit were significantly lower than those of the first visit (P = .01 and .03, respectively), whereas the PedsQL score was significantly higher (P = .0004). Adjustment for the number of sleep clinic visits (fewer than three, and three or more) showed a significant difference from the first to the last visit for the PSQ (P = .04) and PedQL (P = .03), whereas the difference in ESS was not significantly different (P = .32). Sixty-three percent of the patients had a positive PSQ at presentation (PSQ > 0.33), and 46% had a positive ESS at presentation (ESS > 10).
Figure 3. A comparison of questionnaire scores between the first to the last sleep clinic encounter in 34 patients.
Variables are presented as mean ± standard deviation.
Further subgroup analysis was performed in subjects with hypersomnia. For narcolepsy patients (5 out of 6, 1 was lost to follow-up), the median ESS in the first visit was 20 (interquar-tile range [IQR] 16–22), whereas the median EES in the last visit was 9 (IQR 6.5–17.5). A trend toward significant improvement (P = .06) was observed. By parental report, modafinil and stimulants (methylphenidate or amphetamine derivatives) were well tolerated in all five children with narcolepsy. For children with idiopathic hypersomnia, three of four were treated at some points with modafinil. One patient stopped modafinil due to motor tics, whereas the other two responded well. No difference was observed in the ESS of those patients with idiopathic hypersomnia.
DISCUSSION
Our study demonstrates that in children with EDS-hypermobility type presenting to our sleep clinic, multiple types of sleep disorders are diagnosed. The common types include OSA, insomnia, circadian rhythm disorders, PLMD, and hypersomnia. Treatment in our cohort involves both nonpharmacologic and pharmacologic management. Although 26% of the patients had a diagnosis of OSA, all of them had mild to moderate OSA and only two required CPAP. A significant improvement in quality of life, PSQ and ESS questionnaire scores were observed throughout the follow-up period.
OSA was diagnosed in 26% of our subjects. However, because 23% were lost to follow-up, we may be missing some patients with OSA. Most OSA in our cohort was mild (88% of cases), requiring only medical treatment (nasal steroid and or montelukast). It should be noted that the prevalence of OSA in the pediatric population is 1% to 5% (mostly between the ages of 2 to 8 years25) and of habitual snoring is 4% to 34%,26–28 whereas the mean age of our subjects was 13 years. Similar to our findings, a recent study found the prevalence of OSA in adult patients with EDS was 32%.11 Another retrospective study on adults with EDS reported that all received a diagnosis of sleep-disordered breathing.5 The etiology of high prevalence of OSA in EDS is unknown. One possible mechanism is that abnormal cartilaginous growth in adults with EDS can cause abnormal growth of the nasomaxillary complex that could lead to both increased nasal resistance and altered maxillary development.5,29 However, there have been no reports demonstrating an association between craniofacial phenotypes of patients with EDS and the presence of OSA.11 Another possible mechanism is body habitus. However, none of our subjects were obese (BMI z-score > 3).30 Therefore, a mechanism to explain a predisposition for OSA in children with EDS is yet to be determined. Overall, our data suggest that a high index of suspicion should be applied for children with EDS and sleep complaints.
Unexpectedly, we had a significant proportion of patients with hypersomnia (six with narcolepsy and four with idiopathic hypersomnia). For narcolepsy, most of these children were prescribed two to three medications to control their symptoms. This is the first study that demonstrates such a high percentage of narcolepsy in children with EDS. Because the estimated incidence of narcolepsy in children is 0.83 per 100,000 person-years,31 this finding should be examined cautiously and could be an incidental finding based on the small number of study participants.
One-third of our study subjects had insomnia or circadian rhythm disorders. Different studies showed 5% to 20% prevalence of insomnia in the pediatric population,32–34 whereas comorbid insomnia is a much more frequent problem than primary insomnia. Although there are limited data on the association between EDS and insomnia,3 it is a well-known problem in other syndromes that share similar clinical features, such as chronic pain syndromes35 and fibromyalgia.36,37 Previous studies have shown that a reciprocal relationship exists between pain and sleep disturbances and that intervention targeted primarily at insomnia may improve pain.35,38 For this reason, it is possible that appropriate treatment of the underlying sleep disorders could have a dramatic effect on the complaints attributed to chronic pain syndromes.
In our study, most patients were treated with melatonin for insomnia. Other prescribed medications for insomnia included clonazepam, zolpidem, clonidine, and trazodone. Special considerations should be taken when sleep medications are prescribed in EDS. Postural orthostatic tachycardia syndrome (POTS) or dysautonomia occurs in 80% of patients with EDS.39,40 Although medications such as alpha agonists (clonidine),41 benzodiazepine, and melatonin42 treat both POTS and insomnia, tricyclic antidepressants43 can aggravate symptoms of POTS. In addition, psychiatric disorders are common in patients with EDS, and medications that are indicated for both sleep and psychiatric diagnoses should be considered for these patients.
The short-term outcome in our study was assessed by the three different questionnaires. Regarding the quality of life, we showed a significant improvement in the PedsQL scores. A recent publication concerning the natural history of children with EDS and hypermobility syndrome showed a mean score of 67.9 ± 15.5 in males and 61.1 ± 19.2 in females at baseline evaluation.44 Although the sleep aspect was not addressed in that study, our study showed a similar mean PedsQL score in the female group in the last encounter. We also showed improvement in PSQ and ESS that emphasizes the importance of addressing sleep issues in these patients.
Our study has several limitations. First, it is a retrospective study that is susceptible to selection bias and lacks long-term follow-up data for all the subjects. To compensate for the variable follow-up periods for each subject, we added an adjustment to the number of sleep clinic visits. Second, we describe the most common diagnosis of children with EDS who were referred to the sleep clinic; therefore, it may not be applicable to the general population of children with EDS. Moreover, according to the medical records, all our patients received a diagnosis of EDS-hypermobility type. For this reason, our results do not necessarily apply to the other types of EDS. In addition, a response bias due to a “placebo effect” could have been introduced to the short-term outcome assessments by the questionnaire scores. Last, for RLS which a 2% to 4% prevalence was reported in the pediatric population, due to updates in consensus diagnostic criteria throughout the years, some cases of RLS might have been underdiagnosed.45
CONCLUSIONS
To our knowledge, this is the first study that evaluated sleep disorders in children with EDS. Our data indicate a high prevalence of a variety of sleep disorders in late childhood and in adolescent patients with EDS who are referred to the sleep clinic. In addition, management of sleep disorders can improve quality of life. Most children with EDS-hypermobility type seen in our sleep clinic received medical or behavioral therapy for insomnia, circadian rhythm sleep disorders, hypersomnia, PLMD and mild OSA. Many children require complex treatment regimens in order to control their symptoms. A high index of suspicion for sleep disorders is necessary, and referral to the sleep clinic has the potential to improve the clinical symptoms and the quality of life in this population. Further prospective studies to determine the prevalence of sleep disorders in children with EDS are needed.
DISCLOSURE STATEMENT
Work for this study was performed at the Cincinnati Children's Medical Center, Cincinnati, Ohio. All authors have seen and approved the manuscript. The authors report no conflicts of interest.
ABBREVIATIONS
- CPAP
continuous positive airway pressure
- EDS
Ehlers-Danlos Syndrome
- ESS
Epworth Sleepiness Scale
- IQR
interquartile range
- MSLT
Multiple Sleep Latency Test
- oAHI
obstructive apnea-hypopnea index
- OSA
obstructive sleep apnea
- PedsQL
Pediatric Quality of Life Inventory
- PLMD
periodic limb movement disorder
- PLMI
periodic limb movement index
- PLMS
periodic limb movement in sleep
- POTS
postural orthostatic tachycardia syndrome
- PSG
polysomnography
- PSQ
Pediatrics Sleep Questionnaire
- RLS
restless legs syndrome
- SDB
sleep-disordered breathing
- s/p
status post
REFERENCES
- 1.Steinmann B, Royce PM, Superti-Furga A. The Ehlers-Danlos Syndrome. In: Royce PM, Steinmann B, editors. Connective Tissue and its Heritable Disorders: Molecular, Genetic, and Medical Aspects. 2nd ed. New York, NY: Wiley-Liss; 2002. pp. 431–523. [Google Scholar]
- 2.Beighton P, De Paepe A, Steinmann B, Tsipouras P, Wenstrup RJ. Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK) Am J Med Genet. 1998;77(1):31–37. doi: 10.1002/(sici)1096-8628(19980428)77:1<31::aid-ajmg8>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 3.Voermans NC, Knoop H, van de Kamp N, Hamel BC, Bleijenberg G, van Engelen BG. Fatigue is a frequent and clinically relevant problem in Ehlers-Danlos Syndrome. Semin Arthritis Rheum. 2010;40(3):267–274. doi: 10.1016/j.semarthrit.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Hakim A, De Wandele I, O'Callaghan C, Pocinki A, Rowe P. Chronic fatigue in Ehlers-Danlos syndrome-Hypermobile type. Am J Med Genet C Semin Med Genet. 2017;175(1):175–180. doi: 10.1002/ajmg.c.31542. [DOI] [PubMed] [Google Scholar]
- 5.Guilleminault C, Primeau M, Chiu HY, Yuen KM, Leger D, Metlaine A. Sleep-disordered breathing in Ehlers-Danlos syndrome: a genetic model of OSA. Chest. 2013;144(5):1503–1511. doi: 10.1378/chest.13-0174. [DOI] [PubMed] [Google Scholar]
- 6.Rombaut L, Malfait F, Cools A, De Paepe A, Calders P. Musculoskeletal complaints, physical activity and health-related quality of life among patients with the Ehlers-Danlos syndrome hypermobility type. Disabil Rehabil. 2010;32(16):1339–1345. doi: 10.3109/09638280903514739. [DOI] [PubMed] [Google Scholar]
- 7.Voermans NC, Knoop H, Bleijenberg G, van Engelen BG. Pain in ehlersdanlos syndrome is common, severe, and associated with functional impairment. J Pain Symptom Manage. 2010;40(3):370–378. doi: 10.1016/j.jpainsymman.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 8.Stern CM, Pepin MJ, Stoler JM, Kramer DE, Spencer SA, Stein CJ. Musculoskeletal conditions in a pediatric population with Ehlers-Danlos syndrome. J Pediatr. 2017;181:261–266. doi: 10.1016/j.jpeds.2016.10.078. [DOI] [PubMed] [Google Scholar]
- 9.Xu X, Huang H, Sethi S, Zuzuarregui JR, Weinberg J, Hohler AD. A survey based study on sleep disturbance in postural tachycardia syndrome. J Neurol Sci. 2016;365:199–202. doi: 10.1016/j.jns.2016.04.028. [DOI] [PubMed] [Google Scholar]
- 10.Bulbena A, Baeza-Velasco C, Bulbena-Cabre A, et al. Psychiatric and psychological aspects in the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet. 2017;175(1):237–245. doi: 10.1002/ajmg.c.31544. [DOI] [PubMed] [Google Scholar]
- 11.Gaisl T, Giunta C, Bratton DJ, et al. Obstructive sleep apnoea and quality of life in Ehlers-Danlos syndrome: a parallel cohort study. Thorax. 2017;72(8):729–735. doi: 10.1136/thoraxjnl-2016-209560. [DOI] [PubMed] [Google Scholar]
- 12.Verbraecken J, Declerck A, Van de Heyning P, De Backer W, Wouters EF. Evaluation for sleep apnea in patients with Ehlers-Danlos syndrome and Marfan: a questionnaire study. Clin Genet. 2001;60(5):360–365. doi: 10.1034/j.1399-0004.2001.600507.x. [DOI] [PubMed] [Google Scholar]
- 13.Patrick Y, Lee A, Raha O, et al. Effects of sleep deprivation on cognitive and physical performance in university students. Sleep Biol Rhythms. 2017;15(3):217–225. doi: 10.1007/s41105-017-0099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onen SH, Alloui A, Gross A, Eschallier A, Dubray C. The effects of total sleep deprivation, selective sleep interruption and sleep recovery on pain tolerance thresholds in healthy subjects. J Sleep Res. 2001;10(1):35–42. doi: 10.1046/j.1365-2869.2001.00240.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen MC, Burley HW, Gotlib IH. Reduced sleep quality in healthy girls at risk for depression. J Sleep Res. 2012;21(1):68–72. doi: 10.1111/j.1365-2869.2011.00934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howland RH. Sleep interventions for the treatment of depression. J Psychosoc Nurs Ment Health Serv. 2011;49(1):17–20. doi: 10.3928/02793695-20101208-01. [DOI] [PubMed] [Google Scholar]
- 17.Owens J Adolescent Sleep Working Group, Committee on Adolescence. Insufficient sleep in adolescents and young adults: an update on causes and consequences. Pediatrics. 2014;134(3):e921–e932. doi: 10.1542/peds.2014-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Littner MR, Kushida C, Wise M, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28(1):113–121. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- 20.Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000;1(1):21–32. doi: 10.1016/s1389-9457(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 21.Janssen KC, Phillipson S, O'Connor J, Johns MW. Validation of the Epworth Sleepiness Scale for children and adolescents using Rasch analysis. Sleep Med. 2017;33:30–35. doi: 10.1016/j.sleep.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Anderson B, Storfer-Isser A, Taylor HG, Rosen CL, Redline S. Associations of executive function with sleepiness and sleep duration in adolescents. Pediatrics. 2009;123(4):e701–e707. doi: 10.1542/peds.2008-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Medical Care. 2001;39(8):800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Chan S, Kurowski B, Byczkowski T, Timm N. Intravenous migraine therapy in children with posttraumatic headache in the ED. Am J Emerg Med. 2015;33(5):635–639. doi: 10.1016/j.ajem.2015.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):e714–e755. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- 26.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):242–252. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castronovo V, Zucconi M, Nosetti L, et al. Prevalence of habitual snoring and sleep-disordered breathing in preschool-aged children in an Italian community. J Pediatr. 2003;142(4):377–382. doi: 10.1067/mpd.2003.118. [DOI] [PubMed] [Google Scholar]
- 28.Sogut A, Yilmaz O, Dinc G, Yuksel H. Prevalence of habitual snoring and symptoms of sleep-disordered breathing in adolescents. Int J Pediatr Otorhinolaryngol. 2009;73(12):1769–1773. doi: 10.1016/j.ijporl.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 29.Huang YS, Guilleminault C. Pediatric obstructive sleep apnea and the critical role of oral-facial growth: evidences. Front Neurol. 2012;3:184. doi: 10.3389/fneur.2012.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization website. The WHO Child Growth Standards. [Accessed 2017]. http://www.who.int/childgrowth/standards/en/
- 31.Wijnans L, Lecomte C, de Vries C, et al. The incidence of narcolepsy in Europe: before, during, and after the influenza A(H1N1)pdm09 pandemic and vaccination campaigns. Vaccine. 2013;31(8):1246–1254. doi: 10.1016/j.vaccine.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 32.Meltzer LJ, Johnson C, Crosette J, Ramos M, Mindell JA. Prevalence of diagnosed sleep disorders in pediatric primary care practices. Pediatrics. 2010;125(6):e1410–e1418. doi: 10.1542/peds.2009-2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts RE, Roberts CR, Duong HT. Chronic insomnia and its negative consequences for health and functioning of adolescents: a 12-month prospective study. J Adolesc Health. 2008;42(3):294–302. doi: 10.1016/j.jadohealth.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Archbold KH, Pituch KJ, Panahi P, Chervin RD. Symptoms of sleep disturbances among children at two general pediatric clinics. J Pediatr. 2002;140(1):97–102. doi: 10.1067/mpd.2002.119990. [DOI] [PubMed] [Google Scholar]
- 35.Roehrs TA. Does effective management of sleep disorders improve pain symptoms? Drugs. 2009;69(Suppl 2):5–11. doi: 10.2165/11531260-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 36.Wu YL, Chang LY, Lee HC, Fang SC, Tsai PS. Sleep disturbances in fibromyalgia: A meta-analysis of case-control studies. J Psychosom Res. 2017;96:89–97. doi: 10.1016/j.jpsychores.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 37.Rombaut L, Malfait F, De Paepe A, et al. Impairment and impact of pain in female patients with Ehlers-Danlos syndrome: a comparative study with fibromyalgia and rheumatoid arthritis. Arthritis Rheum. 2011;63(7):1979–1987. doi: 10.1002/art.30337. [DOI] [PubMed] [Google Scholar]
- 38.Tang NK, Lereya ST, Boulton H, Miller MA, Wolke D, Cappuccio FP. Nonpharmacological treatments of insomnia for long-term painful conditions: a systematic review and meta-analysis of patient-reported outcomes in randomized controlled trials. Sleep. 2015;38(11):1751–1764. doi: 10.5665/sleep.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gazit Y, Nahir AM, Grahame R, Jacob G. Dysautonomia in the joint hypermobility syndrome. Am J Med. 2003;115(1):33–40. doi: 10.1016/s0002-9343(03)00235-3. [DOI] [PubMed] [Google Scholar]
- 40.Benarroch EE. Postural tachycardia syndrome: a heterogeneous and multifactorial disorder. Mayo Clin Proc. 2012;87(12):1214–1225. doi: 10.1016/j.mayocp.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu WR, Jin HF, Du JB. Pathogenesis and individualized treatment for postural tachycardia syndrome in children. Chin Med J. 2016;129(18):2241–2245. doi: 10.4103/0366-6999.189915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Green EA, Black BK, Biaggioni I, et al. Melatonin reduces tachycardia in postural tachycardia syndrome: a randomized, crossover trial. Cardiovasc Ther. 2014;32(3):105–112. doi: 10.1111/1755-5922.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stage KB. Orthostatic side effects of clomipramine and moclobemide during treatment for depression. Nord J Psychiatry. 2005;59(4):298–301. doi: 10.1080/08039480500213725. [DOI] [PubMed] [Google Scholar]
- 44.Scheper MC, Nicholson LL, Adams RD, Tofts L, Pacey V. The natural history of children with joint hypermobility syndrome and Ehlers-Danlos hypermobility type: a longitudinal cohort study. Rheumatology. 2017;56(12):2073–2083. doi: 10.1093/rheumatology/kex148. [DOI] [PubMed] [Google Scholar]
- 45.Picchietti DL, Bruni O, de Weerd A, et al. Pediatric restless legs syndrome diagnostic criteria: an update by the International Restless Legs Syndrome Study Group. Sleep Med. 2013;14(12):1253–1259. doi: 10.1016/j.sleep.2013.08.778. [DOI] [PubMed] [Google Scholar]