Abstract
Dental pulp stem cells (DPSC) are a relatively new alternative stem cell source for the study of neurogenetic disorders. DPSC can be obtained non-invasively and collected from long-distances remaining viable during transportation. These highly proliferative cells express stem cell markers and retain the ability to differentiate down multiple cell lineages including chondrocytes, adipocytes, osteoblasts, and multiple neuronal cell types. The neural crest origin of DPSC makes them a useful source of primary cells for modeling neurological disorders at the molecular level. In this brief review, we will discuss recent developments in DPSC research that highlight the molecular etiology of DPSC derived neurons and how they may contribute to our understanding of neurogenetic disorders.
Introduction
To understand the complex molecular and physiological pathways underlying neurogenetic syndromes, it is often useful to have an in vitro model that accurately recapitulates the live neurons in the disease state. The use of induced pluripotent stem cells (iPSC) to model the function of cortical neurons from patient derived fibroblasts or blood cells is now well-documented (1–4). However, there are notable drawbacks to using these de-differentiated, reprogrammed cells as in vitro models for molecular studies. Recent studies indicate that iPSC may not accurately represent changes associated with neurological pathogenesis since residual epigenetic marks associated with their original cell type which can lead to inappropriate gene expression in the newly derived iPSC neurons (5). This residual epigenetic signature, along with genomic instability (6), tumorigenic potential (7), and a high mutational load (8) raises concerns for the use of iPSC to model neurogenic disorders that often have complicated genetic and epigenetic etiologies which can alter the molecular changes indicative of the particular syndrome.
Dental pulp stem cells (DPSC) (9,10) provide an alternative to iPSC and overcome some of the problems associated with epigenetic changes and re-programming (11,12). Another significant advantage to using DPSC is that they can be easily obtained from exfoliated or extracted teeth and then transported within 48 h to the laboratory (13). The easy collection and transport means that families of children with various neurogenetic syndromes are able to directly contribute to the investigation of these disorders without ever traveling to the laboratory. It also means that many more teeth representing many more individuals with these syndromes can be collected than for iPSC, which can be difficult to reprogram and often yield clones of a single individual for molecular studies (14–16).
DPSC have been shown to differentiate into functionally active neurons in the presence of varying neurotrophic factors (17–19). DPSC have been shown to differentiate into various neural cell types including dopaminergic cells (20–22), glutamatergic and GABAergic cells (23), as well as glial and Schwann cells (24,25). Additionally, the ability of pre-differentiated DPSC to integrate into the brains of normal rats and a rat model of traumatic cortical lesion which speaks to their therapeutic potential (26). Kiraly et al. showed that these engrafted DPSC retained their neuronal immunohistochemical and electrophysiological characteristics weeks after transplantation. In the traumatic cortical lesion rat model, the pre-differentiated DPSC even migrated to the cortex in response to injury. Pre-differentiated DPSC have also been shown to improve neurological function in a Parkinsonian rat model. These dopaminergic-like neurons increased brain dopamine levels and the neuroprotection of endogenous dopaminergic neurons (27). These studies suggest great promise for using DPSC in translational research.
Stem Cells of the Dental Pulp
Stem cells are classified into three distinct categories: embryonic stem cells (ESC), adult (post-natal) stem cells (ASC), and induced pluripotent stem cells (iPSC). ASC encompass a broad range of different stem cells including neural (NSC) and mesenchymal stem cells (MSC) (28). MSC have been shown to differentiate into several terminal cell types such as osteoblasts, adipocytes, and chondrogenic cells (29), making them prime candidates for cell based therapies. MSC have been isolated from a variety of tissues including bone marrow (BMSC) and dental pulp (DPSC).
DPSC reside deep in the dental pulp at the center of the tooth in the pulp cavity and is a part of the dentin-pulp complex (Fig. 1). The regenerative quality of this complex relies on activity within the dental pulp. Gronthos et al. discovered a unique stem cell population derived from this pulp tissue that resembled BMSC (9). They found that these cells had self-renewal capabilities and were able to differentiate into adipocytes and neural-like cells (10). Following the initial discovery of DPSC, a separate population of stem cells arising from the pulp of deciduous (“baby”) teeth was characterized. Miura et al. isolated stem cells from human exfoliated deciduous teeth (SHED) (30) that shared similarities with MSC found in umbilical cord blood. They also discovered that compared to DPSC, SHED had higher proliferation rates, increased cell population doublings, and a distinct morphology. This difference is not shocking given the different developmental origins between deciduous and adult teeth. Deciduous teeth develop much earlier in the prenatal period than adult teeth. This difference likely effects the stem cell niches in which these cells reside. These microenvironments are controlled by genetic, epigenetic, and environmental forces that regulate the activities of the stem cells (31). Where a particular stem cell resides and the cell type from which it is derived can contribute to the variability in gene expression observed among stem cells isolated from different tissues.
Figure 1.
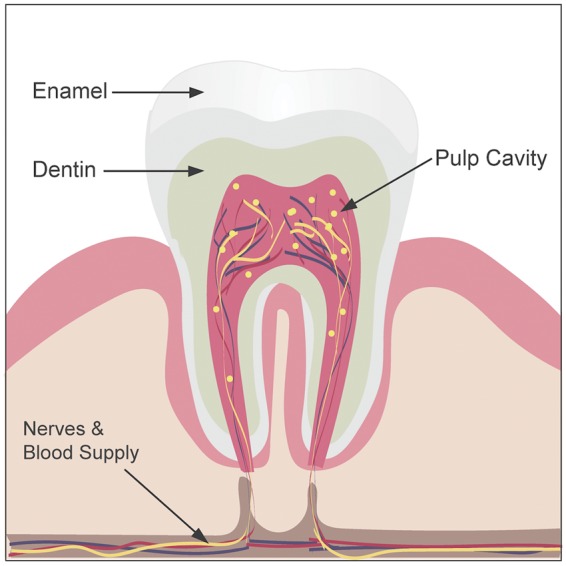
Longitudinal section of a tooth depicting the pulp cavity where the dental pulp resides. DPSC are found within the dental pulp and are represented here as yellow dots.
DPSC have now been compared to other stem cell types used for neurogenetic investigations including ESC, iPSC, and other MSC. Karoaz et al. were among the first to compare DPSC to BMSC (32). They found that DPSC had similar differentiation capabilities as BMSC and expressed both neural and glial specific markers. They also found that DPSC are more developed and metabolically active cells than BMSC, leading to the conclusion that DPSC demonstrate better neural and epithelial stem cell properties. More recently, more robust comparisons utilizing several types of stem cells have been performed. Umbilical cord, dental pulp, and menstrual blood are all relatively accessible sources of MSC. Ren et al. compared these three sources on the basis of morphology, proliferation, and differentiation capabilities (33). Their results show that while stem cells isolated from the umbilical tissue (UC) had higher proliferative capacities, DPSC demonstrated less cellular senescence and higher osteogenic capabilities than either the UC or menstrual blood cells.
Similarly, Kang et al. compared gene expression between DPSC and UC (34). They found that genes related to growth factor activity, receptor activity, and signal transduction were significantly upregulated in DPSC compared to UC. In contrast, genes related to cell proliferation, angiogenesis, and immune responses were expressed in higher levels in the UC. These studies complement each other since the higher proliferation rates of UC correlates with the higher expression of genes related to cell proliferation and the lower senescence of the DPSC likely correlates to the increased expression of genes related to growth factor activity.
Isobe et al. compared the differentiation capacity among DPSC, SHED, BMSC, and synovial fluid cells (SFC), all considered MSC (35). Multipotent capacity was significantly different among these stem cell sources. BMSC and SFC had higher osteogenic and chondrogenic capacity, while DPSC and SHED demonstrated increased neurogenesis. They also observed that SHED showed higher proliferation rates compared to both DPSC and BMSC. Another study comparing DPSC, SHED, and BMSC found that DPSC and SHED express higher levels of embryonic stem cell markers, Oct-4 and STRO-1, than BMSC. As in other studies, BMSC showed higher osteogenic capabilities than SHED and DPSC. They also present notable differences between DPSC and SHED. SHED had higher osteogenic capabilities and expression of embryonic stem cell markers than DPSC. Between DPSC and SHED, SHED demonstrated higher expression of these markers (36). Both studies concluded that SHED likely represent a more primitive cell type than DPSC. These comparison studies both found that the levels of MSC-maker expression between the cell types is similar, but the differentiation potential is significantly different, even between SHED and DPSC.
Comparative studies between DPSC and iPSC, the main stem cell culture used to study neurogenetic syndromes, are still limited. However, there have been multiple studies focused on the efficiency and reliability of re-programming DPSC into iPSC (37–40). DPSC express many ESC markers even prior to reprogramming, thus it has been proposed that these cell types could be a more efficient source of creating iPSC than cells traditionally used for iPSC generation (39). A transcriptomic analysis of iPSC-derived neurons from both SHED and fibroblasts revealed lower expression of genes involved in hindbrain development (HOX and IRX families) and higher expression of genes involved in forebrain development (FOXP2, OTX1, and LHX2) in the SHED derived iPSC neurons (41). FOXP2 is involved in language and communication networks and has been implicated in ASD pathology, leading to the conclusion that DPSC derived iPSC may be appropriate for the study of neurodevelopmental disorders (41).
Analyzing the genetic and functional differences between iPSC and DPSC is crucial for determining the best approach to modeling both molecular and neurophysiological changes that occur in neurons in the disease state (38). Since reprogrammed cells often retain epigenetic marks from their original cell type, understanding the consequences of reprogramming or cellular origin on epigenetic marks is essential while developing reliable disease models. Dunaway et al. analyzed how accurately SHED modeled early life and whole genome DNA methylation patterns compared to iPSC (12). They found that the primitive origin of SHED allows these cells to more accurately model the embryonic inner cellular mass and the placenta. They concluded that compared to iPSC, DPSC are a more reliable model for the study of genome architecture and gene expression changes that occur in neurodevelopmental disease (12).
There is great potential for the use of stem cells to directly treat human disease by returning healthy stem cells with neurogenetic potential to the brain (42,43). In order for stem cells cultured ex vivo to be considered for therapeutic use, however, they must meet certain criteria including assessment of in vivo tumorigenic potential. While the tumorigenic potential of iPSC is well established (44,45), our lab recently showed that immortalized DPSC do not form tumors in vivo (11). The tumorigenic potential of both spontaneous and induced immortalized DPSC was assessed. Karyotype analysis revealed that while the spontaneously immortalized DPSC showed no genomic instability. The genomic instability of spontaneously immortalized stem cells from other tissue sources has been shown in multiple studies (6,46). The absence of this genomic instability and tumorigenic potential adds to the growing evidence that DPSC may represent a better stem cell source for both disease modeling and cell therapies.
Multipotent Differentiation Provides Cellular Specificity for a Variety of Syndromes
As neurodevelopmental disorders often involve multiple neuronal and non-neuronal cell types that can contribute to the phenotype, developing target treatments without access to the specific neuronal cell types can limit the effectiveness of these studies. Neural stem cells (NSC) differentiate into neurons and glia in vivo and in vitro, thus they represent a mixed population of cells that may represent the varied cell types found in the brain. DPSC, which arise for the neural crest, are an excellent proxy for NSC to study in the lab setting. When given the appropriate environmental cues, DPSC differentiate into functional neurons showing neuronal morphology and responsive sodium and potassium channels (47). Arthur et al. first differentiated DPSC into functional neurons using a protocol typically used to differentiate ESC (18). Using fibroblast growth factor or epidermal growth factor signaling, they were able to differentiate DPSC into neurons that exhibited functional sodium channels. Kiraly et al. also developed a protocol for differentiating DPSC into a neural population (19). In their protocol, the simultaneous activation of protein kinase C and cyclic AMP pathways induced neural differentiation. The DPSC-derived neurons had morphological characteristics similar to neurons derived from other sources (Fig. 2). They were able to show genetic markers associated with neural differentiation and functional sodium and potassium channels, demonstrating strong evidence that the DPSC had differentiated into functional neurons. Using the protocol established by Kiraly et al, we assessed the global gene expression changes resulting from DPSC into mixed neuronal cultures (19). We found that the differentiation process significantly changes gene expression and that these expression changes occur through the transcription factor RE1-Silencing Transcription (REST). The differentiated DPSC also positive for the neuronal marker MAP2 and expression for the glial marker GFAP was decreased in the mixed neuron/glia cultures versus undifferentiated DPSC but still present (48).
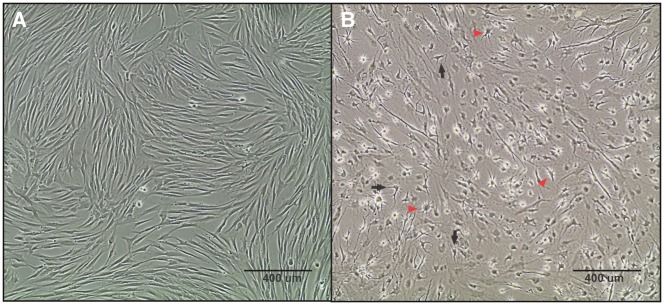
Figure 2.
Morphological changes during neuronal differentiation of DPSC. (A) Undifferentiated DPSC have a spindle-shape similar to fibroblasts. (B) DPSC were differentiated and allowed to mature for 3 weeks, resulting in a mixed culture containing both neuron-like and glial-like cells. The neurons (black arrows) show a pyramidal-like, neuronal morphology with shorter projections similar to dendrites and a longer axonal projection on the opposing side. The glial cells (red arrows) show a typical star-shaped morphology with a rounded cell body and small projections extending from the perimeter. Both images were taken at a 10x magnification using phase-contrast microscopy.
Differentiating DPSC into specific neural subtypes is crucial for modeling neurogenetic disorders as different syndromes affect different neural sub-types in a distinct manner. Several protocols have been published for differentiating DPSC and SHED to dopaminergic cells, or DA neurons. Wang et al. were the first to present a protocol for DA differentiation of SHED (20). This protocol results in about 10% of the cells expressing tyrosine hydroxylase (TH), the enzyme that converts tyrosine to L-DOPA, an essential dopamine precursor. Although dopaminergic induction resulted in a relatively small proportion of TH+ neurons, they also showed that when these DA-like cells were introduced into a Parkinson disease mouse model some Parkinson-like behavioral abnormalities were corrected (20). Kanafi et al. published a two-step protocol using the embryonic midbrain cues, SHH and FGF8 (22). They found that over 70% of cells were TH+ after induction and maturation. In a related study, they found that while this protocol induced the DPSC into functional DA-like cells, it was not efficient in differentiating the SHED to a terminal DA fate (49). Fujii et al. differentiated SHED into DA-like neurons using a BDNF stimulating protocol (27). Compared to other dopaminergic protocols (SHH and FGF8), they discovered that a BDNF protocol resulted in the most TH+ cells (∼75%). Because BDNF specifically is involved in the differentiation of new neurons and synapses, they concluded that a protocol with early neurogenesis signals and later maturation of DA neurons was required for efficient dopaminergic differentiation from SHED. This could provide a potential solution for the differentiation of SHED to DA-like neurons.
Although there is little research published showing differentiation of DPSC along neuronal lineages other than DA, one group has been able to differentiate DPSC into a neuronal population that shows increased glutamatergic and GABAergic-like cells (23). Depletion of PIN1 results in suppression of neurogenesis, while overexpression results in enhanced neurogenesis. PIN1 functions in regulating key signaling molecules involved in cell growth and differentiation. Cho et al. observed that upon differentiation, these neuronal populations displayed increased glutamatergic and GABA-ergic markers, while dopaminergic and glial marker expression was decreased resulting in a more glutamatergic and GABA-ergic like neuronal population (23). These results imply that we may soon develop protocols for a variety of neuronal cell types (dopaminergic, GABAergic, hypothalamic, cortical, etc.), which can be generated from patient-derived DPSC.
Practical Use of Primary DPSC Cultures
All primary cultures, even stem cells, eventually undergo cellular senescence. Long term culturing of primary cells typically causes senescence through telomere shortening or replicative senescence (50), which results in a DNA damage response. Senescent cells remain metabolically active, but undergo irreversible growth arrest that is accompanied by characteristic changes in gene expression. Even in stem cells, cellular senescence occurs during prolonged culture periods and results in the loss of differentiation potential and gene expression changes (51). DPSC also undergoes age-dependent molecular and genetic changes that result in reduced proliferative capacity and differentiation potential (52). Donor age has also been shown to positively correlate with cellular senescence and impaired regenerative abilities. In order for DPSC to be useful for long-term studies and clinical applications, it will be necessary to overcome cellular senescence in primary DPSC cultures.
The introduction of human telomerase reverse transcriptase (hTERT) into cells is one way to immortalize DPSC. In order for hTERT immortalization to be considered a practical solution cellular senescence in DPSC an investigation into how permanent hTERT expression effects gene expression and differentiation potential of DPSC (53). Recently, our lab analyzed the effects of hTERT immortalization on the gene expression of SHED derived neurons (48). Molecular analysis revealed that some of the transcripts that are upregulated during differentiation into neurons were suppressed by constitutive immortalization with hTERT. Thus, constitutive hTERT expression is therefore not ideal for neurogenetic studies. Although the hTERT immortalized SHED demonstrated characteristics of functional neurons, they do not accurately represent the expression patterns of donor neurons. An inducible immortalization system driving hTERT expression in the DPSC stage, but turning it off during neuronal differentiation could be a solution. Identifying a protocol that allows for the immortalization of DPSC without affecting the molecular and genetic characteristics of the neurons derived from these cells is critical for the utility of the DPSC model of disease.
Conclusions
There are several reasons to consider DPSC for neurogenetic studies versus stem cells from other tissue sources. DPSC can be obtained non-invasively and even shipped across long distances, remaining viable in culture media for as long as 72 h (48). Especially for rare genetic syndromes, the ability to collect samples from distant subjects, even from other countries, is invaluable since it is often difficult to obtain large sample sizes for molecular studies of these disorders. More comprehensive comparison studies of various stem cell types, including DPSC, iPSC, ESC, and other MSC, derived from a single donor may help to elucidate the functional and genomic differences between stem cell types. The differentiation potential of DPSC into a variety of neuronal cell types is still largely unexplored. Future studies will require an understanding of the developmental origins and molecular characteristics of DPSC in order to identify the signaling mechanisms needed to differentiate DPSC down different neuronal lineages. Finally, the immortalization of donor DPSC for long-term propagation in vitro is required for scaling up the use of DPSC in the study of various disorders of the nervous system. Although the use of DPSC for the study of neurogenetic disorders is relatively new, there is great potential for this stem cell resource both in the study of disease and potentially as a source for therapeutic stem cell treatments targeted to the nervous system.
Acknowledgements
The authors wish to thank the many families who have donated teeth for the production of DPSC from a wide variety of syndromes.
Conflict of Interest statement. None declared.
Funding
L Reiter through NIH R21NS075709 and a pilot research grant from the Foundation for Prader-Willi Research.
References
- 1. Liu Q., Spusta S.C., Mi R., Lassiter R.N.T., Stark M.R., Hoke A., Rao M.S., Zeng X. (2012) Human neural crest stem cells derived from human ESCs and induced pluripotent stem cells: induction, maintenance, and differentiation into functional Schwann cells. Stem Cells Transl. Med., 2012, 266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen X., Gu Q., Wang X., Ma Q., Tang H., Yan X., Guo X., Yan H., Hao J., Zeng F. (2013) Directed neuronal differentiation of mouse embryonic and induced pluripotent stem cells and their gene expression profiles. Int. J. Mol. Med., 32, 25–34. [DOI] [PubMed] [Google Scholar]
- 3. Badja C., Maleeva G., El-Yazidi C., Barruet E., Lasserre M., Tropel P., Binetruy B., Bregestovski P., Magdinier F. (2014) Efficient and cost-effective generation of mature neurons from human induced pluripotent stem cells. Stem Cells Transl. Med., 3, 1467–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yan Y., Shin S., Jha B., Liu Q., Sheng J., Li F., Zhan M., Davis J., Bharti K., Zeng X.. et al. (2013) Efficient and rapid derivation of primitive neural stem cells and generation of brain subtype neurons from human pluripotent stem cells. Stem Cells Transl. Med., 2, 862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim K., Doi A., Wen B., Ng K., Zhao R., Cahan P., Kim J., Aryee M.J., Ji H., Ehrlich L.I.R.. et al. (2010) Epigenetic memory in induced pluiripotent stem cells. Nature, 467, 285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yoshihara M., Hayashizaki Y., Murakawa Y. (2017) Genomic instability of iPSCs: challenges towards their clinical applications. Stem Cell Rev., 13, 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu Z., Tang Y., L., S., Zhou J., Du Z., Duan C., Li Z., Wang C. (2013) The tumourigenicity of iPS cells and their differentiated derivatives. J. Cell. Mol. Med., 17, 782–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gore A., Li Z., Fung H.-L., Young J., Agarwal S., Antosiewicz-Bourget J., Canto I., Giorgetti A., Israel M., Kiskinis E.. et al. (2011) Somatic coding mutations in human induced pluripotent stem cells. Nature, 471, 63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gronthos S., Mankani M., Brahim J., Gehron Robey P., Shi S. (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl Acad. Sci. U S A., 97, 13625–13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gronthos S., Brahim J., Li W., Fisher L.W., Cherman N., Boyde A., DenBesten P., Robey P.G., Shi S. (2002) Stem cell properties of human dental pulp stem cells. J. Dent. Res., 81, 531–535. [DOI] [PubMed] [Google Scholar]
- 11. Wilson R., Urraca N., Skobowiat C., Hope K.A., Miravalle L., Chamberlin R., Donaldson M., Seagroves T.N., Reiter L.T. (2015) Assessment of the tumorigenic potential of spontaneously immortalized and hTERT immortalized cultured dental pulp stem cells. Stem Cells Transl. Med., 4, 905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dunaway K., Goorha S., Matelski L., Urraca N., Lein P., Korf I., Reiter L., LaSalle J. (2017) Dental pulp stem cells model early life and imprinted DNA methylation patterns. Stem Cells, 35, 981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goorha S., Reiter L. (2017) Culturing and neuronal differentiation of human dental pulp stem cells. Curr. Protoc. Hum. Genet., 92, 21.6.1–21.6.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ohnuki M., Takahashi K. (2015) Present and future challenges of induced pluripotent stem cells. Philos. Trans. R. Soc. Lond. B Biol. Sci., 370, 20140367.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cao S., Loh K., Pei Y., Zhang W., Han J. (2012) Overcoming barriers to the clinical utilization of iPSCs: reprogramming efficiency, safety and quality. Protein Cell, 3, 834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kálmán S., Hathy E., Réthelyi J.M. (2016) A dishful of a troubled mind: induced pluripotent stem cells in psychiatric research. Stem Cells Int., 2016, 7909176.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nosrat I.V., Smith C.A., Mullally P., Olson L., Nosrat C.A. (2004) Dental pulp cells proved neurotrophic support for dopaminergic neurons and differentiate into neurons in vitro; implications for tissue engineering and repair in the nervous system. Eur. J. Neurosci., 19, 2388–2398. [DOI] [PubMed] [Google Scholar]
- 18. Arthur A., Rychkov G., Shi S., Koblar S.A., Gronthos S. (2008) Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells, 2008, 1787–1795. [DOI] [PubMed] [Google Scholar]
- 19. Kiraly M., Porcsalmy B., Pataki A., Kadar K., Jelitai M., Molnar B., Hermann P., Gera I., Grimm W.-D., Ganss B.. et al. (2009) Simultaneous PKC and cAMP activation induces differentiation of human dental pulp stem cells into functionally active neurons. Neurochem. Int., 55, 323–332. [DOI] [PubMed] [Google Scholar]
- 20. Wang J., Wang X., Sun Z., Wang X., Yang H., Shi S., Wang S. (2010) Stem cells from human-exfoliated deciduous teeth can differentiate into dopaminergic neuron-like cells. Stem Cells and Dev., 19, 1375–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chang C.-C., Chang K.-C., Tsai S.-J., Chang H.-H., Lin C.-P. (2014) Neurogenic differentiation of dental pulp stem cells to neuron-like cells in dopaminergic and motor neuronal inductive media. J. Formos. Med. Assoc., 2014, 956–965. [DOI] [PubMed] [Google Scholar]
- 22. Kanafi M., Majumdar D., Bhonde R., Gupta P., Datta I. (2014) Midbrain cues dictate differentiation of human dental pulp stem cells towards functional dopaminergic neurons. J. Cell. Physiol., 229, 1369–1377. [DOI] [PubMed] [Google Scholar]
- 23. Cho Y.-A., Kim D.-S., Song M., Bae W.-J., Lee S., Kim E.-C. (2016) Protein Interacting with never in mitosis A-1 induces glutamatergic and GABAergic neuronal differentiation in human dental pulp stem cells. J. Endod., 42, 1055–1061. [DOI] [PubMed] [Google Scholar]
- 24. Martens W., Sanen K., Georgiou M., Struys T., Bronckaers A., Ameloot M., Phillips J., Lambrichts I. (2014) Human dental pulp stem cells can differentiate into Schwann cells and promote and guide neurite outgrowth in an aligned tissue-engineered collagen construct in vitro. Faseb J., 28, 1634–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Young F.I., Telezhkin V., Youde S.J., Langley M.S., Stack M., Kemp P.J., Waddington R.J., Sloan A.J., Song B. (2016) Clonal heterogeneity in the neuronal and glial differentiation of dental pulp stem/progenitor cells. Stem Cells Int., 2016, Article ID 1290561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kiraly M., Kadar K., Horvathy D.B., Nardai P., Racz G.Z., Lacza Z., Varga G., (2011) Integration of neuronally predifferentiated human dental pulp stem cells into rat brain in vivo. Neurochem. Int, 59, 371–381. [DOI] [PubMed] [Google Scholar]
- 27. Fujii H., Matsubara K., Sakai K., Ito M., Ohno K., Ueda M., Yamamoto A. (2015) Dopaminergic differentiation of stem cells from human deciduous teeth and their therapeutic benefits for Pakinsonian rats. Brain Res., 1613, 59–72. [DOI] [PubMed] [Google Scholar]
- 28. Nuti N., Corallo C., Chan B.M.F., Ferrari M., Gerami-Naini B. (2016) Multipotent differentiation of human dental pulp stem cells: a literature review. Stem Cell Rev. Rep., 2016, 511–523. [DOI] [PubMed] [Google Scholar]
- 29. Bobis S., Jarocha D., Majka M. (2006) Mesenchymal stem cells: characteristics and clinical applications. Folia Histochem. Cytobiol., 44, 215–230. [PubMed] [Google Scholar]
- 30. Miura M., Gronthos S., Zhao M., Lu B., Fisher L.W., Robey P.G., Shi S. (2003) SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl Acad. Sci. U S A., 100, 5807–5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kerkis I., Caplan A.I. (2012) Stem cells in dental pulp of deciduous teeth. Tissue Eng. Part B, Rev., 18, 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karaoz E., Demircan P.C., Saglam O., Aksoy A., Kaymaz F., Duruksu G. (2011) Human dental pulp stem cells demonstrate better neural and epithelial stem cell properties than bone marrow-derived mesenchymal stem cells. Histochem. Cell Biol., 136, 455–473. [DOI] [PubMed] [Google Scholar]
- 33. Ren H., Sang Y., Zhang F., Liu Z., Qi N., Chen Y. (2016) Comparative analysis of human mesenchymal stem cells from umbilical cord, dental pulp, and menstrual blood as sources for cell therapy. Stem Cells Int., 2016, Article ID 3516574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kang C.-M., Kim H., Song J.S., Choi B.-J., Kim S.-O., Jung H.-S., Moon S.-J., Choi H.-J. (2016) Genetic comparison of stemness of human umbilical cord and dental pulp. Stem Cells Int., 2016, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Isobe Y., Koyama N., Nakao K., Osawa K., Ikeno M., Yamanaka S., Okubo Y., Fujimura K., Bessho K. (2016) Comparison of human mesenchymal stem cells derived from bone marrow, synovial fluid, adult dental pulp, and exfoliated deciduous tooth pulp. Int J. Oral Maxillofac. Surg., 45, 124–131. [DOI] [PubMed] [Google Scholar]
- 36. Aghajani F., Hiishmand T., Khanmohammadi M., Khanjani S., Edalatkhah H., Kazemnejad S., Zarnani A.H. (2016) Comparative immunophenotypic characteristics, proliferative features, and osteogenic differentiation of stem cells isolated from human permanent and deciduous teeth with bone marrow. Mol. Biotechnol., 58, 415–427. [DOI] [PubMed] [Google Scholar]
- 37. Chang Y.-C., Li W.-C., Twu N.-F., Li H.-Y., Lo W.-L., Chang Y.-L., Lee Y.-Y., Lin C.-F., Shih Y.-H., Chen M.-T. (2014) Induction of dental pulp-derived induced pluripotent stem cells in the absence of c-Myc for differentiation into neuron-like cells. J. Chin. Med. Assoc., 77, 618–625. [DOI] [PubMed] [Google Scholar]
- 38. Tamaoki N., Takahashi K., Tanaka T., Ichisaka T., Aoki H., Takeda-Kawaguchi T., Iida K., Kunisada T., Shibata T., Yamanaka S.. et al. (2010) Dental pulp cells for induced pluripotent stem cell banking. J. Dent. Res., 89, 773–778. [DOI] [PubMed] [Google Scholar]
- 39. Yan X., Qin H., Qu C., Tuan R.S., Shi S., Huang G.T. (2010) iPS cells reprogrammed from human mesenchymal-like stem/progenitor cells of dental tissue origin. Stem Cells Dev., 19, 469–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kawano E., Toriumi T., Iguchi S., Suzuki D., Sato S., Honda M. (2017) Induction of neural crest cells from human dental pulp-derived induced pluripotent stem cells. Biomed. Res. (Tokyo, Japan), 38, 135–147. [DOI] [PubMed] [Google Scholar]
- 41. Chen J., Lin M., Foxe J.J., Pedrosa E., Hrabovsky A., Carroll R., Zheng D., Lachman H.M. (2013) Transcriptome comparison of human neurons generated using induced pluripotent stem cells derived from dental pulp and skin fibroblasts. PLoS One, 8, e75682.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee H.J., Choi S.S., Lee S.-R., Chang K.-T. (2017) Pham P.V. (ed.), In Neurological Regeneration. Springer International Publishing, Cham, pp. 1–12. [Google Scholar]
- 43. Yoo J., Kim H.-S., Hwang D.-Y. (2013) Stem cells as promising therapeutic options for neurological disorders. J. Cell. Biochem., 114, 743–753. [DOI] [PubMed] [Google Scholar]
- 44. Ben-David U., Benvenisty N. (2011) The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat. Rev. Cancer, 11, 268–277. [DOI] [PubMed] [Google Scholar]
- 45. Liang Y., Zhang H., Feng Q.S., Cai M.B., Deng W., Qin D., Yun J.P., Tsao G.S., Kang T., Esteban M.A.. et al. (2013) The propensity for tumorigenesis in human induced pluripotent stem cells is related with genomic instability. Chin. J. Cancer, 32, 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang Y., Zhang Z., Chi Y., Zhang Q., Xu F., Yang Z., Meng L., Yang S., Yan S., Mao A.. et al. (2013) Long-term cultured mesenchymal stem cells frequently develop genomic mutations but do not undergo malignant transformation. Cell Death Dis., 4, e950.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mayo V., Sawatari Y., Huang C.-Y.C., Garcia-Godoy F. (2014) Neural crest-derived dental stem cells–Where we are and where we are going. J. Dent., 42, 1043–1051. [DOI] [PubMed] [Google Scholar]
- 48. Urraca N., Memon R., El-Iyachi I., Goorha S., Valdez C., Tran Q.T., Scroggs R., Miranda-Carboni G.A., Donaldson M., Bridges D.. et al. (2015) Characterization of neurons from immortalized dental pulp stem cells for the study of neurogenetic disorders. Stem Cell Res., 15, 722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Majumdar D., Kanafi M., Bhonde R., Gupta P., Datta I. (2016) Differential neuronal plasticity of dental pulp stem cells from exfoliated deciduous and permanent teeth towards dopaminergic neurons. J. Cell. Physiol., 231, 2048–2063. [DOI] [PubMed] [Google Scholar]
- 50. Terzi M.Y., Izmirli M., Gogebakan B. (2016) The cell fate: senescence or quiescence. Mol. Biol. Rep., 43, 1213–1220. [DOI] [PubMed] [Google Scholar]
- 51. Turinetto V., Vitale E., Giachino C. (2016) Senescence in human mesenchymal stem cells: functional changes and implications in stem cell-based therapy. Int. J. Mol. Sci., 17, 1164.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yi Q., Liu O., Yan F., Lin X., Diao S., Wang L., Jin L., Wang S., Lu Y., Fan Z. (2016) Analysis of senescence-related differentiation potentials and gene expression profiles in human dental pulp stem cells. Cells Tissues Organs, 2016, PMID: 27627434 [DOI] [PubMed] [Google Scholar]
- 53. Piper S.L., Wang M., Yamamoto A., Malek F., Luu A., Kuo A.C., Kim H.T. (2012) Inducible immortality in hTERT-human mesenchymal stem cells. J. Orthop. Res., 30, 1879–1885. [DOI] [PubMed] [Google Scholar]