Abstract
Unraveling the genetics of the paralytic strabismus syndromes known as congenital cranial dysinnervation disorders (CCDDs) is both informing physicians and their patients and broadening our understanding of development of the ocular motor system. Genetic mutations underlying ocular CCDDs alter either motor neuron specification or motor nerve development, and highlight the importance of modulations of cell signaling, cytoskeletal transport, and microtubule dynamics for axon growth and guidance. Here we review recent advances in our understanding of two CCDDs, congenital fibrosis of the extraocular muscles (CFEOM) and Duane retraction syndrome (DRS), and discuss what they have taught us about mechanisms of axon guidance and selective vulnerability. CFEOM presents with congenital ptosis and restricted eye movements, and can be caused by heterozygous missense mutations in the kinesin motor protein KIF21A or in the β-tubulin isotypes TUBB3 or TUBB2B. CFEOM-causing mutations in these genes alter protein function and result in axon growth and guidance defects. DRS presents with inability to abduct one or both eyes. It can be caused by decreased function of several transcription factors critical for abducens motor neuron identity, including MAFB, or by heterozygous missense mutations in CHN1, which encodes α2-chimaerin, a Rac-GAP GTPase that affects cytoskeletal dynamics. Examination of the orbital innervation in mice lacking Mafb has established that the stereotypical misinnervation of the lateral rectus by fibers of the oculomotor nerve in DRS is secondary to absence of the abducens nerve. Studies of a CHN1 mouse model have begun to elucidate mechanisms of selective vulnerability in the nervous system.
Introduction
Unraveling the genetics of rare Mendelian birth defects not only improves diagnosis, counseling, and care for those affected individuals and their families, but also provides insight into normal development. This has been the case for studies of a series of rare strabismus syndromes in which babies are born with the inability to move one or both eyes in one or more directions of gaze, referred to as the congenital cranial dysinnervation disorders (CCDDs) (1–4).
Proper eye alignment and movement require the coordinated action of the six extraocular muscles (EOM) of each eye, and each EOM requires proper innervation by one of three cranial nerves (Fig. 1A). The oculomotor nerve (cranial nerve III) arises from motor neurons in the midbrain oculomotor nucleus, which send axons ventrally through the midbrain parenchyma to exit into the cranial mesenchyme and extend along a stereotypical trajectory to the orbit. Within the orbit, the oculomotor nerve divides into two main branches again in a stereotypical fashion: the inferior division innervates the medial rectus (MR), inferior rectus (IR), and inferior oblique (IO) muscles, and the superior division innervates the superior rectus (SR) and levator palpebrae superioris (LPS) muscles. The motor neuron cell bodies whose axons form both the inferior and superior divisions are born ipsilateral to the EOM they will innervate. After birth, however, the superior division motor neurons migrate across the midline of the midbrain; those destined to innervate the SR migrate fully across and settle in the contralateral nucleus, while those destined to innervate the LPS migrate to the midline where both left and right motor neurons mix to form a small central caudal nucleus (5,6). Thus, the IR, MR, and IO are innervated by ipsilateral and the SR by contralateral motor neurons, and the LPS is mixed. The trochlear nerve (cranial nerve IV) arises from cell bodies at the midbrain-hindbrain junction. The axons course through the midbrain parenchyma dorsally, cross the midline in the tectum, and exit into the cranial mesenchyme where they turn ventrally towards the orbit and innervate the contralateral SO muscle. The abducens nerve (cranial nerve VI) arises from cell bodies in the hindbrain. Axons travel ventrally through the pontine parenchyma, exit into the cranial mesenchyme, and extend to the orbit, where they innervate the LR muscle. Each ocular cranial nerve develops in a spatially and temporally predictable fashion and is isolated from other nerves along its course. Thus, motor neurons and their axons can be genetically labeled in model organisms and normal and aberrant nerve development can be imaged microscopically (7–9).
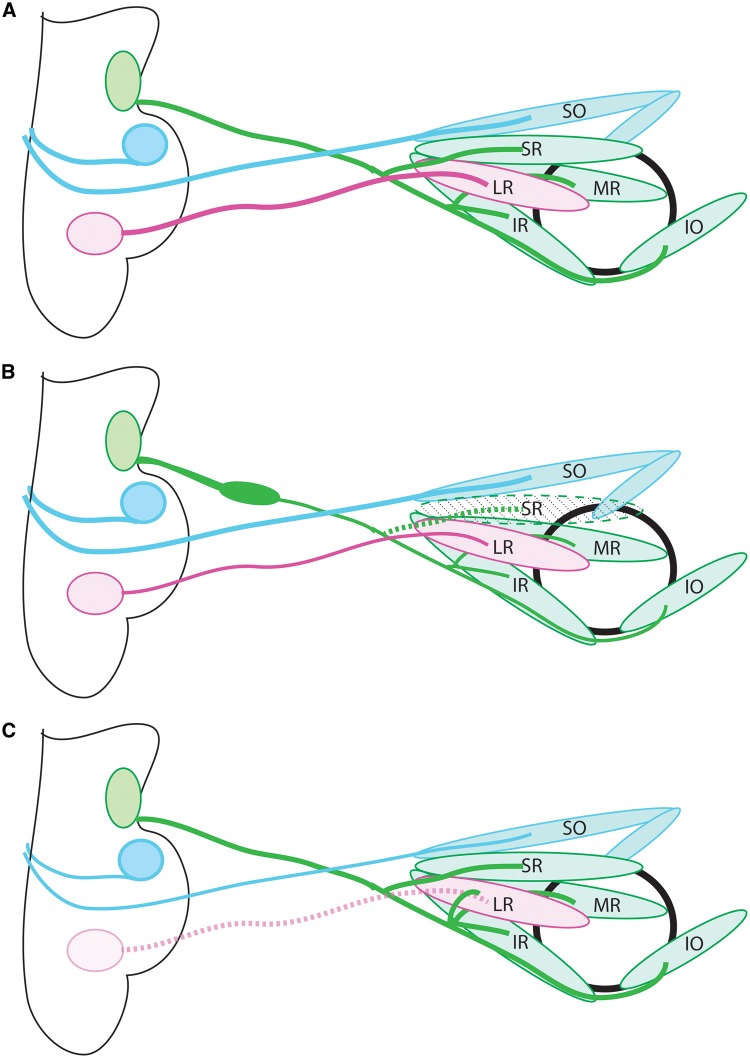
Figure 1.
Anatomy of the ocular motor system. (A) Schematic of the normal anatomy of the ocular motor system. The oculomotor nerve (cranial nerve 3; green) extends from the midbrain to the orbit, where it then divides into superior and inferior divisions and innervates the SR and LPS (not shown), and the IR, MR, IO, respectively. The trochlear nerve (cranial nerve 4; blue) exits the midbrain dorsally, crosses the midline in the tectum, then innervates the contralateral SO. The abducens nerve (cranial nerve 6; pink) exits the hindbrain and innervates the LR. (B) CFEOM1 pathology. The superior division of CN3 is absent, and the nerve displays a proximal bulge formed by stalled, misdirected axons, followed by distal thinning. The SR and LPS (not shown) are hypoplastic. CN4 appears normal and CN6 has mild thinning. (C) Duane syndrome pathology. CN6 is absent, and there is misinnervation of the lateral rectus by axons of CN3. CN3, oculomotor nerve; CN4, trochlear nerve; CN6, abducens nerve; SO, superior oblique; SR, superior rectus; MR, medial rectus; IR, inferior rectus; LR, lateral rectus; IO:= inferior oblique.
Relatively little is known about the cues that guide ocular motor axons to their proper muscle targets. In genetically modified mice lacking EOMs, the three ocular motor nerves have correct trajectories and reach their proper positions within the orbit, indicating that initial guidance decisions do not rely on muscle cues, but rather mesenchymal cues, axon-axon interactions, or cell autonomous processes. Within the orbit, however, the nerves fail to make terminal branches and the superior division of the oculomotor nerve does not form, indicating that muscle-derived cues are important for terminal branching (7). Studies in chick and zebrafish have identified several candidate guidance molecules expressed in EOMs and/or surrounding mesenchyme, including CXCL12, Semaphorins, and Hepatocyte Growth Factor (8,10–12), but their specific roles in ocular motor guidance and terminal innervation remain to be defined.
Genetic and functional studies of the CCDDs are providing insight into our understanding of neuronal development and axon guidance in the ocular motor system and elsewhere in the nervous system. Some CCDDs perturb gene function critical for correct ocular cranial motor neuron specification (13–16), while others perturb gene function necessary for normal axon growth and guidance (17–21). Functional studies of mutant CCDD proteins are also highlighting the importance of specific amino acid residues for critical intramolecular and intermolecular interactions. Here, we review recent work on the CCDDs, focusing on dominant forms of congenital fibrosis of the extraocular muscles (CFEOM) and Duane syndrome, with a special emphasis on what they have taught us about the guidance of ocular motor nerves.
Congenital Fibrosis of the Extraocular Muscles
CFEOM is a congenital, non-progressive disorder characterized by restrictive ophthalmoplegia, eye misalignment, and typically congenital ptosis. Deficits of vertical eye movements, especially upgaze, are prominent, and, coupled with ptosis, result in a prominent chin-up head position. Horizontal eye movement deficits vary, ranging from full horizontal motility to nearly complete ophthalmoplegia (22–24) (Fig. 2A). Three genetic forms of CFEOM have been defined – CFEOM1, CFEOM2, and CFEOM3. CFEOM2 is an autosomal recessive disorder of neuronal specification, caused by homozygous mutations in the transcription factor PHOX2A (13), whereas CFEOM1 and CFEOM3 are autosomal dominant disorders of axon guidance, caused by heterozygous mutations in KIF21A (20) and TUBB3 (19) or TUBB2B (21), respectively.
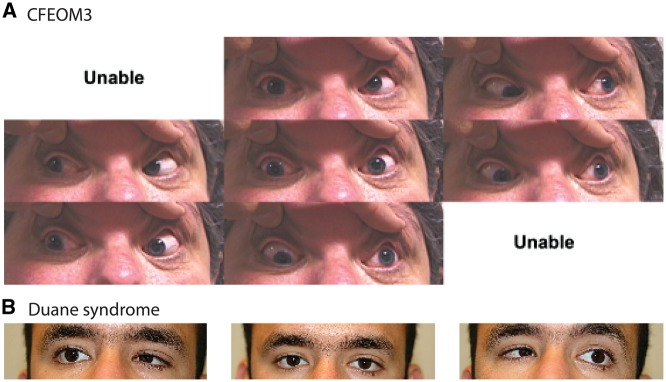
Figure 2.
Clinical photographs of CCDDs. (A) External ocular photographs in different positions of gaze of an individual with TUBB3-CFEOM3. Top row: up gaze, up-left gaze. Central row: right gaze, straight ahead, left gaze, Botton rom: down-right gaze, down gaze. Unable: photographs could not be obtained in up-right gaze, or down-left gaze. Note the prominent ptosis, limited upgaze and limited horizontal motility. This figure was published previously in (31) and copyright belongs to ARVO. (B) External ocular photographs in horizontal gaze positions of an individual with MAFB-DRS. From left to right: right gaze, straight ahead, left gaze. Note the limited abduction of each eye, and the globe retraction on attempted adduction.
KIF21A-CFEOM1
Heterozygous missense mutations in KIF21A are the primary cause of CFEOM1 (20). CFEOM1 is bilateral and fairly symmetrical, with ptosis and the eyes fixed in downgaze, and is not associated with any additional neurologic abnormalities (4,23). Postmortem examination and MRI studies of individuals with KIF21A-CFEOM1 show hypoplasia of the oculomotor nerve, loss of the superior division and its corresponding motor neurons, abnormal innervation patterns in the orbit, and atrophy of the SR and LPS muscles (23,25) (Fig. 1B), supporting a neurogenic rather than myopathic etiology.
KIF21A is a kinesin motor protein that moves cargos along microtubules in an anterograde direction from the cell body to the developing growth cone. It is expressed widely in the cell bodies, axons and dendrites of multiple neuronal populations from early development through maturity, as well as in extraocular and other skeletal muscles (20,26), so it is not immediately obvious why mutations would cause an isolated eye movement disorder. CFEOM-causing mutations are missense, however, and alter specific residues in the third coiled-coil domain of the stalk region or in the motor domain of the protein, supporting an altered or gain-of-function mechanism (20,27).
Consistent with a gain-of-function mechanism, Kif21a-/-mouse embryos have normal oculomotor development, while a mouse model harboring the most common Kif21a R954W amino acid substitution has ptosis and globe retraction, and recapitulates CFEOM1 pathology (17). Embryonic mice form a transient widening (bulge) along the proximal oculomotor nerve that is followed by distal nerve thinning, absence of the superior division, and aberrant branching of the inferior division. The bulge contains the stalled growth cones of superior division oculomotor axons, indicating that the superior division is unable to make the proper pathfinding decisions. The stalling is far proximal to the normal branch point of the superior division from the main trunk of the nerve (Fig. 1B), in contrast to mice which lack extraocular muscles, in which the superior division fails to form and widening of the oculomotor trunk is seen at the normal superior division branch point (7). Thus, the superior division axons in the Kif21a-CFEOM1 mice are likely to stall though a cell-autonomous process or from aberrant response to a mesenchymal cue, and not from aberrant responses to EOM-derived cues.
Studies both in vivo and in vitro established that KIF21A mutations cause CFEOM1 by attenuating an intramolecular interaction between the third coiled-coil and motor domains of KIF21A, critical to its autoinhibition (17,28). This leads to a constitutively active molecule with increased microtubule association (17). Kif21a acts as an inhibitor of microtubule dynamics (28), and interacts with Map1b (microtubule associated protein 1b). Map1b-/-mice also have CFEOM, and double heterozygous mice have increased penetrance of CFEOM (17). Together, these results highlight the importance of cytoskeletal regulation for axon guidance. Moreover, the selective vulnerability of the superior division of the oculomotor nerve to hyperactivation of this widely expressed kinesin motor protein suggests that regulation of the cytoskeleton, through kinesin binding and microtubule associated proteins, differs between different neuronal populations.
TUBB3-CFEOM3
Heterozygous missense mutations in TUBB3 cause CFEOM3 (19). While CFEOM3 is also characterized by restricted upgaze and ptosis, it is a more variable eye phenotype than CFEOM1; it can be unilateral or asymmetric, and may not include ptosis. Moreover, in some instances CFEOM3 is associated with a variety of other neurological abnormalities, including additional cranial nerve defects, intellectual and social disabilities, mild brain malformations, and a progressive peripheral axonal neuropathy (19,29,30). MRI studies of individuals with TUBB3-CFEOM3 show hypoplasia of the oculomotor nerve, thin optic nerve, misinnervation of the lateral rectus by the oculomotor nerve, and variable hypoplasia of the extraocular muscles. With certain mutations, thinning or absence of the facial nerves, brainstem hypoplasia, and additional brain malformations are also seen (19,29–31).
TUBB3 encodes the class III β-tubulin isotype. Multiple different α- and β-tubulin isotypes are encoded by separate genes, and heterodimers of α- and β-tubulin assemble into microtubules. Microtubules are dynamic cytoskeletal polymers that provide structure to cells and axons and act as the highways for motor protein transport. TUBB3, the neuron-specific β isotype, is expressed in all postmitotic neurons in the developing and mature brain (32), although it is not the most abundant isotype in brain (33). Thus, as with KIF21A mutations, the expression pattern does not explain the often predominantly ocular phenotype.
Ten distinct heterozygous missense TUBB3 mutations have been reported to cause CFEOM3, and the resulting amino acid substitutions alter TUBB3 function and result in predictable genotype-phenotype correlations (19,29,30). These mutations are often but not exclusively in the C-terminal domain of the protein, alter amino acid residues shown to interact with motor protein and microtubule associated proteins, and are associated with increased microtubule stability (19,34–36). In Tubb3 R262C knockin mice, the developing oculomotor nerve makes an erroneous turn along its path from the brainstem to the orbit and reaches the wrong position in the orbit, and the trochlear and trigeminal nerves also show growth and branching deficits (19).
TUBB3 R262C, A302T, and R62Q substitutions cause isolated mild to moderately severe CFEOM3. Interestingly, the in vitro folding of these mutant TUBB3 residues is poor, and they incorporate into microtubules less well than wildtype TUBB3 (19,37). This is consistent with an altered- or gain-of-function mechanism: the less mutant tubulin incorporated into the microtubule, the more mild the phenotype. A second series of TUBB3 amino acid substitutions cause more severe eye phenotypes and syndromic CFEOM3 (19,29,30,34), and are associated with better folding and wildtype or near wildtype levels of incorporation of the mutant tubulin into microtubules (19). The most severe phenotypes occur with E410K, R262H, and D417H substitutions (19,30). Depending on the specific mutation, affected individuals may have intellectual disability, autism spectrum disorder, and brain malformations including thin-to-absent anterior commissure and corpus callosum, dysmorphic basal ganglia with fusion between the putamen and caudate, and hypoplastic or absent olfactory sulci and olfactory bulbs. E410K and R262H substitutions cause facial weakness and vocal cord paralysis, and E410K causes facial dysmorphisms, Kallmann’s syndrome (anosmia with hypogonadal hypogonadism), and can lead to cyclic vomiting (19,30). E410K, R262H, D417N and D417H substitutions cause a progressive axonal neuropathy, and R262H and D417H are also associated with congenital joint contractures.
Following the initial description of the TUBB3 mutations in CFEOM, which demonstrated that the substitutions reduced kinesin-microtubule interactions and pathologically stabilized microtubules in yeast, in vitro, and in vivo (19), several additional in vitro studies confirmed and further assessed the effect of the amino acid substitutions on microtubule function. Substitutions at R262, E410, and D417 were confirmed to alter the binding of kinesins or microtubule-associated proteins (36–38), and to alter microtubule polymerization dynamics (36). E410K and D417H were shown to inhibit axonal transport of vesicles and mitochondria (38). R262H and R262A were both demonstrated to reduce kinesin ATPase activity while E410A and R417A did not, implicating R262 in stimulating the ATP-ase function of kinesin, and D417 and E410 in kinesin binding to the microtubule (35,37). Interestingly, mutating kinesin at the residue that interacts with microtubules restores kinesin motility on R262A (not a patient mutation) but not R262H mutant microtubules (37). The human CFEOM3 mutations have thus informed biochemical and biophysical studies of the roles of specific tubulin residues in cytoskeletal function. It remains to be determined exactly why specific amino acid substitutions in a widely expressed protein lead to such specific sets of axon guidance deficits.
Expanding TUBB3 phenotype-genotype correlations even further, nine different heterozygous TUBB3 missense mutations cause malformations of cortical development (MCD) but not CFEOM (39–41). Individuals with these TUBB3-MCD variants have intellectual disability and motor delays, and postmortem and MRI studies reveal mild cortical dysgenesis, cerebellar vermian dysplasia, and hypoplastic brainstem and, in common with TUBB3-CFEOM3 mutations, agenesis or hypogenesis of the corpus callosum and anterior commissure, and dysmorphic basal ganglia with fusion between putamen and caudate (39,41). In contrast to CFEOM-causing substitutions, MCD-causing substitutions are more often in the N-terminal domain of TUBB3 and alter residues that contact the GTP-nucleotide or are involved in intra- and inter-heterodimer interfaces. These mutations thus are more likely to disrupt heterodimer formation and result in decreased microtubule stability (34,41).
CFEOM and MCD were assumed to arise through separate mechanisms until the identification of two additional mutations in the N-terminus of the protein that cause both phenotypes (29). Additionally, a missense mutation in TUBB2B, another β-tubulin isotype, was identified in a family in which affected members had both CFEOM and polymicrogyria (21), while many other pathogenic variants in TUBB2B are associated with polymicrogyria without CFEOM (39,42–44). Remarkably, similar to the CFEOM-causing TUBB3 mutations, the TUBB2B mutation that causes both CFEOM3 and polymicrogyria alters a kinesin binding site, while the other TUBB2B mutations do not.
The range of phenotypes associated with tubulin mutations, and the remarkable genotype-phenotype correlations, show that different neuronal types have differing sensitivity to specific disruptions of tubulin function. The specific reasons for this selective vulnerability remain to be elucidated.
For patients with CFEOM, a molecular diagnosis not only provides valuable information on natural history and associated phenotypes, allowing earlier intervention, but also informs treatments for their ptosis and strabismus, permitting surgeons to tailor their surgical plans based on responses of prior patients with the same molecular defects.
Duane Retraction Syndrome
Duane retraction syndrome (DRS) is characterized by unilateral or bilateral horizontal eye movement limitation, usually of abduction (looking toward the ear), associated with narrowing of the palpebral fissure and retraction of the globe on attempted adduction (looking toward the nose) (Fig. 2B). It is the most common CCDD, with a reported prevalence varying from 1:1000 – 1:10,000 (45–47). Postmortem and MRI studies of patients with DRS have shown hypoplasia or absence of the abducens nerve, and aberrant innervation of the lateral rectus by fibers of the oculomotor nerve (48–50). DRS can occur in isolation or in association with additional birth defects. Most cases of DRS are simplex, but some segregate in families as autosomal dominant traits. To date, four DRS genes have been identified, each of which causes a small proportion of DRS cases. Three of these, MAFB (16), HOXA1 (14), and SALL4 (51,52), are transcription factors that, when mutated, cause syndromic DRS. The fourth, CHN1 (18), alters abducens axon guidance and causes isolated DRS.
MAFB-DRS
MAFB mutations cause Duane syndrome with or without hearing loss (16). In three families with isolated DRS (two transmitted as a dominant trait, one a de novo simplex), heterozygous truncating MAFB mutations that resulted in haploinsufficiency were identified. A fourth family that segregated DRS and sensorineural hearing loss as a dominant trait was found to harbor a truncating MAFB variant that acted in a dominant negative fashion, reducing the activity of the wildtype protein to less than 50% (16). These human findings highlight the different sensitivities of different tissues to loss of MAFB function.
Mafb encodes a transcription factor of the basic leucine zipper family that is expressed in rhombomeres 5 and 6, where the abducens nerve develops, and is required for proper hindbrain patterning (53–57). In the absence of Mafb, abducens motor neurons fail to develop (53,54). By contrast, Mafb is not expressed in the midbrain, and the oculomotor nucleus is not altered by its loss. Orbital dissections of Mafb-/- embryos revealed absence of the abducens nerve and aberrant innervation of the lateral rectus by fibers from the oculomotor nerve (Fig. 1C). Given the absence of Mafb expression in oculomotor neurons, this mouse model established that the aberrant innervation of the lateral rectus by oculomotor axons in DRS is secondary to absent innervation of the lateral rectus by the abducens nerve, and is not a primary deficit of oculomotor development or guidance (16).
CHN1-DRS
Heterozygous missense changes in CHN1 are a rare cause of autosomal dominant, bilateral DRS (18,58). MRI studies of affected individuals show absence or hypoplasia of the sixth nerve, small optic nerves, occasionally small oculomotor nerves, and hypoplasia of the superior oblique muscle (50).
CHN1 encodes the RacGAP proteins α1- and α2-chimaerin. Several DRS-causative mutations are in residues unique to α2-chimaerin, indicating the phenotype is related to the α2-chimaerin isoform. α2-chimaerin has been reported to regulate cytoskeletal dynamics downstream of several axon guidance receptors, including EphA4, TrkB, and Neuropilin1/PlexinA (12,59–64). It is widely expressed embryonically (18), and exists in an autoinhibited form until activated (64,65). Human CHN1 mutations hyperactivate the α2-chimaerin protein, primarily by altering residues critical to the closed autoinhibited conformation, leading to increased RacGTPase activity (18). Overexpression of mutant α2-chimaerin in chick or zebrafish oculomotor neurons causes axon stalling and alters responsiveness to CXCL12 and Sema3A; α2-chimaerin knockdown causes branching defects and occasionally stalling (8,12,66). Mafb mutant mice, however, established that oculomotor innervation of the lateral rectus in DRS is secondary to failure of normal innervation by the abducens nerve, so the relevance of these findings to human development remains undefined (16).
A mouse model harboring a human L20F CHN1-DRS substitution was developed to study the molecular etiology of CHN1-DRS (67). Mutant mice had globe retraction, and examination of developing embryos revealed stalling of abducens axons in the hindbrain mesenchyme, misinnervation of the lateral rectus by axons from the oculomotor nerve, and secondary death of abducens motor neurons (67). Other than the misinnervation of the lateral rectus, the oculomotor nerve developed normally. The trochlear nerve also displayed projection and branching abnormalities, consistent with the vertical movement abnormalities seen in some CHN1-DRS patients (68). By contrast, Chn1-/-mice had defasiculation and wandering of some abducens axons, but a subset of axons innervated the lateral rectus and aberrant innervation by the oculomotor nerve was not seen, consistent with a gain-of-function mechanism in CHN1-DRS. The oculomotor and trochlear nerves developed normally in the absence of α2-chimaerin (67).
Functional studies have begun to provide insights into the selective vulnerability of different neuronal populations in CHN1-DRS. EphA4 had been shown to act upstream of α2-chimaerin in corticospinal and spinal motor neurons (60,62,63), and thus its role in abducens development was examined. EphA4-/-mice were found to have wandering and desfasiculation of the abducens nerve, which resembles the Chn1-/-phenotype, while oculomotor and trochlear nerves developed normally (67). Selective removal of EphA4 in either the motor neurons or the mesenchyme caused abducens nerve phenotypes that were different from each other and from the full EphA4-/-, indicating that abducens neurons use both forward and reverse Eph-ephrin signaling. Finally, mutant α2-chimaerin was demonstrated to act downstream of both forward and reverse Eph-ephrin signaling in abducens nerves. By contrast, cervical spinal nerves were found to use only forward Eph signaling, and in the trochlear nerve, α2-chimaerin functioned through a non-ephrin-mediated pathway (67). These distinct roles of signaling molecules in different neuronal populations begin to explain the selective vulnerability of the abducens nerve to abnormal proteins that are expressed broadly.
Conclusions
Genetic and functional studies of disorders of ocular motility are providing insights into two important aspects of cranial nerve development – motor neuron specification and axon guidance. The CCDDs resulting from disorders of motor neuron specification are generally the result of loss-of-function mutations in transcription factors, and the resulting phenotypes reflect the expression pattern of the mutated gene. The disorders of axon guidance, however, are not easily explained by expression patterns of the mutated genes. Instead, they are typically autosomal dominant disorders that result from specific ‘hot-spot’ amino acid substitutions that result in altered- or gain-of-function. From a molecular standpoint, these disorders provide insight into the critical roles of specific residues for normal protein function. From a developmental standpoint, they provide insights into the selective vulnerability of different axon groups to perturbations in cell signaling and cytoskeletal dynamics. As such, these disorders serve as paradigms for axon guidance mechanisms throughout the nervous system.
Conflict of Interest statement. None declared.
Funding
Harvard-Vision Clinical Scientist Development Program [5K12EY016335], the Knights Templar Eye Foundation [Career Starter Grant], and the Children’s Hospital Ophthalmology Foundation [Faculty Discovery Award]. NIH R01EY012498 and P30HD018655, and the Howard Hughes Medical Institute.
References
- 1. Gutowski N.J., Bosley T.M., Engle E.C. (2003) 110th ENMC International Workshop: the congenital cranial dysinnervation disorders (CCDDs). Naarden, The Netherlands, 25–27 October, 2002. Neuromuscul. Disord., 13, 573–578. [DOI] [PubMed] [Google Scholar]
- 2. Engle E.C. (2002) The molecular basis of the congenital fibrosis syndromes. Strabismus, 10, 125–128. [DOI] [PubMed] [Google Scholar]
- 3. Graeber C.P., Hunter D.G., Engle E.C. (2013) The genetic basis of incomitant strabismus: consolidation of the current knowledge of the genetic foundations of disease. Semin. Ophthalmol., 28, 427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heidary G., Engle E.C., Hunter D.G. (2008) Congenital fibrosis of the extraocular muscles. Semin. Ophthalmol., 23, 3–8. [DOI] [PubMed] [Google Scholar]
- 5. Bienfang D.C. (1975) Crossing axons in the third nerve nucleus. Invest. Ophthalmol., 14, 927–931. [PubMed] [Google Scholar]
- 6. Bjorke B., Shoja-Taheri F., Kim M., Robinson G.E., Fontelonga T., Kim K.T., Song M.R., Mastick G.S. (2016) Contralateral migration of oculomotor neurons is regulated by Slit/Robo signaling. Neural Dev., 11, 18.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Michalak S.M., Whitman M.C., Park J.G., Tischfield M.A., Nguyen E.H., Engle E.C. (2017) Ocular motor nerve development in the presence and absence of extraocular muscle. Invest. Ophthalmol. Vis. Sci., 58, 2388–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clark C., Austen O., Poparic I., Guthrie S. (2013) alpha2-Chimaerin regulates a key axon guidance transition during development of the oculomotor projection. J. Neurosci., 33, 16540–16551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chilton J.K., Guthrie S. (2004) Development of oculomotor axon projections in the chick embryo. J. Comp. Neurol., 472, 308–317. [DOI] [PubMed] [Google Scholar]
- 10. Chilton J.K., Guthrie S. (2003) Cranial expression of class 3 secreted semaphorins and their neuropilin receptors. Dev. Dyn., 228, 726–733. [DOI] [PubMed] [Google Scholar]
- 11. Lerner O., Davenport D., Patel P., Psatha M., Lieberam I., Guthrie S. (2010) Stromal cell-derived factor-1 and hepatocyte growth factor guide axon projections to the extraocular muscles. Dev. Neurobiol., 70, 549–564. [DOI] [PubMed] [Google Scholar]
- 12. Ferrario J.E., Baskaran P., Clark C., Hendry A., Lerner O., Hintze M., Allen J., Chilton J.K., Guthrie S. (2012) Axon guidance in the developing ocular motor system and Duane retraction syndrome depends on Semaphorin signaling via alpha2-chimaerin. Proc. Natl. Acad. Sci. U S A, 109, 14669–14674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakano M., Yamada K., Fain J., Sener E.C., Selleck C.J., Awad A.H., Zwaan J., Mullaney P.B., Bosley T.M., Engle E.C. (2001) Homozygous mutations in ARIX(PHOX2A) result in congenital fibrosis of the extraocular muscles type 2. Nat. Genet., 29, 315–320. [DOI] [PubMed] [Google Scholar]
- 14. Tischfield M.A., Bosley T.M., Salih M.A., Alorainy I.A., Sener E.C., Nester M.J., Oystreck D.T., Chan W.M., Andrews C., Erickson R.P.. et al. (2005) Homozygous HOXA1 mutations disrupt human brainstem, inner ear, cardiovascular and cognitive development. Nat. Genet., 37, 1035–1037. [DOI] [PubMed] [Google Scholar]
- 15. Webb B.D., Shaaban S., Gaspar H., Cunha L.F., Schubert C.R., Hao K., Robson C.D., Chan W.M., Andrews C., MacKinnon S.. et al. (2012) HOXB1 founder mutation in humans recapitulates the phenotype of Hoxb1-/- mice. Am. J. Hum. Genet., 91, 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Park J.G., Tischfield M.A., Nugent A.A., Cheng L., Di Gioia S.A., Chan W.M., Maconachie G., Bosley T.M., Summers C.G., Hunter D.G.. et al. (2016) Loss of MAFB function in humans and mice causes Duane syndrome, aberrant extraocular muscle innervation, and inner-ear defects. Am. J. Hum. Genet., 98, 1220–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cheng L., Desai J., Miranda C.J., Duncan J.S., Qiu W., Nugent A.A., Kolpak A.L., Wu C.C., Drokhlyansky E., Delisle M.M.. et al. (2014) Human CFEOM1 mutations attenuate KIF21A autoinhibition and cause oculomotor axon stalling. Neuron, 82, 334–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miyake N., Chilton J., Psatha M., Cheng L., Andrews C., Chan W.M., Law K., Crosier M., Lindsay S., Cheung M.. et al. (2008) Human CHN1 mutations hyperactivate alpha2-chimaerin and cause Duane's retraction syndrome. Science, 321, 839–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tischfield M.A., Baris H.N., Wu C., Rudolph G., Van Maldergem L., He W., Chan W.M., Andrews C., Demer J.L., Robertson R.L.. et al. (2010) Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell, 140, 74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yamada K., Andrews C., Chan W.M., McKeown C.A., Magli A., de Berardinis T., Loewenstein A., Lazar M., O'Keefe M., Letson R.. et al. (2003) Heterozygous mutations of the kinesin KIF21A in congenital fibrosis of the extraocular muscles type 1 (CFEOM1). Nat. Genet., 35, 318–321. [DOI] [PubMed] [Google Scholar]
- 21. Cederquist G.Y., Luchniak A., Tischfield M.A., Peeva M., Song Y., Menezes M.P., Chan W.M., Andrews C., Chew S., Jamieson R.V.. et al. (2012) An inherited TUBB2B mutation alters a kinesin-binding site and causes polymicrogyria, CFEOM and axon dysinnervation. Hum. Mol. Genet., 21, 5484–5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Engle E.C., Marondel I., Houtman W.A., de Vries B., Loewenstein A., Lazar M., Ward D.C., Kucherlapati R., Beggs A.H. (1995) Congenital fibrosis of the extraocular muscles (autosomal dominant congenital external ophthalmoplegia): genetic homogeneity, linkage refinement, and physical mapping on chromosome 12. Am. J. Hum. Genet., 57, 1086–1094. [PMC free article] [PubMed] [Google Scholar]
- 23. Engle E.C., Goumnerov B.C., McKeown C.A., Schatz M., Johns D.R., Porter J.D., Beggs A.H. (1997) Oculomotor nerve and muscle abnormalities in congenital fibrosis of the extraocular muscles. Ann. Neurol., 41, 314–325. [DOI] [PubMed] [Google Scholar]
- 24. Doherty E.J., Macy M.E., Wang S.M., Dykeman C.P., Melanson M.T., Engle E.C. (1999) CFEOM3: a new extraocular congenital fibrosis syndrome that maps to 16q24.2-q24.3. Invest. Ophthalmol. Vis. Sci., 40, 1687–1694. [PubMed] [Google Scholar]
- 25. Demer J.L., Clark R.A., Engle E.C. (2005) Magnetic resonance imaging evidence for widespread orbital dysinnervation in congenital fibrosis of extraocular muscles due to mutations in KIF21A. Invest. Ophthalmol. Vis. Sci., 46, 530–539. [DOI] [PubMed] [Google Scholar]
- 26. Desai J., Velo M.P., Yamada K., Overman L.M., Engle E.C. (2012) Spatiotemporal expression pattern of KIF21A during normal embryonic development and in congenital fibrosis of the extraocular muscles type 1 (CFEOM1). Gene Expr. Patterns, 12, 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chan W.M., Andrews C., Dragan L., Fredrick D., Armstrong L., Lyons C., Geraghty M.T., Hunter D.G., Yazdani A., Traboulsi E.I.. et al. (2007) Three novel mutations in KIF21A highlight the importance of the third coiled-coil stalk domain in the etiology of CFEOM1. BMC Genet., 8, 26.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van der Vaart B., van Riel W.E., Doodhi H., Kevenaar J.T., Katrukha E.A., Gumy L., Bouchet B.P., Grigoriev I., Spangler S.A., Yu K.L.. et al. (2013) CFEOM1-associated kinesin KIF21A is a cortical microtubule growth inhibitor. Dev. Cell, 27, 145–160. [DOI] [PubMed] [Google Scholar]
- 29. Whitman M.C., Andrews C., Chan W.M., Tischfield M.A., Stasheff S.F., Brancati F., Ortiz-Gonzalez X., Nuovo S., Garaci F., MacKinnon S.E.. et al. (2016) Two unique TUBB3 mutations cause both CFEOM3 and malformations of cortical development. Am. J. Med. Genet. A, 170, 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chew S., Balasubramanian R., Chan W.M., Kang P.B., Andrews C., Webb B.D., MacKinnon S.E., Oystreck D.T., Rankin J., Crawford T.O.. et al. (2013) A novel syndrome caused by the E410K amino acid substitution in the neuronal beta-tubulin isotype 3. Brain, 136, 522–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Demer J.L., Clark R.A., Tischfield M.A., Engle E.C. (2010) Evidence of an asymmetrical endophenotype in congenital fibrosis of extraocular muscles type 3 resulting from TUBB3 mutations. Invest. Ophthalmol. Vis. Sci., 51, 4600–4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leandro-Garcia L.J., Leskela S., Landa I., Montero-Conde C., Lopez-Jimenez E., Leton R., Cascon A., Robledo M., Rodriguez-Antona C. (2010) Tumoral and tissue-specific expression of the major human beta-tubulin isotypes. Cytoskeleton (Hoboken), 67, 214–223. [DOI] [PubMed] [Google Scholar]
- 33. Breuss M., Heng J.I., Poirier K., Tian G., Jaglin X.H., Qu Z., Braun A., Gstrein T., Ngo L., Haas M.. et al. (2012) Mutations in the beta-tubulin gene TUBB5 cause microcephaly with structural brain abnormalities. Cell Rep., 2, 1554–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tischfield M.A., Cederquist G.Y., Gupta M.L. Jr., Engle E.C. (2011) Phenotypic spectrum of the tubulin-related disorders and functional implications of disease-causing mutations. Curr. Opin. Genet. Dev., 21, 286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Uchimura S., Oguchi Y., Hachikubo Y., Ishiwata S., Muto E. (2010) Key residues on microtubule responsible for activation of kinesin ATPase. embo J., 29, 1167–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ti S.C., Pamula M.C., Howes S.C., Duellberg C., Cade N.I., Kleiner R.E., Forth S., Surrey T., Nogales E., Kapoor T.M. (2016) Mutations in human tubulin proximal to the kinesin-binding site alter dynamic instability at microtubule plus- and minus-ends. Dev. Cell, 37, 72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Minoura I., Takazaki H., Ayukawa R., Saruta C., Hachikubo Y., Uchimura S., Hida T., Kamiguchi H., Shimogori T., Muto E. (2016) Reversal of axonal growth defects in an extraocular fibrosis model by engineering the kinesin-microtubule interface. Nat. Commun., 7, 10058.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Niwa S., Takahashi H., Hirokawa N. (2013) beta-Tubulin mutations that cause severe neuropathies disrupt axonal transport. embo J., 32, 1352–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bahi-Buisson N., Poirier K., Fourniol F., Saillour Y., Valence S., Lebrun N., Hully M., Bianco C.F., Boddaert N., Elie C.. et al. (2014) The wide spectrum of tubulinopathies: what are the key features for the diagnosis? Brain, 137, 1676–1700. [DOI] [PubMed] [Google Scholar]
- 40. Folco H.D., Campbell C.S., May K.M., Espinoza C.A., Oegema K., Hardwick K.G., Grewal S.I., Desai A. (2015) The CENP-A N-tail confers epigenetic stability to centromeres via the CENP-T branch of the CCAN in fission yeast. Curr. Biol., 25, 348–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Poirier K., Saillour Y., Bahi-Buisson N., Jaglin X.H., Fallet-Bianco C., Nabbout R., Castelnau-Ptakhine L., Roubertie A., Attie-Bitach T., Desguerre I.. et al. (2010) Mutations in the neuronal ss-tubulin subunit TUBB3 result in malformation of cortical development and neuronal migration defects. Hum. Mol. Genet., 19, 4462–4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jaglin X.H., Poirier K., Saillour Y., Buhler E., Tian G., Bahi-Buisson N., Fallet-Bianco C., Phan-Dinh-Tuy F., Kong X.P., Bomont P.. et al. (2009) Mutations in the beta-tubulin gene TUBB2B result in asymmetrical polymicrogyria. Nat. Genet., 41, 746–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fallet-Bianco C., Laquerriere A., Poirier K., Razavi F., Guimiot F., Dias P., Loeuillet L., Lascelles K., Beldjord C., Carion N.. et al. (2014) Mutations in tubulin genes are frequent causes of various foetal malformations of cortical development including microlissencephaly. Acta Neuropathol. Commun., 2, 69.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Oegema R., Cushion T.D., Phelps I.G., Chung S.K., Dempsey J.C., Collins S., Mullins J.G., Dudding T., Gill H., Green A.J.. et al. (2015) Recognisable cerebellar dysplasia associated with mutations in multiple tubulin genes. Hum. Mol. Genet., 24, 1513–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen X., Fu Z., Yu J., Ding H., Bai J., Chen J., Gong Y., Zhu H., Yu R., Liu H. (2016) Prevalence of amblyopia and strabismus in Eastern China: results from screening of preschool children aged 36-72 months. Br. J. Ophthalmol., 100, 515–519. [DOI] [PubMed] [Google Scholar]
- 46. Murillo-Correa C.E., Kon-Jara V., Engle E.C., Zenteno J.C. (2009) Clinical features associated with an I126M alpha2-chimaerin mutation in a family with autosomal-dominant Duane retraction syndrome. J. Aapos., 13, 245–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gurwood A.S., Terrigno C.A. (2000) Duane's retraction syndrome: literature review. Optometry, 71, 722–726. [PubMed] [Google Scholar]
- 48. Hotchkiss M.G., Miller N.R., Clark A.W., Green W.R. (1980) Bilateral Duane's retraction syndrome. A clinical-pathologic case report. Arch. Ophthalmol., 98, 870–874. [DOI] [PubMed] [Google Scholar]
- 49. Miller N.R., Kiel S.M., Green W.R., Clark A.W. (1982) Unilateral Duane's retraction syndrome (Type 1). Arch. Ophthalmol., 100, 1468–1472. [DOI] [PubMed] [Google Scholar]
- 50. Demer J.L., Clark R.A., Lim K.H., Engle E.C. (2007) Magnetic resonance imaging evidence for widespread orbital dysinnervation in dominant Duane's retraction syndrome linked to the DURS2 locus. Invest. Ophthalmol. Vis. Sci., 48, 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Al-Baradie R., Yamada K., St Hilaire C., Chan W.M., Andrews C., McIntosh N., Nakano M., Martonyi E.J., Raymond W.R., Okumura S.. et al. (2002) Duane radial ray syndrome (Okihiro syndrome) maps to 20q13 and results from mutations in SALL4, a new member of the SAL family. Am. J. Hum. Genet., 71, 1195–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kohlhase J., Heinrich M., Schubert L., Liebers M., Kispert A., Laccone F., Turnpenny P., Winter R.M., Reardon W. (2002) Okihiro syndrome is caused by SALL4 mutations. Hum. Mol. Genet., 11, 2979–2987. [DOI] [PubMed] [Google Scholar]
- 53. Cordes S.P., Barsh G.S. (1994) The mouse segmentation gene kr encodes a novel basic domain-leucine zipper transcription factor. Cell, 79, 1025–1034. [DOI] [PubMed] [Google Scholar]
- 54. Sadl V.S., Sing A., Mar L., Jin F., Cordes S.P. (2003) Analysis of hindbrain patterning defects caused by the kreisler(enu) mutation reveals multiple roles of Kreisler in hindbrain segmentation. Dev. Dyn., 227, 134–142. [DOI] [PubMed] [Google Scholar]
- 55. Kim F.A., Sing I.A., Kaneko T., Bieman M., Stallwood N., Sadl V.S., Cordes S.P. (2005) The vHNF1 homeodomain protein establishes early rhombomere identity by direct regulation of Kreisler expression. Mech. Dev., 122, 1300–1309. [DOI] [PubMed] [Google Scholar]
- 56. Giudicelli F., Gilardi-Hebenstreit P., Mechta-Grigoriou F., Poquet C., Charnay P. (2003) Novel activities of Mafb underlie its dual role in hindbrain segmentation and regional specification. Dev. Biol., 253, 150–162. [DOI] [PubMed] [Google Scholar]
- 57. McKay I.J., Muchamore I., Krumlauf R., Maden M., Lumsden A., Lewis J. (1994) The kreisler mouse: a hindbrain segmentation mutant that lacks two rhombomeres. Development, 120, 2199–2211. [DOI] [PubMed] [Google Scholar]
- 58. Miyake N., Andrews C., Fan W., He W., Chan W.M., Engle E.C. (2010) CHN1 mutations are not a common cause of sporadic Duane's retraction syndrome. Am. J. Med. Genet. A, 152A, 215–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ip J.P., Shi L., Chen Y., Itoh Y., Fu W.Y., Betz A., Yung W.H., Gotoh Y., Fu A.K., Ip N.Y. (2011) alpha2-chimaerin controls neuronal migration and functioning of the cerebral cortex through CRMP-2. Nat. Neurosci., 15, 39–47. [DOI] [PubMed] [Google Scholar]
- 60. Iwasato T., Katoh H., Nishimaru H., Ishikawa Y., Inoue H., Saito Y.M., Ando R., Iwama M., Takahashi R., Negishi M.. et al. (2007) Rac-GAP alpha-chimerin regulates motor-circuit formation as a key mediator of EphrinB3/EphA4 forward signaling. Cell, 130, 742–753. [DOI] [PubMed] [Google Scholar]
- 61. Shi L., Fu W.Y., Hung K.W., Porchetta C., Hall C., Fu A.K., Ip N.Y. (2007) Alpha2-chimaerin interacts with EphA4 and regulates EphA4-dependent growth cone collapse. Proc. Natl. Acad. Sci. U S A, 104, 16347–16352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wegmeyer H., Egea J., Rabe N., Gezelius H., Filosa A., Enjin A., Varoqueaux F., Deininger K., Schnutgen F., Brose N.. et al. (2007) EphA4-dependent axon guidance is mediated by the RacGAP alpha2-chimaerin. Neuron, 55, 756–767. [DOI] [PubMed] [Google Scholar]
- 63. Beg A.A., Sommer J.E., Martin J.H., Scheiffele P. (2007) alpha2-Chimaerin is an essential EphA4 effector in the assembly of neuronal locomotor circuits. Neuron, 55, 768–778. [DOI] [PubMed] [Google Scholar]
- 64. Brown M., Jacobs T., Eickholt B., Ferrari G., Teo M., Monfries C., Qi R.Z., Leung T., Lim L., Hall C. (2004) Alpha2-chimaerin, cyclin-dependent Kinase 5/p35, and its target collapsin response mediator protein-2 are essential components in semaphorin 3A-induced growth-cone collapse. J. Neurosci., 24, 8994–9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Colon-Gonzalez F., Leskow F.C., Kazanietz M.G. (2008) Identification of an autoinhibitory mechanism that restricts C1 domain-mediated activation of the Rac-GAP alpha2-chimaerin. J. Biol. Chem., 283, 35247–35257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chilton J.K., Guthrie S. (2016) Axons get ahead: Insights into axon guidance and congenital cranial dysinnervation disorders. Dev. Neurobiol., doi: 10.1002/dneu.22477. [DOI] [PubMed] [Google Scholar]
- 67. Nugent A.A., Park J.G., Wei Y., Tenney A.P., Gilette N.M., DeLisle M.M., Chan W.M., Cheng L., Engle E.C. (2017) Mutant alpha2-chimaerin signals via bidirectional ephrin pathways in Duane retraction syndrome. J. Clin. Invest., 127, 1664–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Miyake N., Demer J.L., Shaaban S., Andrews C., Chan W.M., Christiansen S.P., Hunter D.G., Engle E.C. (2011) Expansion of the CHN1 strabismus phenotype. Invest. Ophthalmol. Vis. Sci., 52, 6321–6328. [DOI] [PMC free article] [PubMed] [Google Scholar]