Abstract
The optic nerve and the cells that give origin to its 1.2 million axons, the retinal ganglion cells (RGCs), are particularly vulnerable to neurodegeneration related to mitochondrial dysfunction. Optic neuropathies may range from non-syndromic genetic entities, to rare syndromic multisystem diseases with optic atrophy such as mitochondrial encephalomyopathies, to age-related neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease where optic nerve involvement has, until recently, been a relatively overlooked feature. New tools are available to thoroughly investigate optic nerve function, allowing unparalleled access to this part of the central nervous system. Understanding the molecular pathophysiology of RGC neurodegeneration and optic atrophy, is key to broadly understanding the pathogenesis of neurodegenerative disorders, for monitoring their progression in describing the natural history, and ultimately as outcome measures to evaluate therapies. In this review, the different layers, from molecular to anatomical, that may contribute to RGC neurodegeneration and optic atrophy are tackled in an integrated way, considering all relevant players. These include RGC dendrites, cell bodies and axons, the unmyelinated retinal nerve fiber layer and the myelinated post-laminar axons, as well as olygodendrocytes and astrocytes, looked for unconventional functions. Dysfunctional mitochondrial dynamics, transport, homeostatic control of mitobiogenesis and mitophagic removal, as well as specific propensity to apoptosis may target differently cell types and anatomical settings. Ultimately, we can envisage new investigative approaches and therapeutic options that will speed the early diagnosis of neurodegenerative diseases and their cure.
Introduction
It is relatively common for a neurologist looking at the fundus oculi of a patient complaining of visual impairment to recognize a pale optic disc as an isolated feature or associated with a more complex disorder. For a long time the ‘pale optic disc’, evidence of an atrophic optic nerve, has been poorly understood and investigated by the neurologist, or discounted by the ophthalmologist, frustrating the search for answers and therapeutic options.
In the last three decades, tremendous progress has been made in understanding the genetic basis of optic neuropathies and the molecular mechanisms leading to degeneration of retinal ganglion cells (RGCs), the neuronal retinal cell type that projects its axon to the brain, forming the optic nerve (1,2). Currently, the two most frequent non-syndromic inherited optic neuropathies are Leber’s hereditary optic neuropathy (LHON), described by Albrecht von Graefe in 1858 (3) and Theodor Leber in 1871, who left his name attached (4), and dominant optic atrophy (DOA), described by Poul Kjer in 1959 (5). Both disorders are estimated to have a prevalence of approximately 1 in 30,000–65,000, depending on the different studies and countries (6–9), and their molecular bases have been clarified (10–13). However, optic atrophy may also occur for environmental reasons such as deficiencies of vitamin B12 or folic acid, tobacco and alcohol abuse, as well as exposure to toxins and drugs, most frequently antibiotics, in all cases phenocopying the genetic forms, and thus highlighting similar pathogenic mechanisms (14).
Recent technological advances greatly improved the in vivo investigation of RGC function with neurophysiological exams, such as pattern electroretinogram (PERG) and the photopic negative response (PhNR) (15), and most importantly by direct imaging with optical coherence tomography (OCT) (16). OCT allows for the direct assessment of all retinal components, including RGCs and their optic nerve forming axons, by quantitative evaluations of retinal nerve fiber layer (RNFL) thickness, the macular segmentation of the ganglion cell complex (GCC), and, more recently, the vascular components such as the choroid and the retina vasculature by OCT-angiography (17–20). This tool has been fundamental for defining the natural history of inherited optic neuropathies (21,22) and has substantiated the frequent occurrence of optic neuropathy in common age-related diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) (23–25), thus expanding the field of optic neuropathies to the larger area of neurodegeneration (13,26). This holds great promise as we can use the eye as a window on the brain for understanding the basic molecular and cellular mechanisms of neurodegeneration, and as a target for neuroprotective therapies.
Retinal Ganglion Cells Vulnerability
RGCs are the usual target in LHON and DOA patients, as well as in other optic neuropathies most frequently implying an impaired mitochondrial function (1,2,13). In this regard, RGCs obey the paradigm of neurodegenerative disorders where only a subpopulation of neurons is selectively targeted by the pathological mechanism, and RGCs display a particular vulnerability. This vulnerability relates to the peculiar neuronal architecture of RGCs, characterized by unique long axonal segments running unmyelinated in the RNFL to maintain the transparency of the retina, before these fibers turn ninety degrees and become the optic nerve head where they cross the lamina cribrosa, and eventually acquire their myelin sheaths and classic bundle organization as they form the retrobulbar optic nerve (1,2,13).
Retinal Ganglion Cells Metabolic Requirements: Homeostatic Regulation of Mitochondrial Dynamics, Biogenesis and Mitophagy
Not surprisingly, the large majority of inherited optic neuropathies are directly or indirectly related to mitochondrial dysfunction (27,28). LHON is due to mitochondrial DNA (mtDNA) missense mutations affecting the respiratory complex I (1,2) and the pathogenic mechanism is ascribed to a combination of reduced bioenergetic efficiency (29), increased reactive oxygen species (ROS) production (30,31) and special propensity to undergo apoptosis (32–34). DOA is due to heterozygous mutations affecting the OPA1 gene and leading to either haploinsufficiency or a dominant negative effect (1,2,9), which considering the major role played by OPA1 in mitochondrial fusion, results in defective mitochondrial dynamics, altered mitochondrial cristae and reduced bioenergetic efficiency, mostly mediated by complex I (35–37). In both cases, there is defective mitochondrial homeostasis implicating compensatory biogenesis and the removal of damaged mitochondria by mitophagy, altered fission/fusion balance and rates of axonal transport, and ultimately, propensity to apoptotic cell death. These are all themes recapitulating the major mechanisms implicated in neurodegeneration (1,2,13,26,28,38).
Mitochondrial biogenesis and replication of mtDNA are believed to largely occur in the RGC cell body (39), where most of the transcriptional and translational activity is carried out by both nuclear and mitochondrial genomes, although there is increasing evidence of an active axonal transcriptome (40). Mitochondria are then distributed in either the dendritic tree or transported down the long axon to the synaptic bouton (41,42), with the characteristic trafficking of anterograde and retrograde transport, and the asymmetric distribution of organelles, abundant in the unmyelinated proximal axonal segment in the RNFL, drastically reduced in number in the myelinated post-laminar optic nerve (43–45). Mitochondrial biogenesis is balanced with mitochondrial removal by mitophagy in a life cycle regulated by fission and fusion (46). Mitophagy is a hotly debated theme in neurodegeneration, in particular in the field of PD where rare genetic forms due to mutations in the PINK1 and Parkin genes seem to affect the efficiency of removal of damaged mitochondria (47,48). Thus, quality control of the mitochondrial network is tightly linked to mitochondrial biogenesis, as well as to mitochondrial dynamics, with a growing list of proteins that may control multiple pathways in different tissues, including the ATG12-ATG3 complex (49), Parkin (50,51), Rev-erb (52) and TFEB (53,54) to name a few, possibly responsive to the retrograde signaling developed by dysfunctional mitochondria (55). Noticeably, spontaneous compensatory cellular strategies based on activated mitochondrial biogenesis may be set in motion by LHON mutations, whose efficiency might be modulated by specific genetic backgrounds, ultimately driving the incomplete penetrance that characterizes LHON families (56). Estrogens, which amongst other effects can drive mitochondrial biogenesis, may further protect women, thus explaining male prevalence in LHON (57,58). Conversely, accumulating evidence documents excessive mitophagy in RGCs (59) and other cell types in DOA patients with OPA1 mutations, in particular those affecting the GTPase domain leading to the syndromic forms named DOA ‘plus’ (60,61). Interestingly, Parkin also regulates OPA1 through linear ubiquitination of NF-κB essential modulator (NEMO), establishing further links between the quality control PINK1/Parkin axis and mitochondrial dynamics (62). The role played by mitophagy in LHON remains incompletely explored, even if increased mitophagic activity has been described with complex I mtDNA mutations (63). Counter intuitively, excessive mitophagy might play a role in the pathogenic mechanism of RGC neurodegeneration, as opposed to defective mitophagy involved in the PINK1/Parkin genetic PD. Ultimately, the homeostatic balance between mitochondrial biogenesis and mitophagy seems key to the functional architecture of RGCs, involving a tight co-regulation of mitochondrial dynamics and transport along axons and dendrites.
Retinal Ganglion Cells Dendrites: Have We Been Looking in The Right Place?
The canonical view is that the RGC degeneration may affect the soma after axonal involvement in a retrograde march (1,2). However, limitations of in vivo studies may have lead us to miss the early signs of RGC dysfunction that are hard to detect. The availability of OPA1-mutant mouse models recapitulating DOA led to the observation that remodeling of the dendritic tree, with dendritic pruning and loss of synaptic connectivity, may be the earliest manifestations of RGC dysfunction leading, only later, to the stages of neurodegeneration, implying cell and axonal loss (64,65). These findings emphasize the role of mitochondrial dynamics in the maintenance of neuronal architecture. The correct distribution of mitochondria to axons and dendrites is essential and the Ca2+-dependent adaptor Miro1 has been identified as the key protein for mitochondrial transport (66). A recent study shows that Miro1 suppression leads to neurodegeneration by affecting specifically dendritic complexity, but leaving unaltered the axon (67). Interestingly, in another mouse model of complex I deficiency, a knock-out for the nuclear encoded subunit NDUFS4, the first event noticed to precede RGC degeneration was loss of bipolar cells in the retina, which led to deafferentation of RGCs and a reduction of their dendrites (68). This model, which might resemble LHON, highlights again that early pathological events may involve dendrites and their synaptic connections first, and this precedes RGC and optic nerve dysfunction.
Unique Architecture of Retinal Ganglion Cell Axons: Naked Versus Myelination and the Role of Axonal Transport
RGC vulnerability is attributed to the unique axonal structure, characterized by a long intraretinal segment that remains unmyelinated (1,2). This is associated with a very asymmetrical distribution of mitochondria along the fiber, exceedingly abundant in the unmyelinated part, as opposed to the abrupt change in mitochondrial numbers and morphology occurring as the axons exit the eye at the lamina cribrosa and become myelinated (1,2,43–45). Axonal mitochondria move bi-directionally along microtubule tracks thanks to a complex protein machinery, which includes the well-known motor proteins kinesin and dynein, respectively involved in anterograde and retrograde transport, but also myosins and actin, as well as adaptor proteins such as Miro and Milton that in turn interact with the protein machinery involved in fission and fusion, thus strictly connecting axonal transport and mitochondrial dynamics (69). However, we are still missing a detailed description of how this increasingly complex machinery is regulated or what maintains the asymmetrical mitochondrial distribution in RGC axons. In particular, we do not understand how anterograde and retrograde mitochondrial transport equilibrates with the docking and undocking of mitochondria in the RNFL, as well as what controls the levels of fusion/fission cycles and mitophagy in the RGCs. It might be envisaged that the ‘kiss and run’ transient mitochondrial fusion/fission cycles (70) between anterograde and retrograde transported mitochondria may play a major role for their maintenance, also considering distant elements such as the synaptic boutons (71).
A few recent studies, in most cases from the field of neuronal regeneration, point to new and interesting players involved in axonal maintenance (72,73). For example, the Armadillo Repeat Containing, X-Linked 1 (Armcx1) gene encodes a protein that is targeted to the outer mitochondrial membrane, interacts with Miro 1, and promotes neuronal survival and axonal regeneration after injury (72). Remarkably, Armcx1 overexpression enhances mitochondrial transport by recruiting stationary mitochondria in adult RGCs (72). The subacute phase of LHON natural history, preceding the massive loss of macular RGCs and papillomacular axons, is characterized by axonal swelling that may be due to compensatory mitochondrial biogenesis and stalling of axonal transport (1,2). Under such circumstances, Armcx1 might determine the fate of dysfunctional RGCs with axoplasmic flow stasis. Another interesting protein, recently demonstrated to be a regulator of axonal diameter, is the actin-binding protein adducin (73). Abolished expression of adducin led to progressive axonal enlargement in the optic nerve, followed by axonal degeneration and loss in a mouse model (73).
Axonal Myelination: The Oligodendrocytes as Active Players
The role of oligodendrocytes and their myelination of axons remain relatively unexplored in studies of optic neuropathies. Yet, in LHON and DOA the mtDNA or OPA1 mutations also affect oligodendrocytes, possibly altering myelin turnover and ultimately impinging on the pathogenic mechanisms leading to axonal loss (1,14). Evidence of myelin loss and remodeling in the optic nerve comes from very few post-mortem studies of LHON and DOA patients (1,14,74–76) (Fig. 1). Similar features have been recently observed in faithful genetic animal models reproducing LHON and DOA (77,78). Along these lines, various degrees of subclinical white matter pathology have been documented by in-vivo studies of LHON and DOA patients with brain magnetic resonance imaging and spectroscopy (79). Furthermore, in both LHON and DOA a subgroup of patients develops a multiple sclerosis-like illness (80,81), raising the question of whether mitochondrial dysfunction can trigger myelin pathology (82). Optic nerve myelination and brain white matter are vulnerable to complex I dysfunction (83,84), and this is a common biochemical link between LHON and DOA (29,36).
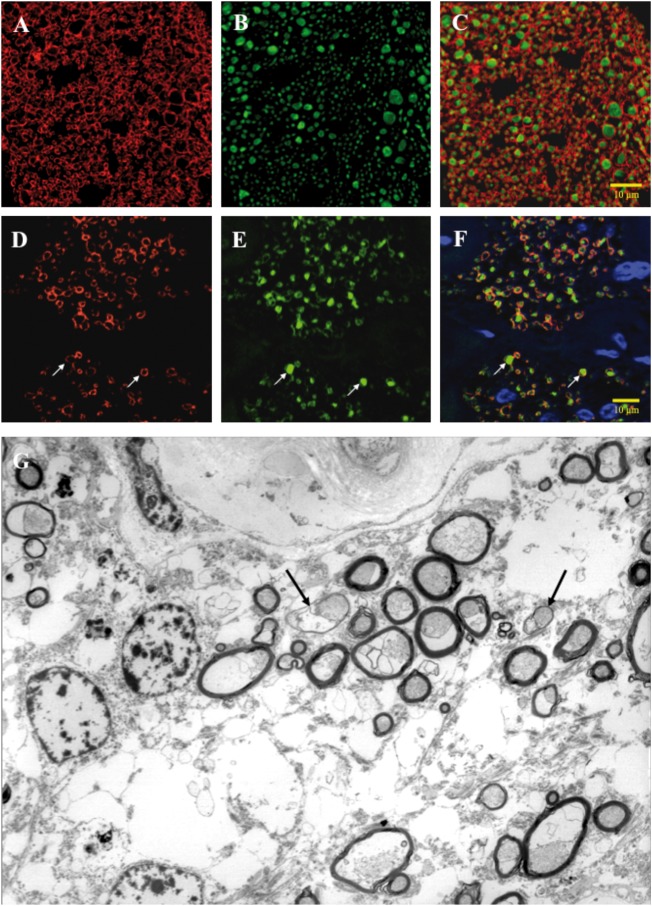
Figure 1.
Immunostained cross-sections of human optic nerve from a healthy control and a LHON patient. (A–F) Formalin-fixed paraffin embedded human optic nerves, cross-sectional profiles, double-immunofluorescence (IF) labeling using confocal microscopy for myelin basic protein (MBP) coupled to TRITC (red) and neurofilament protein (NF) coupled to FITC (green), and counterstained with DAPI (blue) for nuclei. (A–C) Control optic nerve from a 70-year-old female. Panel A demonstrates labeling for MBP, panel B for NF, and panel C merges all three labels. Note the thickness of the myelin and the density of the axons. (D–F) LHON optic nerve from a 68-year-old female. Panel D represents labeling for MBP, panel E for NF, and panel F merges all three labels. Note the decreased number of axons with myelin thinning as illustrated at arrows. Electron microscopy cross-sectional profile of a LHON atrophic optic nerve. (G) Glutaraldehyde-fixed plastic embedded LHON optic nerve, cross-sectional profile, from a 74-year old female, high magnification transmission electron microscopy, demonstrating a decreased density of axons with examples of myelin thinning (arrows).
There is experimental evidence that shows metabolic interactions amongst oligodendrocytes, the myelination of fibers and the axons, and these interactions include mitochondria and energy metabolism that must sustain the functional requirements of myelinated axons (85). Historically, animal models of selective and reversible demyelination without damage of the underlying axons have been based on cyanide (86), cuprizone (87), and ethidium bromide (88) intoxications, all three toxicants also affecting mitochondria by different mechanisms (14). More recent studies of mitochondrial metabolism in oligodendrocytes indicate that their glycolytic lactate production fuels axonal function (89,90). Mitochondria in oligodendrocytes can populate and move into the cytoplasmic tongues of the myelin sheaths (91). Compared to neurons, the oligodendrocyte mitochondria are thinner and shorter, have less cristae, are less motile and are sparsely distributed, indicative of their relatively limited oxidative phosphorylation activity and prevalent glycolytic metabolism (89–91). A new methodological approach recently allowed for monitoring ex-vivo the axonal ATP levels in correlation with firing of axonal action potentials in optic nerves acutely isolated from a transgenic mouse expressing a fluorescent ATP-sensor (92). These experiments demonstrated the high dependence of axons on ATP levels, sustained by both local mitochondrial function and lactate/pyruvate metabolism supported by glycolytic oligodendrocytes (92). It must be noted that some studies, initially prompted by the proteomic evidence that mitochondrial respiratory complexes are apparently expressed in compact myelin, provided some experimental evidence leading to the currently debated proposition that myelin might carry out local aerobic ATP production (93,94). The strict interdependence of axons and oligodendrocytes is further highlighted by the notion that acute demyelination induces an increased mitochondrial population in the denuded portion of the axon as a compensatory mechanism (95,96). Strikingly, the opposite also seems true, as the increased axonal firing rate induces oligodendrocyte precursors to proliferate and differentiate to mature oligodendrocytes, and ultimately increasing myelin thickness (97). All considered, RGCs may be particularly vulnerable to mitochondrial dysfunction because of their special anatomy and physiology. They have segmental myelination that excludes the RNFL, and may be naturally designed to cope by a fine tuning of the axonal bioenergetic needs, but RGCs may also become rapidly imbalanced once the post-laminar myelination undergoes impaired maintenance and turnover, and all this may trigger long-range consequences contributing to neurodegeneration, (1,14).
Different Patterns of Axonal Loss: Small Versus Large
The hallmark of LHON and the other mitochondrial optic neuropathies is the preferential loss of small axons, affecting the papillomacular bundle that originates from RGCs in the macula where small sized RGCs and axons, with thinner myelination, prevail (98). This clinically translates into the characteristic temporal pallor of the optic disc observed at fundus examination, with loss of central vision due to a central scotoma (1,2) (Fig. 2). The axonal caliber determines the firing rate of action potentials and ultimately the energy that is needed as well as that can be provided based on axonal surface and volume respectively (99). Considering that increase of mitochondrial biogenesis and mass is a key compensatory mechanism activated when mitochondria are functionally impaired, a smaller axon is constrained in accommodating these mitochondria. The axonal size, in turn, is a major risk factor that determines the pattern of axonal degeneration, with smaller axons occupying that part of the optic disc most likely to be involved in the neurodegeneration of LHON (98). Recently, this mechanism has been mathematically modeled, demonstrating that the theoretical prediction perfectly fits the histological pattern of axonal loss (100) (Fig. 2). In light of these studies, both proteins above mentioned, Armcx1 (72) and adducin (73), become of great interest for the possible role they might play in LHON, and in particular could their modulation inure optic nerves at risk in LHON from involvement?
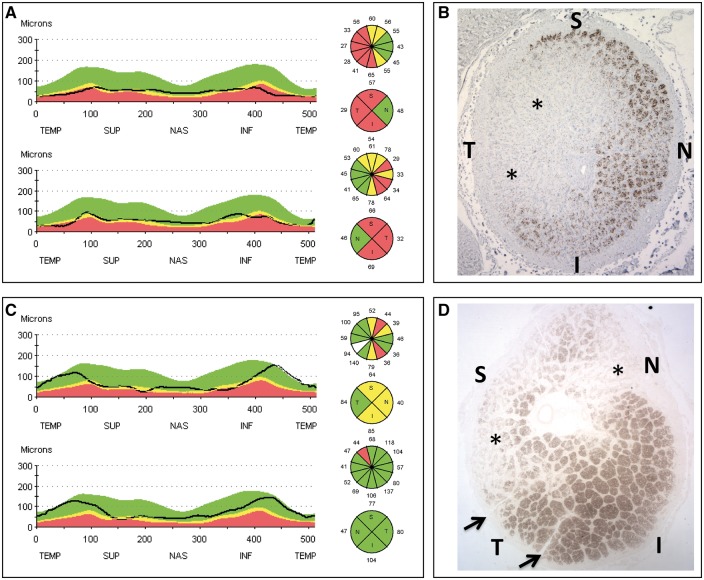
Figure 2.
OCT evaluation of retinal nerve fiber layer and postmortem optic nerve cross-sections from LHON patients (A, B). Panel A shows RNFL thinning at OCT evaluation, more evident on the temporal quadrant, and relative sparing of the nasal quadrant of the optic nerve. Panel B shows an optic nerve cross-sectional profile displaying a classic profound depletion of axons on the temporal aspect of the optic nerve (asterisks) with relative preservation of axons in the supero-nasal sectors, yet with reduced fiber density. OCT evaluation of retinal nerve fiber layer and postmortem optic nerve cross-sections from Alzheimer patients (C, D). Panel C shows RNFL thinning at OCT evaluation, more evident on the supero-nasal quadrants, and sparing of the temporal quadrant of the optic nerve. Panel D shows an optic nerve cross-sectional profile displaying a depletion of axons on the supero-nasal sectors of the optic nerve (asterisks) with preservation of axons in the infero-temporal sectors.
Remarkably, in some of the age-related neurodegenerative diseases such as AD, the pattern of axonal loss in the optic nerve is very different, with the areas of larger sized RGCs with their thicker axons being most affected, and this may indicate a different pathogenic mechanism for AD-related optic neuropathy (13,26) (Fig. 2).
Astrocytes, a Further Player with Some New Roles
Astrocytes have an extraordinary broad number of different roles in the central nervous system (101) and there is mounting evidence of their pathogenic relevance in neurodegenerative disease that is well beyond the scope of the present review (102). However, in the neuroglial crosstalk there is also a novel, non-canonical role for astrocytes that entails a bidirectional exchange of mitochondria between neurons and astrocytes (103,104). It has been reported that at the optic nerve head RGC axons form protrusions through which mitochondria are extruded, then internalized and degraded in adjacent astrocytes by mitophagy, a process that has been named transmitophagy (103). The opposite trafficking may also occur as it has been reported that astrocytes release mitochondria-containing particles that enter nearby neurons amplifying cell survival signals in pathological conditions, such as in focal cerebral ischemia (104). This bidirectional mitochondrial trafficking has obvious broad implications in the pathogenic mechanisms of optic neuropathies, and in general for neurodegeneration.
Melanopsin RGCs Resist Mitochondrial Neurodegeneration
A special case is represented by a special subset of RGCs that constitute about 1% of the total number of RGCs; these are a new class of photoreceptors, the melanopsin-containing RGCs (mRGCs). These cells, first described in 2002 (105,106), are intrinsically photosensitive and particularly sensitive to blue light (470 nm) due to the presence of the photopigment melanopsin (107). They have large soma (up to 25 μm) and dendritic field arborization that creates a photoreceptive net in the retina (108), which uses an ancient phototransduction pathway similar to invertebrates, termed the rhabdomeric system, as opposed to the ciliary visual system of mammals (109). Functionally, mRGCs contribute mainly the non-image forming functions of the eye, playing a crucial role in circadian photoentrainment by projecting to the suprachiasmatic nucleus through the retino-hypothalamic tract, to melatonin synthesis and its suppression, to sleep regulation and mood (110–112). Another mRGC pathway runs primarily through the pretectum and controls the pupillary light response (110–112). Furthermore, there is now evidence that mRGCs may also play a role in visual functions (113).
Interestingly, in inherited optic neuropathies such as LHON and DOA mRGCs are relatively spared (114), as confirmed by the preservation of pupillary light reflex (115). The reasons for this resistance to mitochondrial dysfunction are still unknown, but experimental evidence does not support the melanopsin photopigment itself as key (116). One possible factor protecting mRGCs from mitochondrial dysfunction is their large cellular size (114).
However, in other neurodegenerative disorders such as AD, these mRGCs are preferentially lost and are affected by the typical hallmarks of amyloid pathology, possibly contributing to the circadian imbalance described in AD patients even in the early course of the disease (117). The different profile of RGC loss described in AD, which is more obvious in the superior quadrant of the optic nerve (13,117) (Fig. 2), suggests that larger size RGCs are more vulnerable in AD, possibly explaining why mRGCs are affected by AD pathology (see next paragraph).
Genetically Determined Optic Neuropathies: Are They All Mitochondrial?
The list of inherited optic neuropathies, besides LHON and OPA1-related DOA, is continuously growing with an increasing number of genes involved, in the large majority of cases affecting mitochondrial functions (for reviews see 1,2,13,26,118). Preferential involvement of the small axons is not always the pattern, and other pathogenic mechanisms may occur. Optic neuropathy in Friedreich ataxia, for example, does not obey the classic mitochondrial pattern, even if mitochondrial dysfunction seems to play a role in the pathogenesis of neurodegeneration (119). Wolfram syndrome is due to mutations in two proteins, wolframin 1 and 2, which affect interorganellar communication between mitochondria and endoplasmic reticulum (ER), a hot topic for neurodegeneration (26). Similarly, the recently reported OPA10 gene, RTN4IP1 (reticulon 4 interacting protein 1), is also a protein involved in mitochondrial-ER communication and recessive mutations may lead to isolated or syndromic optic atrophy (120). Optic atrophy with a diffuse pattern of axonal loss is also described in two rare neurodegenerative disorders, autosomal dominant cerebellar ataxia, deafness and narcolepsy (ADCA-DN) and hereditary sensory neuropathy with dementia and hearing loss (HSE-IN), both caused by dominant mutations in the DNA methyltransferase1 (DNMT1) gene, which main function is to maintain genome methylation (121). These latter disorders represent a novel model where global deregulation of gene expression is predicted to occur due to aberrant DNA methylation, possibly affecting some of the about 1,500 mitochondrially related proteins encoded by the nuclear DNA (122).
The Dichotomy of Optic Neuropathy in Parkinson and Alzheimer’s Diseases
With the introduction of OCT, the assessment of optic nerve pathology has been expanded to many neurodegenerative diseases, redefining the ocular phenotype in AD (123,124). It also became clear that different patterns of optic nerve degeneration might be observed. For example, OCT studies in AD described a preferential RNFL thinning in the superior quadrant of the optic nerve, which has a prevalence of large axons, and this corroborates histological studies that showed that large sized RGCs are preferentially lost in AD retinas (117,125,126) (Fig. 2). This pattern resembles that of glaucoma (127) and has also been reported in multiple system atrophy (128). Interestingly, glaucoma patients also suffer sleep and circadian rhythm abnormalities (129) and mRGC loss has been documented in postmortem studies (130).
Unlike AD, PD and Huntington’s disease (HD) seems to involve preferentially the loss of smaller RGCs and axons as demonstrated by selective RNFL thinning in the temporal sector (24,131,132). This pattern is possibly explained by the shared pathophysiological mechanisms of these disorders with inherited mitochondrial optic neuropathies, possibly affecting complex I and mitochondrial quality control in PD (48) and mitochondrial dynamics in HD (133,134).
Conclusions and Future Directions
There has been tremendous progress in the field of inherited optic neuropathies fueled by the great diagnostic power of next generation sequencing. This continuous refinement of the catalog of genetic causes provides newer details on potential pathogenic mechanisms that lead to RGC neurodegeneration. The central focus remains on mitochondria and the disruption of their many signaling and metabolic pathways that can lead to their dysfunction. A deeper understanding for these pathogenic mechanisms is key to designing future therapeutic strategies, and it should be emphasized that, as highlighted in this review, multiple players participate in these pathogenic mechanisms (Fig. 3). If these factors are not properly taken into account, such as with gene therapy designed to correct the mitochondrial genetic defect only in RGCs and not in mutant glial cells, these therapeutic approaches may face a failure in clinical trials.
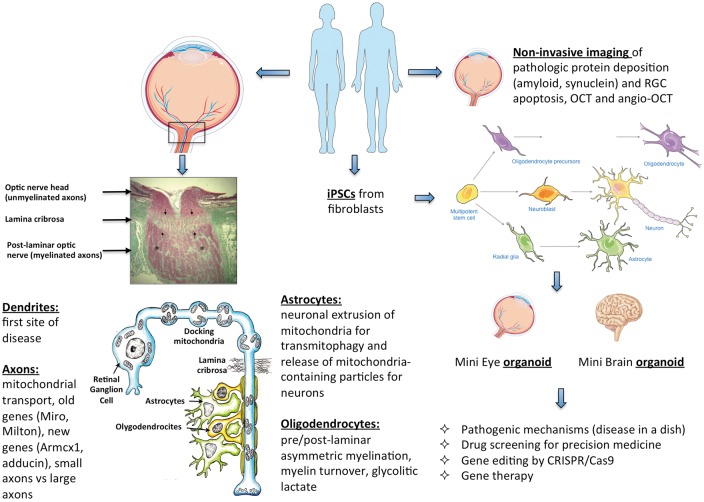
Figure 3.
Summary of the pathogenic mechanisms and future directions in optic nerve neurodegeneration. On the left, the structure of the optic nerve head, lamina cribrosa and post-laminar optic nerve is provided (schematic representation of the RGC is modified from Carelli et al., 2004, (1), with listed all mechanisms discussed involving the different cellular players, such as RGCs with dendrites and axons, oligodendrocytes with myelin sheet, and astrocytes. On the right, the future directions are illustrated in terms of eye imaging with new approaches, as well as the creation of iPSCs and derived organoids aimed at better understanding the pathogenic mechanism and setting therapeutic options.
However, inherited optic neuropathies are relatively rare genetic diseases, which prompted the investigation of optic nerve pathology in the much broader field of neurodegenerative diseases including age-related PD and AD. The evidence of frequent, almost ubiquitous, involvement of the optic nerve in neurodegenerative pathologies provides an unprecedented opportunity to exploit the eye, in particular the neuroretina, as a subclinical biomarker for early detection with which to follow the natural history of these diseases. It is also a potential target/sensor for determining therapeutic efficacy. As example of future directions in this area is the current development of direct and non-invasive imaging of pathologic protein deposition in the retina such as amyloid plaques (135), as well as for apoptotic RGC death (136). This will likely provide key tools for the monitoring and early diagnosis of these diseases. Furthermore, the explosive developing field of induced pluripotent stem cells (iPSCs) reprogrammed from primary patient-derived cell cultures (137) provides the remarkable and unique opportunity to obtain specialized terminally differentiated cells and organoids to study ‘disease in a dish’ models, including mini-eyes and mini-brains (138,139). These approaches hold great promise for high throughput drug screening and the rapid development of gene therapy, taking advantage of the powerful CRISPR/Cas9 editing technologies (Fig. 3). This would allow us to envision a rapid translation of therapeutic options for neurodegenerative diseases, now largely untreatable. In this respect, the eye is in a privileged position for its greater accessibility and potential for easier manipulation for investigations that exploit newly developed technologies and sophisticated tools such as retinal imaging with OCT. The ultimate goal for optic nerve regeneration might not be so intangible as it has appeared until now (140).
Acknowledgements
VC is funded by the Italian Ministries of Health and of Research, Telethon – Italy (Grants no. GGP06233, GGP10005, GGP11182, and GGP14187), e-RARE, ‘Programma di ricerca Regione-Università 2010-2012’ (PRUa1RI-2012-008), and patient-led organisations (IFOND, UMDF, MITOCON, The Poincenot Family, and the Gino Galletti Foundation). CLM receives financial support from the Italian Ministry of Health (GR-2013-02358026). AAS and FNR-C are supported by IFOND and an NIH National Eye Institute Core Grant EY03040. We also thank Servier Medical Art, where the basic artwork elements included in Figure 3 were taken.
Conflict of Interest statement. None declared.
Funding
Italian Ministries of Health and of Research, Telethon – Italy (Grants no. GGP06233, GGP10005, GGP11182, and GGP14187), e-RARE, ‘Programma di ricerca Regione-Università 2010-2012’ (PRUa1RI-2012-008), and patient-led organisations (IFOND, UMDF, MITOCON, The Poincenot Family, and the Gino Galletti Foundation), Italian Ministry of Health (GR-2013-02358026), IFOND and an NIH National Eye Institute Core Grant EY03040. Funding to pay the Open Access publication charges for this article was provided by the Italian region Emilia-Romagna, grant “Programma di ricerca Regione-Università 2010-2012” (PRUa1RI-2012-008).
References
- 1. Carelli V., Ross-Cisneros F.N., Sadun A.A. (2004) Mitochondrial dysfunction as a cause of optic neuropathies. Prog. Ret. Eye Res., 23, 53–89. [DOI] [PubMed] [Google Scholar]
- 2. Yu-Wai-Man P., Griffiths P.G., Chinnery P.F. (2011) Mitochondrial optic neuropathies -Disease mechanisms and therapeutic strategies. Prog. Ret. Eye Res., 30, 81–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Von Graefe A. (1858) Ein ungewohnlicher Fall von hereditarer Amaurose. Arch. Ophthalmol., 4, 266–268. [Google Scholar]
- 4. Leber T. (1871) Uber hereditare und congenital-angelegte Sehnervenleiden. Arch. Ophthalmol., 17, 249–291. [Google Scholar]
- 5. Kjer P. (1959) Infantile optic atrophy with dominant mode of inheritance: a clinical and genetic study of 19 Danish families. Acta Ophthalmol. Scand., 37, 1–146. [PubMed] [Google Scholar]
- 6. Yu-Wai-Man P., Griffiths P.G., Brown D.T., Howell N., Turnbull D.M., Chinnery P.F. (2003) The epidemiology of Leber hereditary optic neuropathy in the North East of England. Am. J. Hum. Genet., 72, 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yu-Wai-Man P., Chinnery P.F. (2013) Dominant optic atrophy: novel OPA1 mutations and revised prevalence estimates. Ophthalmology, 120, 1712–1712.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mascialino B., Leinonen M., Meier T. (2012) Meta-analysis of the prevalence of Leber hereditary optic neuropathy mtDNA mutations in Europe. Eur. J. Ophthalmol., 22, 461–465. [DOI] [PubMed] [Google Scholar]
- 9. Lenaers G., Hamel C., Delettre C., Amati-Bonneau P., Procaccio V., Bonneau D., Reynier P., Milea D. (2012) Dominant optic atrophy. Orphanet J. Rare Dis., 7, 46.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wallace D.C., Singh G., Lott M.T., Hodge J.A., Schurr T.G., Lezza A.M., Elsas L.J. 2nd, Nikoskelainen E.K. (1988) Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science, 242, 1427–1430. [DOI] [PubMed] [Google Scholar]
- 11. Alexander C., Votruba M., Pesch U.E., Thiselton D.L., Mayer S., Moore A., Rodriguez M., Kellner U., Leo-Kottler B., Auburger G.. et al. (2000) OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat. Genet., 26, 211–215. [DOI] [PubMed] [Google Scholar]
- 12. Delettre C., Lenaers G., Griffoin J.M., Gigarel N., Lorenzo C., Belenguer P., Pelloquin L., Grosgeorge J., Turc-Carel C., Perret E.. et al. (2000) Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat. Genet., 26, 207–210. [DOI] [PubMed] [Google Scholar]
- 13. Maresca A., La Morgia C., Caporali L., Valentino M.L., Carelli V. (2013) The optic nerve: a “mito-window” on mitochondrial neurodegeneration. Mol. Cell Neurosci., 55, 62–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carelli V., Ross-Cisneros F.N., Sadun A.A. (2002) Optic nerve degeneration and mitochondrial dysfunction: genetic and acquired optic neuropathies. Neurochem. Int., 40, 573–584. [DOI] [PubMed] [Google Scholar]
- 15. Porciatti V. (2015) Electrophysiological assessment of retinal ganglion cell function. Exp. Eye Res., 141, 164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Popescu D.P., Choo-Smith L.P., Flueraru C., Mao Y., Chang S., Disano J., Sherif S., Sowa M.G. (2011) Optical coherence tomography: fundamental principles, instrumental designs and biomedical applications. Biophys. Rev., 3, 155.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barboni P., Savini G., Valentino M.L., Montagna P., Cortelli P., De Negri A.M., Sadun F., Bianchi S., Longanesi L., Zanini M.. et al. (2005) Retinal nerve fiber layer evaluation by optical coherence tomography in Leber's hereditary optic neuropathy. Ophthalmology, 112, 120–126. [DOI] [PubMed] [Google Scholar]
- 18. Barboni P., Carbonelli M., Savini G., Foscarini B., Parisi V., Valentino M.L., Carta A., De Negri A., Sadun F., Zeviani M.. et al. (2010) OPA1 mutations associated with dominant optic atrophy influence optic nerve head size. Ophthalmology, 117, 1547–1553. [DOI] [PubMed] [Google Scholar]
- 19. Barboni P., Savini G., Cascavilla M.L., Caporali L., Milesi J., Borrelli E., La Morgia C., Valentino M.L., Triolo G., Lembo A.. et al. (2014) Early macular retinal ganglion cell loss in dominant optic atrophy: genotype-phenotype correlation. Am. J. Ophthalmol., 158, 628–636. [DOI] [PubMed] [Google Scholar]
- 20. Borrelli E., Triolo G., Cascavilla M.L., La Morgia C., Rizzo G., Savini G., Balducci N., Nucci P., Giglio R., Darvizeh F.. et al. (2016) Changes in choroidal thickness follow the RNFL changes in Leber's hereditary optic neuropathy. Sci. Rep., 6, 37332.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barboni P., Carbonelli M., Savini G., Ramos Cdo V., Carta A., Berezovsky A., Salomao S.R., Carelli V., Sadun A.A. (2010) Natural history of Leber's hereditary optic neuropathy: longitudinal analysis of the retinal nerve fiber layer by optical coherence tomography. Ophthalmology, 117, 623–627. [DOI] [PubMed] [Google Scholar]
- 22. Balducci N., Savini G., Cascavilla M.L., La Morgia C., Triolo G., Giglio R., Carbonelli M., Parisi V., Sadun A.A., Bandello F.. et al. (2016) Macular nerve fibre and ganglion cell layer changes in acute Leber's hereditary optic neuropathy. Br. J. Ophthalmol., 100, 1232–1237. [DOI] [PubMed] [Google Scholar]
- 23. den Haan J., Verbraak F.D., Visser P.J., Bouwman F.H. (2017) Retinal thickness in Alzheimer's disease: a systematic review and meta-analysis. Alzheimers Dement. (Amst), 6, 162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yu J.G., Feng Y.F., Xiang Y., Huang J.H., Savini G., Parisi V., Yang W.J., Fu X.A. (2014) Retinal nerve fiber layer thickness changes in Parkinson disease: a meta-analysis. PLoS One, 9, e85718.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bodis-Wollner I., Miri S., Glazman S. (2014) Venturing into the no-man's land of the retina in Parkinson's disease. Mov. Disord., 29, 15–22. [DOI] [PubMed] [Google Scholar]
- 26. Yu-Wai-Man P., Votruba M., Burté F., La Morgia C., Barboni P., Carelli V. (2016) A neurodegenerative perspective on mitochondrial optic neuropathies. Acta Neuropathol., 132, 789–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carelli V., La Morgia C., Iommarini L., Carroccia R., Mattiazzi M., Sangiorgi S., Farne S., Maresca A., Foscarini B., Lanzi L.. et al. (2007) Mitochondrial optic neuropathies: how two genomes may kill the same cell type? Biosci. Rep., 27, 173–184. [DOI] [PubMed] [Google Scholar]
- 28. Carelli V., La Morgia C., Valentino M.L., Barboni P., Ross-Cisneros F.N., Sadun A.A. (2009) Retinal ganglion cell neurodegeneration in mitochondrial inherited disorders. Biochim. Biophys. Acta, 1787, 518–528. [DOI] [PubMed] [Google Scholar]
- 29. Baracca A., Solaini G., Sgarbi G., Lenaz G., Baruzzi A., Schapira A.H., Martinuzzi A., Carelli V. (2005) Severe impairment of complex I-driven adenosine triphosphate synthesis in Leber hereditary optic neuropathy cybrids. Arch. Neurol., 62, 730–736. [DOI] [PubMed] [Google Scholar]
- 30. Beretta S., Mattavelli L., Sala G., Tremolizzo L., Schapira A.H., Martinuzzi A., Carelli V., Ferrarese C. (2004) 0 Leber hereditary optic neuropathy mtDNA mutations disrupt glutamate transport in cybrid cell lines. Brain, 127, 2183–2192. [DOI] [PubMed] [Google Scholar]
- 31. Floreani M., Napoli E., Martinuzzi A., Pantano G., De Riva V., Trevisan R., Bisetto E., Valente L., Carelli V., Dabbeni-Sala F. (2005) Antioxidant defences in cybrids harboring mtDNA mutations associated with Leber's hereditary optic neuropathy. FEBS J., 272, 1124–1135. [DOI] [PubMed] [Google Scholar]
- 32. Danielson S.R., Wong A., Carelli V., Martinuzzi A., Schapira A.H., Cortopassi G.A. (2002) Cells bearing mutations causing Leber's hereditary optic neuropathy are sensitized to Fas-Induced apoptosis. J. Biol. Chem., 277, 5810–5815. [DOI] [PubMed] [Google Scholar]
- 33. Ghelli A., Zanna C., Porcelli A.M., Schapira A.H., Martinuzzi A., Carelli V., Rugolo M. (2003) Leber's hereditary optic neuropathy (LHON) pathogenic mutations induce mitochondrial-dependent apoptotic death in transmitochondrial cells incubated with galactose medium. J. Biol. Chem., 278, 4145–4150. [DOI] [PubMed] [Google Scholar]
- 34. Zanna C., Ghelli A., Porcelli A.M., Martinuzzi A., Carelli V., Rugolo M. (2005) Caspase-independent death of Leber's hereditary optic neuropathy cybrids is driven by energetic failure and mediated by AIF and Endonuclease G. Apoptosis, 10, 997–1007. [DOI] [PubMed] [Google Scholar]
- 35. Olichon A., Landes T., Arnaune-Pelloquin L., Emorine L.J., Mils V., Guichet A., Delettre C., Hamel C., Amati-Bonneau P., Bonneau D.. et al. (2007) Effects of OPA1 mutations on mitochondrial morphology and apoptosis: relevance to ADOA pathogenesis. J. Cell. Physiol., 211, 423–430. [DOI] [PubMed] [Google Scholar]
- 36. Zanna C., Ghelli A., Porcelli A.M., Karbowski M., Youle R.J., Schimpf S., Wissinger B., Pinti M., Cossarizza A., Vidoni S.. et al. (2008) OPA1 mutations associated with dominant optic atrophy impair oxidative phosphorylation and mitochondrial fusion. Brain, 131, 352–367. [DOI] [PubMed] [Google Scholar]
- 37. Agier V., Oliviero P., Lainé J., L'Hermitte-Stead C., Girard S., Fillaut S., Jardel C., Bouillaud F., Bulteau A.L., Lombès A. (2012) Defective mitochondrial fusion, altered respiratory function, and distorted cristae structure in skin fibroblasts with heterozygous OPA1 mutations. Biochim. Biophys. Acta, 1822, 1570–1580. [DOI] [PubMed] [Google Scholar]
- 38. Burté F., Carelli V., Chinnery P.F., Yu-Wai-Man P. (2015) Disturbed mitochondrial dynamics and neurodegenerative disorders. Nat. Rev. Neurol., 11, 11–24. [DOI] [PubMed] [Google Scholar]
- 39. Davis A.F., Clayton D.A. (1996) In situ localization of mitochondrial DNA replication in intact mammalian cells. J. Cell Biol., 135, 883–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Deglincerti A., Jaffrey S.R. (2012) Insights into the roles of local translation from the axonal transcriptome. Open Biol., 2, 120079.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li Z., Okamoto K., Hayashi Y., Sheng M. (2004) The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell, 119, 873–887. [DOI] [PubMed] [Google Scholar]
- 42. Faits M.C., Zhang C., Soto F., Kerschensteiner D. (2016) Dendritic mitochondria reach stable positions during circuit development. Elife, 5, e11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Andrews R.M., Griffiths P.G., Johnson M.A., Turnbull D.M. (1999) Histochemical localisation of mitochondrial enzyme activity in human optic nerve and retina. Br. J. Ophthalmol., 83, 231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bristow E.A., Griffiths P.G., Andrews R.M., Johnson M.A., Turnbull D.M. (2002) The distribution of mitochondrial activity in relation to optic nerve structure. Arch. Ophthalmol., 120, 791–796. [DOI] [PubMed] [Google Scholar]
- 45. Barron M.J., Griffiths P., Turnbull D.M., Bates D., Nichols P. (2004) The distributions of mitochondria and sodium channels reflect the specific energy requirements and conduction properties of the human optic nerve head. Br. J. Ophthalmol., 88, 286–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Twig G., Elorza A., Molina A.J., Mohamed H., Wikstrom J.D., Walzer G., Stiles L., Haigh S.E., Katz S., Las G.. et al. (2008) Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J., 27, 433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pickrell A.M., Youle R.J. (2015) The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson's disease. Neuron, 85, 257–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Giannoccaro M.P., La Morgia C., Rizzo G., Carelli V. (2017) Mitochondrial DNA and primary mitochondrial dysfunction in Parkinson's disease. Mov. Disord., 32, 346–363. [DOI] [PubMed] [Google Scholar]
- 49. Radoshevich L., Murrow L., Chen N., Fernandez E., Roy S., Fung C., Debnath J. (2010) ATG12 conjugation to ATG3 regulates mitochondrial homeostasis and cell death. Cell, 142, 590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shin J.H., Ko H.S., Kang H., Lee Y., Lee Y.I., Pletinkova O., Troconso J.C., Dawson V.L., Dawson T.M. (2011) PARIS (ZNF746) repression of PGC-1α contributes to neurodegeneration in Parkinson's disease. Cell, 144, 689–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stevens D.A., Lee Y., Kang H.C., Lee B.D., Lee Y.I., Bower A., Jiang H., Kang S.U., Andrabi S.A., Dawson V.L.. et al. (2015) Parkin loss leads to PARIS-dependent declines in mitochondrial mass and respiration. Proc. Natl. Acad. Sci. USA, 112, 11696–11701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Woldt E., Sebti Y., Solt L.A., Duhem C., Lancel S., Eeckhoute J., Hesselink M.K., Paquet C., Delhaye S., Shin Y.. et al. (2013) Rev-erb-α modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat. Med., 19, 1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Siddiqui A., Bhaumik D., Chinta S.J., Rane A., Rajagopalan S., Lieu C.A., Lithgow G.J., Andersen J.K. (2015) Mitochondrial quality control via the PGC1α-TFEB signaling pathway is compromised by Parkin Q311X mutation but independently restored by Rapamycin. J. Neurosci., 35, 12833–12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mansueto G., Armani A., Viscomi C., D'Orsi L., De Cegli R., Polishchuk E.V., Lamperti C., Di Meo I., Romanello V., Marchet S.. et al. (2017) Transcription Factor EB Controls Metabolic Flexibility during Exercise. Cell Metab., 25, 182–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Quirós P.M., Mottis A., Auwerx J. (2016) Mitonuclear communication in homeostasis and stress. Nat. Rev. Mol. Cell. Biol., 17, 213–226. [DOI] [PubMed] [Google Scholar]
- 56. Giordano C., Iommarini L., Giordano L., Maresca A., Pisano A., Valentino M.L., Caporali L., Liguori R., Deceglie S., Roberti M.. et al. (2014) Efficient mitochondrial biogenesis drives incomplete penetrance in Leber's hereditary optic neuropathy. Brain, 137, 335–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Giordano C., Montopoli M., Perli E., Orlandi M., Fantin M., Ross-Cisneros F.N., Caparrotta L., Martinuzzi A., Ragazzi E., Ghelli A.. et al. (2011) Oestrogens ameliorate mitochondrial dysfunction in Leber's hereditary optic neuropathy. Brain, 134, 220–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chen J.Q., Cammarata P.R., Baines C.P., Yager J.D. (2009) Regulation of mitochondrial respiratory chain biogenesis by estrogens/estrogen receptors and physiological, pathological and pharmacological implications. Biochim. Biophys Acta, 1793, 1540–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. White K.E., Davies V.J., Hogan V.E., Piechota M.J., Nichols P.P., Turnbull D.M., Votruba M. (2009) OPA1 deficiency associated with increased autophagy in retinal ganglion cells in a murine model of dominant optic atrophy. Invest. Ophthalmol. Vis. Sci., 50, 2567–2571. [DOI] [PubMed] [Google Scholar]
- 60. Carelli V., Musumeci O., Caporali L., Zanna C., La Morgia C., Del Dotto V., Porcelli A.M., Rugolo M., Valentino M.L., Iommarini L.. et al. (2015) Syndromic parkinsonism and dementia associated with OPA1 missense mutations. Ann. Neurol., 78, 21–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Liao C., Ashley N., Diot A., Morten K., Phadwal K., Williams A., Fearnley I., Rosser L., Lowndes J., Fratter C.. et al. (2017) Dysregulated mitophagy and mitochondrial organization in optic atrophy due to OPA1 mutations. Neurology, 88, 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Müller-Rischart A.K., Pilsl A., Beaudette P., Patra M., Hadian K., Funke M., Peis R., Deinlein A., Schweimer C., Kuhn P.H.. et al. (2013) The E3 ligase parkin maintains mitochondrial integrity by increasing linear ubiquitination of NEMO. Mol. Cell, 49, 908–921. [DOI] [PubMed] [Google Scholar]
- 63. Dombi E., Diot A., Morten K., Carver J., Lodge T., Fratter C., Ng Y.S., Liao C., Muir R., Blakely E.L.. et al. (2016) The m.13051G>A mitochondrial DNA mutation results in variable neurology and activated mitophagy. Neurology, 86, 1921–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Williams P.A., Morgan J.E., Votruba M. (2010) Opa1 deficiency in a mouse model of dominant optic atrophy leads to retinal ganglion cell dendropathy. Brain, 133, 2942–2951. [DOI] [PubMed] [Google Scholar]
- 65. Williams P.A., Piechota M., von Ruhland C., Taylor E., Morgan J.E., Votruba M. (2012) Opa1 is essential for retinal ganglion cell synaptic architecture and connectivity. Brain, 135, 493–505. [DOI] [PubMed] [Google Scholar]
- 66. Devine M.J., Birsa N., Kittler J.T. (2016) Miro sculpts mitochondrial dynamics in neuronal health and disease. Neurobiol. Dis., 90, 27–34. [DOI] [PubMed] [Google Scholar]
- 67. López-Doménech G., Higgs N.F., Vaccaro V., Roš H., Arancibia-Cárcamo I.L., MacAskill A.F., Kittler J.T. (2016) Loss of dendritic complexity precedes neurodegeneration in a mouse model with disrupted mitochondrial distribution in mature dendrites. Cell Rep., 17, 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Song L., Yu A., Murray K., Cortopassi G. (2017) Bipolar cell reduction precedes retinal ganglion neuron loss in a complex 1 knockout mouse model. Brain Res., 1657, 232–244. [DOI] [PubMed] [Google Scholar]
- 69. Saxton W.M., Hollenbeck P.J. (2012) The axonal transport of mitochondria. J. Cell Sci., 125, 2095–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hyde B.B., Twig G., Shirihai O.S. (2010) Organellar vs cellular control of mitochondrial dynamics. Semin. Cell Dev. Biol., 21, 575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ferree A.W., Trudeau K., Zik E., Benador I.Y., Twig G., Gottlieb R.A., Shirihai O.S. (2013) MitoTimer probe reveals the impact of autophagy, fusion, and motility on subcellular distribution of young and old mitochondrial protein and on relative mitochondrial protein age. Autophagy, 9, 1887–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cartoni R., Norsworthy M.W., Bei F., Wang C., Li S., Zhang Y., Gabel C.V., Schwarz T.L., He Z. (2016) The mammalian-specific protein Armcx1 regulates mitochondrial transport during axon regeneration. Neuron, 92, 1294–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Leite S.C., Sampaio P., Sousa V.F., Nogueira-Rodrigues J., Pinto-Costa R., Peters L.L., Brites P., Sousa M.M. (2016) The actin-binding protein α-Adducin is required for maintaining axon diameter. Cell Rep., 15, 490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kovacs G.G., Hoftberger R., Horvath R., Barsi P., Komoly S., Lassmann H., Budka H., Jakab G. (2005) Neuropathology of white matter disease in Leber’s hereditary optic neuropathy. Brain, 128, 35–41. [DOI] [PubMed] [Google Scholar]
- 75. Kjer P., Jensen O.A., Klinken L. (1983) Histopathology of eye, optic nerve and brain in a case of dominant optic atrophy. Acta Ophthalmol. (Copenh), 61, 300–312. [DOI] [PubMed] [Google Scholar]
- 76. Johnston P.B., Gaster R.N., Smith V.C., Tripathi R.C. (1979) A clinicopathologic study of autosomal dominant optic atrophy. Am. J. Ophthalmol., 88, 868–875. [DOI] [PubMed] [Google Scholar]
- 77. Lin C.S., Sharpley M.S., Fan W., Waymire K.G., Sadun A.A., Carelli V., Ross-Cisneros F.N., Baciu P., Sung E., McManus. et al. (2012) Mouse mtDNA mutant model of Leber hereditary optic neuropathy. Proc. Natl. Acad. Sci. USA, 109, 20065–20070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sarzi E., Angebault C., Seveno M., Gueguen N., Chaix B., Bielicki G., Boddaert N., Mausset-Bonnefont A.L., Cazevieille C., Rigau V.. et al. (2012) The human OPA1delTTAG mutation induces premature age-related systemic neurodegeneration in mouse. Brain, 135, 3599–3613. [DOI] [PubMed] [Google Scholar]
- 79. Manners D.N., Rizzo G., La Morgia C., Tonon C., Testa C., Barboni P., Malucelli E., Valentino M.L., Caporali L., Strobbe D.. et al. (2015) Diffusion tensor imaging mapping of brain white matter pathology in mitochondrial optic neuropathies. Am. J. Neuroradiol., 36, 1259–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Harding A.E., Sweeney M.G., Miller D.H., Mumford C.J., Kellar-Wood H., Menard D., McDonald W.I., Compston D.A. (1992) Occurrence of a multiple sclerosis-like illness in women who have a Leber's hereditary optic neuropathy mitochondrial DNA mutation. Brain, 115, 979–989. [DOI] [PubMed] [Google Scholar]
- 81. Yu-Wai-Man P., Spyropoulos A., Duncan H.J., Guadagno J.V., Chinnery P.F. (2016) A multiple sclerosis-like disorder in patients with OPA1 mutations. Ann. Clin. Transl. Neurol., 3, 723–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Carelli V., Bellan M. (2008) Myelin, mitochondria, and autoimmunity: what's the connection? Neurology, 70, 1075–1076. [DOI] [PubMed] [Google Scholar]
- 83. Marella M., Patki G., Matsuno-Yagi A., Yagi T. (2013) Complex I inhibition in the visual pathway induces disorganization of the node of Ranvier. Neurobiol. Dis., 58, 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Koene S., Rodenburg R.J., van der Knaap M.S., Willemsen M.A., Sperl W., Laugel V., Ostergaard E., Tarnopolsky M., Martin M.A., Nesbitt V.. et al. (2012) Natural disease course and genotype-phenotype correlations in Complex I deficiency caused by nuclear gene defects: what we learned from 130 cases. J. Inherit. Metab. Dis., 35, 737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Harris J.J., Attwell D. (2012) The energetics of CNS white matter. J. Neurosci., 32, 356–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lessell S., Kuwabara T. (1974) Fine structure of experimental cyanide optic neuropathy. Invest. Ophthalmol., 13, 748–756. [PubMed] [Google Scholar]
- 87. Ludwin S.K. (1978) Central nervous system demyelination and remyelination in the mouse: an ultrastructural study of cuprizone toxicity. Lab. Invest., 39, 597–612. [PubMed] [Google Scholar]
- 88. Blakemore W.F. (1982) Ethidium bromide induced demyelination in the spinal cord of the cat. Neuropathol. Appl. Neurobiol., 8, 365–375. [DOI] [PubMed] [Google Scholar]
- 89. Lee Y., Morrison B.M., Li Y., Lengacher S., Farah M.H., Hoffman P.N., Liu Y., Tsingalia A., Jin L., Zhang P.W.. et al. (2012) Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature, 487, 443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Fünfschilling U., Supplie L.M., Mahad D., Boretius S., Saab A.S., Edgar J., Brinkmann B.G., Kassmann C.M., Tzvetanova I.D., Möbius W.. et al. (2012) Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature, 485, 517–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rinholm J.E., Vervaeke K., Tadross M.R., Tkachuk A.N., Kopek B.G., Brown T.A., Bergersen L.H., Clayton D.A. (2016) Movement and structure of mitochondria in oligodendrocytes and their myelin sheaths. Glia, 64, 810–825. [DOI] [PubMed] [Google Scholar]
- 92. Trevisiol A., Saab A.S., Winkler U., Marx G., Imamura H., Möbius W., Kusch K., Nave K.A., Hirrlinger J. (2017) Monitoring ATP dynamics in electrically active white matter tracts. Elife, 6, e24241.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Morelli A., Ravera S., Panfoli I. (2011) Hypothesis of an energetic function for myelin. Cell Biochem. Biophys., 61, 179–187. [DOI] [PubMed] [Google Scholar]
- 94. Harris J., Attwell D. (2013) Is myelin a mitochondrion? J. Cereb. Blood Flow Metab., 33, 33–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Mutsaers S.E., Carroll W.M. (1998) Focal accumulation of intra-axonal mitochondria in demyelination of the cat optic nerve. Acta Neuropathol., 96, 139–143. [DOI] [PubMed] [Google Scholar]
- 96. Mahad D.J., Ziabreva I., Campbell G., Lax N., White K., Hanson P.S., Lassmann H., Turnbull D.M. (2009) Mitochondrial changes within axons in multiple sclerosis. Brain, 132, 1161–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gibson E.M., Purger D., Mount C.W., Goldstein A.K., Lin G.L., Wood L.S., Inema I., Miller S.E., Bieri G., Zuchero J.B.. et al. (2014) Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science, 344, e1252304.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sadun A.A., Win P.H., Ross-Cisneros F.N., Walker S.O., Carelli V. (2000) Leber's hereditary optic neuropathy differentially affects smaller axons in the optic nerve. Trans. Am. Ophthalmol. Soc., 98, 223–232. [PMC free article] [PubMed] [Google Scholar]
- 99. Perge J.A., Niven J.E., Mugnaini E., Balasubramanian V., Sterling P. (2012) Why do axons differ in caliber? J. Neurosci, 32, 626–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Pan B.X., Ross-Cisneros F.N., Carelli V., Rue K.S., Salomao S.R., Moraes-Filho M.N., Moraes M.N., Berezovsky A., Belfort R. Jr, Sadun A.A. (2012) Mathematically modeling the involvement of axons in Leber's hereditary optic neuropathy. Invest. Ophthalmol. Vis. Sci., 53, 7608–7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ben Haim L., Rowitch D.H. (2017) Functional diversity of astrocytes in neural circuit regulation. Nat. Rev. Neurosci., 18, 31–41. [DOI] [PubMed] [Google Scholar]
- 102. Pekny M., Pekna M., Messing A., Steinhäuser C., Lee J.M., Parpura V., Hol E.M., Sofroniew M.V., Verkhratsky A. (2016) Astrocytes: a central element in neurological diseases. Acta Neuropathol., 131, 323–345. [DOI] [PubMed] [Google Scholar]
- 103. Davis C.H., Kim K.Y., Bushong E.A., Mills E.A., Boassa D., Shih T., Kinebuchi M., Phan S., Zhou Y., Bihlmeyer N.A.. et al. (2014) Transcellular degradation of axonal mitochondria. Proc. Natl. Acad. Sci. USA, 111, 9633–9638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hayakawa K., Esposito E., Wang X., Terasaki Y., Liu Y., Xing C., Ji X., Lo E.H. (2016) Transfer of mitochondria from astrocytes to neurons after stroke. Nature, 535, 551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hattar S., Liao H.W., Takao M., Berson D.M., Yau K.W. (2002) Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science, 295, 1065–10670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Berson D.M., Dunn F.A., Takao M. (2002) Phototransduction by retinal ganglion cells that set the circadian clock. Science, 295, 1070–1073. [DOI] [PubMed] [Google Scholar]
- 107. Provencio I., Rodriguez I.R., Jiang G., Hayes W.P., Moreira E.F., Rollag M.D. (2000) A novel human opsin in the inner retina. J. Neurosci., 20, 600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hannibal J., Christiansen A.T., Heegaard S., Fahrenkrug J., Kiilgaard J.F. (2017) Melanopsin expressing human retinal ganglion cells: Subtypes, distribution, and intraretinal connectivity. J. Comp. Neurol., 525, 1934–1961. [DOI] [PubMed] [Google Scholar]
- 109. Graham D.M., Wong K.Y., Shapiro P., Frederick C., Pattabiraman K., Berson D.M. (2008) Melanopsin ganglion cells use a membrane-associated rhabdomeric phototransduction cascade. J. Neurophysiol., 99, 2522–2532. [DOI] [PubMed] [Google Scholar]
- 110. Schmidt T.M., Do M.T., Dacey D., Lucas R., Hattar S., Matynia A. (2011) Melanopsin-positive intrinsically photosensitive retinal ganglion cells: from form to function. J. Neurosci., 31, 16094–16101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hannibal J., Kankipati L., Strang C.E., Peterson B.B., Dacey D., Gamlin P.D. (2014) Central projections of intrinsically photosensitive retinal ganglion cells in the macaque monkey. J. Comp. Neurol., 522, 2231–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hattar S., Kumar M., Park A., Tong P., Tung J., Yau K.W., Berson D.M. (2006) Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J. Comp. Neurol., 497, 326–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Allen A.E., Storchi R., Martial F.P., Bedford R.A., Lucas R.J. (2017) Melanopsin contributions to the representation of images in the early visual system. Curr. Biol., pii:S0960-9822, 30491-30498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. La Morgia C., Ross-Cisneros F.N., Sadun A.A., Hannibal J., Munarini A., Mantovani V., Barboni P., Cantalupo G., Tozer K.R., Sancisi E.. et al. (2010) Melanopsin retinal ganglion cells are resistant to neurodegeneration in mitochondrial optic neuropathies. Brain, 133, 2426–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Moura A.L., Nagy B.V., La Morgia C., Barboni P., Oliveira A.G., Salomão S.R., Berezovsky A., de Moraes-Filho M.N., Chicani C.F., Belfort R. Jr. et al. (2013) The pupil light reflex in Leber's hereditary optic neuropathy: evidence for preservation of melanopsin-expressing retinal ganglion cells. Invest. Ophthalmol. Vis. Sci., 54, 4471–4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Georg B., Ghelli A., Giordano C., Ross-Cisneros F.N., Sadun A.A., Carelli V., Hannibal J., La Morgia C. (2017) Melanopsin-expressing retinal ganglion cells are resistant to cell injury, but not always. Mitochondrion, pii:S1567-7249, 30087-30089. [DOI] [PubMed] [Google Scholar]
- 117. La Morgia C., Ross-Cisneros F.N., Koronyo Y., Hannibal J., Gallassi R., Cantalupo G., Sambati L., Pan B.X., Tozer K.R., Barboni P.. et al. (2016) Melanopsin retinal ganglion cell loss in Alzheimer disease. Ann. Neurol., 79, 90–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Yu-Wai-Man P., Newman N.J. (2017) Inherited eye-related disorders due to mitochondrial dysfunction. Hum. Mol. Genet., [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 119. Fortuna F., Barboni P., Liguori R., Valentino M.L., Savini G., Gellera C., Mariotti C., Rizzo G., Tonon C., Manners D.. et al. (2009) Visual system involvement in patients with Friedreich's ataxia. Brain, 132, 116–123. [DOI] [PubMed] [Google Scholar]
- 120. Angebault C., Guichet P.O., Talmat-Amar Y., Charif M., Gerber S., Fares-Taie L., Gueguen N., Halloy F., Moore D., Amati-Bonneau P.. et al. (2015) Recessive mutations in RTN4IP1 cause isolated and syndromic optic neuropathies. Am. J. Hum. Genet., 97, 754–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Moghadam K.K., Pizza F., La Morgia C., Franceschini C., Tonon C., Lodi R., Barboni P., Seri M., Ferrari S., Liguori R.. et al. (2014) Narcolepsy is a common phenotype in HSAN IE and ADCA-DN. Brain, 137, 1643–1655. [DOI] [PubMed] [Google Scholar]
- 122. Maresca A., Zaffagnini M., Caporali L., Carelli V., Zanna C. (2015) DNA methyltransferase 1 mutations and mitochondrial pathology: is mtDNA methylated? Front. Genet., 6, 90.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Hinton D.R., Sadun A.A., Blanks J.C., Miller C.A. (1986) Optic-nerve degeneration in Alzheimer's disease. N. Engl. J. Med., 315, 485–487. [DOI] [PubMed] [Google Scholar]
- 124. Sadun A.A., Bassi C.J. (1990) Optic nerve damage in Alzheimer's disease. Ophthalmology, 97, 9–17. [DOI] [PubMed] [Google Scholar]
- 125. Coppola G., Di Renzo A., Ziccardi L., Martelli F., Fadda A., Manni G., Barboni P., Pierelli F., Sadun A.A., Parisi V. (2015) Optical coherence tomography in Alzheimer's disease: a meta-analysis. PLoS One, 10, e0134750.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. La Morgia C., Ross-Cisneros F.N., Sadun A.A., Carelli V. (2017) Retinal ganglion cells and circadian rhythms in Alzheimer's disease, Parkinson's disease, and beyond. Front. Neurol., 8, 162.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Quigley H.A., Dunkelberger G.R., Green W.R. (1988) Chronic human glaucoma causing selectively greater loss of large optic nerve fibers. Ophthalmology, 95, 357–363. [DOI] [PubMed] [Google Scholar]
- 128. Mendoza-Santiesteban C.E., Palma J.A., Martinez J., Norcliffe-Kaufmann L., Hedges T.R. 3rd, Kaufmann H. (2015) Progressive retinal structure abnormalities in multiple system atrophy. Mov. Disord., 30, 1944–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Gracitelli C.P., Duque-Chica G.L., Roizenblatt M., Moura A.L., Nagy B.V., Ragot de Melo G., Borba P.D., Teixeira S.H., Tufik S., Ventura D.F., Paranhos A. Jr., (2015) Intrinsically photosensitive retinal ganglion cell activity is associated with decreased sleep quality in patients with glaucoma. Ophthalmology, 122, 1139–1148. [DOI] [PubMed] [Google Scholar]
- 130. Obara E.A., Hannibal J., Heegaard S., Fahrenkrug J. (2016) Loss of melanopsin-expressing retinal ganglion cells in severely staged glaucoma patients. Invest. Ophthalmol. Vis. Sci., 57, 4661–4667. [DOI] [PubMed] [Google Scholar]
- 131. La Morgia C., Barboni P., Rizzo G., Carbonelli M., Savini G., Scaglione C., Capellari S., Bonazza S., Giannoccaro M.P., Calandra-Buonaura G.. et al. (2013) Loss of temporal retinal nerve fibers in Parkinson disease: a mitochondrial pattern? Eur. J. Neurol., 20, 198–201. [DOI] [PubMed] [Google Scholar]
- 132. Kersten H.M., Danesh-Meyer H.V., Kilfoyle D.H., Roxburgh R.H. (2015) Optical coherence tomography findings in Huntington's disease: a potential biomarker of disease progression. J. Neurol., 262, 2457–2465. [DOI] [PubMed] [Google Scholar]
- 133. Song W., Chen J., Petrilli A., Liot G., Klinglmayr E., Zhou Y., Poquiz P., Tjong J., Pouladi M.A., Hayden M.R.. et al. (2011) Mutant huntingtin binds the mitochondrial fission GTPase dynamin-related protein-1 and increases its enzymatic activity. Nat. Med., 17, 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Guedes-Dias P., Pinho B.R., Soares T.R., de Proença J., Duchen M.R., Oliveira J.M. (2016) Mitochondrial dynamics and quality control in Huntington's disease. Neurobiol. Dis., 90, 51–57. [DOI] [PubMed] [Google Scholar]
- 135. Hart N.J., Koronyo Y., Black K.L., Koronyo-Hamaoui M. (2016) Ocular indicators of Alzheimer's: exploring disease in the retina. Acta Neuropathol., 132, 767–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Cordeiro M.F., Normando E.M., Cardoso M.J., Miodragovic S., Jeylani S., Davis B.M., Guo L., Ourselin S., A'Hern R., Bloom P.A. (2017) Real-time imaging of single neuronal cell apoptosis in patients with glaucoma. Brain, 140, 1757–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Shi Y., Inoue H., Wu J.C., Yamanaka S. (2017) Induced pluripotent stem cell technology: a decade of progress. Nat. Rev. Drug Discov., 16, 115–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Dutta D., Heo I., Clevers H. (2017) Disease modeling in stem cell-derived 3D organoid systems. Trends Mol. Med., 23, 393–410. [DOI] [PubMed] [Google Scholar]
- 139. Bredenoord A.L., Clevers H., Knoblich J.A. (2017) Human tissues in a dish: The research and ethical implications of organoid technology. Science, 355, pii:eaaf9414. [DOI] [PubMed] [Google Scholar]
- 140. Laha B., Stafford B.K., Huberman A.D. (2017) Regenerating optic pathways from the eye to the brain. Science, 356, 1031–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]