Introduction
Immune-based therapy is an innovative approach that enhances the human immune response to cancer cells, resulting in elimination of susceptible cancer cells and inhibit tumor growth. This approach has offered new promise in cancer treatment, and can result in prolonged remission of tumors in some patients. The immune system is remarkably equipped to recognize cancer neoantigens as foreign and can develop anti-tumor responses with the potential to eliminate the tumor. The cross-talk between different immune cells in mounting an immune response against the tumor neoantigens is critical to tumor elimination. However, some tumors possess the uncanny ability to produce inhibitory ligands, which engage co-inhibitory receptors, also referred to as immune checkpoints, expressed on tumor-specific immune cells. This engagement induces negative regulatory pathways that limit normal immune responses thus allowing the tumor to evade or dampen the immune response. The most recent immunotherapeutic approaches are aimed at targeting these mechanisms of immune tolerance by blocking immune checkpoints in order to reverse the functionally suppressed immune response, reinvigorate T cells and promote antitumor immunity.
Ipilimumab (anti-CTLA-4 antibody) was the first treatment targeting an immune checkpoint on T cells that could induce durable responses in patients with metastatic melanoma, and was approved by the FDA in 2011 for the treatment of metastatic melanoma. Emerging data, however, indicate that only about 20–30% of ipilimumab-treated patients achieve long-term survival with toxicities occurring in a sizeable population of patients. Initial data from clinical trials with anti-PD-1 therapies (pembrolizumab) suggested that objective anti-tumor response rates may be higher with these agents compared to ipilimumab in metastatic melanoma, but the safety profiles may only be marginally better. Pembrolizumab has received additional FDA approval for treatment of non-small cell lung cancer (NSCLC) and head and neck squamous cell cancer (HNSCC). Nivolumab was the second anti-PD-1 mAb approved by the FDA and has shown efficacy in targeting multiple cancer types including non-small-cell lung cancer (NSCLC), melanoma, and renal cell carcinoma (RCC), with reports of rapid and durable tumor regression in some patients (1). At present, more attempts are being made to design rational combinations of immunotherapeutics that target discreet pathways to achieve synergistic effects in inhibiting tumor growth. A recent study showed that the combination of nivolumab and ipilimumab resulted in a significantly higher objective response rate and significantly longer progression-free survival than ipilimumab alone among previously untreated patients with advanced melanoma (2). However the toxicity issues and adverse autoimmune side effects have to be borne in mind before employing this combination therapeutic regimen. In addition, only about 50% of melanoma patients responded to the combination therapy of anti-PD-1 and anti-CTLA4 with significant side effects related to autoimmune tissue inflammation including colitis and hypophysitis. Furthermore, some tumors, such as colorectal carcinomas, do not respond to either of the checkpoint blockade therapies, raising the issue of whether such tumors might use other checkpoint receptors for inducing T cell dysfunction and inhibiting anti-tumor immunity. In addition to the expression of CTLA-4 and PD-1, tumor infiltrating lymphocytes express a number of other co-inhibitory receptors including Tim-3, Lag-3 and TIGIT, providing additional targets that could be exploited for inducing anti-tumor immune responses. In this review, we will focus on consideration of Tim-3 as a potential target for immunomodulation in cancer immunotherapy.
Tim-3 regulates Th1 and CTL responses
T cell immunoglobulin and mucin-domain containing-3 (Tim-3) is a type I trans-membrane protein that was originally discovered in an effort to identify novel cell surface molecules that would mark IFN-γ-producing Th1 and Tc1 cells (3). Identification of Tim-3 also led to the discovery of the Tim family of genes. The Tim-3 locus, together with other Tim family genes Tim-1 and Tim-4, is located at 11B1.1 in the mouse genome and at 5q33.2 in the human genome, a region that also contains the IL-4 gene cluster that has been linked to both autoimmune and allergic diseases (4). Positional cloning using congenic Balb/c-HBA mice identified significant polymorphisms in both Tim-1 and Tim-3 loci, but not the IL-4 gene cluster, that are associated with airway hyperresponsiveness, supporting a role for the Tim family of molecules in Th1 and Th2 differentiation and induction of autoimmune and allergic diseases (5).
Tim-3 plays a key role in inhibiting Th1 responses and the expression of cytokines such as TNF and INF-γ. Dysregulation of Tim-3 expression has been associated with autoimmune diseases. Early studies on Tim-3 pointed out its inhibitory function in suppressing effector Th1 responses in the mouse autoimmune disease models of multiple sclerosis (EAE) and type 1 diabetes. Blockade of Tim-3 signaling by anti-Tim-3 antibody exacerbated disease progression in EAE (3, 6, 7) and administration of a soluble form of Tim-3, Tim3.Fc, inhibited development of peripheral tolerance with an increase in the production of IFN-γ and TNF from responding T cells. In subsequent human studies, increased IFN-γ production was correlated with decreased Tim-3 expression on T cell clones generated from the cerebrospinal fluid of patients with multiple sclerosis (MS), indicating that compromised T cell tolerance was associated with dysregulated Tim-3 expression (8). T cells from MS patients treated with glatiramer acetate or IFN-β restored Tim-3 expression and restored the inhibitory effects of Tim-3 (9). Similar to the results from MS patients, in vitro activated Th1 cells derived from the intestinal mucosa and peripheral blood from patients with Crohn’s disease (CD) exhibited lower Tim-3 expression compared with the cells from healthy individuals (10). In patients with rheumatoid arthritis (RA), Tim-3 expression on CD4+ and CD8+ T cells in peripheral blood mononuclear cells (PBMCs) and synovial fluid was increased. But the percentage of Tim-3 expression on peripheral CD4+ and CD8+ T cells was negatively correlated with disease severity, as measured by the disease activity score 28 (DAS28) of the patients (11, 12). In addition, the level of Tim-3 expression on T cells was also inversely correlated with plasma TNF levels. Importantly, Tim-3 expression was increased in patients with disease remission after methotrexate or tocilizumab treatment (11). Collectively, the Tim-3 expression level on T cells is negatively correlated with disease progression in autoimmune diseases.
In contrast, high levels of Tim-3 expression correlate with suppression of T cell responses and T cell dysfunction, also referred to as T cell exhaustion, a process of gradual loss of T cell function in an hierarchical manner during chronic viral infections and tumor development (13). A role for Tim-3 in T cell exhaustion was identified first in patients with HIV-1 infection (14), and Tim-3 has been implicated as a checkpoint receptor that controls T cell immunity against a variety of chronic viral infections such as HBV, HCV and Friend virus (15–17). Tim-3 also serves as a checkpoint in tumor immunity, by regulating T cell exhaustion in tumor infiltrating lymphocytes (TILs) from both human and mouse tumors (18, 19). Tim-3 expression on CD8+ TILs is closely associated with PD-1 expression. Tim-3+PD-1+ CD8+ T cells represent a ‘deeply’ exhausted T cell population as compared to PD-1+ single positive CD8+ T cells (19). High levels of Tim-3 expression on CD8+ T cells have been associated with a poor prognosis for tumor progression (20) (21). In addition to effector T cells, Tim-3 has been shown to be expressed on FoxP3+ Tregs and has been shown to enhance the regulatory function of FoxP3+ Tregs (22, 23).
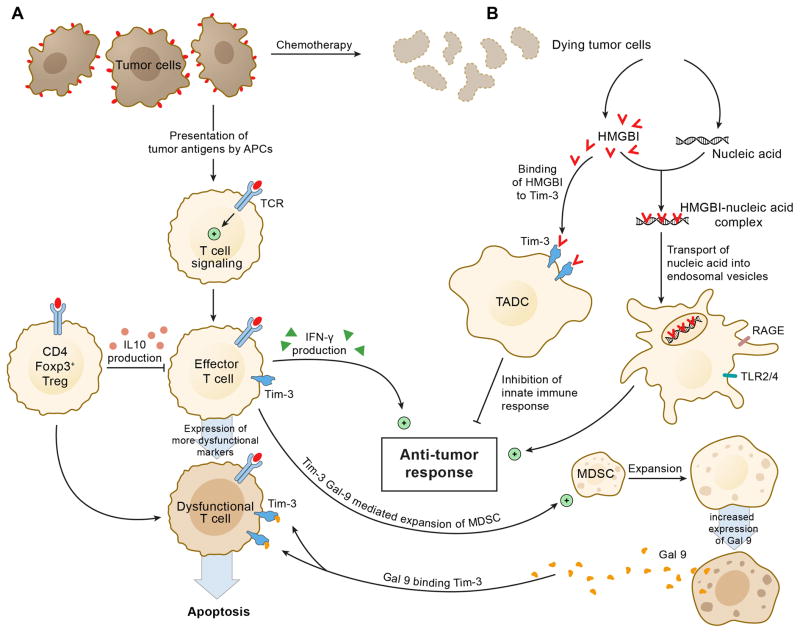
Studies of Tim-3 have been further extended to other immune cells including NK/NKT cells, dendritic cells (DCs), and macrophages. The inhibitory function of Tim-3 in non-T cell populations in the immune system has been found to be consistent with what has been characterized in T cells (Figure 1). This is demonstrated by the expression of Tim-3 on tumor-infiltrating DCs having a critical role in suppressing innate anti-tumor immune responses through the recognition of tumor-derived nucleic acids (24). Thus, Tim-3 emerges as a negative regulatory checkpoint associated with both T cell exhaustion and suppression of innate immune responses.
Figure 1.
Tim-3 serves as a major regulator of immunity in the lymphoid (A) and myeloid (B) compartments. In DCs (dendritic cells), HMGB1 plays a critical role in the transport of nucleic acids into enodosomal vesicles, which is a key step for DCs to sense tumor-derived stress factors or pathogen-associated molecular patterns (PAMPs) and to generate protective immune responses to tumors. In tumor microenvironments, the Tim-3 molecules expressed on the tumor-infiltrating DCs (TADC), bind to HMGB1 to block the transport of nucleic acids into endosomes, thereby suppressing pattern-recognition receptor (PPR)-mediated innate immune responses to tumor-derived nucleic acids.
The Interferon-γ production by effector T cells promotes anti-tumor response but also drives expansion of myeloid-derived suppressor cells. The latter produce increased Galectin-9 (Gal9) molecules, which then bind to Tim-3 molecules expressed on Tim-3 expressing effector CD8+ T cells in the tumor microenvironment, leading to apoptosis of effector T cells. Further, Tim-3+FoxP3+ Tregs present within the tumor express high amounts of Treg effector molecules (IL-10, etc.) and inhibit effector T cells.
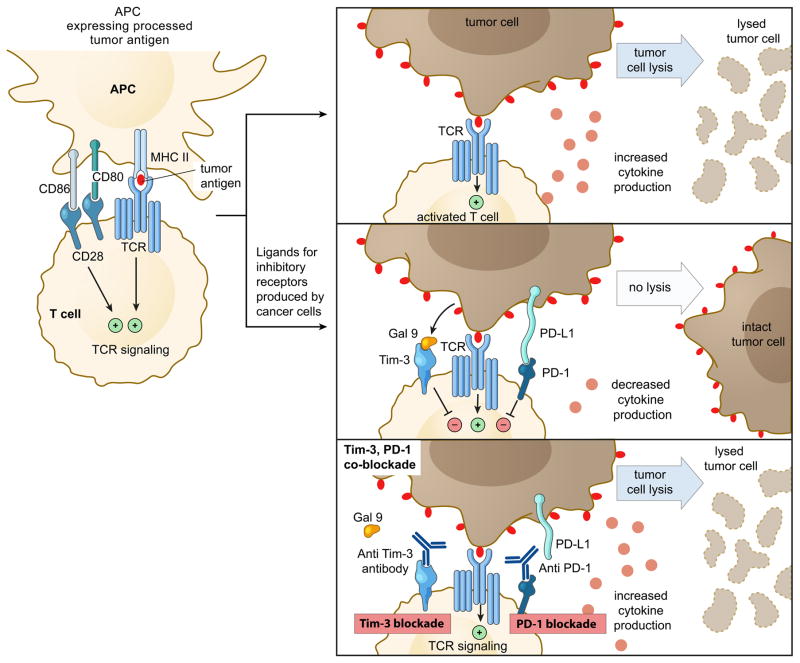
Consistent with a key regulatory role in both innate and adaptive immune responses, blockade of Tim-3 signaling in preclinical tumor models exhibited therapeutic benefit as cancer immunotherapy. Combination therapies based on blockade of Tim-3 and other inhibitory molecules, such as PD-1 exhibited synergistic effects in restoring anti-tumor immunity in preclinical animal models (Figure 2) (19, 25, 26). However, Tim-3 likely plays a more complex role in regulating anti-tumor responses, as some of the data indicate that Tim-3 can function as a co-stimulatory receptor to enhance CTL and other immune cell responses under certain in vivo and in vitro settings (27–30). However, it is not yet clear under what circumstances Tim-3 exhibits inhibitory effects or shows co-stimulatory effects. Whether its inhibitory effects are dependent on the co-expression of other molecules like Ceacam-1 or ligand dependent cross-linking of Tim-3 needs to be further elucidated.
Figure 2.
Expression of ligands for PD-1 and Tim-3 by tumor cells leads to the ligation of PDL1 with PD1 (programmed cell death protein 1) and galectin9 with Tim-3 molecules causing down-regulation of T cell function, essentially creating a negative feedback loop that dampens anti-tumor immunity. Co-blockade of both PD-1 and Tim-3 leads to synergistic efficacy and restoration of T cell effector function and tumor cell killing.
Tim-3 protein Structure
Tim-3 is expressed by multiple murine and human immune cell types. Mouse Tim-3 consists of 281 amino acid residues, while its human homologue consists of 302 amino acid residues with 63% identity with mouse Tim-3 (3). Tim-3 belongs to the immunoglobulin superfamily (IgSF), with an extracellular domain consisting of a membrane-distal N-terminal Immunoglobulin (IgV) domain, followed by a membrane-proximal mucin domain containing potential sites of O-linked glycosylation. The stalk domain lying between the mucin and transmembrane domain has sites for N-linked sugars, which is followed by a transmembrane domain and a cytoplasmic tail (4).
The Tim-3 gene consists of 7 exons encoding its full-length protein sequence. An alternatively spliced form that lacks exons 3 to 5 was identified in concanavalin A-activated mouse spleen cells. This short Tim-3 variant lacks the entire coding sequence for the mucin and transmembrane domains (31). Since the alternatively spliced variant does not encode the hydrophobic transmembrane domain, its translational product is likely a soluble form that can be secreted outside of cells. However, this spliced variant only exists in mouse, and no human homolog has been identified as yet.
In humans, a soluble form of Tim-3 can be released by a disintegrin and metalloprotease (ADAM)-mediated ectodomain shedding. ADAM 10 and ADAM17 are 2 major sheddases that specifically cut Glu181-Asp190 of the stalk region of Tim-3 (32). Interestingly, Tim-3 cannot be efficiently cleaved in the absence of the intracellular domain indicating that the ectodomain shedding maybe controlled by cell signaling events. Shedding of the Tim-3 ectodomain is found in human CD14+ monocytes after LPS stimulation and Tim-3-expressing T cells in patients with graft-versus-host disease (GVHD) after allogeneic hematopoietic cell transplantation. Such a process abrogates Tim-3-mediated signal transduction (32, 33). However, its biological significance is not fully understood. Down-regulation of Tim-3 signaling in the cell may be only one outcome of enzymatic digestion, as the released intact ectodomain of Tim-3 may retain signaling function.
The crystal structure of the IgV domain of Tim-3 shows the presence of two anti-parallel β sheets, which are tethered by a disulphide bond. Two additional disulfide bonds formed by four non-canonical cysteines stabilize the IgV domain and reorient a CC′ loop towards a FG loop thereby forming a “cleft” structure that is thought to be involved in ligand binding, and is not found in other IgSF members. Instead, this “cleft” assembly is the signature structure that is identified in all Tim family proteins including Tim-1 and Tim-4 (34–36). The engagement of the IgV domain by appropriate ligands has been found to be important for the immune-modulatory role of Tim-3, and instrumental for induction of peripheral tolerance and suppression of anti-tumor immunity (6, 24, 25).
The Tim-3 cytoplasmic tail adjacent to the trans-membrane domain is devoid of ITIMs or ITSMs, the classical inhibitory and switch motifs found in other inhibitory receptors, but contains a conserved region of five tyrosine residues, two of which (Tyr 256, 263 in mouse and 265, 272 in human) have been found to be critically important for coupling to downstream signaling pathways. The peptide sequences surrounding these two tyrosine residues are highly conserved between human and mouse and function as SH2 domain-binding motifs, where multiple SH2 domain-containing kinases including Fyn, Lck, PI3K p85, and Itk are found to bind (27, 37). Many of these molecules are key components of the T cell receptor (TCR) signaling pathway, indicating a functional relationship between Tim-3 and the TCR pathway. Importantly, the SH2 domain-binding motif is also a trans-regulatory site for controlling Tim-3 signal transduction (38)
Tim-3 ligands
Galectin-9
Initial efforts to characterize Tim-3 ligands by biochemical approaches identified two extracellular membrane-associated proteins with molecular weights around 35 kD and 60 kD binding to the Tim-3 IgV domain. The 35 kD candidate was characterized biochemically as C-type lectin galectin-9 (6). Galectin-9, a soluble protein containing two tandemly linked carbohydrate recognition domains, specifically recognizes the structure of N-linked sugar chains in the Tim-3 IgV domain, but not those in other Tim proteins such as Tim-2 and Tim-4. The interaction between the Tim-3 IgV domain and galectin 9 was the highest affinity as compared with interactions of the Tim-3 IgV domain and other galectins including galectin-1, galectin-3, and galectin-4, suggesting a relatively specific binding interaction between galectin-9 and Tim-3. Interaction between galectin-9 and Tim-3 triggers cell death in effector Th1 cells thereby dampening tissue inflammation and inhibition of autoimmune disease EAE (6). Similarly, galectin-9 also induces cell death in Tim-3+CD8+ TIL in colon cancer (39). However, not all galectin-9-Tim-3 interactions result in cell death, as galectin-9 was found to increase Tim-3-mediated IFN-γ production in an NK cell line (29). In human THP-1 myeloid cells, galectin-9 activated Tim-3-mediated PI3K-mTOR signaling without inducing cell death (40).
The interaction of galectin-9 and Tim-3 on monocytes/macrophages may result in alterations in cytokine production that affect the Th1 or Th17 response by alterations in IL-12 and IL-23 production (41–43).
The Tim-3-galectin-9 interaction can also transduce reverse signaling. Studies in a mouse tuberculosis (TB) model demonstrated that Tim-3 is critical for induction of IL-1β and for enhancing anti-mycobacterial activity in macrophages via a galectin-9 dependent mechanism in both mouse and human cells (44, 45). Fewer CFU were recovered when Mycobacterium tuberculosis (Mtb)-infected macrophages were treated with Tim-3-Ig fusion protein. Interestingly, Tim-3-Ig treatment controlled Mtb replication equally well in WT and Tim-3−/− macrophages, but the Tim-3-Ig anti-Mtb effect was abrogated in galectin-9−/− macrophages. Thus, endogenous Tim-3 expression on macrophages was not required for anti-Mtb activity, whereas the trans-interaction between Tim-3-Ig and galectin-9 on macrophages was critical in controlling Mtb replication inside the macrophages. In addition, Tim-3 T cell-transgenic (tg) CD4+ T cells but not Tim-3−/− CD4+ T cells controlled Mtb replication in galectin-9-expressing macrophages, further confirming that Tim-3-galectin-9 trans-interaction-mediated reverse signaling is critical for anti-Mtb activity in macrophages. This reverse signaling pathway plays an important role in controlling Mtb growth in HIV-infected individuals who have increased expression of Tim-3 on T cells (45). Collectively, the Tim-3-galectin-9 reverse signaling indicates a crosstalk between effector T cells and macrophages that must have evolved to control intracellular pathogens by Th1 and Tc1 cells in infected macrophages so as to clear infection. As IFN-γ is critical for the induction of galectin-9 expression, this suggests a mechanism by which IFN-γ induced galectin-9 may promote clearance of intracellular pathogens from macrophages, while also engaging Tim-3 on T cells to ensure clonal contraction of responding Th1 cells (Figure 1).
Ceacam1
The second Tim-3 ligand candidate with a molecular weight around 60 kD was recently characterized as carcinoembryonic antigen cell adhesion molecule 1 (Ceacam1) (25). The membrane-distal IgV domains of Ceacam1 and Tim-3 share structural similarities, and interact along their FG-CC′ interface, a highly conserved structure that was predicted as a ligand binding site (25, 34). The co-expression of Ceacam1 is required for Tim-3 glycosylation and protein stability, and the inhibitory function of Tim-3 is compromised in the absence of Ceacam1 expression. This dependence of Tim-3 function on Ceacam1 co-expression is based on the cis-interaction between these two proteins. In addition, a Ceacam1-Tim-3 trans-interaction suppresses effector T cell function and is required for maintaining T cell tolerance. Galectin-9 and Ceacam1 bind to different regions in the IgV domain of Tim-3 (25, 34) and both Ceacam1-Tim-3 and galectin-9-Tim-3 interactions result in similar downstream events, in which Bat3, an inhibitory regulator of the Tim-3 signaling pathway, is released from its binding site on the Tim-3 cytoplasmic tail (25, 38). Thus these two ligands might have cooperative effects in regulating Tim-3 signaling.
HMGB1
Chiba and colleagues recently identified high-mobility group box 1 (HMGB1) as another Tim-3 ligand. HMGB1 is a damage-associated molecular pattern (DAMP) protein that senses endogenous danger signals. HMGB1 can be actively released from activated DCs to promote T cell and B cell responses (46). In DCs, HMGB1 plays a critical role in the transport of nucleic acids into enodosomal vesicles, which is a key step for DCs to sense tumor-derived stress factors or pathogen-associated molecular patterns (PAMPs) and to generate protective immune responses to tumors or pathogen infections. In tumor microenvironments, the tumor-infiltrating DCs express higher levels of Tim-3 than DCs in normal tissues. Tim-3 binds to HMGB1 to block the transport of nucleic acids into endosomes, thereby suppressing pattern-recognition receptor (PPR)-mediated innate immune responses to tumor-derived nucleic acids (Figure 1) (24). Thus, blockade of Tim-3 mediated suppression of the nucleic acid-sensing system could potentially enhance DNA vaccine development and cytotoxic chemotherapy. Interestingly, the HMGB1 binding epitope on Tim-3 is largely overlapping with Ceacam1 binding epitopes at the FG-CC′ loop region in the IgV domain of Tim-3. Q62 (E62 for human) in the FG-CC loop is the essential amino acid residue for the interaction to both HMGB1 and Ceacam1 (24, 25), raising a question of potential competitive binding to Tim-3 between HMGB1 and Ceacam1. Whether this indicates a functional redundancy between HMGB1 and Ceacam1-mediated Tim-3 signaling, or it represents a cell type-specific ligand-receptor signaling is currently unknown.
A study by Dolina et al reported that liver-primed Tim-3+CD8+ T cells during adenovirus infection exhibited suppressive “Treg” function. Tim-3 on these suppressive CD8+ T cells acts as “sink” to sequester HMGB1, preventing HMGB1 from activating hepatic CD8+ T cells (47). Given the fact that HMGB1 has multiple high affinity-binding partners, such as IL-1β, LPS, DNA, nucleosomes and multiple receptors, such as TLR2, TLR4, CXCR4, the precise role of Tim-3 in acting as a specific neutralizer to prevent HMGB1-mediated T cell activation and to dampen hepatic T cell responses is not clear.
PtdSer
Unlike all other Tim-3 ligands, phosphatidylserine (PtdSer) is a non-protein ligand for Tim-3 (48, 49). PtsSer binds to the FG-CC′ loops of the Tim-3 IgV domain. Since the cleft formed by the FG-CC′ loop is the signature structure shared by all of the Tim proteins, PtdSer is also a ligand for other Tim family proteins. (35, 36, 49). The binding of PtdSer to Tim proteins has been implicated in apoptotic cell uptake (34–36). Mouse Tim-3 can exist as allelic variants and these polymorphic variants can differ functionally in their recognition of PtdSer and clearance of apoptotic cells (49). The biological relevance of the Tim-3-PtdSer interaction in T cells is unknown, as T cells do not play a role in clearing apoptotic bodies. However, interaction of Tim-3 with PtdSer may result in the induction of IL-10 in T cells, since IL-10 has been shown to co-expressed with T cells that express Tim-3.
Due to the overlapping binding sites at the FG-CC′ loop in the Tim-3 IgV domain, it is important to understand how Ceacam1, HMGB1, and PtdSer coordinate their interactions with Tim-3 and regulate the function of specific types of immune cells.
Tim-3 signaling
Tim-3 has been found to play a role as a checkpoint receptor on T cells. This has been evidenced by the fact that Tim-3 has been found to be a negative regulator of IFN-γ-secreting CD4+ Th1, CD8+ T cells and an important player in T cell exhaustion in multiple settings. These findings were further supported in vivo by showing that blocking Tim-3 could reverse the dysfunctional phenotype in T cells. This underscores the importance of mechanistic insights into Tim-3 signaling pathways and their coupling to TCR signaling to pave the way for understanding Tim-3-mediated inhibition in the disease context and also for advancing rational targeting of Tim-3 for immunotherapy. Though several studies aimed at understanding Tim-3 signaling pathways have been carried out, many aspects of Tim-3-interactions with its intracellular partners that mediate inhibitory function remain largely unexplored. Studies aimed at establishing links between Tim-3 and intracellular signaling pathways are somewhat conflicting, with some suggesting an inhibitory role for Tim-3 and others suggesting a stimulatory role. As discussed above, Tim-3 does not have a classical ITIM (immunoreceptor tyrosine-based inhibition) or an ITSM (immunoreceptor tyrosine-based switch) motif in its cytoplasmic tail and apparently has no structural provision for recruitment of inhibitory phosphatases, which may be associated with inactivation of phosphorylated proteins involved in proximal T cell signaling pathways. Rather, the cytoplasmic tails of both mouse and human Tim-3 contain five conserved tyrosine residues, phosphorylation of two of which, Y256 and Y263 in mouse (Y265, 272 in human) have been shown to be critically important for coupling to downstream signaling pathways. An early study by Anderson et al. (50) showed that treatment of cloned Th1 cells or the DC line D2SC1 with anti-Tim-3 antibodies could induce tyrosine phosphorylation of several proteins and activation of Erk and degradation of IκB. In the case of the D2SC1 cells, these pro-stimulatory effects correlated with increased TNF secretion. Much in the same light, a study in Jurkat cells demonstrated that ectopic expression of Tim-3 on Jurkat T cells followed by T cell receptor and CD28 engagement leads to enhancement of Src family kinase Fyn or Lck-mediated phosphorylation of Y256 and Y263 residues of Tim-3. The phosphorylation of Y256 and Y263 enables the recruitment of one or more SH2 domain-containing proteins, including the p85 of PI3K and PLC-γ1 to the phosphorylated Tim-3 tail. The interaction of Tim-3 with components of the TCR signaling pathway, including Zap 70 and SLP-76 further augments NFAT and NF-κB activation, leading to increased T cell signaling (27). Van de Wryer and his colleagues have also demonstrated that ITK-mediated specific phosphorylation of Y265 of Tim-3 occurs in the presence of galectin-9, probably through a receptor-ligand interaction. Phosphorylation of Y265, which is located in a highly conserved SH2 domain binding motif, could result in the recruitment of SH2 domain-containing proteins and trigger downstream signaling events, which may include negative regulators of cytokine signaling, such as members of the suppressor of cytokine signaling (SOCS) family under conditions in which Tim-3 plays an immunosuppressive role, thereby regulating the function of Tim-3 expressing T-cells (37). However, it should be pointed out that many of these studies have utilized artificial in vitro over-expression systems using cell lines. Whether this is also true in vivo where multiple cell types express Tim-3 cannot be addressed until conditional ‘knock-out” mice are available for in vivo studies.
Additionally, studies by Lee et al demonstrated that Jurkat T cells expressing Tim-3, as well as Tim-3-expressing human primary CD4+ T cells, produce less IL-2 when stimulated with a phorbol ester and a calcium ionophore that mimic TCR stimulation. These effects were attributed to impaired activation of the transcription factor NFAT and reduced expression of the transcription factors c-Fos and c-Jun (51). Another work exemplifying the immune-suppressive role of Tim-3 in human primary CD8+ T cells showed that Tim-3 interacts with multiple signaling molecules at the immunological synapse between CD8 T cells and target cells and further co-localizes with phosphatases that can suppress T cell receptor (TCR) signaling (52). Taken together, these data support the conclusion that the engagement of Tim-3 may facilitate its interaction with multiple partners, thereby promoting cross-talk with immune cell signaling components leading to either a state of immune activation or immunosuppression mediated by its interaction with molecules that activate or suppress immune-mediated signaling.
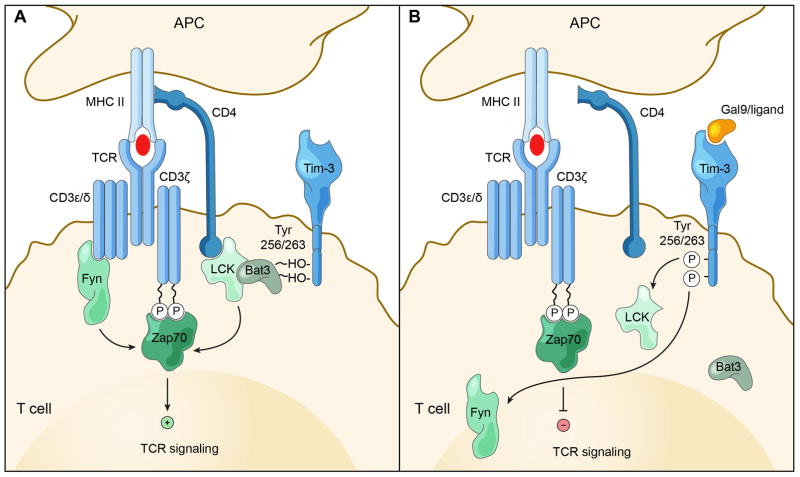
In contrast to the above findings by Anderson et al and Lee et al which established a pro-stimulatory role for Tim-3, Zhu et al. demonstrated that the Tim-3 ligand galectin-9-induced intracellular calcium flux, aggregation and death of Th1 cells are Tim-3-dependent in vitro. Furthermore, administration of galectin-9 in vivo causes down-regulation of Th1-dependent immune responses with selective loss of interferon-γ-producing cells (6). A later investigation by Rangachari et al illustrated that the Y256 and Y263 are critical for the binding of HLA-B associated transcript 3 (Bat3), to the C-terminal tail of Tim-3 (38). In its unactivated state, Bat3 recruits the catalytically active form of Lck, thereby forming an intracellular molecular complex with Tim-3 that preserves and potentially promotes T cell signaling and represses Tim-3–mediated cell death and exhaustion. Interactions between Tim-3 with its ligands, galectin-9 and Ceacam-1, results in phosphorylation of Y256 and Y263 and release of Bat-3 from the Tim-3 tail, thereby promoting Tim-3-mediated T cell inhibitory function by allowing binding of SH2 domain-containing Src kinases and subsequent regulation of TCR signaling (Figure 3). Because Fyn and Bat3 bind to the same domain in the Tim-3 cytoplasmic tail, a likely molecular switch between Tim-3-Bat3 and Tim-3-Fyn might trigger the switch of Tim-3 function from being permissive to TCR signaling to inhibition of proximal TCR signaling (38). Thus, this study suggests that Tim-3 function is altered by ligand binding through regulation of Bat3 binding to the cytoplasmic tail of Tim-3. Ligand binding releases Bat3, preventing recruitment of activated LCK and allowing phosphorylation of Y256 and Y263 by Fyn or other kinases, and signaling of Tim-3 to promote Tim-3 mediated T cell inhibition.
Figure 3.
(A) In the absence of ligand-mediated Tim-3 signaling the Tyr 256 and Tyr 263 in the C-terminal tail of Tim-3 bind to Bat3. In this state, Bat3 recruits the catalytically active form of Lck, thereby forming an intracellular molecular complex with Tim-3 that preserves and potentially promotes T cell signaling and represses Tim-3–mediated cell death and exhaustion.
(B) Interactions between Tim-3 ligands (Galectin 9), and Tim-3 leads to phosphorylation of Tyr 256 and Tyr 263 and release of Bat-3 from the Tim-3 tail, thereby promoting Tim-3-mediated T cell inhibition by allowing binding of SH2 domain-containing Src kinases (Lck/Fyn/Other proteins) and subsequent regulation of TCR signaling. Because Fyn and Bat3 bind to the same domain in the Tim-3 cytoplasmic tail, a likely molecular switch between Tim-3-Bat3 and Tim-3-Fyn probably triggers the switch of Tim-3 function from being permissive to TCR signaling to inhibition of proximal TCR signaling.
Saresella et al analyzed the role of Tim-3 in MS patients with a diagnosis of stable relapsing-remitting MS (RRMS), primary progressive MS (PPMS), benign MS (BEMS), and healthy control (HC) subjects. High levels of Tim-3 and galectin-9 expression on CD4+ T cells were found in the patients with BEMS. In contrast, increased Bat3 and decreased Tim-3 expression on T cells were found in the patients with PPMS. Blockade of the galectin-9-Tim-3 interaction reversed Tim-3-mediated suppression in vitro, including reduced T cell apoptosis and increased proinflammatory cytokine production, in the BEMS, RRMS, and HC groups, but not in the PPMS group where Bat3 expression is high. In addition, the galection9-Tim-3 interaction favored apoptosis of MBP-specific T cells in BEMS, but not in PPMS (53). This study supports the role of Bat3 as another layer of regulation in Tim-3 signaling. Increased Bat3 expression blocks Tim-3-mediated inhibitory signaling and enhances effector T cell function. By contrast, reduced Bat3 expression would shift the balance to stronger Tim-3-mediated inhibitory signaling. Indeed, significantly reduced Bat3 expression was detected in exhausted Tim-3+PD-1+ TILs, but not Tim-3-PD-1+ TILs, isolated from mice implanted with CT26 colorectal carcinomas. Importantly, adoptive transfer of myelin antigen specific T cell receptor (TCR) tg (2D2) Th1 cells in which Bat3 expression was inhibited resulted in a reduced severity and incidence of EAE as compared with intact 2D2 Th1 cells. In fact, these Bat3-deficient 2D2 Th1 cells developed an exhaustion phenotype with increased Tim-3 and IL-10 but decreased IFN-γ and TNF expression even under the autoimmune disease setting (38). These results strongly suggest a key role for Bat3 in the regulation of Tim-3 signaling. Bat3 deficiency in Th1 and CD8 T cells permits the inhibitory function of Tim-3 and drives the autoreactive T cells into a stage of functional exhaustion.
The outcome of ligand binding to Tim-3 depends on the Tim-3 ligands involved, the cellular context, including the level of Bat3, and the biological state of the cell either under acute or chronic conditions. In this context it has been suggested that the Tim-3-mediated pro-stimulatory effects may be indirect, acting through the effects on APCs. Tim-3 is constitutively expressed by some monocytic cells, including dendritic cells (DCs) and microglia, and ligation of Tim- 3 on these cells can increase their expression of costimulatory receptors and cytokines (50). Furthermore, Tim-3-mediated chronic stimulation or over-activation of T cells observed under certain conditions discussed above may push the cells to immune dysfunction or exhaustion.
Tim-3 expression in the immune system and its functional indications
Tim-3 and effector T cells
The expression of Tim-3 was originally found to be associated with IFN-γ-producing CD4+ and CD8+ T cells (3). Transient Tim-3 expression is also detected on activated T cells both in vitro and in vivo (6, 54), but persistent expression is only observed following chronic stimulation (54). Because Th1 cells usually exhibit stabilized, high-level expression of Tim-3 after several rounds of in vitro polarization, Tim-3 is also defined as a marker for terminally differentiated effector Th1 cells (3, 54). Similarly, during infection by Listeria monocytogenes, Tim-3 expression is preferentially associated with KLRG1+CD127− effector precursor but not KLRG1−CD127+ memory precursor CD8+ T cells, supporting role of Tim-3 in marking effector T cells. Indeed, Tim-3 deficient mice display compromised primary and secondary CD8+ T cell responses (28). In addition, active TB infection in humans is associated with the up-regulation of Tim-3 on both CD4+ and CD8+ T cells and these Tim-3+ T cells display more robust anti-Mtb responses (55, 56).
The major function of Tim-3 is an inhibitory receptor that suppresses CTL and effector Th1 cell function. In agreement with this hypothesis, expression of Tim-3 on Th1 cells was found to have an important role in regulating T cell tolerance. Loss of Tim-3 abrogates induction of high-dose tolerance (31). Importantly, Tim-3 is one of the signature genes whose expression is induced by autoantigen myelin basic protein (MBP) N-terminal peptide in a dose-dependent manner during peripheral tolerance induction to protect animals from EAE induction (57). Therefore, Tim-3 activation could serve as a mediator of antigen-specific immunotherapy in the treatment of autoimmune diseases. The expression of Tim-3 on alloreactive CD4+ and CD8+ T cells helps to suppress alloimmune responses and to promote immunological tolerance in different models of transplantation (54, 58, 59). Up-regulation of Tim-3 has been observed on decidual CD8+ T cells where it has been suggested to play a key role in mediating maternal-fetal immune tolerance. The authors suggest that co-expression of Tim-3 and PD-1 on CD8+ T cells marks an intermediate memory like cells that exhibits high proliferation and Th2 type cytokine production and functions to maintain normal pregnancy. Blockade of Tim-3 and/or PD-1 function using antibodies increases the risk of miscarriage (60).
One of the most significant findings in Tim-3 biology was that a highly exhausted subset of T cells from patients with chronic HIV infection expressed high levels of Tim-3 (14). Sakuishi et al made a similar observation subsequently on TILs in their study on animal tumor models (19). Importantly, the expression of Tim-3 on TILs is usually associated with that of PD-1, a prototypic checkpoint inhibitor that marks T cell exhaustion. Studies from both chronic viral infections and cancers suggest that antigen-specific T cells with Tim-3-PD-1 co-expression are functionally more “exhausted” than those expressing PD-1 alone (19, 61).
Expression of Tim-3 on T cells also plays a critical role in the generation of CD11b+Gr1+ Ly6G+ MDSCs that is required for maintaining the immunosuppressive environment. Tim-3-mediated MDSC generation is dependent on the Tim-3-galectin-9 interaction (62). Both Tim-3 T cell-tg mice and galectin-9 tg mice exhibit increased MDSC population. Importantly, when galectin-9 tg mice were crossed with Tim-3−/− mice, the CD11b+ Gr1+Ly6G+ population in galectin-9 tg x Tim-3−/− mice was restored to a WT level (62). Since tumor-bearing Tim-3 T cell-tg mice have increased CD11b+Gr1+Ly6G+ cells, transgenic Tim-3 expression on TILs seems to have a direct role for MDSC differentiation in the tumor microenvironment to suppress anti-tumor immunity. Indeed, Tim-3 blockade in both monotherapy and combination therapy in syngeneic mouse tumor models results in decreased MDSCs at the tumor sites (62, 63)
Tim-3 and FoxP3+ Treg cells
In addition to its expression on Th1 cells and CD8+ T cells, Tim-3 is also expressed on CD4+FoxP3+ Treg cells. The Tim-3 expression level on FoxP3+ Treg cells is significantly up-regulated, particularly in inflamed tissues, such as on graft-infiltrating FoxP3+ Treg cells in an allograft rejection animal model (22) and on tumor-infiltrating Treg cells in both humans and mice (23, 64–66).
The immunoregulatory function of FoxP3+ Treg cells is critical in restraint of allograft rejection. The expression of Tim-3 marks highly active, apoptotic FoxP3+ Treg cells in tissue allografts. These Tim-3+FoxP3+ Treg cells are derived from proliferating Tim-3− FoxP3+ Treg cells and exhibit an enhanced regulatory function that is critical in maintenance of tolerance to allografts. Tim-3+FoxP3+ Treg cells are functionally superior to Tim-3−FoxP3+ Treg cells in in vitro suppression assays, and they exhibit more robust IL-10, CD39, CD73, and TGF-β expression, and also express high levels of other checkpoint receptors including CTLA-4, Lag3, and PD-1. Collectively, Tim-3 marks a population of FoxP3+ Treg cells with enhanced suppressor function (22, 67). In vivo Tim-3 blockade during transplantation abrogated donor-derived FoxP3+ Treg inhibition and accelerated graft rejection (68). In tumor settings, Tim-3+ Tregs also represent T cells with the most potent regulatory function. Whereas Tim-3 is rarely found on Tregs in the peripheral immune compartment, the vast majority of tumor infiltrating FoxP3+ Tregs express Tim-3 and comprise a special type of tissue Tregs. Tim-3+ FoxP3+ Treg appear in the tumor tissue prior to development of exhaustion in CD8 T cells and depletion of FoxP3+ Tregs at this stage reverses development of T cell exhaustion in effector T cells. These studies suggest that Tim-3+ FoxP3+ Tregs represent specialized tumor resident FoxP3+ Tregs that may have a role in promoting the developing T dysfunction that is frequently observed in tumor infiltrating T cells (23). Additionally, Tim-3 expression on these tumor-associated FoxP3+ Treg cells might also represent a key mechanism that contributes to the more potent suppressive function of CD8+ TILs than their peripheral counterparts (69).
Regulation of Tim-3 expression in T cells
As Tim-3 is preferentially associated with IFN-γ-producing T cells, cytokines that are involved in effector T cell subset differentiation were believed to have important roles in the induction of Tim-3 expression. Although IL-12 clearly drives Tim-3 expression on Th1 cells, T-bet but not STAT4 was found to be critical for its transcriptional regulation. This result suggests that IL-12 signaling could be dispensable for induction of Tim-3 on T cells (70). Indeed, a subsequent study by Zhu and colleagues demonstrated that IL-27 actually transduces a more robust signal than IL-12 to induce Tim-3 expression in both CD4+ and CD8+ T cells (71). IL-27 not only strongly induces T-bet expression, but also is critical for the induction of NFIL3, another key transcription factor that induces Tim-3 expression. Importantly, T-bet and NFIL3 represent two functionally non-redundant transcription factors that are activated by STAT1 and STAT3 respectively downstream of IL-27 signaling (71). As discussed above, Tim-3 expression on autoantigen-specific T cells in MS patients is significantly lower than on cells from healthy individuals (8). IFN-β treatment promotes Tim-3 expression on the T cells from MS patients and Tim-3 expression closely correlates with response to therapy (9). As the therapeutic effect of IFN-β in MS therapy is closely dependent on its induction of IL-27 (72), it is tempting to hypothesize that the induction of Tim-3 and other anti-inflammatory molecules such as IL-10 is a key mechanism by which IL-27 mediates the therapeutic effects of IFN-β. In mice, IFN-β can directly induce Tim-3 expression on Th1 cells (73), and we have observed that IL-27 and IFN-β synergize in inducing Tim-3 and IL-10 from responding CD4+ T cells. Therefore, IFN-β could induce Tim-3 expression directly or indirectly via IL-27. Further, common γ-chain cytokines IL-2, IL-7, IL-15 and IL-21 were reported to induce Tim-3 expression on human peripheral T cells (74). Paradoxically, the lack of common-γ chain cytokine signaling either by reduced cytokine production or by down-regulated expression of their receptors has been associated with T cell exhaustion in chronic viral infections and cancers where elevated Tim-3 expression is observed on exhausted T cells (75–77).
Tim-3 and innate immune cells
In human PBMCs, the highest Tim-3 expression is found on NK cells (78). Specifically, Tim-3 is expressed on all mature CD56dimCD16+ NK cells, and its expression can be induced on immature CD56brightCD16− NK cells after stimulation by IL-12, IL-15, and IL-18, suggesting that Tim-3 is a maturation marker of NK cells (79). In vitro observations suggest that Tim-3 acts as a co-receptor on NK cells to promote IFN-γ production (29, 79). Unlike its role in inducing cell death among Tim-3-expressing Th1 cells, galectin-9 is required for Tim-3-mediated IFN-γ production in NK cells. Anti-Tim-3 antibody blockade or diminished Tim-3 expression correlates with impaired IFN-γ production (29). Paradoxically, NK cells from advanced melanoma patients express high levels of Tim-3 and are functionally exhausted. The up-regulation of Tim-3 in NK cells is associated with a poor prognosis. In vitro anti-Tim-3 antibody treatment actually reverses the exhaustion phenotype in melanoma donor NK cells (80). Similarly, increased Tim-3 expression in peripheral NK cells (CD3−CD56+ and CD3+CD56dim) predicts a poor prognosis and Tim-3 blockade improves NK cell-mediated cytotoxicity in human lung adenocarcinoma. Blockade of Tim-3 on NK cells from patients with lung adenocarcinoma results in increased cytotoxicity and IFN-γ production (81). Taken together, current studies from different cancers suggest that the elevated expression of Tim-3 on NK cells, similar to its expression on CD8+ T cells, suppresses their anti-tumor function and that Tim-3 serves as a key molecule to drive functional exhaustion in NK cells.
Tim-3 is constitutively expressed on DCs and macrophages in both humans and mice. Mouse macrophages in steady state expresses much lower Tim-3 compared to its expression on DCs (24, 50, 82). In human, constitutive expression of Tim-3 on monocyte/macrophages suppresses IL-12 expression (82). Similarly, Tim-3 expression on mouse macrophages is also associated with an inhibitory function in different disease models (83, 84). As we discussed above, the interaction between PtdSer and Tim-3 is critical for apoptotic cell uptake by phagocytic cells. It is likely that PtdSer-Tim-3 signaling is important to maintain immune tolerance by eliminating apoptotic bodies.
Tim-3 expression on bone marrow-derived DCs can be synergistically stimulated by IL-10 and VEGF-A (24). In dendritic cells, Tim-3 inhibits DC activation and maturation by blocking NF-κB signaling via a Btk-c-Src signaling-dependent mechanism (85). As discussed above, Tim-3 can interfere with the ability of cytoplasmic TLRs to sense immunogenic nucleic acids via a HMGB1-dependent mechanism, preventing activation of tumor associated dendritic cells, and thereby suppressing anti-tumor immunity.
Tim-3 and tumor
Tim-3 polymorphism and cancer
In addition to increased Tim-3 expression in immune cells and tumors, genetic polymorphisms in Tim-3 have also been associated with cancers. Multiple polymorphisms in the promoter region (−574G/T, −882C/T, −1516G/T, and −1541C/T) and in the coding region (+4259T/G, amino acid substitution: Arg to Leu) within the Tim-3 gene were identified in a Chinese population (86–88). Subjects carrying the +4259TG genotype had a 2.81-fold increased risk of NSCLC and a significantly shorter survival time as compared with the controls (86). The prevalence of the +4259TG genotype and the +4259G allele were significantly increased in pancreatic cancer cases as compared with controls (87). SNPs located at the promoter region were also associated with cancers.
Tim-3 as a potential prognostic biomarker for cancers
The expression of Tim-3 has been found in tumors, including on cancer cells, tumor infiltrating lymphocytes and endothelial cells. It has been well-documented that the protein and/or mRNA expression of Tim-3 is significantly up-regulated in tumor tissue samples and is associated with poor prognosis of patients with prostate cancer (89), ccRCC (90), colon cancer (91), bladder urothelial carcinoma (92), cervical cancer (93), and gastric cancer (94). The increased Tim-3 expression on tumor antigen-specific CD8+ T cells is also correlated with dysfunction of CD8+ T cell and poor prognosis in HBV-associated hepatocellular carcinoma (20) and prostate cancer patients (21). Similarly, increased Tim-3 expression on NK cells from patients with gastric cancer is associated with tumor progression (95). Collectively, increased expression of Tim-3 in human tumors, particularly on immune cells, could serve as a potential prognostic biomarker for a variety of tumors.
Tim-3 in the regulation of T cells in tumor
Studies carried out in different cancer settings conclusively establish that Tim-3 acts as a negative regulator of anti-tumor immunity due to its association with T cell exhaustion in the tumor. In patients with advanced melanoma, cancer-germline antigen NY-ESO-1–specific Tim-3+PD-1+ CD8+ T cells represent a highly dysfunctional population of tumor-induced T cells and approximately 30% of NYESO-1–specific CD8+ T cells express Tim-3 (18). Similarly, in patients with NSCLC, approximately one third of CD8+ tumor-infiltrating lymphocytes (TIL) express Tim-3 (64). In these cancers, the Tim-3+ T cells co-express PD-1 and exhibit defects in proliferation and effector cytokine production.
In addition to its key role in regulating CD8+ T-cell effector function in cancer, recent studies demonstrate the role of Tim-3 in intratumoral and in vitro activated human Tim3+FoxP3+ Treg cells. The presence of Tim-3+FoxP3+ Treg cells seems to be a significant common feature across multiple forms of cancer including hepatocellular, ovarian, colon, and cervical carcinomas (65). In patients with NSCLC, approximately 60% of CD4+FoxP3+ TILs express Tim-3. The presence of Tim-3+FoxP3+ Treg cells in TILs correlates with the presence of nodal metastases and advanced cancer stage (64). The role of Tim-3 in FoxP3+ Treg cells is further substantiated by the presence of Tim-3+ FoxP3+ Treg cells in tumor tissue in multiple preclinical models, including both transplantable and de novo tumors. The tumor tissues in these models exhibit the presence of a predominant Tim-3+FoxP3+ Treg population, which is more immunosuppressive than the Tim-3−FoxP3+ Treg population, possibly owing to increased production of IL-10. In these models, the Tim-3+FoxP3+ Treg cells have also been suggested to promote the development of a dysfunctional phenotype in CD8+ TILs (23). One could further hypothesize that the tumor microenvironment drives FoxP3+ Treg cell activation and that increased Tim-3 expression contributes to more robust immunosuppression of tumor infiltrating CD8+ T cells. Initial studies from our laboratory (23) suggest that indeed Tregs present in the tumor microenvironment contributes to development of T cell exhaustion, but what role does Tim-3 play has not been elucidated.
Tim-3 in the regulation of innate immune cells in tumor
In contrast to its inducible expression on T cells, constitutive expression of Tim-3 has been found on innate immune cells. The majority of in vivo studies carried out so far have established that Tim-3 suppresses innate anti-tumor immunity.
Tim-3 has been shown to have a direct inhibitory role in human monocytes. It is constitutively expressed on unstimulated peripheral blood CD14+ monocytes but the expression level decreases rapidly upon TLR stimulation with concomitant increase in IL-12 production. Blockade of Tim-3 leads to an increase in the production of both IL-12 and IL-10 in monocytes following stimulation through TLR 4/7 (96).
A recent investigation showed that Tim-3 expression on tumor associated macrophages (TAM) in hepatocellular carcinoma (HCC) is induced by tumor-derived signals, including TGF-β. This further promotes TAM-mediated growth of HCC due to secretion of soluble factors such as IL-6. Moreover, Tim-3 expression inhibits the activation of tumor-specific CD8+ T cells in this model (97). Recent studies show that the M1 macrophages that perform diverse functions, such as phagocytosis, antigen presentation and production of pro-inflammatory cytokines, and serve to eliminate cancer cells, have a low expression of Tim-3. This exemplifies the immune suppressive role of Tim-3 in multiple cell types and its role in regulating immune cell cross-talk in the tumor setting.
Tim-3 and tumor cells
The expression of Tim-3 is not just restricted to immune cells. Accumulating evidence suggests that Tim-3 is also expressed on cancer cells, such as melanoma (98), osteosarcoma (99), cervical cancer (93), and clear cell renal cell carcinoma (ccRCC) (100). Two recent studies demonstrated that Tim-3 is highly expressed on acute myeloid leukemia stem cells (LSCs) but not on normal hematopoietic stem cells (HSCs). Since acute myeloid leukemia (AML) patients contain both LSCs and HSCs, Tim-3 could serve as a marker for LSCs (101, 102). In addition, the expression of Tim-3 was also found on lymphoma-derived endothelial cells (ECs) (103).
The ubiquitous expression of Tim-3 on multiple tumor cells strongly indicates its potential role in in tumor progression. Recent studies suggested that Tim-3 can promote tumor progression through different mechanisms, including facilitating tumor cell migration and invasion (93), directly suppressing CD4+ T cells through activation of the IL-6-STAT3 pathway to inhibit Th1 polarization (103), or activating mTOR function in AML cells (104).
Since the Tim-3-galectin-9 reverse signaling pathway is critical for MDSC expansion as discussed above, it is also likely that the expression of Tim-3 on tumor cells, similar to its expression on TILs, may have a key role in immune escape of tumors by shaping the immunosuppressive function of MDSCs through the galectin-9 dependent mechanism. Further investigations are needed to better understand the Tim-3-mediated cross talk between immune cells and tumor cells.
Rationale for targeting Tim-3
A firm connection between elevated Tim-3 expression and exhausted CD8+ T cells was initially established in multiple chronic viral infections including HIV, HCV, HBV, Friend virus, and LCMV in either humans or mice (15, 16, 61, 105, 106). Studies carried out in different preclinical cancer settings conclusively establish that Tim-3 acts as a checkpoint inhibitor of anti-tumor immunity, likely due to its association with T cell exhaustion in cancer.
Although tumor outgrowth was linked to the exhaustion of CD8+ T cells potentially mediated by the expression of inhibitory markers, including PD-1 in TILs, blockade of the PD-1 pathway could only partially restore the CTL function of exhausted tumor antigen-specific CD8+ T cells (107–110). Interestingly, Tim-3 expression on exhausted T cells usually is associated with PD-1 expression and marks the deeply exhausted T cells from both human and animal studies, supporting the functional correlation between Tim-3 and PD-1 during the development of T cells exhaustion (18, 19, 111). The combination of Tim-3 blockade with PD-1 pathway blockade is remarkably more effective in these models, leading to greater tumor regression with higher frequency than with blockade of either the Tim-3 or PD-1 pathway alone (19, 112). These observations support the potential of combinatorial therapeutic approaches involving the PD-1 and Tim-3 pathway blockade for effective treatment of various types of cancer (Figure 2).
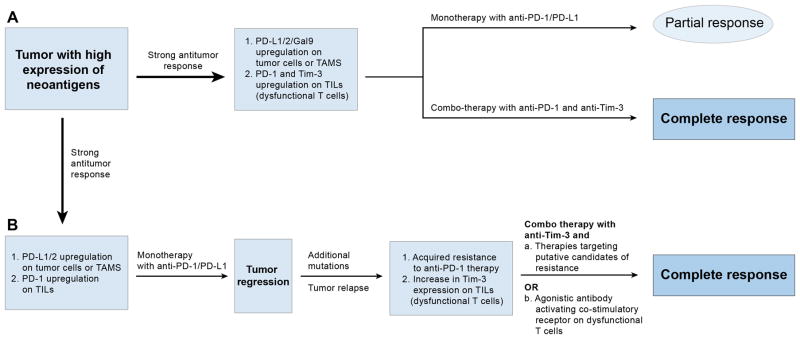
One of the major challenges in immunotherapy is to improve the proportion of patients who may respond to the treatment (113). Targeting the PD-1 pathway may not be sufficient to break dysfunction in exhausted T cells, as complex cross-regulation exists between PD-1 and other checkpoint inhibitors to restrain anti-tumor T cell immunity, and in certain cases PD-1 blockade may be followed by development of adaptive resistance. This has been established by Koyama et al who demonstrated in a mouse model of lung adenocarcinoma that failure of PD-1 monotherapy blockade or PD-1 adaptive resistance is associated with up-regulation of alternative immune checkpoint molecules particularly Tim-3 (114). Thus, combined blockade of PD-1 and other checkpoint inhibitors, including Tim-3 or use of Tim-3 blockade following adaptive resistance to PD-1 therapy could be an effective therapeutic solution to treat patients that develop resistance to anti-PD-1 therapy. This therapeutic regimen could be particularly relevant to cancers that exhibit key mutations associated with acquired resistance and immune escape to PD-1 blockade (115) (Figure 4).
Figure 4.
(A) A tumor with high expression of neoantigens will evoke a strong anti-tumor response, which the tumor evades by upregulating immune-checkpoint ligands (PD-L1, Galectin 9). Monotherapy with anti-PD-1/PD-L1 yields a partial response while a combo therapy with anti- PD-1 plus Tim-3 may be effective in generating a complete response.
(B) Following initial tumor regression, there may be delayed tumor relapse following additional mutations in tumor, leading to acquired resistance to further PD-1 blockade. The resistance may be associated with upregulation of Tim-3 or other checkpoint molecules on TILs (tumor infiltrating lymphocytes). A second-line therapy involving a combo therapy of anti-Tim-3 and an agent targeting resistance pathways or an agonistic antibody to activate co-stimulatory receptors on T cells may efficiently induce an effective anti-tumor response to clear the tumor.
These studies exemplify the potential benefit of using anti-Tim-3 therapeutic approaches, particularly in specific contexts and in certain stages of cancer progression that are associated with adaptive resistance to other checkpoint inhibition. In this context, targeting Tim-3 sparks interest due to the positive response generated in many preclinical cancer models, including cancers largely refractory to CTLA-4 and PD-1 blockade, such as colorectal cancer.
As emerging new modalities in the field of cancer immunotherapy are being developed, certain key features characteristic of the checkpoint receptors, including their pattern of expression in the host, need to be considered. Therapeutic blockade of checkpoint receptors could lead to exacerbations of underlying autoimmune conditions, a consideration which is particularly relevant to blockade of checkpoint receptors that are widely expressed. PD-1 and CTLA-4 are upregulated on all effector T cells, and blocking these receptors under disease conditions may disrupt the balance between Treg cells and effector T cells that recognize autoantigens. Indeed, autoimmune-like toxicities are commonly observed in patients treated with anti–CTLA-4 antibody (116), and in patients treated with anti–PD-1 antibodies (1, 117). Hence, targeting Tim-3 may be advantageous as it is not expressed on all T cells, being selectively expressed on T cells that have differentiated toward an IFN-γ–producing phenotype (3), and in patients with cancer, Tim-3 seems to be expressed primarily in intratumoral T cells (64, 118). The likelihood of Tim-3 blockade playing an adverse role in the regulation of T-cell responses outside of tumor tissue is far less when compared to blockade of either CTLA-4 or PD-1. This is corroborated by the observations that Tim-3–deficient mice do not exhibit autoimmunity (31), unlike both CTLA-4–deficient and PD-1–deficient mice (119, 120), and that tumor-bearing mice treated with anti–Tim-3 antibody do not exhibit autoimmunity (112). This indicates that targeting Tim-3 is less likely to be associated with adverse autoimmune-like toxicities. Moreover, the mechanism by which Tim-3 targets T cell responses varies widely from the mechanism of inhibition by classical inhibitory molecules such as PD-1, which exploits its ITIM/ITSM motifs that recruit phosphatases and dephosphorylate key TCR signaling intermediates, or CTLA-4 which competes with CD28 on the T cell surface for binding with shared co-stimulatory signals and relies on phosphatase PP2A for exerting inhibitory signals. Though the Tim-3 interacting partners remain largely unexplored, experiments have shown that Tim-3 has key tyrosine residues in its tail that can be phosphorylated by Src family tyrosine kinases such as Lck and Fyn. The key phosphorylated residues, in turn, interact with SH2 domain containing proteins, including PI3K, PLC-g1or Ras-GAP1, leading to activation of NFAT/AP-1 or NF-κB activity, which may lead to T cell activation and may finally accelerate the acquisition of the exhausted phenotype. Taken together, these mechanistic insights suggest that immunotherapeutic strategies combining blockade of Tim-3 along with PD-1 or CTLA-4 may have efficacy and safety advantages, since the Tim-3-mediated signaling pathway of T cell inhibition is not likely to be functionally redundant with those mediated by CTLA-4 or PD-1.
Combined use of antagonistic anti-Tim-3 antibodies with agonistic antibodies to pro-stimulatory molecules on tumor-specific T cells, such as CD137, has demonstrated long-term protection in a murine model of ovarian cancer (63). This therapeutic regimen of using Tim-3 blockade with activation of a pro-stimulatory molecule on T cells could be exploited in the future to obtain objective anti-tumor responses in patients (Figure 4). One could expect that further exploratory studies would reveal additional exciting anti-Tim-3 immunotherapeutic approaches to treat cancers.
Concluding remarks: Tim-3 – a target for cancer immunotherapy
A growing body of evidence supports the relevance of targeting Tim-3 in human cancer. It is now well established that Tim-3 along with PD-1 marks dysfunctional T cells in multiple cancer types both in experimental models and in humans. Moreover, the immune-suppressive effects of Tim-3 have been observed in multiple cell types, including effector T cells, Tregs and innate immune cells. These findings underscore the importance of targeting Tim-3 in these cells and since Tim-3 is expressed in non-T cells compartment as well, this blocking strategy could potentially convert the tumor microenvironment from an immune-suppressive state to an immune activating, anti-tumor state. The Tim-3 pathway is a highly suitable target for anti-cancer immunotherapy due to its expression on both dysfunctional CD8+ T cells and FoxP3+ Treg cells—two key immune cell populations that promote an immunosuppressive environment in tumor tissue, and also on other cell types including myeloid cells, NK cells, cancer stem cells and lastly, the cancer cells themselves, where Tim-3 is associated with an immune-suppressive and tumor-promoting role.
Thus, targeting Tim-3 along with other checkpoint inhibitors or combining Tim-3 inhibition with new immunotherapeutic approaches that activate cancer-specific T-cell stimulatory molecules has high potential for developing modalities with durable clinical benefit. In addition, Tim-3 blockade may also induce an anti-tumor immune response and mediate tumor regression in situations where anti-PD-1 or anti-CTLA4 does not work, such as in colorectal carcinomas. The key challenge in moving forward is to develop reliable strategies and indications under which combination therapies should be tested. Given the fact that PD-1 blockade may lead to up-regulation of Tim-3, blockade of Tim-3 following development of adaptive resistance to PD-1 or other checkpoint inhibitors holds promise in developing combination therapies in which anti-Tim-3 is a key component.
References
- 1.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Postow MA, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. The New England journal of medicine. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monney L, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 4.Meyers JH, Sabatos CA, Chakravarti S, Kuchroo VK. The TIM gene family regulates autoimmune and allergic diseases. Trends Mol Med. 2005;11:362–369. doi: 10.1016/j.molmed.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 5.McIntire JJ, et al. Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked Tim gene family. Nature immunology. 2001;2:1109–1116. doi: 10.1038/ni739. [DOI] [PubMed] [Google Scholar]
- 6.Zhu C, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nature immunology. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 7.Kanzaki M, et al. Galectin-9 and T cell immunoglobulin mucin-3 pathway is a therapeutic target for type 1 diabetes. Endocrinology. 2012;153:612–620. doi: 10.1210/en.2011-1579. [DOI] [PubMed] [Google Scholar]
- 8.Koguchi K, Anderson DE, Yang L, O’Connor KC, Kuchroo VK, Hafler DA. Dysregulated T cell expression of TIM3 in multiple sclerosis. The Journal of experimental medicine. 2006;203:1413–1418. doi: 10.1084/jem.20060210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang L, Anderson DE, Kuchroo J, Hafler DA. Lack of TIM-3 immunoregulation in multiple sclerosis. Journal of immunology. 2008;180:4409–4414. doi: 10.4049/jimmunol.180.7.4409. [DOI] [PubMed] [Google Scholar]
- 10.Morimoto K, et al. Dysregulated upregulation of T-cell immunoglobulin and mucin domain-3 on mucosal T helper 1 cells in patients with Crohn’s disease. Scand J Gastroenterol. 2011;46:701–709. doi: 10.3109/00365521.2011.568518. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, et al. Increased Tim-3 expression on peripheral lymphocytes from patients with rheumatoid arthritis negatively correlates with disease activity. Clin Immunol. 2010;137:288–295. doi: 10.1016/j.clim.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Li S, et al. Expression of TIM-3 on CD4+ and CD8+ T cells in the peripheral blood and synovial fluid of rheumatoid arthritis. APMIS. 2014;122:899–904. doi: 10.1111/apm.12228. [DOI] [PubMed] [Google Scholar]
- 13.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nature reviews Immunology. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones RB, et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. The Journal of experimental medicine. 2008;205:2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takamura S, et al. Premature terminal exhaustion of Friend virus-specific effector CD8+ T cells by rapid induction of multiple inhibitory receptors. Journal of immunology. 2010;184:4696–4707. doi: 10.4049/jimmunol.0903478. [DOI] [PubMed] [Google Scholar]
- 16.Golden-Mason L, et al. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. Journal of virology. 2009;83:9122–9130. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu W, Shi Y, Li J, Chen F, Chen Z, Zheng M. Tim-3 expression on peripheral T cell subsets correlates with disease progression in hepatitis B infection. Virol J. 2011;8:113. doi: 10.1186/1743-422X-8-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fourcade J, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. The Journal of experimental medicine. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. The Journal of experimental medicine. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H, et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology. 2012;56:1342–1351. doi: 10.1002/hep.25777. [DOI] [PubMed] [Google Scholar]
- 21.Japp AS, et al. Dysfunction of PSA-specific CD8+ T cells in prostate cancer patients correlates with CD38 and Tim-3 expression. Cancer Immunol Immunother. 2015;64:1487–1494. doi: 10.1007/s00262-015-1752-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta S, et al. Allograft rejection is restrained by short-lived TIM-3+PD-1+Foxp3+ Tregs. The Journal of clinical investigation. 2012;122:2395–2404. doi: 10.1172/JCI45138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakuishi K, et al. TIM3FOXP3 regulatory T cells are tissue-specific promoters of T-cell dysfunction in cancer. Oncoimmunology. 2013;2:e23849. doi: 10.4161/onci.23849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiba S, et al. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nature immunology. 2012;13:832–842. doi: 10.1038/ni.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang YH, et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature. 2015;517:386–390. doi: 10.1038/nature13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurtulus S, et al. TIGIT predominantly regulates the immune response via regulatory T cells. The Journal of clinical investigation. 2015;125:4053–4062. doi: 10.1172/JCI81187. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Lee J, et al. Phosphotyrosine-dependent coupling of Tim-3 to T-cell receptor signaling pathways. Molecular and cellular biology. 2011;31:3963–3974. doi: 10.1128/MCB.05297-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gorman JV, et al. Tim-3 directly enhances CD8 T cell responses to acute Listeria monocytogenes infection. Journal of immunology. 2014;192:3133–3142. doi: 10.4049/jimmunol.1302290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gleason MK, et al. Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9. Blood. 2012;119:3064–3072. doi: 10.1182/blood-2011-06-360321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakae S, et al. TIM-1 and TIM-3 enhancement of Th2 cytokine production by mast cells. Blood. 2007;110:2565–2568. doi: 10.1182/blood-2006-11-058800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabatos CA, et al. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nature immunology. 2003;4:1102–1110. doi: 10.1038/ni988. [DOI] [PubMed] [Google Scholar]
- 32.Moller-Hackbarth K, et al. A disintegrin and metalloprotease (ADAM) 10 and ADAM17 are major sheddases of T cell immunoglobulin and mucin domain 3 (Tim-3) The Journal of biological chemistry. 2013;288:34529–34544. doi: 10.1074/jbc.M113.488478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansen JA, et al. A novel soluble form of Tim-3 associated with severe graft-versus-host disease. Biol Blood Marrow Transplant. 2013;19:1323–1330. doi: 10.1016/j.bbmt.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao E, et al. T cell immunoglobulin mucin-3 crystal structure reveals a galectin-9-independent ligand-binding surface. Immunity. 2007;26:311–321. doi: 10.1016/j.immuni.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 35.Santiago C, et al. Structures of T cell immunoglobulin mucin protein 4 show a metal-Ion-dependent ligand binding site where phosphatidylserine binds. Immunity. 2007;27:941–951. doi: 10.1016/j.immuni.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santiago C, Ballesteros A, Tami C, Martinez-Munoz L, Kaplan GG, Casasnovas JM. Structures of T Cell immunoglobulin mucin receptors 1 and 2 reveal mechanisms for regulation of immune responses by the TIM receptor family. Immunity. 2007;26:299–310. doi: 10.1016/j.immuni.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van de Weyer PS, Muehlfeit M, Klose C, Bonventre JV, Walz G, Kuehn EW. A highly conserved tyrosine of Tim-3 is phosphorylated upon stimulation by its ligand galectin-9. Biochemical and biophysical research communications. 2006;351:571–576. doi: 10.1016/j.bbrc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 38.Rangachari M, et al. Bat3 promotes T cell responses and autoimmunity by repressing Tim-3-mediated cell death and exhaustion. Nature medicine. 2012;18:1394–1400. doi: 10.1038/nm.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang CW, et al. Apoptosis of tumor infiltrating effector TIM-3+CD8+ T cells in colon cancer. Sci Rep. 2015;5:15659. doi: 10.1038/srep15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prokhorov A, et al. The immune receptor Tim-3 mediates activation of PI3 kinase/mTOR and HIF-1 pathways in human myeloid leukaemia cells. The international journal of biochemistry & cell biology. 2015;59:11–20. doi: 10.1016/j.biocel.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 41.Wang JM, et al. Tim-3 alters the balance of IL-12/IL-23 and drives TH17 cells: role in hepatitis B vaccine failure during hepatitis C infection. Vaccine. 2013;31:2238–2245. doi: 10.1016/j.vaccine.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang JM, et al. Differential regulation of interleukin-12 (IL-12)/IL-23 by Tim-3 drives T(H)17 cell development during hepatitis C virus infection. Journal of virology. 2013;87:4372–4383. doi: 10.1128/JVI.03376-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma CJ, et al. Cis association of galectin-9 with Tim-3 differentially regulates IL-12/IL-23 expressions in monocytes via TLR signaling. PLoS One. 2013;8:e72488. doi: 10.1371/journal.pone.0072488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jayaraman P, et al. Tim3 binding to galectin-9 stimulates antimicrobial immunity. The Journal of experimental medicine. 2010;207:2343–2354. doi: 10.1084/jem.20100687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sada-Ovalle I, et al. Tim-3 blocking rescue macrophage and T cell function against Mycobacterium tuberculosis infection in HIV+ patients. J Int AIDS Soc. 2015;18:20078. doi: 10.7448/IAS.18.1.20078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li G, Liang X, Lotze MT. HMGB1: The Central Cytokine for All Lymphoid Cells. Frontiers in immunology. 2013;4:68. doi: 10.3389/fimmu.2013.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dolina JS, Braciale TJ, Hahn YS. Liver-primed CD8+ T cells suppress antiviral adaptive immunity through galectin-9-independent T-cell immunoglobulin and mucin 3 engagement of high-mobility group box 1 in mice. Hepatology. 2014;59:1351–1365. doi: 10.1002/hep.26938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakayama M, et al. Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation. Blood. 2009;113:3821–3830. doi: 10.1182/blood-2008-10-185884. [DOI] [PubMed] [Google Scholar]
- 49.DeKruyff RH, et al. T cell/transmembrane, Ig, and mucin-3 allelic variants differentially recognize phosphatidylserine and mediate phagocytosis of apoptotic cells. Journal of immunology. 2010;184:1918–1930. doi: 10.4049/jimmunol.0903059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson AC, et al. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 2007;318:1141–1143. doi: 10.1126/science.1148536. [DOI] [PubMed] [Google Scholar]
- 51.Lee MJ, Woo MY, Chwae YJ, Kwon MH, Kim K, Park S. Down-regulation of interleukin-2 production by CD4(+) T cells expressing TIM-3 through suppression of NFAT dephosphorylation and AP-1 transcription. Immunobiology. 2012;217:986–995. doi: 10.1016/j.imbio.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 52.Clayton KL, et al. T cell Ig and mucin domain-containing protein 3 is recruited to the immune synapse, disrupts stable synapse formation, and associates with receptor phosphatases. Journal of immunology. 2014;192:782–791. doi: 10.4049/jimmunol.1302663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saresella M, et al. A role for the TIM-3/GAL-9/BAT3 pathway in determining the clinical phenotype of multiple sclerosis. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2014;28:5000–5009. doi: 10.1096/fj.14-258194. [DOI] [PubMed] [Google Scholar]
- 54.Sanchez-Fueyo A, et al. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nature immunology. 2003;4:1093–1101. doi: 10.1038/ni987. [DOI] [PubMed] [Google Scholar]
- 55.Qiu Y, et al. Tim-3-expressing CD4+ and CD8+ T cells in human tuberculosis (TB) exhibit polarized effector memory phenotypes and stronger anti-TB effector functions. PLoS Pathog. 2012;8:e1002984. doi: 10.1371/journal.ppat.1002984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jayaraman P, et al. TIM3 Mediates T Cell Exhaustion during Mycobacterium tuberculosis Infection. PLoS Pathog. 2016;12:e1005490. doi: 10.1371/journal.ppat.1005490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burton BR, et al. Sequential transcriptional changes dictate safe and effective antigen-specific immunotherapy. Nature communications. 2014;5:4741. doi: 10.1038/ncomms5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oikawa T, et al. Preferential involvement of Tim-3 in the regulation of hepatic CD8+ T cells in murine acute graft-versus-host disease. Journal of immunology. 2006;177:4281–4287. doi: 10.4049/jimmunol.177.7.4281. [DOI] [PubMed] [Google Scholar]
- 59.Veenstra RG, et al. Contrasting acute graft-versus-host disease effects of Tim-3/galectin-9 pathway blockade dependent upon the presence of donor regulatory T cells. Blood. 2012;120:682–690. doi: 10.1182/blood-2011-10-387977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang SC, et al. PD-1 and Tim-3 pathways are associated with regulatory CD8+ T-cell function in decidua and maintenance of normal pregnancy. Cell Death Dis. 2015;6:e1738. doi: 10.1038/cddis.2015.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jin HT, et al. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dardalhon V, et al. Tim-3/galectin-9 pathway: regulation of Th1 immunity through promotion of CD11b+Ly-6G+ myeloid cells. Journal of immunology. 2010;185:1383–1392. doi: 10.4049/jimmunol.0903275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo Z, et al. Combined TIM-3 blockade and CD137 activation affords the long-term protection in a murine model of ovarian cancer. J Transl Med. 2013;11:215. doi: 10.1186/1479-5876-11-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao X, et al. TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression. PLoS One. 2012;7:e30676. doi: 10.1371/journal.pone.0030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yan J, Zhang Y, Zhang JP, Liang J, Li L, Zheng L. Tim-3 expression defines regulatory T cells in human tumors. PLoS One. 2013;8:e58006. doi: 10.1371/journal.pone.0058006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jie HB, et al. Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. Br J Cancer. 2013;109:2629–2635. doi: 10.1038/bjc.2013.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gautron AS, Dominguez-Villar M, de Marcken M, Hafler DA. Enhanced suppressor function of TIM-3+ FoxP3+ regulatory T cells. European journal of immunology. 2014;44:2703–2711. doi: 10.1002/eji.201344392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang F, Wan L, Zhang C, Zheng X, Li J, Chen ZK. Tim-3-Galectin-9 pathway involves the suppression induced by CD4+CD25+ regulatory T cells. Immunobiology. 2009;214:342–349. doi: 10.1016/j.imbio.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 69.Bu M, et al. Ovarian carcinoma-infiltrating regulatory T cells were more potent suppressors of CD8(+) T cell inflammation than their peripheral counterparts, a function dependent on TIM3 expression. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2016;37:3949–3956. doi: 10.1007/s13277-015-4237-x. [DOI] [PubMed] [Google Scholar]
- 70.Anderson AC, et al. T-bet, a Th1 transcription factor regulates the expression of Tim-3. European journal of immunology. 2010;40:859–866. doi: 10.1002/eji.200939842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu C, et al. An IL-27/NFIL3 signalling axis drives Tim-3 and IL-10 expression and T-cell dysfunction. Nature communications. 2015;6:6072. doi: 10.1038/ncomms7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Axtell RC, et al. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nature medicine. 2010;16:406–412. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boivin N, Baillargeon J, Doss PM, Roy AP, Rangachari M. Interferon-beta suppresses murine Th1 cell function in the absence of antigen-presenting cells. PLoS One. 2015;10:e0124802. doi: 10.1371/journal.pone.0124802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mujib S, et al. Antigen-independent induction of Tim-3 expression on human T cells by the common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 is associated with proliferation and is dependent on the phosphoinositide 3-kinase pathway. Journal of immunology. 2012;188:3745–3756. doi: 10.4049/jimmunol.1102609. [DOI] [PubMed] [Google Scholar]
- 75.Wherry EJ. T cell exhaustion. Nature immunology. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 76.Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324:1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pellegrini M, et al. IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell. 2011;144:601–613. doi: 10.1016/j.cell.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 78.Khademi M, et al. T Cell Ig- and mucin-domain-containing molecule-3 (TIM-3) and TIM-1 molecules are differentially expressed on human Th1 and Th2 cells and in cerebrospinal fluid-derived mononuclear cells in multiple sclerosis. Journal of immunology. 2004;172:7169–7176. doi: 10.4049/jimmunol.172.11.7169. [DOI] [PubMed] [Google Scholar]
- 79.Ndhlovu LC, et al. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood. 2012;119:3734–3743. doi: 10.1182/blood-2011-11-392951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.da Silva IP, et al. Reversal of NK-cell exhaustion in advanced melanoma by Tim-3 blockade. Cancer Immunol Res. 2014;2:410–422. doi: 10.1158/2326-6066.CIR-13-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu L, et al. Increased Tim-3 expression in peripheral NK cells predicts a poorer prognosis and Tim-3 blockade improves NK cell-mediated cytotoxicity in human lung adenocarcinoma. Int Immunopharmacol. 2015;29:635–641. doi: 10.1016/j.intimp.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 82.Zhang Y, et al. Tim-3 negatively regulates IL-12 expression by monocytes in HCV infection. PLoS One. 2011;6:e19664. doi: 10.1371/journal.pone.0019664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Frisancho-Kiss S, et al. Cutting edge: T cell Ig mucin-3 reduces inflammatory heart disease by increasing CTLA-4 during innate immunity. Journal of immunology. 2006;176:6411–6415. doi: 10.4049/jimmunol.176.11.6411. [DOI] [PubMed] [Google Scholar]
- 84.Jiang X, et al. Tim-3 promotes intestinal homeostasis in DSS colitis by inhibiting M1 polarization of macrophages. Clin Immunol. 2015;160:328–335. doi: 10.1016/j.clim.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 85.Maurya N, Gujar R, Gupta M, Yadav V, Verma S, Sen P. Immunoregulation of dendritic cells by the receptor T cell Ig and mucin protein-3 via Bruton’s tyrosine kinase and c-Src. Journal of immunology. 2014;193:3417–3425. doi: 10.4049/jimmunol.1400395. [DOI] [PubMed] [Google Scholar]
- 86.Bai J, Li X, Tong D, Shi W, Song H, Li Q. T-cell immunoglobulin- and mucin-domain-containing molecule 3 gene polymorphisms and prognosis of non-small-cell lung cancer. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2013;34:805–809. doi: 10.1007/s13277-012-0610-1. [DOI] [PubMed] [Google Scholar]
- 87.Tong D, et al. T cell immunoglobulin- and mucin-domain-containing molecule 3 gene polymorphisms and susceptibility to pancreatic cancer. Mol Biol Rep. 2012;39:9941–9946. doi: 10.1007/s11033-012-1862-y. [DOI] [PubMed] [Google Scholar]
- 88.Cao B, et al. Genetic variations and haplotypes in TIM-3 gene and the risk of gastric cancer. Cancer Immunol Immunother. 2010;59:1851–1857. doi: 10.1007/s00262-010-0910-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Piao YR, Piao LZ, Zhu LH, Jin ZH, Dong XZ. Prognostic value of T cell immunoglobulin mucin-3 in prostate cancer. Asian Pac J Cancer Prev. 2013;14:3897–3901. doi: 10.7314/apjcp.2013.14.6.3897. [DOI] [PubMed] [Google Scholar]
- 90.Yuan J, Jiang B, Zhao H, Huang Q. Prognostic implication of TIM-3 in clear cell renal cell carcinoma. Neoplasma. 2013 [PubMed] [Google Scholar]
- 91.Zhou E, et al. Up-regulation of Tim-3 is associated with poor prognosis of patients with colon cancer. Int J Clin Exp Pathol. 2015;8:8018–8027. [PMC free article] [PubMed] [Google Scholar]
- 92.Yang M, et al. T-cell immunoglobulin mucin-3 expression in bladder urothelial carcinoma: Clinicopathologic correlations and association with survival. J Surg Oncol. 2015;112:430–435. doi: 10.1002/jso.24012. [DOI] [PubMed] [Google Scholar]
- 93.Cao Y, et al. Tim-3 expression in cervical cancer promotes tumor metastasis. PLoS One. 2013;8:e53834. doi: 10.1371/journal.pone.0053834. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 94.Jiang J, et al. Decreased galectin-9 and increased Tim-3 expression are related to poor prognosis in gastric cancer. PLoS One. 2013;8:e81799. doi: 10.1371/journal.pone.0081799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang Z, et al. The Clinical Significance of Abnormal Tim-3 Expression on NK Cells from Patients with Gastric Cancer. Immunol Invest. 2015;44:578–589. doi: 10.3109/08820139.2015.1052145. [DOI] [PubMed] [Google Scholar]
- 96.Zhang Y, et al. Tim-3 regulates pro- and anti-inflammatory cytokine expression in human CD14+ monocytes. J Leukoc Biol. 2012;91:189–196. doi: 10.1189/jlb.1010591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yan W, et al. Tim-3 fosters HCC development by enhancing TGF-beta-mediated alternative activation of macrophages. Gut. 2015;64:1593–1604. doi: 10.1136/gutjnl-2014-307671. [DOI] [PubMed] [Google Scholar]
- 98.Wiener Z, et al. TIM-3 is expressed in melanoma cells and is upregulated in TGF-beta stimulated mast cells. J Invest Dermatol. 2007;127:906–914. doi: 10.1038/sj.jid.5700616. [DOI] [PubMed] [Google Scholar]
- 99.Shang Y, Li Z, Li H, Xia H, Lin Z. TIM-3 expression in human osteosarcoma: Correlation with the expression of epithelial-mesenchymal transition-specific biomarkers. Oncol Lett. 2013;6:490–494. doi: 10.3892/ol.2013.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Komohara Y, et al. The Coordinated Actions of TIM-3 on Cancer and Myeloid Cells in the Regulation of Tumorigenicity and Clinical Prognosis in Clear Cell Renal Cell Carcinomas. Cancer Immunol Res. 2015;3:999–1007. doi: 10.1158/2326-6066.CIR-14-0156. [DOI] [PubMed] [Google Scholar]
- 101.Kikushige Y, et al. TIM-3 is a promising target to selectively kill acute myeloid leukemia stem cells. Cell Stem Cell. 2010;7:708–717. doi: 10.1016/j.stem.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 102.Jan M, et al. Prospective separation of normal and leukemic stem cells based on differential expression of TIM3, a human acute myeloid leukemia stem cell marker. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5009–5014. doi: 10.1073/pnas.1100551108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huang X, et al. Lymphoma endothelium preferentially expresses Tim-3 and facilitates the progression of lymphoma by mediating immune evasion. The Journal of experimental medicine. 2010;207:505–520. doi: 10.1084/jem.20090397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Goncalves Silva I, Gibbs BF, Bardelli M, Varani L, Sumbayev VV. Differential expression and biochemical activity of the immune receptor Tim-3 in healthy and malignant human myeloid cells. Oncotarget. 2015;6:33823–33833. doi: 10.18632/oncotarget.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nebbia G, et al. Upregulation of the Tim-3/galectin-9 pathway of T cell exhaustion in chronic hepatitis B virus infection. PLoS One. 2012;7:e47648. doi: 10.1371/journal.pone.0047648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu W, et al. Blockade of Tim-3 signaling restores the virus-specific CD8(+) T-cell response in patients with chronic hepatitis B. European journal of immunology. 2012;42:1180–1191. doi: 10.1002/eji.201141852. [DOI] [PubMed] [Google Scholar]
- 107.Blank C, et al. Blockade of PD-L1 (B7-H1) augments human tumor-specific T cell responses in vitro. Int J Cancer. 2006;119:317–327. doi: 10.1002/ijc.21775. [DOI] [PubMed] [Google Scholar]
- 108.Ahmadzadeh M, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yamamoto R, et al. PD-1-PD-1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphoma. Blood. 2008;111:3220–3224. doi: 10.1182/blood-2007-05-085159. [DOI] [PubMed] [Google Scholar]
- 110.Mumprecht S, Schurch C, Schwaller J, Solenthaler M, Ochsenbein AF. Programmed death 1 signaling on chronic myeloid leukemia-specific T cells results in T-cell exhaustion and disease progression. Blood. 2009;114:1528–1536. doi: 10.1182/blood-2008-09-179697. [DOI] [PubMed] [Google Scholar]
- 111.Zhou Q, et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117:4501–4510. doi: 10.1182/blood-2010-10-310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-gamma-mediated antitumor immunity and suppresses established tumors. Cancer research. 2011;71:3540–3551. doi: 10.1158/0008-5472.CAN-11-0096. [DOI] [PubMed] [Google Scholar]
- 113.Hellmann MD, Friedman CF, Wolchok JD. Combinatorial Cancer Immunotherapies. Adv Immunol. 2016;130:251–277. doi: 10.1016/bs.ai.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 114.Koyama S, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nature communications. 2016;7:10501. doi: 10.1038/ncomms10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zaretsky JM, et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. The New England journal of medicine. 2016;375:819–829. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hamid O, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. The New England journal of medicine. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang ZZ, et al. IL-12 upregulates TIM-3 expression and induces T cell exhaustion in patients with follicular B cell non-Hodgkin lymphoma. The Journal of clinical investigation. 2012;122:1271–1282. doi: 10.1172/JCI59806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 120.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]