Abstract
Background
Many patients with major depressive disorder (MDD) who experience full symptomatic remission after antidepressant treatment still have residual depressive symptoms. We describe the types and frequency of residual depressive symptoms and their relationship to subsequent depressive relapse after treatment with citalopram in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial.
Method
Participants in primary (n = 18) and psychiatric (n = 23) practice settings were openly treated with citalopram using measurement-based care for up to 14 weeks and follow-up for up to 1 year. We assessed 943 (32.8% of 2876) participants who met criteria for remission to determine the proportions with individual residual symptoms and any of the nine DSM-IV criterion symptom domains to define a major depressive episode. At each visit, the 16-item Quick Inventory of Depressive Symptomatology, Self-Report (QIDS-SR16) and the self-report Frequency, Intensity, and Burden of Side Effects Rating (FIBSER) scale were used to assessed depressive symptoms and side-effects respectively.
Results
More than 90% of remitters had at least one residual depressive symptom (median = 3). The most common were weight increase (71.3%) and mid-nocturnal insomnia (54.9 %). The most common residual symptom domains were sleep disturbance (71.7%) and appetite/weight disturbance (35.9 %). Those who remitted before 6 weeks had fewer residual symptoms at study exit than did later remitters. Residual sleep disturbance did not predict relapse during follow-up. Having a greater number of residual symptom domains was associated with a higher probability of relapse.
Conclusions
Patients with remission of MDD after treatment with citalopram continue to experience selected residual depressive symptoms, which increase the risk of relapse.
Keywords: Major depression, remission, residual symptoms
Introduction
Residual depressive symptoms (Fava et al. 2002; Carney et al. 2007) after remission (Nierenberg & Wright, 1999; Rush et al. 2006b) (typically defined as ⩽7 on the 17-item Hamilton Depression Rating Scale (HAMD17; Hamilton, 1960, 1967) or ⩽5 on the 16-item Quick Inventory of Depressive Symptomatology, Self-Report (QIDS-SR16; Rush et al. 2003, 2006a; Trivedi et al. 2004) or response (typically defined as 50% improvement in depression rating scale scores) have been associated with continued impaired psychosocial functioning (Mintz et al. 1992; Kennedy & Paykel, 2004; Fava et al. 2007; Zimmerman et al. 2007), a lack of feeling well (Fava et al. 2007), and an increased risk of subsequent depressive relapse and recurrence (Judd et al. 1998a, b; Kanai et al. 2003; Bockting et al. 2006). However, only a few studies have focused on specific residual symptoms after remission (Kennedy & Paykel, 2004; Zimmerman et al. 2007), and most studies of residual symptoms after pharmacological treatment include only participants who meet narrow inclusion and exclusion criteria for acute randomized controlled trials. Thus, little is known about residual symptoms that could occur in representative patients seeking treatment in typical practice settings.
In addition to the core DSM-IV depressive symptoms such as sad mood, fatigue, persistent insomnia, guilt and lowered self-esteem, patients can experience residual symptoms of anxiety, irritability, excessive reactivity to environmental stressors, pessimism, hopelessness, and impaired functioning at work (Fava et al. 2002). These associated symptoms may be transient for some patients because symptoms fade over time. For others, however, these symptoms may persist despite ongoing treatment. Epidemiological evidence shows that many people with major depressive disorder (MDD) have residual depressive symptoms that persist for more than a year after an index depressive episode resolves, although these data may include those who are no longer in a depressive episode and who may or may not be in remission (Mojtabai, 2001).
The aim of this report was to assess the frequency and types of residual symptoms and their relationship to subsequent depressive relapse for a large representative group of remitters who had participated in the National Institute of Mental Health (NIMH)-sponsored Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study and were treated vigorously with the selective serotonin reuptake inhibitor (SSRI) citalopram using measurement-based care (Trivedi et al. 2006).
Method
This report is based on data collected in the STAR*D study, which was designed to assess the effectiveness of medications or cognitive therapy for out-patients who did not have a satisfactory response to an initial or subsequent prospective treatment. The rationale, design and methods for STAR*D have been detailed elsewhere (Fava et al. 2003; Rush et al. 2004).
Participants
The Institutional Review Boards at the National Coordinating Center, the Data Coordinating Center, each Regional Center and relevant Clinical Sites, and the Data Safety and Monitoring Board of the NIMH (Bethesda, USA) approved and monitored the protocol. Following a complete description of the study, participants provided written informed consent at study enrollment.
Between July 2001 and April 2004, STAR*D enrolled 4041 out-patients aged 18–75 years from primary (n = 18) and psychiatric (n = 23) practice settings serving both public and private sector patients. Advertising was proscribed. Enrollment required a primary clinical diagnosis of non-psychotic MDD based on the DSM-IV confirmed by a checklist completed by the Clinical Research Coordinators (CRCs) located at each Clinical Site. Broad inclusion and minimal exclusion criteria aimed to maximize the generalizability of findings.
All STAR*D participants entered the first treatment step with the SSRI citalopram. Remission was defined as a score ⩽5 on the 16-item Quick Inventory of Depressive Symptomatology, Clinician-rated (QIDS-C16; Rush et al. 2003, 2006a; Trivedi et al. 2004).
Protocol for acute treatment
To mimic clinical practice, enhance safety, and ensure vigorous dosing, participants and treating clinicians were not masked to either treatment assignment or dose. A clinical treatment manual (www.star-d.org) was used to deliver measurement-based care (Trivedi et al. 2006) that recommended starting doses and dose changes for each medication treatment. These recommendations were guided by symptom and side-effect ratings obtained at each treatment visit using the QIDS-C16 and the Frequency, Intensity, and Burden of Side Effects Rating (FIBSER) scale (Wisniewski et al. 2006). In addition, didactic instruction, CRC support, and a centralized monitoring system with feedback constituted intense efforts to assure timely dose increases when inadequate symptom reduction occurred in the context of acceptable side-effects. Clinical management aimed to achieve symptom remission (QIDS-C16 rating ⩽5 at treatment exit). The protocol recommended treatment clinic visits at weeks 0, 2, 4, 6, 9 and 12, but allowed for flexibility (e.g. the week 2 visit could be held within ±6 days of week 2). Extra visits could be held if needed. For participants who experienced a response or remission only at week 12, treatment could be extended for up to two additional weeks (14 weeks total) to determine whether that status was sustained.
Concomitant treatments
Stimulants, anticonvulsants, antipsychotics, mood stabilizers, non-protocol antidepressant medications and potential antidepressant augmenting agents (e.g. buspirone) were proscribed. Otherwise, any concomitant medication was allowed for managing concurrent general medical conditions or protocol antidepressant side-effects (e.g. sexual dysfunction), as were anxiolytics (except alprazolam) and sedative hypnotics (including trazodone ⩽200 mg/day for sleep).
Protocol for follow-up treatment
Those participants who responded (with a 50% improvement in baseline QIDS-SR16 scores) or who remitted (with a QIDS-SR16 score of ⩽5) after acute treatment with citalopram, and who elected to continue to be followed, were eligible for a year of free continuation/maintenance treatment. Their clinicians recommended that they continue with their acute dose of citalopram. Treatment itself, however, was naturalistic and ultimately decided upon by the participant and their clinician. Changes in the dose of citalopram and changes in concomitant medications were not dictated by the protocol, but instead by clinical need. Minimal levels of compliance with taking medication were not required to continue in the protocol.
Measures
The QIDS-SR16 was completed by participants at baseline and at every visit to assess depressive symptoms. The self-report FIBSER was completed by participants after every visit to assess side-effects. Both measures were gathered within 72 h of each visit using a telephone-based interactive voice response (IVR) system.
Definition of residual symptoms and relapse
Because the most complete data available with the least missing data points were gathered using the QIDS-SR16, the presence of individual or domain residual symptoms was categorized using the QIDS-SR16 with a score ⩾1 defining the minimal and a score ⩾2 the moderate boundary between presence and absence of residual symptoms. The QIDS-SR16 items range from 0 to 3, so a threshold score of ⩾1 identifies even the mildest of symptoms and a threshold of ⩾2 identifies those symptoms that would meet the threshold for DSM-IV criteria. Residual DSM-IV symptom domains obtained from the QIDS-SR16 (sleep disturbance, sad mood, appetite/weight, concentration, outlook, suicidal ideation, involvement, energy/fatigue, psychomotor) were also examined. Response was defined as ⩾50% reduction in the baseline QIDS-SR16 by the end of citalopram treatment, whereas remission was defined as a QIDS-SR16 score ⩽5 at treatment exit. Relapse was defined when the QIDS-SR16 score obtained from the IVR during the naturalistic follow-up phase was ⩾11 (corresponding to an HAMD17 score ⩾14; see Rush et al. 2003). As participants were evaluated once a month with the IVR, data were not available to assess, beyond symptom severity, whether clinical exacerbation of depression met full criteria for another DSM-IV episode.
Statistical methods
Analyses are primarily descriptive in nature. Means and standard deviations are presented for continuous characteristics and percentages for discrete characteristics. Statistical tests (χ2, t test) were conducted to compare the characteristics of remitters with no residual symptoms to remitters with at least one residual symptom. For those in follow-up, Kaplan–Meier curves were generated and a log-rank statistic was used to compare the cumulative probability of relapse between those with and without sleep disturbance as a residual symptom domain, and between those with different numbers of residual symptom domains (0–5).
Results
The evaluable sample included the 2876 participants who contributed to the overall results of the open trial with citalopram (Trivedi et al. 2006). About 32% (943) met criteria for remission, with an exit mean dose of citalopram of 39.8 ± 15.4 mg.
Ninety-two of 943 (9.8%) remitters were completely free of any QIDS-SR16 residual symptoms (total QIDS-SR16 = 0) at treatment exit. These 91 remitters had slightly higher mean baseline QIDS-SR16 scores (16.2 ± 4.2) compared to those remitters with at least one residual symptom (15.0 ± 4.1, p = 0.008), and were younger (36.6 ± 12.5 v. 40.5 ± 12.9 years, p = 0.006). No other statistically or clinically significant differences in baseline variables were associated between remitters with no residual symptoms (n = 92) and those who had at least one residual symptom.
Table 1 shows the frequency of individual residual symptoms for remitters based on the QIDS-SR16 at the end of acute treatment, including the proportions of those with at least minimal (QIDS-SR16 ⩾1) or moderate (QIDS-SR16 ⩾2) levels of residual symptoms. Remitters had a range from 0 to 8 residual symptoms. Among the 16 symptoms with at least a minimal level (⩾1), the most frequent were weight increase (71.3%), mid-nocturnal insomnia (54.9%), increased appetite (50.6%), sleep onset insomnia (29.5%), and sad mood (27.1%). When the threshold for having a residual symptom was increased to at least a moderate level (QIDS-SR16 ⩾2), the most common symptoms were mid-nocturnal insomnia (40.5%) and weight increase (21.7%).
Table 1.
Proportion of remitters with at least mild or moderate levels of residual symptoms
| Residual QIDS-SR16 items ⩾1 or ⩾2 (n = 943) |
% with at least mild symptomsa |
% with at least moderate symptomsb |
|---|---|---|
| Sleep onset insomnia | 29.5 | 9.7 |
| Mid-nocturnal insomnia | 54.9 | 40.5 |
| Early morning insomnia | 16.6 | 6.8 |
| Hypersomnia | 24.0 | 2.4 |
| Sad mood | 27.1 | 0.4 |
| Decreased appetite | 12.2 | 0.6 |
| Increased appetite | 50.6 | 9.5 |
| Weight decrease | 16.7 | 4.5 |
| Weight increase | 71.3 | 21.7 |
| Concentration/ decision making |
20.9 | 0.9 |
| Outlook self | 6.8 | 0.4 |
| Suicidal ideation | 1.3 | 0.3 |
| Involvement | 9.4 | 1.8 |
| Energy | 22.5 | 1.7 |
| Slowed down | 5.8 | 0.3 |
| Restless | 15.2 | 0.9 |
QIDS-SR16, 16-item Quick Inventory of Depressive Symptomatology, Self-Report.
Any QIDS-SR16 item ⩾1.
Any QIDS-SR16 item ⩾2.
Of those with baseline suicidal ideation, 2.4% continued to have this symptom after remission. Of the 12 remitted participants who had the most severe baseline level of suicidal ideation (QIDS-SR16 item no. 12 rated as 3; those who endorsed at baseline that they think of suicide or death several times a day or made plans for suicide or had in fact tried to take their life), all had complete resolution at exit. Of the 88 participants who rated QIDS-SR16 item no. 12 at 2 (‘I think of suicide or death several times a week for several minutes’), 96.6% had complete resolution, 1.1% continued at the same level, and 2.3% went down to a QIDS-SR16 suicide item score = 1. Of the 367 participants with a baseline suicide item score = 1, 97.8% had complete resolution, 1.6% stayed at 1, and 0.5% had an increase to 2.
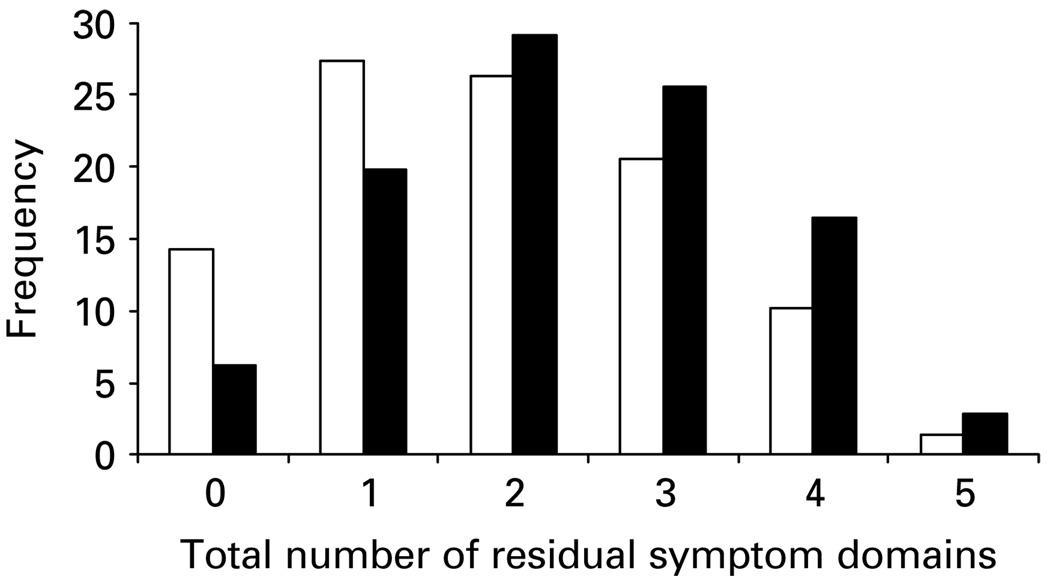
As symptoms observed after treatment could result from either persistent symptoms that were present at baseline (i.e. residual symptoms) or those that arose during treatment (i.e. treatment-emergent symptoms), it is important to differentiate between true residual symptoms and treatment-emergent symptoms. Data on residual and treatment-emergent symptoms are listed in Table 2. Participants who reached remission within the first 6 weeks had fewer residual symptoms compared to those who reached remission after 6 weeks (Fig. 1).
Table 2.
Proportion of remitters with persistent baseline symptoms and treatment-emergent symptoms
| QIDS-SR16 item | n | % with symptom at baseline |
% with persistent baseline symptoms |
% without symptom at baseline, who had it at remission |
|---|---|---|---|---|
| Sleep onset insomnia | 943 | 76.4 | 35.8 | 9.0 |
| Mid-nocturnal insomnia | 943 | 88.9 | 58.8 | 23.8 |
| Early morning insomnia | 942 | 59.5 | 21.1 | 10.0 |
| Hypersomnia | 943 | 32.8 | 44.3 | 14.2 |
| Sad mood | 943 | 97.8 | 27.7 | 4.8 |
| Decreased appetite | 940 | 48.9 | 10.9 | 8.8 |
| Increased appetite | 939 | 26.7 | 12.0 | 9.6 |
| Decreased weight | 938 | 36.5 | 15.5 | 10.7 |
| Increased weight | 942 | 28.0 | 20.8 | 16.1 |
| Concentration/ decision making |
942 | 93.1 | 22.0 | 6.2 |
| Self-view | 942 | 84.0 | 7.5 | 3.3 |
| Suicidal ideation | 942 | 49.6 | 2.4 | 0.2 |
| General interest | 942 | 92.0 | 9.8 | 5.3 |
| Energy | 942 | 92.8 | 23.0 | 5.3 |
| Slowed down | 942 | 76.2 | 6.3 | 4.5 |
| Restlessness | 942 | 66.5 | 18.9 | 8.2 |
For example, 76.4% of participants had sleep onset insomnia at baseline. Of these, 35.8% continued to have sleep onset insomnia at exit. Of all participants who had sleep onset insomnia at exit, 9.0% did not have it at baseline.
Fig. 1.
Frequency distribution of total number of residual domains of the 16-item Quick Inventory of Depressive Symptomatology – Self-Report (QIDS-SR16) by time to remit status. □, <6 weeks; ■, ⩾6 weeks.
With regard to treatment-emergent symptoms, almost 25% of participants without mid-nocturnal insomnia at baseline developed it by exit. Other notable treatment-emergent symptoms included hypersomnia, early morning insomnia, changes in appetite and weight, decreased concentration and interest, and fatigue or decreased energy. Treatment-emergent suicidal ideation was found in 0.2%, with all of these 12 participants scoring 1 on the QIDS-SR16 suicide item (‘I feel that life is empty or wonder if it’s worth living’); none had thoughts of suicide or death, or made specific plans.
Table 3 shows data on residual symptoms at exit by QIDS-SR16 symptom domain. Remitters had a range from 0 to 6 residual domains. Of the nine domains, the most frequent were sleep disturbance (71.7%), appetite/weight disturbance (35.9%), sad mood (27.1%), fatigue or decreased energy (22.9%), and decreased concentration (20.9%). Table 3 also shows the percentages of participants with one domain of residual symptoms at exit who also had another symptom domain. For example, of 676 participants with residual sleep disturbance, 35.8% had appetite/weight disturbances, 28.1% had sad mood, 24.7% had fatigue or decreased energy, and 21.9% had decreased concentration. Most participants with any residual symptom domain had other associated symptom domains.
Table 3.
Proportion of remitters with any given residual symptoms by domain who had another symptom domain at exit (n = 943)
| Symptom domains | Sleep disturbance (n = 676) 71.7% |
Sad mood (n = 256) 27.1% |
Appetite/weight (n = 339) 35.9% |
Concentration (n = 197) 20.9% |
Outlook (n = 64) 6.8% |
Suicidal ideation (n = 12) 1.3% |
Involvement (n = 89) 9.4% |
Energy/fatigability (n = 212) 22.9% |
Psychomotor (n = 180) 19.1% |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sleep | 676 | (100) | 190 | (74.2) | 242 | (71.4) | 148 | (75.1) | 45 | (70.3) | 6 | (50.0) | 65 | (73.0) | 167 | (78.8) | 130 | (72.2) |
| Sad mood | 190 | (28.1) | 256 | (100) | 77 | (22.7) | 73 | (37.1) | 33 | (51.6) | 10 | (83.3) | 33 | (37.1) | 64 | (30.2) | 57 | (31.7) |
| Appetite/weight | 242 | (35.8) | 77 | (30.1) | 339 | (100) | 59 | (30.0) | 14 | (21.9) | 3 | (25.0) | 25 | (28.1) | 77 | (36.3) | 62 | (34.4) |
| Concentration | 148 | (21.9) | 73 | (28.5) | 59 | (17.4) | 197 | (100) | 20 | (31.3) | 2 | (16.7) | 26 | (29.2) | 64 | (30.2) | 64 | (35.6) |
| Outlook | 45 | (6.7) | 33 | (12.9) | 14 | (4.1) | 20 | (10.2) | 64 | (100) | 3 | (25.0) | 10 | (11.2) | 12 | (5.7) | 16 | (8.9) |
| Suicidal ideation | 6 | (0.9) | 10 | (3.9) | 3 | (0.9) | 2 | (1.0) | 3 | (4.7) | 12 | (100) | 0 | (0.0) | 3 | (1.4) | 1 | (0.6) |
| Involvement | 65 | (9.6) | 33 | (12.9) | 25 | (7.4) | 26 | (13.2) | 10 | (15.6) | 0 | (0.0) | 89 | (100) | 34 | (16.0) | 19 | (10.6) |
| Energy/fatigability | 167 | (24.7) | 64 | (25.0) | 77 | (22.7) | 64 | (32.5) | 12 | (18.8) | 3 | (25.0) | 34 | (38.2) | 212 | (100) | 53 | (29.4) |
| Psychomotor | 130 | (19.2) | 57 | (22.3) | 62 | (18.3) | 64 | (32.5) | 16 | (25.0) | 1 | (8.3) | 19 | (21.4) | 53 | (25.0) | 180 | (100) |
Values given as n (%).
Each column is the residual symptom domain. Each row is the number and proportion of patients who also have another symptom domain. For example, of 676 patients who had residual insomnia, 190 (28.1%) also had sad mood.
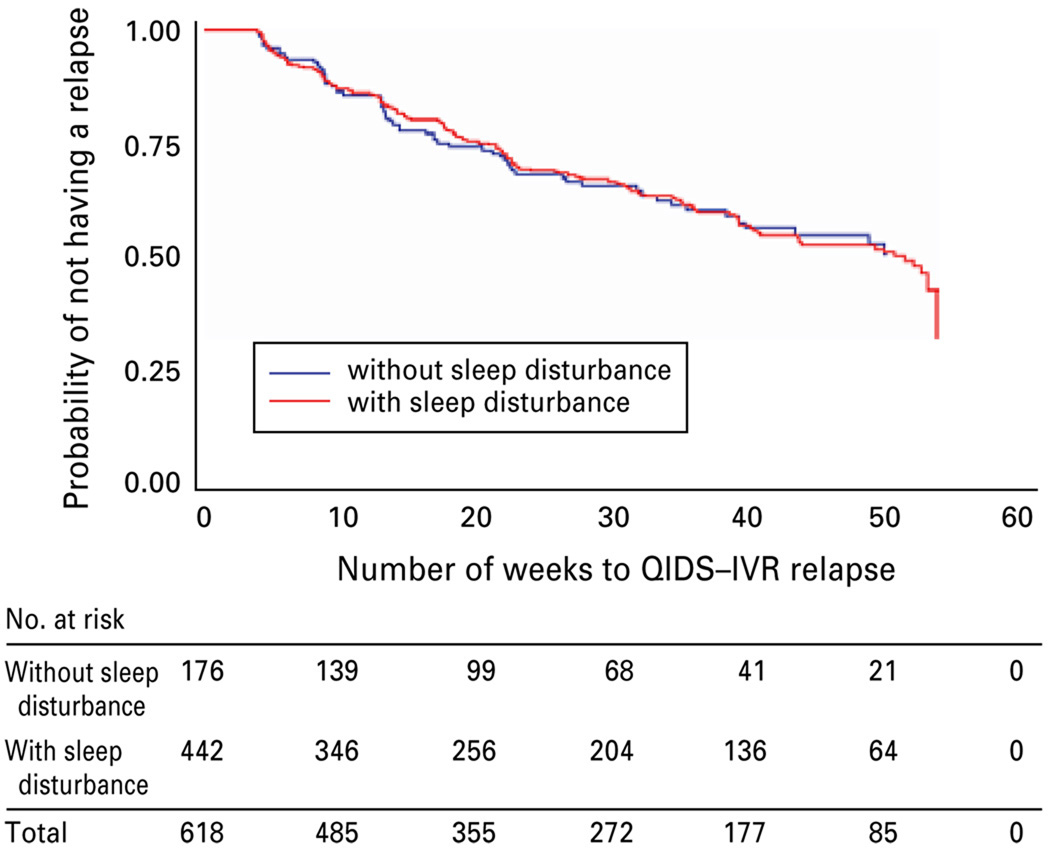
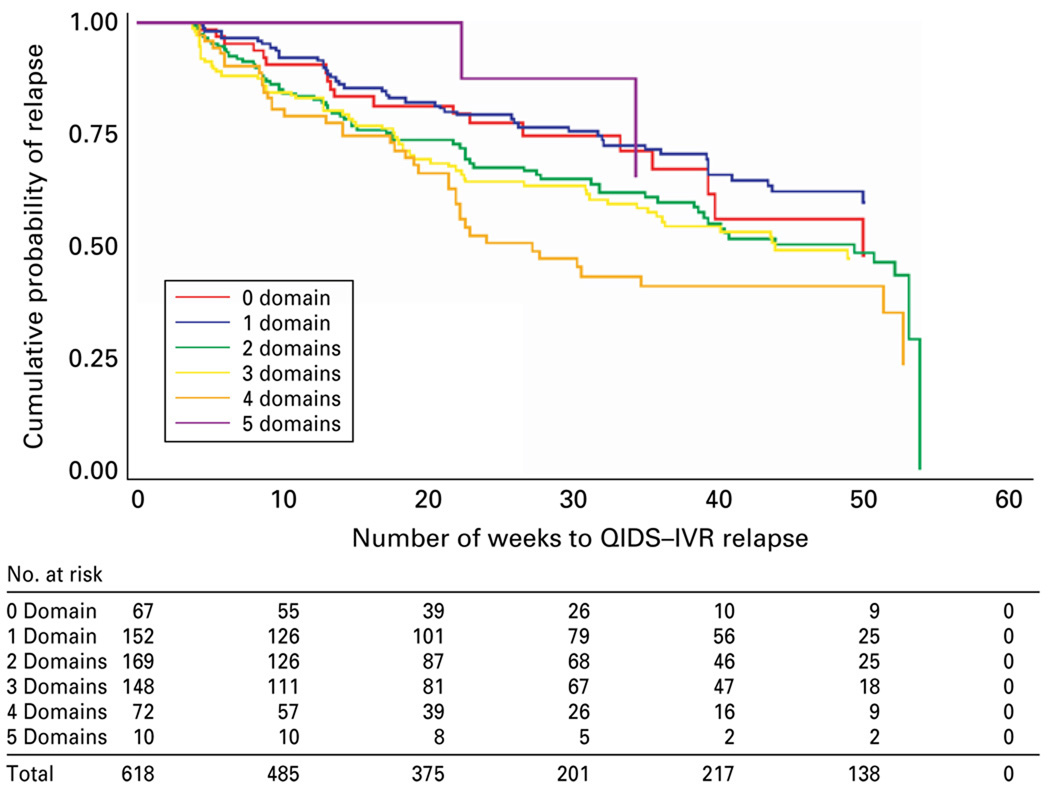
Participants who remitted with citalopram were invited to participate in a monthly follow-up phase for 12 months of naturalistic treatment. We examined the effect of the residual sleep disturbance domain, and also of the number of residual domains, on depressive relapse. No difference was found for those with or without sleep disturbance [χ2(1) = 0.0007, p = 0.9794; Fig. 2]. Those with a greater number of residual symptom domains had a greater probability of relapse [χ2(5) = 17.7155, p = 0.0033], with the exception that those with five domains did not (note that this group consisted of only 10 participants; Fig. 3).
Fig. 2.
Kaplan–Meier survival curve for those with and without the domain of residual sleep disturbance in the year following acute remission with citalopram.
Fig. 3.
Kaplan–Meier survival curve of major depressive disorder relapse with the number of residual symptom domains in the year following acute remission with citalopram.
Discussion
This is the first study to describe residual symptoms and their impact on depressive relapse after measurement-based treatment with an SSRI in a large generalizable population of out-patients with non-psychotic MDD. By using measurement-based care, clinicians titrated the dose of citalopram vigorously, systematically tracked depressive symptoms and adverse events, and extended the duration of acute treatment for up to 14 weeks. Additionally, clinicians could use ancillary treatments to manage anxiety and insomnia. However, even with optimized SSRI antidepressant treatment, 90% of participants who reached remission experienced at least one residual symptom.
Remitters, who by definition should be within the normal range of depressive symptoms, had a surprisingly large burden of residual depressive symptoms. The most common residual symptom domains for remitters were sleep disturbance (especially mid-nocturnal insomnia), appetite/weight disturbance, sad mood, decreased energy, and decreased concentration. Over 70% of the remitters had at least moderate sleep disturbance, and over 35% had at least moderate problems with appetite/weight disturbance. Although sleep disturbance was the most common symptom domain to emerge during treatment, most of those who had these symptoms at the end of treatment also had them at baseline. We expected that overall baseline severity of depression would be associated with residual symptoms, but it was not. Perhaps this lack of association is related to the greater responsiveness of more severe depression to pharmacological intervention.
Prior studies of residual depressive symptoms have shown high rates of insomnia, fatigue, concentration and weight changes after successful treatment (Nierenberg et al. 1999; Fava et al. 2007). Rates of overall residual insomnia reported in remitters to pharmacotherapy range from 44% (Nierenberg et al. 1999) to 53% (Carney et al. 2007). We found that 29.5, 54.9 and 16.6% of remitters had at least mild onset, mid-nocturnal or early morning insomnia respectively, and 9.7, 40.5 and 6.8% respectively had these symptoms at least at a moderate level. Unlike other studies that examined residual symptoms in patients who were not allowed to use ‘rescue’ medications, STAR*D participants could receive medications for insomnia, including hypnotics or low-dose trazodone. Even with the option of using these adjunctive medications, however, only 21 of 943 (2.6%) remitters took hypnotics and 24 of 943 (2.6%) took adjunctive trazodone. In the context of minimal use of these additional medications, residual insomnia persisted as a problem. These data suggest that residual insomnia occurs frequently, and few patients take adjunctive treatment. We were surprised to find that sleep disturbance was not associated with relapse. One possible explanation is that residual sleep disturbance may be a highly sensitive but relatively non-specific indicator of residual depression (i.e. with a very low threshold).
As might be expected, about a quarter of the remitters with residual sleep disturbance also had residual fatigue and decreased energy (24.7%) and decreased concentration (21.9%), problems that could conceivably result from sleep disturbance. An alternative explanation for the presence of residual sleep disturbance is that, even though these symptoms were present at baseline and endpoint, sleep disturbance (and other residual symptoms) could just as plausibly be present due to the side-effects of citalopram or due to concomitant general medical conditions or other medications being taken for these conditions. This study does not allow us to make this distinction.
Fatigue has been the focus of several reports of residual symptoms and their treatment in partial responders and remitters (Nierenberg et al. 1999; DeBattista et al. 2003; Stahl et al. 2003; Fava et al. 2005; Thase et al. 2006). A prior study of remitters with fluoxetine found 38% had residual fatigue (Nierenberg et al. 1999). We found that 22.5% of remitters with citalopram had residual fatigue. It is possible that fewer remitters had residual fatigue in this study because of the vigorous dosing, careful monitoring, and extended duration of treatment with citalopram. Additionally, some prior reports of residual fatigue included responders without remission whereas this report focuses on remitters only.
The emergence of suicidal ideation with antidepressant treatment has been the focus of multiple studies and meta-analyses (e.g. Simon, 2006; Leon, 2007; Leon et al. 2007), but these have not explored suicidal ideation in remitters. In our study, of those remitters who did not have any suicidal ideation at baseline, 0.2% had very mild residual suicidal ideation after remission. The 12 patients with residual suicidal ideation also had continued sad mood (10/12) and insomnia (6/12). It might be speculated that treatment of insomnia would have led to further improvements in sad mood and suicidal ideation. Those with the most severe suicidal ideation at baseline, however, had robust improvements, with complete resolution of suicidal ideation and, of the less severe groups, only one participant had a slight worsening. Thus, suicidal ideation was highly responsive to treatment in remitters. Only a very small minority had either persistent or treatment-emergent suicidal ideation.
A shorter time to remission (<6 weeks) was associated with having fewer residual symptom domains (see Fig. 1). One possible explanation for this is that remitters who have their remission occur within the first 6 weeks of treatment have a more robust remission. Alternatively, those with later remissions may not have the time for their residual symptoms to fully resolve and just need more time. Follow-up analyses of the STAR*D data can address these issues.
Finally, even though sleep disturbance was not associated with relapse, an increased number of residual domains was associated with relapse. Although prior studies of naturalistic treatment found that residual symptoms predict relapse (Judd et al. 1998a), our findings regarding relapse are unique because of the combination of measurement-based care and uniformity of treatment with citalopram, as well as the generalizability of the participants.
The strengths of this study include the large representative sample of out-patients with MDD who had a full range of concurrent psychiatric and general medical conditions and were treated in primary and psychiatric care settings. Treatment was administered using measurement-based care (Trivedi et al. 2006) so that antidepressant treatment was optimized.
This study has several limitations. It was not designed to assess residual symptoms and the results are based on a post-hoc analysis. Treatment was provided openly to patients so that a placebo effect was probably included, but, if anything, open treatment would be expected to minimize residual symptoms. The categorical definition of the presence of any residual symptom was set at a minimal level (i.e. any QIDS-SR16 item above zero). It could be argued that a higher threshold for residual symptoms could have been set (e.g. QIDS-SR16 symptom scores above 1 or 2). Criteria for relapse were, likewise, set at a minimal QIDS-SR16 score. It is possible that these clinical exacerbations could or could not have met full criteria for another depressive episode.
In summary, among participants who reached remission after acute-phase depression treatment, residual symptoms are common; less than 10% of full remitters to citalopram were entirely free of residual depressive symptoms. Sleep disturbance was the most common residual symptom domain, followed by appetite/weight disturbance, persistent sad mood, fatigue or decreased energy, and decreased concentration. In general, the more residual symptom domains present after acute-phase treatment, the higher the risk of relapse, but residual sleep disturbance alone is not a significant predictor of relapse. Further studies are needed to assess the time course of individual residual symptoms during longer-term treatment, and their relationship to depressive relapse and dysfunction.
Acknowledgements
This project was funded by the National Institute of Mental Health under Contract N01MH90003 to UT Southwestern Medical Center at Dallas (PI: A. J. Rush). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. We appreciate the support of Bristol–Myers Squibb, Forest Laboratories, GlaxoSmithKline, King Pharmaceuticals, Organon, Pfizer, and Wyeth for providing medications at no cost for this trial. We also acknowledge the editorial support of Jon Kilner, M.S., M.A. [Trial registration: National Institute of Mental Health (NIMH), ClinicalTrials.gov identifier and link: NCT00021528.]
Footnotes
Declaration of Interest
Andrew A. Nierenberg, M.D. Research support: Bristol–Myers Squibb Company; Cederroth; Cyberonics, Inc.; Forest Pharmaceuticals Inc.; GlaxoSmithKline; Janssen Pharmaceutica; Lichtwer Pharma; Eli Lilly & Company; PamLabs; Pfizer Inc.; National Institute of Mental Health; National Alliance for Research in Schizophrenia and Depression, Stanley Foundation; Wyeth-Ayerst Laboratories. Advisory/Consulting: AstraZeneca; Basilea Pharmaceutica; Brain Cells, Inc., Bristol–Myers Squibb Company; Dainippon Sumitomo; Eli Lilly & Company; EpiQ; Genaissance; GlaxoSmithKline; Jazz Pharmaceuticals; Innapharma; Merck; Neuronetics; Novartis; Pfizer, Inc.; PGx Health; Sepracor; Shire; Targacept; Takeda. Speaking: Eli Lilly & Company; GlaxoSmithKline; Organon, Inc.; Wyeth-Ayerst Laboratories; Massachusetts General Psychiatry Academy [MGHPA talks are supported through Independent Medical Education (IME) grants from the following pharmaceutical companies in 2008: Astra Zeneca, Eli Lilly, and Janssen Pharmaceuticals]. No other speaker bureaus since 2003. Equity Holdings (exclude mutual funds/blinded trusts): Appliance Computing, Inc. Mustafa M. Husain, M.D. Research Support: National Institute of Mental Health; Stanley Medical Research Institute; Cyberonics, Inc.; Pfizer, Inc. (in process); Neuronetics, Inc.; Magstim; Medtronics, Inc. (potential research sponsor). Advisory/Consulting: AstraZeneka; VersusMed; Avinar; Boston Scientific; MEASURE; Bristol–Myers Squibb; Clinical Advisors. Speaking: Cyberonics, Inc.; Avinar, Inc.; Cerebrio, Inc.; AstraZeneka; Bristol-Meyers-Squibb; Optima/Forrest Pharmaceuticals; Glaxo-Smith-Kline; Forrest Pharmaceuticals; Janssen. Madhukar H. Trivedi, M.D. Research support: Bristol–Myers Squibb Company; Cephalon, Inc.; Corcept Therapeutics, Inc.; Eli Lilly & Company; GlaxoSmithKline; Janssen Pharmaceutica; National Institute of Mental Health; National Alliance for Research in Schizophrenia and Depression; Pfizer Inc.; Predix Pharmaceuticals; Wyeth-Ayerst Laboratories. Advisory/Consulting: Abbott Laboratories, Inc.; Akzo (Organon Pharmaceuticals Inc.); Bayer; Bristol–Myers Squibb Company; Cyberonics, Inc.; Forest Pharmaceuticals; GlaxoSmithKline; Janssen Pharma-ceutica Products, LP; Johnson & Johnson PRD; Eli Lilly & Company; Meade Johnson; Parke-Davis Pharmaceuticals, Inc.; Pfizer, Inc.; Pharmacia & Upjohn; Sepracor; Solvay Pharmaceuticals, Inc.; Wyeth-Ayerst Laboratories. Speaking: Akzo (Organon Pharmaceuticals Inc.); Bristol–Myers Squibb Company; Cyberonics, Inc.; Forest Pharmaceuticals; Janssen Pharmaceutica Products, LP; Eli Lilly & Company; Pharmacia & Upjohn; Solvay Pharmaceuticals, Inc.; Wyeth–Ayerst Laboratories. Equity Holdings (exclude mutual funds/blinded trusts): None. Maurizio Fava, M.D. Research support: Abbott Laboratories; Alkermes; Aspect Medical Systems; Astra-Zeneca; Bristol–Myers Squibb Company; Cephalon; Forest Pharmaceuticals Inc.; GlaxoSmithKline; J & J Pharmaceuticals; Lichtwer Pharma GmbH; Eli Lilly & Company; Lorex Pharmaceuticals; Novartis; Organon Inc.; PamLab, LLC; Pfizer Inc.; Pharmavite; Roche; Sanofi/Synthelabo; Solvay Pharmaceuticals, Inc.; Wyeth-Ayerst Laboratories. Advisory/Consulting: Aspect Medical Systems; Astra-Zeneca; Bayer AG; Biovail Pharmaceuticals, Inc.; BrainCells, Inc.; Bristol–Myers Squibb Company; Cephalon; Compellis; Cypress Pharmaceuticals; Dov Pharmaceuticals; EPIX Pharmaceuticals; Fabre-Kramer Pharmaceuticals, Inc.; Forest Pharmaceuticals Inc.; GlaxoSmithKline; Grunenthal GmBH; J & J Pharmaceuticals; Janssen Pharmaceutica; Jazz Pharmaceuticals; Knoll Pharmaceutical Company; Eli Lilly & Company; Lundbeck; MedAvante, Inc.; Novartis; Nutrition 21; Organon Inc.; PamLab, LLC; Pfizer Inc.; PharmaStar; Pharmavite; Roche; Sanofi/Synthelabo; Sepracor; Solvay Pharmaceuticals, Inc.; Somerset Pharmaceuticals; Wyeth-Ayerst Laboratories. Speaking: AstraZeneca; Bristol–Myers Squibb Company; Cephalon; Forest Pharmaceuticals Inc.; GlaxoSmithKline; Eli Lilly & Company; Novartis; Organon Inc.; Pfizer Inc.; PharmaStar; Wyeth-Ayerst Laboratories. Equity Holdings (exclude mutual funds/blinded trusts): Compellis; MedAvante. Diane Warden, Ph.D, M.B.A. Research Support: National Institute of Mental Health; National Institute of Drug Abuse; NARSAD. Equity holdings: Pfizer; Bristol–Myers Squibb. Stephen R. Wisniewski, Ph.D. Research support: National Institute of Mental Health. Advisory/Consulting: Cyberonics, Inc. Equity Holdings (exclude mutual funds/blinded trusts): None. A. John Rush, M.D. Research support: Robert Wood Johnson Foundation; the National Institute of Mental Health; the Stanley Medical Research Institute. Advisory/Consulting: Advanced Neuronetic Systems, Inc.; AstraZeneca; Best Practice Project Management, Inc.; Bristol–Myers Squibb Company; Cyberonics, Inc.; Forest Pharmaceuticals, Inc.; Gerson Lehman Group; GlaxoSmith-Kline; Healthcare Technology Systems, Inc.; Jazz Pharmaceuticals; Eli Lilly & Company; Magellan Health Services; Merck & Co., Inc.; Neuronetics; Ono Pharmaceutical; Organon USA Inc.; Personality Disorder Research Corp.; Pfizer Inc.; The Urban Institute; Wyeth-Ayerst Laboratories Inc. Speaking: Cyberonics, Inc.; Forest Pharmaceuticals, Inc.; GlaxoSmithKline; Eli Lilly & Company; Merck & Co., Inc. Equity Holdings (exclude mutual funds/blinded trusts): Pfizer Inc. Royalty/patent, other income: Guilford Publications; Healthcare Technology Systems, Inc.
References
- Bockting C, Spinhoven P, Koeter M, Wouters L, Schene A Depression Evaluation Longitudinal Therapy Assessment (DELTA) Study Group. Prediction of recurrence in recurrent depression and the influence of consecutive episodes on vulnerability for depression: a 2-year prospective study. Journal of Clinical Psychiatry. 2006;67:747–755. [PubMed] [Google Scholar]
- Carney CE, Segal ZV, Edinger JD, Krystal AD. A comparison of rates of residual insomnia symptoms following pharmacotherapy or cognitive-behavioral therapy for major depressive disorder. Journal of Clinical Psychiatry. 2007;68:254–260. doi: 10.4088/jcp.v68n0211. [DOI] [PubMed] [Google Scholar]
- DeBattista C, Doghramji K, Menza M, Rosenthal M, Fieve R. Adjunct modafinil for the short-term treatment of fatigue and sleepiness in patients with major depressive disorder: a preliminary double-blind, placebo-controlled study. Journal of Clinical Psychiatry. 2003;64:1057–1064. doi: 10.4088/jcp.v64n0911. [DOI] [PubMed] [Google Scholar]
- Fava G, Ruini C, Belaise C. The concept of recovery in major depression. Psychological Medicine. 2007;37:307–317. doi: 10.1017/S0033291706008981. [DOI] [PubMed] [Google Scholar]
- Fava GA, Fabbri S, Sonino N. Residual symptoms in depression: an emerging therapeutic target. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2002;26:1019–1027. doi: 10.1016/s0278-5846(02)00226-9. [DOI] [PubMed] [Google Scholar]
- Fava M, Rush AJ, Trivedi MH, Nierenberg AA, Thase ME, Sackeim HA, Quitkin FM, Wisniewski S, Lavori PW, Rosenbaum JF, Kupfer DJ. Background and rationale for the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study. Psychiatric Clinics of North America. 2003;26:457–494. doi: 10.1016/s0193-953x(02)00107-7. [DOI] [PubMed] [Google Scholar]
- Fava M, Thase ME, DeBattista C. A multicenter, placebo-controlled study of modafinil augmentation in partial responders to selective serotonin reuptake inhibitors with persistent fatigue and sleepiness. Journal of Clinical Psychiatry. 2005;66:85–93. doi: 10.4088/jcp.v66n0112. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–61. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. British Journal of Social and Clinical Psychology. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W, Paulus MP, Kunovac JL, Leon AC, Mueller TI, Rice JA, Keller MB. Major depressive disorder: a prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. Journal of Affective Disorders. 1998a;50:97–108. doi: 10.1016/s0165-0327(98)00138-4. [DOI] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W, Paulus MP, Kunovac JL, Leon AC, Mueller TI, Rice JA, Keller MB. A prospective 12-year study of subsyndromal and syndromal depressive symptoms in unipolar major depressive disorders. Archives of General Psychiatry. 1998b;55:694–700. doi: 10.1001/archpsyc.55.8.694. [DOI] [PubMed] [Google Scholar]
- Kanai T, Takeuchi H, Furukawa TA, Yoshimura R, Imaizumi T, Kitamura T, Takahashi K. Time to recurrence after recovery from major depressive episodes and its predictors. Psychological Medicine. 2003;33:839–845. doi: 10.1017/s0033291703007827. [DOI] [PubMed] [Google Scholar]
- Kennedy N, Paykel E. Residual symptoms at remission from depression: impact on long-term outcome. Journal of Affective Disorders. 2004;80:135–144. doi: 10.1016/S0165-0327(03)00054-5. [DOI] [PubMed] [Google Scholar]
- Leon AC. The revised warning for antidepressants and suicidality: unveiling the black box of statistical analyses. American Journal of Psychiatry. 2007;164:1786–1789. doi: 10.1176/appi.ajp.2007.07050775. [DOI] [PubMed] [Google Scholar]
- Leon AC, Marzuk PM, Tardiff K, Bucciarelli A, Stajic M, Piper TM, Galea S. Antidepressants in adult suicides in New York City: 2001–2004. Journal of Clinical Psychiatry. 2007;68:1399–1403. doi: 10.4088/jcp.v68n0911. [DOI] [PubMed] [Google Scholar]
- Mintz J, Mintz LI, Arruda MJ, Hwang SS. Treatments of depression and the functional capacity to work. Archives of General Psychiatry. 1992;49:761–768. doi: 10.1001/archpsyc.1992.01820100005001. [DOI] [PubMed] [Google Scholar]
- Mojtabai R. Residual symptoms and impairment in major depression in the community. American Journal of Psychiatry. 2001;158:1645–1651. doi: 10.1176/appi.ajp.158.10.1645. [DOI] [PubMed] [Google Scholar]
- Nierenberg AA, Keefe BR, Leslie VC, Alpert JE, Pava JA, Worthington JJ, Rosenbaum JF, Fava M. Residual symptoms in depressed patients who respond acutely to fluoxetine. Journal of Clinical Psychiatry. 1999;60:221–225. doi: 10.4088/jcp.v60n0403. [DOI] [PubMed] [Google Scholar]
- Nierenberg AA, Wright EC. Evolution of remission as the new standard in the treatment of depression. Journal of Clinical Psychiatry. 1999;60:7–11. [PubMed] [Google Scholar]
- Rush AJ, Bernstein IH, Trivedi MH, Carmody TJ, Wisniewski S, Mundt JC, Shores-Wilson K, Biggs MM, Woo A, Nierenberg AA, Fava M. An evaluation of the Quick Inventory of Depressive Symptomatology and the Hamilton Rating Scale for Depression: a Sequenced Treatment Alternatives to Relieve Depression trial report. Biological Psychiatry. 2006a;59:493–501. doi: 10.1016/j.biopsych.2005.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Fava M, Wisniewski SR, Lavori PW, Trivedi MH, Sackeim HA, Thase ME, Nierenberg AA, Quitkin FM, Kashner TM, Kupfer DJ, Rosenbaum JF, Alpert J, Stewart JW, McGrath PJ, Biggs MM, Shores-Wilson K, Lebowitz BD, Ritz L, Niederehe G. Sequenced Treatment Alternatives to Relieve Depression (STAR*D): rationale and design. Controlled Clinical Trials. 2004;25:119–142. doi: 10.1016/s0197-2456(03)00112-0. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Kraemer HC, Sackeim HA, Fava M, Trivedi MH, Frank E, Ninan PT, Thase ME, Gelenberg AJ, Kupfer DJ, Regier DA, Rosenbaum JF, Ray O, Schatzberg AF. Report by the ACNP Task Force on Response and Remission in Major Depressive Disorder. Neuropsychopharmacology. 2006b;31:1841–1853. doi: 10.1038/sj.npp.1301131. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biological Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- Simon GE. How can we know whether antidepressants increase suicide risk? American Journal of Psychiatry. 2006;163:1861–1863. doi: 10.1176/ajp.2006.163.11.1861. [DOI] [PubMed] [Google Scholar]
- Stahl SM, Zhang LS, Damatarca C, Grady M. Brain-circuits determine destiny in depression: a novel approach to the psychopharmacology of wakefulness, fatigue, and executive dysfunction in major depressive disorder. Journal of Clinical Psychiatry. 2003;64:6–17. [PubMed] [Google Scholar]
- Thase ME, Fava M, DeBattista C, Arora S, Hughes RJ. Modafinil augmentation of SSRI therapy in patients with major depressive disorder and excessive sleepiness and fatigue: a 12-week, open-label, extension study. CNS Spectrums. 2006;11:93–102. doi: 10.1017/s1092852900010622. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Ibrahim HM, Carmody TJ, Biggs MM, Suppes T, Crismon ML, Shores-Wilson K, Toprac MG, Dennehy EB, Witte B, Kashner TM. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychological Medicine. 2004;34:73–82. doi: 10.1017/s0033291703001107. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. American Journal of Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- Wisniewski SR, Rush AJ, Balasubramani GK, Trivedi MH, Nierenberg AA for the STAR*D Investigators. Self-rated global measure of the frequency, intensity, and burden of side effects. Journal of Psychiatric Practice. 2006;12:71–79. doi: 10.1097/00131746-200603000-00002. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Posternak MA, Chelminski I. Heterogeneity among depressed outpatients considered to be in remission. Comprehensive Psychiatry. 2007;48:113–117. doi: 10.1016/j.comppsych.2006.10.005. [DOI] [PubMed] [Google Scholar]