Abstract
The ability of different CD4+ T cell subsets to help a CD8+ T cell response is not fully understood. Here, we found that Th17 cells induced by IL-1β, unlike Th1, were not effective helpers for antiviral CD8 responses as measured by IFNγ-producing cells or protection against virus infection. However, they skewed CD8 responses to a Tc17 phenotype. Thus, the apparent lack of help was actually immune deviation. This skewing depended on both IL-21 and IL-23. To overcome this effect, we inhibited Th17 induction by blocking TGF-β. Anti-TGF-β allowed the IL-1β adjuvant to enhance CD8+ T cell responses without skewing the phenotype to Tc17, thereby providing an approach to harness the benefit of common IL-1-inducing adjuvants like alum without immune deviation.
Introduction
Studies of CD4+ T cell help for CD8+ T cell responses has mostly been conducted under conditions in which the CD4+ T cells are predominantly of the Th1 subset. However, little is known about the ability of other T helper subsets to help a CD8+ T cell response. As the importance of other CD4+ T cell subsets in disease is rapidly emerging, their role in helping or perturbing CD8+ T cell responses becomes critical to understand.
IL-1 orchestrates the differentiation and function of innate and adaptive immune systems. Through the induction of adhesion molecules in endothelial cells and chemokines it amplifies leukocyte recruitment to sites of infection and stimulates the effector function of the recruited innate cells, resulting in enhanced immune response to infections. Together with other cytokines from the activated innate cells, IL-1 promotes the antigen-capturing cells such as macrophages and dendritic cells (DC) to become efficient antigen-presenting cells to adaptive lymphocytes (1). IL-1 strikingly enhances expansion of antigen-primed CD8 and CD4 T cells in vivo (2-4). In addition, during immunization IL-1 increases the frequency of IL-17- and IFN-γ-producing cells among primed CD4 cells and the frequency of granzyme B-expressing, IFN-γ-producing and cytotoxic cells among primed CD8 cells. Thus, IL-1β enhances antigen-primed CD4 and CD8 T-cell expansion, differentiation, and migration to the periphery and memory, the specific functions required for generation of effective protective immune responses (2-4).
In the course of studying IL-1β as an adjuvant, which has been shown to enhance CD4+ T cell responses but skew them toward Th17 phenotype (2), and more recently to enhance the number and functional activity of CD8+ T cells (3), we found a surprising paradox. IL-1 promoted a T cell response and protection against a vaccinia virus infection (which is IFNγ dependent) when the vaccine was a minimal CD8+ T cell epitope (P18-I10, RGPGRAFVTI), but surprisingly not when the vaccine was a fusion peptide of a helper epitope (PCLUS3) and the P18-I10 CD8 epitope, PCLUS3-P18-I10). Indeed, IL-1 as an adjuvant actually negated the improved response achieved with the helper epitope. Because it was known that IL-1β could skew the CD4+ T cell response toward Th17 cells (2, 4-7), we asked whether the loss of help for a CD8+ T cell response was due to an inability of Th17 cells to provide help for a CD8+ T cell response. This possibility was unexpected because Th17 cells had been shown to be good helpers for B cell responses (8). We investigated the mechanism of this Th17-mediated help or lack of help in terms of the roles of dendritic cells and of the cytokines that alter the CD8+ T cell response. Our study identified an approach to block the impaired anti-viral protection in the presence of Th17 cell help that may have clinical utility, especially in view of the facts that the most commonly-used adjuvants such as alum can induce IL-1 production through activation of the NALP3 inflammasome (9) and that in chronic infections continuous IL-1 secretion may alter the desired protective immune response.
Results
IL-1β as an adjuvant enhances CD8 T cell responses but diminishes CD4 help
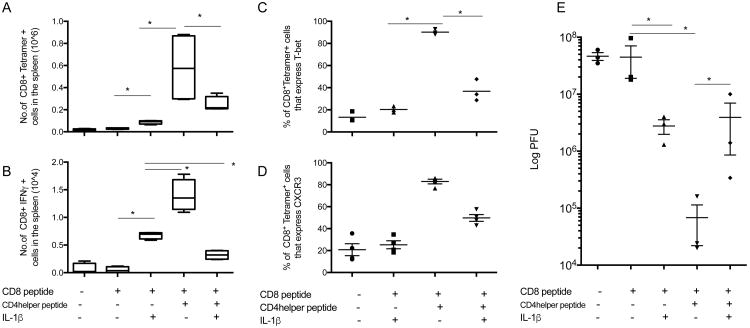
IL-1β is well known for its inflammatory properties (reviewed in (10)). Here, we examined the effect of IL-1 on the development of a CD8+ T cell response in the presence or absence of CD4+ T cell help. In animals immunized with P18-I10 peptide (dominant CD8 epitope) mixed in DOTAP as an adjuvant, IL-1β treatment enhanced both the proportion of tetramer-binding CD8+ T cells and the antigen-specific IFN-γ expression (Fig. 1A, B) Animals immunized with PCLUS3-P18-I10 peptide containing both CD8 and CD4 helper epitopes displayed a greater CD8+ T cell response as measured by tetramer binding and increased expression of CXCR3 and IFN-γ compared with animals immunized with the P18-I10 peptide alone (Fig. 1A-D). We previously found that physical linkage of the helper and CTL epitopes was critical to obtaining optimal CD4 help for a CD8 T cell response (11). Surprisingly, treatment with IL-1β in the animals that were given the PCLUS3-P18-I10 peptide actually reduced the frequency of IFN-γ expressing CD8+ T cells to levels found in animals immunized with the minimal P18-I10 peptide and IL-1, without CD4 help (Fig. 1B). Note that there was a reduction both in the absolute number of tetramer-positive CD8+ T cells (about 2-fold) when IL-1β was administered with the CD4/CD8 epitope vaccine (Fig 1A)and also in the fraction of those remaining tetramer-positive T cells that expressed Tbet (Fig 1C). Thus it appears that the IL-1β is altering not only the magnitude of the T cell response but also its phenotype. In corroboration, in animals immunized with P18-I10 peptide, IL-1β enhanced protection against systemic challenge with vaccinia virus, whereas in animals immunized with PCLUS3-P18-I10 peptide, IL-1β treatment reduced the higher level of protection against virus challenge afforded by immunization in the presence of CD4 help (Fig. 1E).
Figure 1.
IL-1β abrogates CD4+ T cell help for a protective IFN-γ secreting CD8+ T cell response. Mice were immunized with P18-I10 (CD8 epitope) or PCLUS3-18IIIB (CD4 helper + CD8 epitope), 7 days later, spleens were removed and expression of P18-I10 tetramer, and CXCR3 and T-bet on live tetramer specific CD3+CD8+ T cells was analyzed (A, C, D). Splenocytes were stimulated in vitro with P18-I10 peptide overnight and expression of IFN-γ was measured in CD8+ T cells (B). 10 days post-vaccination, mice were infected i.p. with 2 × 107pfu of vPE16. 5 days later viral pfu was determined in the ovaries (E). Each experiment was performed three times. Mann whitney test was used to determine significance. * indicates P<0.05.
IL-1-induced skewing of CD4 T cells to Th17 causes immune deviation of CD8 T cells to Tc17
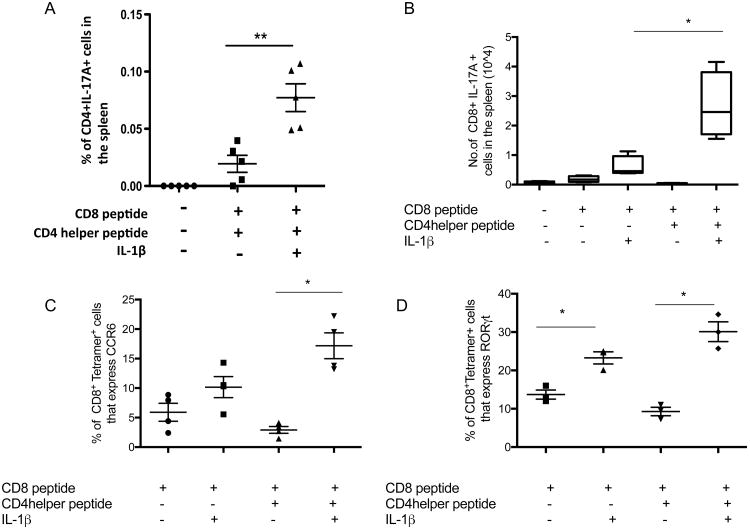
There are accumulating data demonstrating that IL-1β promotes development of Th17 responses in vivo (2,4-6) Therefore, we hypothesized that in the animals given IL-1β and the peptide vaccine containing CD4 helper epitopes, the vaccine-induced CD4 response may be of predominantly IL-17-secreting cells and that these Th17 cells may not be effective at providing help for the development of an IFN-γ-secreting CD8+ T cell response. Consistent with prior studies (2, 4-6), we found that IL-1β promotes the development of Th17 cells (Fig. 2A). Interestingly we also observed an increase in the expression of IL-17A, RORγt and CCR6 by antigen specific CD8+ T cells in both groups of immunized animals that received IL-1 (Fig. 2B, C, D). Note that in these cases, IL-1β increases the CD8+ T cell response even without the presence of stimulated CD4+ T cell help, as we have seen previously (3), but it is the surprising role of CD4+ T cell help in the presence of IL-1 that we interpret as Th17 help in the CD8+ T cell response that is the key unexpected observation coming from (Fig. 1A-E).
Figure 2.
IL-1β enhances development of Tc17 response. Mice were immunized with P18-I10 (CD8 epitope) or PCLUS3-18IIIB (CD4 helper epitope + CD8 epitope), 7 days later, spleens were removed and were stimulated in vitro with PCLUS3-18IIIB peptide overnight and expression of IL-17A was measured in both CD4+ and CD8+ T cells (A. B). The percentage of live P18-I10 tetramer-positive specific CD3+CD8+ T cells expressing CCR6 (Panel C) or RORγt (Panel D) was analyzed. Each experiment was performed three times. Mann Whitney test was used to determine significance. * P<0.05, and **P<0.01.
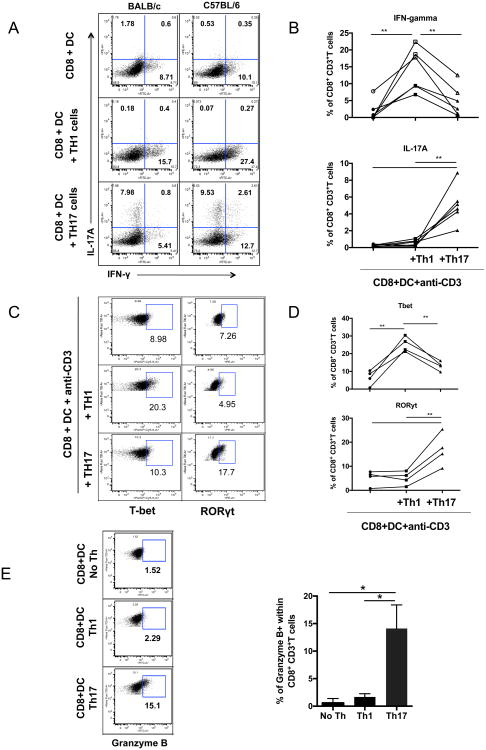
To test whether the Th17 cells induced were simply poor helpers for a CD8+ T cell response or they selectively induced a different kind of CD8+ T cell response instead of a Tc1 (IFNγ-producing) response, we asked whether Th17 cells provide help for the development of a Tc17 response. Purified CD8+ T cells co-cultured with splenic DCs and in vitro derived Th1 cells (Supplemental Figure 2A) resulted in a high proportion of CD8+ T cells expressing IFN-γ and T-bet (Fig 3A-C). In contrast, CD8+ T cells co-cultured with in vitro-derived Th17 CD4+ T cells (Supplemental Figure 2A) differentiated into a population of CD8+ T cells that expressed IL-17A and RORγt, indicative of Tc17 cells (Fig. 3A-C). From the line graphs (panels B and D), the Th17 cells induced several-fold more IL-17-producing and RORγt expressing CD8+ T cells than did the Th1 cells (p < 0.01) and several fold fewer IFNγ-producing and Tbet+ cells than the Th1 cells induced (p < 0.01). IL-2 production by CD8+ T cells cultured with Th1 or Th17 cells was not significantly different, although there was a tendency to be higher with Th1 stimulation (Supplemental Fig 2B). We tested this finding with cells from both BALB/c and C57BL/6 mice (Fig. 3A) and cells from OT-I and OT-II transgenic mice (data not shown) and we obtained similar results in all cases. Surprisingly, we found that although only a minority (15%) of CD8+ T cells stimulated by Th17 cells expressed granzyme B, this percentage was higher than among CD8+ T cells helped by Th1 cells (Fig. 3E). It is possible that this subset is similar to the novel cytotoxic Th17 cells described by Tajima et al (12) that express granzyme B dependent on IL-12. Thus, overall, the data indicate that Th17 are poorer helpers for CD8+ T cell responses than Th1 cells and what help they provide mainly induces immune deviation toward Tc17 CD8+ T cells, except for the induction of granzyme B, in which activity they are superior.
Figure 3.
T helper 17 cells provide help for Tc17 response but not a Tc1 response. A-C. negatively selected purified CD8+ T cells (10̂6) were cocultured with syngeneic DCs (2×10̂5), and 10̂6 polarized CD4+ Th1 or Th17 cells prepared as described in Materials and Methods, along with anti-CD3 antibody in solution. A. BALB/c (left column) and C57BL.6 (right column)CD8+ T cells receiving help from Th1 cells (middle row) or Th17 cells (bottom row) were analyzed for IL-17 and IFN-γ production. B. Multiple similar experiments in BALB/c mice were compared to show statistics. C. In a similar culture experiment, the cells were permeabilized and stained for intracellular T-bet and RORγt, transcription factors for IFN-γ and IL-17, respectively. D. In a similar culture, the CD8+ T cells cultured with Th1, Th17 or no Th were stained for granzyme B expression. Each experiment was performed three times. Wilcoxon rank test was used to determine significance. * P<0.05, and **P<0.01.
Mechanistic differences in Th1 vs Th17 help for CD8 T cells
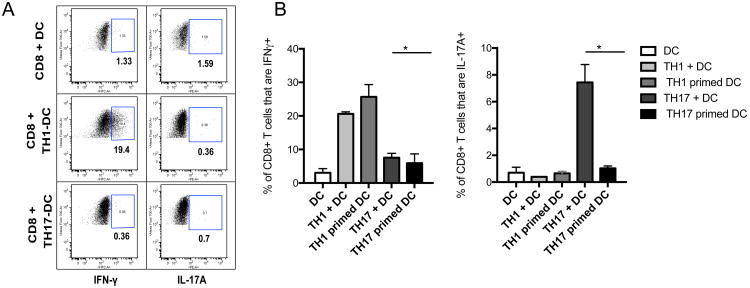
The mechanism by which Th1 cells provide help for Tc1 cells has been well characterized and involves DC activation or “licensing” through the CD40-CD40L and upregulation of IL-12 production(13-17). However, the mechanism by which Th17 cells provide help for Tc17 cells remains unknown. We first examined whether DC activated by either Th1 or Th17 cells could promote either IFN-γ or IL-17A secretion in CD8+ T cells. The CD8+ T cells were OT-I TCR transgenic T cells specific for the SIINFEKL epitope of ovalbumin, and the CD4+ T cells used were OT-II TCR transgenic T cells specific for another epitope of ovalbumin, and the DCs were pulsed with ovalbumin or SIINFEKL peptide. As shown previously by multiple groups (13-15, 18), DCs activated by Th1 cells were sufficient to promote IFN-γ expression by CD8+ T cells (Fig. 4A). However, DCs activated by Th17 cells did not enhance IL-17A expression by the CD8+ T cells (Fig. 4A), suggesting that there maybe different mechanisms by which different CD4 subsets provide help to CD8+ T cells. In other words, in contrast to the case of Th1 help, the actual presence of Th17 cells was necessary for the help (Fig 3A), and the DCs conditioned by Th17 cells were not sufficient by themselves (Fig 4A, B). The mechanism of this difference is beyond the scope of this study.
Figure 4.
Th1 cells provide help for Tc1 via dendritic cell priming, whereas Th17 cell help for Tc17 response requires both the helper cell and dendritic cell in the culture. A. Purified splenic DCs from B6 mice were cocultured with polarized Th1 or Th17 helper OT-II T cells, prepared as described in Materials and Methods, in the presence of ovalbumin antigen. After 16 hours, CD11c+ DCs were reisolated using magnetic beads and cultured with OT-I CD8+ T cells for 18 hours. The cells were intracellularly stained for IFN-γ or IL-17A, and positive cells within the CD8+ gate enumerated. B. Bar graph shows mean and standard deviation of 3 replicate cultures. Each experiment was performed three times. Mann Whitney test was used to determine significance. * P<0.05, **P<0.01, and **** P<0.0001.
Role of cytokines in immune deviation of CD8 T cells
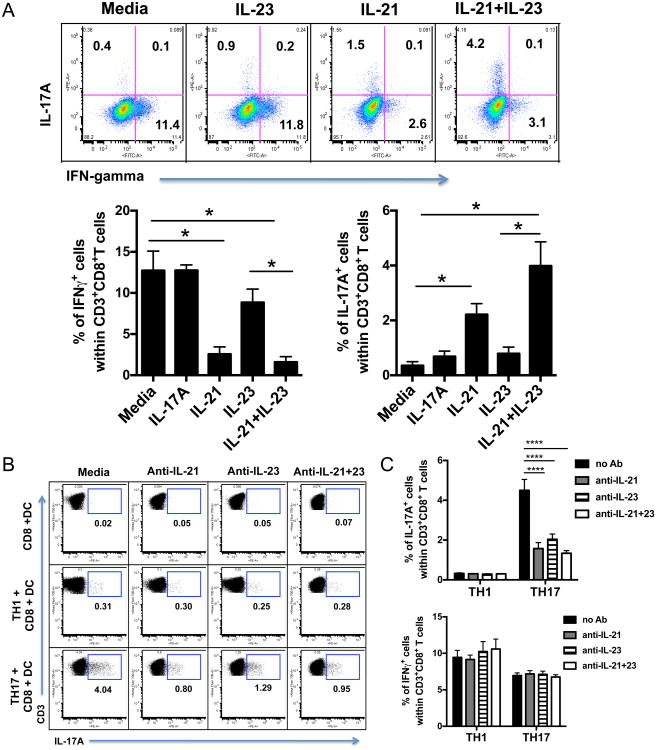
To address the mechanism of immune deviation, we examined the effect of exogenous Th17-associated cytokines on induction of IL-17-expressing CD8+T cells by adding different combinations of IL-17A, IL-17F, IL-21, and IL-23 to purified OT-1 cells co-cultured with splenic DCs loaded with SIINFEKL peptide. The combination of IL-21 and IL-23 induced the highest proportion of CD8+ T cells expressing IL-17A (Fig. 5A). No significant increases in IL-17A-expressing CD8+ T cells were observed when we used IL-17A, IL-17F in combination or singly (data not shown). In agreement with other work (19), we show that IL-21 substantially inhibits IFN-γ expression in the activated CD8+ T cells (Fig. 5A), indicating that IL-21 is sufficient to inhibit IFN-γ, although it may not be the only cytokine to do so (see below).To determine the role of IL-21 (probably produced by the Th17 cells as reported (20))and IL-23 (likely produced by the DCs as it has been reported that DCs stimulated by IL-1β make IL-23 (21)) in the Th17-mediated help for Tc17 induction, we co-cultured purified CD8+ T cells with DCs and either Th1 or Th17 cells in the presence of blocking antibodies to IL-21 and IL-23. We found a similar reduction in the population of CD8+ T cells expressing IL-17A in the cultures in which we blocked either IL-21 or IL-23, and blocking both is no more effective than blocking either alone (Fig 5B), indicating that both cytokines are necessary, and confirming that neither alone is sufficient for maximal CD8+ T cell IL-17 induction. In contrast, no effect of blocking either cytokine or both was observed on the production of IFNγ (Fig 5C), indicating that under these conditions, levels of IL-21 may not be sufficient to suppress IFN-γ production or other factors suppressing IFN-γ production (such as TGF-β) may also be present.
Figure 5.
IL-21 and IL-23 contribute to the induction of Tc17 cells by Th17. Purified dendritic cells, CD8+ T cells and CD4+ T cells were cocultured using a combination of cytokines and blocking antibodies. For Th1, recombinant mouse IL-12 was used and for Th17, recombinant mouse IL-6, IL-23 and TGF-β and a blocking antibody to IFN-γ were used. The polarized cells were washed but not rested before being cocultured. Purified CD8+ T cells from OT-1 mice were cultured in vitro with SIINFEKL peptide and IL-17A, IL-21 and IL-23 (A). To block, IL-17A, IL-21 and IL-23, antibodies against these cytokines were used (B). Cells were gated on CD3+CD8+ T cells. In (C), Upper panel shows effect of IL-21 and /or Il-23 on IL-17 producing CD8+ T cells and lower panel shows similar comparison for IFN-γ production. Each experiment was performed three times. Mann Whitney test was used to determine significance. * P<0.05, and ****P<0.0001.
Overcoming immune deviation by TGF-β blockade to allow help for protective CD8 T cells
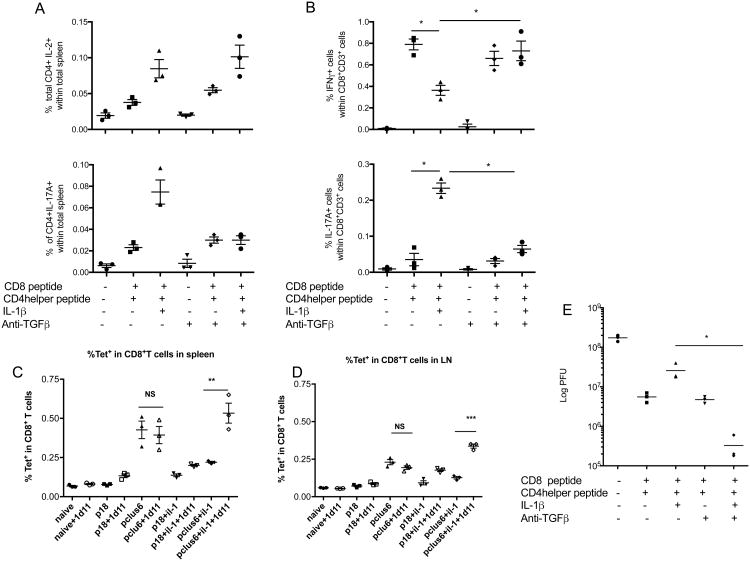
In order to use IL-1β as an effective adjuvant, while maintaining induction of Tc1 CD8+ T cells, we asked how we could block the skewing toward Th17 helper cells. We did not want to block downstream mediators like IL-21 because these are needed for other functions such as T follicular cell help for B cells. From our in vitro data, we hypothesized that if we block Th17 induction in the IL-1β-treated animals that were also immunized with both CD4 helper epitopes and the CD8 epitope, then we may restore the Th1 and Tc1 response. Since TGF-β plays an important albeit controversial role for the induction of Th17 cells (7, 22) (reviewed in (5)), to block Th17 induction we pretreated the animals with a monoclonal antibody (1D11) that has been shown previously to specifically block active TGF-β in vivo(23, 24), one day prior to immunization and every other day for one week. In the animals that received the blocking antibody, the induction of antigen-specific Th17 cells was abolished, whereas the overall magnitude of the CD4+ T cell response as measured by IL-2 secreting CD4+ T cells was not affected by the TGF-β blockade (Fig. 6 A) In addition, we also observed a decrease in the proportion of antigen specific IL-17-expressing CD8+ T cells and a concomitant increase in IFN-γ expressing CD8+ T cells (Fig. 6 B). The number of tetramer-positive CD8+ cells was not affected by the 1D11 treatment in the absence of IL-1, but when IL-1 was given, then the 1D11 antibody blockade of TGF-beta significantly increased the number of tetramer-positive T cells in response to PCLUS6.1-18 peptide in the spleen (Fig 6 C) and lymph node (Fig.6D). We have previously seen that this treatment does not affect the number or function of Foxp3+ Treg cells. (25) Strikingly, when we challenged the animals with vaccinia virus, the animals that received the TGF-β blockade in addition to IL-1β had enhanced viral clearance, which coincides with an increase in IFN-γ secreting T cells and decreased IL-17A secreting T cells (Fig. 6 E). Thus, TGF-β blockade allows the use of IL-1β as an adjuvant without skewing the helper response to Th17 or the CD8+ T cell response towards Tc17.
Figure 6.
Blockade of TGF-β inhibits induction of Th17 and Tc17 response by IL-1β and restores protective Tc1 response. Mice were administered intraperitoneally an anti-TGF-β antibody one day prior to being immunized with P18-I10 (CD8 epitope) or PCLUS3-18IIIB (CD4 helper + CD8 epitope) and were given this antibody every other day. Seven days post-immunization, spleens were removed and stimulated in vitro with P18-I10 peptide or PCLUS3-18IIIB peptide overnight and expression of IL-2 and IL-17A was measured in CD4+ T cells (A)and IFN-γ and IL-17A was measured in CD8+ T cells (B). % of CD8+ T cells that were positive for P18-I10-H2Dd tetramers were also stained for each group in the spleen (C) and lymph nodes (D). 10 days post-vaccination, mice were infected i.p. with 2 × 107pfu of vPE16. 5 days later viral pfu were determined in the ovaries (E). Mann Whitney test was used to determine significance between groups of mice. Data shown are representative of two independent experiments. * P<0.05.
Discussion
Here we have addressed the open question whether other helper T cell phenotypes besides Th1, in particular Th17 cells, can provide help for a CD8+ T cell response, and whether they perturb the phenotype of that response. As emerging data consistently demonstrate the importance of different CD4+ T cell subsets in different disease settings, it becomes increasingly important to understand their effect on CD8+ T cells. Although it was known that Th17 cells could help for B cell responses (8), this question for CD8+ T cell responses had never been examined. This question became important when we found that IL-1β, which skewed helper T cell responses toward Th17 phenotype, was not effective for inducing helper-dependent CD8+ responses. We had been measuring CD8+ T cell responses as commonly done by IFNγ-producing CD8+ T cells, as well as by protection against virus infection. By these criteria, indeed, Th17 cells were not good helpers for a protective CD8+ T cell response. However, what appeared to be loss or inhibition of help for a CD8+ T cell response turned out to be actually largely immune deviation of the CD8 response to Tc17 cells producing IL-17 but not IFNγ, although there was also some diminution of help in general by Th17 cells. Thus the readout was critical. This finding was surprising in view of the ability of Th17 cells to help B cell responses (8). In a previous study, we found that IL-1β could improve a CD8+ T cell response largely independent of CD4 help, but the role of help was not studied (3). Likewise, the clinical impact in the impaired clearance of a viral infection was also due to the same immune deviation, because clearance of vaccinia virus (like many viruses) is dependent on IFNγ (26). However, the ability of Th17 to protect depends on the disease studied, as Th17 were found to protect against melanoma tumors (27) and against Trypanosoma cruzi parasitic infection (28), where IL-17-producing T cells are more critical. This finding has some analogy with much earlier findings that what had appeared to be suppression of T-cell proliferative or delayed type hypersensitivity responses was really immune deviation from Th1 to Th2 phenotype (29-31). Because IFNγ can be made by both CD4+ and CD8+ T cells, immune deviation of both populations could play a role in the diminished protection. However, it is clear that the CD8+ T cells can provide some protection alone when no CD4 epitope is included (Fig. 1 and (3)), and that the major effect of IL-1 was to decrease the benefit of CD4 help on both CD8+ T cell production of IFNγ and on protection, which paralleled one another.
Because it is known that CD4+ T cell help for CD8+ T cell responses is dependent largely on activation or “licensing” of DCs (13-15, 18), we asked whether DCs also played a role in the immune deviation of CD8+ T cells mediated by Th17 cells. Unlike the induction of IFNγ-producing Tc1 CD8+ T cells by Th1 cells, which can be mediated by DCs activated by Th1 cells (presumably involving IL-12, reviewed in (17)), pre-treatment of the DCs with Th17 cells and antigen was not sufficient to allow the DCs to skew the CD8+phenotype to Tc17 (partially explained by requirement for cytokines like IL-21 made by the CD4+ T cells as we found subsequently). Although IL-17 itself did not seem to play a role, IL-21 made by the CD4+ T cells (20) and IL-23 made by the DCs (21) were both individually necessary. Neither alone was sufficient. This finding for Tc17 parallels earlier evidence for IL-21 involvement in Th17 production (32) and Tc17 induction (33). We have seen that naive CD8 T cells express IL-21R because they respond to IL-21 (34), and although naïve CD8 T cells do not express IL-23R, they rapidly upregulate it on activation (35). The transcriptional pathways by which IL-21 and IL-23 induce Th17 phenotype have been worked out and involve STAT3 and RORγt activation(20, 36).
Because commonly used adjuvants such as alum are known to induce IL-1β production through activation of the NLRP3 inflammasome (9), this effect may have clinical importance in vaccinology beyond the use of IL-1β itself as an adjuvant. Alum is not known to be the most effective adjuvant for inducing CD8-mediated T cell immunity. To overcome this effect and promote a Tc1 type CD8+ response that may be clinically important in controlling virus infections, we sought to find an approach that would not require blocking IL-21 production, as this is a key cytokine produced by T follicular helper (Tfh) cells needed for B-cell help (37). It has been shown that induction of Th17 cells is dependent on a combination of TGF-β and inflammatory cytokines such as IL-1β or IL-6 (5, 22, 32, 38). Therefore, we tested the effect of a TGF-β-neutralizing antibody, 1D11 (23, 24), on immune deviation of CD8+ T cells to Tc17 phenotype. Indeed, this antibody overcame the immune deviation and allowed an IL-1-based adjuvant to improve not only the Tc1 response but also the protection against a recombinant vaccinia virus infection. The even greater protection seen in Fig.6E with the combination of IL-1β and anti-TGF-β than with anti-TGF-β alone may be the result of greater granzyme B production with IL-1β (as seen in Fig.3E) in addition to the greater IFN-γ production, as the latter also occurred with anti-TGF-β alone (Fig. 6B), and could also be due to the suppression of the Tc17 response.
Anti-TGF-β can also affect Treg formation and/or function, and it has been reported that CD4+ T cell help through CD40L interaction with CD40 is less critical in the absence of Treg cells (39). However, it is unlikely that the effect of anti-TGF-β is primarily through inhibiting T reg, for several reasons. We immunized in the presence of IL-1, which upregulates expression of CD40 on DCs (40) via TRAF6 signaling (41). TRAF6 is a critical factor for DC maturation and also induces expression of CD40L (CD154) on T cells (42). Under these circumstances, T reg activity should be largely precluded. Consequently, neutralization of TGF-β should have either no effect via this mechanism or might have increased the Th17 response by reducing any residual Treg function, but instead we observed that Th17 responses were suppressed while the overall CD4 response measured by IL-2 production was not diminished, and Th1 cells actually increased. Furthermore, in another study of anti-TGF-β antibodies in cancer-bearing mice, we observed no effect on Treg cell numbers or function (25). Thus, our results are most likely due to neutralization of the known activity of TGF-β in induction or differentiation of Th17 cells through SOCS3 (43).
Thus, these findings about the effect of IL-1 on immune deviation not only of CD4+ T cells but also CD8+ T cell responses may have important clinical utility in vaccine design, not only for direct use of IL-1 but also for widely used adjuvants such as alum that induce IL-1 production. Findings in this study may help explain why alum is not generally a good adjuvant for inducing a CD8+ T cell response. Overall, our understanding of the ability of different CD4+ T helper subsets to provide help for CD8+ T cells and to otherwise alter their response phenotype is critical for vaccine design as well as for understanding both immune protection and immunopathology in many diseases that are now known to affect the balance of CD4+ T cell subsets.
Materials and Methods
Animals and immunizations
Mice were immunized subcutaneously (s.c.) with an immunodominant CTL epitope presented by H-2Dd in BALB/c mice, P18-I10 (RGPGRAFVTI) from the V3 loop of the IIIB strain of HIV-1 or PCLUS3-P18-I10 which contains both a CD4 helper epitope and CTL epitope (11) complexed in DOTAP. IL-1β (2 μg) was injected s.c.daily for 4 days where indicated(4). For the in vitro stimulation assays 6-12 week old C57BL/6 (Charles River), OT-I (Taconic) and OT-II (Taconic) mice were used.
Flow cytometry and Intracellular cytokine staining
Seven days post-vaccination animals were sacrificed and spleens and draining LNs removed and processed as previously described (44). Cells were stimulated with P18-I10 peptide or PCLUS3-P18-I10 peptide for 2 hrs at 37°C. Golgiplug was then added (0.25ul/well) for 12-16 hrs. The next morning, cells were labeled with antibodies to surface molecules CD3 (Pacific Blue), CD4 (PerCPCy5.5), CD8 (Alexa 700) and Yellow viability dye (Life technologies), before being fixed and permeabilized and labeled with antibodies to IFN-γ (FITC), IL-17A (PE) and IL-2 (PeCy7). For staining of cells with P18-10 loaded tetramer (NIH tetramer facility), cells were labeled ex vivo with tetramer and with a viability dye and antibodies against CD3 (Pacific Blue), CD8 (Alexa 700), CCR6, CXCR3 (PerCPCy5.5), RORγt (PE) and t-Bet (PerCPCy5.5). Following culture of DCs with different populations of CD4+ T cells, DCs were labeled with viability dye and antibodies against CD11c, CD80, CD86, CD40 and CD8α. Representative gating strategies are shown in Supplemental Figure 1.
Cell isolations and co-culture assays
Purified cell populations were isolated from the spleen using magnetic bead separation (Miltenyi Biotec). DCs were isolated using positive selection of anti-CD11c antibody; CD8+ T cells and CD4+ T cells were isolated using negative selection to obtain T cells lacking antibodies on their surface. Th1 and Th17 cells subsets were polarized in vitro using cytokines and blocking antibodies. For Th1, recombinant mouse IL-12 (20 ng/mL) was used and for Th17 recombinant mouse IL-6 (20 ng/mL), IL-23 (30 ng/mL) and TGF-beta (10 ng/mL) and a blocking antibody to IFN-gamma (10 ug/mL). The cytokine profiles of these polarized Th1 and Th17 cells are shown in Supplemental Figure 2A. In some experiments, purified CD8+ T cells from OT-I mice were cultured in vitro with SIINFEKL ovalbumin peptide and different combinations of IL-17A (20 ng/mL), IL-21 (20 ng/mL) and IL-23 (20 ng/mL). To block cytokines IL-17A, IL-21 and IL-23, anti-bodies against these cytokines were used at 20 ug/mL.
Virus infection
Mice were challenged intraperitoneally with 2 × 107 pfu of recombinant vaccinia virus expressing gp160IIIB (vPE16) (45). Six days after challenge both ovaries were homogenized in PBS, sonicated and assayed for viral titers by culturing 10-fold serial dilutions with BSC-1 cells (46). After 48 hrs of culture, crystal violet (0.1% in 20% Ethanol) was added to the cells and the number of plaques were counted and multiplied by the dilution factor.
Statistics
We performed statistical analyses with Prism (Graph Pad). A two-sided significance level of 0.05 was used for all analyses. The Mann-Whitney, or Wilcoxon non-parametric tests were used as indicated.
Supplementary Material
Supplemental Figure 1. Gating strategies for intracellular straining of T cells for IFNγ tetramer, RORγt, t-bet, CXCR3 and CCR6. In each set of 4 flow plots, the gates are sequential from upper left to upper right to lower left to lower right, except for IL-17A where we show both CD8+ T cells on the upper row and CD4+ T cells on the lower row.
Supplemental Figure 2. CD4 cells polarized to Th1 or Th17: cytokine profiles and help for CD8+ T cells. A. 2D plots of Th1-polarized cells (polarized as in Methods) in the upper row and Th17-polarized cells (polarized as in Methods) in the lower row staining for IFNγ, IL-17A and IL-2. B. Induction of IL-2-producing CD8+ T cells by culture of CD8+ T cells with Th1 or Th17 cells and DCs and anti-CD3 similar to Fig 3 A and B.
Footnotes
This work is dedicated to the memory of Dr. William E. Paul, an outstanding scientist and mentor and a wonderful human being.
References
- 1.Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39:1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ben-Sasson SZ, et al. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc Natl Acad Sci U S A. 2009;106:7119–7124. doi: 10.1073/pnas.0902745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Sasson SZ, et al. IL-1 enhances expansion, effector function, tissue localization, and memory response of antigen-specific CD8 T cells. J Exp Med. 2013;210:491–502. doi: 10.1084/jem.20122006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung Y, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 6.Zielinski CE, et al. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature. 2012;484:514–518. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]
- 7.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1 beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17–producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 8.Mitsdoerffer M, et al. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc Natl Acad Sci U S A. 2010;107:14292–14297. doi: 10.1073/pnas.1009234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: 10 years of progress. Immunol Rev. 2008;226:10–18. doi: 10.1111/j.1600-065X.2008.00701.x. [DOI] [PubMed] [Google Scholar]
- 11.Shirai M, et al. Helper-CTL determinant linkage required for priming of anti-HIV CD8+ CTL in vivo with peptide vaccine constructs. J Immunol. 1994;152:549–556. [PubMed] [Google Scholar]
- 12.Tajima M, Wakita D, Satoh T, Kitamura H, Nishimura T. IL-17/IFN-gamma double producing CD8+ T (Tc17/IFN-gamma) cells: a novel cytotoxic T-cell subset converted from Tc17 cells by IL-12. Int Immunol. 2011;23:751–759. doi: 10.1093/intimm/dxr086. [DOI] [PubMed] [Google Scholar]
- 13.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 14.Schoenberger SP, Toes REM, van der Voort EIH, Offringa R, Melief CJM. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 15.Bennett SRM, et al. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 16.Kubin M, Kamoun M, Trinchieri G. Interleukin 12 synergizes with B7/CD28 interaction in inducing efficient proliferation and cytokine production of human T cells. J Exp Med. 1994;180:211–222. doi: 10.1084/jem.180.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trinchieri G. Interleukin-12: A cytokine at the interface of inflammation and immunity. Adv Immunol. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- 18.Janssen EM, et al. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 19.Huber M, et al. A Th17-like developmental process leads to CD8(+) Tc17 cells with reduced cytotoxic activity. Eur J Immunol. 2009;39:1716–1725. doi: 10.1002/eji.200939412. [DOI] [PubMed] [Google Scholar]
- 20.Wei L, Laurence A, Elias KM, O'Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem. 2007;282:34605–34610. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wesa A, Galy A. Increased production of pro-inflammatory cytokines and enhanced T cell responses after activation of human dendritic cells with IL-1 and CD40 ligand. BMC Immunol. 2002;3:14. doi: 10.1186/1471-2172-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008 doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dasch JR, et al. Monoclonal antibodies recognizing transforming growth factor-beta. Bioactivity neutralization and transforming growth factor beta 2 affinity purification. J Immunol. 1989;142:1536–1541. [PubMed] [Google Scholar]
- 24.Terabe M, et al. Transforming Growth Factor-b production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block Cytotoxic T Lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741–1752. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terabe M, et al. Synergistic enhancement of CD8+ T cell-mediated tumor vaccine efficacy by an anti-transforming growth factor-beta monoclonal antibody. Clin Cancer Res. 2009;15:6560–6569. doi: 10.1158/1078-0432.CCR-09-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris N, Buller RM, Karupiah G. Gamma interferon-induced, nitric oxide-mediated inhibition of vaccinia virus replication. J Virol. 1995;69:910–915. doi: 10.1128/jvi.69.2.910-915.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin-Orozco N, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai CW, Blase JR, Zhang X, Eickhoff CS, Hoft DF. Th17 Cells Are More Protective Than Th1 Cells Against the Intracellular Parasite Trypanosoma cruzi. PLoS Pathog. 2016;12:e1005902. doi: 10.1371/journal.ppat.1005902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mosmann TR, Coffman RL. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- 30.Mosmann TR, Coffman RL. TH1 and TH2 cells: Different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 31.Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, Locksley RM. Reciprocal expression of interferon g or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang L, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ciric B, El-behi M, Cabrera R, Zhang GX, Rostami A. IL-23 drives pathogenic IL-17-producing CD8+ T cells. J Immunol. 2009;182:5296–5305. doi: 10.4049/jimmunol.0900036. [DOI] [PubMed] [Google Scholar]
- 34.Zeng R, et al. Synergy of IL-21 and IL-15 in Regulating CD8+ T-Cell Expansion and Function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanden Eijnden S, Goriely S, De Wit D, Willems F, Goldman M. IL-23 up-regulates IL-10 and induces IL-17 synthesis by polyclonally activated naive T cells in human. Eur J Immunol. 2005;35:469–475. doi: 10.1002/eji.200425677. [DOI] [PubMed] [Google Scholar]
- 36.Ivanov II, Zhou L, Littman DR. Transcriptional regulation of Th17 cell differentiation. Semin Immunol. 2007;19:409–417. doi: 10.1016/j.smim.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 38.Mangan PR, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 39.Ballesteros-Tato A, Leon B, Lund FE, Randall TD. CD4+ T helper cells use CD154-CD40 interactions to counteract T reg cell-mediated suppression of CD8+ T cell responses to influenza. J Exp Med. 2013;210:1591–1601. doi: 10.1084/jem.20130097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamada N, Katz SI. Generation of mature dendritic cells from a CD14+ cell line (XS52) by IL-4, TNF-alpha, IL-1 beta, and agonistic anti-CD40 monoclonal antibody. J Immunol. 1999;163:5331–5337. [PubMed] [Google Scholar]
- 41.Kobayashi T, et al. TRAF6 is a critical factor for dendritic cell maturation and development. Immunity. 2003;19:353–363. doi: 10.1016/s1074-7613(03)00230-9. [DOI] [PubMed] [Google Scholar]
- 42.Nakae S, Asano M, Horai R, Sakaguchi N, Iwakura Y. IL-1 enhances T cell-dependent antibody production through induction of CD40 ligand and OX40 on T cells. J Immunol. 2001;167:90–97. doi: 10.4049/jimmunol.167.1.90. [DOI] [PubMed] [Google Scholar]
- 43.Qin H, et al. TGF-beta promotes Th17 cell development through inhibition of SOCS3. J Immunol. 2009;183:97–105. doi: 10.4049/jimmunol.0801986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oh S, et al. Human CTL to wild type and enhanced epitopes of a novel prostate and breast tumor-associated protein, TARP, lyse human breast cancer cells. Cancer Research. 2004;64:2610–2618. doi: 10.1158/0008-5472.can-03-2183. [DOI] [PubMed] [Google Scholar]
- 45.Earl PL, Hugin AW, Moss B. Removal of cryptic poxvirus transcription termination signals from the human immunodeficiency virus type 1 envelope gene enhances expression and immunogenicity of a recombinant vaccinia virus. J Virol. 1990;64:2448–2451. doi: 10.1128/jvi.64.5.2448-2451.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alexander-Miller MA, Leggatt GR, Berzofsky JA. Selective expansion of high or low avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc Natl Acad Sci USA. 1996;93:4102–4107. doi: 10.1073/pnas.93.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Gating strategies for intracellular straining of T cells for IFNγ tetramer, RORγt, t-bet, CXCR3 and CCR6. In each set of 4 flow plots, the gates are sequential from upper left to upper right to lower left to lower right, except for IL-17A where we show both CD8+ T cells on the upper row and CD4+ T cells on the lower row.
Supplemental Figure 2. CD4 cells polarized to Th1 or Th17: cytokine profiles and help for CD8+ T cells. A. 2D plots of Th1-polarized cells (polarized as in Methods) in the upper row and Th17-polarized cells (polarized as in Methods) in the lower row staining for IFNγ, IL-17A and IL-2. B. Induction of IL-2-producing CD8+ T cells by culture of CD8+ T cells with Th1 or Th17 cells and DCs and anti-CD3 similar to Fig 3 A and B.