Abstract
Background
Traumatic brain injury (TBI) is a devastating and costly acquired condition that affects individuals of all ages, races, and geographies via a number of mechanisms. The effects of TBI on melatonin receptors remains unknown.
Purpose
The purpose of this study is to explore whether endogenous changes in two melatonin receptor subtypes (MT1 and MT2) occur after experimental TBI.
Sample
A total of 25 adult male Sprague Dawley rats were used with 6 or 7 rats per group.
Methods
Rats were randomly assigned to receive either TBI modeled using controlled cortical impact or sham surgery and to be sacrificed at either 6- or 24- hours post-operatively. Brains were harvested, dissected, and flash frozen until whole cell lysates were prepared, and the supernatant fluid aliquoted and used for western blotting. Primary antibodies were used to probe for melatonin receptors (MT1 and MT2), and beta actin for a loading control. ImageJ and Image Lab software were used to quantify the data which was analyzed using t-tests to compare means.
Results
Melatonin receptors levels were reduced in a brain region- and time point-dependent manner. Both MT1 and MT2 were reduced in the frontal cortex at 24 hours and in the hippocampus at both 6 hours and 24 hours.
Discussion
MT1 and MT2 are less abundant after injury, which may alter response to MEL therapy. Studies characterizing MT1 and MT2 after TBI are needed, including exploration of the time course and regional patterns, replication in diverse samples, and use of additional variables, especially sleep-related outcomes.
Conclusion
TBI in rats resulted in lower levels of MT1 and MT2; replication of these findings is necessary as is evaluation of the consequences of lower receptor levels.
Keywords: Traumatic brain injury (TBI), brain trauma, controlled cortical impact (CCI), rat, melatonin, receptors
Introduction
TBI is a devastating condition that globally affects individuals at all stages of life [1,2]. In the United States of America (USA) alone, a recent estimate values the direct and indirect costs of TBI at a staggering $76.5 billion annually [3]. Unfortunately, acute and chronic disability remains common [4]. No therapy has demonstrated sufficient safety and efficacy to warrant translation to TBI clinical care [5]. Thus, the quest to identify effective therapies for TBI remains a worldwide initiative. Many major barriers to identification of new effective therapies exist. For example, TBI is characterized by a wide variety of cellular and histopathological changes [6], suggesting drugs with multiple mechanisms of action or a combination of therapies may be necessary [7]. Also, in order for a TBI therapeutic to be effective, it must be able to reach and exert its effects in the brain; however, many pharmaceutical compounds lack the necessary properties (e.g. small; lipophilic) to efficiently cross the blood-brain-barrier [8] via natural (i.e. unassisted) mechanisms.
One promising potential TBI therapeutic is melatonin (MEL), which readily and rapidly crosses the blood-brain-barrier [9]. MEL is produced throughout the body, with the primary site of production being the pineal gland. MEL is available as a medication and over-the-counter supplement and has a known low toxicity profile in both human and animal studies with few reported adverse effects even at very high doses [10]. Existing evidence shows endogenous MEL levels are altered in TBI-survivors in a time-point and biosample-dependent manner [11,12]; thus, MEL may be important in the body’s response to TBI and there may be an opportunity to improve outcomes via therapeutic administration of MEL. Pre-clinical studies have found MEL leads to attenuation of one or more of the histopathological and functional consequences of TBI [13,14]. Moreover, published evidence suggests that MEL has many mechanisms of action, including anti-apoptotic [15,16], anti-oxidative [17], and anti-mitophagic [18] properties; notably, the abovementioned pathways are all well-established as implicated in TBI-pathology.
Despite the beneficial characteristics of MEL, current evidence remains largely limited to a small number of preclinical studies which yielded conflicting evidence. More concerning, none of the studies to date have confirmed that a major target for MEL therapy (MEL-specific receptors, MT1 and MT2) remains unaltered by TBI. This pilot study is the first to explore MT1 and MT2 levels after TBI, thereby addressing a major gap in the knowledge. Hypothesis: decreased levels of MEL-specific receptors (MT1 and MT2) may occur in response to TBI. Lower levels of MEL receptors have previously been reported in an animal model of depression [19] and following treatment with the melatonin receptor antagonist luzindole [20]. In this study western blot was used to semi-quantify melatonin-specific receptor levels (MT1 and MT2) within the hippocampus and frontal cortex during the acute (6 hr and 24 hr) period post-TBI.
Materials and Methods
Methods Overview
All experimental procedures were approved by the Institutional Animal Care & Use Committee prior to beginning study activities. Prior to the enrollment, and throughout the duration of the study, test animals were kept in a climate-controlled housing facility on a 12 hour light/dark cycle. Rats were randomly assigned to be subjected to TBI using the controlled cortical impact (CCI) model or sham surgery and then be humanely euthanized at either 6 hr or 24 hr after surgery. Brain tissue was harvested, the ipsilateral (i.e. injured) hippocampus and frontal cortex dissected, and flash frozen; whole cell lysates were stored at −80 °C until later processed for whole cell lysates and used for western blotting.
Sample
In this pilot study, the sample was comprised of male Sprague Dawley rats (Harlan, Indianapolis, IN, USA); at the time of surgery all test animals were young adults (10–14 week old), weighing 275–375g. The rationale for the chosen sample demographics was to control for the confounding effects of age, brain development, and sex on TBI outcomes [21–24]. In total, 25 rats were included in this exploratory pilot study, resulting in a 6 or 7 rats per group across the 4 groups: (1) CCI with 6 hr sacrifice (n= 6), (2) sham with 6 hr sacrifice (n= 6), (3) CCI with 24 hr sacrifice (n= 6), and (4) sham with 24 hr sacrifice (n= 7).
Surgery
Prior to surgery, the CCI device was examined and test fired to ensure proper functioning (e.g. the piston fires freely). Rats received inhaled anesthetic immediately prior to and throughout surgery. Each rat was placed in an anesthesia induction chamber and given 4.0% isoflurane in a 2:1 mixture of N2O:O2. Once sedated, the rat was intubated and placed into a stereotaxic frame, secured using bilateral ear bars and a single incisor bar. Isoflurane levels were reduced to a maintenance dose (2.0%) throughout the surgery, unless the rat showed signs of regaining consciousness, in which case the dose was increased. The head was shaved with electric trimmers and the surgical site prepared using betadine and sterile gauze. A scalpel was used to make a midline incision approximately 20mm in length. The muscles were gently separated and the skin and fascia reflected using sterile surgical tools and cotton-tipped applicators. A pneumatic drill was used to make a craniectomy on the exposed skull between the lambda and bregma (anterior-to-posterior) and also between the coronal ridge and sagittal suture (medial-to-lateral). The window was approximately 7 mm, just large enough for unobstructed clearance of the 6 mm tip. The detached bone flap was carefully removed using microdissecting forceps so as to not breach the dura and subsequently discarded.
The piston was gently lowered to ensure that it was centered within the bone window and to confirm unobstructed clearance for the 6 mm diameter rigid, flat-beveled tip. The device was zeroed to the cortical surface and gently withdrawn to avoid surgical site disruption. The piston assembly was adjusted to reflect the desired impact parameters: depth of 2.8 mm, velocity of 4 m/s, and dwell time (i.e. duration) of 150 ms. At this point the device was actuated to induce TBI. In both CCI- and sham-exposed rats, the surgical site was sutured closed, topical anesthetic applied, and anesthesia discontinued. The animal was removed from the stereotaxic frame, extubated, and assessed for righting reflex. Following return of spontaneous locomotion, regular housing and husbandry were resumed. Animals were monitored for evidence of pain and distress and analgesic was administered per institutional protocol. Sham control rats received identical surgical and post-surgical treatment to TBI-exposed animals but were not be exposed to CCI.
Sacrifice
Animals were humanely euthanized at one of two post-surgery time points: 6 hr or 24 hr. At the time of sacrifice, animals were injected with Fatal Plus (0.25 mL per rat) and decapitated by guillotine. Brains were rapidly harvested and the ipsilateral fontal cortex and hippocampus dissected over ice, placed in microcentrifuge tubes, and flash frozen in liquid nitrogen. Tubes containing dissected tissue were stored at −80° C until processed for analysis.
Tissue Processing
A lysis buffer was prepared, composed of: 0.01M Tris-Cl/0.1M NaCl, 0.001M ethylenediaminetetraacetic acid (EDTA), 1 μg/mL aprotinin, and 100 μg/mL phenylmethylsulfonyl fluoride (PMSF). Specific volumes of lysis buffer were pipetted onto the brain tissues (200 μL for frontal cortex; 100 μL for hippocampus). A sonicator was used to homogenize the tissue and generate whole cell lysates, which were centrifuged at high speed in a cold (4°) room for 30 minutes. Following separation of the layers, the supernatant fluid was collected into a microcentrifuge tube, vortexed to homogenize, and aliquoted out into smaller tubes to minimize the effects of freeze/thaw cycles.
BCA Assay
On the day the gel was to subject to electrophoresis, the protein content of the samples was determined using a Pierce bicinchoninic acid (BCA) assay (Thermo Fisher Waltham, MA, USA). Samples were diluted five-fold and loaded in duplicate into a 96 well plate. For comparison, 8 standards of known protein concentration were loaded in triplicate. A spectrophotometer (Molecular Devices, Sunnyvale, CA, USA), and associated Softmax Pro software (Molecular Devices, Sunnyvale, CA, USA) were used to determine the volume of supernatant fluid needed to load a consistent mass of 20 μg total protein per well.
Sample Preparation and Wet Laboratory Methods
Samples were prepared by combining the volume of sample required for the desired mass of protein, with Bolt™ Sample Reducing Agent (Thermo Fisher Scientific, Waltham, MA, USA), and Bolt™ LDS Sample Buffer (Thermo Fisher Scientific, Waltham, MA, USA). The mixture was centrifuged briefly before boiling for 10 minutes; boiled samples were allowed to cool and were re-centrifuged at room temperature. Prepared samples were loaded into a Bolt™ 4–12% Bis Tris Plus 15 well gel (Thermo Fisher Scientific, Waltham, MA, USA) along with a SeeBlue® Plus2 Pre-stained Protein Standard ladder (Thermo Fisher Scientific, Waltham, MA, USA). The gel was electrophoresed at a constant 165 volts for approximately 30 minutes.
A first generation Invitrogen Bolt™ semi-dry transfer system (Thermo Fisher Scientific, Waltham, MA, USA) was used in accordance with the manufacturer’s instructions orienting the anode stack on the bottom, the polyvinylidene fluoride (PVDF) membrane in the middle, and the cathode stack on top. The transfer program was run for a total semi-dry transfer time of 7 minutes. Immediately following transfer, the gel was retrieved and placed in a tray and a small volume (~5 mL) of GelCode™ Blue Stain Reagent (Thermo Fisher Scientific, Waltham, MA, USA) was poured over the gel and allowed to incubate on a rocker for at least 1 hour to ensure that there were no issues during electrophoresis.
Next, membranes were labeled, rinsed with deionized water, and then rinsed with methanol. Membranes were then washed in Tris-Buffered Saline and Tween 20 (TBS-T) for 3 washes of 5 minutes each. Next, membranes were blocked for 30 minutes in 5% blotting-grade non-fat dry milk (BioRad, Hercules, California, USA) and then incubated overnight in 5% milk with the primary antibodies as described in additional detail below. Following incubation with the primary antibody, membranes were re-washed for 15 minutes (3 washes at 5 minutes each) in TBS-T. Membranes were then incubated in 1% milk with the corresponding secondary antibody described below. A commercially available (Super Signal West Femto Maximum Sensitivity Substrate) two-component chemiluminescent solution (Thermo Fisher Scientific, Waltham, MA, USA) was applied to the membrane (total volume = 1.0 mL per membrane, with the two parts in equal volume). The membrane was imaged using a digital imager (BioRad, Hercules, California, USA). Following imaging, and prior to repeating the blocking, staining, and imaging steps for the remaining antibodies, the membranes were washed (15 minutes, as before), stripped with Restore™ PLUS Western Blot Stripping Buffer (Thermo Fisher Scientific, Waltham, MA, USA) for 15 minutes and rewashed (15 minutes, as before).
First, membranes were probed for MT1 (ab184013, 1:1000, Abcam, Cambridge, UK) with goat-anti-rabbit secondary antibody (#31460, 1:5000, Thermo Scientific, Waltham, MA, USA). Second, membranes were probed for MT2 (ab203346, 1:1000, Abcam, Cambridge, UK) with (#31460, 1:5000, Thermo Scientific, Waltham, MA, USA) goat-anti-rabbit secondary antibody. Finally, membranes were probed for beta actin (a2066, 1:2500, Sigma Aldrich, St. Louis, MO, USA) with (#31460, 1:5000, Thermo Scientific, Waltham, MA, USA) goat-anti-rabbit secondary antibody, in an effort to control for the possibility of differential protein loading.
Analysis
Image J software (National Institutes of Health, Bethesda, MD, USA) was used in combination with Image Lab software (Bio-Rad, Hercules, CA, USA) to quantify data for analysis. Melatonin receptor levels were normalized to beta actin levels for the same test animal to control for the possibility unequal protein loading. All analysis was conducted using SPSS version 24 (IBM, Armonk, CA, USA) statistical software. Preliminary analysis was completed as follows: t-tests to compare protein levels of sham vs. injured rats at a single time point of either 6 hr or 24 hr post-operatively.
Results
Post-Operative Outcomes
In this study, there was a 0% mortality rate associated with experimental procedures. Moreover, neither sham surgery nor CCI caused significant morbidity (e.g. seizures) that would have necessitated a test animal being prematurely euthanized. Results from western blot analysis are summarized below, with composite gels provided in Figure 1, and a graphical group comparison displayed in Figure 2. The criteria for statistical significance was p < 0.05 (Note: in figure 2, which does not provide exact p-values, statistical significance is denoted as follows: *p < 0.05; ** p < 0.01).
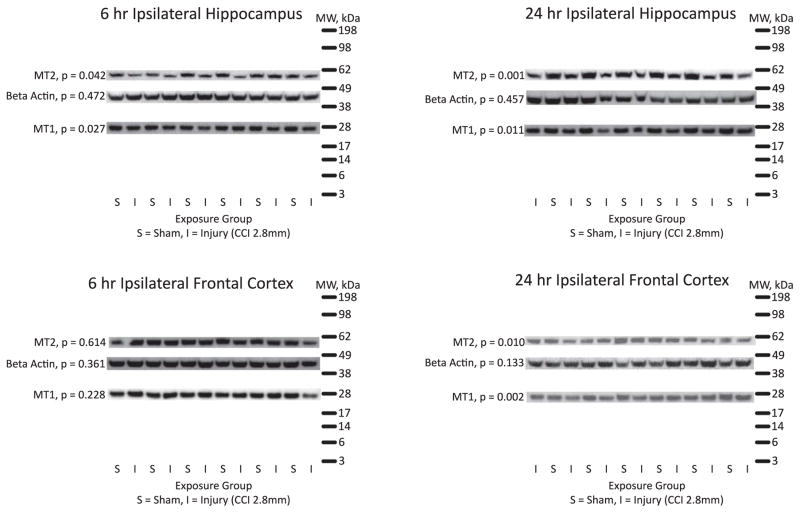
Figure 1.
Composite figure of western blot results with molecular weight ladder.
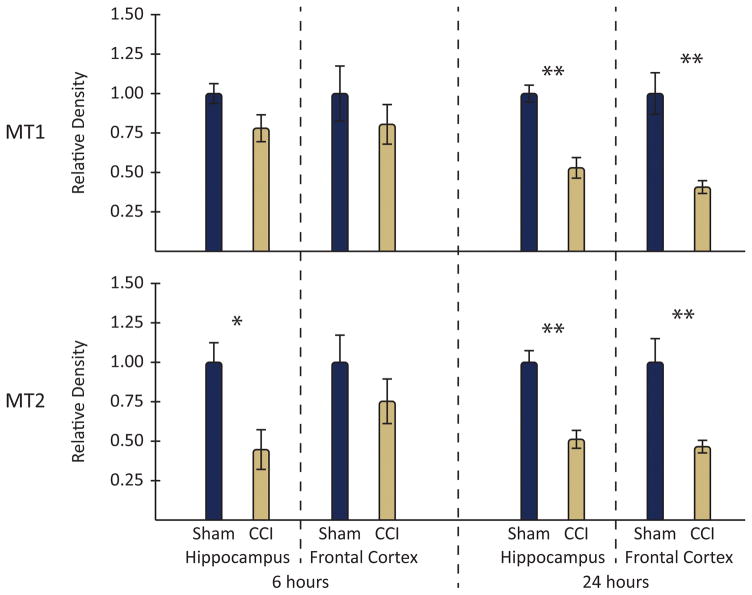
Figure 2.
Graphical comparison of CCI and sham animal western blot results by protein and brain region.
MT1 Levels
When whole cell lysates from ipsilateral frontal cortex of rats exposed to CCI (vs. sham) were compared using western blot, MT1 levels were reduced at 24 hr (p= 0.002), though they were unchanged from sham levels at 6 hr. Moreover, in the hippocampus, MT1 levels were reduced at both 6 hr (p= 0.027) and 24 hr (p= 0.011).
MT2 Levels
As with the cortical MT1, cortical MT2 levels were reduced at 24 hr post-injury (p= 0.010), but unchanged from sham levels at 6 hr. Likewise, in the hippocampus, MT2 levels were reduced at both 6 hr (p= 0.042) and 24 hr (p= 0.001) post-injury.
Actin
In all brain regions and time points examined in this study, there was no statistically significant change in beta actin levels after TBI (compared to sham). This is consistent with what has been reported previously [25–27]. This supports the use of actin to normalize the results of this study in an attempt to control for any inconsistencies in loading samples.
Discussion
Novel Contribution to the Literature and Relationship to Other Published Findings
This study is the first to report a reduction in levels of melatonin receptor subtypes 1 and 2 (i.e. MT1 and MT2) after TBI with evidence of time point- and brain region-specific differences. This reduction in MEL receptors may affect the efficacy of MEL therapy after TBI, though this remains to be empirically tested. Notably, past attempts to treat experimental TBI with MEL therapy have yielded inconsistent results, with many studies showing neuroprotective effects after TBI for at least one of the regimens tested [13,16,28,29], one study showing no effect of therapy [30], and a few studies showing adverse effects of one or more of the therapeutic regimens tested [15,31]. Interestingly, many of the studies that reported beneficial effects of melatonin therapy tested the therapeutic effects in reducing oxidative stress [28,31–36], which rely on melatonin’s receptor-independent free radical scavenging properties, rather than receptor-dependent effects. Thus, the importance of melatonin-specific receptors may have been obscured by studies whose endpoints resulted from receptor-independent activities. Moreover, there is known genetic variation in [37] or near [38] genes encoding MEL receptors, the effect of which was controlled for in this study with the use of congenic (i.e. inbred) rats. Replication in different strains of test animal would strengthen the available evidence, as would clinical studies exploring the effects of MEL receptor polymorphisms on TBI outcomes as well as response to therapy. This emerging line of inquiry may prove relevant for precision therapy initiatives and identification of the subset of patients most likely to benefit from melatonin therapy.
Limitations and Future Directions
All pre-clinical studies have limited clinical applicability and require replication in pre-clinical models before clinical trials can be justified. Importantly, many of the therapies that show success in pre-clinical studies, even when replicated, do not demonstrate therapeutic effects in clinical trials. Moreover, the focal nature of TBI induced using the CCI model means that the results may not hold true when diffuse brain injury and/or polytrauma is present; similarly, these results may not reflect the effects of milder brain injury including closed head injury. Replication using CCI of varying severity along with other injury models (e.g. fluid percussion; blast-induced TBI) is needed. The generalizability of this study is further limited by the homogenous nature of the sample, which was restricted to young adult male rats. Since sex is known to be an important factor in brain trauma [39–41], validation of study findings in female animals is necessary. Likewise, replicating the study using pediatric and aging mice would strengthen the evidence base.
Several specific limitations should be acknowledged and considered when interpreting the results of this pre-clinical study. Western blot analysis of MT1 and MT2 receptor levels provides preliminary evidence of lower numbers of MT1 and MT2 receptors after injury, but additional research using more sophisticated techniques (e.g. immunohistochemistry; gene expression studies) is necessary. Future studies should identify the reason for the reduced number of receptors detectable using western blot after TBI (e.g. Are they being down-regulated? Is the protein itself being damaged/altered by injury?). Moreover, exploring additional time points and brain regions will enhance our understanding of these MEL receptor changes. For example, the exclusion of behavioral endpoints represents a significant limitation in this study and although CCI-exposed animals had lower hippocampal and cortical levels of MT1 and MT2 at 24 hours post-injury, this may not result in changes in symptom profiles.
One important behavioral endpoint worth examining in future studies is sleep. Further inquiry should seek to relate the findings of the present study to sleep-related outcomes after TBI, considering melatonin’s well-established release following diurnal rhythms as well as its’ critical role in maintaining circadian rhythms across the phylogenetic tree [42–49]. Moreover, the relationship between MT1 and MT2 protein expression/levels in biological fluids and circadian rhythms as well as sleep-wake cycles has also been reported [50–52] as have the utility of melatonin receptor agonists at improving sleep-related outcomes [53–57]. Taken together, existing evidence suggests that the findings of reduced abundance of melatonin receptors after TBI in this study may alter sleep-related outcomes that were unstudied in the present pilot project. This is especially of interest, consider TBI is known to result in altered circadian rhythms [58] as well as sleep-related problems including but not limited to insomnia, hypersomnia, altered sleep timing, difficulty maintaining sleep, sleep-disordered breathing, and nightmares [12,59–66] that may be due in part to changes within the melatonergic system, especially the receptors, that are yet to be characterized.
There are also some practical limitations of this study that should be acknowledged. For example, whole cell lysates were generated, the supernatant fluid collected, and the resulting pellet disposed of. Thus, it was not possible to use tissue subcellular fractionation techniques with differential centrifugation to evaluate the membrane and nuclear MEL receptors in isolation [67]. A related consideration is that while the use of actin to normalize comparisons of melatonin receptor concentrations between sham and injured animals is consistent with most western blot research, the method has limitations. Specifically, there can be regional changes in the ratio of cell types that could confound the interpretation of findings in this and other studies. For example, in a situation characterized by neuronal death accompanied by increased gliosis, actin levels in total protein would appear unchanged, but fail to account for the loss of cells that would otherwise express MT1 and/or MT2 within injured brain regions. Not only would this obscure the ability to accurately interpret the cause of melatonin receptor loss, it would also complicate attempts to therapeutically target these changes.
Conclusions
This study is the first to demonstrate that MEL receptors are affected by TBI. Specifically, time point- and region-specific decreases in both MT1 and MT2 levels occurred after TBI. Replication of these results is necessary using more diverse pre-clinical samples (e.g. other strains/species, females, older/younger animals) and studies with additional cellular and behavioral endpoints. Clinical research exploring the effects of TBI on MEL receptors and trialing the effects of therapeutic MEL may be warranted. Overall, the results of this study, along with the existing literature, suggest the melatonergic system is implicated in TBI pathology and/or recovery and is worth further study.
Acknowledgments
This work was supported by the National Institutes of Health [T32NR009759, F31NR014957]; The Copeland Award of The Pittsburgh Foundation; the International Society of Nurses in Genetics; The Neurotrauma Nursing Foundation/American Association of Neuroscience Nursing; and Sigma Theta Tau International Eta Chapter. In addition, we would like to thank Michael Farmer for his assistance with the figures.
References
- 1.Faul M, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Centers Dis Control Prev Natl Cent Inj Prev Control. 2010 http://www.cdc.gov/traumaticbraininjury/pdf/blue_book.pdf.
- 2.Colantonio A, Saverino C, Zagorski B, Swaine B, Lewko J, Jaglal S, Vernich L. Hospitalizations and emergency department visits for TBI in Ontario. Can J Neurol Sci. 2010;37:783–790. doi: 10.1017/s0317167100051441. N17W524410H20708 [pii] [DOI] [PubMed] [Google Scholar]
- 3.Coronado VG, McGuire LC, Faul M. Sugarman, Pearson, The Epidemiology and Prevention of TBI. 2012 [Google Scholar]
- 4.Corrigan JD, Selassie AW, Orman JA. The epidemiology of traumatic brain injury. J Head Trauma Rehabil. 2010;25:72–80. doi: 10.1097/HTR.0b013e3181ccc8b4. [DOI] [PubMed] [Google Scholar]
- 5.Gold EM, Su D, López-Velázquez L, Haus DL, Perez H, Lacuesta GA, Anderson AJ, Cummings BJ. Functional assessment of long-term deficits in rodent models of traumatic brain injury. Regen Med. 2013;8:483–516. doi: 10.2217/rme.13.41. [DOI] [PubMed] [Google Scholar]
- 6.Osier N, Carlson SW, DeSana AJ, Dixon CE. Chronic Histopathological and Behavioral Outcomes of Experimental Traumatic Brain Injury in Adult Male Animals. J Neurotrauma. 2014;1882:1–85. doi: 10.1089/neu.2014.3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kline AE, Leary JB, Radabaugh HL, Cheng JP, Bondi CO. Combination therapies for neurobehavioral and cognitive recovery after experimental traumatic brain injury: Is more better? Prog Neurobiol. 2016 doi: 10.1016/j.pneurobio.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nau R, Sorgel F, Eiffert H. Penetration of Drugs through the Blood-Cerebrospinal Fluid/Blood-Brain Barrier for Treatment of Central Nervous System Infections. Clin Microbiol Rev. 2010;23:858–883. doi: 10.1128/CMR.00007-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Bars D, Thivolle P, Vitte PA, Bojkowski C, Chazot G, Arendt J, Frackowiak RS, Claustrat B. PET and plasma pharmacokinetic studies after bolus intravenous administration of [11C]melatonin in humans. Int J Rad Appl Instrum B. 1991;18:357–62. doi: 10.1016/0883-2897(91)90132-5. [DOI] [PubMed] [Google Scholar]
- 10.Wiechmann AF, Chignell CF, Roberts JE. Influence of dietary melatonin on photoreceptor survival in the rat retina: an ocular toxicity study. Exp Eye Res. 2008;86:241–50. doi: 10.1016/j.exer.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seifman MA, Adamides AA, Nguyen PN, Vallance Sa, Cooper DJ, Kossmann T, Rosenfeld JV, Morganti-Kossmann MC. Endogenous melatonin increases in cerebrospinal fluid of patients after severe traumatic brain injury and correlates with oxidative stress and metabolic disarray. J Cereb Blood Flow Metab. 2008;28:684–96. doi: 10.1038/sj.jcbfm.9600603. [DOI] [PubMed] [Google Scholar]
- 12.Shekleton JA, Parcell DL, Redman JR, Phipps-Nelson J, Ponsford JL, Rajaratnam SMW. Sleep disturbance and melatonin levels following traumatic brain injury. Neurology. 2010;74:1732–8. doi: 10.1212/WNL.0b013e3181e0438b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babaee A, Eftekhar-Vaghefi SH, Asadi-Shekaari M, Shahrokhi N, Soltani SD, Malekpour-Afshar R, Basiri M. Melatonin treatment reduces astrogliosis and apoptosis in rats with traumatic brain injury. Iran J Basic Med Sci. 2015;18:867–72. [PMC free article] [PubMed] [Google Scholar]
- 14.Kelestemur T, Yulug B, Caglayan AB, Beker MC, Kilic U, Caglayan B, Yalcin E, Gundogdu RZ, Kilic E. Targeting different pathophysiological events after traumatic brain injury in mice: Role of melatonin and memantine. Neurosci Lett. 2016;612:92–97. doi: 10.1016/j.neulet.2015.11.043. [DOI] [PubMed] [Google Scholar]
- 15.Jadhav V, Lee S, Ayer RE, Rojas H, Hyong A, Lekic T, Tang J, Zhang JH, Jadhav V, Ayer RE, Rojas H, Hyong A, Lekic T, Tang J, Zhang JH, Lee S, Ayer RE, Rojas H, Hyong A, Lekic T, Tang J, Zhang JH. Dual effects of melatonin on oxidative stress after surgical brain injury in rats. J Pineal Res. 2009;46:43–8. doi: 10.1111/j.1600-079X.2008.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campolo M, Ahmad A, Crupi R, Impellizzeri D, Morabito R, Esposito E, Cuzzocrea S. Combination therapy with melatonin and dexamethasone in a mouse model of traumatic brain injury. J Endocrinol. 2013;217:291–301. doi: 10.1530/JOE-13-0022. [DOI] [PubMed] [Google Scholar]
- 17.Tamura H, Takasaki A, Taketani T, Tanabe M, Kizuka F, Lee L, Tamura I, Maekawa R, Asada H, Yamagata Y, Sugino N. Melatonin as a free radical scavenger in the ovarian follicle. Endocr J. 2013;60:1–13. doi: 10.1507/endocrj.ej12-0263. [DOI] [PubMed] [Google Scholar]
- 18.Lin C, Chao H, Li Z, Xu X, Liu Y, Hou L, Liu N, Ji J. Melatonin Attenuates Traumatic Brain Injury-induced Inflammation: A Possible Role for Mitophagy. J Pineal Res. 2016 doi: 10.1111/jpi.12337. [DOI] [PubMed] [Google Scholar]
- 19.Wang S, Tian Y, Song L, Lim G, Tan Y, You Z, Chen L, Mao J. Exacerbated mechanical hyperalgesia in rats with genetically predisposed depressive behavior: role of melatonin and NMDA receptors. Pain. 2012;153:2448–57. doi: 10.1016/j.pain.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kokkola T, Vaittinen M, Laitinen JT. Inverse agonist exposure enhances ligand binding and G protein activation of the human MT1 melatonin receptor, but leads to receptor down-regulation. J Pineal Res. 2007;43:255–62. doi: 10.1111/j.1600-079X.2007.00470.x. [DOI] [PubMed] [Google Scholar]
- 21.Yakovlev AG, Ota K, Wang G, Movsesyan V, Bao WL, Yoshihara K, Faden AI. Differential expression of apoptotic protease-activating factor-1 and caspase-3 genes and susceptibility to apoptosis during brain development and after traumatic brain injury. J Neurosci. 2001;21:7439–46. doi: 10.1523/JNEUROSCI.21-19-07439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandhir R, Berman NEJ. Age-dependent response of CCAAT/enhancer binding proteins following traumatic brain injury in mice. Neurochem Int. 2010;56:188–93. doi: 10.1016/j.neuint.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mannix RC, Zhang J, Park J, Zhang X, Bilal K, Walker K, Tanzi RE, Tesco G, Whalen MJ. Age-dependent effect of apolipoprotein E4 on functional outcome after controlled cortical impact in mice. J Cereb Blood Flow Metab. 2011;31:351–61. doi: 10.1038/jcbfm.2010.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor SR, Smith C, Harris BT, Costine BA, Duhaime AC. Maturation-dependent response of neurogenesis after traumatic brain injury in children. J Neurosurg Pediatr. 2013;12:545–54. doi: 10.3171/2013.8.PEDS13154. [DOI] [PubMed] [Google Scholar]
- 25.Budinich CS, Chen H, Lowe D, Rosenberger JG, Bernstock JD, McCabe JT. Mouse Brain PSA-NCAM Levels Are Altered by Graded-Controlled Cortical Impact Injury. Neural Plast. 2012;2012:378307. doi: 10.1155/2012/378307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang G, Qian P, Xu Z, Zhang J, Wang Y, Cheng S, Cai W, Qian G, Wang C, DeCoster MA. Regulatory effects of the JAK3/STAT1 pathway on the release of secreted phospholipase A2-IIA in microvascular endothelial cells of the injured brain. J Neuroinflammation. 2012;9:170. doi: 10.1186/1742-2094-9-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borán MS, García A. The cyclic GMP-protein kinase G pathway regulates cytoskeleton dynamics and motility in astrocytes. J Neurochem. 2007;102:216–230. doi: 10.1111/j.1471-4159.2007.04464.x. [DOI] [PubMed] [Google Scholar]
- 28.Beni SM, Kohen R, Reiter RJ, Tan DX, Shohami E. Melatonin-induced neuroprotection after closed head injury is associated with increased brain antioxidants and attenuated late-phase activation of NF-kappaB and AP-1. FASEB J. 2004;18:149–51. doi: 10.1096/fj.03-0323fje. [DOI] [PubMed] [Google Scholar]
- 29.Yürüker V, Naz M, Nilgün Ş. Reduction in traumatic brain injury-induced oxidative stress, apoptosis, and calcium entry in rat hippocampus by melatonin: Possible involvement of TRPM2 channels. 2014 doi: 10.1007/s11011-014-9623-3. [DOI] [PubMed] [Google Scholar]
- 30.Kelso ML, Scheff NN, Scheff SW, Pauly JR. Melatonin and minocycline for combinatorial therapy to improve functional and histopathological deficits following traumatic brain injury. Neurosci Lett. 2011;488:60–4. doi: 10.1016/j.neulet.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cirak B, Rousan N, Kocak A, Palaoglu O, Palaoglu S, Kilic K. Melatonin as a free radical scavenger in experimental head trauma. Pediatr Neurosurg. 1999;31:298–301. doi: 10.1159/000028879. 28879. [DOI] [PubMed] [Google Scholar]
- 32.Ozdemir D, Uysal N, Gonenc S, Acikgoz O, Sonmez A, Topcu A, Ozdemir N, Duman M, Semin I, Ozkan H. Effect of melatonin on brain oxidative damage induced by traumatic brain injury in immature rats. Physiol Res. 2005;54:631–7. [PubMed] [Google Scholar]
- 33.Ates O, Cayli S, Gurses I, Yucel N, Iraz M, Altinoz E, Kocak A, Yologlu S. Effect of pinealectomy and melatonin replacement on morphological and biochemical recovery after traumatic brain injury. Int J Dev Neurosci. 2006;24:357–63. doi: 10.1016/j.ijdevneu.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Dehghan F, Khaksari Hadad M, Asadikram G, Najafipour H, Shahrokhi N. Effect of melatonin on intracranial pressure and brain edema following traumatic brain injury: role of oxidative stresses. Arch Med Res. 2013;44:251–8. doi: 10.1016/j.arcmed.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Senol N, Nazıroğlu M. Melatonin reduces traumatic brain injury-induced oxidative stress in the cerebral cortex and blood of rats. Neural Regen Res. 2014;9:1112–6. doi: 10.4103/1673-5374.135312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding K, Wang H, Xu J, Li T, Zhang L, Ding Y, Zhu L, He J, Zhou M. Melatonin stimulates antioxidant enzymes and reduces oxidative stress in experimental traumatic brain injury: The Nrf2-ARE signaling pathway as a potential mechanism. Free Radic Biol Med. 2014;73:1–11. doi: 10.1016/j.freeradbiomed.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 37.Natarajan R, Einarsdottir E, Riutta A, Hagman S, Raunio M, Mononen N, Lehtimäki T, Elovaara I. Melatonin pathway genes are associated with progressive subtypes and disability status in multiple sclerosis among Finnish patients. J Neuroimmunol. 2012;250:106–10. doi: 10.1016/j.jneuroim.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 38.Chambers JC, Zhang W, Zabaneh D, Sehmi J, Jain P, McCarthy MI, Froguel P, Ruokonen A, Balding D, Jarvelin MR, Scott J, Elliott P, Kooner JS. Common Genetic Variation Near Melatonin Receptor MTNR1B Contributes to Raised Plasma Glucose and Increased Risk of Type 2 Diabetes Among Indian Asians and European Caucasians. Diabetes. 2009;58:2703–2708. doi: 10.2337/db08-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ratcliff JJ, Greenspan AI, Goldstein FC, Stringer AY, Bushnik T, Hammond FM, Novack TA, Whyte J, Wright DW. Gender and traumatic brain injury: do the sexes fare differently? Brain Inj. 2007;21:1023–30. doi: 10.1080/02699050701633072. [DOI] [PubMed] [Google Scholar]
- 40.Slewa-Younan S, Green AM, Baguley IJ, Gurka JA, Marosszeky JE. Sex differences in injury severity and outcome measures after traumatic brain injury. Arch Phys Med Rehabil. 2004;85:376–379. doi: 10.1016/j.apmr.2003.05.007. [DOI] [PubMed] [Google Scholar]
- 41.O’Connor CA, Cernak I, Johnson F, Vink R. Effects of progesterone on neurologic and morphologic outcome following diffuse traumatic brain injury in rats. Exp Neurol. 2007;205:145–53. doi: 10.1016/j.expneurol.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 42.Reiter R. The melatonin rhythm: both a clock and a calendar. Experientia. 1993;49:654–664. doi: 10.1007/BF01923947. [DOI] [PubMed] [Google Scholar]
- 43.Pandi-Perumal SR, Srinivasan V, Maestroni GJM, Cardinali DP, Poeggeler B, Hardeland R. Melatonin: Nature’s most versatile biological signal? FEBS J. 2006;273:2813–38. doi: 10.1111/j.1742-4658.2006.05322.x. [DOI] [PubMed] [Google Scholar]
- 44.Solis-Chagoyan H, Mendoza-Vargas L, Fuentes-Pardo B. Melatonin modulates the ERG circadian rhythm in crayfish. Comp Biochem Physiol A Mol Integr Physiol. 2008;149:373–379. doi: 10.1016/j.cbpa.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 45.Yellon SM, Tamarkin L, Pratt BL, Goldman BD. Pineal melatonin in the Djungarian hamster: photoperiodic regulation of a circadian rhythm. Endocrinology. 1982;111:488–492. doi: 10.1210/endo-111-2-488. [DOI] [PubMed] [Google Scholar]
- 46.Wetterberg L, Hayes DK, Halberg F. Circadian rhythm of melatonin in the brain of the face fly, Musca autumnalis De Geer. Chronobiologia. 1987;14:377–381. [PubMed] [Google Scholar]
- 47.Murakami N, Kawano T, Nakahara K, Nasu T, Shiota K. Effect of melatonin on circadian rhythm, locomotor activity and body temperature in the intact house sparrow, Japanese quail and owl. Brain Res. 2001;889:220–224. doi: 10.1016/s0006-8993(00)03205-4. [DOI] [PubMed] [Google Scholar]
- 48.Miranda-Anaya M, Bartell PA, Menaker M. Circadian rhythm of iguana electroretinogram: the role of dopamine and melatonin. J Biol Rhythms. 2002;17:526–538. doi: 10.1177/0748730402238235. [DOI] [PubMed] [Google Scholar]
- 49.Kennaway DJ, Voultsios A. Circadian rhythm of free melatonin in human plasma. J Clin Endocrinol Metab. 1998;83:1013–1015. doi: 10.1210/jcem.83.3.4636. [DOI] [PubMed] [Google Scholar]
- 50.Masana MI, Benloucif S, Dubocovich ML. Circadian rhythm of mt1 melatonin receptor expression in the suprachiasmatic nucleus of the C3H/HeN mouse. J Pineal Res. 2000;28:185–192. doi: 10.1034/j.1600-079x.2001.280309.x. [DOI] [PubMed] [Google Scholar]
- 51.Dubocovich M, Rivera-Bermudez M, Gerdin M, Masana M. Molecular pharmacology, regulation and function of mammalian melatonin receptors. Front Biosci. 2003;8:d1093–108. doi: 10.2741/1089. [DOI] [PubMed] [Google Scholar]
- 52.Harrington M. Specific melatonin receptor promotes a deeper sleep. Lab Anim (NY) 2012;41:31. doi: 10.1038/laban0212-31b. [DOI] [PubMed] [Google Scholar]
- 53.Roth T, Stubbs C, Walsh JK. Ramelteon (TAK-375), a selective MT1/MT2-receptor agonist, reduces latency to persistent sleep in a model of transient insomnia related to a novel sleep environment. Sleep. 2005;28:303–307. [PubMed] [Google Scholar]
- 54.Miyamoto M. Pharmacology of ramelteon, a selective MT1/MT2 receptor agonist: a novel therapeutic drug for sleep disorders. CNS Neurosci Ther. 2009;15:32–51. doi: 10.1111/j.1755-5949.2008.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fisher SP, Sugden D. Sleep-promoting action of IIK7, a selective MT2 melatonin receptor agonist in the rat. Neurosci Lett. 2009;457:93–96. doi: 10.1016/j.neulet.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ochoa-Sanchez R, Comai S, Lacoste B, Bambico FR, Dominguez-Lopez S, Spadoni G, Rivara S, Bedini A, Angeloni D, Fraschini F, Mor M, Tarzia G, Descarries L, Gobbi G. Promotion of non-rapid eye movement sleep and activation of reticular thalamic neurons by a novel MT2 melatonin receptor ligand. J Neurosci. 2011;31:18439–18452. doi: 10.1523/JNEUROSCI.2676-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yanagihara M, Nakamura M, Usui A, Nishida S, Ito E, Okawa M, Inoue Y. The melatonin receptor agonist is effective for free-running type circadian rhythm sleep disorder: case report on two sighted patients. Tohoku J Exp Med. 2014;234:123–128. doi: 10.1620/tjem.234.123. [DOI] [PubMed] [Google Scholar]
- 58.Grima NA, Ponsford JL, St Hilaire MA, Mansfield D, Rajaratnam SM. Circadian Melatonin Rhythm Following Traumatic Brain Injury. Neurorehabil Neural Repair. 2016 doi: 10.1177/1545968316650279. [DOI] [PubMed] [Google Scholar]
- 59.Hou L, Han X, Sheng P, Tong W, Li Z, Xu D, Yu M, Huang L, Zhao Z, Lu Y, Dong Y. Risk factors associated with sleep disturbance following traumatic brain injury: clinical findings and questionnaire based study. PLoS One. 2013;8:e76087. doi: 10.1371/journal.pone.0076087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Theadom A, Cropley M, Parmar P, Barker-Collo S, Starkey N, Jones K, Feigin VL BIONIC Research Group. Sleep difficulties one year following mild traumatic brain injury in a population-based study. Sleep Med. 2015;16:926–32. doi: 10.1016/j.sleep.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 61.Castriotta RJ, Wilde MC, Lai JM, Atanasov S, Masel BE, Kuna ST. Prevalence and consequences of sleep disorders in traumatic brain injury. J Clin Sleep Med. 2007;3:349–56. [PMC free article] [PubMed] [Google Scholar]
- 62.Duclos C, Dumont M, Wiseman-Hakes C, Arbour C, Mongrain V, Gaudreault PO, Khoury S, Lavigne G, Desautels A, Gosselin N. Sleep and wake disturbances following traumatic brain injury. Pathol Biol (Paris) 2014;62:252–61. doi: 10.1016/j.patbio.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 63.Ayalon L, Borodkin K, Dishon L, Kanety H, Dagan Y. Circadian rhythm sleep disorders following mild traumatic brain injury. Neurology. 2007;68:1136–40. doi: 10.1212/01.wnl.0000258672.52836.30. [DOI] [PubMed] [Google Scholar]
- 64.Steele DL, Rajaratnam SMW, Redman JR, Ponsford JL. The effect of traumatic brain injury on the timing of sleep. Chronobiol Int. 2005;22:89–105. doi: 10.1081/cbi-200042428. [DOI] [PubMed] [Google Scholar]
- 65.Moeller DR, Duffey JM, Goolsby AM, Gallimore JT. Use of a Removable Mandibular Neuroprosthesis for the Reduction of Posttraumatic Stress Disorder (PTSD) and Mild Traumatic Brain Injury/PTSD/Associated Nightmares, Headaches, and Sleep Disturbances. J Spec Oper Med a Peer Rev J SOF Med Prof. 2014;14:64–73. doi: 10.55460/MHVO-MN5Q. [DOI] [PubMed] [Google Scholar]
- 66.Farrell-Carnahan L, Barnett S, Lamberty G, Hammond FM, Kretzmer TS, Franke LM, Geiss M, Howe L, Nakase-Richardson R. Insomnia symptoms and behavioural health symptoms in veterans 1 year after traumatic brain injury. Brain Inj. 2015;29:1400–1408. doi: 10.3109/02699052.2015.1063161. [DOI] [PubMed] [Google Scholar]
- 67.Carrillo-Vico A, García-Pergañeda A, Naji L, Calvo JR, Romero MP, Guerrero JM. Expression of membrane and nuclear melatonin receptor mRNA and protein in the mouse immune system. Cell Mol Life Sci. 2003;60:2272–8. doi: 10.1007/s00018-003-3207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]